Head and Neck Malignancies in Autoimmune Polyendocrine Syndrome Type 1 (APS-1/APECED): A Scoping Review of Molecular Pathogenesis, Clinical Features, and Outcomes
Abstract
1. Background
2. Results
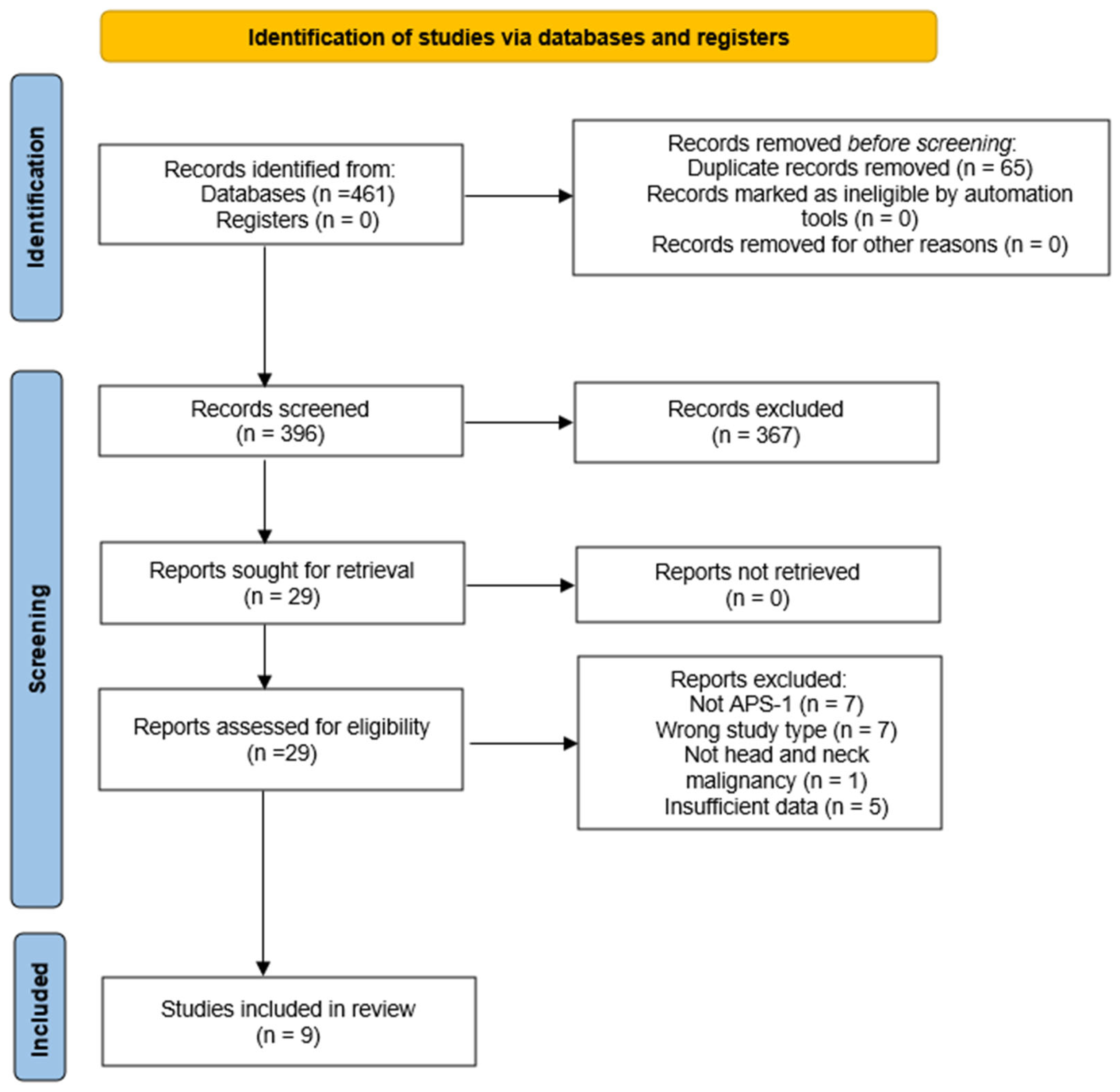
2.1. Study Selection and Characteristics of Included Studies
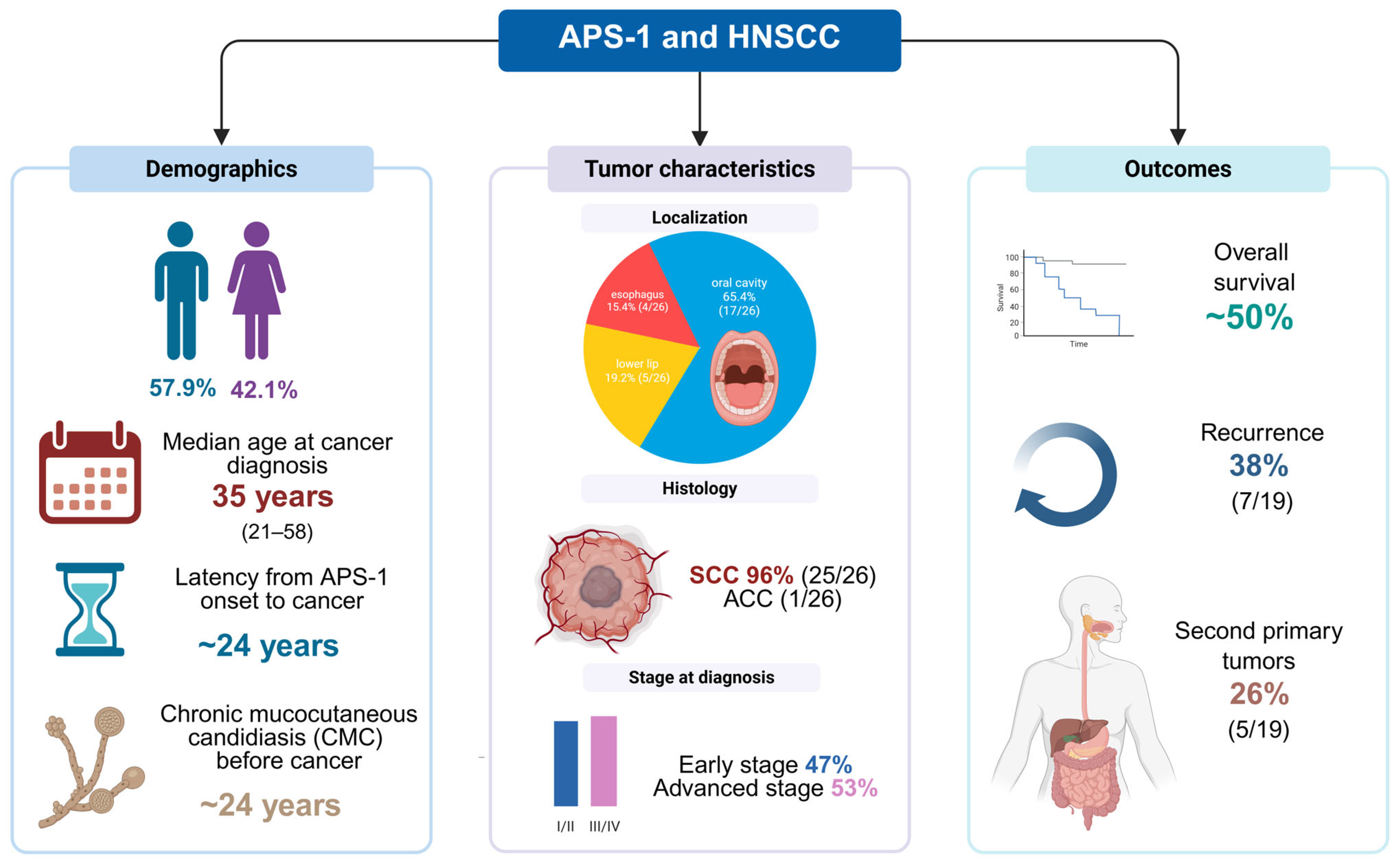
2.2. Demographic and Clinical Characteristics
2.3. Clinical Features of APS-1
2.4. Genetics and Autoantibodies
2.5. Tumor Location, Grade and Stage
2.6. Conventional Head and Neck Cancer Risk Factors
2.7. Treatment Modalities and Adjunctive Antifungal Therapy
2.8. Outcomes, Follow-Up, Recurrence and Second Primary Tumors
2.9. Additional Italian Cohort Data
2.10. Summary Table
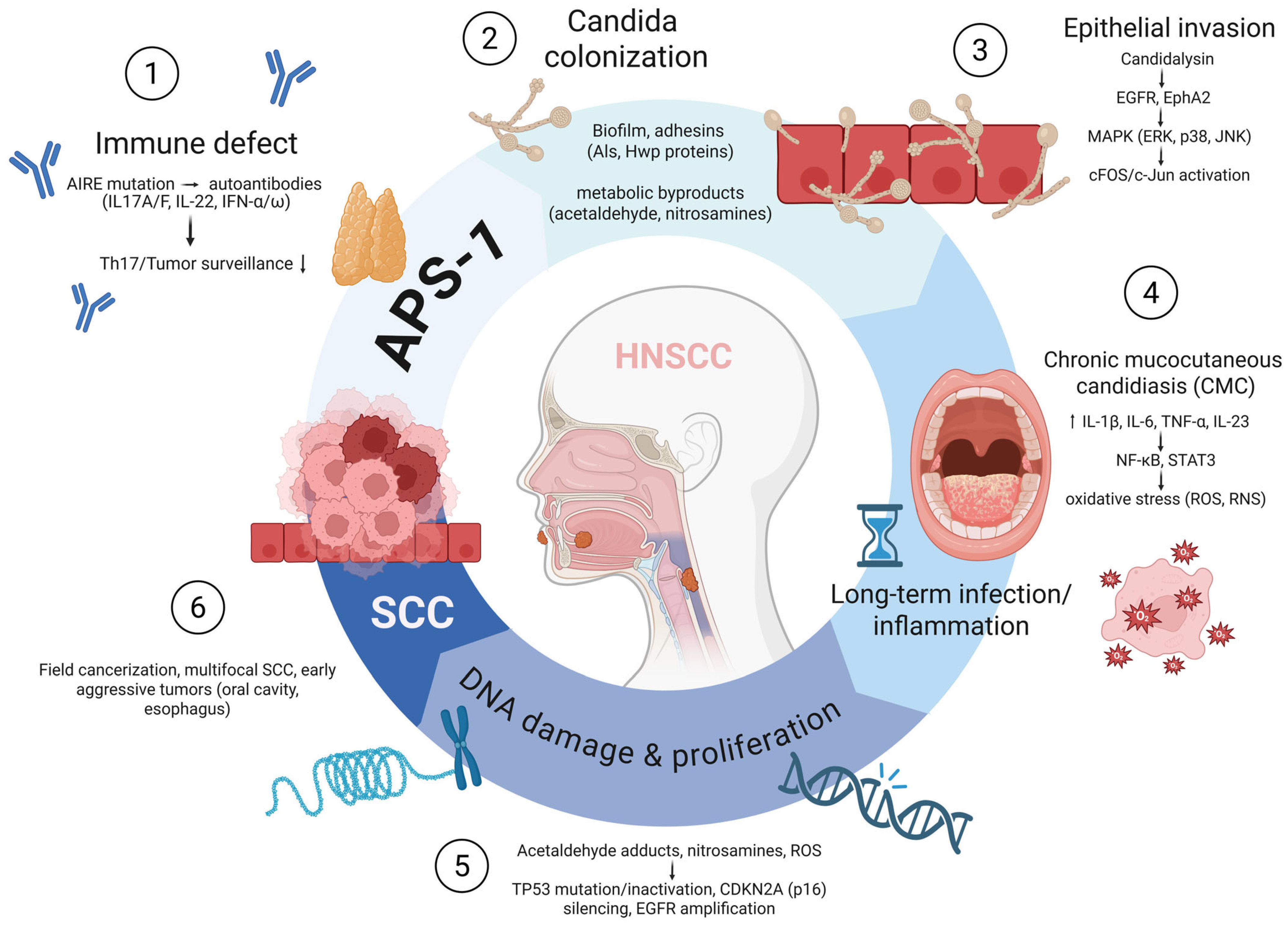
3. Discussion
4. Materials and Methods
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| APS-1 | Autoimmune Polyendocrine Syndrome Type 1 |
| APECED | Autoimmune Polyendocrinopathy–Candidiasis–Ectodermal Dystrophy |
| AIRE | Autoimmune Regulator |
| CMC | Chronic Mucocutaneous Candidiasis |
| SCC | Squamous Cell Carcinoma |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| IL | Interleukin |
| IFN | Interferon |
| EGFR | Epidermal Growth Factor Receptor |
| MAPK | Mitogen-Activated Protein Kinase |
| NF-κB | Nuclear Factor Kappa-light-chain-enhancer of activated B cells |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TP53 | Tumor Protein p53 |
| CDKN2A | Cyclin Dependent Kinase Inhibitor 2A (p16INK4a) |
| PICOS | Population, Intervention, Comparator, Outcome, Study Design |
| PRISMA-ScR | Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Re-views |
| ROB | Risk of Bias |
| ROS | Reactive Oxygen Species |
| RNS | Reactive Nitrogen Species |
| OLP | Oral Lichen Planus |
| SEER | Surveillance, Epidemiology, and End Results Program |
References
- Orlova, E.M.; Sozaeva, L.S.; Kareva, M.A.; Oftedal, B.E.; Wolff, A.S.B.; Breivik, L.; Zakharova, E.Y.; Ivanova, O.N.; Kampe, O.; Dedov, I.I.; et al. Expanding the Phenotypic and Genotypic Landscape of Autoimmune Polyendocrine Syndrome Type 1. J. Clin. Endocrinol. Metab. 2017, 102, 3546–3556. [Google Scholar] [CrossRef]
- Bjorklund, G.; Pivin, M.; Hangan, T.; Yurkovskaya, O.; Pivina, L. Autoimmune polyendocrine syndrome type 1: Clinical manifestations, pathogenetic features, and management approach. Autoimmun. Rev. 2022, 21, 103135. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.S.; Su, M.A. AIRE expands: New roles in immune tolerance and beyond. Nat. Rev. Immunol. 2016, 16, 247–258. [Google Scholar] [CrossRef]
- Sng, J.; Ayoglu, B.; Chen, J.W.; Schickel, J.N.; Ferre, E.M.N.; Glauzy, S.; Romberg, N.; Hoenig, M.; Cunningham-Rundles, C.; Utz, P.J.; et al. AIRE expression controls the peripheral selection of autoreactive B cells. Sci. Immunol. 2019, 4, eaav6778. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.J.; Leung, P.S.C.; Zhang, W.; Ma, X.; Gershwin, M.E. The immunobiology and clinical features of type 1 autoimmune polyglandular syndrome (APS-1). Autoimmun. Rev. 2018, 17, 78–85. [Google Scholar] [CrossRef]
- Wu, H.; Mo, Y.; Yu, S.; Ye, X.; Lu, Y.; Wang, C.; Shan, X. Novel homozygous mutations in AIRE leading to APS-1 and potential mechanisms based on bioinformatics analysis. Heliyon 2024, 10, e28037. [Google Scholar] [CrossRef]
- Husebye, E.S.; Anderson, M.S.; Kampe, O. Autoimmune Polyendocrine Syndromes. N. Engl. J. Med. 2018, 378, 1132–1141. [Google Scholar] [CrossRef]
- Ahonen, P.; Myllarniemi, S.; Sipila, I.; Perheentupa, J. Clinical variation of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) in a series of 68 patients. N. Engl. J. Med. 1990, 322, 1829–1836. [Google Scholar] [CrossRef]
- Bruserud, O.; Oftedal, B.E.; Landegren, N.; Erichsen, M.M.; Bratland, E.; Lima, K.; Jorgensen, A.P.; Myhre, A.G.; Svartberg, J.; Fougner, K.J.; et al. A Longitudinal Follow-up of Autoimmune Polyendocrine Syndrome Type 1. J. Clin. Endocrinol. Metab. 2016, 101, 2975–2983. [Google Scholar] [CrossRef]
- Dos Santos, E.S.; Perez-de-Oliveira, M.E.; Normando, A.G.C.; Gueiros, L.A.M.; Rogatto, S.R.; Vargas, P.A.; Lopes, M.A.; da Silva Guerra, E.N.; Leme, A.F.P.; Santos-Silva, A.R. Systemic conditions associated with increased risk to develop oral squamous cell carcinoma: Systematic review and meta-analysis. Head Neck 2022, 44, 2925–2937. [Google Scholar] [CrossRef]
- Nokovitch, L.; Maquet, C.; Crampon, F.; Taihi, I.; Roussel, L.M.; Obongo, R.; Virard, F.; Fervers, B.; Deneuve, S. Oral Cavity Squamous Cell Carcinoma Risk Factors: State of the Art. J. Clin. Med. 2023, 12, 3264. [Google Scholar] [CrossRef]
- Puel, A.; Doffinger, R.; Natividad, A.; Chrabieh, M.; Barcenas-Morales, G.; Picard, C.; Cobat, A.; Ouachee-Chardin, M.; Toulon, A.; Bustamante, J.; et al. Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. J. Exp. Med. 2010, 207, 291–297. [Google Scholar] [CrossRef]
- Yu, D.; Liu, Z. The research progress in the interaction between Candida albicans and cancers. Front. Microbiol. 2022, 13, 988734. [Google Scholar] [CrossRef] [PubMed]
- Ho, J.; Yang, X.; Nikou, S.A.; Kichik, N.; Donkin, A.; Ponde, N.O.; Richardson, J.P.; Gratacap, R.L.; Archambault, L.S.; Zwirner, C.P.; et al. Candidalysin activates innate epithelial immune responses via epidermal growth factor receptor. Nat. Commun. 2019, 10, 2297. [Google Scholar] [CrossRef] [PubMed]
- Hirano, T. IL-6 in inflammation, autoimmunity and cancer. Int. Immunol. 2021, 33, 127–148. [Google Scholar] [CrossRef]
- Meager, A.; Visvalingam, K.; Peterson, P.; Moll, K.; Murumagi, A.; Krohn, K.; Eskelin, P.; Perheentupa, J.; Husebye, E.; Kadota, Y.; et al. Anti-interferon autoantibodies in autoimmune polyendocrinopathy syndrome type 1. PLoS Med. 2006, 3, e289. [Google Scholar] [CrossRef]
- Garelli, S.; Dalla Costa, M.; Sabbadin, C.; Barollo, S.; Rubin, B.; Scarpa, R.; Masiero, S.; Fierabracci, A.; Bizzarri, C.; Crino, A.; et al. Autoimmune polyendocrine syndrome type 1: An Italian survey on 158 patients. J. Endocrinol. Investig. 2021, 44, 2493–2510. [Google Scholar] [CrossRef]
- Borchers, J.; Pukkala, E.; Makitie, O.; Laakso, S. Patients With APECED Have Increased Early Mortality Due to Endocrine Causes, Malignancies and infections. J. Clin. Endocrinol. Metab. 2020, 105, e2207–e2213. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA Extension for Scoping Reviews (PRISMA-ScR): Checklist and Explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Richman, R.A.; Rosenthal, I.M.; Solomon, L.M.; Karachorlu, K.V. Candidiasis and multiple endocrinopathy. With oral squamous cell carcinoma complications. Arch. Dermatol. 1975, 111, 625–627. [Google Scholar] [CrossRef]
- Firth, N.A.; O’Grady, J.F.; Reade, P.C. Oral squamous cell carcinoma in a young person with candidosis endocrinopathy syndrome: A case report. Int. J. Oral Maxillofac. Surg. 1997, 26, 42–44. [Google Scholar] [CrossRef]
- Rautemaa, R.; Hietanen, J.; Niissalo, S.; Pirinen, S.; Perheentupa, J. Oral and oesophageal squamous cell carcinoma--a complication or component of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED, APS-I). Oral Oncol. 2007, 43, 607–613. [Google Scholar] [CrossRef]
- Bockle, B.C.; Wilhelm, M.; Muller, H.; Gotsch, C.; Sepp, N.T. Oral mucous squamous cell carcinoma-an anticipated consequence of autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED). J. Am. Acad. Dermatol. 2010, 62, 864–868. [Google Scholar] [CrossRef] [PubMed]
- Shephard, M.K.; Schifter, M.; Palme, C.E. Multiple oral squamous cell carcinomas associated with autoimmune polyendocrinopathy-candidiasis-ectodermal dystrophy. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2012, 114, e36–e42. [Google Scholar] [CrossRef] [PubMed]
- Mc Cormack, O.; Timlin, M.; Mc Gowan, A.; Healy, M.L.; Ravi, N.; Reynolds, J.V. Management of squamous cell cancer of the oesophagus in a patient with a polyglandular endocrinopathy (APECED) and achalasia. J. Gastrointest. Surg. 2012, 16, 1963–1966. [Google Scholar] [CrossRef]
- Awad, S.F.M.; Kämpe, O.; Gustafsson, J.; Rorsman, F.; Hallberg, P. Fatal Esophageal Squamous Cell Carcinoma at a Young Age as a Complication of Autoimmune Polyendocrinopathy-Candidiasis-Ectodermal Dystrophy. AACE Clin. Case Rep. 2015, 1, e240–e244. [Google Scholar] [CrossRef][Green Version]
- Bruserud, O.; Costea, D.E.; Laakso, S.; Garty, B.Z.; Mathisen, E.; Makitie, A.; Makitie, O.; Husebye, E.S. Oral Tongue Malignancies in Autoimmune Polyendocrine Syndrome Type 1. Front. Endocrinol. 2018, 9, 463. [Google Scholar] [CrossRef]
- Johnson, D.E.; Burtness, B.; Leemans, C.R.; Lui, V.W.Y.; Bauman, J.E.; Grandis, J.R. Head and neck squamous cell carcinoma. Nat. Rev. Dis. Primers 2020, 6, 92. [Google Scholar] [CrossRef]
- Fasano, M.; D’Onofrio, I.; Belfiore, M.P.; Angrisani, A.; Caliendo, V.; Della Corte, C.M.; Pirozzi, M.; Facchini, S.; Caterino, M.; Guida, C.; et al. Head and Neck Squamous Cell Carcinoma in Elderly Patients: Role of Radiotherapy and Chemotherapy. Cancers 2022, 14, 472. [Google Scholar] [CrossRef]
- Tarle, M.; Luksic, I. Pathogenesis and Therapy of Oral Carcinogenesis. Int. J. Mol. Sci. 2024, 25, 6343. [Google Scholar] [CrossRef]
- Stepan, K.O.; Mazul, A.L.; Larson, J.; Shah, P.; Jackson, R.S.; Pipkorn, P.; Kang, S.Y.; Puram, S.V. Changing Epidemiology of Oral Cavity Cancer in the United States. Otolaryngol. Head Neck Surg. 2023, 168, 761–768. [Google Scholar] [CrossRef]
- Chen, Y.K.; Huang, H.C.; Lin, L.M.; Lin, C.C. Primary oral squamous cell carcinoma: An analysis of 703 cases in southern Taiwan. Oral Oncol. 1999, 35, 173–179. [Google Scholar] [CrossRef]
- Desai, M.K.; Brinton, R.D. Autoimmune Disease in Women: Endocrine Transition and Risk Across the Lifespan. Front. Endocrinol. 2019, 10, 265. [Google Scholar] [CrossRef] [PubMed]
- Kronzer, V.L.; Bridges, S.L., Jr.; Davis, J.M., 3rd. Why women have more autoimmune diseases than men: An evolutionary perspective. Evol. Appl. 2021, 14, 629–633. [Google Scholar] [CrossRef] [PubMed]
- Tran, Q.; Maddineni, S.; Arnaud, E.H.; Divi, V.; Megwalu, U.C.; Topf, M.C.; Sunwoo, J.B. Oral cavity cancer in young, non-smoking, and non-drinking patients: A contemporary review. Crit. Rev. Oncol. Hematol. 2023, 190, 104112. [Google Scholar] [CrossRef]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef]
- Di Cosola, M.; Cazzolla, A.P.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Santacroce, L. Candida albicans and Oral Carcinogenesis. A Brief Review. J. Fungi 2021, 7, 476. [Google Scholar] [CrossRef]
- Simple, M.; Suresh, A.; Das, D.; Kuriakose, M.A. Cancer stem cells and field cancerization of oral squamous cell carcinoma. Oral Oncol. 2015, 51, 643–651. [Google Scholar] [CrossRef]
- Mello, F.W.; Melo, G.; Pasetto, J.J.; Silva, C.A.B.; Warnakulasuriya, S.; Rivero, E.R.C. The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 2849–2859. [Google Scholar] [CrossRef]
- Kolegova, E.S.; Patysheva, M.R.; Larionova, I.V.; Fedorova, I.K.; Kulbakin, D.E.; Choinzonov, E.L.; Denisov, E.V. Early-onset oral cancer as a clinical entity: Aetiology and pathogenesis. Int. J. Oral Maxillofac. Surg. 2022, 51, 1497–1509. [Google Scholar] [CrossRef]
- Bruserud, O.; Oftedal, B.E.; Wolff, A.B.; Husebye, E.S. AIRE-mutations and autoimmune disease. Curr. Opin. Immunol. 2016, 43, 8–15. [Google Scholar] [CrossRef]
- Kaleviste, E.; Ruhlemann, M.; Karner, J.; Haljasmagi, L.; Tserel, L.; Org, E.; Trebusak Podkrajsek, K.; Battelino, T.; Bang, C.; Franke, A.; et al. IL-22 Paucity in APECED Is Associated With Mucosal and Microbial Alterations in Oral Cavity. Front. Immunol. 2020, 11, 838. [Google Scholar] [CrossRef]
- Philippot, Q.; Casanova, J.L.; Puel, A. Candidiasis in patients with APS-1: Low IL-17, high IFN-gamma, or both? Curr. Opin. Immunol. 2021, 72, 318–323. [Google Scholar] [CrossRef] [PubMed]
- Vadovics, M.; Ho, J.; Igaz, N.; Alfoldi, R.; Rakk, D.; Veres, E.; Szucs, B.; Horvath, M.; Toth, R.; Szucs, A.; et al. Candida albicans Enhances the Progression of Oral Squamous Cell Carcinoma In Vitro and In Vivo. mBio 2021, 13, e0314421. [Google Scholar] [CrossRef]
- Talapko, J.; Mestrovic, T.; Dmitrovic, B.; Juzbasic, M.; Matijevic, T.; Bekic, S.; Eric, S.; Flam, J.; Belic, D.; Petek Eric, A.; et al. A Putative Role of Candida albicans in Promoting Cancer Development: A Current State of Evidence and Proposed Mechanisms. Microorganisms 2023, 11, 1476. [Google Scholar] [CrossRef]
- Engku Nasrullah Satiman, E.A.F.; Ahmad, H.; Ramzi, A.B.; Abdul Wahab, R.; Kaderi, M.A.; Wan Harun, W.H.A.; Dashper, S.; McCullough, M.; Arzmi, M.H. The role of Candida albicans candidalysin ECE1 gene in oral carcinogenesis. J. Oral Pathol. Med. 2020, 49, 835–841. [Google Scholar] [CrossRef]
- Ayuningtyas, N.F.; Mahdani, F.Y.; Pasaribu, T.A.S.; Chalim, M.; Ayna, V.K.P.; Santosh, A.B.R.; Santacroce, L.; Surboyo, M.D.C. Role of Candida albicans in Oral Carcinogenesis. Pathophysiology 2022, 29, 650–662. [Google Scholar] [CrossRef]
- Gainza-Cirauqui, M.L.; Nieminen, M.T.; Novak Frazer, L.; Aguirre-Urizar, J.M.; Moragues, M.D.; Rautemaa, R. Production of carcinogenic acetaldehyde by Candida albicans from patients with potentially malignant oral mucosal disorders. J. Oral Pathol. Med. 2013, 42, 243–249. [Google Scholar] [CrossRef]
- Yang, D.; Zhang, M.; Su, C.; Dong, B.; Lu, Y. Candida albicans exploits N-acetylglucosamine as a gut signal to establish the balance between commensalism and pathogenesis. Nat. Commun. 2023, 14, 3796. [Google Scholar] [CrossRef]
- Shukla, K.; Vun, I.; Lov, I.; Laparidis, G.; McCamley, C.; Ariyawardana, A. Role of Candida Infection in the Malignant Transformation of Oral Leukoplakia: A Systematic Review of Observational Studies. Transl. Res. Oral Oncol. 2019, 4, 2057178X19828229. [Google Scholar] [CrossRef]
- Okada, S.; Puel, A.; Casanova, J.L.; Kobayashi, M. Chronic mucocutaneous candidiasis disease associated with inborn errors of IL-17 immunity. Clin. Transl. Immunol. 2016, 5, e114. [Google Scholar] [CrossRef]
- Eyerich, K.; Foerster, S.; Rombold, S.; Seidl, H.P.; Behrendt, H.; Hofmann, H.; Ring, J.; Traidl-Hoffmann, C. Patients with chronic mucocutaneous candidiasis exhibit reduced production of Th17-associated cytokines IL-17 and IL-22. J. Investig. Dermatol. 2008, 128, 2640–2645. [Google Scholar] [CrossRef]
- Kucuka, I.; Iraji, D.; Braun, S.; Breivik, L.; Wolff, A.S.B.; Husebye, E.S.; Oftedal, B.E. Longitudinal Immune Profiling in Autoimmune Polyendocrine Syndrome Type 1. Scand. J. Immunol. 2025, 101, e70021. [Google Scholar] [CrossRef] [PubMed]
- Hetemaki, I.; Saari, V.; Yohannes, D.A.; Holopainen, E.; Holster, T.; Jokiranta, S.; Mayranpaa, M.I.; Virtanen, S.; Makitie, O.; Kekalainen, E.; et al. Increased type 1 inflammation in gynecologic cervicovaginal samples in patients with APS-1. J. Allergy Clin. Immunol. 2024, 153, 1736–1742. [Google Scholar] [CrossRef] [PubMed]
- Clarke, A.E.; Pooley, N.; Marjenberg, Z.; Langham, J.; Nicholson, L.; Langham, S.; Embleton, N.; Wang, X.; Desta, B.; Barut, V.; et al. Risk of malignancy in patients with systemic lupus erythematosus: Systematic review and meta-analysis. Semin. Arthritis Rheum. 2021, 51, 1230–1241. [Google Scholar] [CrossRef]
- Pasoto, S.G.; Adriano de Oliveira Martins, V.; Bonfa, E. Sjogren’s syndrome and systemic lupus erythematosus: Links and risks. Open Access Rheumatol. 2019, 11, 33–45. [Google Scholar] [CrossRef]
- Huh, G.; Kim, D.; Lee, K.N.; Han, K.; Cho, J.H. Risk of Head and Neck Cancer in Patients with Psoriasis: A Nationwide Population-based Study. Acta Derm. Venereol. 2024, 104, adv18487. [Google Scholar] [CrossRef]
- Trafford, A.M.; Parisi, R.; Kontopantelis, E.; Griffiths, C.E.M.; Ashcroft, D.M. Association of Psoriasis With the Risk of Developing or Dying of Cancer: A Systematic Review and Meta-analysis. JAMA Dermatol. 2019, 155, 1390–1403. [Google Scholar] [CrossRef]
- Ladjevac, N.; Milovanovic, M.; Jevtovic, A.; Arsenijevic, D.; Stojanovic, B.; Dimitrijevic Stojanovic, M.; Stojanovic, B.; Arsenijevic, N.; Arsenijevic, A.; Milovanovic, J. The Role of IL-17 in the Pathogenesis of Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 9874. [Google Scholar] [CrossRef]
- Watanabe, Y.; Yamaguchi, Y.; Komitsu, N.; Ohta, S.; Azuma, Y.; Izuhara, K.; Aihara, M. Elevation of serum squamous cell carcinoma antigen 2 in patients with psoriasis: Associations with disease severity and response to the treatment. Br. J. Dermatol. 2016, 174, 1327–1336. [Google Scholar] [CrossRef]
- Honma, M.; Nozaki, H. Molecular Pathogenesis of Psoriasis and Biomarkers Reflecting Disease Activity. J. Clin. Med. 2021, 10, 3199. [Google Scholar] [CrossRef]
- Teh, L.S.; Lai, J.C.; Lian, J.C. Rapidly Developed Multiple Face and Neck Skin Cancers in a Patient with Sjogren’s Syndrome: A Case Report. Am. J. Case Rep. 2017, 18, 347–350. [Google Scholar] [CrossRef]
- Yoo, W.H.; Min Kim, K.; Choi, Y. The hidden oncological challenge in Sjogren’s syndrome with a focus on pharyngeal cancer. Arch. Rheumatol. 2024, 39, 471–473. [Google Scholar] [CrossRef]
- Blachut, D.; Przywara-Chowaniec, B.; Tomasik, A. Pathogenesis, Epidemiology, and Risk Factors of Malignant Tumors in Systemic Lupus Erythematosus. Rheumato 2024, 4, 209–221. [Google Scholar] [CrossRef]
- Aghbari, S.M.H.; Abushouk, A.I.; Attia, A.; Elmaraezy, A.; Menshawy, A.; Ahmed, M.S.; Elsaadany, B.A.; Ahmed, E.M. Malignant transformation of oral lichen planus and oral lichenoid lesions: A meta-analysis of 20095 patient data. Oral Oncol. 2017, 68, 92–102. [Google Scholar] [CrossRef] [PubMed]
- Li, J.-W.; Li, K.Y.; Chan, B.W.A.; McGrath, C.P.; Zheng, L.-W. Rate of Malignant Transformation Differs Based on Diagnostic Criteria for Oral Lichenoid Conditions: A Systematic Review and Meta-Analysis of 24,277 Patients. Cancers 2023, 15, 2537. [Google Scholar] [CrossRef]
- Lüdecke, C.; Neumann, H.; Remmerbach, T.W. The Early Detection of Malignant Transformation of Potentially Malignant Disorders: Oral Lichen Planus. Cancers 2025, 17, 1489. [Google Scholar] [CrossRef]
- Gonzalez-Moles, M.A.; Ruiz-Avila, I.; Gonzalez-Ruiz, L.; Ayen, A.; Gil-Montoya, J.A.; Ramos-Garcia, P. Malignant transformation risk of oral lichen planus: A systematic review and comprehensive meta-analysis. Oral Oncol. 2019, 96, 121–130. [Google Scholar] [CrossRef]
- Tota, J.E.; Best, A.F.; Zumsteg, Z.S.; Gillison, M.L.; Rosenberg, P.S.; Chaturvedi, A.K. Evolution of the Oropharynx Cancer Epidemic in the United States: Moderation of Increasing Incidence in Younger Individuals and Shift in the Burden to Older Individuals. J. Clin. Oncol. 2019, 37, 1538–1546. [Google Scholar] [CrossRef]
- Joseph, L.J.; Goodman, M.; Higgins, K.; Pilai, R.; Ramalingam, S.S.; Magliocca, K.; Patel, M.R.; El-Deiry, M.; Wadsworth, J.T.; Owonikoko, T.K.; et al. Racial disparities in squamous cell carcinoma of the oral tongue among women: A SEER data analysis. Oral Oncol. 2015, 51, 586–592. [Google Scholar] [CrossRef]
- Uddin, S.; Singh, A.; Mishra, V.; Agrawal, N.; Gooi, Z.; Izumchenko, E. Molecular drivers of oral cavity squamous cell carcinoma in non-smoking and non-drinking patients: What do we know so far? Oncol. Rev. 2022, 16, 549. [Google Scholar] [CrossRef]
- Grigolato, R.; Accorona, R.; Lombardo, G.; Corrocher, G.; Garagiola, U.; Massari, F.; Nicoli, S.; Rossi, S.; Calabrese, L. Oral cancer in non-smoker non-drinker patients. Could comparative pet oncology help to understand risk factors and pathogenesis? Crit. Rev. Oncol. Hematol. 2021, 166, 103458. [Google Scholar] [CrossRef]
- Valero, C.; Yuan, A.; Zanoni, D.K.; Lei, E.; Dogan, S.; Shah, J.P.; Morris, L.G.T.; Wong, R.J.; Mizrachi, A.; Patel, S.G.; et al. Young non-smokers with oral cancer: What are we missing and why? Oral Oncol. 2022, 127, 105803. [Google Scholar] [CrossRef] [PubMed]
- Pillai, A.; Adilbay, D.; Matsoukas, K.; Ganly, I.; Patel, S.G. Autoimmune disease and oral squamous cell carcinoma: A systematic review. J. Oral Pathol. Med. 2021, 50, 855–863. [Google Scholar] [CrossRef] [PubMed]
- Humbert, L.; Cornu, M.; Proust-Lemoine, E.; Bayry, J.; Wemeau, J.L.; Vantyghem, M.C.; Sendid, B. Chronic Mucocutaneous Candidiasis in Autoimmune Polyendocrine Syndrome Type 1. Front. Immunol. 2018, 9, 2570. [Google Scholar] [CrossRef]
- Pfaller, M.A.; Diekema, D.J. Epidemiology of invasive candidiasis: A persistent public health problem. Clin. Microbiol. Rev. 2007, 20, 133–163. [Google Scholar] [CrossRef]
- Zhang, M.R.; Zhao, F.; Wang, S.; Lv, S.; Mou, Y.; Yao, C.L.; Zhou, Y.; Li, F.Q. Molecular mechanism of azoles resistant Candida albicans in a patient with chronic mucocutaneous candidiasis. BMC Infect. Dis. 2020, 20, 126. [Google Scholar] [CrossRef]
- Zhu, F.; Willette-Brown, J.; Song, N.Y.; Lomada, D.; Song, Y.; Xue, L.; Gray, Z.; Zhao, Z.; Davis, S.R.; Sun, Z.; et al. Autoreactive T Cells and Chronic Fungal Infection Drive Esophageal Carcinogenesis. Cell Host Microbe 2017, 21, 478–493.e477. [Google Scholar] [CrossRef]
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef]
- Rupa, D.; Chuang, H.W.; Hu, C.E.; Su, W.M.; Wu, S.R.; Lee, H.S.; Yuan, T.C. ACSL4 upregulates IFI44 and IFI44L expression and promotes the proliferation and invasiveness of head and neck squamous cell carcinoma cells. Cancer Sci. 2024, 115, 3026–3040. [Google Scholar] [CrossRef]
- Civico-Ortega, J.L.; Gonzalez-Ruiz, I.; Ramos-Garcia, P.; Cruz-Granados, D.; Samayoa-Descamps, V.; Gonzalez-Moles, M.A. Prognostic and Clinicopathological Significance of Epidermal Growth Factor Receptor (EGFR) Expression in Oral Squamous Cell Carcinoma: Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2023, 24, 1888. [Google Scholar] [CrossRef] [PubMed]
- Tarle, M.; Raguž, M.; Muller, D.; Lukšić, I. Nuclear Epidermal Growth Factor Receptor Overexpression as a Survival Predictor in Oral Squamous Cell Carcinoma. Int. J. Mol. Sci. 2023, 24, 5816. [Google Scholar] [CrossRef] [PubMed]
- Satgunaseelan, L.; Porazinski, S.; Strbenac, D.; Istadi, A.; Willet, C.; Chew, T.; Sadsad, R.; Palme, C.E.; Lee, J.H.; Boyer, M.; et al. Oral Squamous Cell Carcinoma in Young Patients Show Higher Rates of EGFR Amplification: Implications for Novel Personalized Therapy. Front. Oncol. 2021, 11, 750852. [Google Scholar] [CrossRef] [PubMed]
- Leemans, C.R.; Snijders, P.J.F.; Brakenhoff, R.H. The molecular landscape of head and neck cancer. Nat. Rev. Cancer 2018, 18, 269–282. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Moher, D. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ 2020, 372, n71. [Google Scholar] [CrossRef]
| Case | Source | Age (y) | Sex | Country | APS-1 Onset (y) | APS-1 Components (Chronological) | AIRE Mutation | Autoantibodies | CMC Duration (y) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Richman et al., 1975 [20] | 26 | M | USA | 22 | HT(2), CMC(3), PA, PAI(22), GF(23), DM | NR | NR | 23 |
| 2 | Firth et al., 1997 [21] | 21 | F | Australia | 16 | HP(2), PA(8), PAI(16), POF, CMC | NR | NR | NR |
| 3 | Rautemaa et al., 2006 [22] | 44 | M | Finland | NR | A(18), AZ(27) | NR | NR | None |
| 4 | Rautemaa et al., 2006 [22] | 33 | M | Finland | NR | HP(9), PAI(9), K(10), CMC(14), PA(16) | NR | NR | 19 |
| 5 | Rautemaa et al., 2006 [22] | 34 | F | Finland | NR | CMC(0), HP(5), PAI(7), POF(27) | NR | NR | 34 |
| 6 | Rautemaa et al., 2006 [22] | 29 | F | Finland | NR | HP(1.9), PAI(5.4), A(10), POF(14.7), IN(19.3), CMC(13.4) | R257X/ R257X | NR | 15.6 |
| 7 | Rautemaa et al., 2006 [22] | 40 | M | Finland | NR | CMC(3.3), A(6), K(7), PAI(9.7), HP(16.4), DM(19.3) | NR | NR | 36.7 |
| 8 | Rautemaa et al., 2006 [22] | 42 | M | Finland | NR | HP(1.7), CMC(3.3.), K(9.7), V(11) | NR | NR | 38.7 |
| 9 | Böckle et al., 2010 [23] | 40 | F | Austria | 40 | HP(5), CMC(18), PAI(33), A(35), V(35) | c.967_979del13/ c.967_979del13 | NR | 22 |
| 10 | Shephard et al., 2012 [24] | 35 | F | Australia | 10 | AS(0), HP(7), PAI(10), CMC | NR | NR | 25+ |
| 11 | Cormack et al., 2012. [25] | 37 | M | Ireland | 27 | HP(27), PAI(27), CMC(<29) | NR | NR | 8+ |
| 12 | Awad et al., 2015 [26] | 34 | F | Canada | 0–6 | CMC(0), ED(2), EH(2), K(2), HP(4), PAI(9), V(9), A(9), PA(18), HT(19), POF(19), DM(31) | NR | NR | 34 |
| 13 | Bruserud et al., 2016 [9] | 35 | M | Norway | 4 | HP(4), PAI(4), CMC(26), EH, A | c.967_979del13/ c.967_979del13 | IFN-ω+, 21OH, AADC, IL-22, PCA, SCC, TGM4, TH | 30 |
| 14 | Bruserud et al., 2016 [9] | 58 | M | Norway | NR | HP, PAI(13), A | c.22C>T/ c.402delC | IFN-ω+, 21OH, 17OH, SCC, SOX10, TPH1 | NR |
| 15 | Bruserud et al., 2016 [9] | 35 | M | Norway | 12 | HP(12), PAI(12), EH, B12(59) | c.967_979del13/ c.967_979del13 | IFN-ω+, 17OH, IL-22, NALP5, SCC, TPH1 | 15+ |
| 16 | Bruserud et al., 2018 [27] | 45 | F | Finland | 1 | HP(1), CMC(3), EH(6), HG(13), V(13), PAI(16), A(27), B12(38), M(30), DM1(31), AS(39), AT(47) | R257X/R257X | SCC, NALP5, IFN-ω | 42 |
| 17 | Bruserud et al., 2018 [27] | 31 | F | Finland | 2 | HP(2), PAI(5), EH(5), A(10), CMC(10), H(15), TN(19), AT(32) | R257X/R257X | 17OH, SCC, NALP5, IL-22, IFN-ω | 21 |
| 18 | Bruserud et al., 2018 [27] | 21 | M | Norway | 11 | H(0), M(0), A, CMC(~3), PAI(11) | c.967_979del13/ c.967_979del13 | 21OH, 17OH, AADC, IL-22, SCC, TPH1, IFN-ω | 15+ |
| 19 | Bruserud et al., 2018 [27] | 38 | M | Israel | 3 | A(3), CMC, H, V, AS, HP (5), PAI | A374G/A374G | 21OH, TPO | 30+ |
| Case | Tumor Site | Histology | Smoking/Alcohol | Treatment | Outcome | Follow-Up (mo) | Recurrence | SPT |
|---|---|---|---|---|---|---|---|---|
| 1 | lower lip, buccal mucosa, palate, gingiva | SCC Grade 1 | NR | RT + CT | Died | 8 | Yes | Yes |
| 2 | buccal mucosa | SCC Grade 1, T4N0 | No/Yes (minimal alcohol) | Primary RT; AF (ketoconazole, nystatin, amphotericin B) | Alive | 60 | No | No |
| 3 | buccal mucosa | SCC Grade 1, T2N1 | Yes/Yes | Neoadjuvant RT; Surgery (IOE + ND) | Died | 6.5 | Yes (after 2.5 months) | No |
| 4 | buccal mucosa | SCC Grade 2, T1N2 | Yes/NR | Palliative RT | Died | 7 | No | No |
| 5 | buccal mucosa | SCC Grade 1, T2N2 | No/Yes (minimal alcohol) | Surgery (IOE + bilateral ND; AF (ketoconazole) | Died | 24 | Yes | No |
| 6 | lateral tongue | SCC Grade 1, T1N0 | No/No | Surgery (IOE), AF (miconazole, fluconazole) | Alive | 307 | Yes (after 1 year) | No |
| 7 | esophagus | SCC Grade 1, T3N1 | Yes/Yes | Surgery (esophagectomy), adjuvant CRT; AF (miconazole, flucoazole, ketoconazole) | Died | 18 | No | No |
| 8 | mandibular gingiva | SCC Grade 3, T4N1 | Yes/Yes | CRT—refused radical surgery; AF (fluconazole) | Alive (terminal stage) | 19 | No | No |
| 9 | buccal mucosa, lower lip | SCC Grade 2, T2N0 SCC Grade 2, T1N0 | No/No | Serial surgical excisions (laser resection & ablation); AF (amphotericin B, fluconazol) | Alive | 12 | Yes /after 2 months) | Yes |
| 10 | buccal mucosa | SCC Grade 1, T2N0 | No/Yes (minimal alcohol) | Laser surgical excisions + ND; AF (amphotericin B, fluconazole, voriconazole) | Alive | 48 | No | Yes (lower lip, after 1 y; CIS- lip commissure, after 3 y) |
| 11 | esophagus | SCC Grade 2 | NR | Neoadjuvant CRT; oesophagetomy | Alive | 6 | No | No |
| 12 | esophagus | SCC Grade 3 | No/No | AF (long-term fluconazol, nystatin, micafungin); supportive care (advanced disease) | Died | 5 | No | No |
| 13 | tongue | SCC | NR | NR | Died | NR | No | |
| 14 | esophagus | AC Grade 3, T3N3M1 | NR | NR | Died | NR | No | Yes (rectum) |
| 15 | lower lip | SCC | NR | NR | Alive | NR | Yes | |
| 16 | lateral tongue | SCC Grade 1, T1N0 | Yes (~1–4 cigarettes/day) /Yes (minimal alcohol) | Surgery (IOE) | Alive | 60 | No | No |
| 17 | lateral tongue | SCC Grade 1, T1N0 | No/Yes (~4 units/week) | Surgery (hemiglossectomy) | Alive | 192 | No | Yes/mandibular gingiva (PDT) |
| 18 | lateral tongue | SCC Grade 3, T3N1 | No/No | Surgery (hemiglossectomy + ND), adjuvant CRT | Alive | 7 | No | No |
| 19 | lateral tongue | SCC T2N0 | No/No | Surgery (partial glossectomy + SND); adjuvant RT; AF (nystatin, ketoconazol, fluconazole) | Died | 27 | Yes (after 2 years) | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarle, M.; Raguž, M.; Lukšić, I. Head and Neck Malignancies in Autoimmune Polyendocrine Syndrome Type 1 (APS-1/APECED): A Scoping Review of Molecular Pathogenesis, Clinical Features, and Outcomes. Int. J. Mol. Sci. 2025, 26, 8969. https://doi.org/10.3390/ijms26188969
Tarle M, Raguž M, Lukšić I. Head and Neck Malignancies in Autoimmune Polyendocrine Syndrome Type 1 (APS-1/APECED): A Scoping Review of Molecular Pathogenesis, Clinical Features, and Outcomes. International Journal of Molecular Sciences. 2025; 26(18):8969. https://doi.org/10.3390/ijms26188969
Chicago/Turabian StyleTarle, Marko, Marina Raguž, and Ivica Lukšić. 2025. "Head and Neck Malignancies in Autoimmune Polyendocrine Syndrome Type 1 (APS-1/APECED): A Scoping Review of Molecular Pathogenesis, Clinical Features, and Outcomes" International Journal of Molecular Sciences 26, no. 18: 8969. https://doi.org/10.3390/ijms26188969
APA StyleTarle, M., Raguž, M., & Lukšić, I. (2025). Head and Neck Malignancies in Autoimmune Polyendocrine Syndrome Type 1 (APS-1/APECED): A Scoping Review of Molecular Pathogenesis, Clinical Features, and Outcomes. International Journal of Molecular Sciences, 26(18), 8969. https://doi.org/10.3390/ijms26188969








