Genes Encoding Heat Shock Proteins Are Associated with Risk and Clinical Course of Severe COVID-19: A Pilot Study
Abstract
1. Introduction
2. Results
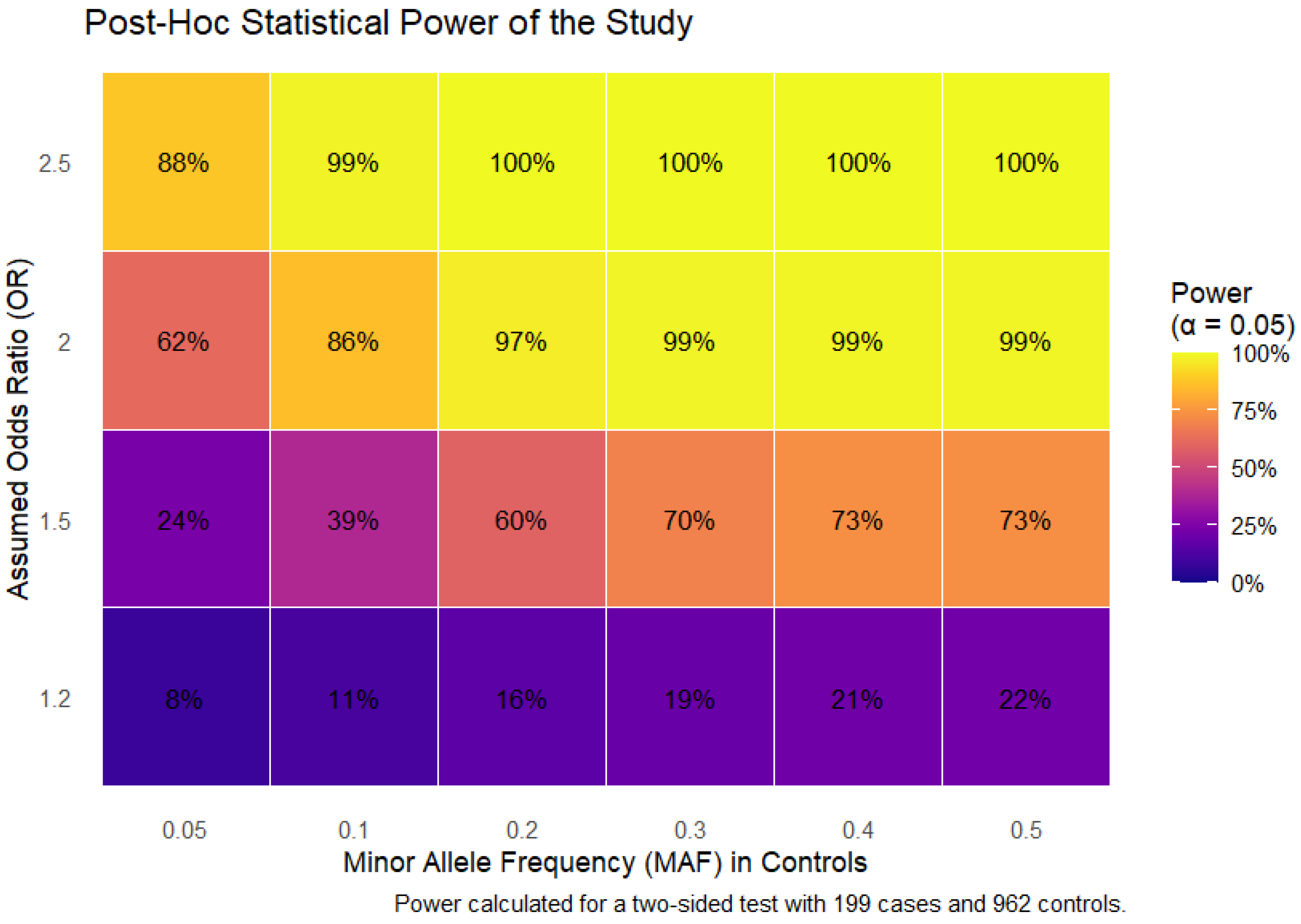
2.1. Quality Control and Post Hoc Power Analysis
2.2. HSPs SNPs and the Risk of Severe COVID-19
2.3. Associations Between HSPs SNPs and the Clinical and Biochemical Parameters of Patients with Severe COVID-19
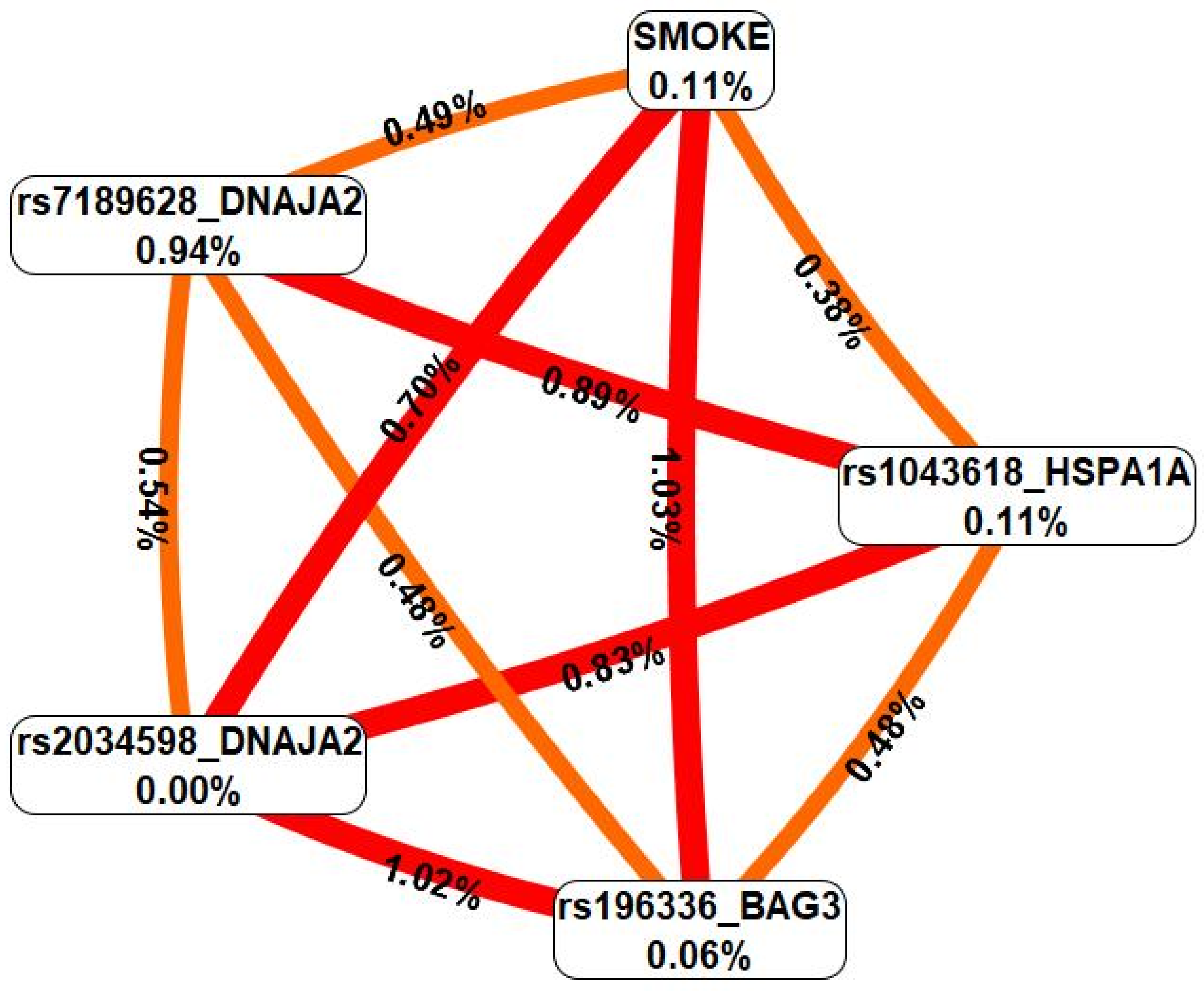
2.4. Gene-Gene Interactions Associated with Severe COVID-19 (MB-MDR, MDR Modeling)
2.5. Gene-Environment Interactions Associated with Severe COVID-19 (MB-MDR, MDR Modeling)
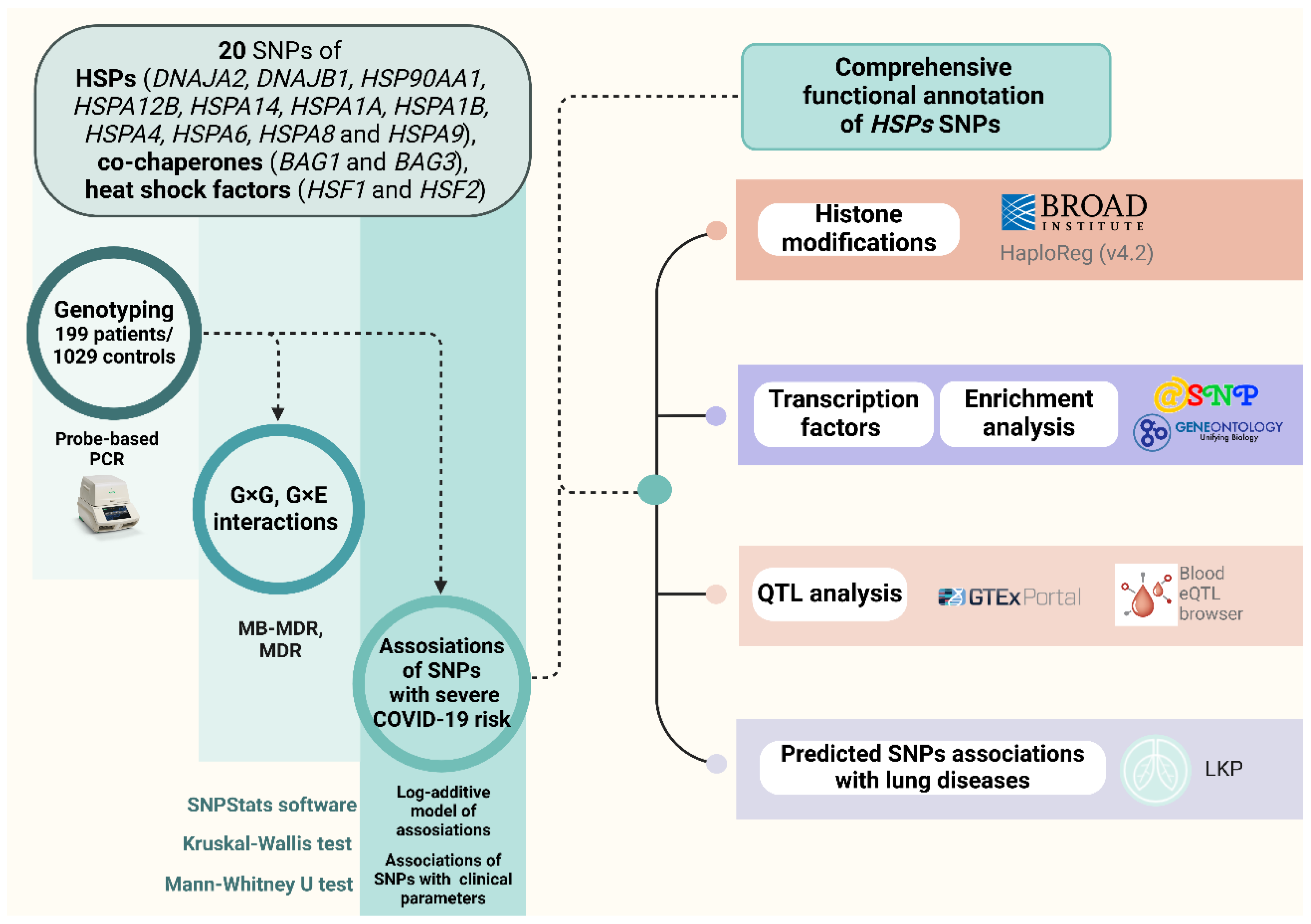
2.6. Functional Annotation
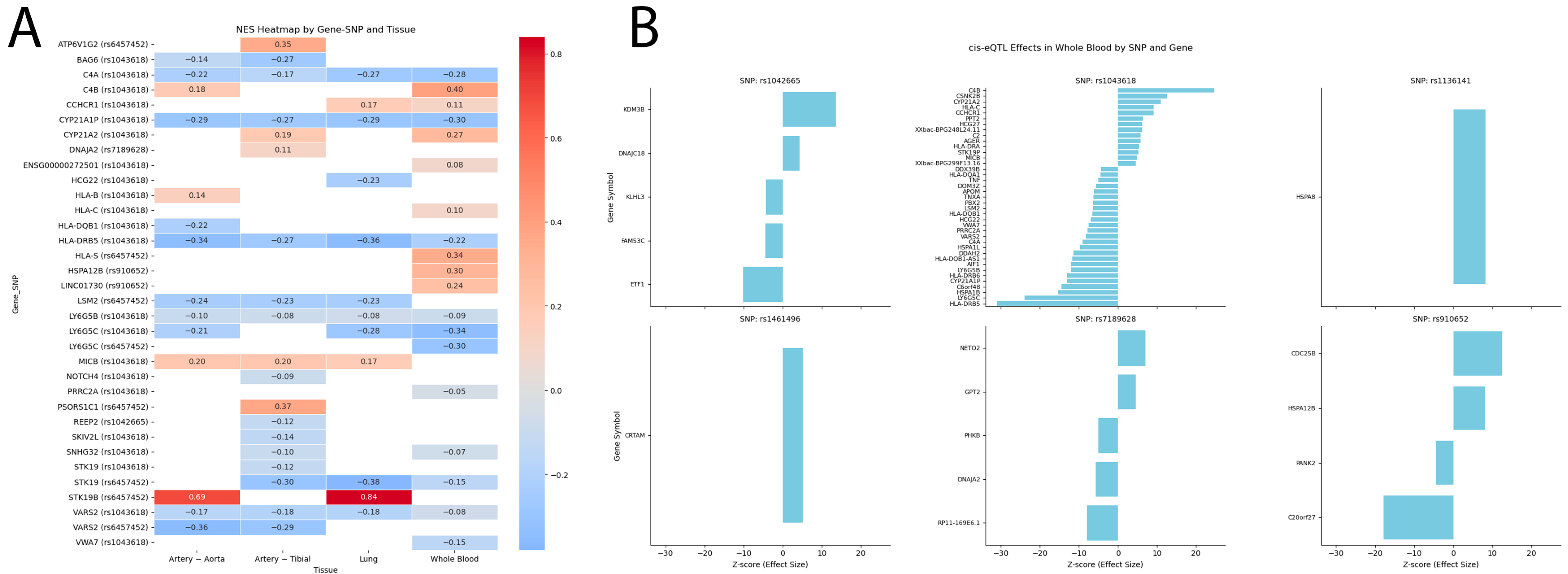
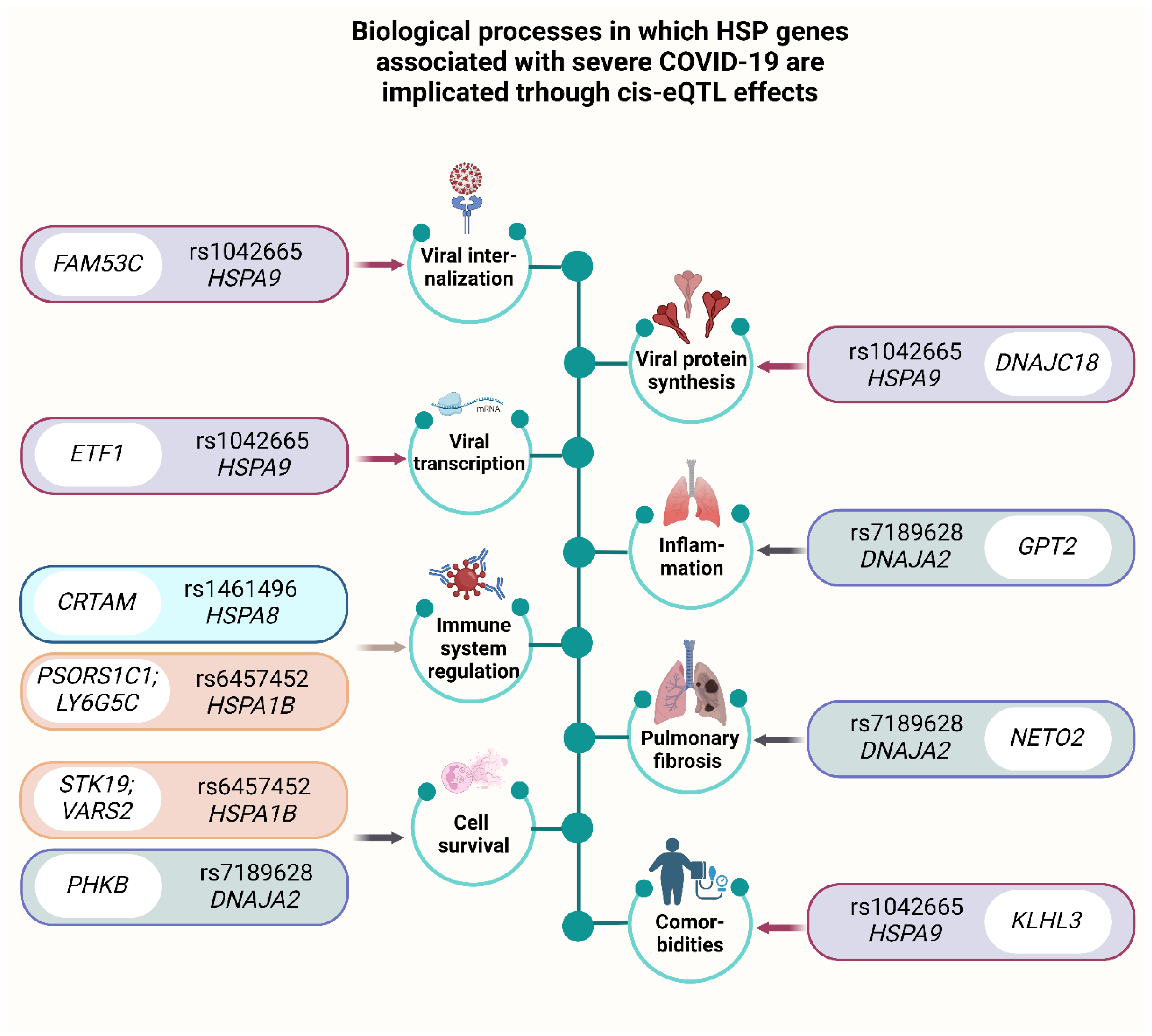
2.6.1. eQTL Effects
2.6.2. Histone Modifications
2.6.3. Bioinformatic Analysis of the Associations of the Studied SNPs with COVID-19 and Related Phenotypes
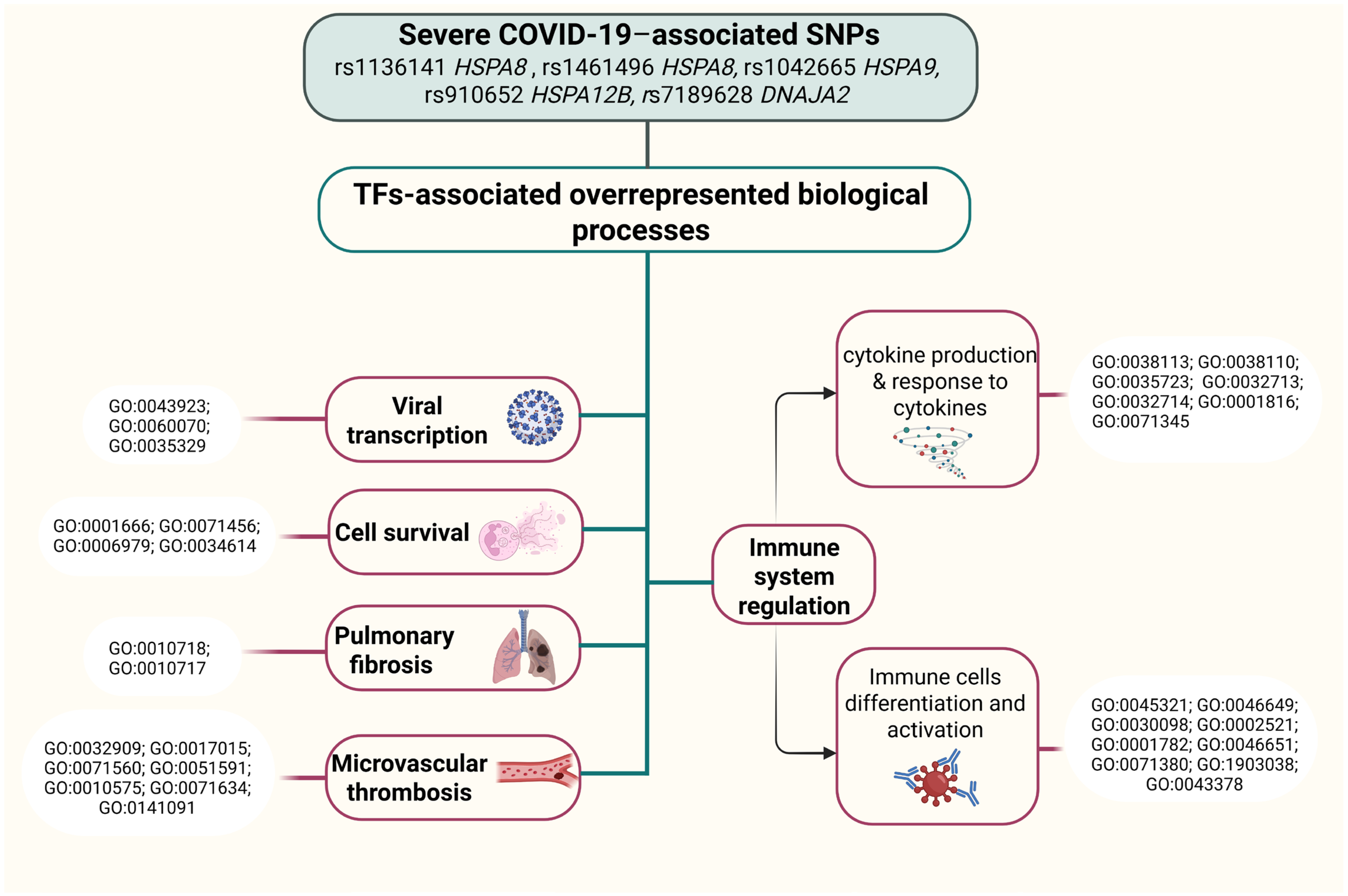
2.6.4. Analysis of Transcription Factors
3. Discussion
4. Materials and Methods
4.1. Study Participants
4.2. Genetic Analysis
4.3. Thrombodynamics Analysis
4.4. Statistical and Bioinformatic Analysis
- GTExportal (http://www.gtexportal.org/, accessed on 15 February 2025) was used to analyze the expression of quantitative trait loci (eQTLs) in blood, vessels, and lungs [108].
- eQTLGen (https://www.eqtlgen.org/, accessed on 15 February 2025) was employed for examination of HSPs SNPs that bind to eQTLs in peripheral blood [109]. To illustrate cis-eQTL associations, we used custom scripts in Python 3.12 (pandas, seaborn, matplotlib). Heatmaps were generated to display NES values across SNP-gene-tissue combinations, with color scale centered at zero to indicate direction of effect. For eQTLGen results, Z-scores of gene expression associations in whole blood were visualized using faceted bar plots, with genes sorted by effect size within each SNP for readability. Full association tables are provided in the Tables S9 and S10.
- HaploReg (v4.2) (https://pubs.broadinstitute.org/mammals/haploreg/haploreg.php, accessed on 15 February 2025) was utilized to assess the associations between HSPs SNPs and specific histone modifications (acetylation of lysine residues at positions 27 and 9 of the histone H3 protein (H3K27ac, H3K9ac), monomethylation at position 4 (H3K4me1) and trimethylation at position 4 (H3K4me3) of the histone H3 protein). Additionally, the tool was applied to investigate the positioning of SNPs in DNase-1 hypersensitive regions [110].
- The atSNP function prediction online tool (http://atsnp.biostat.wisc.edu/search, accessed on 15 February 2025) was used to evaluate the impact of HSPs SNPs on gene affinity to transcription factors (TFs) [111].
- Gene Ontology (http://geneontology.org/, accessed on 15 February 2025) was employed to analyze the joint involvement of TFs linked to the reference/SNP alleles in overrepresented biological processes directly related to the pathogenesis of COVID-19 [112].
- The Lung Disease Knowledge Portal (LKP) (https://cd.hugeamp.org/, accessed on 15 February 2025) was used for bioinformatic analyses of the associations of SNPs with lung diseases, intermediate phenotypes, and risk factors for COVID-19 (such as FEV1 and the FEV1-to-FVC ratio).
5. Conclusions
Study Limitations
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- da Rosa Mesquita, R.; Francelino Silva Junior, L.C.; Santos Santana, F.M.; Farias de Oliveira, T.; Campos Alcântara, R.; Monteiro Arnozo, G.; Rodrigues da Silva Filho, E.; Galdino Dos Santos, A.G.; Oliveira da Cunha, E.J.; Salgueiro de Aquino, S.H.; et al. Clinical Manifestations of COVID-19 in the General Population: Systematic Review. Wien. Klin. Wochenschr. 2021, 133, 377–382. [Google Scholar] [CrossRef]
- Tsai, P.-H.; Lai, W.-Y.; Lin, Y.-Y.; Luo, Y.-H.; Lin, Y.-T.; Chen, H.-K.; Chen, Y.-M.; Lai, Y.-C.; Kuo, L.-C.; Chen, S.-D.; et al. Clinical Manifestation and Disease Progression in COVID-19 Infection. J. Chin. Med. Assoc. 2021, 84, 3–8. [Google Scholar] [CrossRef]
- COVID-19 Cases|WHO COVID-19 Dashboard. Available online: https://data.who.int/dashboards/covid19/cases (accessed on 30 May 2024).
- Richter, K.; Haslbeck, M.; Buchner, J. The Heat Shock Response: Life on the Verge of Death. Mol. Cell 2010, 40, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Hartl, F.U.; Bracher, A.; Hayer-Hartl, M. Molecular Chaperones in Protein Folding and Proteostasis. Nature 2011, 475, 324–332. [Google Scholar] [CrossRef]
- Saibil, H. Chaperone Machines for Protein Folding, Unfolding and Disaggregation. Nat. Rev. Mol. Cell Biol. 2013, 14, 630–642. [Google Scholar] [CrossRef]
- Krishnan-Sivadoss, I.; Mijares-Rojas, I.A.; Villarreal-Leal, R.A.; Torre-Amione, G.; Knowlton, A.A.; Guerrero-Beltrán, C.E. Heat Shock Protein 60 and Cardiovascular Diseases: An Intricate Love-Hate Story. Med. Res. Rev. 2021, 41, 29–71. [Google Scholar] [CrossRef]
- Dukay, B.; Csoboz, B.; Tóth, M.E. Heat-Shock Proteins in Neuroinflammation. Front. Pharmacol. 2019, 10, 920. [Google Scholar] [CrossRef]
- Guo, H.; Yi, J.; Wang, F.; Lei, T.; Du, H. Potential Application of Heat Shock Proteins as Therapeutic Targets in Parkinson’s Disease. Neurochem. Int. 2023, 162, 105453. [Google Scholar] [CrossRef] [PubMed]
- Kobzeva, K.A.; Shilenok, I.V.; Belykh, A.E.; Gurtovoy, D.E.; Bobyleva, L.A.; Krapiva, A.B.; Stetskaya, T.A.; Bykanova, M.A.; Mezhenskaya, A.A.; Lysikova, E.A.; et al. C9orf16 (BBLN) Gene, Encoding a Member of Hero Proteins, Is a Novel Marker in Ischemic Stroke Risk. Res. Results Biomed. 2022, 8, 278–292. [Google Scholar] [CrossRef]
- Belykh, A.E.; Soldatov, V.O.; Stetskaya, T.A.; Kobzeva, K.A.; Soldatova, M.O.; Polonikov, A.V.; Deykin, A.V.; Churnosov, M.I.; Freidin, M.B.; Bushueva, O.Y. Polymorphism of SERF2, the Gene Encoding a Heat-Resistant Obscure (Hero) Protein with Chaperone Activity, Is a Novel Link in Ischemic Stroke. IBRO Neurosci. Rep. 2023, 14, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Shilenok, I.; Kobzeva, K.; Stetskaya, T.; Freidin, M.; Soldatova, M.; Deykin, A.; Soldatov, V.; Churnosov, M.; Polonikov, A.; Bushueva, O. SERPINE1 mRNA Binding Protein 1 Is Associated with Ischemic Stroke Risk: A Comprehensive Molecular–Genetic and Bioinformatics Analysis of SERBP1 SNPs. Int. J. Mol. Sci. 2023, 24, 8716. [Google Scholar] [CrossRef]
- Cappello, F.; Marino Gammazza, A.; Dieli, F.; Conway de Macario, E.; Macario, A.J. Does SARS-CoV-2 Trigger Stress-InducedAutoimmunity by Molecular Mimicry? A Hypothesis. J. Clin. Med. 2020, 9, 2038. [Google Scholar] [CrossRef]
- Ehrenfeld, M.; Tincani, A.; Andreoli, L.; Cattalini, M.; Greenbaum, A.; Kanduc, D.; Alijotas-Reig, J.; Zinserling, V.; Semenova, N.; Amital, H.; et al. Covid-19 and Autoimmunity. Autoimmun. Rev. 2020, 19, 102597. [Google Scholar] [CrossRef]
- Hall, B.G. Stress Proteins as Predictors of COVID-19 Outcomes. Cell Stress Chaperones 2021, 26, 287–288. [Google Scholar] [CrossRef]
- Marino Gammazza, A.; Légaré, S.; Lo Bosco, G.; Fucarino, A.; Angileri, F.; Conway de Macario, E.; Macario, A.J.; Cappello, F. Human Molecular Chaperones Share with SARS-CoV-2 Antigenic Epitopes Potentially Capable of Eliciting Autoimmunity against Endothelial Cells: Possible Role of Molecular Mimicry in COVID-19. Cell Stress Chaperones 2020, 25, 737–741. [Google Scholar] [CrossRef]
- Lucchese, G.; Flöel, A. SARS-CoV-2 and Guillain-Barré Syndrome: Molecular Mimicry with Human Heat Shock Proteins as Potential Pathogenic Mechanism. Cell Stress Chaperones 2020, 25, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.Z.; Fernandez-Prada, C.M.; Bhattacharjee, A.K.; Hoover, D.L. Over-Expression of Hsp-70 Inhibits Bacterial Lipopolysaccharide-Induced Production of Cytokines in Human Monocyte-Derived Macrophages. Cytokine 2001, 16, 210–219. [Google Scholar] [CrossRef]
- Wei, R.; Zhou, B.; Li, S.; Zhong, D.; Li, B.; Qin, J.; Zhao, L.; Qin, L.; Hu, J.; Wang, J.; et al. Plasma Gp96 Is a Novel Predictive Biomarker for Severe COVID-19. Microbiol. Spectr. 2021, 9, e0059721. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, M.; Traxler, D.; Bekos, C.; Simader, E.; Mueller, T.; Graf, A.; Lainscak, M.; Marčun, R.; Košnik, M.; Fležar, M.; et al. Heat Shock Protein 27 as a Predictor of Prognosis in Patients Admitted to Hospital with Acute COPD Exacerbation. Cell Stress Chaperones 2020, 25, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Navhaya, L.T.; Blessing, D.M.; Yamkela, M.; Godlo, S.; Makhoba, X.H. A Comprehensive Review of the Interaction between COVID-19 Spike Proteins with Mammalian Small and Major Heat Shock Proteins. Biomol. Concepts 2024, 15, 20220027. [Google Scholar] [CrossRef]
- Loktionov, A.V.; Kobzeva, K.A.; Karpenko, A.R.; Sergeeva, V.A.; Orlov, Y.L.; Bushueva, O.Y. GWAS-Significant Loci and Severe COVID-19: Analysis of Associations, Link with Thromboinflammation Syndrome, Gene-Gene, and Gene-Environmental Interactions. Front. Genet. 2024, 15, 1434681. [Google Scholar] [CrossRef]
- Degenhardt, F.; Ellinghaus, D.; Juzenas, S.; Lerga-Jaso, J.; Wendorff, M.; Maya-Miles, D.; Uellendahl-Werth, F.; ElAbd, H.; Rühlemann, M.C.; Arora, J.; et al. Detailed Stratified GWAS Analysis for Severe COVID-19 in Four European Populations. Hum. Mol. Genet. 2022, 31, 3945–3966. [Google Scholar] [CrossRef]
- Pairo-Castineira, E.; Rawlik, K.; Bretherick, A.D.; Qi, T.; Wu, Y.; Nassiri, I.; McConkey, G.A.; Zechner, M.; Klaric, L.; Griffiths, F.; et al. GWAS and Meta-Analysis Identifies 49 Genetic Variants Underlying Critical COVID-19. Nature 2023, 617, 764–768. [Google Scholar] [CrossRef]
- Venediktov, A.A.; Bushueva, O.Y.; Kudryavtseva, V.A.; Kuzmin, E.A.; Moiseeva, A.V.; Baldycheva, A.; Meglinski, I.; Piavchenko, G.A. Closest Horizons of Hsp70 Engagement to Manage Neurodegeneration. Front. Mol. Neurosci. 2023, 16, 1230436. [Google Scholar] [CrossRef]
- Shilenok, I.; Kobzeva, K.; Soldatov, V.; Deykin, A.; Bushueva, O. C11orf58 (Hero20) Gene Polymorphism: Contribution to Ischemic Stroke Risk and Interactions with Other Heat-Resistant Obscure Chaperones. Biomedicines 2024, 12, 2603. [Google Scholar] [CrossRef] [PubMed]
- Shilenok, I.; Kobzeva, K.; Deykin, A.; Pokrovsky, V.; Patrakhanov, E.; Bushueva, O. Obesity and Environmental Risk Factors Significantly Modify the Association between Ischemic Stroke and the Hero Chaperone C19orf53. Life 2024, 14, 1158. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhang, H.; Xiao, B. C9orf16 Represents the Aberrant Genetic Programs and Drives the Progression of PDAC. BMC Cancer 2022, 22, 1102. [Google Scholar] [CrossRef]
- Gao, W.; Li, J.Z.-H.; Chen, S.-Q.; Chu, C.-Y.; Chan, J.Y.-W.; Wong, T.-S. BEX3 Contributes to Cisplatin Chemoresistance in Nasopharyngeal Carcinoma. Cancer Med. 2017, 6, 439–451. [Google Scholar] [CrossRef] [PubMed]
- Soldatov, V.; Venediktov, A.; Belykh, A.; Piavchenko, G.; Naimzada, M.D.; Ogneva, N.; Kartashkina, N.; Bushueva, O. Chaperones vs. Oxidative Stress in the Pathobiology of Ischemic Stroke. Front. Mol. Neurosci. 2024, 17, 1513084. [Google Scholar] [CrossRef]
- Pockley, A.G. Heat Shock Proteins as Regulators of the Immune Response. Lancet 2003, 362, 469–476. [Google Scholar] [CrossRef]
- Fodor, A.; Tiperciuc, B.; Login, C.; Orasan, O.H.; Lazar, A.L.; Buchman, C.; Hanghicel, P.; Sitar-Taut, A.; Suharoschi, R.; Vulturar, R.; et al. Endothelial Dysfunction, Inflammation, and Oxidative Stress in COVID-19-Mechanisms and Therapeutic Targets. Oxid. Med. Cell Longev. 2021, 2021, 8671713. [Google Scholar] [CrossRef]
- Alwazeer, D.; Liu, F.F.-C.; Wu, X.Y.; LeBaron, T.W. Combating Oxidative Stress and Inflammation in COVID-19 by Molecular Hydrogen Therapy: Mechanisms and Perspectives. Oxidative Med. Cell. Longev. 2021, 2021, 5513868. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Jiang, L.; He, T.; Liu, J.; Fan, J.; Xu, X.; Tang, B.; Shi, Y.; Zhao, Y.; Qian, F.; et al. NETO2 Promotes Invasion and Metastasis of Gastric Cancer Cells via Activation of PI3K/Akt/NF-κB/Snail Axis and Predicts Outcome of the Patients. Cell Death Dis. 2019, 10, 162. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, X.; Deng, X.; Wang, P.; Mo, Y.; Zhang, Y.; Tong, X. Cytokines as Drivers: Unraveling the Mechanisms of Epithelial-Mesenchymal Transition in COVID-19 Lung Fibrosis. Biochem. Biophys. Res. Commun. 2023, 686, 149118. [Google Scholar] [CrossRef] [PubMed]
- Acloque, H.; Adams, M.S.; Fishwick, K.; Bronner-Fraser, M.; Nieto, M.A. Epithelial-Mesenchymal Transitions: The Importance of Changing Cell State in Development and Disease. J. Clin. Investig. 2009, 119, 1438–1449. [Google Scholar] [CrossRef]
- Lee, H.-W.; Jose, C.C.; Cuddapah, S. Epithelial-Mesenchymal Transition: Insights into Nickel-Induced Lung Diseases. Semin. Cancer Biol. 2021, 76, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Saifi, M.A.; Bansod, S.; Godugu, C. COVID-19 and Fibrosis: Mechanisms, Clinical Relevance, and Future Perspectives. Drug Discov. Today 2022, 27, 103345. [Google Scholar] [CrossRef]
- Kim, M.; Gwak, J.; Hwang, S.; Yang, S.; Jeong, S.M. Mitochondrial GPT2 Plays a Pivotal Role in Metabolic Adaptation to the Perturbation of Mitochondrial Glutamine Metabolism. Oncogene 2019, 38, 4729–4738. [Google Scholar] [CrossRef]
- Niayesh-Mehr, R.; Kalantar, M.; Bontempi, G.; Montaldo, C.; Ebrahimi, S.; Allameh, A.; Babaei, G.; Seif, F.; Strippoli, R. The Role of Epithelial-Mesenchymal Transition in Pulmonary Fibrosis: Lessons from Idiopathic Pulmonary Fibrosis and COVID-19. Cell Commun. Signal 2024, 22, 542. [Google Scholar] [CrossRef]
- Spittler, A.; Holzer, S.; Oehler, R.; Boltz-Nitulescu, G.; Roth, E. A Glutamine Deficiency Impairs the Function of Cultured Human Monocytes. Clin. Nutr. 1997, 16, 97–99. [Google Scholar] [CrossRef]
- Yaqoob, P.; Calder, P.C. Cytokine Production by Human Peripheral Blood Mononuclear Cells: Differential Senstivity to Glutamine Availability. Cytokine 1998, 10, 790–794. [Google Scholar] [CrossRef]
- Murphy, C.; Newsholme, P. Importance of Glutamine Metabolism in Murine Macrophages and Human Monocytes to L-Arginine Biosynthesis and Rates of Nitrite or Urea Production. Clin Sci. 1998, 95, 397–407. [Google Scholar] [CrossRef]
- Akaberi, D.; Krambrich, J.; Ling, J.; Luni, C.; Hedenstierna, G.; Järhult, J.D.; Lennerstrand, J.; Lundkvist, Å. Mitigation of the Replication of SARS-CoV-2 by Nitric Oxide in Vitro. Redox Biol. 2020, 37, 101734. [Google Scholar] [CrossRef]
- Shen, B.; Yi, X.; Sun, Y.; Bi, X.; Du, J.; Zhang, C.; Quan, S.; Zhang, F.; Sun, R.; Qian, L.; et al. Proteomic and Metabolomic Characterization of COVID-19 Patient Sera. Cell 2020, 182, 59–72.e15. [Google Scholar] [CrossRef]
- Wu, P.; Chen, D.; Ding, W.; Wu, P.; Hou, H.; Bai, Y.; Zhou, Y.; Li, K.; Xiang, S.; Liu, P.; et al. The Trans-Omics Landscape of COVID-19. Nat. Commun. 2021, 12, 4543. [Google Scholar] [CrossRef] [PubMed]
- Atila, A.; Alay, H.; Yaman, M.E.; Akman, T.C.; Cadirci, E.; Bayrak, B.; Celik, S.; Atila, N.E.; Yaganoglu, A.M.; Kadioglu, Y.; et al. The Serum Amino Acid Profile in COVID-19. Amino Acids 2021, 53, 1569–1588. [Google Scholar] [CrossRef] [PubMed]
- Thomas, T.; Stefanoni, D.; Reisz, J.A.; Nemkov, T.; Bertolone, L.; Francis, R.O.; Hudson, K.E.; Zimring, J.C.; Hansen, K.C.; Hod, E.A.; et al. COVID-19 Infection Alters Kynurenine and Fatty Acid Metabolism, Correlating with IL-6 Levels and Renal Status. JCI Insight 2020, 5, e140327. [Google Scholar] [CrossRef]
- Masoodi, M.; Peschka, M.; Schmiedel, S.; Haddad, M.; Frye, M.; Maas, C.; Lohse, A.; Huber, S.; Kirchhof, P.; Nofer, J.-R.; et al. Disturbed Lipid and Amino Acid Metabolisms in COVID-19 Patients. J. Mol. Med. 2022, 100, 555–568. [Google Scholar] [CrossRef]
- Rees, C.A.; Rostad, C.A.; Mantus, G.; Anderson, E.J.; Chahroudi, A.; Jaggi, P.; Wrammert, J.; Ochoa, J.B.; Ochoa, A.; Basu, R.K.; et al. Altered Amino Acid Profile in Patients with SARS-CoV-2 Infection. Proc. Natl. Acad. Sci. USA 2021, 118, e2101708118. [Google Scholar] [CrossRef]
- Durante, W. Glutamine Deficiency Promotes Immune and Endothelial Cell Dysfunction in COVID-19. Int. J. Mol. Sci. 2023, 24, 7593. [Google Scholar] [CrossRef]
- Wei, X.; Yi, X.; Liu, J.; Sui, X.; Li, L.; Li, M.; Lv, H.; Yi, H. Circ-Phkb Promotes Cell Apoptosis and Inflammation in LPS-Induced Alveolar Macrophages via the TLR4/MyD88/NF-kB/CCL2 Axis. Respir. Res. 2024, 25, 62. [Google Scholar] [CrossRef]
- Tu, F.; Wang, X.; Zhang, X.; Ha, T.; Wang, Y.; Fan, M.; Yang, K.; Gill, P.S.; Ozment, T.R.; Dai, Y.; et al. Novel Role of Endothelial Derived Exosomal HSPA12B in Regulating Macrophage Inflammatory Responses in Polymicrobial Sepsis. Front. Immunol. 2020, 11, 825. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Li, X.; Huang, L.; Jiang, S.; Tu, F.; Zhang, X.; Ma, H.; Li, R.; Li, C.; Li, Y.; et al. HSPA12B Inhibits Lipopolysaccharide-Induced Inflammatory Response in Human Umbilical Vein Endothelial Cells. J. Cell Mol. Med. 2015, 19, 544–554. [Google Scholar] [CrossRef]
- Hou, Y.; Yang, Z.; Xiang, B.; Liu, J.; Geng, L.; Xu, D.; Zhan, M.; Xu, Y.; Zhang, B. Metformin Is a Potential Therapeutic for COVID-19/LUAD by Regulating Glucose Metabolism. Sci. Rep. 2024, 14, 12406. [Google Scholar] [CrossRef]
- Basile, M.S.; Cavalli, E.; McCubrey, J.; Hernández-Bello, J.; Muñoz-Valle, J.F.; Fagone, P.; Nicoletti, F. The PI3K/Akt/mTOR Pathway: A Potential Pharmacological Target in COVID-19. Drug Discov. Today 2022, 27, 848–856. [Google Scholar] [CrossRef] [PubMed]
- de Antonellis, P.; Ferrucci, V.; Miceli, M.; Bibbo, F.; Asadzadeh, F.; Gorini, F.; Mattivi, A.; Boccia, A.; Russo, R.; Andolfo, I.; et al. Targeting ATP2B1 Impairs PI3K/Akt/FOXO Signaling and Reduces SARS-COV-2 Infection and Replication. EMBO Rep. 2024, 25, 2974–3007. [Google Scholar] [CrossRef]
- Gupta, A.; Jayakumar, M.N.; Saleh, M.A.; Kannan, M.; Halwani, R.; Qaisar, R.; Ahmad, F. SARS-CoV-2 Infection- Induced Growth Factors Play Differential Roles in COVID-19 Pathogenesis. Life Sci. 2022, 304, 120703. [Google Scholar] [CrossRef]
- Zhang, Y.; Gao, Z.; Jiang, F.; Yan, H.; Yang, B.; He, Q.; Luo, P.; Xu, Z.; Yang, X. JAK-STAT Signaling as an ARDS Therapeutic Target: Status and Future Trends. Biochem. Pharmacol. 2023, 208, 115382. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Bai, M.; You, Q. Associations between Serum Interleukins (IL-1β, IL-2, IL-4, IL-6, IL-8, and IL-10) and Disease Severity of COVID-19: A Systematic Review and Meta-Analysis. BioMed Res. Int. 2022, 2022, 2755246. [Google Scholar] [CrossRef]
- Li, F.; Boon, A.C.M.; Michelson, A.P.; Foraker, R.E.; Zhan, M.; Payne, P.R.O. Estrogen Hormone Is an Essential Sex Factor Inhibiting Inflammation and Immune Response in COVID-19. Sci. Rep. 2022, 12, 9462. [Google Scholar] [CrossRef]
- Ricke-Hoch, M.; Stelling, E.; Lasswitz, L.; Gunesch, A.P.; Kasten, M.; Zapatero-Belinchón, F.J.; Brogden, G.; Gerold, G.; Pietschmann, T.; Montiel, V.; et al. Impaired Immune Response Mediated by Prostaglandin E2 Promotes Severe COVID-19 Disease. PLoS ONE 2021, 16, e0255335. [Google Scholar] [CrossRef]
- Tavasolian, F.; Rashidi, M.; Hatam, G.R.; Jeddi, M.; Hosseini, A.Z.; Mosawi, S.H.; Abdollahi, E.; Inman, R.D. HLA, Immune Response, and Susceptibility to COVID-19. Front. Immunol. 2021, 11, 601886. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Xiao, B.; Deng, J.; Gong, L.; Li, Y.; Li, J.; Zhong, Y. The Role of Cytochrome P450 Enzymes in COVID-19 Pathogenesis and Therapy. Front. Pharmacol. 2022, 13, 791922. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.; Kong, H.; Yan, M.; Shen, C.; Xu, G.; Zhang, D.; Zhang, K.; Zheng, H.; Liu, X. Inhibition of Orf Virus Replication in Goat Skin Fibroblast Cells by the HSPA1B Protein, as Demonstrated by iTRAQ-Based Quantitative Proteome Analysis. Arch. Virol. 2020, 165, 2561–2587. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, Y.; Zhou, Y.; Xiao, Y.; Huang, W.; Zhou, Q.; Tu, Y.; Zhao, Y.; Zhang, S.; Dai, L.; et al. STK19 Is a DNA/RNA-Binding Protein Critical for DNA Damage Repair and Cell Proliferation. J. Cell Biol. 2024, 223, e202301090. [Google Scholar] [CrossRef] [PubMed]
- Kayvanpour, E.; Wisdom, M.; Lackner, M.K.; Sedaghat-Hamedani, F.; Boeckel, J.-N.; Müller, M.; Eghbalian, R.; Dudek, J.; Doroudgar, S.; Maack, C.; et al. VARS2 Depletion Leads to Activation of the Integrated Stress Response and Disruptions in Mitochondrial Fatty Acid Oxidation. Int. J. Mol. Sci. 2022, 23, 7327. [Google Scholar] [CrossRef]
- Upadhyay, G. Emerging Role of Lymphocyte Antigen-6 Family of Genes in Cancer and Immune Cells. Front. Immunol. 2019, 10, 819. [Google Scholar] [CrossRef]
- Sun, H.; Xia, Y.; Wang, L.; Wang, Y.; Chang, X. PSORS1C1 May Be Involved in Rheumatoid Arthritis. Immunol. Lett. 2013, 153, 9–14. [Google Scholar] [CrossRef]
- Watanabe, K.; Fuse, T.; Asano, I.; Tsukahara, F.; Maru, Y.; Nagata, K.; Kitazato, K.; Kobayashi, N. Identification of Hsc70 as an Influenza Virus Matrix Protein (M1) Binding Factor Involved in the Virus Life Cycle. FEBS Lett. 2006, 580, 5785–5790. [Google Scholar] [CrossRef]
- Zhu, P.; Lv, C.; Fang, C.; Peng, X.; Sheng, H.; Xiao, P.; Kumar Ojha, N.; Yan, Y.; Liao, M.; Zhou, J. Heat Shock Protein Member 8 Is an Attachment Factor for Infectious Bronchitis Virus. Front. Microbiol. 2020, 11, 1630. [Google Scholar] [CrossRef]
- Zárate, S.; Cuadras, M.A.; Espinosa, R.; Romero, P.; Juárez, K.O.; Camacho-Nuez, M.; Arias, C.F.; López, S. Interaction of Rotaviruses with Hsc70 during Cell Entry Is Mediated by VP5. J. Virol. 2003, 77, 7254–7260. [Google Scholar] [CrossRef] [PubMed]
- Salinas, E.; Byrum, S.D.; Moreland, L.E.; Mackintosh, S.G.; Tackett, A.J.; Forrest, J.C. Identification of Viral and Host Proteins That Interact with Murine Gammaherpesvirus 68 Latency-Associated Nuclear Antigen during Lytic Replication: A Role for Hsc70 in Viral Replication. J. Virol. 2016, 90, 1397–1413. [Google Scholar] [CrossRef]
- Alqutami, F.; Senok, A.; Hachim, M. COVID-19 Transcriptomic Atlas: A Comprehensive Analysis of COVID-19 Related Transcriptomics Datasets. Front. Genet. 2021, 12, 755222. [Google Scholar] [CrossRef] [PubMed]
- Vallée, A.; Lecarpentier, Y.; Vallée, J.-N. Interplay of Opposing Effects of the WNT/β-Catenin Pathway and PPARγ and Implications for SARS-CoV2 Treatment. Front. Immunol. 2021, 12, 666693. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, R.; Chathambath, A.; Al-Sehemi, A.G.; Pannipara, M.; Unnikrishnan, M.K.; Aleya, L.; Raghavan, R.P.; Mathew, B. Critical Role of Nitric Oxide in Impeding COVID-19 Transmission and Prevention: A Promising Possibility. Environ. Sci. Pollut. Res. 2022, 29, 38657–38672. [Google Scholar] [CrossRef]
- Zivancevic-Simonovic, S.; Minic, R.; Cupurdija, V.; Stanojevic-Pirkovic, M.; Milosevic-Djordjevic, O.; Jakovljevic, V.; Mihaljevic, O. Transforming Growth Factor Beta 1 (TGF-Β1) in COVID-19 Patients: Relation to Platelets and Association with the Disease Outcome. Mol. Cell Biochem. 2023, 478, 2461–2471. [Google Scholar] [CrossRef]
- Garcia, G., Jr.; Jeyachandran, A.V.; Wang, Y.; Irudayam, J.I.; Cario, S.C.; Sen, C.; Li, S.; Li, Y.; Kumar, A.; Nielsen-Saines, K.; et al. Hippo Signaling Pathway Activation during SARS-CoV-2 Infection Contributes to Host Antiviral Response. PLOS Biol. 2022, 20, e3001851. [Google Scholar] [CrossRef]
- Zlamal, J.; Althaus, K.; Jaffal, H.; Häberle, H.; Pelzl, L.; Singh, A.; Witzemann, A.; Weich, K.; Bitzer, M.; Malek, N.; et al. Upregulation of cAMP Prevents Antibody-Mediated Thrombus Formation in COVID-19. Blood Adv. 2022, 6, 248–258. [Google Scholar] [CrossRef]
- Shan, Y.; Cortopassi, G. Mitochondrial Hspa9/Mortalin Regulates Erythroid Differentiation via Iron-Sulfur Cluster Assembly. Mitochondrion 2016, 26, 94–103. [Google Scholar] [CrossRef]
- Maio, N.; Lafont, B.A.P.; Sil, D.; Li, Y.; Bollinger, J.M.; Krebs, C.; Pierson, T.C.; Linehan, W.M.; Rouault, T.A. Fe-S Cofactors in the SARS-CoV-2 RNA-Dependent RNA Polymerase Are Potential Antiviral Targets. Science 2021, 373, 236–241. [Google Scholar] [CrossRef]
- An, M.-J.; Kim, D.-H.; Kim, C.-H.; Kim, M.; Rhee, S.; Seo, S.-B.; Kim, J.-W. Histone Demethylase KDM3B Regulates the Transcriptional Network of Cell-Cycle Genes in Hepatocarcinoma HepG2 Cells. Biochem. Biophys. Res. Commun. 2019, 508, 576–582. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Kim, K.-B.; Eom, G.H.; Choe, N.; Kee, H.J.; Son, H.-J.; Oh, S.-T.; Kim, D.-W.; Pak, J.H.; Baek, H.J.; et al. KDM3B Is the H3K9 Demethylase Involved in Transcriptional Activation of Lmo2 in Leukemia. Mol. Cell. Biol. 2012, 32, 2917–2933. [Google Scholar] [CrossRef]
- Frolova, L.; Le Goff, X.; Rasmussen, H.H.; Cheperegin, S.; Drugeon, G.; Kress, M.; Arman, I.; Haenni, A.L.; Celis, J.E.; Philippe, M. A Highly Conserved Eukaryotic Protein Family Possessing Properties of Polypeptide Chain Release Factor. Nature 1994, 372, 701–703. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Zhu, L.; Wang, Y.; Li, P.; Gao, Y. Translational Control of COVID-19 and Its Therapeutic Implication. Front. Immunol. 2022, 13, 857490. [Google Scholar] [CrossRef]
- Miyata, Y.; Nishida, E. Identification of FAM53C as a Cytosolic-Anchoring Inhibitory Binding Protein of the Kinase DYRK1A. Life Sci. Alliance 2023, 6, e202302129. [Google Scholar] [CrossRef]
- Strine, M.S.; Cai, W.L.; Wei, J.; Alfajaro, M.M.; Filler, R.B.; Biering, S.B.; Sarnik, S.; Chow, R.D.; Patil, A.; Cervantes, K.S.; et al. DYRK1A Promotes Viral Entry of Highly Pathogenic Human Coronaviruses in a Kinase-Independent Manner. PLOS Biol. 2023, 21, e3002097. [Google Scholar] [CrossRef] [PubMed]
- Jang, J.; Lee, J.W.; Cho, M.J.; Hwang, B.; Kwon, M.-G.; Kim, D.-H.; Lee, N.-K.; Lee, J.; Park, Y.-J.; Yang, Y.R.; et al. KLHL3 Deficiency in Mice Ameliorates Obesity, Insulin Resistance, and Nonalcoholic Fatty Liver Disease by Regulating Energy Expenditure. Exp. Mol. Med. 2022, 54, 1250–1261. [Google Scholar] [CrossRef] [PubMed]
- Louis-Dit-Picard, H.; Barc, J.; Trujillano, D.; Miserey-Lenkei, S.; Bouatia-Naji, N.; Pylypenko, O.; Beaurain, G.; Bonnefond, A.; Sand, O.; Simian, C.; et al. KLHL3 Mutations Cause Familial Hyperkalemic Hypertension by Impairing Ion Transport in the Distal Nephron. Nat. Genet. 2012, 44, 456–460. [Google Scholar] [CrossRef]
- Bagchi, P.; Walczak, C.P.; Tsai, B. The Endoplasmic Reticulum Membrane J Protein C18 Executes a Distinct Role in Promoting Simian Virus 40 Membrane Penetration. J. Virol. 2015, 89, 4058–4068. [Google Scholar] [CrossRef]
- Rosado-Olivieri, E.A.; Razooky, B.; Le Pen, J.; De Santis, R.; Barrows, D.; Sabry, Z.; Hoffmann, H.-H.; Park, J.; Carroll, T.S.; Poirier, J.T.; et al. Organotypic Human Lung Bud Microarrays Identify BMP-Dependent SARS-CoV-2 Infection in Lung Cells. Stem Cell Rep. 2023, 18, 1107–1122. [Google Scholar] [CrossRef]
- Loktionov, A.; Kobzeva, K.; Dorofeeva, A.; Sergeeva, V.; Bushueva, O. GWAS-Identified Loci Are Associated with Obesity and Type 2 Diabetes Mellitus in Patients with Severe COVID-19. FBS 2024, 16, 14. [Google Scholar] [CrossRef]
- Loktionov, A.; Kobzeva, K.; Dorofeeva, A.; Babkina, M.; Kolodezhnaya, E.; Bushueva, O. A Comprehensive Genetic and Bioinformatic Analysis Provides Evidence for the Engagement of COVID-19 GWAS-Significant Loci in the Molecular Mechanisms of Coronary Artery Disease and Stroke. J. Mol. Pathol. 2024, 5, 385–404. [Google Scholar] [CrossRef]
- Bushueva, O.Y. Single Nucleotide Polymorphisms in Genes Encoding Xenobiotic Metabolizing Enzymes Are Associated with Predisposition to Arterial Hypertension. Res. Results Biomed. 2020, 6, 447–456. [Google Scholar] [CrossRef]
- Bushueva, O.Y.; Bulgakova, I.V.; Ivanov, V.P.; Polonikov, A.V. Association of Flavin Monooxygenase Gene E158K Polymorphism with Chronic Heart Disease Risk. Bull. Exp. Biol. Med. 2015, 159, 776–778. [Google Scholar] [CrossRef]
- World Health Organization. Diet, Nutrition, and the Prevention of Chronic Diseases: Report of a Joint WHO/FAO Expert Consultation; World Health Organization: Geneva, Switzerland, 2003; Volume 916, ISBN 92-4-120916-X. [Google Scholar]
- Bull, F.C.; Al-Ansari, S.S.; Biddle, S.; Borodulin, K.; Buman, M.P.; Cardon, G.; Carty, C.; Chaput, J.-P.; Chastin, S.; Chou, R.; et al. World Health Organization 2020 Guidelines on Physical Activity and Sedentary Behaviour. Br. J. Sports Med. 2020, 54, 1451–1462. [Google Scholar] [CrossRef] [PubMed]
- Koressaar, T.; Remm, M. Enhancements and Modifications of Primer Design Program Primer3. Bioinformatics 2007, 23, 1289–1291. [Google Scholar] [CrossRef]
- Kobzeva, K.A.; Soldatova, M.O.; Stetskaya, T.A.; Soldatov, V.O.; Deykin, A.V.; Freidin, M.B.; Bykanova, M.A.; Churnosov, M.I.; Polonikov, A.V.; Bushueva, O.Y. Association between HSPA8 Gene Variants and Ischemic Stroke: A Pilot Study Providing Additional Evidence for the Role of Heat Shock Proteins in Disease Pathogenesis. Genes 2023, 14, 1171. [Google Scholar] [CrossRef]
- Kobzeva, K.A.; Gurtovoy, D.E.; Polonikov, A.V.; Pokrovsky, V.M.; Patrakhanov, E.A.; Bushueva, O.Y. Polymorphism in Genes Encoding HSP40 Family Proteins Is Associated with Ischemic Stroke Risk and Brain Infarct Size: A Pilot Study. JIN 2024, 23, 211. [Google Scholar] [CrossRef]
- Kobzeva, K.; Ivenkov, M.; Gromov, R.; Bushueva, O. HSP90 Family Members, Their Regulators and Ischemic Stroke Risk: A Comprehensive Molecular-Genetics and Bioinformatics Analysis. FBS 2024, 16, 19. [Google Scholar] [CrossRef]
- Che, R.; Jack, J.R.; Motsinger-Reif, A.A.; Brown, C.C. An Adaptive Permutation Approach for Genome-Wide Association Study: Evaluation and Recommendations for Use. BioData Min. 2014, 7, 9. [Google Scholar] [CrossRef]
- Polonikov, A.V.; Samgina, T.A.; Nazarenko, P.M.; Bushueva, O.Y.; Ivanov, V.P. Alcohol Consumption and Cigarette Smoking Are Important Modifiers of the Association Between Acute Pancreatitis and the PRSS1-PRSS2 Locus in Men. Pancreas 2017, 46, 230–236. [Google Scholar] [CrossRef]
- Bushueva, O.; Solodilova, M.; Ivanov, V.; Polonikov, A. Gender-Specific Protective Effect of the −463G>A Polymorphism of Myeloperoxidase Gene against the Risk of Essential Hypertension in Russians. J. Am. Soc. Hypertens. 2015, 9, 902–906. [Google Scholar] [CrossRef]
- Ivanova, T.A. Sex-Specific Features of Interlocus Interactions Determining Susceptibility to Hypertension. Res. Results Biomed. 2024, 10, 53–68. [Google Scholar] [CrossRef]
- Choi, J.; Park, T. Multivariate Generalized Multifactor Dimensionality Reduction to Detect Gene-Gene Interactions. BMC Syst. Biol. 2013, 7, S15. [Google Scholar] [CrossRef]
- Stetskaya, T.A.; Kobzeva, K.A.; Zaytsev, S.M.; Shilenok, I.V.; Komkova, G.V.; Goryainova, N.V.; Bushueva, O.Y. HSPD1 Gene Polymorphism Is Associated with an Increased Risk of Ischemic Stroke in Smokers. Res. Results Biomed. 2024, 10, 175–186. [Google Scholar] [CrossRef]
- The GTEX Consortium. The GTEx Consortium Atlas of Genetic Regulatory Effects across Human Tissues. Science 2020, 369, 1318–1330. [Google Scholar] [CrossRef]
- Võsa, U.; Claringbould, A.; Westra, H.-J.; Bonder, M.J.; Deelen, P.; Zeng, B.; Kirsten, H.; Saha, A.; Kreuzhuber, R.; Kasela, S. Unraveling the Polygenic Architecture of Complex Traits Using Blood eQTL Metaanalysis. bioRxiv 2018. [Google Scholar] [CrossRef]
- Ward, L.D.; Kellis, M. HaploReg: A Resource for Exploring Chromatin States, Conservation, and Regulatory Motif Alterations within Sets of Genetically Linked Variants. Nucleic Acids Res. 2012, 40, D930–D934. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Hudson, R.; Harrison, C.; Craven, M.; Keleş, S. atSNP Search: A Web Resource for Statistically Evaluating Influence of Human Genetic Variation on Transcription Factor Binding. Bioinformatics 2019, 35, 2657–2659. [Google Scholar] [CrossRef]
- The Gene Ontology Consortium. The Gene Ontology Resource: 20 Years and Still GOing Strong. Nucleic Acids Res. 2019, 47, D330–D338. [Google Scholar] [CrossRef] [PubMed]


| Genetic Variant | Effect Allele | Other Allele | N | OR [95% CI] 1 | p 2 | pperm 3 |
|---|---|---|---|---|---|---|
| rs753856 HSPA6 | G | C | 1146 | 1.13 [0.78–1.64] | 0.51 | 0.64 |
| rs13161158 HSPA4 | C | T | 1159 | 1.14 [0.71–1.82] | 0.60 | 0.55 |
| rs1042665 HSPA9 | C | T | 1153 | 1.33 [1.01–1.75] | 0.04 | 0.06 |
| rs1043618 HSPA1A | C | G | 1157 | 1.07 [0.84–1.36] | 0.59 | 0.75 |
| rs6457452 HSPA1B | T | C | 1143 | 1.27 [0.90–1.80] | 0.18 | 0.16 |
| rs6909985 HSF2 | T | G | 1153 | 1.15 [0.83–1.59] | 0.41 | 0.46 |
| rs4279640 HSF1 | C | T | 1145 | 0.93 [0.73–1.18] | 0.54 | 0.70 |
| rs706121 BAG1 | C | T | 1151 | 1.17 [0.87–1.59] | 0.30 | 0.28 |
| rs17155992 HSPA14 | A | G | 1144 | 1.24 [0.81-1.91] | 0.32 | 0.33 |
| rs196336 BAG3 | T | C | 1155 | 1.01 [0.80–1.28] | 0.94 | 1.00 |
| rs196329 BAG3 | A | G | 1155 | 0.92 [0.70–1.21] | 0.56 | 0.75 |
| rs1461496 HSPA8 | A | G | 1156 | 1.11 [0.87–1.41] | 0.42 | 0.42 |
| rs10892958 HSPA8 | G | C | 1154 | 1.03 [0.76–1.38] | 0.87 | 0.86 |
| rs1136141 HSPA8 | A | G | 1108 | 1.25 [0.91–1.72] | 0.17 | 0.27 |
| rs7155973 HSP90AA1 | A | G | 1149 | 0.96 [0.59–1.57] | 0.88 | 1.00 |
| rs2034598 DNAJA2 | G | A | 1155 | 0.94 [0.72–1.22] | 0.64 | 0.75 |
| rs7189628 DNAJA2 | T | C | 1138 | 2.02 [1.26–3.24] | 0.003 | 0.002 |
| rs4926222 DNAJB1 | G | A | 1156 | 0.96 [0.68–1.35] | 0.81 | 0.86 |
| rs862832 HSPA12B | T | C | 1148 | 0.82 [0.57–1.18] | 0.28 | 0.25 |
| rs910652 HSPA12B | C | T | 1149 | 0.70 [0.53–0.92] | 0.01 | 0.01 |
| Genetic Variant | Effect Allele | Other Allele | N | OR [95% CI] 1 | p 2 (pbonf) | pperm 3 (pbonf) | N | OR [95% CI] 1 | p 2 (pbonf) | pperm 3 (pbonf) |
|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | |||||||||
| rs910652 HSPA12B | C | T | 481 | 0.91 [0.63–1.31] | 0.60 | 0.45 | 668 | 0.68 [0.47–0.98] | 0.04 | 0.04 |
| rs7189628 DNAJA2 | T | C | 473 | 3.53 [1.9–6.56] | 6.8 × 10−5 | 7.6 × 10−5 | 665 | 1.56 [0.84–2.9] | 0.16 | 0.15 |
| Smokers | Nonsmokers | |||||||||
| rs7189628 DNAJA2 | T | C | 307 | 3.99 [1.92–8.29] | 0.0002 | 0.0003 | 811 | 1.58 [0.9–2.78] | 0.11 | 0.14 |
| Low physical activity level | Normal physical activity level | |||||||||
| rs910652 HSPA12B | C | T | 1061 | 0.96 [0.69–1.33] | 0.79 (1.0) | 0.75 (1.0) | 1047 | 0.58 [0.39–0.88] | 0.009 (0.02) | 0.007 (0.01) |
| rs6457452 HSPA1B | T | C | 1058 | 1.6 [1.08–2.37] | 0.02 (0.04) | 0.02 (0.04) | 1045 | 0.69 [0.38–1.25] | 0.22 (0.44) | 0.25 (0.5) |
| rs1042665 HSPA9 | C | T | 1067 | 1.17 [0.83–1.66] | 0.36 (0.72) | 0.39 (0.78) | 1050 | 1.47 [1.03–2.1] | 0.03 (0.06) | 0.02 (0.04) |
| rs7189628 DNAJA2 | T | C | 1050 | 1.88 [1.05–3.34] | 0.03 (0.06) | 0.02 (0.04) | 1037 | 2.71 [1.52–4.84] | 0.0007 (0.001) | 0.0009 (0.002) |
| Low fruit and vegetable intake | Normal fruit and vegetable intake | |||||||||
| rs1136141 HSPA8 | A | G | 1039 | 1.69 [1.2–2.36] | 0.002 (0.004) | 0.002 (0.004) | 987 | 0.6 [0.32–1.12] | 0.11 (0.22) | 0.14 (0.28) |
| rs1042665 HSPA9 | C | T | 1083 | 1.12 [0.81–1.55] | 0.50 (1.0) | 0.52 (1.0) | 1034 | 1.67 [1.14–2.46] | 0.009 (0.02) | 0.009 (0.02) |
| rs7189628 DNAJA2 | T | C | 1068 | 2.39 [1.45–3.95] | 0.0007 (0.001) | 0.0008 (0.002) | 1019 | 1.92 [0.94–3.91] | 0.07 (0.14) | 0.05 (0.1) |
| Age < 68 | Age ≥ 68 | |||||||||
| rs1461496 HSPA8 | A | G | 952 | 0.91 [0.66–1.26] | 0.57 | 0.56 | 204 | 1.59 [1.05–2.4] | 0.03 | 0.03 |
| rs1136141 HSPA8 | A | G | 914 | 1.55 [1.06–2.28] | 0.02 | 0.02 | 194 | 0.9 [0.5–1.61] | 0.71 | 0.75 |
| rs1043618 HSPA1A | C | G | 954 | 0.89 [0.64–1.23] | 0.48 | 0.35 | 203 | 1.56 [1.04–2.35] | 0.03 | 0.03 |
| rs6457452 HSPA1B | T | C | 946 | 0.96 [0.59–1.55] | 0.85 | 0.86 | 197 | 2.29 [1.16–4.54] | 0.02 | 0.01 |
| rs7189628 DNAJA2 | T | C | 939 | 2.02 [1.08–3.75] | 0.03 | 0.02 | 199 | 2.04 [0.96–4.36] | 0.06 | 0.09 |
| Gene-Gene Interaction Models | NH | Beta H | WH | NL | Beta L | WL | Wmax | pperm |
|---|---|---|---|---|---|---|---|---|
| The best two-locus models of intergenic interactions (for G×G models with pmin. < 5 × 10−5, 1000 permutations) | ||||||||
| rs7189628 DNAJA2 × rs2034598 DNAJA2 | 2 | 0.2570 | 19.42 | 0 | NA | NA | 19.42 | <0.001 |
| rs7189628 DNAJA2 × rs10892958 HSPA8 | 2 | 0.2718 | 18.06 | 0 | NA | NA | 18.06 | 0.001 |
| rs7189628 DNAJA2 × rs706121 BAG1 | 3 | 0.3072 | 17.94 | 1 | −0.04071 | 3.210 | 17.94 | 0.001 |
| The best three-locus models of intergenic interactions (for G×G models with pmin. < 1 × 10−8, 1000 permutations) | ||||||||
| rs7189628 DNAJA2 × rs10892958 HSPA8 × rs2034598 DNAJA2 | 2 | 0.5336 | 34.64 | 1 | −0.16146 | 3.275 | 34.64 | <0.001 |
| The best four-locus models of gene-gene interactions (for G×G models with pmin. < 1 × 10−6, 1000 permutations) | ||||||||
| rs4279640 HSF1 × rs7189628 DNAJA2 × rs10892958 HSPA8 × rs706121 BAG1 | 8 | 0.6104 | 60.27 | 1 | −0.16122 | 3.061 | 60.27 | <0.001 |
| rs4279640 HSF1 × rs7189628 DNAJA2 × rs706121 BAG1 × rs1136141 HSPA8 | 9 | 0.6407 | 60.23 | 0 | NA | NA | 60.23 | <0.001 |
| Gene-Environmental Interaction Models | NH | Beta H | WH | NL | Beta L | WL | Wmax | pperm |
|---|---|---|---|---|---|---|---|---|
| The best two-order models of gene-interactions (for G×E models with pmin. < 0.001, 1000 permutations) | ||||||||
| rs7189628 DNAJA2 × SMOKE | 3 | 0.2551 | 17.09 | 0 | NA | NA | 17.09 | <0.001 |
| The best three-order models of gene-interactions (for G×E models with pmin. < 1 × 10−7, 1000 permutations) | ||||||||
| rs7189628 DNAJA2 × rs2034598 DNAJA2 × SMOKE | 3 | 0.5505 | 32.01 | 0 | NA | NA | 32.01 | <0.001 |
| rs7189628 DNAJA2 × rs196329 BAG3 × SMOKE | 4 | 0.3641 | 29.19 | 0 | NA | NA | 29.19 | <0.001 |
| rs7189628 DNAJA2 × rs4926222 DNAJB1 × SMOKE | 6 | 0.2854 | 28.81 | 0 | NA | NA | 28.81 | <0.001 |
| The best four-order models of gene-interactions (for G×E models with pmin. < 1 × 10−12, 1000 permutations) | ||||||||
| rs4279640 HSF1 × rs7189628 DNAJA2 × rs196329 BAG3 × SMOKE | 5 | 0.6674 | 59.44 | 0 | NA | NA | 59.44 | <0.001 |
| rs7189628 DNAJA2 × rs2034598 DNAJA2 × rs1043618 HSPA1A × SMOKE | 5 | 0.6554 | 53.12 | 0 | NA | NA | 53.12 | <0.001 |
| rs7189628 DNAJA2 × rs1042665 HSPA9 × rs1043618 HSPA1A × SMOKE | 8 | 0.4561 | 52.91 | 0 | NA | NA | 52.91 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karpenko, A.R.; Kobzeva, K.A.; Orlov, Y.L.; Bushueva, O.Y. Genes Encoding Heat Shock Proteins Are Associated with Risk and Clinical Course of Severe COVID-19: A Pilot Study. Int. J. Mol. Sci. 2025, 26, 8967. https://doi.org/10.3390/ijms26188967
Karpenko AR, Kobzeva KA, Orlov YL, Bushueva OY. Genes Encoding Heat Shock Proteins Are Associated with Risk and Clinical Course of Severe COVID-19: A Pilot Study. International Journal of Molecular Sciences. 2025; 26(18):8967. https://doi.org/10.3390/ijms26188967
Chicago/Turabian StyleKarpenko, Andrey R., Ksenia A. Kobzeva, Yuriy L. Orlov, and Olga Yu. Bushueva. 2025. "Genes Encoding Heat Shock Proteins Are Associated with Risk and Clinical Course of Severe COVID-19: A Pilot Study" International Journal of Molecular Sciences 26, no. 18: 8967. https://doi.org/10.3390/ijms26188967
APA StyleKarpenko, A. R., Kobzeva, K. A., Orlov, Y. L., & Bushueva, O. Y. (2025). Genes Encoding Heat Shock Proteins Are Associated with Risk and Clinical Course of Severe COVID-19: A Pilot Study. International Journal of Molecular Sciences, 26(18), 8967. https://doi.org/10.3390/ijms26188967







