Sex Steroids in COVID-19 Patients with Hypertension: An Exploratory Study
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics
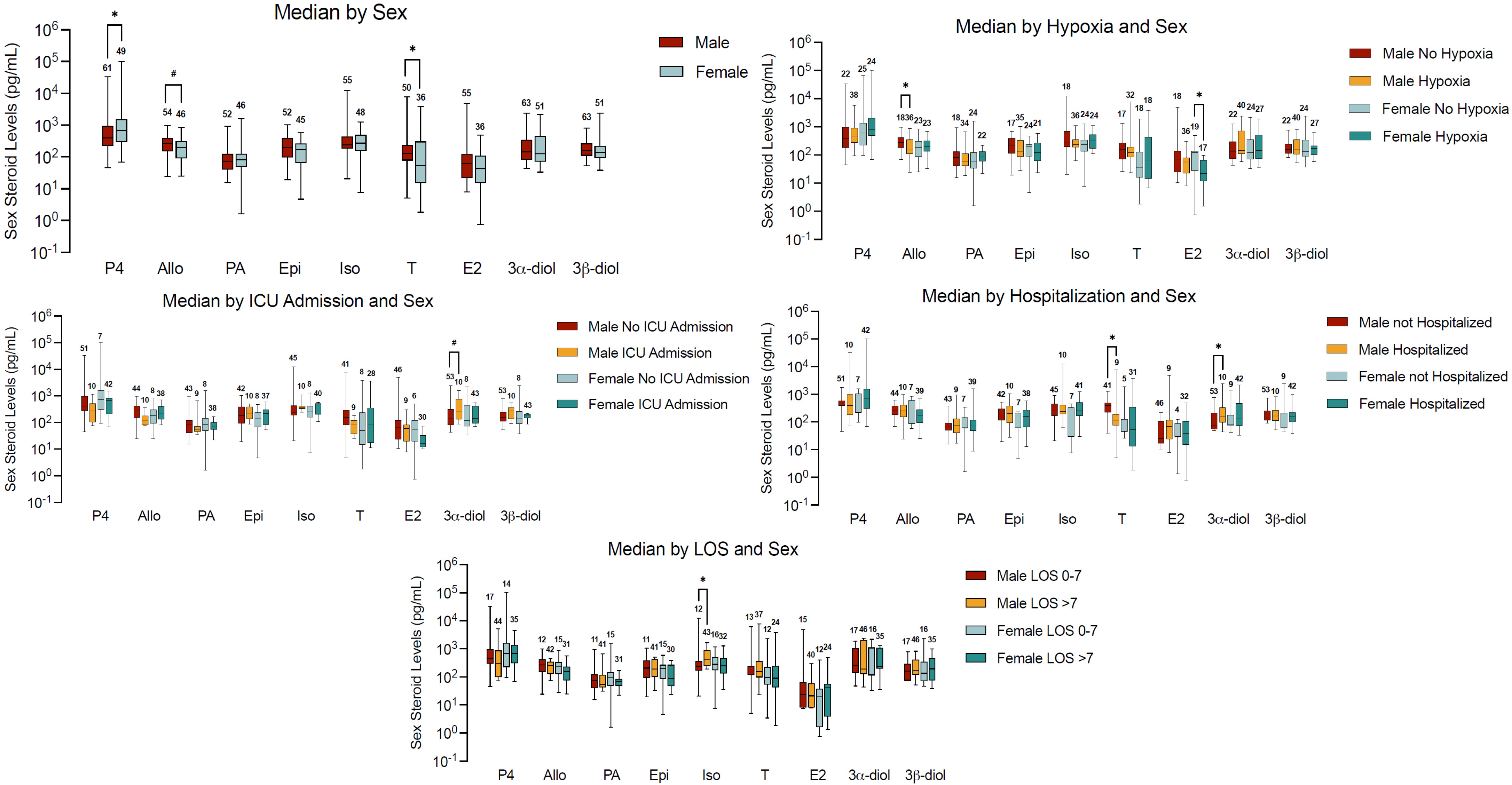
2.2. Sex Steroid Levels in Male and Females with Hypertension and COVID-19 Infection
2.3. Sex Steroid Levels and Clinical Outcomes in Subjects with Hypertension and COVID-19 Infection
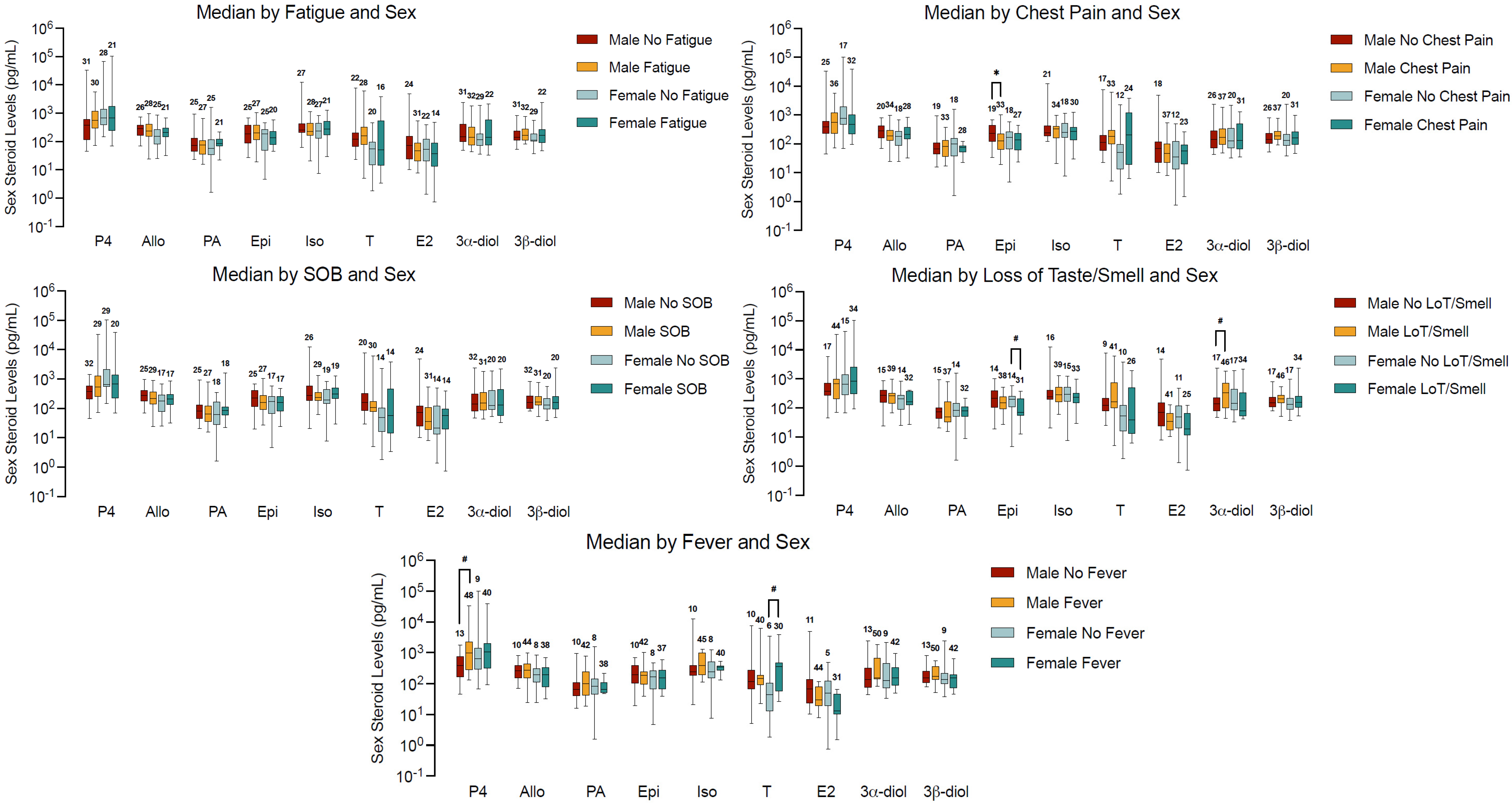
2.4. Sex Steroid Concentrations and Symptoms in Male and Females with Hypertension and COVID-19 Infection
2.5. Sex Dimorphism in Steroid Levels and Hospitalization and ICU Admission
3. Discussion
4. Methods
4.1. Study Population
4.2. Gas Chromatography-Mass Spectrometry Neuroactive Steroid Assays
4.3. Analytic Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, G.; Volgman, A.S.; Michos, E.D. Sex Differences in Mortality from COVID-19 Pandemic: Are Men Vulnerable and Women Protected? JACC Case Rep. 2020, 2, 1407–1410. [Google Scholar] [CrossRef]
- Capuano, A.; Rossi, F.; Paolisso, G. COVID-19 Kills More Men Than Women: An Overview of Possible Reasons. Front. Cardiovasc. Med. 2020, 7, 131. [Google Scholar] [CrossRef]
- Pradhan, A.; Olsson, P.-E. Sex differences in severity and mortality from COVID-19: Are males more vulnerable? Biol. Sex Differ. 2020, 11, 53. [Google Scholar] [CrossRef] [PubMed]
- Mauvais-Jarvis, F.; Merz, N.B.; Barnes, P.J.; Brinton, R.D.; Carrero, J.-J.; DeMeo, D.L.; De Vries, G.J.; Epperson, C.N.; Govindan, R.; Klein, S.L.; et al. Sex and gender: Modifiers of health, disease, and medicine. Lancet 2020, 396, 565–582. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Guastamacchia, E.; Magrone, T.; Jirillo, E.; Lisco, G.; De Pergola, G.; Triggiani, V. Worse progression of COVID-19 in men: Is testosterone a key factor? Andrology 2021, 9, 53–64. [Google Scholar] [CrossRef]
- Stasi, V.D.; Rastrelli, G. The Role of Sex Hormones in the Disparity of COVID-19 Outcomes Based on Gender. J. Sex. Med. 2021, 18, 1950–1954. [Google Scholar] [CrossRef]
- Scully, E.P.; Haverfield, J.; Ursin, R.L.; Tannenbaum, C.; Klein, S.L. Considering how biological sex impacts immune responses and COVID-19 outcomes. Nat. Rev. Immunol. 2020, 20, 442–447. [Google Scholar] [CrossRef]
- Mauvais-Jarvis, F. Do Anti-androgens Have Potential as Therapeutics for COVID-19? Endocrinology 2021, 162, bqab114. [Google Scholar] [CrossRef]
- Lanser, L.; Burkert, F.R.; Thommes, L.; Egger, A.; Hoermann, G.; Kaser, S.; Pinggera, G.M.; Anliker, M.; Griesmacher, A.; Weiss, G.; et al. Testosterone Deficiency Is a Risk Factor for Severe COVID-19. Front. Endocrinol. 2021, 12, 694083. [Google Scholar] [CrossRef]
- Cinislioglu, A.E.; Cinislioglu, N.; Demirdogen, S.O.; Sam, E.; Akkas, F.; Altay, M.S.; Utlu, M.; Sen, I.A.; Yildirim, F.; Kartal, S.; et al. The relationship of serum testosterone levels with the clinical course and prognosis of COVID-19 disease in male patients: A prospective study. Andrology 2021, 10, 24–33. [Google Scholar] [CrossRef]
- Pinna, G. Sex and COVID-19: A Protective Role for Reproductive Steroids. Trends Endocrinol. Metabolism 2021, 32, 3–6. [Google Scholar] [CrossRef]
- Dhindsa, S.; Zhang, N.; McPhaul, M.J.; Wu, Z.; Ghoshal, A.K.; Erlich, E.C.; Mani, K.; Randolph, G.J.; Edwards, J.R.; Mudd, P.A.; et al. Association of Circulating Sex Hormones With Inflammation and Disease Severity in Patients with COVID-19. JAMA Netw. Open 2021, 4, e2111398. [Google Scholar] [CrossRef]
- Bupp, M.R.G.; Potluri, T.; Fink, A.L.; Klein, S.L. The Confluence of Sex Hormones and Aging on Immunity. Front. Immunol. 2018, 9, 1269. [Google Scholar] [CrossRef]
- Dhindsa, S.; Reddy, A.; Karam, J.S.; Bilkis, S.; Chaurasia, A.; Mehta, A.; Raja, K.P.; Batra, M.; Dandona, P. Prevalence of subnormal testosterone concentrations in men with type 2 diabetes and chronic kidney disease. Eur. J. Endocrinol. 2015, 173, 359–366. [Google Scholar] [CrossRef]
- Dhindsa, S.; Ghanim, H.; Batra, M.; Dandona, P. Hypogonadotropic hypogonadism in men with diabesity. Diabetes Care 2018, 41, 1516–1525. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Naing, S. Hypogonadism in chronic obstructive pulmonary disease: Incidence and effects. Curr. Opin. Pulm. Med. 2012, 18, 112–117. [Google Scholar] [CrossRef]
- Rastrelli, G.; Di Stasi, V.; Inglese, F.; Beccaria, M.; Garuti, M.; Di Costanzo, D.; Spreafico, F.; Greco, G.F.; Cervi, G.; Pecoriello, A.; et al. Low testosterone levels predict clinical adverse outcomes in SARS-CoV-2 pneumonia patients. Andrology 2021, 9, 88–98. [Google Scholar] [CrossRef]
- Çayan, S.; Uğuz, M.; Saylam, B.; Akbay, E. Effect of serum total testosterone and its relationship with other laboratory parameters on the prognosis of coronavirus disease 2019 (COVID-19) in SARS-CoV-2 infected male patients: A cohort study. Aging Male 2020, 23, 1493–1503. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, T.; Iwasaki, A. Sex differences in immune responses. Science 2021, 371, 347–348. [Google Scholar] [CrossRef] [PubMed]
- Di Florio, D.N.; Sin, J.; Coronado, M.J.; Atwal, P.S.; Fairweather, D. Sex differences in inflammation, redox biology, mitochondria and autoimmunity. Redox Biol. 2020, 31, 101482. [Google Scholar] [CrossRef] [PubMed]
- Peng, M.; He, J.; Xue, Y.; Yang, X.; Liu, S.; Gong, Z. Role of Hypertension on the Severity of COVID-19: A Review. J. Cardiovasc. Pharmacol. 2021, 78, e648–e655. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reckelhoff, J.F. Gender differences in the regulation of blood pressure. Hypertension 2001, 37, 1199–1208. [Google Scholar] [CrossRef]
- Caulin-Glaser, T.; García-Cardeña, G.; Sarrel, P.; Sessa, W.C.; Bender, J.R. 17 beta-estradiol regulation of human endothelial cell basal nitric oxide release, independent of cytosolic Ca2+ mobilization. Circ. Res. 1997, 81, 885–892. [Google Scholar] [CrossRef]
- Schrör, K.; Morinelli, T.A.; Masuda, A.; Matsuda, K.; Mathur, R.S.; Halushka, P.V. Testosterone treatment enhances thromboxane A2 mimetic induced coronary artery vasoconstriction in guinea pigs. Eur. J. Clin. Investig. 1994, 24, 50–52. [Google Scholar] [CrossRef]
- Chou, T.M.; Sudhir, K.; Hutchison, S.J.; Ko, E.; Amidon, T.M.; Collins, P.; Chatterjee, K. Testosterone induces dilation of canine coronary conductance and resistance arteries in vivo. Circulation 1996, 94, 2614–2619. [Google Scholar] [CrossRef] [PubMed]
- Hughes, G.S.; Mathur, R.S.; Margolius, H.S. Sex steroid hormones are altered in essential hypertension. J. Hypertens. 1989, 7, 181–187. [Google Scholar] [CrossRef]
- Sutton-Tyrrell, K.; Wildman, R.P.; Matthews, K.A.; Chae, C.; Lasley, B.L.; Brockwell, S.; Pasternak, R.C.; Lloyd-Jones, D.; Sowers, M.F.; TorrénS, J.I. Sex-hormone-binding globulin and the free androgen index are related to cardiovascular risk factors in multiethnic premenopausal and perimenopausal women enrolled in the Study of Women Across the Nation (SWAN). Circulation 2005, 111, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Phillips, G.B.; Jing, T.Y.; Laragh, J.H. Serum sex hormone levels in postmenopausal women with hypertension. J. Hum. Hypertens. 1997, 11, 523–526. [Google Scholar] [CrossRef]
- Prendergast, H.M.; Kotini-Shah, P.; Pobee, R.; Richardson, M.; Ardati, A.; Darbar, D.; Khosla, S. Association of Angiotensin Converting Enzyme Phenotypes and Polymorphisms with Clinical Outcomes in SARS-CoV2 Patients with Hypertension in an Urban Emergency Department. Curr. Hypertens. Rev. 2025, 20, 166–175. [Google Scholar]
- Wagner, A.K.; McCullough, E.H.; Niyonkuru, C.; Ozawa, H.; Loucks, T.L.; Dobos, J.A.; Brett, C.A.; Santarsieri, M.; Dixon, C.E.; Berga, S.L.; et al. Acute serum hormone levels: Characterization and prognosis after severe traumatic brain injury. J. Neurotrauma 2011, 28, 871–888. [Google Scholar] [CrossRef]
- Wagner, J.; Dusick, J.R.; McArthur, D.L.; Cohan, P.; Wang, C.; Swerdloff, R.; Boscardin, W.J.; Kelly, D.F. Acute gonadotroph and somatotroph hormonal suppression after traumatic brain injury. J. Neurotrauma 2010, 27, 1007–1019. [Google Scholar] [CrossRef]
- Dossett, L.A.; Swenson, B.R.; Evans, H.L.; Bonatti, H.; Sawyer, R.G. Serum estradiol concentration as a predictor of death in critically ill and injured adults. Surg. Infect. 2008, 9, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Dossett, L.A.; Swenson, B.R.; Heffernan, D.; Bonatti, H.; Metzger, R.; Sawyer, R.G.; May, A.K. High levels of endogenous estrogens are associated with death in the critically injured adult. J. Trauma 2008, 64, 580–585. [Google Scholar] [CrossRef][Green Version]
- Christeff, N.; Benassayag, C.; Carli-Vielle, C.; Carli, A.; Nunez, E.A. Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. J. Steroid Biochem. 1988, 29, 435–440. [Google Scholar] [CrossRef]
- Gee, A.; Sawai, R.; Differding, J.; Muller, P.; Underwood, S.; Schreiber, M. The influence of sex hormone on coagulation and inflammation in the trauma patients. Shock 2008, 29, 334–341. [Google Scholar] [CrossRef]
- Di Stasi, V.; Rastrelli, G.; Inglese, F.; Beccaria, M.; Garuti, M.; Di Costanzo, D.; Spreafico, F.; Cervi, G.; Greco, G.F.; Pecoriello, A.; et al. Higher testosterone is associated with increased inflammatory markers in women with SARS-CoV-2 pneumonia: Preliminary results from an observational study. J. Endocrinol. Investig. 2022, 45, 639–648. [Google Scholar] [CrossRef]
- Van den Berghe, G.; Weekers, F.; Baxter, R.C.; Wouters, P.; Iranmanesh, A.; Bouillon, R.; Veldhuis, J.D. Fiveday pulsatile gonadotropin-releasing hormone administration unveils combined hypothalamic–pituitary–gonadal defects underlying profound hypoandrogenism in men with prolongedcritical illness. J. Clin. Endocrinol. Metab. 2001, 86, 3217–3226. [Google Scholar]
- Kalantaridou, S.N.; Makrigiannakis, A.; Zoumakis, E.; Chrousos, G.P. Stress and the female reproductive system. J. Reprod. Immunol. 2004, 62, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Mastorakos, G.; Pavlatou, M. Exercise as a stress model and the interplay between the hypothalamus–pituitary–adrenal and the hypothalamus–pituitary–thyroid axes. Horm. Metab. Res. 2005, 37, 577–584. [Google Scholar] [CrossRef]
- Locci, A.; Pinna, G. Neurosteroid biosynthesis down-regulation and changes in GABAA receptor subunit composition: A biomarker axis in stress-induced cognitive and emotional impairment. Br. J. Pharmacol. 2017, 174, 3226–3241. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Dichtel, L.E.; Lawson, E.A.; Schorr, M.; Meenaghan, E.; Paskal, M.L.; Eddy, K.T.; Pinna, G.; Nelson, M.; Rasmusson, A.M.; Klibanski, A.; et al. Neuroactive Steroids and Affective Symptoms in Women Across the Weight Spectrum. Neuropsychopharmacology 2018, 43, 1436–1444. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Rasmusson, A.M.; King, M.W.; Valovski, I.; Gregor, K.; Scioli-Salter, E.; Pineles, S.L.; Hamouda, M.; Nillni, Y.I.; Anderson, G.M.; Pinna, G. Relationships between cerebrospinal fluid GABAergic neurosteroid levels and symptom severity in men with PTSD. Psychoneuroendocrinology 2019, 102, 95–104. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinna, G. Allopregnanolone (1938-2019): A trajectory of 80 years of outstanding scientific achievements. Neurobiol. Stress 2020, 13, 100246. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Balan, I.; Beattie, M.C.; O’Buckley, T.K.; Aurelian, L.; Morrow, A.L. Endogenous Neurosteroid (3α,5α)3-Hydroxypregnan-20-one Inhibits Toll-like-4 Receptor Activation and Pro-inflammatory Signaling in Macrophages and Brain. Sci. Rep. 2019, 9, 1220. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pinna, G.; Uzunova, V.; Matsumoto, K.; Puia, G.; Mienville, J.M.; Costa, E.; Guidotti, A. Brain allopregnanolone regulates the potency of the GABA(A) receptor agonist muscimol. Neuropharmacology 2000, 39, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Osborne, L.M.; Etyemez, S.; Pinna, G.; Alemani, R.; Standeven, L.R.; Wang, X.Q.; Payne, J.L. Neuroactive steroid biosynthesis during pregnancy predicts future postpartum depression: A role for the 3α and/or 3β-HSD neurosteroidogenic enzymes? Neuropsychopharmacology 2025, 50, 904–912. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
| Variables | Categories | Overall n = 116 | Female n = 53 | Male n = 63 |
|---|---|---|---|---|
| Age; Mean (SD) | 62.1 (13.1) | 64.3 (10.1) | 60.3 (15.0) | |
| Age group | ≥65 | 55 (47.4%) | 28 (52.8%) | 27 (42.9%) |
| <65 | 61 (52.6%) | 25 (47.2%) | 36 (57.1%) | |
| Sex | Male | 63 (54.3%) | - | 63 (100%) |
| Female | 53 (45.7%) | 53 (100%) | - | |
| Race/Ethnicity | NH-Black | 64 (55.2%) | 32 (60.4%) | 32 (50.8%) |
| Hispanic/Latino | 40 (34.5%) | 17 (32.1%) | 23 (36.5%) | |
| NH-White | 6 (5.2%) | |||
| Other * | 6 (5.2%) | |||
| Fever ** | Yes | 22 (19.0%) | 9 (17.0%) | 13 (20.6%) |
| No | 94 (81.0%) | 44 (83.0%) | 50 (79.4%) | |
| Shortness of Breath | Yes | 64 (55.2%) | 32 (60.4%) | 32 (50.8%) |
| No | 52 (44.8%) | 21 (39.6%) | 31 (49.2%) | |
| Chest Pain/tightness | Yes | 46 (39.7%) | 20 (37.7%) | 26 (41.3%) |
| No | 70 (60.3%) | 33 (62.3%) | 37 (58.7%) | |
| Loss of smell/taste | Yes | 35 (30.2%) | 18 (34.0%) | 17 (27.0%) |
| No | 81 (69.8%) | 35 (66.0%) | 46 (73.0%) | |
| Fatigue | Yes | 62 (53.4%) | 31 (58.5%) | 31 (49.2%) |
| No | 54 (46.6%) | 22 (41.5%) | 32 (50.8%) | |
| Hospitalized | Yes | 97 (83.6%) | 44 (83.0%) | 53 (84.1%) |
| No | 19 (16.4%) | 9 (17.0%) | 10 (15.9%) | |
| ICU admit | Yes | 18 (15.5%) | 8 (15.1%) | 10 (15.9%) |
| No | 98 (84.5%) | 45 (84.9%) | 53 (84.1%) | |
| Length of Stay | >7 days | 33 (28.4%) | 16 (30.2%) | 17 (27.0%) |
| 0–7 days | 83 (71.6%) | 37 (69.8%) | 46 (73.0%) | |
| Ventilator support | Yes | 4 (3.5%) | ||
| No | 111 (96.5%) | |||
| Hypoxia | Yes | 48 (41.7%) | 26 (49.1%) | 22 (35.5%) |
| No | 67 (58.3%) | 27 (50.9%) | 40 (64.5%) | |
| Vasopressor use | Yes | 6 (5.2%) | ||
| No | 110 (94.8%) |
| Overall | Female n = 53 | Male n = 63 | |
|---|---|---|---|
| Cardiovascular disease | |||
| Yes | 32 (27.6%) | 10 (18.9%) | 22 (34.9%) |
| No | 84 (72.4%) | 43 (81.1%) | 41 (65.1%) |
| Prior history of atrial fibrillation/atrial flutter/atrial tachycardia/VT/Vfib/SVT AND/OR new onset arrhythmia during index presentation | |||
| Yes | 20 (17.2%) | 9 (17.0%) | 11 (17.5%) |
| No | 96 (82.8%) | 44 (83.0%) | 52 (82.5%) |
| Any QTc 500 during index presentation | |||
| Yes | 7 (6.0%) | 1 (1.9%) | 6 (9.5%) |
| No | 109 (94.0%) | 52 (98.1%) | 57 (90.5%) |
| Elevated Cardiac Markers > 0.04 | |||
| Yes | 18 (15.5%) | 9 (17.0%) | 9 (14.3%) |
| No | 98 (84.5%) | 44 (83.0%) | 54 (85.7%) |
| a Significant valve disease | |||
| Yes | 6 (5.2%) | 1 (1.9%) | 5 (7.9%) |
| No | 110 (94.8%) | 52 (98.1%) | 58 (92.1%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kotini-Shah, P.; Khosla, S.; Almeida, F.B.; Santovito, L.S.; Prendergast, H.; Pinna, G. Sex Steroids in COVID-19 Patients with Hypertension: An Exploratory Study. Int. J. Mol. Sci. 2025, 26, 8976. https://doi.org/10.3390/ijms26188976
Kotini-Shah P, Khosla S, Almeida FB, Santovito LS, Prendergast H, Pinna G. Sex Steroids in COVID-19 Patients with Hypertension: An Exploratory Study. International Journal of Molecular Sciences. 2025; 26(18):8976. https://doi.org/10.3390/ijms26188976
Chicago/Turabian StyleKotini-Shah, Pavitra, Shaveta Khosla, Felipe Borges Almeida, Luca Spiro Santovito, Heather Prendergast, and Graziano Pinna. 2025. "Sex Steroids in COVID-19 Patients with Hypertension: An Exploratory Study" International Journal of Molecular Sciences 26, no. 18: 8976. https://doi.org/10.3390/ijms26188976
APA StyleKotini-Shah, P., Khosla, S., Almeida, F. B., Santovito, L. S., Prendergast, H., & Pinna, G. (2025). Sex Steroids in COVID-19 Patients with Hypertension: An Exploratory Study. International Journal of Molecular Sciences, 26(18), 8976. https://doi.org/10.3390/ijms26188976








