Actinidia eriantha Benth. Root as a New Phytomedicine Inhibits Non-Small Cell Lung Cancer by Regulating·TGF-β/FOXO/mTOR
Abstract
1. Introduction
2. Results
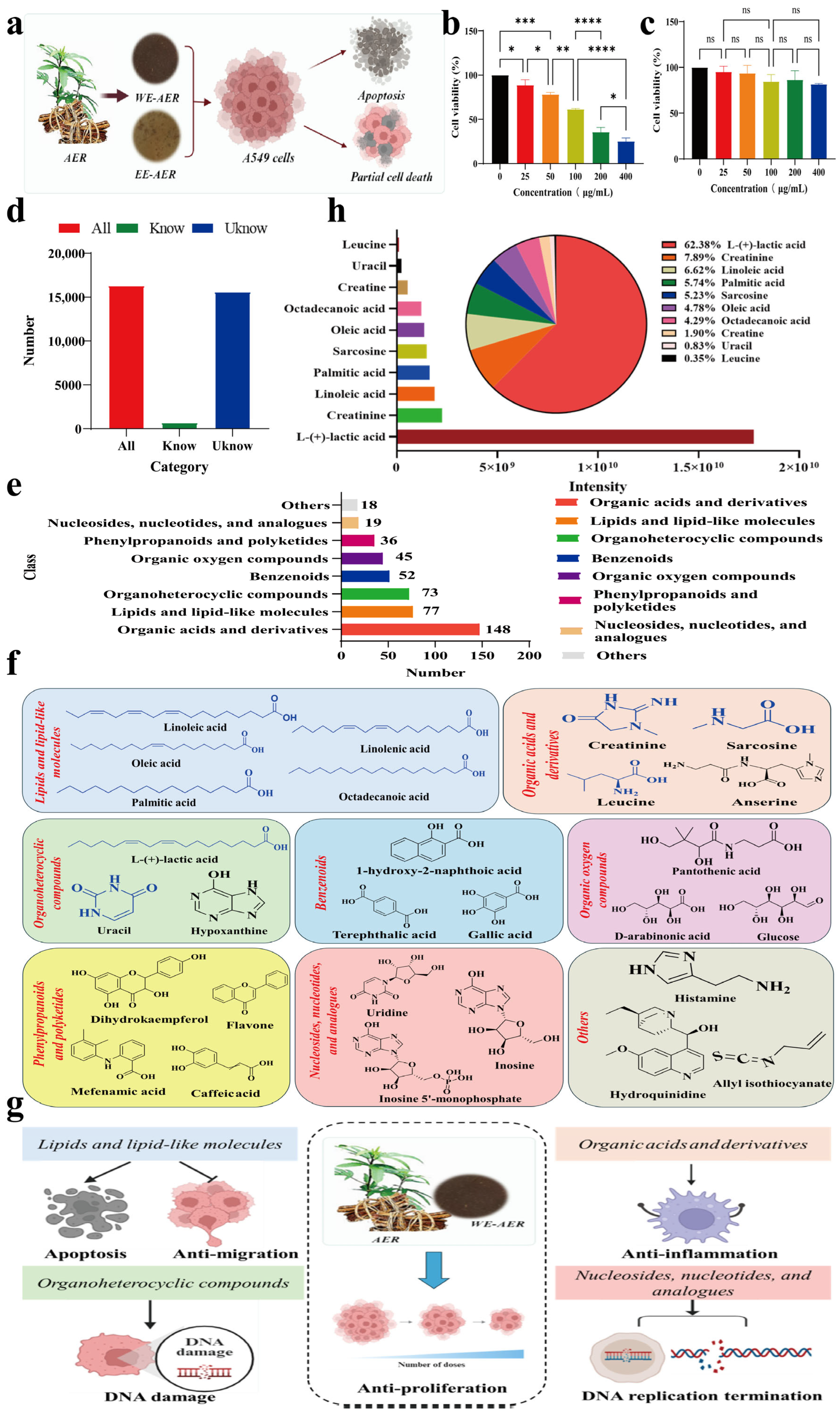
2.1. Characterization and Bioactivities Screening of the ARE Extracts
2.2. Effects of WE-AER on the Migration, Invasion Inhibition, and Pro-Apoptosis of A549 Cells
2.3. Anticancer Mechanism of WE-AER
2.4. Anticancer Effects of WE-AER in Nude Mice with Xenograft Tumors
2.5. The WE-AER with High Biosafety In Vivo
3. Discussion
4. Materials and Methods
4.1. Chemical Reagents and Materials
4.2. Extraction and Purification of Extracts from AER
4.3. Cell Viability Assay
4.4. Cell Migration and Invasion Assays
4.5. Cell Apoptosis Assay
4.6. Molecular Mechanism Analysis
4.7. Western Blot Analysis
4.8. Establishment of Xenograft Tumor Model in BALB/c Nude Mice
4.9. Immunofluorescence Tissue Staining
4.10. H&E Staining
4.11. Cytokine Level Analysis
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AE | Actinidia eriantha Benth |
| AER | Actinidia eriantha Benth. root |
| CD31 | platelet endothelial cell adhesion molecule-1 |
| CDDP | cisplatincisplatin |
| EE-AER | ethanol extract of Actinidia eriantha Benth. root |
| H&E | hematoxylin and eosin |
| LUAD | histologic subtypes of lung adenocarcinoma |
| LUSC | lung squamous cell carcinoma |
| NSCLC | non-small cell lung cancer |
| PTX | paclitaxel |
| TCM | traditional Chinese medicine |
| VEGF | vascular endothelial growth factor |
| WE-AER | aqueous extract of Actinidia eriantha Benth. root |
References
- Ferlay, J.; Soerjomataram, I.; Dikshit, R.; Eser, S.; Mathers, C.; Rebelo, M.; Parkin, D.M.; Forman, D.; Bray, F. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 2015, 136, E359–E386. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, M.; Yang, K.; Tian, J. 6,6′-Bieckol induces apoptosis and suppresses TGF-β-induced epithelial-mesenchymal transition in non-small lung cancer cells. Chin. Herb. Med. 2022, 14, 254–262. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, B.; Xu, H.; Gong, Y.; Hu, W.; Jin, Z.; Wu, X.; Chen, X.; Li, M.; Shi, L.; et al. Cinobufagin induces FOXO1-regulated apoptosis, proliferation, migration, and invasion by inhibiting G9a in non-small-cell lung cancer A549 cells. J. Ethnopharmacol. 2022, 291, 115095. [Google Scholar] [CrossRef] [PubMed]
- Li, J.W.; Zheng, G.; Kaye, F.J.; Wu, L. PROTAC therapy as a new targeted therapy for lung cancer. Mol. Ther. J. Am. Soc. Gene Ther. 2023, 31, 647–656. [Google Scholar] [CrossRef]
- Yang, G.; Li, X.; Li, X.; Wang, L.; Li, J.; Song, X.; Chen, J.; Guo, Y.; Sun, X.; Wang, S.; et al. Traditional Chinese Medicine in Cancer Care: A Review of Case Series Published in the Chinese Literature. Evid.-Based Complement. Altern. Med. 2012, 2012, 751046. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, S.; Du, K.; Liang, C.; Wang, S.; Boadi, E.O.; Li, J.; Pang, X.; He, J.; Chang, Y.-X. Traditional herbal medicine: Therapeutic potential in rheumatoid arthritis. J. Ethnopharmacol. 2021, 279, 114368. [Google Scholar] [CrossRef]
- Xie, Y.; Gan, C.; Liu, H.; Hou, Y.; Su, X.; Xue, T.; Wang, D.; Li, P.; Yue, L.; Qiu, Q.; et al. Polyphyllin VI Ameliorates Pulmonary Fibrosis by Suppressing the MAPK/ERK and PI3K/AKT Signaling Pathways via Upregulating DUSP6. Phytother. Res. 2024, 38, 5930–5948. [Google Scholar] [CrossRef]
- Huang, P.H.; Duan, X.B.; Tang, Z.Z.; Zou, Z.X.; Song, W.M.; Gao, G.; Li, D.; Nie, F.Q.; Yan, X.; Fu, Y.X.; et al. Betulinaldehyde exhibits effective anti-tumor effects in A549 cells by regulating intracellular autophagy. Sci. Rep. 2023, 13, 743. [Google Scholar] [CrossRef]
- Peng, L.H.; Zhang, Y.H.; Han, L.J.; Zhang, C.Z.; Wu, J.H.; Wang, X.R.; Gao, J.Q.; Mao, Z.W. Cell Membrane Capsules for Encapsulation of Chemotherapeutic and Cancer Cell Targeting in Vivo. ACS Appl. Mater. Interfaces 2015, 7, 18628–18637. [Google Scholar] [CrossRef]
- Campbell, J.D.; Alexandrov, A.; Kim, J.; Wala, J.; Berger, A.H.; Pedamallu, C.S.; Shukla, S.A.; Guo, G.; Brooks, A.N.; Murray, B.A.; et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat. Genet. 2016, 48, 607. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Feng, J.; Zhu, X.; Feng, D.; Zheng, L. Plant-derived vesicle-like nanoparticles: A new tool for inflammatory bowel disease and colitis-associated cancer treatment. Mol. Ther. J. Am. Soc. Gene Ther. 2024, 32, 890–909. [Google Scholar] [CrossRef]
- He, Y. Progress in using combination of Chinese drug with chemotherapy to treat cancer. J. Tradit. Chin. Med. 2004, 24, 153–157. [Google Scholar]
- Fu, W.; Shentu, C.; Chen, D.; Qiu, J.; Zong, C.; Yu, H.; Zhang, Y.; Chen, Y.; Liu, X.; Xu, T. Network pharmacology combined with affinity ultrafiltration to elucidate the potential compounds of Shaoyao Gancao Fuzi Decoction for the treatment of rheumatoid arthritis. J. Ethnopharmacol. 2024, 330, 118268. [Google Scholar] [CrossRef]
- Kong, F.; Wang, C.; Zhao, L.; Liao, D.; Wang, X.; Sun, B.; Yang, P.; Jia, Y. Traditional Chinese medicines for non-small cell lung cancer: Therapies and mechanisms. Chin. Herb. Med. 2023, 15, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.H.; Wang, M.Z.; Chu, Y.; Zhang, L.; Niu, J.; Shao, H.T.; Yuan, T.J.; Jiang, Z.H.; Gao, J.Q.; Ning, X.H. Engineering bacterial outer membrane vesicles as transdermal nanoplatforms for photo-TRAIL-programmed therapy against melanoma. Sci. Adv. 2020, 6, eaba2735. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yang, K.; Cui, S.; Liu, X.; Chen, J.; Li, J.; Yu, H.; Fu, X. Exploration on Medication Rules of Chinese Marine Materia Medica Prescriptions based on Literature Recordings. Mod. Tradit. Chin. Med. Mater. Medica—World Sci. Technol. 2017, 19, 414–418. [Google Scholar]
- Xu, H.; Yao, L.; Sun, H.; Wu, Y. Chemical composition and antitumor activity of different polysaccharides from the roots of Actinidia eriantha. Carbohydr. Polym. 2009, 78, 316–322. [Google Scholar] [CrossRef]
- Du, J.; Chen, X.; Wang, C.; Sun, H. Pathway analysis of global gene expression change in dendritic cells induced by the polysaccharide from the roots of Actinidia eriantha. J. Ethnopharmacol. 2018, 214, 141–152. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Chen, F.; Chen, X.; Zhou, Z.; Wang, H. Activation of RAW264.7 macrophages by the polysaccharide from the roots of Actinidia eriantha and its molecular mechanisms. Carbohydr. Polym. 2015, 121, 388–402. [Google Scholar] [CrossRef]
- Xu, H.-S.; Wu, Y.-W.; Xu, S.-F.; Sun, H.-X.; Chen, F.-Y.; Yao, L. Antitumor and immunomodulatory activity of polysaccharides from the roots of Actinidia eriantha. J. Ethnopharmacol. 2009, 125, 310–317. [Google Scholar] [CrossRef]
- Nava Lauson, C.B.; Tiberti, S.; Corsetto, P.A.; Conte, F.; Tyagi, P.; Machwirth, M.; Ebert, S.; Loffreda, A.; Scheller, L.; Sheta, D.; et al. Linoleic acid potentiates CD8+ T cell metabolic fitness and antitumor immunity. Cell Metab. 2023, 35, 633–650. [Google Scholar] [CrossRef]
- Kim, D.; Koo, S. Concise and Practical Total Synthesis of (+)-Abscisic Acid. ACS Omega 2020, 5, 13296–13302. [Google Scholar] [CrossRef]
- Wang, M.Z.; Gao, J.; Chu, Y.; Niu, J.; Chen, M.; Shang, Q.; Peng, L.H.; Jiang, Z.H. Synthesis of crocetin derivatives and their potent inhibition in multiple tumor cells proliferation and inflammatory property of macrophage. BMC Complement. Med. Ther. 2020, 20, 29. [Google Scholar] [CrossRef]
- Ji, Y.; Dou, Y.-N.; Zhao, Q.-W.; Zhang, J.-Z.; Yang, Y.; Wang, T.; Xia, Y.-F.; Dai, Y.; Wei, Z.-F. Paeoniflorin suppresses TGF-β mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol. Sin. 2016, 37, 794–804. [Google Scholar] [CrossRef] [PubMed]
- Manola, M.S.; Gumeni, S.; Trougakos, I.P. Differential Dose- and Tissue-Dependent Effects of foxo on Aging, Metabolic and Proteostatic Pathways. Cells 2021, 10, 3577. [Google Scholar] [CrossRef]
- Yan, G.; Xiao, Q.; Zhao, J.; Chen, H.; Xu, Y.; Tan, M.; Peng, L. Brucea javanica derived exosome-like nanovesicles deliver miRNAs for cancer therapy. J. Control. Release 2024, 367, 425–440. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wei, Y.; Yin, S.; Li, W.; Wang, Y.; Pi, C.; Zeng, M.; Wang, X.; Chen, L.; Liu, F.; et al. Construction and Evaluation of BAL-PTX Co-Loaded Lipid Nanosystem for Promoting the Anti-Lung Cancer Efficacy of Paclitaxel and Reducing the Toxicity of Chemotherapeutic Drugs. Int. J. Nanomed. 2024, 19, 7775–7797. [Google Scholar] [CrossRef]
- Jia, X.-B.; Zhang, Q.; Xu, L.; Yao, W.-J.; Wei, L. Lotus leaf flavonoids induce apoptosis of human lung cancer A549 cells through the ROS/p38 MAPK pathway. Biol. Res. 2021, 54, 7. [Google Scholar] [CrossRef]
- Wu, C.-F.; Wu, C.-Y.; Chiou, R.Y.Y.; Yang, W.-C.; Lin, C.-F.; Wang, C.-M.; Hou, P.-H.; Lin, T.-C.; Kuo, C.-Y.; Chang, G.-R. The Anti-Cancer Effects of a Zotarolimus and 5-Fluorouracil Combination Treatment on A549 Cell-Derived Tumors in BALB/c Nude Mice. Int. J. Mol. Sci. 2021, 22, 4562. [Google Scholar] [CrossRef]
- Shashni, B.; Nishikawa, Y.; Nagasaki, Y. Management of tumor growth and angiogenesis in triple-negative breast cancer by using redox nanoparticles. Biomaterials 2021, 269, 120645. [Google Scholar] [CrossRef] [PubMed]
- Linderholm, B.K.; Hellborg, H.; Johansson, U.; Elmberger, G.; Skoog, L.; Lehtio, J.; Lewensohn, R. Significantly higher levels of vascular endothelial growth factor (VEGF) and shorter survival times for patients with primary operable triple-negative breast cancer. Ann. Oncol. 2009, 20, 1639–1646. [Google Scholar] [CrossRef]
- Propper, D.J.; Balkwill, F.R. Harnessing cytokines and chemokines for cancer therapy. Nat. Rev. Clin. Oncol. 2022, 19, 237–253. [Google Scholar] [CrossRef]
- Lan, T.; Chen, L.; Wei, X. Inflammatory Cytokines in Cancer: Comprehensive Understanding and Clinical Progress in Gene Therapy. Cells 2021, 10, 100. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, F.; Hughes, T.; Yu, J. FOXOs in cancer immunity: Knowns and unknowns. Semin. Cancer Biol. 2018, 50, 53–64. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, M.; Peng, J.; Wang, X.; Huang, T.; Li, S.; Lin, M.; Un, H.; Xu, Y.; Li, J.; et al. Baicalein inhibits migration and invasion of gastric cancer cells through suppression of the TGF-β signaling pathway. Mol. Med. Rep. 2014, 10, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Farkhondeh, T.; Pourbagher-Shahri, A.M.; Samarghandian, S. The role of curcumin in aging and senescence: Molecular mechanisms. Biomed. Pharmacother. 2021, 134, 111119. [Google Scholar] [CrossRef] [PubMed]
- Yip, P.Y. Phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin (PI3K-Akt-mTOR) signaling pathway in non-small cell lung cancer. Transl. Lung Cancer Res. 2015, 4, 165–176. [Google Scholar] [PubMed]
- Zhao, S.; Tang, Y.; Wang, R.; Najafi, M. Mechanisms of cancer cell death induction by paclitaxel: An updated review. Apoptosis 2022, 27, 647–667. [Google Scholar] [CrossRef]
- Rugo, H.S.; Barry, W.T.; Moreno-Aspitia, A.; Lyss, A.P.; Cirrincione, C.; Leung, E.; Mayer, E.L.; Naughton, M.; Toppmeyer, D.; Carey, L.A.; et al. Randomized Phase III Trial of Paclitaxel Once Per Week Compared With Nanoparticle Albumin-Bound Nab-Paclitaxel Once Per Week or Ixabepilone with Bevacizumab as First-Line Chemotherapy for Locally Recurrent or Metastatic Breast Cancer: CALGB 40502/NCCTG N063H (Alliance). J. Clin. Oncol. 2015, 33, 2361–2369. [Google Scholar]
- Zhang, L.; Zhu, L.; Yao, X.; Lou, X.; Wan, J.; Duan, X.; Pan, L.; Li, A.; Gu, Z.; Wang, M.; et al. Paclitaxel treatment enhances lymphatic metastasis of B16F10 melanoma cells via CCL21/CCR7 axis. Int. J. Biol. Sci. 2022, 18, 1476–1490. [Google Scholar] [CrossRef]
- Kim, D.H.; Lee, S.M.; Lee, Y.J.; Yoon, J.J.; Tan, R.; Yu, Y.C.; Kang, D.G.; Lee, H.S. Effect of Paeotang on tumor necrosis factor alpha-induced vascular inflammation in human umbilical vein endothelial cells. Chin. J. Integr. Med. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, T.; Ito, H.; Mukainaka, T.; Tokuda, H.; Nishino, H.; Yoshida, T. Anti-tumor promoting effect of glycosides from Prunus persica seeds. Biol. Pharm. Bull. 2003, 26, 271–273. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhao, X.; Sun, Q.; Jiang, Y.; Zhang, W.; Luo, J.; Li, Y. Synergic effect of PD-1 blockade and endostar on the PI3K/AKT/mTOR-mediated autophagy and angiogenesis in Lewis lung carcinoma mouse model. Biomed. Pharmacother. 2020, 125, 109746. [Google Scholar] [CrossRef] [PubMed]






| Groups | Cardiac Index (g/g) | Liver Index (g/g) | Splenic Index (g/g) | Lung Index (g/g) | Renal Index (g/g) |
|---|---|---|---|---|---|
| CON | 0.006 ± 0.001 | 0.067 ± 0.006 | 0.006 ± 0.001 | 0.007 ± 0.001 | 0.016 ± 0.002 |
| PTX | 0.005 ± 0.000 | 0.042 ± 0.003 **** | 0.004 ± 0.003 | 0.007 ± 0.001 | 0.013 ± 0.001 * |
| WE-AER | 0.006 ± 0.000 | 0.065 ± 0.002 | 0.005 ± 0.001 | 0.006 ± 0.001 | 0.016 ± 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, X.; Xiao, Q.; Chen, H.; Yang, S.; Li, Q.; Peng, L. Actinidia eriantha Benth. Root as a New Phytomedicine Inhibits Non-Small Cell Lung Cancer by Regulating·TGF-β/FOXO/mTOR. Int. J. Mol. Sci. 2025, 26, 8957. https://doi.org/10.3390/ijms26188957
Zhang X, Xiao Q, Chen H, Yang S, Li Q, Peng L. Actinidia eriantha Benth. Root as a New Phytomedicine Inhibits Non-Small Cell Lung Cancer by Regulating·TGF-β/FOXO/mTOR. International Journal of Molecular Sciences. 2025; 26(18):8957. https://doi.org/10.3390/ijms26188957
Chicago/Turabian StyleZhang, Xuan, Qiyao Xiao, Haoran Chen, Shaoming Yang, Qingli Li, and Lihua Peng. 2025. "Actinidia eriantha Benth. Root as a New Phytomedicine Inhibits Non-Small Cell Lung Cancer by Regulating·TGF-β/FOXO/mTOR" International Journal of Molecular Sciences 26, no. 18: 8957. https://doi.org/10.3390/ijms26188957
APA StyleZhang, X., Xiao, Q., Chen, H., Yang, S., Li, Q., & Peng, L. (2025). Actinidia eriantha Benth. Root as a New Phytomedicine Inhibits Non-Small Cell Lung Cancer by Regulating·TGF-β/FOXO/mTOR. International Journal of Molecular Sciences, 26(18), 8957. https://doi.org/10.3390/ijms26188957






