One Syndrome, Many Faces: A Unified Perspective on Heart Failure Phenotypes
Abstract
1. Introduction: The Complexity of Heart Failure Classification
- (A)
- EF > 50% (heart failure with preserved EF, HFpEF);
- (B)
- EF between 41–49% (heart failure with mildly reduced EF, HFmrEF);
- (C)
- EF < 40% (heart failure with reduced EF, HFrEF).
2. HFpEF: A Syndrome or an Epiphenomenon?
2.1. The Role of the ‘Mosaic Theory’ in Hypertension and HF
2.2. Is Left Atrial Enlargement a Diagnostic Clue or a Nonspecific Marker?
2.3. Aging and Diagnostic Ambiguities
2.4. HFpEF as a Systemic Syndrome with Multiple Contributors
- Aging-related;
- Cardiometabolic;
- Hypertension-related;
- Associated with pulmonary arterial hypertension;
- Coronary artery disease-related;
- Left atrial myopathy [20].
3. Rethinking Heart Failure as a Unified Syndrome
3.1. A Common Pathophysiological Foundation
3.2. The Limitations of Ejection Fraction: What Is Normal?
4. Pathophysiological Mechanisms of Heart Failure
4.1. Disease Progression and Homeostatic Imbalance
4.2. Neurohormonal Activation Across Phenotypes
4.2.1. Sympathetic–Parasympathetic Imbalance
4.2.2. Vasopressin System Dysregulation
5. Inflammation and Immune Dysregulation
5.1. Inflammation as a Shared Pathogenic Process
5.2. Sterile Inflammation and Pattern Recognition
5.3. Immune Response and Comorbidity-Driven Inflammation
6. Myocardial Cellular Changes in Heart Failure
6.1. Fibrosis and Cellular Remodeling
6.2. Patterns of Myocardial Cell Death
7. Mitochondrial Dysfunction and Oxidative Stress
7.1. Central Role of Mitochondria
7.2. Mitochondrial Defense and Organelle Crosstalk
7.3. Reactive Oxygen Species and Oxidative Stress
8. Molecular Pathways in HF (AMPK, MMP, cGMP–PKG)
9. Advanced Imaging and Biomarkers in Unifying Heart Failure Diagnosis
10. The Role of Comorbidities in the HF Phenotype Spectrum
11. Therapeutic Convergence: Are We Moving Towards a Universal HF Treatment Model?
11.1. Limitations and Gaps in Literature
11.2. Future Perspectives
12. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ACC | American College of Cardiology |
| ADP | Adenosine Diphosphate |
| AHA | American Heart Association |
| AMPK | AMP-Activated Protein Kinase |
| ARNIs | Angiotensin Receptor–Neprilysin Inhibitors |
| ATP | Adenosine Triphosphate |
| AVP | Arginine Vasopressin |
| cGMP | Cyclic Guanosine Monophosphate |
| CMR | Cardiac Magnetic Resonance |
| CRP | C Reactive Protein |
| DAMPs | Damage-Associated Molecular Patterns |
| Drp1 | Dynamin-Related Protein 1 |
| ECM | Extracellular Matrix |
| ECV | Extracellular Volume |
| EF | Ejection Fraction |
| ER | Endoplasmic Reticulum |
| ESC | European Society of Cardiology |
| GDF-15 | Growth Differentiation Factor-15 |
| GLS | Global Longitudinal Strain |
| HF | Heart Failure |
| HFmrEF | Heart Failure with Mildly Reduced Ejection Fraction |
| HFpEF | Heart Failure with Preserved Ejection Fraction |
| HFrEF | Heart Failure with Reduced Ejection Fraction |
| HMGB1 | High-Mobility Group Box 1 |
| IL-6 | Interleukin-6 |
| LV | Left Ventricle/Left Ventricular |
| Mfn1/2 | Mitofusins 1/2 |
| MMPs | Matrix Metalloproteinases |
| MRAs | Mineralocorticoid Receptor Antagonists |
| mtROS | Mitochondrial Reactive Oxygen Species |
| NF-κB | Nuclear Factor κB |
| NLRs | NOD-Like Receptors |
| NO | Nitric Oxide |
| NT-proBNP | N-terminal pro-B-type Natriuretic Peptide |
| NYHA | New York Heart Association |
| Opa1 | Optic Atrophy Protein 1 |
| PCr | Phosphocreatine |
| PDE5 | Phosphodiesterase Type 5 |
| PGC-1α | Peroxisome Proliferator-Activated Receptor Gamma Coactivator-1α |
| PKG | Protein Kinase G |
| PRRs | Pattern Recognition Receptors |
| RAAS | Renin–Angiotensin–Aldosterone System |
| ROS | Reactive Oxygen Species |
| sGC | Soluble Guanylate Cyclase |
| SGLT2 | Sodium-Glucose Cotransporter-2 |
| SNS | Sympathetic Nervous System |
| ST2 | Suppression of Tumorigenicity 2 Protein |
| TGF-β1 | Transforming Growth Factor Beta 1 |
| TIMPs | Tissue Inhibitor of Metalloproteinases |
| TLRs | Toll-Like Receptors |
| TNF-α | Tumor Necrosis Factor-α |
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2023 Focused Update of the 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. J. Heart Fail. 2024, 26, 5–17. [Google Scholar] [PubMed]
- Pieske, B.; Tschöpe, C.; de Boer, R.A.; Fraser, A.G.; Anker, S.D.; Donal, E.; Edelmann, F.; Fu, M.; Guazzi, M.; Lam, C.S.P.; et al. How to diagnose heart failure with preserved ejection fraction: The HFA-PEFF diagnostic algorithm: A consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur. Heart J. 2019, 40, 3297–3317. [Google Scholar] [CrossRef] [PubMed]
- Bozkurt, B.; Coats, A.J.; Tsutsui, H.; Abdelhamid, M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal definition and classification of heart failure: A report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. Eur. J. Heart Fail. 2021, 23, 352–380. [Google Scholar] [PubMed]
- Kodur, N.; Tang, W.H.W. Myocardial recovery and relapse in heart failure with improved ejection fraction. Curr. Treat. Options Cardiovasc. Med. 2024, 26, 139–160. [Google Scholar] [CrossRef]
- Heidenreich, P.A.; Bozkurt, B.; Aguilar, D.; Allen, L.A.; Byun, J.J.; Colvin, M.M.; Deswal, A.; Drazner, M.H.; Dunlay, S.M.; Evers, L.R.; et al. 2022 AHA/ACC/HFSA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation 2022, 145, e895–e1032, Erratum in: Circulation 2023, 147, e674. [Google Scholar] [CrossRef] [PubMed]
- Borlaug, B.A. Evaluation and management of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2020, 17, 559–573. [Google Scholar] [CrossRef]
- Rosano, G.M.C.; Teerlink, J.R.; Kinugawa, K.; Bayes-Genis, A.; Chioncel, O.; Fang, J.; Greenberg, B.; Ibrahim, N.E.; Imamura, T.; Inomata, T.; et al. The use of left ventricular ejection fraction in the diagnosis and management of heart failure. A clinical consensus statement of the Heart Failure Association (HFA) of the ESC, the Heart Failure Society of America (HFSA), and the Japanese Heart Failure Society (JHFS). Eur. J. Heart Fail. 2025, 27, 1174–1187. [Google Scholar] [CrossRef]
- von Haehling, S.; Assmus, B.; Bekfani, T.; Dworatzek, E.; Edelmann, F.; Hashemi, D.; Hellenkamp, K.; Kempf, T.; Raake, P.; Schütt, K.A.; et al. Heart failure with preserved ejection fraction: Diagnosis, risk assessment, and treatment. Clin. Res. Cardiol. 2024, 113, 1287–1305. [Google Scholar] [CrossRef]
- Ren, J.; Wu, N.N.; Wang, S.; Sowers, J.R.; Zhang, Y. Obesity cardiomyopathy: Evidence, mechanisms, and therapeutic implications. Physiol. Rev. 2021, 101, 1745–1807. [Google Scholar] [CrossRef] [PubMed]
- Salvatore, T.; Pafundi, P.C.; Galiero, R.; Albanese, G.; Di Martino, A.; Caturano, A.; Vetrano, E.; Rinaldi, L.; Sasso, F.C. The Diabetic Cardiomyopathy: The Contributing Pathophysiological Mechanisms. Front. Med. 2021, 8, 695792. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Sarafidis, P.; Briasoulis, A.; Magouliotis, D.E.; Athanasiou, T.; Skoularigis, J.; Xanthopoulos, A. Hypertensive Heart Failure. J. Clin. Med. 2023, 12, 5090. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, B.A.; Zhao, X.; Heidenreich, P.A.; Peterson, E.D.; Bhatt, D.L.; Cannon, C.P.; Hernandez, A.F.; Fonarow, G.C.; Get with the Guidelines Scientific Advisory Committee and Investigators. Trends in patients hospitalized with heart failure and preserved left ventricular ejection fraction: Prevalence, therapies, and outcomes. Circulation 2012, 126, 65–75. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin–Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef] [PubMed]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Claggett, B.; de Boer, R.A.; DeMets, D.; Hernandez, A.F.; Inzucchi, S.E.; Kosiborod, M.N.; Lam, C.S.P.; Martinez, F.; et al. Dapagliflozin in Heart Failure with Mildly Reduced or Preserved Ejection Fraction. N. Engl. J. Med. 2022, 387, 1089–1098. [Google Scholar] [CrossRef]
- Reddy, Y.N.V.; Carter, R.E.; Obokata, M.; Redfield, M.M.; Borlaug, B.A. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018, 138, 861–870. [Google Scholar] [CrossRef]
- Harrison, D.G.; Coffman, T.M.; Wilcox, C.S. Pathophysiology of Hypertension: The Mosaic Theory and Beyond. Circ. Res. 2021, 128, 847–863. [Google Scholar] [CrossRef]
- Tana, M.; Piccinini, R.; Moffa, L.; Tana, C. Heart Failure with Preserved Ejection Fraction and Cardiac Amyloidosis in the Aging Heart. Int. J. Mol. Sci. 2024, 25, 11519. [Google Scholar] [CrossRef] [PubMed]
- Campbell, P.; Rutten, F.H.; Lee, M.M.Y.; Hawkins, N.M.; Petrie, M.C. Heart failure with preserved ejection fraction: Everything the clinician needs to know. Lancet 2024, 403, 1083–1092, Erratum in: Lancet 2024, 403, 1026. [Google Scholar] [CrossRef] [PubMed]
- Stoicescu, L.; Crisan, D.; Morgovan, C.; Avram, L.; Ghibu, S. Heart Failure with Preserved Ejection Fraction: The Pathophysiological Mechanisms behind the Clinical Phenotypes and the Therapeutic Approach. Int. J. Mol. Sci. 2024, 25, 794. [Google Scholar] [CrossRef]
- Packer, M. Left Ventricular Ejection Fraction in Heart Failure: Crazy, Stupid Love—And Maybe, Redemption. J. Am. Heart Assoc. 2024, 13, e034642. [Google Scholar] [CrossRef]
- Gonzalez, A.; Ravassa, S.; Beaumont, J.; Lopez, B.; Díez, J. New Targets to Treat the Structural Remodeling of the Myocardium. J. Am. Coll. Cardiol. 2011, 58, 1833–1843. [Google Scholar] [CrossRef]
- Basuray, A.; French, B.; Ky, B.; Vorovich, E.; Olt, C.; Sweitzer, N.K.; Cappola, T.P.; Fang, J.C. Heart failure with recovered ejection fraction. Circulation 2014, 129, 2380–2387. [Google Scholar] [CrossRef]
- Cheng, L.; Hammersley, D.; Ragavan, A.; Javed, S.; Mukhopadhyay, S.; Gregson, J.; Han, J.; Khalique, Z.; Lota, A.; Pantazis, A.; et al. Long-term follow-up of the TRED-HF trial: Implications for therapy in patients with dilated cardiomyopathy and heart failure remission. Eur. J. Heart Fail. 2025, 7, 113–123. [Google Scholar] [CrossRef]
- Kodur, N.; Tang, W.H.W. Management of Heart Failure with Improved Ejection Fraction: Current Evidence and Controversies. JACC Heart Fail. 2025, 13, 537–553. [Google Scholar] [CrossRef] [PubMed]
- Aimo, A.; Vergaro, G.; Castiglione, V.; Barison, A.; Pasanisi, E.; Petersen, C.; Chubuchny, V.; Giannoni, A.; Poletti, R.; Maffei, S.; et al. Effect of Sex on Reverse Remodeling in Chronic Systolic Heart Failure. J. Am. Coll. Cardiol. Heart Fail. 2017, 5, 735–742. [Google Scholar] [CrossRef] [PubMed]
- Kapłon-Cieślicka, A.; Benson, L.; Chioncel, O.; Crespo-Leiro, M.G.; Coats, A.J.S.; Anker, S.D.; Filippatos, G.; Ruschitzka, F.; Hage, C.; Drożdż, J.; et al. A comprehensive characterization of acute heart failure with preserved versus mildly reduced versus reduced ejection fraction–insights from the ESC-HFA EORP Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2022, 24, 335–350, Erratum in: Eur. J. Heart Fail. 2024, 26, 193. [Google Scholar] [CrossRef] [PubMed]
- Manolis, A.A.; Manolis, T.A.; Manolis, A.S. Neurohumoral Activation in Heart Failure. Int. J. Mol. Sci. 2023, 24, 15472. [Google Scholar] [CrossRef]
- Castiglione, V.; Gentile, F.; Ghionzoli, N.; Chiriacò, M.; Panichella, G.; Aimo, A.; Vergaro, G.; Giannoni, A.; Passino, C.; Emdin, M. Pathophysiological Rationale and Clinical Evidence for Neurohormonal Modulation in Heart Failure with Preserved Ejection Fraction. Card. Fail. Rev. 2023, 5, e09. [Google Scholar] [CrossRef]
- Gorini, S.; Marzolla, V.; Mammi, C.; Armani, A.; Caprio, M. Mineralocorticoid Receptor and Aldosterone-Related Biomarkers of End-Organ Damage in Cardiometabolic Disease. Biomolecules 2018, 8, 96. [Google Scholar] [CrossRef]
- Vergaro, G.; Aimo, A.; Prontera, C.; Ghionzoli, N.; Arzilli, C.; Zyw, L.; Taddei, C.; Gabutti, A.; Poletti, R.; Giannoni, A.; et al. Sympathetic and renin-angiotensin-aldosterone system activation in heart failure with preserved, mid-range and reduced ejection fraction. Int. J. Cardiol. 2019, 296, 91–97. [Google Scholar] [CrossRef]
- Ke, B.; Tan, X.; Ren, L.; Fan, Y.; Zhang, Y.; Li, F.; Sun, Q.; Liu, T.; Jia, L.; Wang, Y.; et al. Aldosterone dysregulation predicts the risk of mortality and rehospitalization in heart failure with a preserved ejection fraction. Sci. China Life Sci. 2022, 65, 631–642. [Google Scholar] [CrossRef] [PubMed]
- Binder, C.; Poglitsch, A.; Duca, F.; Rettl, R.; Dachs, T.M.; Dalos, D.; Schrutka, L.; Seirer, B.; Ligios, L.C.; Capelle, C.; et al. Renin feedback is an independent predictor of outcome in HFpEF. J. Pers. Med. 2021, 11, 370. [Google Scholar] [CrossRef] [PubMed]
- McHugh, K.; DeVore, A.D.; Wu, J.; Matsouaka, R.A.; Fonarow, G.C.; Heidenreich, P.A.; Yancy, C.W.; Green, J.B.; Altman, N.; Hernandez, A.F. Heart failure with preserved ejection fraction and diabetes: JACC state-of-the-art review. J. Am. Coll. Cardiol. 2019, 73, 602–611. [Google Scholar] [CrossRef] [PubMed]
- Badrov, M.B.; Mak, S.; Floras, J.S. Cardiovascular autonomic disturbances in heart failure with preserved ejection fraction. Can. J. Cardiol. 2021, 37, 609–620. [Google Scholar] [CrossRef]
- Kaye, D.M.; Nanayakkara, S.; Wang, B.; Shihata, W.; Marques, F.Z.; Esler, M.; Lambert, G.; Mariani, J. Characterization of cardiac sympathetic nervous system and inflammatory activation in HFpEF patients. JACC Basic Transl. Sci. 2022, 7, 116–127. [Google Scholar] [CrossRef]
- Jimenez-Marrero, S.; Moliner, P.; Rodríguez-Costoya, I.; Enjuanes, C.; Alcoberro, L.; Yun, S.; Gonzalez-Costello, J.; Garay, A.; Tajes, M.; Calero, E.; et al. Sympathetic activation and outcomes in chronic heart failure: Does the neurohormonal hypothesis apply to mid-range and preserved ejection fraction patients? Eur. J. Intern. Med. 2020, 81, 60–66. [Google Scholar] [CrossRef]
- Buijs, R.M.; Hurtado-Alvarado, G.; Soto-Tinoco, E. Vasopressin: An output signal from the suprachiasmatic nucleus to prepare physiology and behaviour for the resting phase. J. Neuroendocrinol. 2021, 33, e12998. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E. The Heart as a Target of Vasopressin and Other Cardiovascular Peptides in Health and Cardiovascular Diseases. Int. J. Mol. Sci. 2022, 23, 14414. [Google Scholar] [CrossRef]
- Szczepanska-Sadowska, E.; Wsol, A.; Cudnoch-Jedrzejewska, A.; Czarzasta, K.; Zera, T. Multiple aspects of inappropriate action of renin-angiotensin, vasopressin, and oxytocin systems in neuropsychiatric and neurodegenerative diseases. J. Clin. Med. 2022, 11, 908. [Google Scholar] [CrossRef]
- Wsol, A.; Wojno, O.; Puchalska, L.; Wrzesien, R.; Szczepanska-Sadowska, E.; Cudnoch-Jedrzejewska, A. Impaired hypotensive effects of centrally acting oxytocin in SHR and WKY rats exposed to chronic mild stress. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2020, 318, R160–R172. [Google Scholar] [CrossRef]
- Urbach, J.; Goldsmith, S.R. Vasopressin antagonism in heart failure: A review of the hemodynamic studies and major clinical trials. Ther. Adv. Cardiovasc. Dis. 2021, 15, 1753944720977741. [Google Scholar] [CrossRef]
- Iovino, M.; Iacoviello, M.; De Pergola, G.; Licchelli, B.; Iovino, E.; Guastamacchia, E.; Giagulli, V.A.; Triggiani, V. Vasopressin in Heart Failure. Endocr. Metab. Immune Disord. Drug Targets 2018, 18, 458–465. [Google Scholar] [CrossRef]
- D’Acierno, M.; Fenton, R.A.; Hoorn, E.J. The biology of water homeostasis. Nephrol. Dial. Transplant. 2025, 40, 632–640. [Google Scholar] [CrossRef]
- Goldsmith, S.R. Arginine vasopressin antagonism in heart failure: Current status and possible new directions. J. Cardiol. 2019, 74, 49–52. [Google Scholar] [CrossRef]
- Chirinos, J.A.; Sardana, M.; Oldland, G.; Ansari, B.; Lee, J.; Hussain, A.; Mustafa, A.; Akers, S.R.; Wei, W.; Lakatta, E.G.; et al. Association of arginine vasopressin with low atrial natriuretic peptide levels, left ventricular remodeling, and outcomes in adults with and without heart failure. ESC Heart Fail. 2018, 5, 912–920. [Google Scholar]
- Pugliese, N.R.; Pellicori, P.; Filidei, F.; De Biase, N.; Maffia, P.; Guzik, T.J.; Masi, S.; Taddei, S.; Cleland, J.G.F. Inflammatory pathways in heart failure with preserved left ventricular ejection fraction: Implications for future interventions. Cardiovasc. Res. 2023, 118, 3536–3555. [Google Scholar] [CrossRef]
- Lam, C.S.P.; Voors, A.A.; de Boer, R.A.; Solomon, S.D.; van Veldhuisen, D.J. Heart failure with preserved ejection fraction: From mechanisms to therapies. Eur. Heart J. 2018, 39, 2780–2792, Erratum in: Eur. Heart J. 2019, 40, 528. [Google Scholar] [CrossRef] [PubMed]
- Lourenço, A.P.; Leite-Moreira, A.F.; Balligand, J.L.; Bauersachs, J.; Dawson, D.; de Boer, R.A.; de Windt, L.J.; Falcão-Pires, I.; Fontes-Carvalho, R.; Franz, S.; et al. An integrative translational approach to study heart failure with preserved ejection fraction: A position paper from the Working Group on Myocardial Function of the European Society of Cardiology. Eur. J. Heart Fail. 2018, 20, 216–227. [Google Scholar] [CrossRef] [PubMed]
- Schumacher, S.M.; Naga Prasad, S.V. Tumor Necrosis Factor-α in Heart Failure: An Updated Review. Curr. Cardiol. Rep. 2018, 20, 117. [Google Scholar] [CrossRef] [PubMed]
- Adamo, L.; Rocha-Resende, C.; Prabhu, S.D.; Mann, D.L. Reappraising the role of inflammation in heart failure. Nat. Rev. Cardiol. 2020, 17, 269–285, Erratum in: Nat. Rev. Cardiol. 2021, 18, 735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bauersachs, J.; Langer, H.F. Immune mechanisms in heart failure. Eur. J. Heart Fail. 2017, 19, 1379–1389. [Google Scholar] [CrossRef]
- Antonello, J.; Roy, P. Damage-associated molecular patterns (DAMPs) in vascular diseases. J. Biol. Chem. 2025, 301, 110241. [Google Scholar] [CrossRef]
- Lai, S.L.; Marín-Juez, R.; Stainier, D.Y.R. Immune responses in cardiac repair and regeneration: A comparative point of view. Cell Mol. Life Sci. 2019, 76, 1365–1380. [Google Scholar] [CrossRef]
- Nakahira, K.; Hisata, S.; Choi, A.M. The Roles of Mitochondrial Damage-Associated Molecular Patterns in Diseases. Antioxid. Redox Signal. 2015, 23, 1329–1350. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Jiang, W.; Zhou, R. DAMPs and DAMP-sensing receptors in inflammation and diseases. Immunity 2024, 57, 752–771. [Google Scholar] [CrossRef]
- Lin, H.; Xiong, W.; Fu, L.; Yi, J.; Yang, J. Damage-associated molecular patterns (DAMPs) in diseases: Implications for therapy. Mol. Biomed. 2025, 6, 60. [Google Scholar] [CrossRef]
- Paraskevaidis, I.; Farmakis, D.; Papingiotis, G.; Tsougos, E. Inflammation and Heart Failure: Searching for the Enemy—Reaching the Entelechy. J. Cardiovasc. Dev. Dis. 2023, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Zhang, Y.; Li, H.; Wang, F.; Yao, S. Toll-like receptor 4: A potential therapeutic target for multiple human diseases. Biomed. Pharmacother. 2023, 166, 115338. [Google Scholar] [CrossRef]
- Duan, T.; Du, Y.; Xing, C.; Wang, H.Y.; Wang, R.F. Toll-Like Receptor Signaling and Its Role in Cell-Mediated Immunity. Front. Immunol. 2022, 13, 812774. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Xu, W.; Zhou, R. NLRP3 inflammasome activation and cell death. Cell Mol. Immunol. 2021, 18, 2114–2127. [Google Scholar] [CrossRef]
- Hahn, V.S.; Knutsdottir, H.; Luo, X.; Bedi, K.; Margulies, K.B.; Haldar, S.M.; Stolina, M.; Yin, J.; Khakoo, A.Y.; Vaishnav, J.; et al. Myocardial gene expression signatures in human heart failure with preserved ejection fraction. Circulation 2021, 143, 120–134, Erratum in: Circulation 2021, 143, e1027. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liu, R.; Nikolajczyk, B.S. Tissue immune cells fuel obesity-associated inflammation in adipose tissue and beyond. Front. Immunol. 2019, 10, 1587. [Google Scholar] [CrossRef]
- Drummond, G.R.; Vinh, A.; Guzik, T.J.; Sobey, C.G. Immune mechanisms of hypertension. Nat. Rev. Immunol. 2019, 19, 517–532. [Google Scholar] [CrossRef]
- Alcaide, P.; Kallikourdis, M.; Emig, R.; Prabhu, S.D. Myocardial inflammation in heart failure with reduced and preserved ejection fraction. Circ. Res. 2024, 134, 1752–1766. [Google Scholar] [CrossRef] [PubMed]
- Roman-Pepine, D.; Serban, A.M.; Capras, R.D.; Cismaru, C.M.; Filip, A.G. A Comprehensive Review: Unraveling the Role of Inflammation in the Etiology of Heart Failure. Heart Fail. Rev. 2025, 30, 931–954. [Google Scholar] [CrossRef]
- Li, G.; He, W.; Wang, D.W. Immune cell dynamics in heart failure: Implicated mechanisms and therapeutic targets. ESC Heart Fail. 2025, 12, 1739–1758. [Google Scholar] [CrossRef]
- Bermea, K.; Bhalodia, A.; Huff, A.; Rousseau, S.; Adamo, L. The Role of B Cells in Cardiomyopathy and Heart Failure. Curr. Cardiol. Rep. 2022, 24, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Simmonds, S.J.; Cuijpers, I.; Heymans, S.; Jones, E.A.V. Cellular and molecular differences between HFpEF and HFrEF: A step ahead in an improved pathological understanding. Cells 2020, 9, 242. [Google Scholar] [CrossRef] [PubMed]
- Morishita, T.; Uzui, H.; Mitsuke, Y.; Amaya, N.; Kaseno, K.; Ishida, K.; Fukuoka, Y.; Ikeda, H.; Tama, N.; Yamazaki, T.; et al. Association between matrix metalloproteinase-9 and worsening heart failure events in patients with chronic heart failure. ESC Heart Fail. 2017, 4, 321–330. [Google Scholar] [CrossRef]
- Zile, M.R.; Baicu, C.F.; Ikonomidis, J.S.; Stroud, R.E.; Nietert, P.J.; Bradshaw, A.D.; Slater, R.; Palmer, B.M.; Van Buren, P.; Meyer, M.; et al. Myocardial stiffness in patients with heart failure and a preserved ejection fraction: Contributions of collagen and titin. Circulation 2015, 131, 1247–1259. [Google Scholar] [CrossRef]
- Mishra, S.; Kass, D.A. Cellular and molecular pathobiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2021, 18, 400–423, Erratum in: Nat. Rev. Cardiol. 2021, 18, 735. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Jankowski, J.; Kozub, K.O.; Kleibert, M.; Camlet, K.; Kleibert, K.; Cudnoch-Jedrzejewska, A. The role of programmed types of cell death in pathogenesis of heart failure with preserved ejection fraction. Int. J. Mol. Sci. 2024, 25, 9921. [Google Scholar] [CrossRef]
- Kostin, S.; Pool, L.; Elsasser, A.; Hein, S.; Drexler, H.C.; Arnon, E.; Hayakawa, Y.; Zimmermann, R.; Bauer, E.; Klovekorn, W.P.; et al. Myocytes die by multiple mechanisms in failing human hearts. Circ. Res. 2003, 92, 715–724. [Google Scholar] [CrossRef]
- Wencker, D.; Chandra, M.; Nguyen, K.; Miao, W.; Garantziotis, S.; Factor, S.M.; Shirani, J.; Armstrong, R.C.; Kitsis, R.N. A mechanistic role for cardiac myocyte apoptosis in heart failure. J. Clin. Investig. 2003, 111, 1497–1504. [Google Scholar] [CrossRef] [PubMed]
- Corsetti, G.; Chen-Scarabelli, C.; Romano, C.; Pasini, E.; Dioguardi, F.S.; Onorati, F.; Knight, R.; Patel, H.; Saravolatz, L.; Faggian, G.; et al. Autophagy and oncosis/necroptosis are enhanced in cardiomyocytes from heart failure patients. Med. Sci. Monit. Basic Res. 2019, 25, 33–44. [Google Scholar] [CrossRef]
- Paraskevaidis, I.; Kourek, C.; Farmakis, D.; Tsougos, E. Heart failure: A deficiency of energy—A path yet to discover and walk. Biomedicines 2024, 12, 2589. [Google Scholar] [CrossRef]
- Zhang, J.J.; Cheng, L.; Qiao, Q.; Xiao, X.L.; Lin, S.J.; He, Y.F.; Sha, R.L.; Sha, J.; Ma, Y.; Zhang, H.L.; et al. Adenosine triphosphate-induced cell death in heart failure: Is there a link? World J. Cardiol. 2025, 17, 105021. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Houston, B. A comprehensive review of the bioenergetics of fatty acid and glucose metabolism in the healthy and failing heart in nondiabetic condition. Heart Fail. Rev. 2017, 22, 825–842. [Google Scholar] [CrossRef] [PubMed]
- Karwi, Q.G.; Uddin, G.M.; Ho, K.L.; Lopaschuk, G.D. Loss of metabolic flexibility in the failing heart. Front. Cardiovasc. Med. 2018, 6, 68. [Google Scholar] [CrossRef]
- Zong, Y.; Li, H.; Liao, P.; Chen, L.; Pan, Y.; Zheng, Y.; Zhang, C.; Liu, D.; Zheng, M.; Gao, J. Mitochondrial dysfunction: Mechanisms and advances in therapy. Signal Transduct. Target Ther. 2024, 9, 124. [Google Scholar] [CrossRef]
- Wang, N.; Wang, X.; Lan, B.; Gao, Y.; Cai, Y. DRP1, fission and apoptosis. Cell Death Discov. 2025, 11, 150. [Google Scholar] [CrossRef]
- Song, Z.; Ghochani, M.; McCaffery, J.M.; Frey, T.G.; Chan, D.C. Mitofusins and OPA1 mediate sequential steps in mitochondrial membrane fusion. Mol. Biol. Cell 2009, 20, 3525–3532. [Google Scholar] [CrossRef]
- Ali, S.; McStay, G.P. Regulation of Mitochondrial Dynamics by Proteolytic Processing and Protein Turnover. Antioxidants 2018, 7, 15. [Google Scholar] [CrossRef]
- Schwartz, B.; Gjini, P.; Gopal, D.M.; Fetterman, J.L. Inefficient Batteries in Heart Failure: Metabolic Bottlenecks Disrupting the Mitochondrial Ecosystem. JACC Basic Transl. Sci. 2022, 7, 1161–1179. [Google Scholar] [CrossRef] [PubMed]
- Ashrafi, G.; Schwarz, T.L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death Differ. 2013, 20, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, N.; Scott, I. In the heart and beyond: Mitochondrial dysfunction in heart failure with preserved ejection fraction (HFpEF). Curr. Opin. Pharmacol. 2024, 76, 102461. [Google Scholar] [CrossRef] [PubMed]
- Scheffer, D.D.L.; Garcia, A.A.; Lee, L.; Mochly-Rosen, D.; Ferreira, J.C.B. Mitochondrial Fusion, Fission, and Mitophagy in Cardiac Diseases: Challenges and Therapeutic Opportunities. Antioxid. Redox Signal. 2022, 36, 844–863. [Google Scholar] [CrossRef] [PubMed]
- Paraskevaidis, I.; Kourek, C.; Farmakis, D.; Tsougos, E. Mitochondrial dysfunction in cardiac disease: The fort fell. Biomolecules 2024, 14, 1534. [Google Scholar] [CrossRef]
- Rostamzadeh, F.; Najafipour, H.; Aminizadeh, S.; Jafari, E. Therapeutic effects of the combination of moderate-intensity endurance training and MitoQ supplementation in rats with isoproterenol-induced myocardial injury: The role of mitochondrial fusion, fission, and mitophagy. Biomed. Pharmacother. 2024, 170, 116020. [Google Scholar] [CrossRef]
- Lin, J.; Duan, J.; Wang, Q.; Xu, S.; Zhou, S.; Yao, K. Mitochondrial dynamics and mitophagy in cardiometabolic disease. Front. Cardiovasc. Med. 2022, 9, 917135. [Google Scholar] [CrossRef]
- Yoshii, A.; McMillen, T.S.; Wang, Y.; Zhou, B.; Chen, H.; Banerjee, D.; Herrero, M.; Wang, P.; Muraoka, N.; Wang, W.; et al. Blunted cardiac mitophagy in response to metabolic stress contributes to HFpEF. Circ. Res. 2024, 135, 1004–1017, Erratum in: Circ. Res. 2024, 135, e154. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Martinez, C.S.; Zheng, A.; Xiao, Q. Mitochondrial reactive oxygen species dysregulation in heart failure with preserved ejection fraction: A fraction of the whole. Antioxidants 2024, 13, 1330. [Google Scholar] [CrossRef] [PubMed]
- Gorica, E.; Geiger, M.A.; Di Venanzio, L.; Atzemian, N.; Kleeberger, J.A.; Grigorian, D.; Mongelli, A.; Emini Veseli, B.; Mohammed, S.A.; Ruschitzka, F.; et al. Cardiometabolic heart failure with preserved ejection fraction: From molecular signatures to personalized treatment. Cardiovasc. Diabetol. 2025, 24, 265. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; Spanos, M.; Zhao, C.; Wan, W.; Cui, C.; Wang, L.; Xiao, J. Mitochondrial Dysfunction in HFpEF: Potential Interventions Through Exercise. J. Cardiovasc. Transl. Res. 2025, 18, 442–456. [Google Scholar] [CrossRef] [PubMed]
- Belli, M.; Barone, L.; Bellia, A.; Sergi, D.; Lecis, D.; Prandi, F.R.; Milite, M.; Galluccio, C.; Muscoli, S.; Romeo, F.; et al. Treatment of HFpEF beyond the SGLT2-Is: Does the Addition of GLP-1 RA Improve Cardiometabolic Risk and Outcomes in Diabetic Patients? Int. J. Mol. Sci. 2022, 23, 14598. [Google Scholar] [CrossRef]
- Echouffo-Tcheugui, J.B.; Lewsey, S.C.; Weiss, R.G. SGLT2 inhibitors: Further evidence for heart failure with preserved ejection fraction as a metabolic disease? J. Clin. Investig. 2021, 131, e156309. [Google Scholar] [CrossRef]
- Lei, Y.; Gan, M.; Qiu, Y.; Chen, Q.; Wang, X.; Liao, T.; Zhao, M.; Chen, L.; Zhang, S.; Zhao, Y.; et al. The role of mitochondrial dynamics and mitophagy in skeletal muscle atrophy: From molecular mechanisms to therapeutic insights. Cell Mol. Biol. Lett. 2024, 29, 59. [Google Scholar] [CrossRef]
- Ren, J.; Bi, Y.; Sowers, J.; Hetz, C.; Zhang, Y. Endoplasmic reticulum stress and unfolded protein response in cardiovascular diseases. Nat. Rev. Cardiol. 2021, 18, 499–521. [Google Scholar] [CrossRef]
- Zhou, H.; Wang, S.; Hu, S.; Chen, Y.; Ren, J. ER mitochondria microdomains in cardiac ischemia-reperfusion injury: A fresh perspective. Front. Physiol. 2018, 9, 755. [Google Scholar] [CrossRef]
- Gao, P.; Yan, Z.; Zhu, Z. Mitochondria-associated endoplasmic reticulum membranes in cardiovascular diseases. Front. Cell Dev. Biol. 2020, 8, 604240. [Google Scholar] [CrossRef] [PubMed]
- Bou-Teen, D.; Kaludercic, N.; Weissman, D.; Turan, B.; Maack, C.; Di Lisa, F.; Ruiz-Meana, M. Mitochondrial ROS and mitochondria-targeted antioxidants in the aged heart. Free Radic. Biol. Med. 2021, 167, 109–124. [Google Scholar] [CrossRef] [PubMed]
- Paulus, W.J.; Tschope, C. A novel paradigm for heart failure with preserved ejection fraction: Comorbidities drive myocardial dysfunction and remodeling through coronary microvascular endothelial inflammation. J. Am. Coll. Cardiol. 2013, 62, 263–271. [Google Scholar] [CrossRef]
- Liu, S.; Liu, J.; Wang, Y.; Deng, F.; Deng, Z. Oxidative Stress: Signaling Pathways, Biological Functions, and Disease. MedComm (2020) 2025, 6, e70268. [Google Scholar] [CrossRef]
- Mongirdienė, A.; Skrodenis, L.; Varoneckaitė, L.; Mierkytė, G.; Gerulis, J. Reactive oxygen species induced pathways in heart failure pathogenesis and potential therapeutic strategies. Biomedicines 2022, 10, 602. [Google Scholar] [CrossRef]
- Marino, A.; Hausenloy, D.J.; Andreadou, I.; Horman, S.; Bertrand, L.; Beauloye, C. AMP-activated protein kinase: A remarkable contributor to preserve a healthy heart against ROS injury. Free Radic. Biol. Med. 2021, 166, 238–254. [Google Scholar] [CrossRef]
- Ribeiro Junior, R.F.; Dabkowski, E.R.; Shekar, K.C.; O’Connell, K.A.; Hecker, P.A.; Murphy, M.P. MitoQ improves mitochondrial dysfunction in heart failure induced by pressure overload. Free Radic. Biol. Med. 2018, 117, 18–29. [Google Scholar] [CrossRef]
- Pieske, B.; Butler, J.; Filippatos, G.; Lam, C.; Maggioni, A.P.; Ponikowski, P.; Shah, S.; Solomon, S.; Kraigher-Krainer, E.; Samano, E.T.; et al. Rationale and design of the SOluble guanylate Cyclase stimulatoR in heArT failurE Studies (SOCRATES). Eur. J. Heart Fail. 2014, 16, 1026–1038. [Google Scholar] [CrossRef] [PubMed]
- Schiattarella, G.G.; Rodolico, D.; Hill, J.A. Metabolic inflammation in heart failure with preserved ejection fraction. Cardiovasc. Res. 2021, 117, 423–434. [Google Scholar] [CrossRef]
- Li, X.; Liu, J.; Lu, Q.; Ren, D.; Sun, X.; Rousselle, T.; Tan, Y.; Li, J. AMPK: A therapeutic target of heart fail-ure-not only metabolism regulation. Biosci. Rep. 2019, 39, BSR20181767. [Google Scholar] [CrossRef] [PubMed]
- Lopaschuk, G.D.; Karwi, Q.G.; Tian, R.; Wende, A.R.; Abel, E.D. Cardiac Energy Metabolism in Heart Failure. Circ. Res. 2021, 128, 1487–1513. [Google Scholar] [CrossRef] [PubMed]
- Spinale, F.G.; Janicki, J.S.; Zile, M.R. Membrane-associated matrix proteolysis and heart failure. Circ. Res. 2013, 112, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, K.E.; Pontes, M.H.B.; Cantão, M.B.S.; Prado, A.F. The role of matrix metalloproteinase-9 in cardiac remodeling and dysfunction and as a possible blood biomarker in heart failure. Pharmacol. Res. 2024, 206, 107285. [Google Scholar] [CrossRef]
- Krebber, M.M.; van Dijk, C.G.M.; Vernooij, R.W.M.; Brandt, M.M.; Emter, C.A.; Rau, C.D.; Fledderus, J.O.; Duncker, D.J.; Verhaar, M.C.; Cheng, C.; et al. Matrix Metalloproteinases and Tissue Inhibitors of Metalloproteinases in Extracellular Matrix Remodeling during Left Ventricular Diastolic Dysfunction and Heart Failure with Preserved Ejection Fraction: A Systematic Review and Meta-Analysis. Int. J. Mol. Sci. 2020, 21, 6742. [Google Scholar] [CrossRef]
- Greenberg, B. Angiotensin Receptor-Neprilysin Inhibition (ARNI) in Heart Failure. Int. J. Heart Fail. 2020, 2, 73–90. [Google Scholar] [CrossRef]
- Bann, G.G. Antagonizing HFpEF by Targeting Fibrosis. Circulation 2025, 151, 396–399. [Google Scholar] [CrossRef]
- Taylor, E.B.; Hall, J.E.; Mouton, A.J. Current anti-inflammatory strategies for treatment of heart failure: From innate to adaptive immunity. Pharmacol. Res. 2025, 216, 107761. [Google Scholar] [CrossRef]
- Cai, Z.; Wu, C.; Xu, Y.; Cai, J.; Zhao, M.; Zu, L. The NO-cGMP-PKG Axis in HFpEF: From Pathological Mechanisms to Potential Therapies. Aging Dis. 2023, 14, 46–62. [Google Scholar] [CrossRef]
- Allbritton-King, J.D.; García-Cardeña, G. Endothelial cell dysfunction in cardiac disease: Driver or consequence? Front. Cell Dev. Biol. 2023, 11, 1278166. [Google Scholar] [CrossRef] [PubMed]
- Greene, S.J.; Gheorghiade, M.; Borlaug, B.A.; Pieske, B.; Vaduganathan, M.; Burnett, J.C., Jr.; Roessig, L.; Stasch, J.P.; Solomon, S.D.; Paulus, W.J.; et al. The cGMP signaling pathway as a therapeutic target in heart failure with preserved ejection fraction. J. Am. Heart Assoc. 2013, 2, e000536. [Google Scholar] [CrossRef]
- Volpe, M.; Carnovali, M.; Mastromarino, V. The natriuretic peptides system in the pathophysiology of heart failure: From molecular basis to treatment. Clin. Sci. 2016, 130, 57–77. [Google Scholar] [CrossRef]
- Kansakar, S.; Guragain, A.; Verma, D.; Sharma, P.; Dhungana, B.; Bhattarai, B.; Yadav, S.; Gautam, N. Soluble Guanylate Cyclase Stimulators in Heart Failure. Cureus 2021, 13, e17781. [Google Scholar] [CrossRef]
- Kourek, C.; Briasoulis, A.; Papamichail, A.; Xanthopoulos, A.; Tsougos, E.; Farmakis, D.; Paraskevaidis, I. Beyond quadruple therapy and current therapeutic strategies in heart failure with reduced ejection fraction: Medical therapies with potential to become part of the therapeutic armamentarium. Int. J. Mol. Sci. 2024, 25, 3113. [Google Scholar] [CrossRef]
- Ipek, R.; Holland, J.; Cramer, M.; Rider, O. CMR to characterize myocardial structure and function in heart failure with preserved left ventricular ejection fraction. Eur. Heart J. Cardiovasc. Imaging 2024, 25, 1491–1504. [Google Scholar] [CrossRef] [PubMed]
- Tao, M.; Dhaliwal, S.; Ghosalkar, D.; Sheng, S.; Dianati-Maleki, N.; Tam, E.; Rahman, T.; Mann, N.; Kort, S. Utility of native T1 mapping and myocardial extracellular volume fraction in patients with nonischemic dilated cardiomyopathy: A systematic review and meta-analysis. Int. J. Cardiol. Heart Vasc. 2024, 51, 101339. [Google Scholar] [CrossRef] [PubMed]
- Moustafa, A.; Khan, M.S.; Alsamman, M.A.; Jamal, F.; Atalay, M.K. Prognostic significance of T1 mapping parameters in heart failure with preserved ejection fraction: A systematic review. Heart Fail. Rev. 2021, 26, 1325–1331. [Google Scholar] [CrossRef]
- Park, J.J.; Park, J.B.; Park, J.H.; Cho, G.Y. Global longitudinal strain to predict mortality in patients with acute heart failure. J. Am. Coll. Cardiol. 2018, 71, 1947–1957. [Google Scholar] [CrossRef]
- Maia, R.J.C.; Brandão, S.C.S.; Leite, J.; Parente, G.B.; Pinheiro, F.; Araújo, B.T.S.; Aguiar, M.I.R.; Martins, S.M.; Brandão, D.C.; Andrade, A.D. Global longitudinal strain predicts poor functional capacity in patients with systolic heart failure. Arq. Bras. Cardiol. 2019, 113, 188–194. [Google Scholar] [CrossRef]
- Kuster, N.; Huet, F.; Dupuy, A.M.; Akodad, M.; Battistella, P.; Agullo, A.; Leclercq, F.; Kalmanovich, E.; Meilhac, A.; Aguilhon, S.; et al. Multimarker approach including CRP, sST2 and GDF-15 for prognostic stratification in stable heart failure. ESC Heart Fail. 2020, 7, 2230–2239. [Google Scholar] [CrossRef] [PubMed]
- Clemente, G.; Soldano, J.S.; Tuttolomondo, A. Heart failure: Is there an ideal biomarker? Rev. Cardiovasc. Med. 2023, 24, 310. [Google Scholar] [CrossRef]
- Mouton, A.J.; Li, X.; Hall, M.E.; Hall, J.E. Obesity, hypertension, and cardiac dysfunction: Novel roles of immunometabolism in macrophage activation and inflammation. Circ. Res. 2020, 126, 789–806. [Google Scholar] [CrossRef]
- Ndumele, C.E.; Rangaswami, J.; Chow, S.L.; Neeland, I.J.; Tuttle, K.R.; Khan, S.S.; Coresh, J.; Mathew, R.O.; Baker-Smith, C.M.; Carnethon, M.R.; et al. Cardiovascular-kidney-metabolic health: A presidential advisory from the American Heart Association. Circulation 2023, 148, 1606–1635, Erratum in: Circulation 2024, 149, e1023. [Google Scholar] [CrossRef] [PubMed]
- D’Amario, D.; Migliaro, S.; Borovac, J.A.; Restivo, A.; Vergallo, R.; Galli, M.; Leone, A.M.; Montone, R.A.; Niccoli, G.; Aspromonte, N.; et al. Microvascular dysfunction in heart failure with preserved ejection fraction. Front. Physiol. 2019, 10, 1347. [Google Scholar] [CrossRef] [PubMed]
- Boutouyrie, P.; Chowienczyk, P.; Humphrey, J.D.; Mitchell, G.F. Arterial stiffness and cardiovascular risk in hypertension. Circ. Res. 2021, 128, 864–886. [Google Scholar] [CrossRef] [PubMed]
- Røe, Å.T.; Aronsen, J.M.; Skårdal, K.; Hamdani, N.; Linke, W.A.; Danielsen, H.E.; Sejersted, O.M.; Sjaastad, I.; Louch, W.E. Increased passive stiffness promotes diastolic dysfunction despite improved Ca2+ handling during left ventricular concentric hypertrophy. Cardiovasc. Res. 2017, 113, 1161–1172. [Google Scholar] [CrossRef]
- Ketema, E.B.; Lopaschuk, G.D. The impact of obesity on cardiac energy metabolism and efficiency in heart failure with preserved ejection fraction. Can. J. Cardiol. 2025, in press. [Google Scholar] [CrossRef]
- Borlaug, B.A.; Jensen, M.D.; Kitzman, D.W.; Lam, C.S.P.; Obokata, M.; Rider, O.J. Obesity and heart failure with preserved ejection fraction: New insights and pathophysiological targets. Cardiovasc. Res. 2023, 118, 3434–3450. [Google Scholar] [CrossRef]
- Li, C.; Qin, D.; Hu, J.; Yang, Y.; Hu, D.; Yu, B. Inflamed adipose tissue: A culprit underlying obesity and heart failure with preserved ejection fraction. Front. Immunol. 2022, 13, 947147. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Clinical update: Cardiovascular disease in diabetes mellitus—Atherosclerotic cardiovascular disease and heart failure in type 2 diabetes mellitus—Mechanisms, management, and clinical considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Junho, C.V.C.; Frisch, J.; Soppert, J.; Wollenhaupt, J.; Noels, H. Cardiomyopathy in chronic kidney disease: Clinical features, biomarkers and the contribution of murine models in understanding pathophysiology. Clin. Kidney J. 2023, 16, 1786–1803. [Google Scholar] [CrossRef]
- Wojtaszek, E.; Oldakowska-Jedynak, U.; Kwiatkowska, M.; Glogowski, T.; Malyszko, J. Uremic toxins, oxidative stress, atherosclerosis in chronic kidney disease, and kidney transplantation. Oxid. Med. Cell. Longev. 2021, 2021, 6651367. [Google Scholar] [CrossRef] [PubMed]
- Carlisle, M.A.; Fudim, M.; DeVore, A.D.; Piccini, J.P. Heart failure and atrial fibrillation, like fire and fury. JACC Heart Fail. 2019, 7, 447–456. [Google Scholar] [CrossRef]
- Scholten, M.; Davidge, J.; Agvall, B.; Halling, A. Comorbidities in heart failure patients that predict cardiovascular readmissions within 100 days—An observational study. PLoS ONE 2024, 19, e0296527. [Google Scholar] [CrossRef]
- Screever, E.M.; van der Wal, M.H.L.; van Veldhuisen, D.J.; Jaarsma, T.; Koops, A.; van Dijk, K.S.; Warink-Riemersma, J.; Coster, J.E.; Westenbrink, B.D.; van der Meer, P.; et al. Comorbidities complicating heart failure: Changes over the last 15 years. Clin. Res. Cardiol. 2023, 112, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Crispino, S.P.; Segreti, A.; Nafisio, V.; Valente, D.; Crisci, F.; Ferro, A.; Cavallari, I.; Nusca, A.; Ussia, G.P.; Grigioni, F. The role of SGLT2-inhibitors across all stages of heart failure and mechanisms of early clinical benefit: From prevention to advanced heart failure. Biomedicines 2025, 13, 608. [Google Scholar] [CrossRef] [PubMed]
- Talha, K.M.; Anker, S.D.; Butler, J. SGLT-2 inhibitors in heart failure: A review of current evidence. Int. J. Heart Fail. 2023, 5, 82–90. [Google Scholar] [CrossRef]
- Xiang, B.; Zhang, R.; Wu, X.; Zhou, X. Optimal pharmacologic treatment of heart failure with preserved and mildly reduced ejection fraction: A meta-analysis. JAMA Netw. Open 2022, 5, e2231963. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, L.; Sun, W.; Zhu, Y.; Zhang, Z.; Chen, L.; Xie, M.; Zhang, L. Artificial intelligence in diagnosis of heart failure. J. Am. Heart Assoc. 2025, 14, e039511. [Google Scholar] [CrossRef]
- Salih, A.; Boscolo Galazzo, I.; Gkontra, P.; Lee, A.M.; Lekadir, K.; Raisi-Estabragh, Z.; Petersen, S.E. Explainable artificial intelligence and cardiac imaging: Toward more interpretable models. Circ. Cardiovasc. Imaging 2023, 16, e014519. [Google Scholar] [CrossRef]
- Lteif, C.; Huang, Y.; Guerra, L.A.; Gawronski, B.E.; Duarte, J.D. Using omics to identify novel therapeutic targets in heart failure. Circ. Genom. Precis. Med. 2024, 17, e004398. [Google Scholar] [CrossRef] [PubMed]
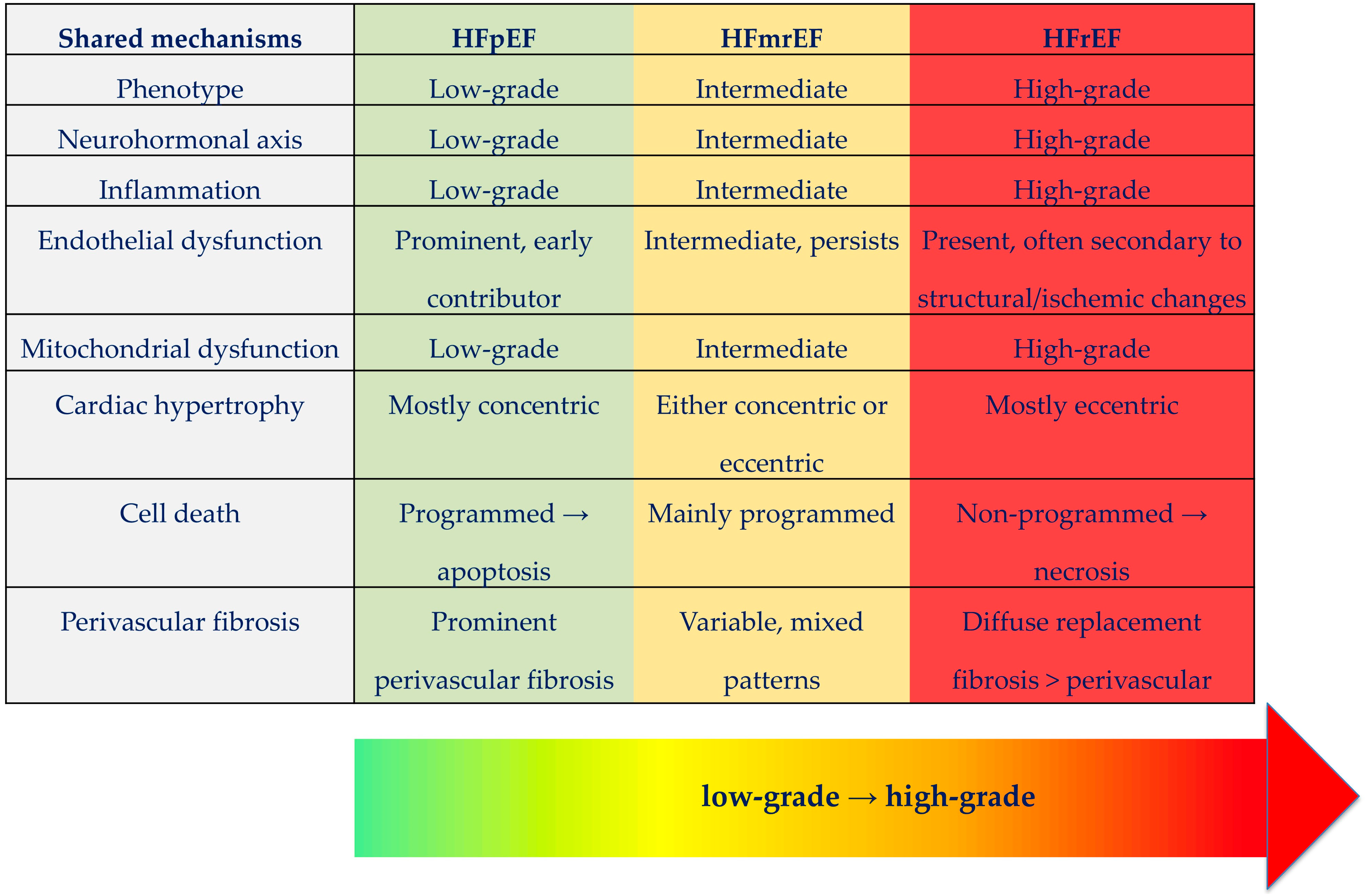


| Pathway/ Mechanism | HFpEF | HFrEF | Therapeutic Implications |
|---|---|---|---|
| AMPK signaling | Suppressed due to metabolic comorbidities (obesity, diabetes, hypertension) → impaired substrate flexibility | Partially activated as compensatory response to energy depletion | AMPK activators may restore metabolic efficiency in HFpEF; supportive role in HFrEF |
| MMPs activity | Subtle upregulation; altered MMP/TIMP balance; promotes fibrosis | Strongly upregulated; drives ECM degradation, adverse remodeling, dilation | MMPs inhibitors/ARNIs more effective in HFrEF; antifibrotic strategies needed in HFpEF |
| cGMP–PKG signaling | Impaired due to endothelial dysfunction, reduced NO bioavailability, microvascular rarefaction | Relatively preserved via natriuretic peptide–driven cGMP, but insufficient to fully counter remodeling | sGC stimulators or PDE5 inhibitors promising in HFpEF; ARNIs beneficial in HFrEF |
| Biomarker | Pathophysiological Role in HF | Expression Across Phenotypes | Clinical Relevance |
|---|---|---|---|
| C-reactive protein (CRP) | Marker of systemic inflammation | Elevated in HFpEF, HFmrEF, HFrEF | Prognostic of adverse outcomes, reflects low-grade systemic inflammation |
| Interleukin-6 (IL-6) | Pro-inflammatory cytokine | Increased in all phenotypes, higher in advanced HFrEF | Associated with remodeling, progression, and mortality |
| Tumor necrosis factor-α (TNF-α) | Cytokine driving apoptosis, cachexia, remodeling | Prominent in HFrEF; also elevated in HFpEF | Linked to cachexia, systolic dysfunction, poor prognosis |
| Soluble ST2 (sST2) | Biomarker of myocardial stress and fibrosis | Elevated in all phenotypes | Strong prognostic marker, guides risk stratification |
| NT-proBNP/BNP | Reflects wall stress | Elevated in all phenotypes, higher in HFrEF | Widely used diagnostic/prognostic tool |
| Troponins (hs-cTnI/T) | Indicator of ongoing myocardial injury | Elevated in HFrEF and myocardial injury; lower but detectable in HFpEF | Predicts adverse events, reflects ongoing cell damage |
| Galectin-3 | Marker of fibrosis and inflammation | Higher in HFpEF | Predicts adverse remodeling and outcomes |
| Therapy | Primary Target/ Mechanism | HFpEF | HFrEF | Convergence/ Divergence |
|---|---|---|---|---|
| ARNI (sacubitril/valsartan) | Neurohormonal modulation, natriuretic peptides | Limited benefit (selected patients) | Strong evidence, improves survival | Unified neurohormonal pathway, stronger in HFrEF |
| SGLT2 inhibitors | Metabolic modulation, improved energetics, anti-inflammatory | Strong evidence for reduced hospitalization | Strong evidence for mortality and hospitalization reduction | Convergent benefit across phenotypes |
| Beta-blockers | Sympathetic inhibition | Symptom control, limited outcome benefit | Clear mortality benefit | More effective in HFrEF |
| Mineralocorticoid receptor antagonists (MRAs) | Aldosterone inhibition, antifibrotic | Mixed results | Strong survival benefit | Divergent effect strength |
| sGC stimulators (vericiguat, riociguat) | Enhance cGMP–PKG signaling | Promising in HFpEF with endothelial dysfunction | Moderate evidence in HFrEF | Phenotype-guided targeting |
| AMPK activators/metabolic modulators | Restore energy homeostasis, improve substrate use | Strong rationale, ongoing trials | Supportive role | More phenotype-specific for HFpEF |
| MMP inhibitors/antifibrotics | Extracellular matrix regulation | Potential to limit fibrosis | Potential to limit remodeling | Pathway present in both, but dominant in HFrEF |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paraskevaidis, I.; Tsougos, E.; Kourek, C. One Syndrome, Many Faces: A Unified Perspective on Heart Failure Phenotypes. Int. J. Mol. Sci. 2025, 26, 8960. https://doi.org/10.3390/ijms26188960
Paraskevaidis I, Tsougos E, Kourek C. One Syndrome, Many Faces: A Unified Perspective on Heart Failure Phenotypes. International Journal of Molecular Sciences. 2025; 26(18):8960. https://doi.org/10.3390/ijms26188960
Chicago/Turabian StyleParaskevaidis, Ioannis, Elias Tsougos, and Christos Kourek. 2025. "One Syndrome, Many Faces: A Unified Perspective on Heart Failure Phenotypes" International Journal of Molecular Sciences 26, no. 18: 8960. https://doi.org/10.3390/ijms26188960
APA StyleParaskevaidis, I., Tsougos, E., & Kourek, C. (2025). One Syndrome, Many Faces: A Unified Perspective on Heart Failure Phenotypes. International Journal of Molecular Sciences, 26(18), 8960. https://doi.org/10.3390/ijms26188960







