Mitochondria, Sex, and Cardiovascular Disease: A Complex Interplay
Abstract
1. Introduction
1.1. General Background and Objective
1.2. Method of Review
2. Sex and Cardiovascular Disease
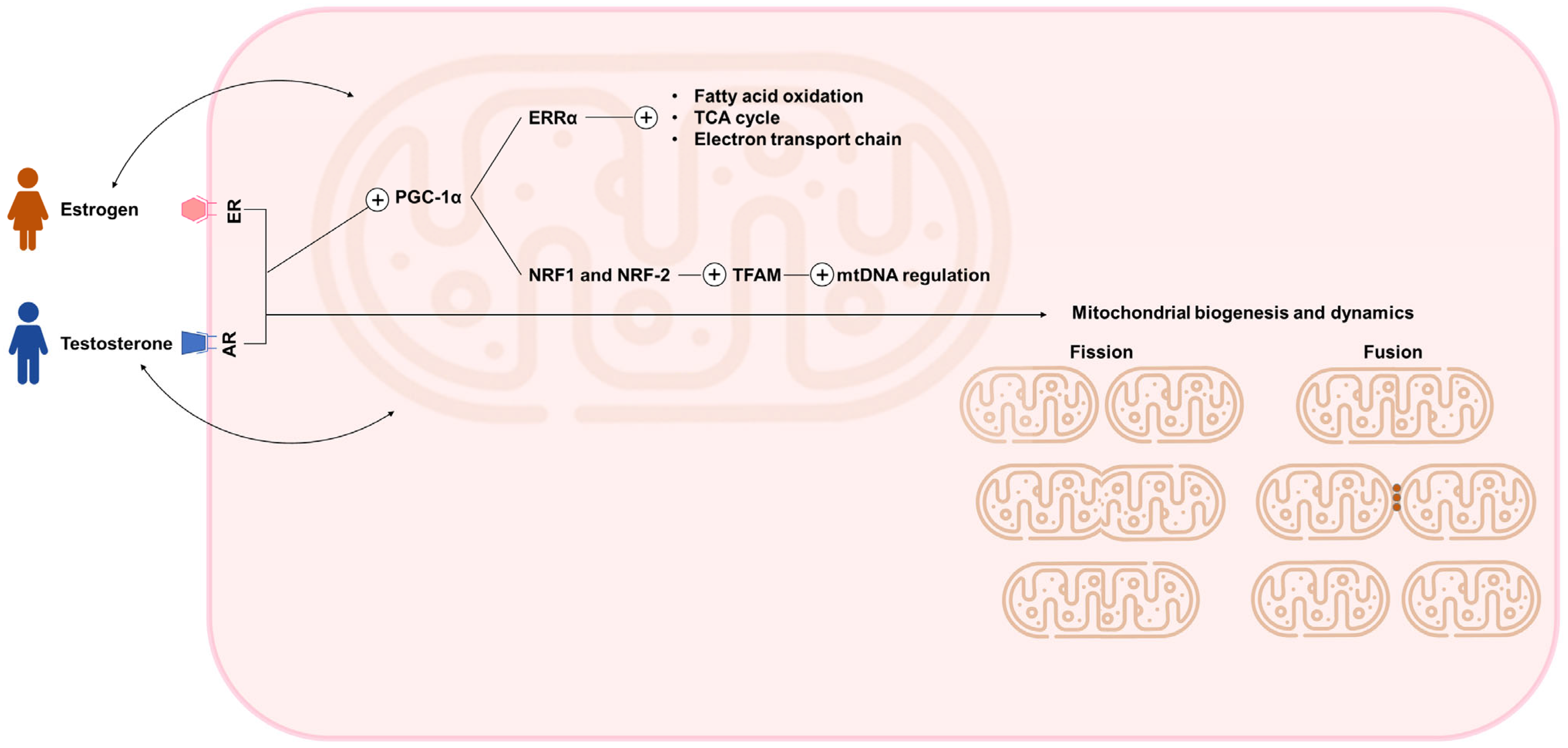
3. Sex, Hormonal Regulation, and Mitochondrial Fitness
4. Mitochondrial Function in Cardiac and Vascular Tissues
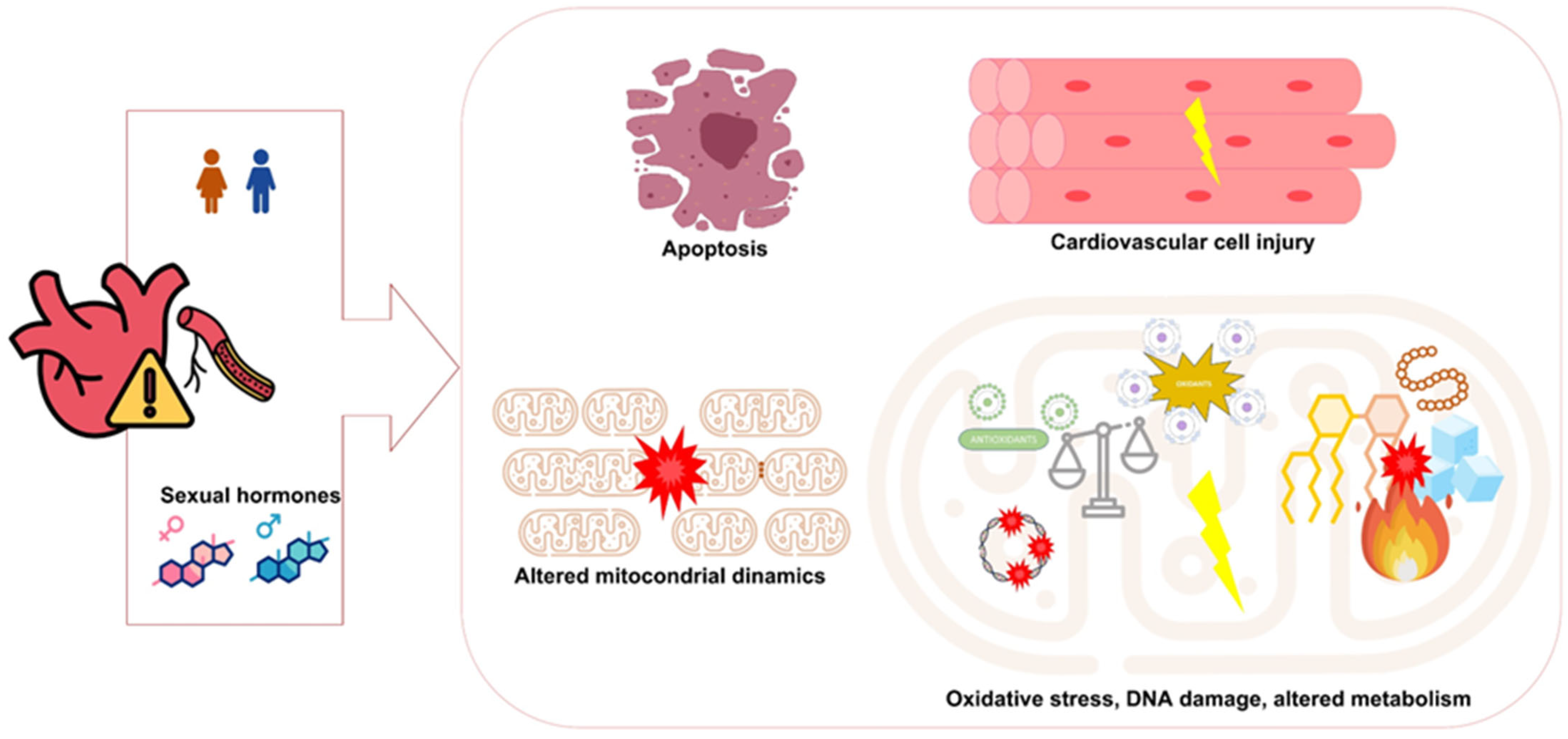
5. Cardiovascular Disease, Mitochondria, and Sex-Specific Factors
6. Therapeutic Strategies Targeting Mitochondrial Dysfunction
7. Integrating Evidence of Sex-Specific Treatment Responses
8. Challenges and Opportunities in Translational Research
9. Implications for Precision Cardiovascular Medicine
10. Future Directions in Translational Cardiovascular Research
11. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chong, B.; Jayabaskaran, J.; Jauhari, S.M.; Chan, S.P.; Goh, R.; Kueh, M.T.W.; Li, H.; Chin, Y.H.; Kong, G.; Anand, V.V.; et al. Global burden of cardiovascular diseases: Projections from 2025 to 2050. Eur. J. Prev. Cardiol. 2024, 32, 1001–1015. [Google Scholar] [CrossRef]
- Cardiovascular Diseases (CVDs). Available online: https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds) (accessed on 4 August 2025).
- Di Cesare, M.; Perel, P.; Taylor, S.; Kabudula, C.; Bixby, H.; Gaziano, T.A.; McGhie, D.V.; Mwangi, J.; Pervan, B.; Narula, J.; et al. The heart of the world. Glob Heart. 2024, 19, 11. [Google Scholar] [CrossRef]
- Park, J.B.; Avolio, A. Arteriosclerosis and atherosclerosis assessment in clinical practice: Methods and significance. Pulse 2023, 11, 1–8. [Google Scholar] [CrossRef]
- Pahwa, R.; Jialal, I. Atherosclerosis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Frąk, W.; Wojtasińska, A.; Lisińska, W.; Młynarska, E.; Franczyk, B.; Rysz, J. Pathophysiology of cardiovascular diseases: New insights into molecular mechanisms of atherosclerosis, arterial hypertension, and coronary artery disease. Biomedicines 2022, 10, 1938. [Google Scholar] [CrossRef]
- Chen, W.; Li, Z.; Zhao, Y.; Chen, Y.; Huang, R. Global and National Burden of Atherosclerosis from 1990 to 2019: Trend analysis based on the global burden of disease study 2019. Chin. Med. J. 2023, 136, 2442–2450. [Google Scholar] [CrossRef]
- Crea, F. The burden of cardiovascular risk factors: A global perspective. Eur. Heart J. 2022, 43, 2817–2820. [Google Scholar] [CrossRef] [PubMed]
- Colom, B.; Oliver, J.; Garcia-Palmer, F.J. Sexual dimorphism in the alterations of cardiac muscle mitochondrial bioenergetics associated to the ageing process. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2015, 70, 1360–1369. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Vergnes, L.; Wang, Y.-C.; Pan, C.; Chella Krishnan, K.; Moore, T.M.; Rosa-Garrido, M.; Kimball, T.H.; Zhou, Z.; Charugundla, S.; et al. Sex differences in heart mitochondria regulate diastolic dysfunction. Nat. Commun. 2022, 13, 3850. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Pan, C.-H.; Yin, F.; Peng, J.; Yang, L. The Role of Estrogen in Mitochondrial Disease. Cell. Mol. Neurobiol. 2025, 45, 68. [Google Scholar] [CrossRef]
- Murphy, E.; Steenbergen, C. Gender-based differences in mechanisms of protection in myocardial ischemia–reperfusion injury. Cardiovasc. Res. 2007, 75, 478–486. [Google Scholar] [CrossRef]
- Vogel, B.; Acevedo, M.; Appelman, Y.; Bairey Merz, C.N.; Chieffo, A.; Figtree, G.A.; Guerrero, M.; Kunadian, V.; Lam, C.S.P.; Maas, A.H.E.M.; et al. The Lancet women and cardiovascular disease commission: Reducing the global burden by 2030. Lancet 2021, 397, 2385–2438. [Google Scholar] [CrossRef]
- Hiremath, P.G.; Aversano, T.; Spertus, J.A.; Lemmon, C.C.; Naiman, D.Q.; Czarny, M.J. Sex differences in health status and clinical outcomes after nonprimary percutaneous coronary intervention. Circ. Cardiovasc. Interv. 2022, 15, e011308. [Google Scholar] [CrossRef]
- Gauci, S.; Cartledge, S.; Redfern, J.; Gallagher, R.; Huxley, R.; Lee, C.M.Y.; Vassallo, A.; O’Neil, A. Biology, Bias, or Both? The contribution of sex and gender to the disparity in cardiovascular outcomes between women and men. Curr. Atheroscler. Rep. 2022, 24, 701–708. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Gebhard, C. Gender Medicine: Effects of Sex and Gender on Cardiovascular Disease Manifestation and Outcomes. Nat. Rev. Cardiol. 2023, 20, 236–247. [Google Scholar] [CrossRef] [PubMed]
- Hartman, R.J.G.; Owsiany, K.; Ma, L.; Koplev, S.; Hao, K.; Slenders, L.; Civelek, M.; Mokry, M.; Kovacic, J.C.; Pasterkamp, G.; et al. Sex-Stratified Gene Regulatory Networks Reveal Female Key Driver Genes of Atherosclerosis Involved in Smooth Muscle Cell Phenotype Switching. Circulation 2021, 143, 713–726. [Google Scholar] [CrossRef] [PubMed]
- Shufelt, C.L.; Pacheco, C.; Tweet, M.S.; Miller, V.M. Sex-Specific Physiology and Cardiovascular Disease. In Sex-Specific Analysis of Cardiovascular Function; Kerkhof, P.L.M., Miller, V.M., Eds.; Springer: Cham, Switzerland, 2018; Volume 1065, pp. 433–454. ISBN 978-3-319-77932-4. [Google Scholar]
- Timmis, A.; Aboyans, V.; Vardas, P.; Townsend, N.; Torbica, A.; Kavousi, M.; Boriani, G.; Huculeci, R.; Kazakiewicz, D.; Scherr, D.; et al. European Society of Cardiology: The 2023 Atlas of cardiovascular disease statistics. Eur. Heart J. 2024, 45, 4019–4062. [Google Scholar] [CrossRef]
- Alonso, C.; Díaz Molina, B.; Tamargo, J.; Sambola, A. Aspectos diferenciales en la insuficiencia cardiaca en la mujer. REC CardioClinics. 2019, 54, 253–261. [Google Scholar] [CrossRef]
- Sayago-Silva, I.; García-López, F.; Segovia-Cubero, J. Epidemiología de la insuficiencia cardiaca en España en los últimos 20 años. Rev. Esp. Cardiol. 2013, 66, 649–656. [Google Scholar] [CrossRef]
- Ventura-Clapier, R.; Piquereau, J.; Garnier, A.; Mericskay, M.; Lemaire, C.; Crozatier, B. Gender issues in cardiovascular diseases. Focus on energy metabolism. Biochim. Biophys. Acta-Mol. Basis Dis. 2020, 1866, 165722. [Google Scholar] [CrossRef]
- Vijay, V.; Han, T.; Moland, C.L.; Kwekel, J.C.; Fuscoe, J.C.; Desai, V.G. Sexual dimorphism in the expression of mitochondria-related genes in rat heart at different ages. PLoS ONE 2015, 10, e0117047. [Google Scholar] [CrossRef]
- Golob, M.J.; Tian, L.; Wang, Z.; Zimmerman, T.A.; Caneba, C.A.; Hacker, T.A.; Song, G.; Chesler, N.C. Mitochondria DNA mutations cause sex-dependent development of hypertension and alterations in cardiovascular function. J. Biomech. 2015, 48, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Ventura-Clapier, R.; Moulin, M.; Piquereau, J.; Lemaire, C.; Mericskay, M.; Veksler, V.; Garnier, A. Mitochondria: A central target for sex differences in pathologies. Clin. Sci. 2017, 131, 803–822. [Google Scholar] [CrossRef] [PubMed]
- Guajardo-Correa, E.; Silva-Agüero, J.F.; Calle, X.; Chiong, M.; Henríquez, M.; García-Rivas, G.; Latorre, M.; Parra, V. Estrogen signaling as a bridge between the nucleus and mitochondria in cardiovascular diseases. Front. Cell Dev. Biol. 2022, 10, 968373. [Google Scholar] [CrossRef] [PubMed]
- Knowlton, A.A.; Lee, A.R. Estrogen and the cardiovascular system. Pharmacol. Ther. 2012, 135, 54–70. [Google Scholar] [CrossRef]
- Chung, E.; Joiner, H.E.; Skelton, T.; Looten, K.D.; Manczak, M.; Reddy, P.H. Maternal exercise upregulates mitochondrial gene expression and increases enzyme activity of fetal mouse hearts. Physiol. Rep. 2017, 5, e13184. [Google Scholar] [CrossRef]
- John, C.; Grune, J.; Ott, C.; Nowotny, K.; Deubel, S.; Kühne, A.; Schubert, C.; Kintscher, U.; Regitz-Zagrosek, V.; Grune, T. Sex differences in cardiac mitochondria in the New Zealand obese mouse. Front. Endocrinol. 2018, 9, 732. [Google Scholar] [CrossRef]
- Hellgren, K.T.; Premanandhan, H.; Quinn, C.J.; Trafford, A.W.; Galli, G.L.J. Sex-dependent effects of developmental hypoxia on cardiac mitochondria from adult murine offspring. Free Radic. Biol. Med. 2021, 162, 490–499. [Google Scholar] [CrossRef]
- Dos Santos, R.L.; Da Silva, F.B.; Ribeiro, R.F.; Stefanon, I. Sex hormones in the cardiovascular system. Horm. Mol. Biol. Clin. Investig. 2014, 18, 89–103. [Google Scholar] [CrossRef]
- Basaria, S.; Harman, S.M.; Travison, T.G.; Hodis, H.; Tsitouras, P.; Budoff, M.; Pencina, K.M.; Vita, J.; Dzekov, C.; Mazer, N.A.; et al. Effects of testosterone administration for 3 years on subclinical atherosclerosis progression in older men with low or low-normal testosterone levels: A randomized clinical trial. JAMA 2015, 314, 570–579. [Google Scholar] [CrossRef]
- Corona, G.; Rastrelli, G.; Sparano, C.; Carinci, V.; Casella, G.; Vignozzi, L.; Sforza, A.; Maggi, M. Cardiovascular safety of testosterone replacement therapy in men: An updated systematic review and meta-analysis. Expert Opin. Drug Saf. 2024, 23, 565–579. [Google Scholar] [CrossRef]
- Streed, C.G.; Harfouch, O.; Marvel, F.; Blumenthal, R.S.; Martin, S.S.; Mukherjee, M. Cardiovascular disease among transgender adults receiving hormone therapy: A narrative review. Ann. Intern. Med. 2017, 167, 256–267. [Google Scholar] [CrossRef]
- Murphy, C.N.; Delles, C.; Davies, E.; Connelly, P.J. Cardiovascular disease in transgender individuals. Atherosclerosis 2023, 384, 117282. [Google Scholar] [CrossRef] [PubMed]
- Glintborg, D.; Rubin, K.H.; Petersen, T.G.; Lidegaard, Ø.; T’Sjoen, G.; Hilden, M.; Andersen, M.S. Cardiovascular risk in danish transgender persons: A matched historical cohort study. Eur. J. Endocrinol. 2022, 187, 463–477. [Google Scholar] [CrossRef] [PubMed]
- De Blok, C.J.; Wiepjes, C.M.; Van Velzen, D.M.; Staphorsius, A.S.; Nota, N.M.; Gooren, L.J.; Kreukels, B.P.; Den Heijer, M. Mortality trends over five decades in adult transgender people receiving hormone treatment: A report from the Amsterdam cohort of gender dysphoria. Lancet Diabetes Endocrinol. 2021, 9, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Clements, R.T.; Terentyeva, R.; Hamilton, S.; Janssen, P.M.L.; Roder, K.; Martin, B.Y.; Perger, F.; Schneider, T.; Nichtova, Z.; Das, A.S.; et al. Sexual dimorphism in bidirectional sr-mitochondria crosstalk in ventricular cardiomyocytes. Basic Res. Cardiol. 2023, 118, 15. [Google Scholar] [CrossRef]
- Crescioli, C. The Role of Estrogens and vitamin d in cardiomyocyte protection: A female perspective. Biomolecules 2021, 11, 1815. [Google Scholar] [CrossRef]
- Merz, A.A.; Cheng, S. Sex differences in cardiovascular ageing. Heart 2016, 102, 825–831. [Google Scholar] [CrossRef]
- Arain, F.A.; Kuniyoshi, F.H.; Abdalrhim, A.D.; Miller, V.M. Sex/gender medicine. The biological basis for personalized care in cardiovascular medicine. Circ. J. 2009, 73, 1774–1782. [Google Scholar] [CrossRef]
- Miller, W.L. Steroid hormone synthesis in mitochondria. Mol. Cell. Endocrinol. 2013, 379, 62–73. [Google Scholar] [CrossRef]
- Vassalle, C.; Simoncini, T.; Chedraui, P.; Pérez-López, F.R. Why sex matters: The biological mechanisms of cardiovascular disease. Gynecol. Endocrinol. 2012, 28, 746–751. [Google Scholar] [CrossRef]
- Araujo, A.B.; Wittert, G.A. Endocrinology of the aging male. Best Pract. Res. Clin. Endocrinol. Metab. 2011, 25, 303–319. [Google Scholar] [CrossRef]
- Lynch, S.; Boyett, J.E.; Smith, M.R.; Giordano-Mooga, S. Sex hormone regulation of proteins modulating mitochondrial metabolism, dynamics and inter-organellar cross talk in cardiovascular disease. Front. Cell Dev. Biol. 2021, 8, 610516. [Google Scholar] [CrossRef]
- Yin, L.; Qi, S.; Zhu, Z. Advances in mitochondria-centered mechanism behind the roles of androgens and androgen receptor in the regulation of glucose and lipid metabolism. Front. Endocrinol. 2023, 14, 1267170. [Google Scholar] [CrossRef] [PubMed]
- Acaz-Fonseca, E.; Ortiz-Rodriguez, A.; Garcia-Segura, L.M.; Astiz, M. Sex differences and gonadal hormone regulation of brain cardiolipin, a key mitochondrial phospholipid. J. Neuroendocrinol. 2020, 32, e12774. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Newell-Fugate, A.E. Role of androgens and androgen receptor in control of mitochondrial function. Am. J. Physiol. Cell Physiol. 2022, 323, C835–C846. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Ma, J.; Zhang, J.; Zhao, H.; Zhu, Y.; Qi, J.; Liu, L.; Zhu, L.; Jiang, Y.; Tang, G.; et al. Testosterone deficiency caused by castration modulates mitochondrial biogenesis through the AR/PGC1α/TFAM Pathway. Front. Genet. 2019, 10, 505. [Google Scholar] [CrossRef]
- Usui, T.; Kajita, K.; Kajita, T.; Mori, I.; Hanamoto, T.; Ikeda, T.; Okada, H.; Taguchi, K.; Kitada, Y.; Morita, H.; et al. Elevated mitochondrial biogenesis in skeletal muscle is associated with testosterone-induced body weight loss in male mice. FEBS Lett. 2014, 588, 1935–1941. [Google Scholar] [CrossRef]
- Samanta, K.; Douglas, S.; Parekh, A.B. Mitochondrial calcium uniporter MCU supports cytoplasmic Ca2+ oscillations, store-operated Ca2+ entry and Ca2+-dependent gene expression in response to receptor stimulation. PLoS ONE 2014, 9, e101188. [Google Scholar] [CrossRef]
- Tatarkova, Z.; Kolisek, M.; Pilchova, I.; Racay, P.; Kaplan, P. Interplay between mitochondrial proteins and age-associated risk of cardiovascular diseases. In Mitochondrial Diseases; Taskin, E., Guven, C., Sevgiler, Y., Eds.; InTech: Bolton, UK, 2018; ISBN 978-1-78923-675-0. [Google Scholar] [CrossRef]
- Tashkandi, A.J.; Gorman, A.; McGoldrick Mathers, E.; Carney, G.; Yacoub, A.; Setyaningsih, W.A.W.; Kuburas, R.; Margariti, A. Metabolic and mitochondrial dysregulations in diabetic cardiac complications. Int. J. Mol. Sci. 2025, 26, 3016. [Google Scholar] [CrossRef]
- Fajardo, G.; Coronado, M.; Matthews, M.; Bernstein, D. Mitochondrial quality control in the heart: The balance between physiological and pathological stress. Biomedicines 2022, 10, 1375. [Google Scholar] [CrossRef]
- Quiles, J.M.; Gustafsson, Å.B. The role of mitochondrial fission in cardiovascular health and disease. Nat. Rev. Cardiol. 2022, 19, 723–736. [Google Scholar] [CrossRef]
- Zhao, R.; Jiang, S.; Zhang, L.; Yu, Z. Mitochondrial electron transport chain, ros generation and uncoupling. Int. J. Mol. Med. 2019, 44, 3–15. [Google Scholar] [CrossRef]
- Endlicher, R.; Drahota, Z.; Štefková, K.; Červinková, Z.; Kučera, O. The mitochondrial permeability transition pore—Current knowledge of its structure, function, and regulation, and optimized methods for evaluating its functional state. Cells 2023, 12, 1273. [Google Scholar] [CrossRef] [PubMed]
- Werbner, B.; Tavakoli-Rouzbehani, O.M.; Fatahian, A.N.; Boudina, S. The dynamic interplay between cardiac mitochondrial health and myocardial structural remodeling in metabolic heart disease, aging, and heart failure. J. Cardiovasc. Aging 2023, 3, 9. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Karwi, Q.G.; Wong, N.; Lopaschuk, G.D. Advances in myocardial energy metabolism: Metabolic remodelling in heart failure and beyond. Cardiovasc. Res. 2024, 120, 1996–2016. [Google Scholar] [CrossRef] [PubMed]
- Tower, J. Mitochondrial maintenance failure in aging and role of sexual dimorphism. Arch. Biochem. Biophys. 2015, 576, 17–31. [Google Scholar] [CrossRef]
- Klinge, C.M. Estrogenic control of mitochondrial function. Redox Biol. 2020, 31, 101435. [Google Scholar] [CrossRef]
- Rovira-Llopis, S.; Bañuls, C.; De Marañon, A.M.; Diaz-Morales, N.; Jover, A.; Garzon, S.; Rocha, M.; Victor, V.M.; Hernandez-Mijares, A. Low testosterone levels are related to oxidative stress, mitochondrial dysfunction and altered subclinical atherosclerotic markers in type 2 diabetic male patients. Free Radic. Biol. Med. 2017, 108, 155–162. [Google Scholar] [CrossRef]
- Cardenas, M.; Alvarez, F.; Cabrera-Orefice, A.; Paredes-Carbajal, C.; Silva-Palacios, A.; Uribe-Carvajal, S.; García-Trejo, J.J.; Pavón, N. Cross-sex hormonal replacement: Some effects over mitochondria. J. Steroid Biochem. Mol. Biol. 2024, 244, 106595. [Google Scholar] [CrossRef]
- Yin, L.; Luo, M.; Wang, R.; Ye, J.; Wang, X. Mitochondria in sex hormone-induced disorder of energy metabolism in males and females. Front. Endocrinol. 2021, 12, 749451. [Google Scholar] [CrossRef]
- Shinlapawittayatorn, K.; Pongkan, W.; Sivasinprasasn, S.; Chattipakorn, S.C.; Chattipakorn, N. Sexual dimorphism in cardiometabolic and cardiac mitochondrial function in obese rats following sex hormone deprivation. Nutr. Diabetes. 2022, 12, 11. [Google Scholar] [CrossRef] [PubMed]
- Do Val Lima, P.R.; Ronconi, K.S.; Morra, E.A.; Rodrigues, P.L.; Ávila, R.A.; Merlo, E.; Graceli, J.B.; Simões, M.R.; Stefanon, I.; Ribeiro Júnior, R.F. Testosterone deficiency impairs cardiac interfibrillar mitochondrial function and myocardial contractility while inducing oxidative stress. Front. Endocrinol. 2023, 14, 1206387. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.-X.; Fu, L.; Li, Y.; Lin, Z.-B.; Liu, X.; Wang, J.-F.; Chen, Y.-X.; Wang, Z.-P.; Zhang, X.; Ou, Z.-J.; et al. The cardioprotective effect of vitamin e (alpha-tocopherol) is strongly related to age and gender in mice. PLoS ONE 2015, 10, e0137405. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, Y.; Akahoshi, T.; Eto, H.; Hyodo, F.; Murata, M.; Tokuda, K.; Eto, M.; Yamaura, K. Noninvasive diagnosis of the mitochondrial function of doxorubicin-induced cardiomyopathy using in vivo dynamic nuclear polarization–magnetic resonance imaging. Antioxidants 2022, 11, 1454. [Google Scholar] [CrossRef]
- Yang, H.-M. Mitochondrial dysfunction in cardiovascular diseases. Int. J. Mol. Sci. 2025, 26, 1917. [Google Scholar] [CrossRef]
- Kavousi, M.; Bielak, L.F.; Peyser, P.A. Genetic research and women’s heart disease: A primer. Curr. Atheroscleros. Rep. 2016, 18, 67. [Google Scholar] [CrossRef]
- Siasos, G.; Tsigkou, V.; Kosmopoulos, M.; Theodosiadis, D.; Simantiris, S.; Tagkou, N.M.; Tsimpiktsioglou, A.; Stampouloglou, P.K.; Oikonomou, E.; Mourouzis, K.; et al. Mitochondria and cardiovascular diseases—From pathophysiology to treatment. Ann. Transl. Med. 2018, 6, 256. [Google Scholar] [CrossRef]
- Oskuye, Z.Z.; Mehri, K.; Mokhtari, B.; Bafadam, S.; Nemati, S.; Badalzadeh, R. Cardioprotective effect of antioxidant combination therapy: A highlight on MitoQ plus alpha-lipoic acid beneficial impact on myocardial ischemia-reperfusion injury in aged rats. Heliyon 2024, 10, e28158. [Google Scholar] [CrossRef]
- Ribeiro Junior, R.F.; Dabkowski, E.R.; Shekar, K.C.; O’Connell, K.A.; Hecker, P.A.; Murphy, M.P. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic. Biol. Med. 2018, 117, 18–29. [Google Scholar] [CrossRef]
- Rius-Pérez, S.; Torres-Cuevas, I.; Millán, I.; Ortega, Á.L.; Pérez, S. PGC-1α, inflammation, and oxidative stress: An integrative view in metabolism. Oxidative Med. Cell. Longev. 2020, 2020, 1452696. [Google Scholar] [CrossRef]
- Kunkel, G.H.; Chaturvedi, P.; Tyagi, S.C. Mitochondrial pathways to cardiac recovery: TFAM. Heart Fail. Rev. 2016, 21, 499–517. [Google Scholar] [CrossRef]
- Brinton, R.D. Estrogen regulation of glucose metabolism and mitochondrial function: Therapeutic implications for prevention of alzheimer’s disease. Adv. Drug Deliv. Rev. 2008, 60, 1504–1511. [Google Scholar] [CrossRef]
- Kuzmicic, J.; Del Campo, A.; López-Crisosto, C.; Morales, P.E.; Pennanen, C.; Bravo-Sagua, R.; Hechenleitner, J.; Zepeda, R.; Castro, P.F.; Verdejo, H.E.; et al. Mitochondrial dynamics: A potential new therapeutic target for heart failure. Rev. Esp. Cardiol. 2011, 64, 916–923. [Google Scholar] [CrossRef]
- Miotto, P.M.; McGlory, C.; Holloway, T.M.; Phillips, S.M.; Holloway, G.P. Sex differences in mitochondrial respiratory function in human skeletal muscle. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 314, R909–R915. [Google Scholar] [CrossRef] [PubMed]
- Yoon, T.K.; Lee, C.H.; Kwon, O.; Kim, M.-S. Exercise, mitohormesis, and mitochondrial ORF of the 12S rRNA type-C (MOTS-c). Diabetes Metab. J. 2022, 46, 402–413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhang, Y.; Zhang, J.; Jia, D. Exercise alleviates cardiovascular diseases by improving mitochondrial homeostasis. J. Am. Heart Assoc. 2024, 13, e036555. [Google Scholar] [CrossRef] [PubMed]
- Benítez-Muñoz, J.A.; Guisado-Cuadrado, I.; Rojo-Tirado, M.Á.; Alcocer-Ayuga, M.; Romero-Parra, N.; Peinado, A.B.; Cupeiro, R. Females have better metabolic flexibility in different metabolically challenging stimuli. Appl. Physiol. Nutr. Metab. 2025, 50, 1–12. [Google Scholar] [CrossRef]
- Kleis-Olsen, A.S.; Farlov, J.E.; Petersen, E.A.; Schmücker, M.; Flensted-Jensen, M.; Blom, I.; Ingersen, A.; Hansen, M.; Helge, J.W.; Dela, F.; et al. Metabolic flexibility in postmenopausal women: Hormone replacement therapy is associated with higher mitochondrial content, respiratory capacity, and lower total fat mass. Acta Physiol. 2024, 240, e14117. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ Collaboration; Baigent, C.; Blackwell, L.; Emberson, J.; Holland, L.E.; Reith, C.; Bhala, N.; Peto, R.; Collins, R.; Landray, M.J.; et al. Efficacy and safety of cholesterol-lowering treatment: Prospective meta-analysis of data from 90,056 participants in 14 randomised trials of statins. Lancet 2005, 366, 1267–1278. [Google Scholar] [CrossRef]
- Cholesterol Treatment Trialists’ (CTT) Collaboration; Fulcher, J.; O’Connell, R.; Voysey, M.; Emberson, J.; Blackwell, L.; Mihaylova, B.; Simes, J.; Collins, R.; Kirby, A.; et al. Efficacy and safety of LDL-lowering therapy among men and women: Meta-analysis of individual data from 174,000 participants in 27 randomised trials. Lancet 2015, 385, 1397–1405. [Google Scholar] [CrossRef]
- Peters, S.A.E.; Huxley, R.R.; Woodward, M. Do women gain the same benefit as men from cardiovascular drug treatments? Systematic review and meta-analysis of randomized controlled trials. Eur. Heart J. 2014, 35, 1914–1922. [Google Scholar] [CrossRef]
- Hunt, N.B.; Emmens, J.E.; Irawati, S.; de Vos, S.; Bos, J.H.J.; Wilffert, B.; Hak, E.; de Boer, R.A. Sex disparities in the effect of statins on lipid parameters: The PharmLines Initiative. Medicine 2022, 101, e28394. [Google Scholar] [CrossRef]
- Kim, C.J.; Park, M.W.; Kim, M.C.; Choo, E.H.; Hwang, B.H.; Lee, K.Y.; Choi, Y.S.; Kim, H.Y.; Yoo, K.D.; Jeon, D.S.; et al. TALOS-AMI Investigators. Unguided de-escalation from ticagrelor to clopidogrel in stabilised patients with acute myocardial infarction undergoing percutaneous coronary intervention (TALOS-AMI): An investigator-initiated, open-label, multicentre, non-inferiority, randomised trial. Lancet 2021, 398, 1305–1316. [Google Scholar] [CrossRef]
- Palmerini, T.; Sangiorgi, D.; Valgimigli, D.; Biondi-Zoccai, F.; Feres, F.; Abizaid, A.; Costa, R.A.; Hong, M.K.; Kim, B.K.; Jang, Y.; et al. Comparison of clinical outcomes with drug-eluting and bare-metal stents in patients with ST-segment elevation myocardial infarction: A meta-analysis of randomized controlled trials. J. Am. Coll. Cardiol. 2015, 65, 1092–1102. [Google Scholar] [CrossRef]
- Piccolo, R.; Laino, A.; Vitale, A.P.; Franzone, A.; Esposito, G. Sex-related differences in efficacy and safety of antithrombotic therapy in patients with coronary artery disease: Systematic review and meta-analysis. BMJ 2025, 390, e082974. [Google Scholar] [CrossRef]
- Franzone, A.; Piccolo, R.; Gargiulo, G.; Ariotti, S.; Marino, M.; Santucci, A.; Capodanno, D.; Gatto, L.; Colonna, G.; Tamburino, C.; et al. Prolonged versus short duration of dual antiplatelet therapy after percutaneous coronary intervention in patients with or without peripheral arterial disease: A subgroup analysis of the PRODIGY randomized clinical trial. JAMA Cardiol. 2016, 1, 795–803. [Google Scholar] [CrossRef]
- Boersma, E.; Harrington, R.A.; Moliterno, D.J.; White, H.; Théroux, P.; Van de Werf, F.; de Torbal, A.; Armstrong, P.W.; Wallentin, L.C.; Wilcox, R.G.; et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: A meta-analysis of all major randomised clinical trials. Lancet 2002, 359, 189–198. [Google Scholar] [CrossRef]
- Scantlebury, D.C.; Borlaug, B.A. Why are women more likely than men to develop heart failure with preserved ejection fraction? Curr. Opin. Cardiol. 2011, 26, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Stehli, J.; Martin, C.; Brennan, A.; Dinh, D.T.; Lefkovits, J.; Zaman, S. Sex Differences Persist in Time to Presentation, Revascularization, and Mortality in Myocardial Infarction Treated With Percutaneous Coronary Intervention. J. Am. Heart Assoc. 2019, 8, e012161. [Google Scholar] [CrossRef] [PubMed]
- Vranckx, P.; Valgimigli, M.; Windecker, S.; Steg, P.G.; Hamm, C.; Jüni, P.; Garcia-Garcia, H.M.; van Es, G.A.; Serruys, P.W. Long-term ticagrelor monotherapy versus standard dual antiplatelet therapy followed by aspirin monotherapy in patients undergoing biolimus-eluting stent implantation: Rationale and design of the GLOBAL LEADERS trial. EuroIntervention 2015, 10, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Regitz-Zagrosek, V.; Kararigas, G. Mechanistic pathways of sex differences in cardiovascular disease. Physiol. Rev. 2016, 97, 1–37. [Google Scholar] [CrossRef]
| Condition | Men | Women |
|---|---|---|
| Overall CVD prevalence |
|
|
| Age of CVD onset |
|
|
| Heart failure |
|
|
| Myocardial infarction |
|
|
| Valvular heart disease Mitral valve Aortic valve |
|
|
| Peripheral artery disease |
|
|
| Stroke |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iboleon-Jimenez, A.; Contreras-Muñoz, A.; Peláez-Berdún, C.; Franco-Hita, R.; Sesmero, A.; Robles-Mezcua, A.; García-Pinilla, J.M.; Jimenez-Navarro, M.; Murri, M. Mitochondria, Sex, and Cardiovascular Disease: A Complex Interplay. Int. J. Mol. Sci. 2025, 26, 8971. https://doi.org/10.3390/ijms26188971
Iboleon-Jimenez A, Contreras-Muñoz A, Peláez-Berdún C, Franco-Hita R, Sesmero A, Robles-Mezcua A, García-Pinilla JM, Jimenez-Navarro M, Murri M. Mitochondria, Sex, and Cardiovascular Disease: A Complex Interplay. International Journal of Molecular Sciences. 2025; 26(18):8971. https://doi.org/10.3390/ijms26188971
Chicago/Turabian StyleIboleon-Jimenez, Andrea, Alberto Contreras-Muñoz, Cristian Peláez-Berdún, Rafael Franco-Hita, Alba Sesmero, Ainhoa Robles-Mezcua, Jose M. García-Pinilla, Manuel Jimenez-Navarro, and Mora Murri. 2025. "Mitochondria, Sex, and Cardiovascular Disease: A Complex Interplay" International Journal of Molecular Sciences 26, no. 18: 8971. https://doi.org/10.3390/ijms26188971
APA StyleIboleon-Jimenez, A., Contreras-Muñoz, A., Peláez-Berdún, C., Franco-Hita, R., Sesmero, A., Robles-Mezcua, A., García-Pinilla, J. M., Jimenez-Navarro, M., & Murri, M. (2025). Mitochondria, Sex, and Cardiovascular Disease: A Complex Interplay. International Journal of Molecular Sciences, 26(18), 8971. https://doi.org/10.3390/ijms26188971







