Efficient Recovery of Valeric Acid Using Phosphonium-Based Ionic Liquids
Abstract
1. Introduction
2. Results
2.1. Experimental Results
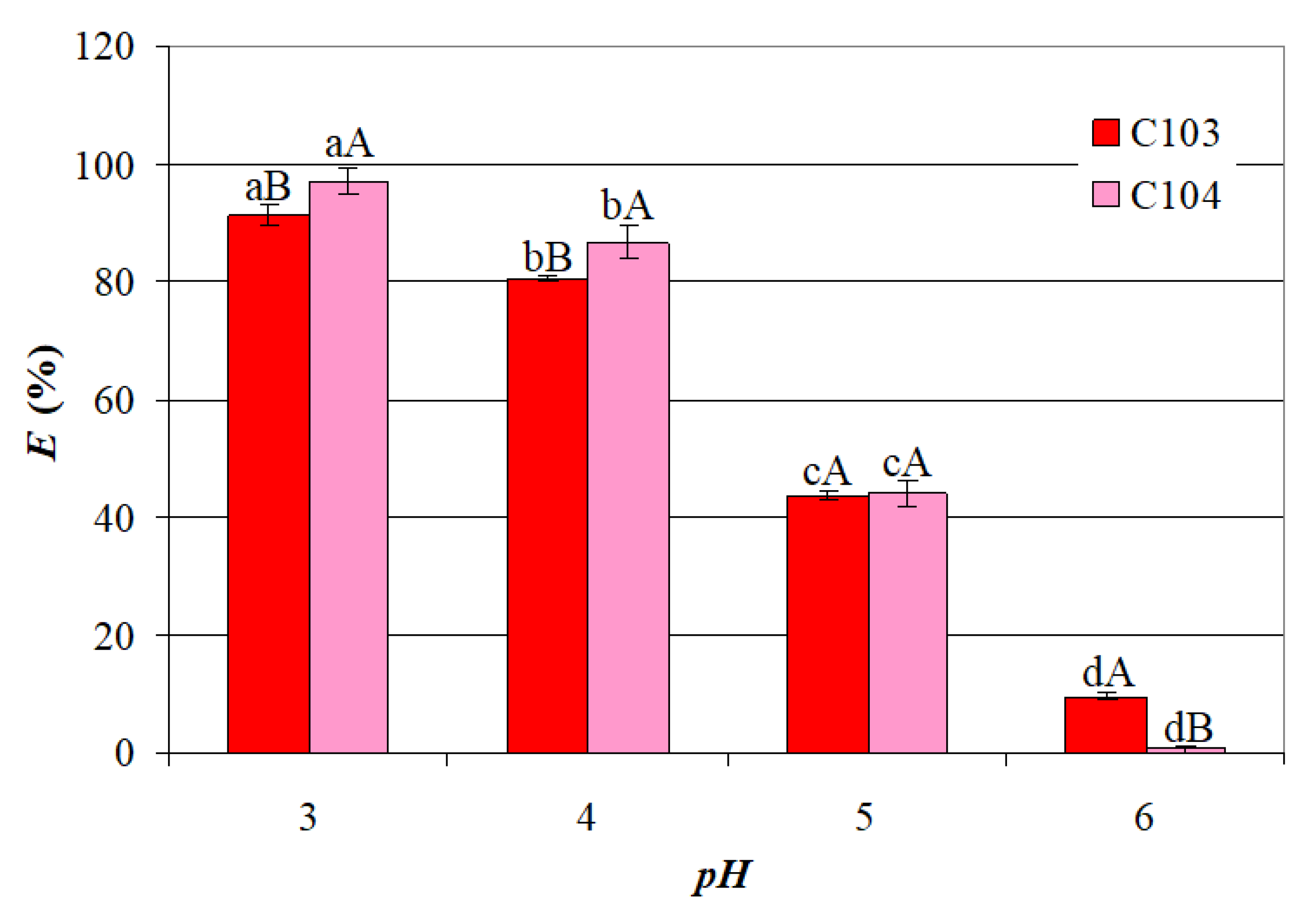
- A significant decrease in the mean values of E with an increase in pH from 3 to 6, i.e., from 91.21% to 9.489% for C103 and from 96.95% to 0.694% for C104;
- Significantly higher mean values of EC104 than those of EC103 for pH = 3 and pH = 4, similar mean values of EC103 and EC104 for pH = 5, and significantly higher mean value of EC103 than that of EC104 for pH = 6.
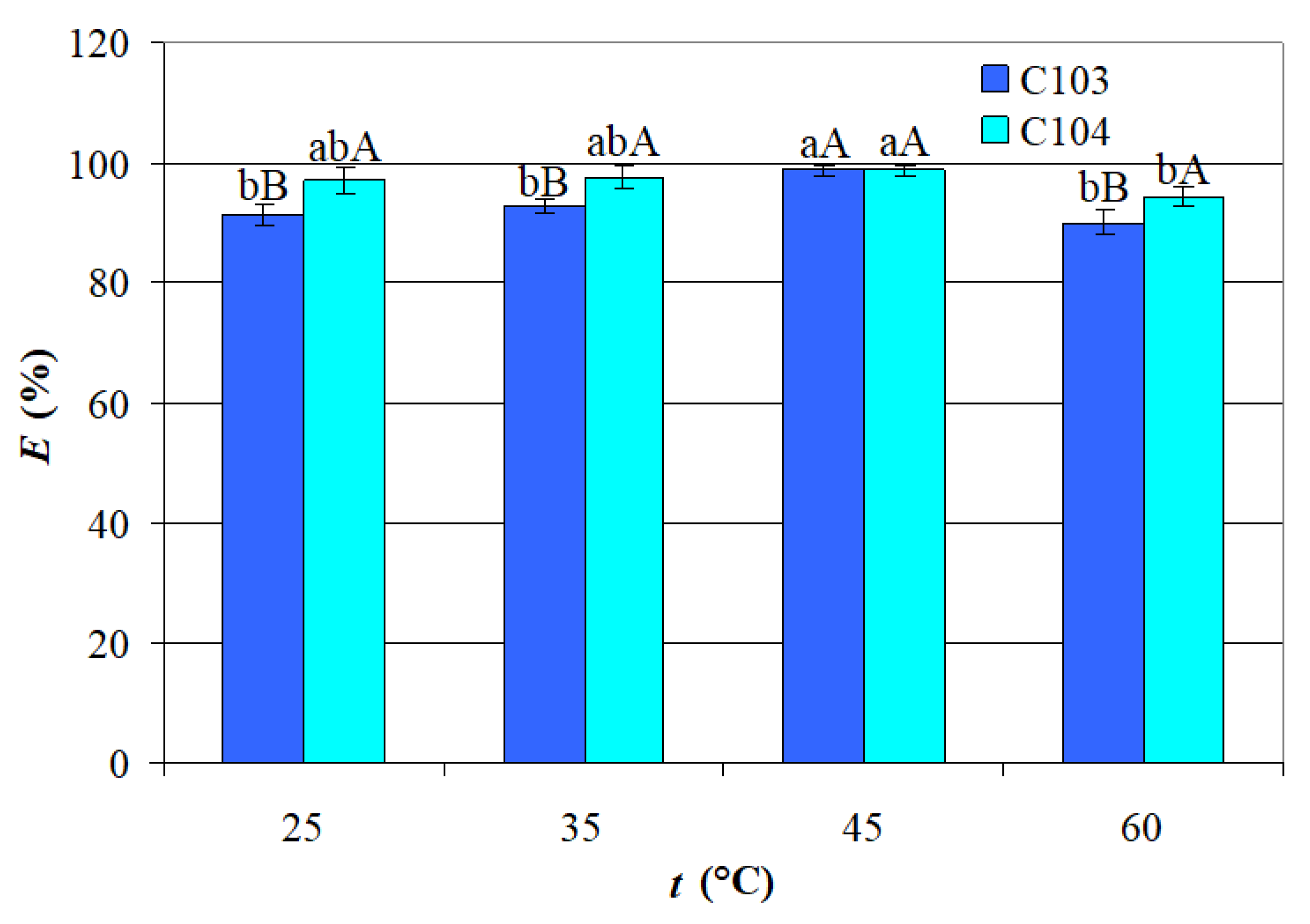
- The mean value of EC103 obtained at t = 45 °C (98.59%) was significantly higher than the mean values of EC103 attained at the other t levels (90.05–92.89%), which were not significantly different; this trend may be attributed to the system approaching equilibrium conditions, where complexation between the acid and ILs occurs predominantly at the organic-aqueous interface; these complexation reactions are generally exothermic and sensitive to thermal variations; as temperature increases, the kinetic energy of molecules also rises, potentially disrupting weaker interactions while promoting the formation of more stable acid–IL ILs complexes; additionally, the exothermic nature of hydrogen bonding involved in complex formation may contribute to a reduction in system entropy, further influencing extraction behavior [30].
- The mean value of EC104 obtained at t = 45 °C (98.68%) was significantly higher than that attained at t = 60 °C (94.23%);
- The mean values of EC103 and EC104 reached at t = 45 °C were similar, whereas the mean values of EC104 obtained at the other t levels (94.23–97.49%) were significantly higher than those of EC103 (90.05–92.89%).
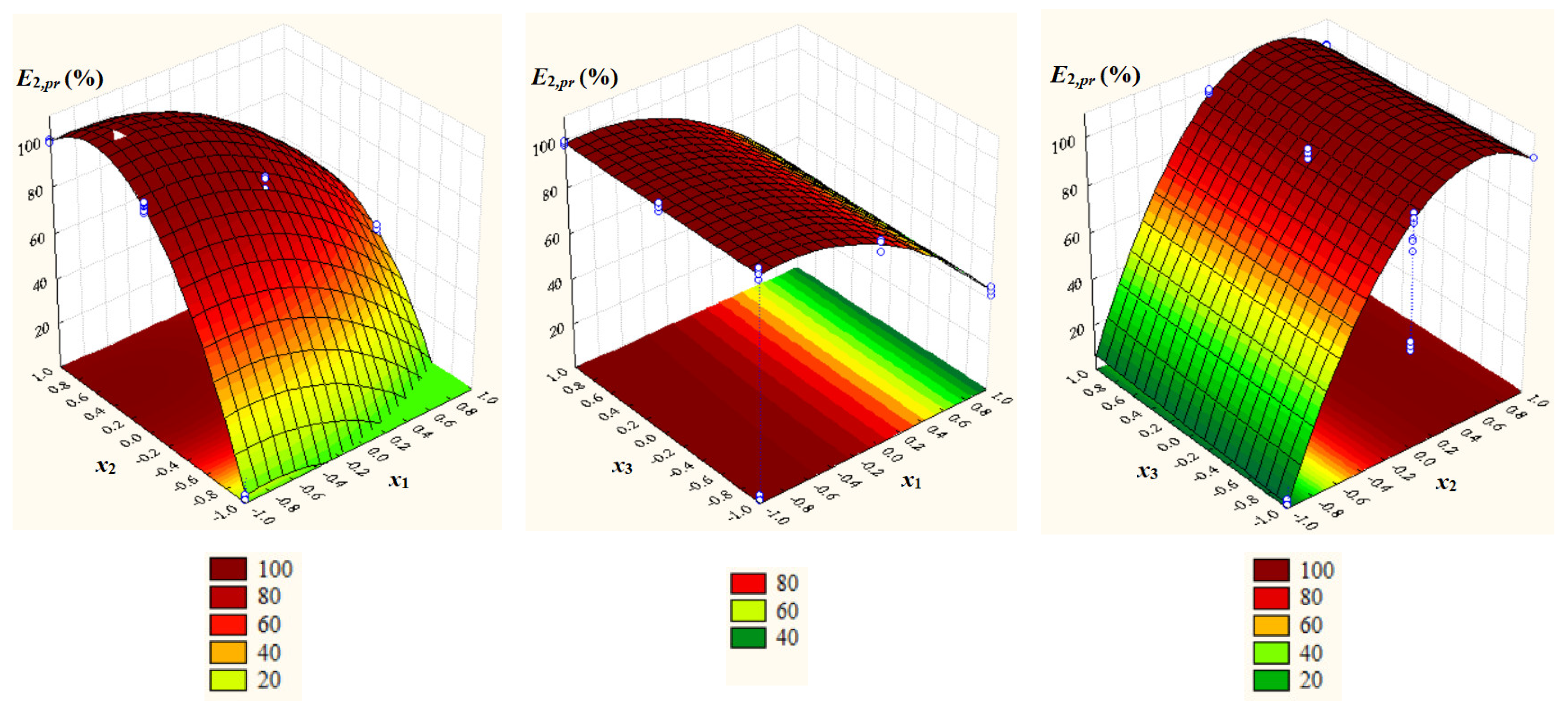
2.2. Statistical Models
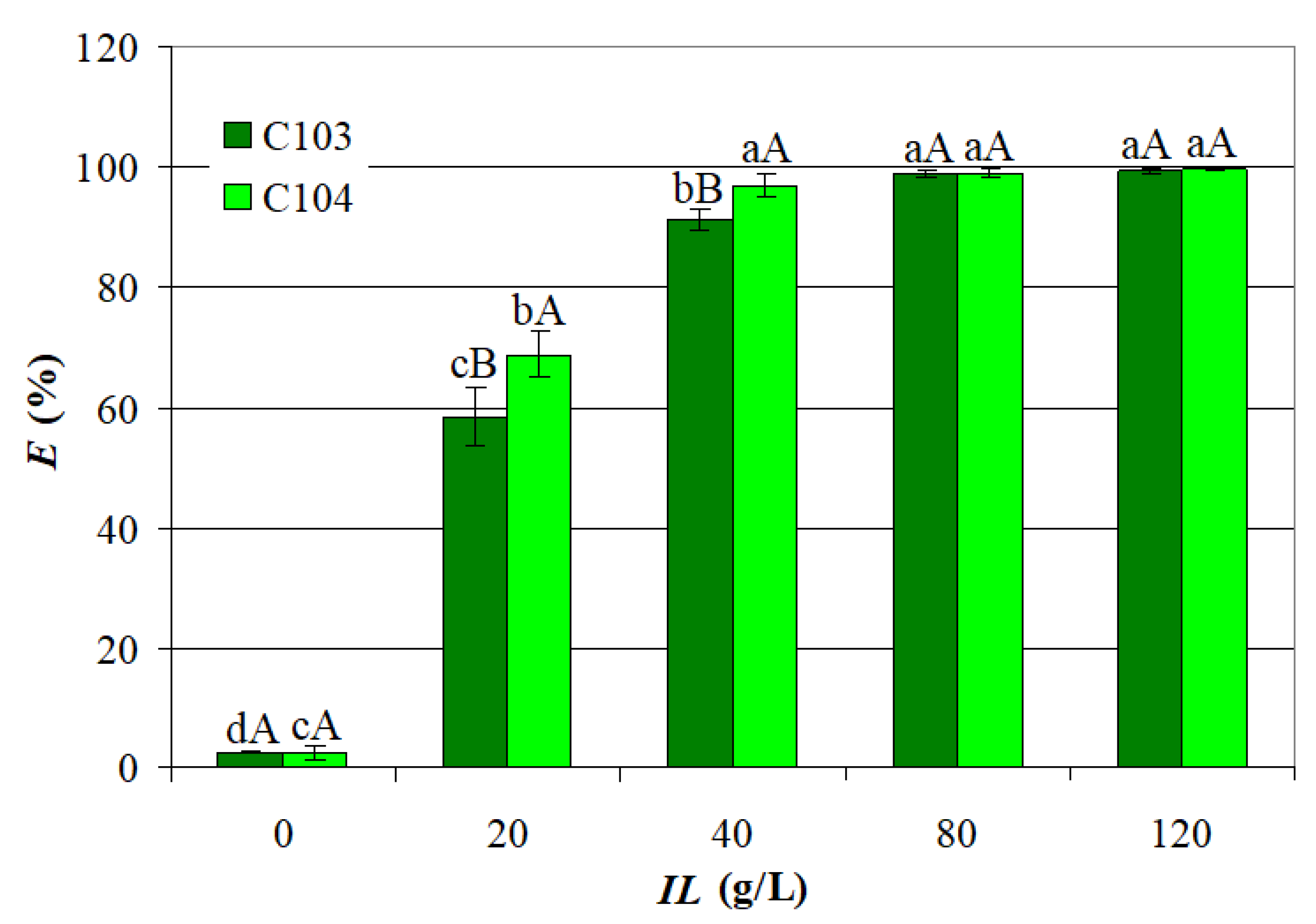
- E1 = EC103 ranged from 2.370% to 99.78% (72.58 ± 34.51%), and E2 = EC104 from 1.431% to 99.76% (75.05 ± 35.68%); MN1 = 72.58% and MN2 = 75.05% were not significantly different, i.e., p = 0.41 (one-tail) and p = 0.82 (two-tail);
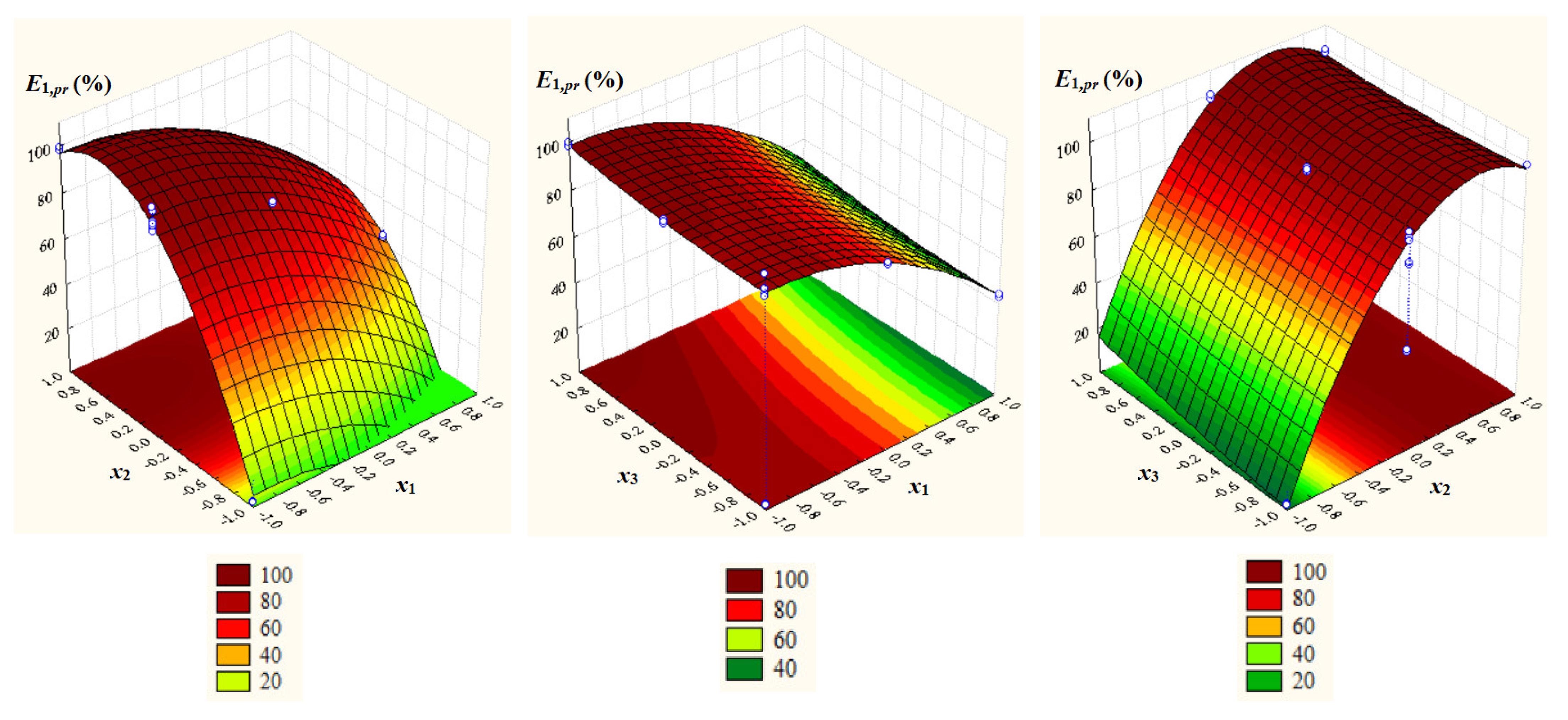
- Significant positive effects of dimensionless concentration of IL (x2), dimensionless temperature (x3), and x32 as well as significant negative effects of dimensionless pH (x1), x12, x22, and x2x3 on E1,pr = EC103,pr;
- Significant positive effect of x2 and significant negative effects of x1, x12, and x22 on E2,pr = EC104,pr;
- A very good agreement between experimental and predicted values of process response variables (Rj2 ≥ 0.9983, Rj,adj2 ≥ 0.9974, Fj ≥ 1117.4, and pj = 0.0000 for j = 1, 2).
2.3. Optimization of Process Factors
3. Discussion
- At low Z values (Z < 0.5), a 1:1 acid-to-extractant complex is typically formed;
- At intermediate Z values (0.5 < Z < 1), 2:1 or 1:2 acid-to-extractant complexes may form in the organic phase;
- At high Z values (Z > 1), a 2:1 acid-to-extractant complex may form.
4. Materials and Methods
4.1. Chemicals and Procedures
4.2. Statistical Analysis, Modeling, and Factor Optimization
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
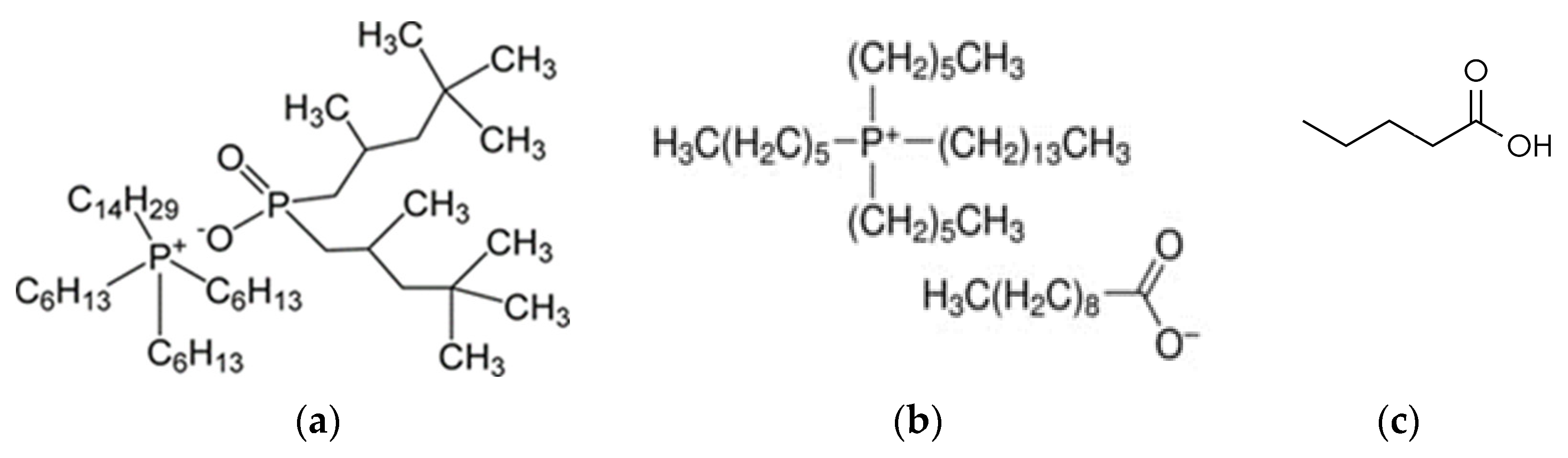
| C103 | trihexyl(tetradecyl)phosphonium decanoate |
| C104 | trihexyl(tetradecyl)phosphonium bis(2,4,4-trimethylpentyl)phosphinate |
| VA | valeric acid |
| IL | ionic liquid |
References
- Jiang, J.; Zhang, Y.; Li, K.; Wang, Q.; Gong, C.; Li, M. Volatile fatty acids production from food waste: Effects of pH, temperature, and organic loading rate. Bioresour. Technol 2013, 143, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Chen, Y.; Zheng, X. Enhancement of waste activated sludge protein conversion and volatile fatty acids accumulation during waste activated sludge anaerobic fermentation by carbohydrate substrate addition: The effect of pH. Environ. Sci. Technol. 2009, 43, 4373–4380. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Chen, H.; Hu, L.; Yu, L.; Chen, Y.; Gu, G. Pilot-scale waste activated sludge alkaline fermentation, fermentation liquid separation, and application of fermentation liquid to improve biological nutrient removal. Environ. Sci. Technol. 2011, 45, 1834–1839. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.; Chua, H.; Zhao, Q.; Shirley, S.N.; Ren, J. Optimal production of polyhydroxyalkanoates (PHA) in activated sludge fed by volatile fatty acids (VFAs) generated from alkaline excess sludge fermentation. Bioresour. Technol. 2009, 100, 1399–1405. [Google Scholar] [CrossRef]
- Chen, H.; Meng, H.; Nie, Z.; Zhang, M. Polyhydroxyalkanoate production from fermented volatile fatty acids: Effect of pH and feeding regimes. Bioresour. Technol. 2013, 128, 533–538. [Google Scholar] [CrossRef]
- Shi, B.; Huang, J.; Lin, Y.; Han, W.; Qiu, S.; Zhang, D.; Tang, J.; Hou, P. Towards Valeric Acid Production from Riboflavin-Assisted Waste Sludge: pH-Dependent Fermentation and Microbial Community. Waste Biomass Valor. 2023, 14, 833–845. [Google Scholar] [CrossRef]
- Sukphun, P.; Sittijunda, S.; Reungsang, A. Volatile fatty acid production from organic waste with the emphasis on membrane-based recovery. Fermentation 2021, 7, 159. [Google Scholar] [CrossRef]
- Cavinato, C.; Da Ros, C.; Pavan, P.; Bolzonella, D. Influence of temperature and hydraulic retention on the production of volatile fatty acids during anaerobic fermentation of cow manure and maize silage. Bioresour. Technol. 2017, 223, 59–64. [Google Scholar] [CrossRef]
- Goldberg, I.; Rokem, J.S. Organic and Fatty Acid Production, Microbial. In Encyclopedia of Microbiology, 3rd ed.; Elsevier: Amsterdam, The Netherlands, 2009; pp. 421–442. [Google Scholar] [CrossRef]
- Saravanapriya, P.; Devi, K.P. Plant extracts with putative hepatotoxicity activity. In Influence of Nutrients, Bioactive Compounds, and Plant Extracts in Liver Diseases; Elsevier: Amsterdam, The Netherlands, 2021; pp. 259–287. [Google Scholar] [CrossRef]
- Bhanuchander, P.; Samudrala, S.; Putrakumar, B.; Vijayanand, P.; Kumar, B.; Chary, V. Hydrogenation of levulinic acid to valeric acid over platinum–tungsten catalysts supported on γ-Al2O3. New J. Chem. 2019, 43, 18003–18011. [Google Scholar] [CrossRef]
- Ganigue, R.; Naert, P.; Candry, P.; Smedt, J.; Stevens, C.; Rabaey, K. Fruity flavors from waste: A novel process to upgrade crude glycerol to ethyl valerate. Bioresour. Technol. 2019, 289, 121574. [Google Scholar] [CrossRef]
- Gebresillase, M.N.; Hong, D.H.; Lee, J.-H.; Cho, E.-B.; Seo, J.G. Direct solvent-free selective hydrogenation of levulinic acid to valeric acid over multi-metal [NixCoyMnzAlw]-doped mesoporous silica catalysts. Chem. Eng. J. 2023, 472, 144591. [Google Scholar] [CrossRef]
- Yin, J.; Liu, J.; Chen, T.; Long, Y.; Shen, D. Influence of melanoidins on acidogenic fermentation of food waste to produce volatility fatty acids. Bioresour. Technol. 2019, 284, 121–127. [Google Scholar] [CrossRef]
- Ye, M.; Luo, J.; Zhang, S.; Yang, H.; Li, Y.; Liu, J. In-situ ammonia stripping with alkaline fermentation of waste activated sludge to improve short-chain fatty acids production and carbon source availability. Bioresour. Technol. 2020, 301, 122782. [Google Scholar] [CrossRef]
- Liu, X.; Du, M.; Yang, J.; Wu, Y.; Xu, Q.; Wang, D.; Yang, Q.; Yang, G.; Li, X. Sulfite serving as a pretreatment method for alkaline fermentation to enhance short-chain fatty acid production from waste activated sludge. Chem. Eng. J. 2020, 385, 123991. [Google Scholar] [CrossRef]
- Huang, X.; Shen, C.; Liu, J.; Lu, L. Improved volatile fatty acid production during waste activated sludge anaerobic fermentation by different bio-surfactants. Chem. Eng. J. 2015, 264, 280–290. [Google Scholar] [CrossRef]
- Wu, Q.; Guo, W.; Bao, X.; Yin, R.; Feng, X.; Zheng, H.; Luo, H.; Ren, N. Enhancing sludge biodegradability and volatile fatty acid production by tetrakis hydroxymethyl phosphonium sulfate pretreatment. Bioresour. Technol. 2017, 239, 518–522. [Google Scholar] [CrossRef]
- Kaur, G.; Garcia-Gonzalez, L.; Elst, K.; Truzzi, F.; Bertin, L.; Kaushik, A.; Balakrishnan, M.; De Wever, H. Reactive extraction for in-situ carboxylate recovery from mixed culture fermentation. Biochem. Eng. J. 2020, 16, 107641. [Google Scholar] [CrossRef]
- Alkaya, E.; Kaptan, S.; Ozkan, L.; Uludag-Demirer, S.; Demirer, G.N. Recovery of acids from anaerobic acidification broth by liquid–liquid extraction. Chemosphere 2009, 77, 1137–1142. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.A.A.; Raeissi, S.; Hage, P.; Weggemans, W.M.A.; van Spronsen, J.; Peters, C.J.; Kroon, M.C. Recovery of volatile fatty acids from water using medium-chain fatty acids and a cosolvent. Chem. Eng. Sci. 2017, 165, 74–80. [Google Scholar] [CrossRef]
- Begum, S.; Arelli, V.; Anupoju, G.R.; Sridhar, S.; Bhargava, S.K.; Eshtiaghi, N. Optimization of feed and extractant concentration for the liquid–liquid extraction of volatile fatty acids from synthetic solution and landfill leachate. J. Ind. Eng. Chem. 2020, 90, 190–202. [Google Scholar] [CrossRef]
- Reyhanitash, E.; Fufachev, E.; van Munster, K.D.; van Beek, M.B.M.; Sprakel, L.M.J.; Edelijn, C.N.; Weckhuysen, B.M.; Kersten, S.R.A.; Bruijnincx, P.C.A.; Schuur, B. Recovery and conversion of acetic acid from a phosphonium phosphinate ionic liquid to enable valorization of fermented wastewater. Green Chem. 2019, 21, 2023–2034. [Google Scholar] [CrossRef]
- Singh, R.; Kumar, N.; Parameswaran, P.; Simmons, B.A.; Sale, K.; Sun, N. Volatile fatty acid extraction from fermentation broth using a hydrophobic ionic liquid and in situ enzymatic esterification. RSC Sustain. 2025, 3, 311–322. [Google Scholar] [CrossRef]
- Andersen, S.J.; Berton, J.K.; Naert, P.; Gildemyn, S.; Rabaey, K.; Stevens, C.V. Extraction and esterification of low-titer short-chain volatile fatty acids from anaerobic fermentation with ionic liquids. ChemSusChem 2016, 9, 2059–2063. [Google Scholar] [CrossRef] [PubMed]
- Xing, T.; Yu, S.; Tang, J.; Liu, H.; Zhen, F.; Sun, Y.; Kong, X. Liquid–Liquid extraction of volatile fatty acids from anaerobic acidification broth using ionic liquids and cosolvent. Energies 2023, 16, 785. [Google Scholar] [CrossRef]
- Tönjes, S.; Uitterhaegen, E.; De Winter, K.; Soetaert, W. Reactive extraction technologies for organic acids in industrial fermentation processes—A review. Sep. Purif. Technol. 2025, 356, 129881. [Google Scholar] [CrossRef]
- Žilnik, L.F.; Likozar, B. Back-extraction process operation and modeling through thermodynamic equilibrium solubility of valeric acid in aqueous and organic phase mixtures. Sep. Purif. Technol. 2019, 222, 125–135. [Google Scholar] [CrossRef]
- Yudaev, P.A.; Chistyakov, E.M. Ionic liquids as components of systems for metal extraction. ChemEngineering 2022, 6, 6. [Google Scholar] [CrossRef]
- Inyang, V.; Lokhat, D. Reactive extraction of malic acid using trioctylamine in 1–decanol: Equilibrium studies by response surface methodology using Box Behnken optimization technique. Sci. Rep. 2020, 10, 2400. [Google Scholar] [CrossRef]
- Skoronski, E.; Fernandes, M.; Malaret, F.J.; Hallett, J.P. Use of phosphonium ionic liquids for highly efficient extraction of phenolic compounds from water. Sep. Purif. Technol. 2020, 248, 117069. [Google Scholar] [CrossRef]
- Çevik, A.; Aşçı, Y.S.; Lalikoglu, M. Investigation of the effects of ionic liquid as diluent in separation of lactic acid from aqueous media by reactive extraction. Biomass Conv. Bioref. 2022, 12, 1323–1330. [Google Scholar] [CrossRef]
- Eda, S.; Borra, A.; Parthasarathy, R.; Bankupalli, S.; Bhargava, S.; Thella, P.K. Recovery of levulinic acid by reactive extraction using tri-n-octylamine in methyl isobutyl ketone: Equilibrium and thermodynamic studies and optimization using Taguchi multivariate approach. Sep. Purif. Technol. 2018, 197, 314–324. [Google Scholar] [CrossRef]
- Antony, F.M.; Wasewar, K. Effect of temperature on equilibria for physical and reactive extraction of protocatechuic acid. Heliyon 2020, 6, e03664. [Google Scholar] [CrossRef]
- Uslu, H.; Datta, D.; Bamufleh, H.S. Reactive extraction of phenol from aqueous solution using tri-octylamine dissolved in alkanes and alcohols. J. Mol. Liq. 2015, 212, 430–435. [Google Scholar] [CrossRef]
- Marták, J.; Schlosser, S. Extraction of lactic acid by phosphonium ionic liquids. Sep. Purif. Technol. 2007, 57, 483–494. [Google Scholar] [CrossRef]
- Baylan, N. Separation of valeric acid from aqueous solutions by reactive extraction using 1-hexyl-3-methylimidazolium hexafluorophosphate. Desalination Water Treat. 2019, 172, 184–189. [Google Scholar] [CrossRef]
- Firdous, K.; Ahmad, S.A. Reactive extraction of valeric acid with a tributyl phosphate + diluent system. J. Chem. Eng. Data 2020, 65, 5096–5100. [Google Scholar] [CrossRef]
- Senol, A.; Bilgin, M.; Baslioglu, B. Optimal reactive extraction of valeric acid from aqueous solutions using tri-n-propyl amine/diluent and dibenzyl amine/diluent Systems. Chem. Biochem. Eng. Q. 2016, 30, 317–330. [Google Scholar] [CrossRef]
- Mukherjee, S.; Munshi, B. Recovery of valeric acid using green solvents. Can. J. Chem. Eng. 2023, 101, 1633–1644. [Google Scholar] [CrossRef]
- Drăghici-Popa, A.M.; Boscornea, A.C.; Brezoiu, A.M.; Tomas, Ș.T.; Pârvulescu, O.C.; Stan, R. Effects of extraction process factors on the composition and antioxidant activity of blackthorn (Prunus spinosa L.) fruit extracts. Antioxidants 2023, 12, 1897. [Google Scholar] [CrossRef]
| Pretreatment | VA (mg COD/g VSS) | VA (% COD) | VFA Production (mg COD/g VSS) | Reference |
|---|---|---|---|---|
| In situ ammonia stripping | 77.2 | 25 | 308 | [15] |
| Sulfite | 16 | 4.9 | 324 | [16] |
| Riboflavin | 220.1 | 62.8 | 355 | [6] |
| Saponin | 113.8 | 40 | 292 | [17] |
| Tetrakis hydroxymethyl phosphonium sulfate | 72.7 | 37.5 | 194 | [18] |
| No. | X1 = pH | X2 = IL (g/L) | X3 = t (°C) | x1 | x2 | x3 |
|---|---|---|---|---|---|---|
| 1 | 3 | 40 | 25 | −1 | 0 | −1 |
| 2 | 4 | 40 | 25 | 0 | 0 | −1 |
| 3 | 5 | 40 | 25 | 1 | 0 | −1 |
| 4 | 3 | 0 | 25 | −1 | −1 | −1 |
| 5 | 3 | 80 | 25 | −1 | 1 | −1 |
| 6 | 3 | 40 | 35 | −1 | 0 | 0 |
| 7 | 3 | 40 | 45 | −1 | 0 | 1 |
| i | 1 | 2 | 3 | 1 | 2 | 3 |
| MINi | 3 | 0 | 25 | −1 | −1 | −1 |
| MAXi | 5 | 80 | 45 | 1 | 1 | 1 |
| MNi | 4 | 40 | 35 | 0 | 0 | 0 |
| Experimental Extraction Efficiency | Ej (%) | |
|---|---|---|
| j | 1 (C103) | 2 (C104) |
| Minimum value, MINj | 2.370 | 1.431 |
| Maximum value, MAXj | 99.78 | 99.76 |
| Mean value, MNj | 72.58 | 75.05 |
| Standard deviation, SDj | 34.51 | 35.68 |
| Coefficient of variation, CVj = 100SDj/MNj | 47.55 | 47.54 |
| Predicted extraction efficiency | Ej,pr (%) | |
| Regression coefficients | ||
| α0j | 82.111 | 87.155 |
| α1j | −23.851 | −26.500 |
| α11j | −13.077 | −16.166 |
| α2j | 44.444 | 47.202 |
| α22j | −40.515 | −45.790 |
| α3j | 3.6882 | 0.8649 |
| α33j | 2.0150 | 0.3300 |
| α12j | 0.0000 | 0.0000 |
| α13j | 0.0000 | 0.0000 |
| α23j | −3.6882 | −1.3974 |
| Coefficient of determination, adjusted coefficient of determination, F statistic and its pj-value | ||
| Rj2 | 0.9994 | 0.9983 |
| Rj,adj2 | 0.9991 | 0.9974 |
| Fj | 3264.6 | 1117.4 |
| pj | 0.0000 | 0.0000 |
| j | x1,opt | x2,opt | x3,opt | pHopt | ILopt (g/L) | topt (°C) | Ej,pr,opt (%) | Ej,m,opt ± SDj (%) |
|---|---|---|---|---|---|---|---|---|
| 1 | −0.2 | 0.5 | −1 | 3.8 | 60 | 25 | 98.62 | 98.61 ± 0.18 |
| 2 | 0 | 0.5 | −1 | 4 | 60 | 25 | 99.47 | ±0.19 |
| Extractant | C103 | C104 | ||
|---|---|---|---|---|
| IL (g/L) | IL (mol/L) | Z | IL (mol/L) | Z |
| 20 | 0.031 | 1.88 | 0.025 | 2.61 |
| 40 | 0.061 | 1.46 | 0.051 | 1.83 |
| 80 | 0.122 | 0.79 | 0.103 | 0.94 |
| 120 | 0.183 | 0.53 | 0.155 | 0.63 |
| Extractant | C103 | C104 | ||||||
|---|---|---|---|---|---|---|---|---|
| t (°C) | Em (%) | Kd | Z | Ke | Em (%) | Kd | Z | Ke |
| 25 | 91.21 | 10.18 | 1.57 | 70.74 | 96.95 | 31.31 | 1.83 | 532.93 |
| 35 | 92.89 | 13.06 | 1.58 | 114.05 | 97.49 | 38.82 | 1.85 | 814.26 |
| 45 | 98.59 | 73.33 | 1.60 | 3388.06 | 98.68 | 74.97 | 1.87 | 3000.17 |
| 60 | 90.05 | 9.22 | 1.57 | 58.64 | 94.23 | 16.01 | 1.78 | 143.48 |
| Study | Extractant System | Valeric Acid Concentration (mol/L) | Max. Extraction Efficiency (%) |
|---|---|---|---|
| Baylan (2019) [37] | [HMIM][PF6] + TBP | 0.10–0.30 | 87.96 |
| Firdous & Ahmad (2020) [38] | TBP + kerosene | 0.05–0.13 | 97.64 |
| Senol (2015) [39] | TPA + ethyl valerate | 0.10 | 99.07 |
| Mukherjee & Munshi (2022) [40] | 40% TBP + sunflower oil | 0.01–0.10 | 96.42 |
| 40% TBP + soybean oil | 0.01–0.10 | 96.18 | |
| This work | C103/C104 + heptane | 0.097 | 98.61/99.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaga, A.C.; Parvulescu, O.C.; Cascaval, D.; Galaction, A.I. Efficient Recovery of Valeric Acid Using Phosphonium-Based Ionic Liquids. Int. J. Mol. Sci. 2025, 26, 8970. https://doi.org/10.3390/ijms26188970
Blaga AC, Parvulescu OC, Cascaval D, Galaction AI. Efficient Recovery of Valeric Acid Using Phosphonium-Based Ionic Liquids. International Journal of Molecular Sciences. 2025; 26(18):8970. https://doi.org/10.3390/ijms26188970
Chicago/Turabian StyleBlaga, Alexandra Cristina, Oana Cristina Parvulescu, Dan Cascaval, and Anca Irina Galaction. 2025. "Efficient Recovery of Valeric Acid Using Phosphonium-Based Ionic Liquids" International Journal of Molecular Sciences 26, no. 18: 8970. https://doi.org/10.3390/ijms26188970
APA StyleBlaga, A. C., Parvulescu, O. C., Cascaval, D., & Galaction, A. I. (2025). Efficient Recovery of Valeric Acid Using Phosphonium-Based Ionic Liquids. International Journal of Molecular Sciences, 26(18), 8970. https://doi.org/10.3390/ijms26188970









