Induction of Sperm DNA Fragmentation by Cryopreservation and In Vitro Incubation: Comparison of TUNEL, SCSA, SCD Test and COMET Assay
Abstract
1. Introduction
2. Results
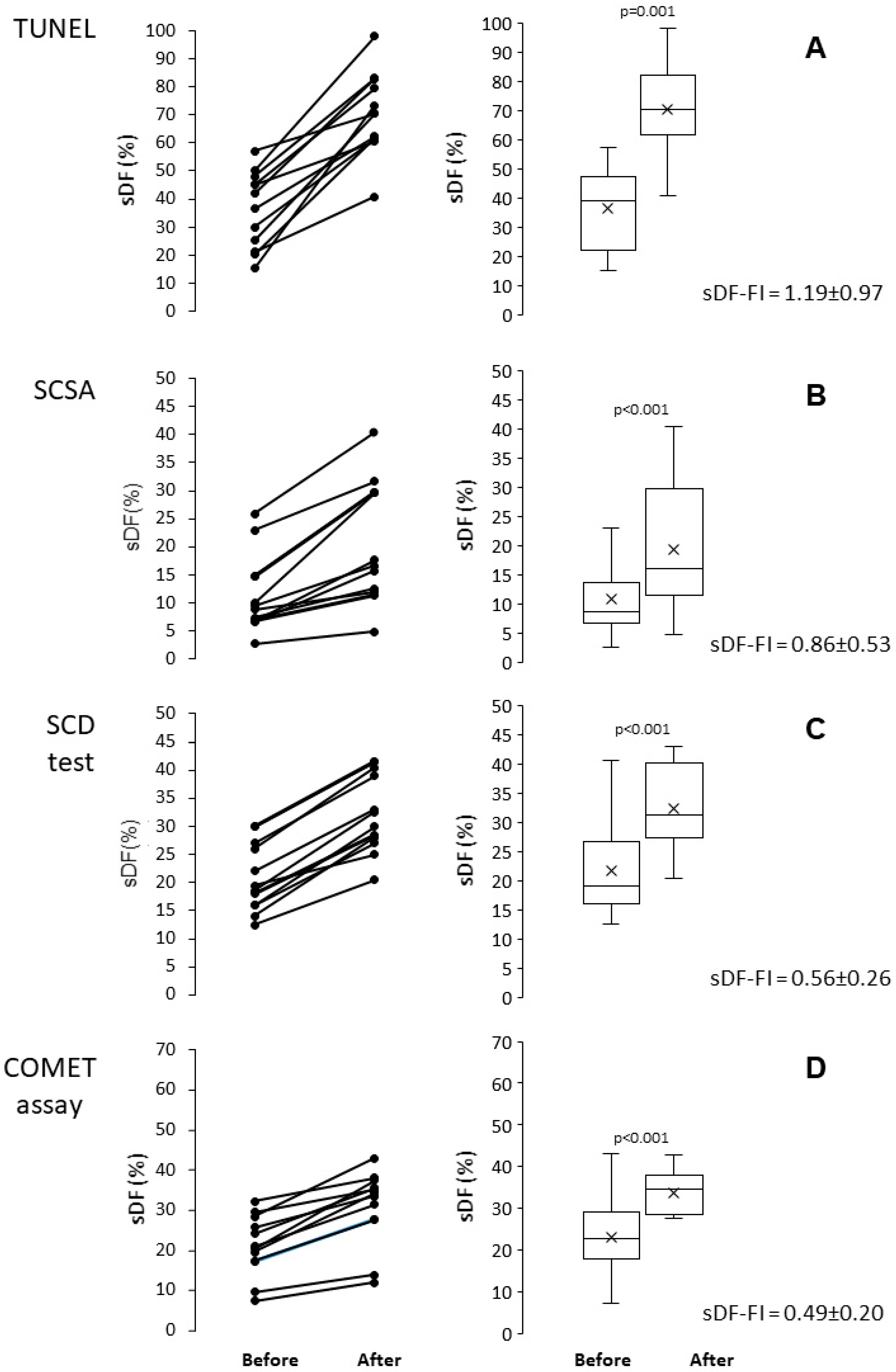
2.1. Induction of sDF by Cryopreservation
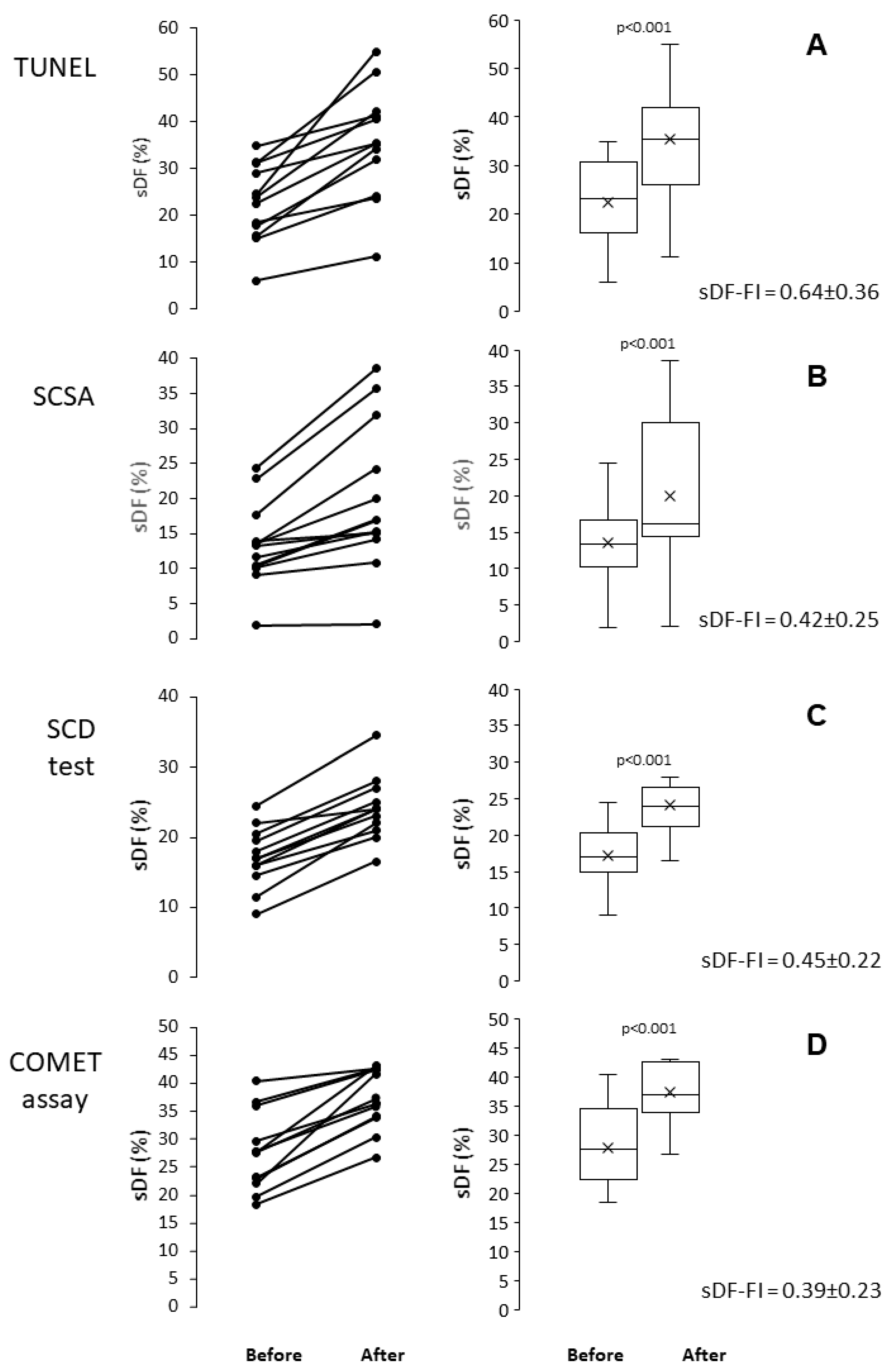
2.2. Induction of sDF by In Vitro Incubation
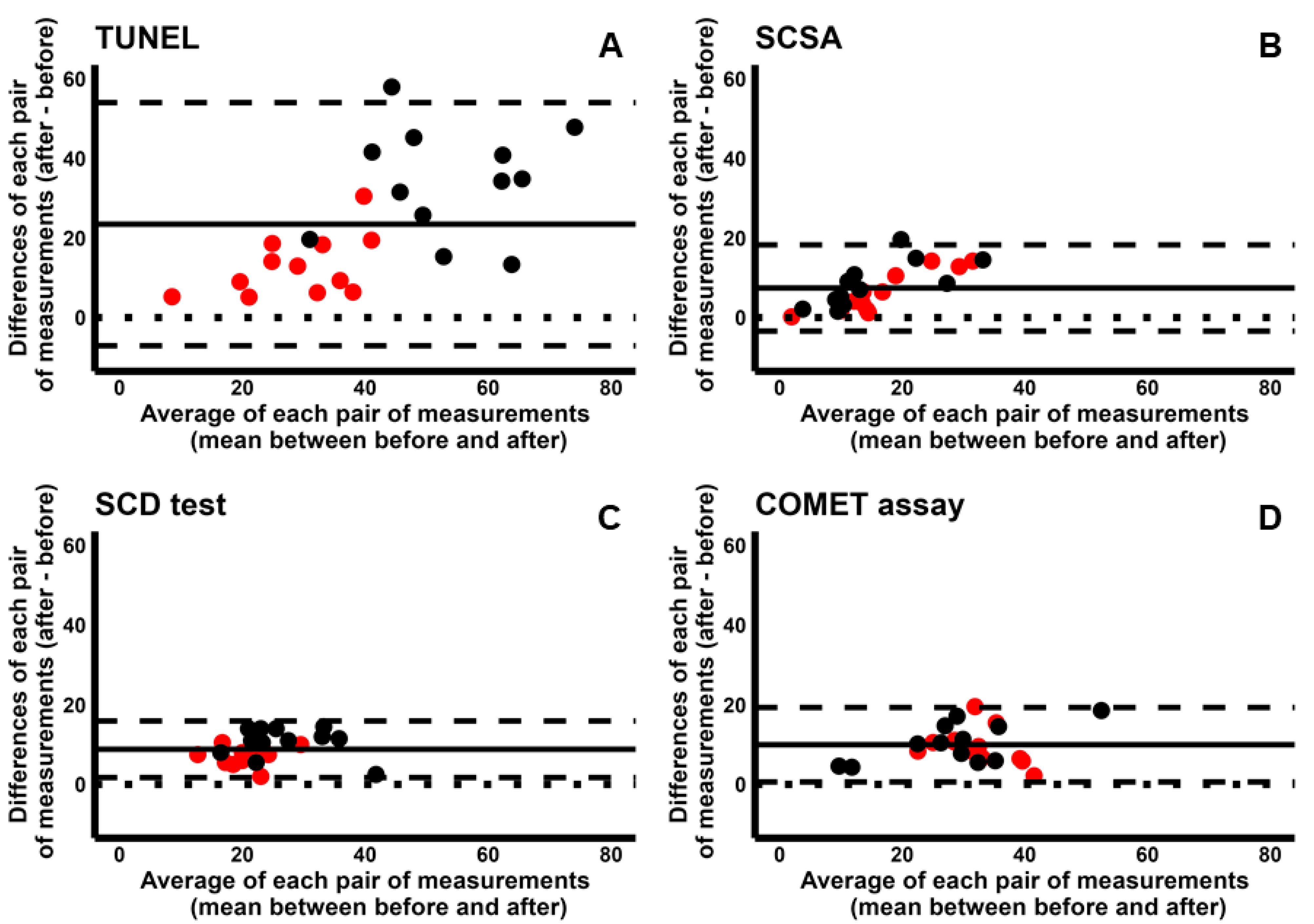
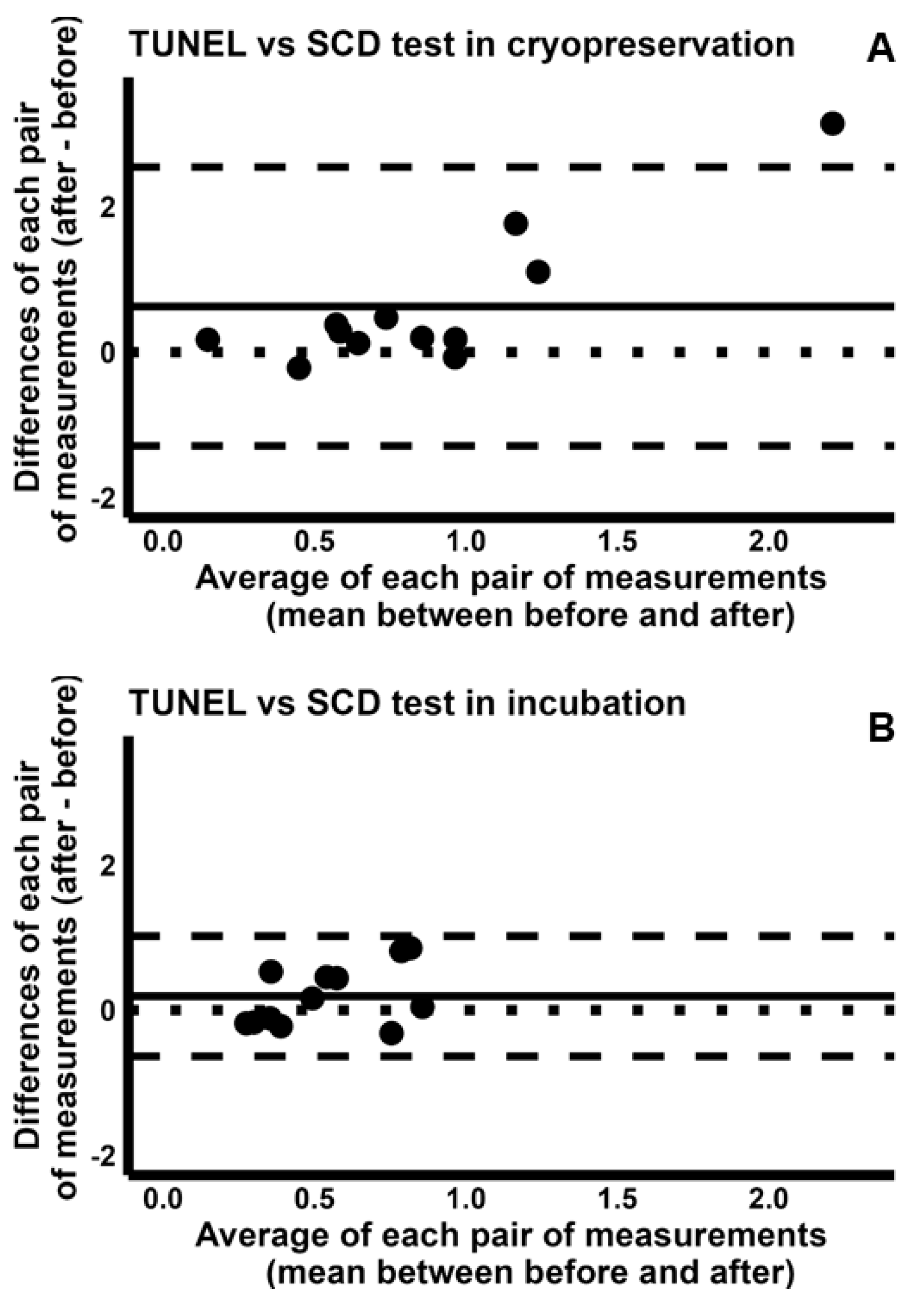
2.3. Comparison Between Cryopreservation and In Vitro Incubation
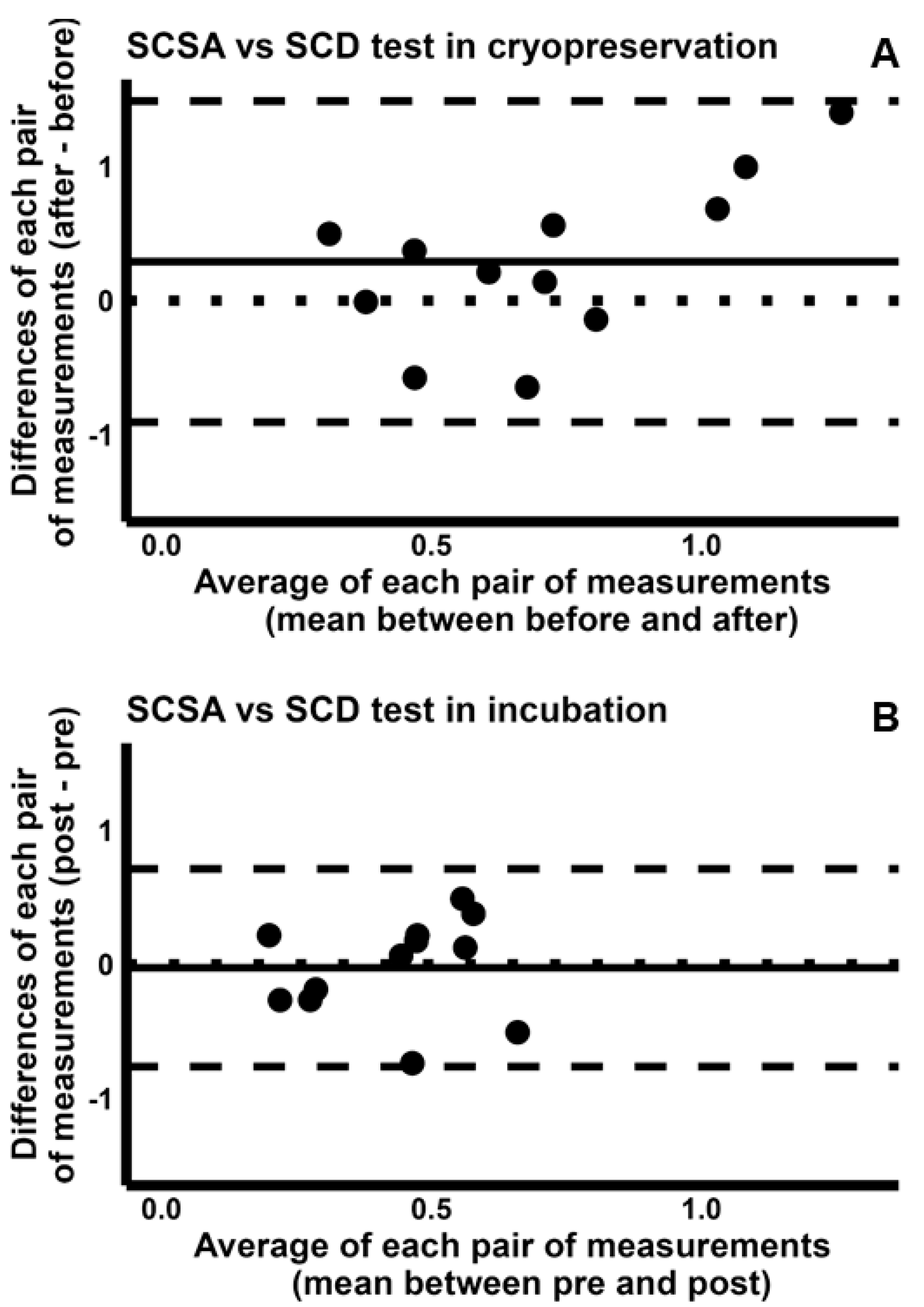
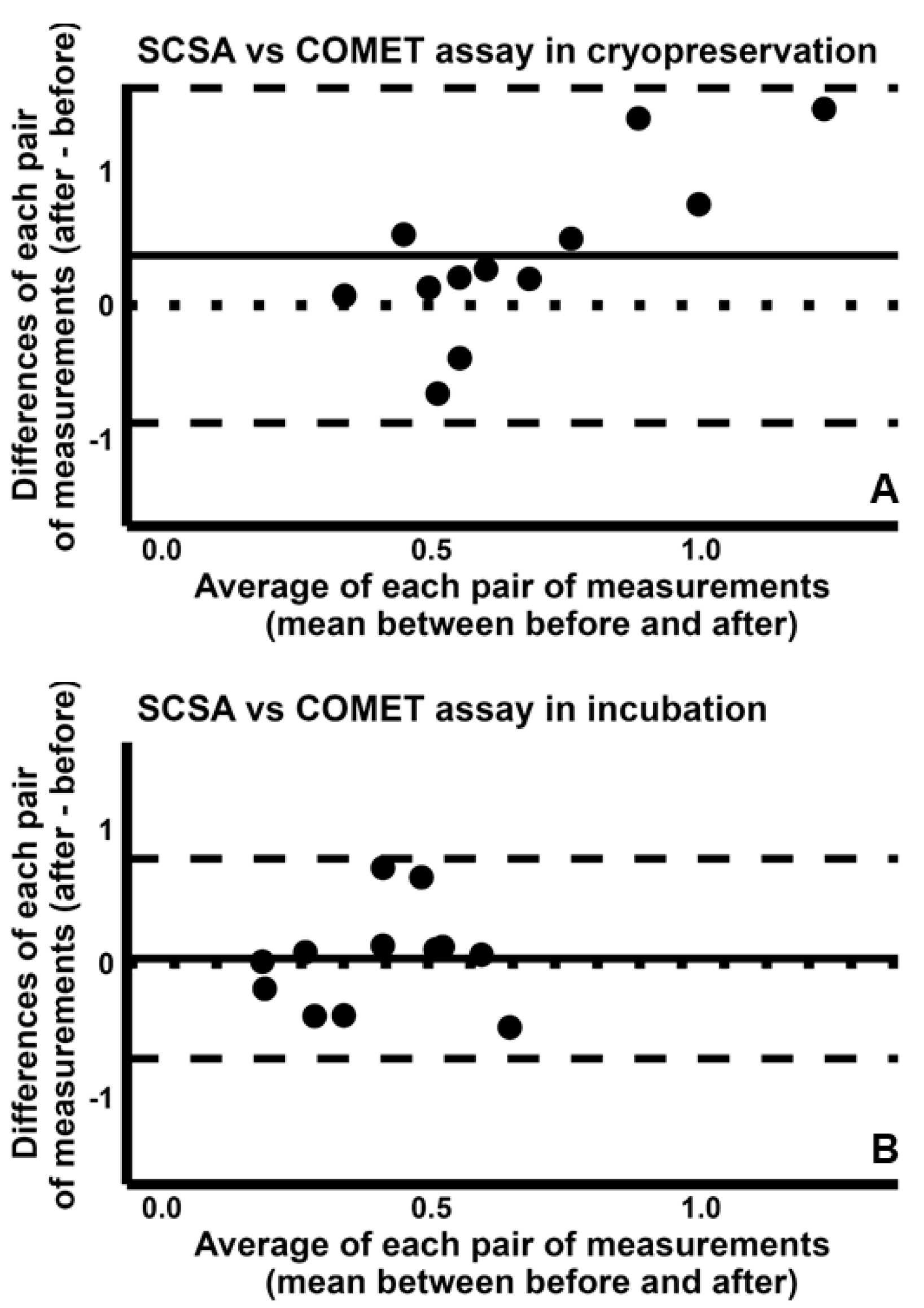
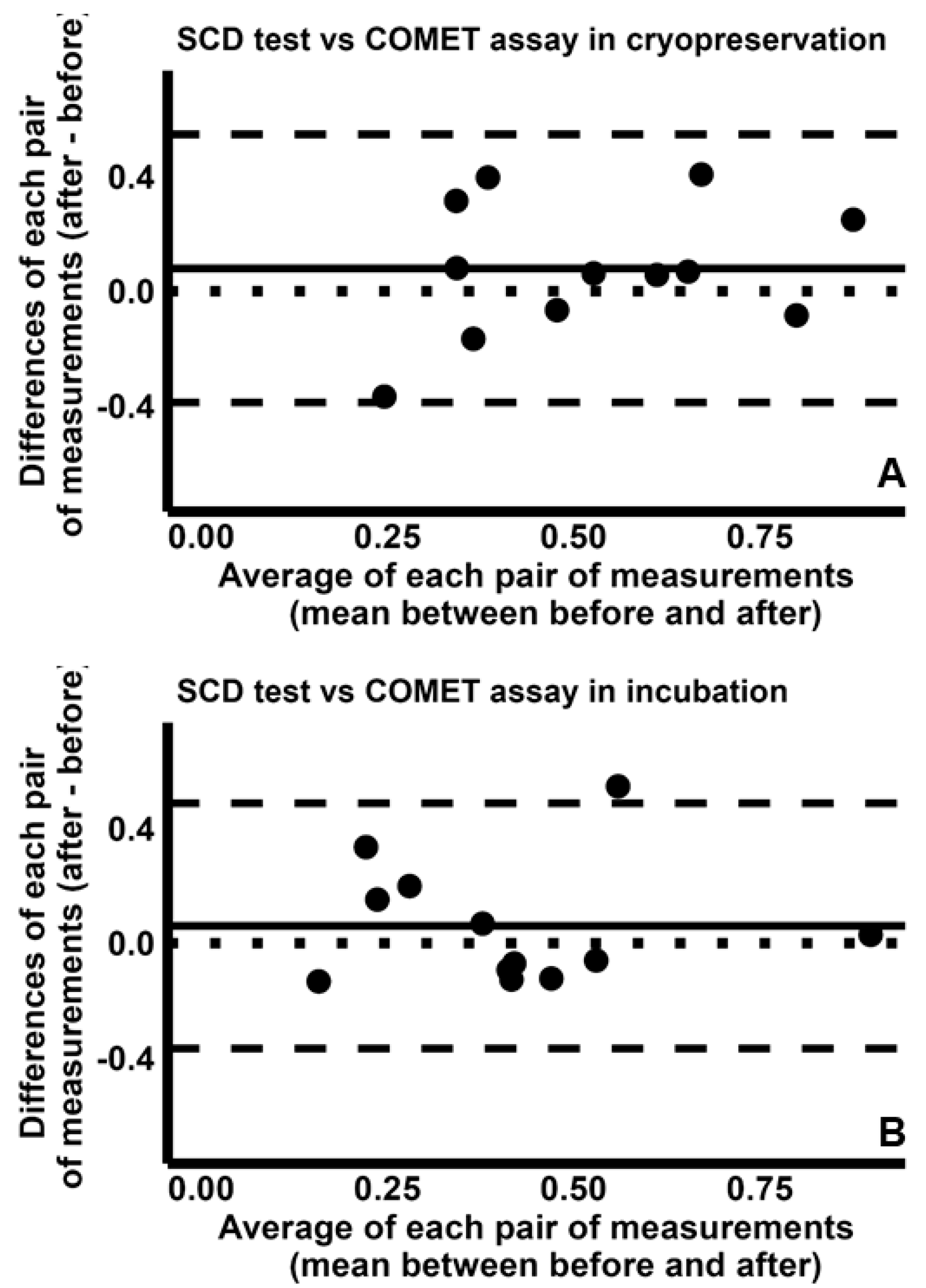
2.4. Pairwise Comparison Among the Four Tests
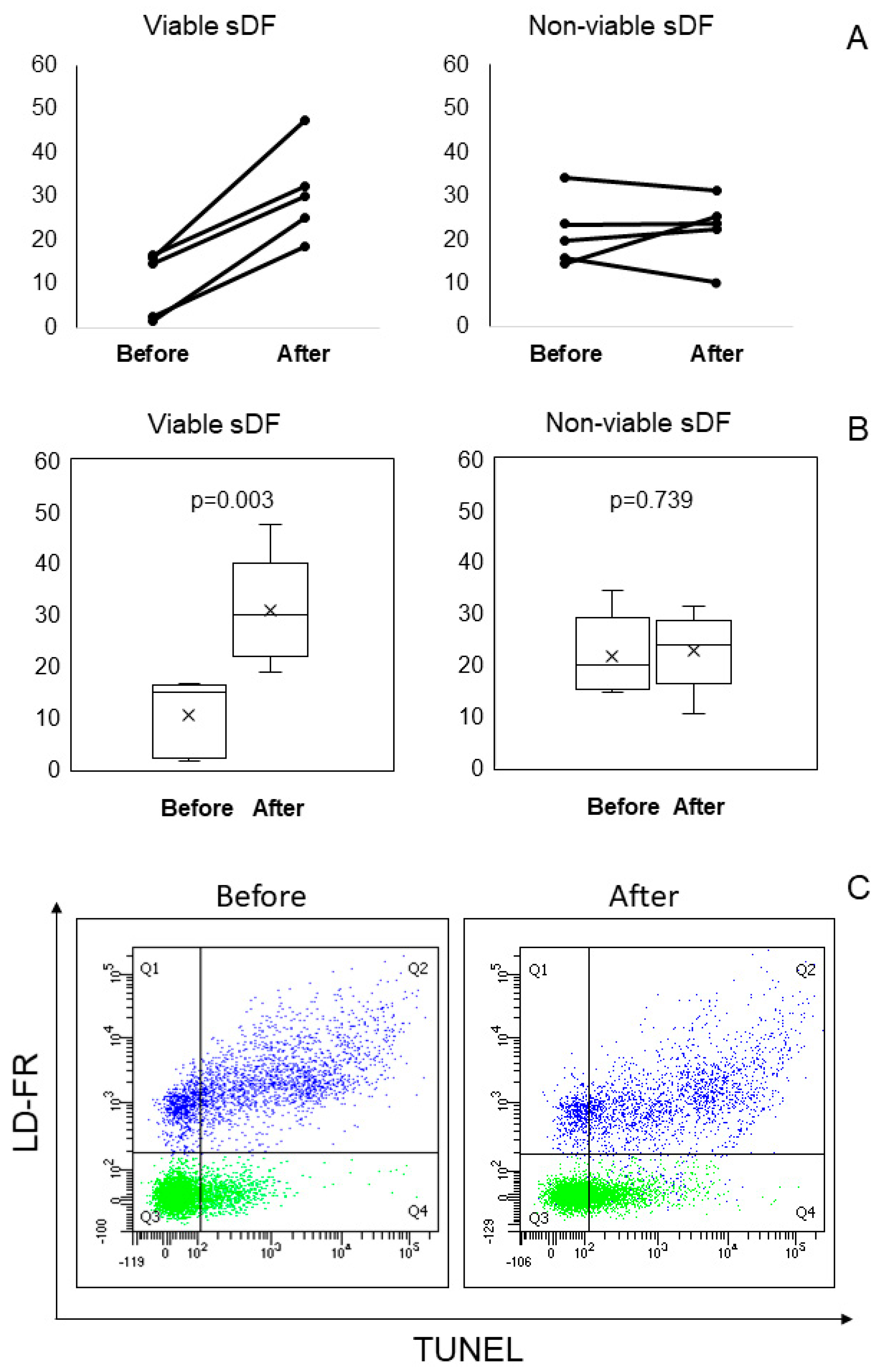
2.5. LiveTUNEL Before and After Cryopreservation
3. Discussion
4. Materials and Methods
4.1. Reagents and Media
4.2. Sample Collection
4.3. Cryopreservation
4.4. In Vitro Incubation
4.5. TUNEL
4.6. SCSA
4.7. SCD Test
4.8. COMET Assay
4.9. LiveTUNEL
4.10. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Mindlis, I.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis. Hum. Reprod. Update 2017, 23, 646–659. [Google Scholar] [CrossRef]
- Levine, H.; Jørgensen, N.; Martino-Andrade, A.; Mendiola, J.; Weksler-Derri, D.; Jolles, M.; Pinotti, R.; Swan, S.H. Temporal Trends in Sperm Count: A Systematic Review and Meta-Regression Analysis of Samples Collected Globally in the 20th and 21st Centuries. Hum. Reprod. Update 2023, 29, 157–176. [Google Scholar] [CrossRef]
- Tournaye, H.; Krausz, C.; Oates, R.D. Novel Concepts in the Aetiology of Male Reproductive Impairment. Lancet Diabetes Endocrinol. 2017, 5, 544–553. [Google Scholar] [CrossRef]
- Santi, D.; Spaggiari, G.; Simoni, M. Sperm DNA Fragmentation Index as a Promising Predictive Tool for Male Infertility Diagnosis and Treatment Management—Meta-Analyses. Reprod. Biomed. Online 2018, 37, 315–326. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Cambi, M.; Lotti, F.; Natali, I.; Filimberti, E.; Noci, I.; Forti, G.; Maggi, M.; et al. DNA Fragmentation in Brighter Sperm Predicts Male Fertility Independently from Age and Semen Parameters. Fertil. Steril. 2015, 104, 582–590.e4. [Google Scholar] [CrossRef]
- Pu, Y.; Yan, L.; Lu, S.; Guo, Y.; Zhu, X. Sperm DNA Fragmentation Index with Unexplained Recurrent Spontaneous Abortion: A Systematic Review and Meta-Analysis. J. Gynecol. Obstet. Hum. Reprod. 2020, 49, 101740. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Liu, J.; Yuan, E.; Li, Y.; Shi, Y.; Zhang, L. Relationship Among Traditional Semen Parameters, Sperm DNA Fragmentation, and Unexplained Recurrent Miscarriage: A Systematic Review and Meta-Analysis. Front. Endocrinol. 2022, 12, 802632. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Zini, A.; Dyachenko, A.; Ciampi, A.; Carrell, D.T. A Systematic Review and Meta-Analysis to Determine the Effect of Sperm DNA Damage on in Vitro Fertilization and Intracytoplasmic Sperm Injection Outcome. Asian J. Androl. 2017, 19, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; Yeste, M.; Becerra-Tomás, N.; Aston, K.I.; James, E.R.; Salas-Huetos, A. Clinical Implications of Sperm DNA Damage in IVF and ICSI: Updated Systematic Review and Meta-Analysis. Biol. Rev. Camb. Philos. Soc. 2021, 96, 1284–1300. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination and Processing of Human Semen, 6th ed.; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Conti, D.; Calamai, C.; Muratori, M. Sperm DNA Fragmentation in Male Infertility: Tests, Mechanisms, Meaning and Sperm Population to Be Tested. J. Clin. Med. 2024, 13, 5309. [Google Scholar] [CrossRef]
- Muratori, M.; Pellegrino, G.; Mangone, G.; Azzari, C.; Lotti, F.; Tarozzi, N.; Boni, L.; Borini, A.; Maggi, M.; Baldi, E. DNA Fragmentation in Viable and Non-Viable Spermatozoa Discriminates Fertile and Subfertile Subjects with Similar Accuracy. J. Clin. Med. 2020, 9, 1341. [Google Scholar] [CrossRef]
- Aravindan, G.R.; Bjordahl, J.; Jost, L.K.; Evenson, D.P. Susceptibility of Human Sperm to in Situ DNA Denaturation Is Strongly Correlated with DNA Strand Breaks Identified by Single-Cell Electrophoresis. Exp. Cell Res. 1997, 236, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Ribas-Maynou, J.; García-Peiró, A.; Fernández-Encinas, A.; Abad, C.; Amengual, M.J.; Prada, E.; Navarro, J.; Benet, J. Comprehensive Analysis of Sperm DNA Fragmentation by Five Different Assays: TUNEL assay, SCSA, SCD test and Alkaline and Neutral Comet Assay. Andrology 2013, 1, 715–722. [Google Scholar] [CrossRef]
- Simon, L.; Liu, L.; Murphy, K.; Ge, S.; Hotaling, J.; Aston, K.I.; Emery, B.; Carrell, D.T. Comparative Analysis of Three Sperm DNA Damage Assays and Sperm Nuclear Protein Content in Couples Undergoing Assisted Reproduction Treatment. Hum. Reprod. 2014, 29, 904–917. [Google Scholar] [CrossRef] [PubMed]
- Javed, A.; Talkad, M.S.; Ramaiah, M.K. Evaluation of Sperm DNA Fragmentation Using Multiple Methods: A Comparison of Their Predictive Power for Male Infertility. Clin. Exp. Reprod. Med. 2019, 46, 14–21, Erratum in Clin. Exp. Reprod. Med. 2019, 46, 211. [Google Scholar] [CrossRef]
- Pérez-Cerezales, S.; Miranda, A.; Gutiérrez-Adán, A. Comparison of Four Methods to Evaluate Sperm DNA Integrity between Mouse Caput and Cauda Epididymidis. Asian J. Androl. 2012, 14, 335–337. [Google Scholar] [PubMed]
- Mohammadi, Z.; Tavalaee, M.; Gharagozloo, P.; Drevet, J.R.; Nasr-Esfahani, M.H. Could High DNA Stainability (HDS) Be a Valuable Indicator of Sperm Nuclear Integrity? Basic Clin. Androl. 2020, 30, 12. [Google Scholar] [CrossRef]
- Arciero, V.; Ammar, O.; Maggi, M.; Vignozzi, L.; Muratori, M.; Dabizzi, S. Vapour Fast Freezing with Low Semen Volumes Can Highly Improve Motility and Viability or DNA Quality of Cryopreserved Human Spermatozoa. Andrology 2022, 10, 1123–1133. [Google Scholar] [CrossRef]
- Riley, L.; Ammar, O.; Mello, T.; Giovannelli, L.; Vignozzi, L.; Muratori, M. Novel Methods to Detect ROS in Viable Spermatozoa of Native Semen Samples. Reprod. Toxicol. 2021, 106, 51–60. [Google Scholar] [CrossRef]
- Ortiz, I.; Urbano, M.; Dorado, J.; Morrell, J.M.; Al-Essawe, E.; Johannisson, A.; Hidalgo, M. Comparison of DNA Fragmentation of Frozen-Thawed Epididymal Sperm of Dogs Using Sperm Chromatin Structure Analysis and Sperm Chromatin Dispersion test. Anim. Reprod. Sci. 2017, 187, 74–78. [Google Scholar] [CrossRef]
- Kunkitti, P.; Sjödahl, A.; Bergqvist, A.S.; Johannisson, A.; Axnér, E. Comparison of DNA Fragmentation Assay in Frozen-Thawed Cat Epididymal Sperm. Reprod. Domest. Anim. 2016, 51, 618–622. [Google Scholar] [CrossRef] [PubMed]
- Serafini, R.; Love, C.C.; Coletta, A.; Mari, G.; Mislei, B.; Caso, C.; Di Palo, R. Sperm DNA Integrity in Frozen-Thawed Semen from Italian Mediterranean Buffalo Bulls and its Relationship to in Vivo Fertility. Anim. Reprod. Sci. 2016, 172, 26–31. [Google Scholar] [CrossRef] [PubMed]
- Lusignan, M.F.; Li, X.; Herrero, B.; Delbes, G.; Chan, P.T.K. Effects of Different Cryopreservation Methods on DNA Integrity and Sperm Chromatin Quality in Men. Andrology 2018, 6, 829–835. [Google Scholar] [CrossRef] [PubMed]
- West, R.; Coomarasamy, A.; Frew, L.; Hutton, R.; Kirkman-Brown, J.; Lawlor, M.; Lewis, S.; Partanen, R.; Payne-Dwyer, A.; Román-Montañana, C.; et al. Sperm Selection with Hyaluronic Acid Improved Live Birth Outcomes among Older Couples and Was Connected to Sperm DNA Quality, Potentially Affecting all Treatment Outcomes. Hum. Reprod. 2022, 37, 1106–1125. [Google Scholar] [CrossRef]
- Bucak, M.N.; Keskin, N.; Bodu, M.; Bülbül, B.; Kırbaş, M.; Öztürk, A.E.; Frootan, F.; İli, P.; Özkan, H.; Başpınar, N.; et al. Combination of Trehalose and Low Boron in Presence of Decreased Glycerol Improves Post-Thawed Ram Sperm Parameters: A Model Study in Boron Research. Andrology 2022, 10, 585–594. [Google Scholar] [CrossRef]
- Bucak, M.N.; Keskin, N.; Taşpınar, M.; Çoyan, K.; Başpınar, N.; Cenariu, M.C.; Bilgili, A.; Öztürk, C.; Kurşunlu, A.N. Raffinose and Hypotaurine Improve the Post-Thawed Merino Ram Sperm Parameters. Cryobiology 2013, 67, 34–39. [Google Scholar] [CrossRef]
- Öztürk, A.E.; Bodu, M.; Bucak, M.N.; Ağır, V.; Özcan, A.; Keskin, N.; İli, P.; Topraggaleh, T.R.; Sidal, H.; Başpınar, N.; et al. The Synergistic Effect of Trehalose and Low Concentrations of Cryoprotectants Can Improve Post-Thaw Ram Sperm Parameters. Cryobiology 2020, 95, 157–163. [Google Scholar] [CrossRef]
- Muratori, M.; Tamburrino, L.; Tocci, V.; Costantino, A.; Marchiani, S.; Giachini, C.; Laface, I.; Krausz, C.; Meriggiola, M.C.; Forti, G.; et al. Small Variations in Crucial Steps of TUNEL Assay Coupled to Flow Cytometry Greatly Affect Measures of Sperm DNA Fragmentation. J. Androl. 2010, 31, 336–345. [Google Scholar] [CrossRef]
- Ribeiro, S.C.; Muratori, M.; De Geyter, M.; De Geyter, C. TUNEL Labeling with BrdUTP/Anti-BrdUTP Greatly Underestimates the Level of Sperm DNA Fragmentation in Semen Evaluation. PLoS ONE 2017, 12, e0181802. [Google Scholar] [CrossRef]
- Smith, T.B.; Dun, M.D.; Smith, N.D.; Curry, B.J.; Connaughton, H.S.; Aitken, R.J. The Presence of a Truncated Base Excision Repair Pathway in Human Spermatozoa that Is Mediated by OGG1. J. Cell Sci. 2013, 126, 1488–1497. [Google Scholar] [CrossRef]
- Aitken, R.J.; Smith, T.B.; Jobling, M.S.; Baker, M.A.; De Iuliis, G.N. Oxidative Stress and Male Reproductive Health. Asian J. Androl. 2014, 16, 31–38. [Google Scholar] [CrossRef]
- Aitken, R.J.; Baker, M.A. Causes and consequences of apoptosis in spermatozoa; contributions to infertility and impacts on development. Int. J. Dev. Biol. 2013, 57, 265–272. [Google Scholar] [CrossRef]
- Mahmoodi, M.; Shojafar, E.; Dastjani-Farahani, M. Incubation of semen with human follicular fluid improves the antioxidant status and quality of spermatozoa after freezing-thawing. Reprod. Fertil. 2025, 6, e240056. [Google Scholar] [CrossRef]
- Zekri, S.; Bahiraei, R.; Ghezelayagh, Z.; Maghami, P.; Shahverdi, A.; Sabbagian, M.; Ebrahimi, B. Effects of 6-Gingerol Supplementation in Cryopreservation on Human Sperm Parameters, DNA Fragmentation, and Apoptosis Incidence. Urol. J. 2025, 22, 8352. [Google Scholar]
- Cui, Z.L.; Zheng, D.Z.; Liu, Y.H.; Chen, L.Y.; Lin, D.H.; Lan, F.-H. Diagnostic Accuracies of the TUNEL, SCD, and Comet Based Sperm DNA Fragmentation Assays for Male Infertility: A Meta-analysis Study. Clin. Lab. 2015, 61, 525–535. [Google Scholar] [CrossRef]
- Cissen, M.; Wely, M.V.; Scholten, I.; Mansell, S.; Bruin, J.P.; Mol, B.W.; Braat, D.; Repping, S.; Hamer, G. Measuring Sperm DNA Fragmentation and Clinical Outcomes of Medically Assisted Reproduction: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0165125. [Google Scholar] [CrossRef] [PubMed]
- Caglar, G.S.; Köster, F.; Schöpper, B.; Asimakopoulos, B.; Nehls, B.; Nikolettos, N.; Diedrich, K.; Al-Hasani, S. Semen DNA fragmentation index, evaluated with both TUNEL and Comet assay, and the ICSI outcome. In Vivo 2007, 21, 1075–1080. [Google Scholar]
- Castro, L.S.; Siqueira, A.F.P.; Hamilton, T.R.S.; Mendes, C.M.; Visintin, J.A.; Assumpção, M.E.O.A. Effect of bovine sperm chromatin integrity evaluated using three different methods on in vitro fertility. Theriogenology 2018, 107, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Bibi, R.; Jahan, S.; Razak, S.; Hammadeh, M.E.; Almajwal, A.; Amor, H. Protamines and DNA integrity as a biomarkers of sperm quality and assisted conception outcome. Andrologia 2022, 54, e14418. [Google Scholar] [CrossRef]
- Muratori, M.; Marchiani, S.; Tamburrino, L.; Tocci, V.; Failli, P.; Forti, G.; Baldi, E. Nuclear Staining Identifies Two Populations of Human Sperm with Different DNA Fragmentation Extent and Relationship with Semen Parameters. Hum. Reprod. 2008, 23, 1035–1043. [Google Scholar] [CrossRef] [PubMed]
- Spanò, M.; Toft, G.; Hagmar, L.; Eleuteri, P.; Rescia, M.; Rignell-Hydbom, A.; Tyrkiel, E.; Zvyezday, V.; Bonde, J.P. Exposure to PCB and p, p′-DDE in European and Inuit Populations: Impact on Human Sperm Chromatin Integrity. Hum. Reprod. 2005, 20, 3488–3499. [Google Scholar] [CrossRef] [PubMed]
- Evenson, D.P.; Larson, K.L.; Jost, L.K. Sperm Chromatin Structure Assay: Its Clinical Use for Detecting Sperm DNA Fragmentation in Male Infertility and Comparisons with Other Techniques. J. Androl. 2002, 23, 25–43. [Google Scholar] [CrossRef]
- Fernandez, J.L.; Muriel, L.; Rivero, M.T.; Goyanes, V.; Vazquez, R.; Alvarez, J.G. The sperm chromatin dispersion test: A simple method for the determination of sperm DNA fragmentation. J. Androl. 2003, 24, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Carrell, D.T. Sperm DNA Damage Measured by Comet Assay. Methods Mol. Biol. 2013, 927, 137–146. [Google Scholar] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calamai, C.; Tanturli, M.; Conti, D.; Leter, G.; Vignozzi, L.; Giovannelli, L.; Muratori, M. Induction of Sperm DNA Fragmentation by Cryopreservation and In Vitro Incubation: Comparison of TUNEL, SCSA, SCD Test and COMET Assay. Int. J. Mol. Sci. 2025, 26, 8978. https://doi.org/10.3390/ijms26188978
Calamai C, Tanturli M, Conti D, Leter G, Vignozzi L, Giovannelli L, Muratori M. Induction of Sperm DNA Fragmentation by Cryopreservation and In Vitro Incubation: Comparison of TUNEL, SCSA, SCD Test and COMET Assay. International Journal of Molecular Sciences. 2025; 26(18):8978. https://doi.org/10.3390/ijms26188978
Chicago/Turabian StyleCalamai, Costanza, Michele Tanturli, Donata Conti, Giorgio Leter, Linda Vignozzi, Lisa Giovannelli, and Monica Muratori. 2025. "Induction of Sperm DNA Fragmentation by Cryopreservation and In Vitro Incubation: Comparison of TUNEL, SCSA, SCD Test and COMET Assay" International Journal of Molecular Sciences 26, no. 18: 8978. https://doi.org/10.3390/ijms26188978
APA StyleCalamai, C., Tanturli, M., Conti, D., Leter, G., Vignozzi, L., Giovannelli, L., & Muratori, M. (2025). Induction of Sperm DNA Fragmentation by Cryopreservation and In Vitro Incubation: Comparison of TUNEL, SCSA, SCD Test and COMET Assay. International Journal of Molecular Sciences, 26(18), 8978. https://doi.org/10.3390/ijms26188978







