Neonatal Urine Metabolic Signature Reflects Multisystemic Adaptations Linked to Preterm Birth
Abstract
1. Introduction
2. Results
2.1. Demographic Characteristics of the Study Populations
2.1.1. Mothers
2.1.2. Neonates
2.2. Urine Metabolomics
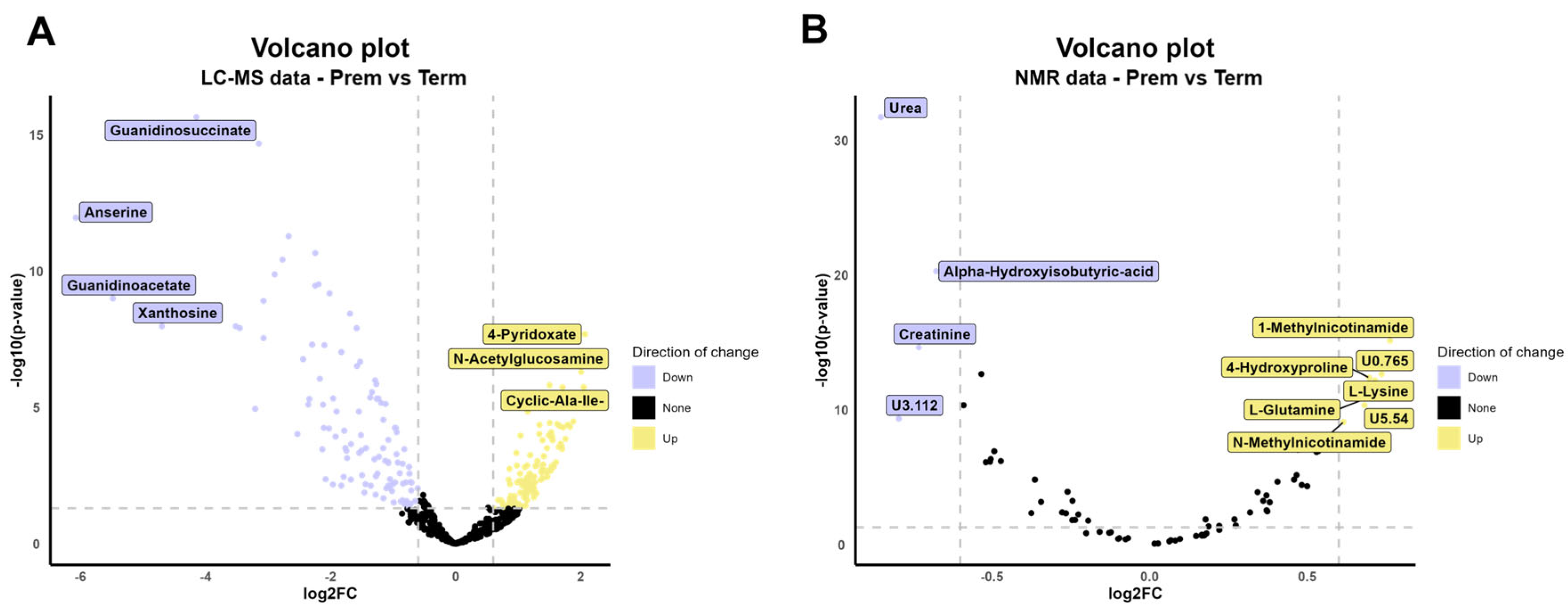
2.2.1. Univariate Analysis (UVA) of Metabolites Identified
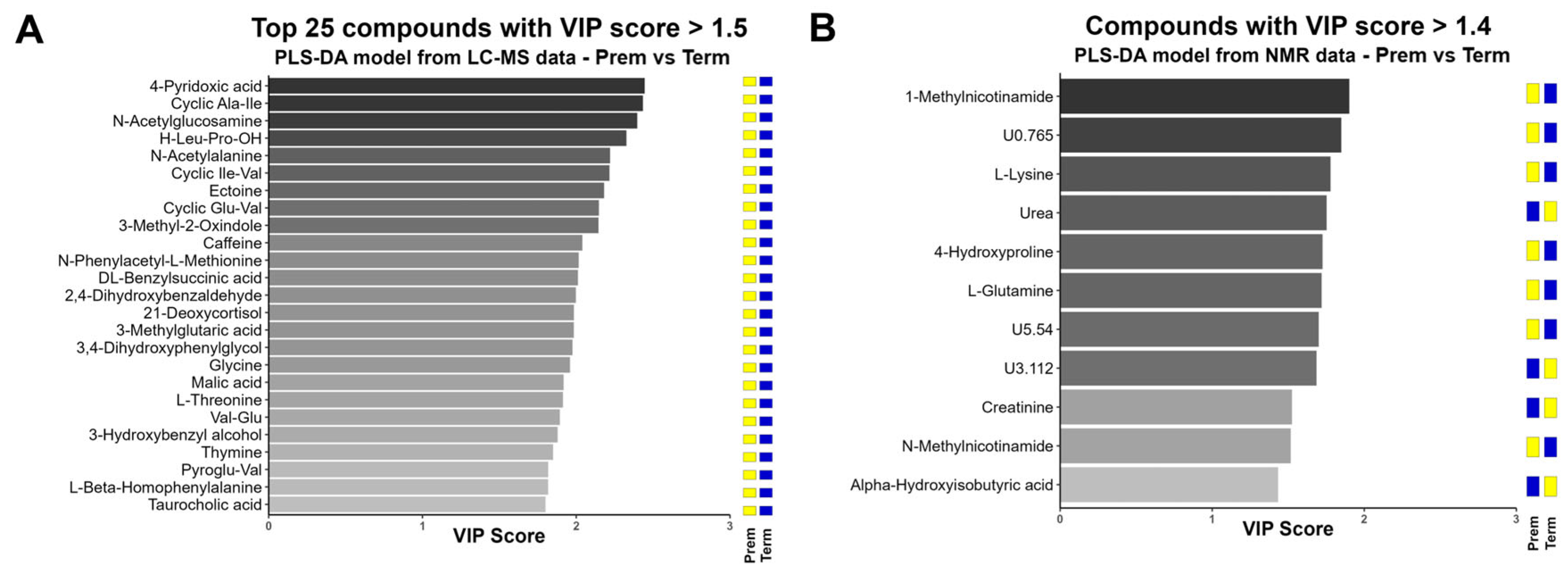
2.2.2. Multivariate Analysis (MVA) of Urinary Metabolome
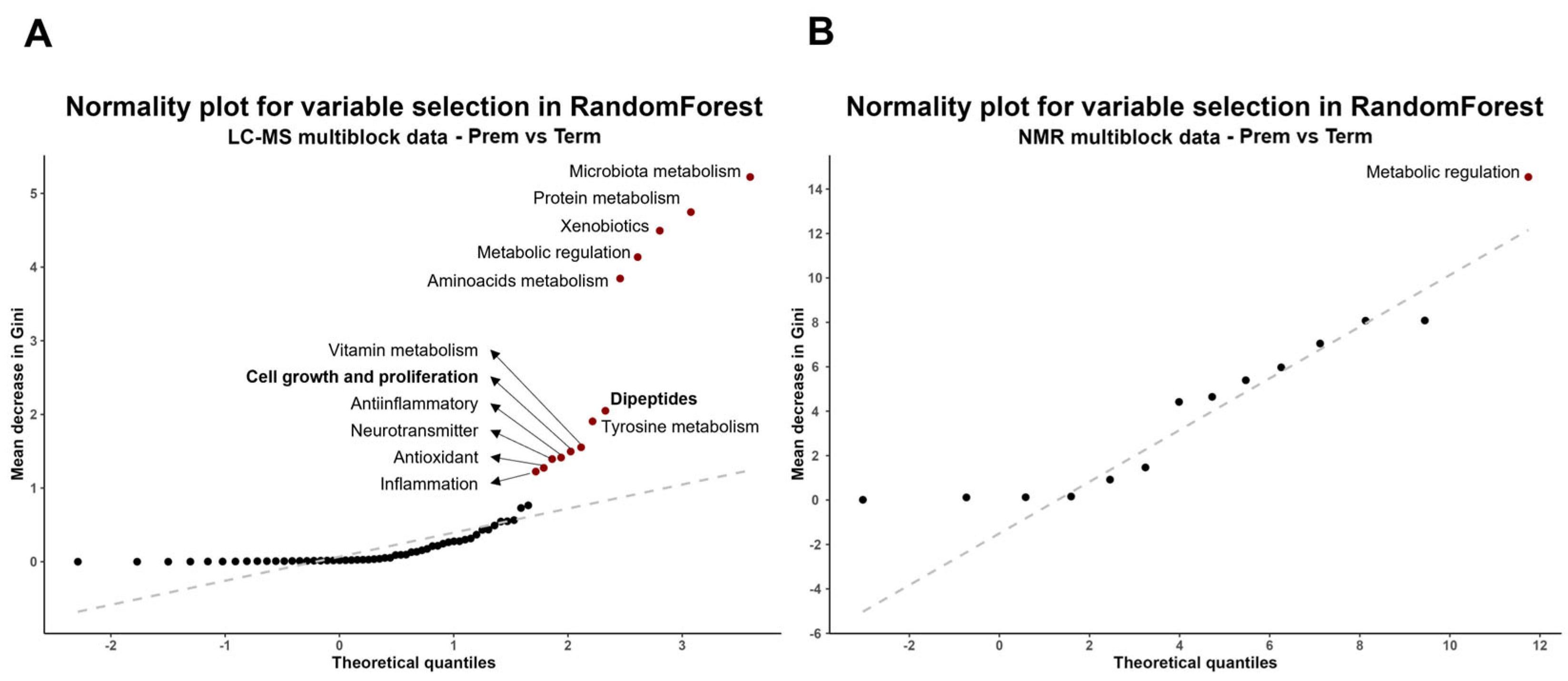
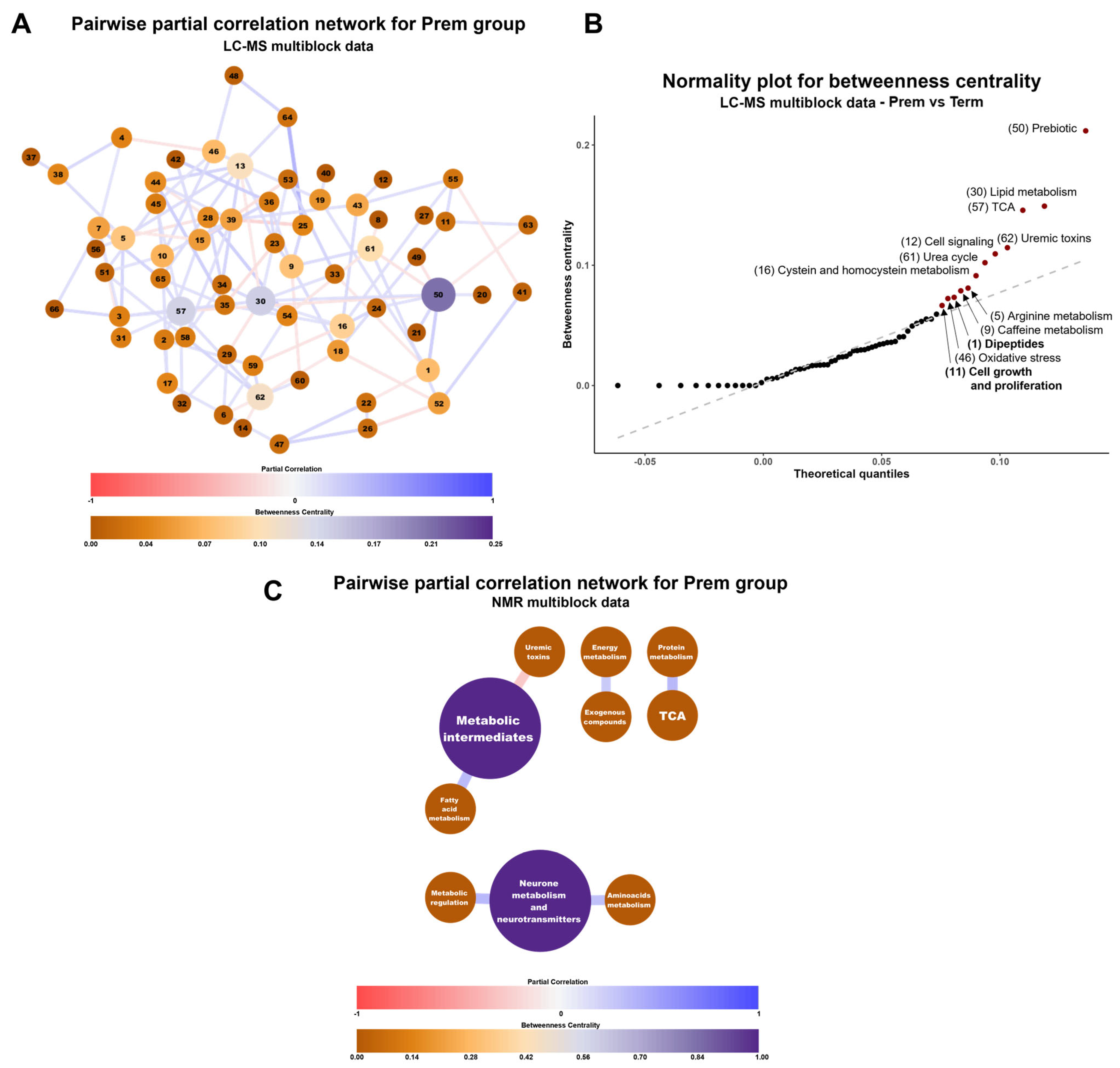
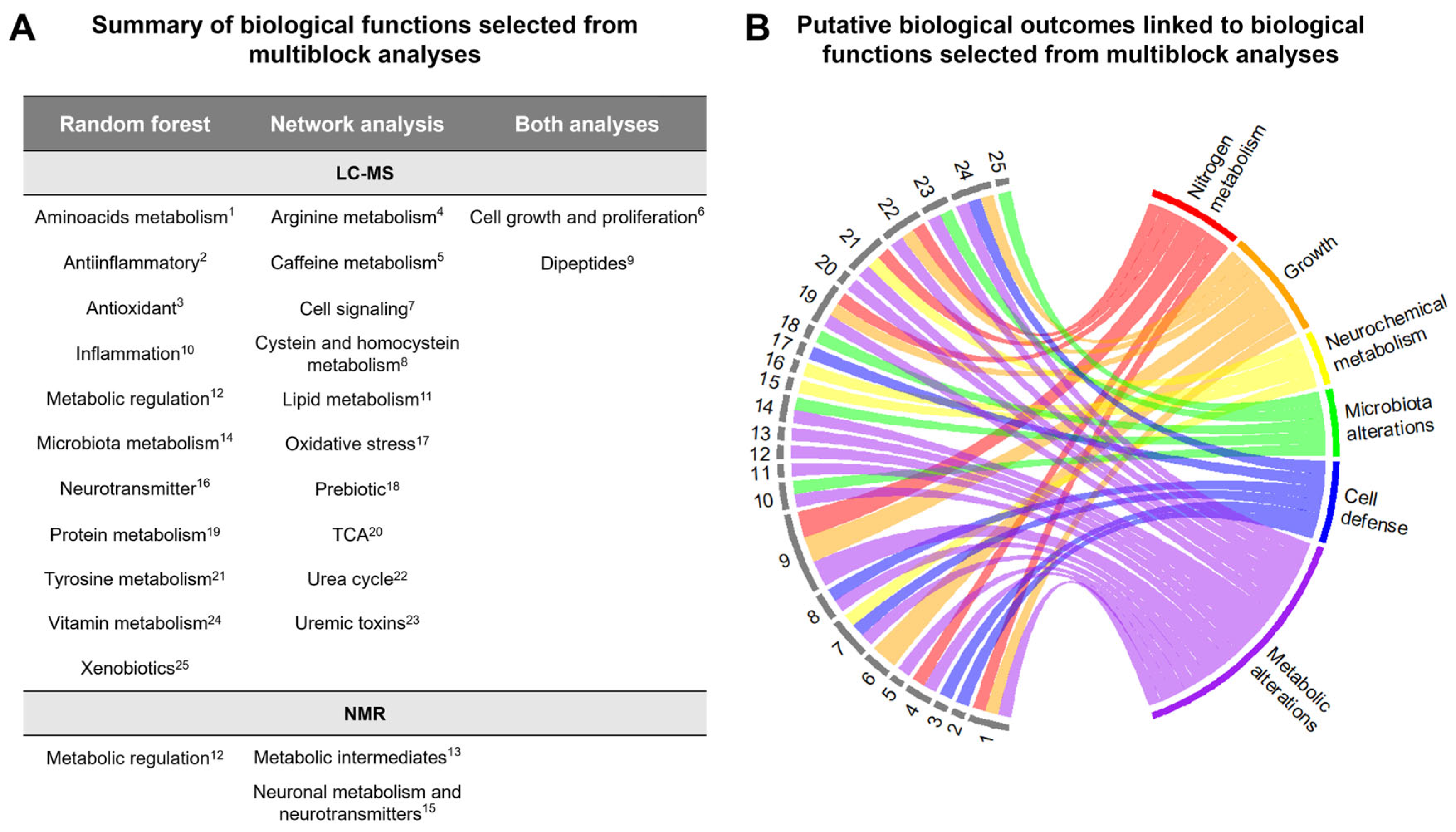
2.2.3. Multiblock Analysis of Urinary Metabolome
3. Discussion
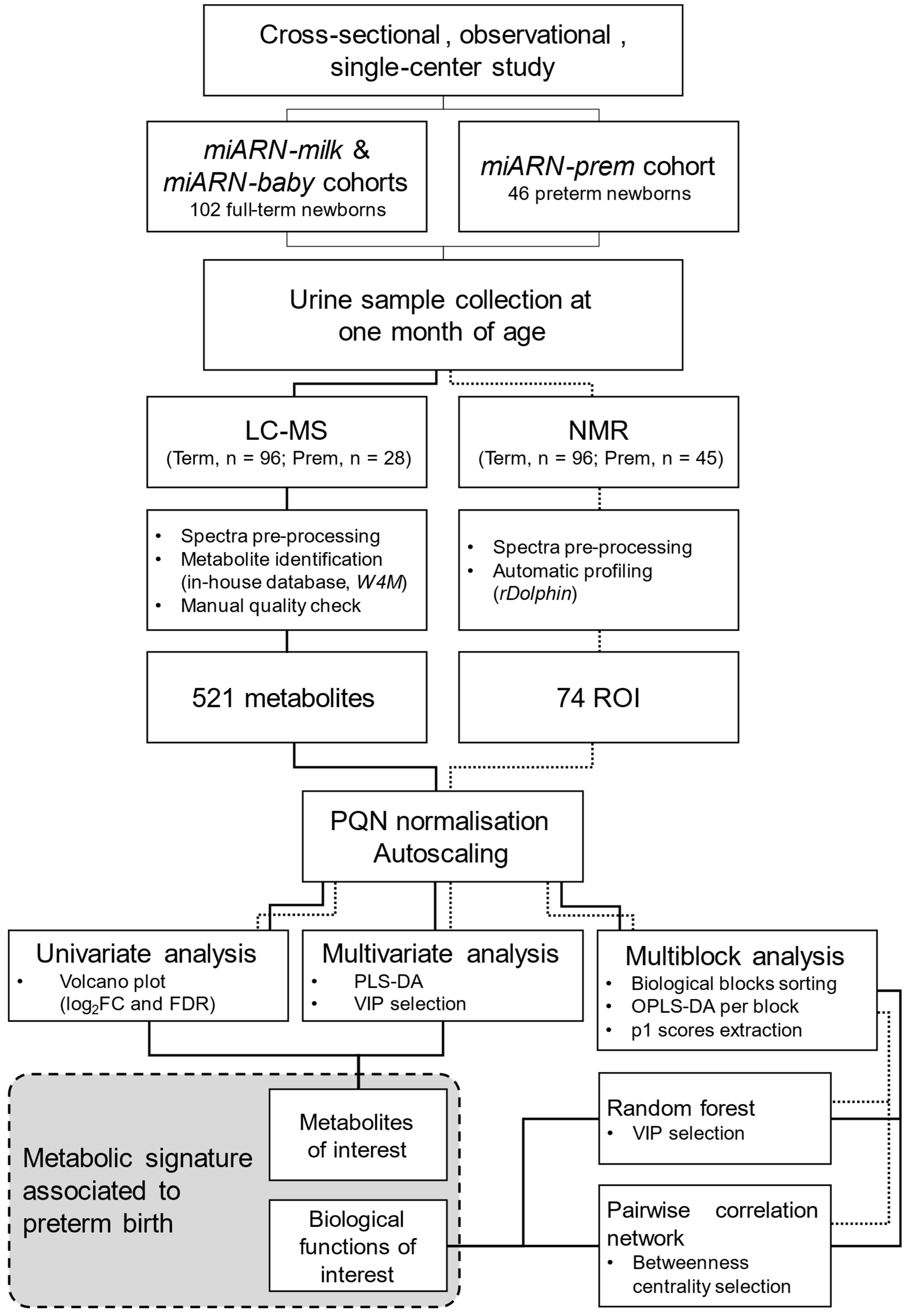
4. Materials and Methods
4.1. Subjects
4.2. Sample Acquisition and Anthropometrical Measurements
4.3. LC-MS Analysis
4.4. NMR Analysis
4.5. Data Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BFP | Body Fat Percentage |
| CEI-IB | Comité de Ética de Investigación de las Islas Baleares |
| DOHaD | Developmental Origins of Health and Disease |
| FC | Fold Change |
| FDR | False Discovery Rate |
| HMDB | Human Metabolome Database |
| LC-MS | Liquid Chromatography coupled to Mass Spectrometry |
| MMP | Muscle Mass Percentage |
| MS/MS | Tandem Mass Spectrometry |
| MVA | Multivariate Analysis |
| NMR | Nuclear Magnetic Resonance |
| OPLS-DA | Orthogonal Partial Least Squares Discriminant Analysis |
| PLS-DA | Partial Least Squares Discriminant Analysis |
| PQN | Probabilistic Quotient Normalization |
| QC | Quality Control |
| TCA | Tricarboxylic acid cycle |
| UVA | Univariate Analysis |
| VIP | Variable Importance in Projection |
References
- World Health Organization (WHO). Preterm Birth. Available online: https://www.who.int/news-room/fact-sheets/detail/preterm-birth (accessed on 30 August 2023).
- World Health Organization. Born too Soon Decade of Action on Preterm Birth; World Health Organization: Geneva, Switzerland, 2023. [Google Scholar]
- Institute of Medicine (US); Committee on Understanding Premature Birth and Assuring Healthy Outcomes; Behrman, R.E.; Butler, A.S. Mortality and Acute Complications in Preterm Infants. In Preterm Birth: Causes, Consequences, and Prevention; National Academies Press (US): Washington, DC, USA, 2007. [Google Scholar]
- Crump, C. An Overview of Adult Health Outcomes after Preterm Birth. Early Hum. Dev. 2020, 150, 105187. [Google Scholar] [CrossRef]
- Luu, T.M.; Rehman Mian, M.O.; Nuyt, A.M. Long-Term Impact of Preterm Birth: Neurodevelopmental and Physical Health Outcomes. Clin. Perinatol. 2017, 44, 305–314. [Google Scholar] [CrossRef]
- Jańczewska, I.; Wierzba, J.; Jańczewska, A.; Szczurek-Gierczak, M.; Domżalska-Popadiuk, I. Prematurity and Low Birth Weight and Their Impact on Childhood Growth Patterns and the Risk of Long-Term Cardiovascular Sequelae. Children 2023, 10, 1599. [Google Scholar] [CrossRef]
- Hanson, M.A.; Gluckman, P.D. Early Developmental Conditioning of Later Health and Disease: Physiology or Pathophysiology? Physiol. Rev. 2014, 94, 1027–1076. [Google Scholar] [CrossRef]
- Nuyt, A.M.; Lavoie, J.C.; Mohamed, I.; Paquette, K.; Luu, T.M. Adult Consequences of Extremely Preterm Birth: Cardiovascular and Metabolic Diseases Risk Factors, Mechanisms, and Prevention Avenues. Clin. Perinatol. 2017, 44, 315–332. [Google Scholar] [CrossRef]
- Bouatra, S.; Aziat, F.; Mandal, R.; Guo, A.C.; Wilson, M.R.; Knox, C.; Bjorndahl, T.C.; Krishnamurthy, R.; Saleem, F.; Liu, P.; et al. The Human Urine Metabolome. PLoS ONE 2013, 8, e73076. [Google Scholar] [CrossRef]
- Atzori, L.; Antonucci, R.; Barberini, L.; Locci, E.; Marincola, F.C.; Scano, P.; Cortesi, P.; Agostiniani, R.; Defraia, R.; Weljie, A.; et al. 1H NMR-Based Metabolomic Analysis of Urine from Preterm and Term Neonates. Front. Biosci. (Elite Ed) 2011, 3, 1005–1012. [Google Scholar] [CrossRef]
- Diaz, S.O.; Pinto, J.; Barros, A.S.; Morais, E.; Duarte, D.; Negrao, F.; Pita, C.; Almeida, M.D.C.; Carreira, I.M.; Spraul, M.; et al. Newborn Urinary Metabolic Signatures of Prematurity and Other Disorders: A Case Control Study. J. Proteome Res. 2016, 15, 311–325. [Google Scholar] [CrossRef] [PubMed]
- Foxall, P.J.D.; Bewley, S.; Neild, G.H.; Rodeck, C.H.; Nicholson, J.K. Analysis of Fetal and Neonatal Urine Using Proton Nuclear Magnetic Resonance Spectroscopy. Arch. Dis. Child. Fetal Neonatal Ed. 1995, 73, F153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Morniroli, D.; Dessì, A.; Giannì, M.L.; Roggero, P.; Noto, A.; Atzori, L.; Lussu, M.; Fanos, V.; Mosca, F. Is the Body Composition Development in Premature Infants Associated with a Distinctive Nuclear Magnetic Resonance Metabolomic Profiling of Urine? J. Matern.-Fetal Neonatal Med. 2019, 32, 2310–2318. [Google Scholar] [CrossRef] [PubMed]
- Trump, S.; Laudi, S.; Unruh, N.; Goelz, R.; Leibfritz, D. 1H-NMR Metabolic Profiling of Human Neonatal Urine. Magn. Reson. Mater. Phys. Biol. Med. 2006, 19, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Marino, L.V.; Paulson, S.; Ashton, J.J.; Weeks, C.; Young, A.; Pappachan, J.V.; Swann, J.; Johnson, M.J.; Beattie, R.M. A Scoping Review: Urinary Markers of Metabolic Maturation in Preterm Infants and Future Interventions to Improve Growth. Nutrients 2022, 14, 3957. [Google Scholar] [CrossRef] [PubMed]
- Heino, A.; Gissler, M.; Hindori-Mohangoo, A.D.; Blondel, B.; Klungsøyr, K.; Verdenik, I.; Mierzejewska, E.; Velebil, P.; Ólafsdóttir, H.S.; Macfarlane, A.; et al. Variations in Multiple Birth Rates and Impact on Perinatal Outcomes in Europe. PLoS ONE 2016, 11, e0149252. [Google Scholar] [CrossRef]
- INE Movimiento Natural de La Población/Indicadores Demográficos Básico. Año 2023. Available online: https://www.ine.es/dyngs/INEbase/es/operacion.htm?c=Estadistica_C&cid=1254736177007&menu=ultiDatos&idp=1254735573002 (accessed on 24 March 2025).
- Delnord, M.; Blondel, B.; Drewniak, N.; Klungsøyr, K.; Bolumar, F.; Mohangoo, A.; Gissler, M.; Szamotulska, K.; Lack, N.; Nijhuis, J.; et al. Varying Gestational Age Patterns in Cesarean Delivery: An International Comparison. BMC Pregnancy Childbirth 2014, 14, 321. [Google Scholar] [CrossRef]
- Lechosa-Muñiz, C.; Paz-Zulueta, M.; Sota, S.M.; de Adana Herrero, M.S.; del Rio, E.C.; Llorca, J.; Cabero-Perez, M.J. Factors Associated with Duration of Breastfeeding in Spain: A Cohort Study. Int. Breastfeed. J. 2020, 15, 79. [Google Scholar] [CrossRef]
- Donangelo, C.M.; Bezerra, F.F. Pregnancy: Metabolic Adaptations and Nutritional Requirements. In Encyclopedia of Food and Health; Academic Press: Cambridge, MA, USA, 2016; pp. 484–490. ISBN 9780123849533. [Google Scholar]
- Wickham, H. Ggplot2: Elegant Graphics for Data Analysis; Springer: New York, NY, USA, 2016; ISBN 978-3-319-24277-4. [Google Scholar]
- Kamil Slowikowski Ggrepel: Automatically Position Non-Overlapping Text Labels with “Ggplot2”. Available online: https://ggrepel.slowkow.com/ (accessed on 7 March 2025).
- Thévenot, E.A.; Roux, A.; Xu, Y.; Ezan, E.; Junot, C. Analysis of the Human Adult Urinary Metabolome Variations with Age, Body Mass Index, and Gender by Implementing a Comprehensive Workflow for Univariate and OPLS Statistical Analyses. J. Proteome Res. 2015, 14, 3322–3335. [Google Scholar] [CrossRef]
- Almeida, A.; Loy, A.; Hofmann, H. Ggplot2 Compatible Quantile-Quantile Plots in R. R J. 2019, 10, 248–261. [Google Scholar] [CrossRef]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A Software Environment for Integrated Models of Biomolecular Interaction Networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef] [PubMed]
- Gu, Z.; Gu, L.; Eils, R.; Schlesner, M.; Brors, B. Circlize Implements and Enhances Circular Visualization in R. Bioinformatics 2014, 30, 2811–2812. [Google Scholar] [CrossRef]
- Illsinger, S.; Schmidt, K.H.; Lücke, T.; Vaske, B.; Bohnhorst, B.; Das, A.M. Plasma and Urine Amino Acid Pattern in Preterm Infants on Enteral Nutrition: Impact of Gestational Age. Amino Acids 2010, 38, 959–972. [Google Scholar] [CrossRef]
- Rudar, M.; Naberhuis, J.K.; Suryawan, A.; Nguyen, H.V.; Stoll, B.; Style, C.C.; Verla, M.A.; Olutoye, O.O.; Burrin, D.G.; Fiorotto, M.L.; et al. Prematurity Blunts the Insulin- and Amino Acid-Induced Stimulation of Translation Initiation and Protein Synthesis in Skeletal Muscle of Neonatal Pigs. Am. J. Physiol. Endocrinol. Metab. 2021, 320, E551–E565. [Google Scholar] [CrossRef]
- Rudar, M.; Naberhuis, J.K.; Suryawan, A.; Nguyen, H.V.; Fiorotto, M.L.; Davis, T.A. Prematurity Blunts Protein Synthesis in Skeletal Muscle Independently of Body Weight in Neonatal Pigs. Pediatr. Res. 2023, 94, 143–152. [Google Scholar] [CrossRef]
- Kalhan, S.C.; Bier, D.M. Protein and Amino Acid Metabolism in the Human Newborn. Annu. Rev. Nutr. 2008, 28, 389–410. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.J.; Wootton, S.A.; Leaf, A.A.; Jackson, A.A. Preterm Birth and Body Composition at Term Equivalent Age: A Systematic Review and Meta-Analysis. Pediatrics 2012, 130, e640–e649. [Google Scholar] [CrossRef] [PubMed]
- Greer, F.R. Long-Term Adverse Outcomes of Low Birth Weight, Increased Somatic Growth Rates, and Alterations of Body Composition in the Premature Infant: Review of the Evidence. J. Pediatr. Gastroenterol. Nutr. 2007, 45, S147–S151. [Google Scholar] [CrossRef]
- Deutsch, L.; Debevec, T.; Millet, G.P.; Osredkar, D.; Opara, S.; Šket, R.; Murovec, B.; Mramor, M.; Plavec, J.; Stres, B. Urine and Fecal1H-NMR Metabolomes Differ Significantly between Pre-Term and Full-Term Born Physically Fit Healthy Adult Males. Metabolites 2022, 12, 536. [Google Scholar] [CrossRef]
- Perrone, S.; Negro, S.; Laschi, E.; Calderisi, M.; Giordano, M.; De Bernardo, G.; Parigi, G.; Toni, A.L.; Esposito, S.; Buonocore, G. Metabolomic Profile of Young Adults Born Preterm. Metabolites 2021, 11, 697. [Google Scholar] [CrossRef]
- Moltu, S.J.; Sachse, D.; Blakstad, E.W.; Strømmen, K.; Nakstad, B.; Almaas, A.N.; Westerberg, A.C.; Rønnestad, A.; Brække, K.; Veierød, M.B.; et al. Urinary Metabolite Profiles in Premature Infants Show Early Postnatal Metabolic Adaptation and Maturation. Nutrients 2014, 6, 1913–1930. [Google Scholar] [CrossRef]
- Embleton, N.D.; Jennifer Moltu, S.; Lapillonne, A.; Van Den Akker, C.H.P.; Carnielli, V.; Fusch, C.; Gerasimidis, K.; Van Goudoever, J.B.; Haiden, N.; Iacobelli, S.; et al. Enteral Nutrition in Preterm Infants (2022): A Position Paper from the ESPGHAN Committee on Nutrition and Invited Experts. J. Pediatr. Gastroenterol. Nutr. 2023, 76, 248–268. [Google Scholar] [CrossRef]
- Zhou, S.-S.; Zhou, Y. Excess Vitamin Intake: An Unrecognized Risk Factor for Obesity. World J. Diabetes 2014, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Toftlund, L.H.; Halken, S.; Agertoft, L.; Zachariassen, G. Catch-Up Growth, Rapid Weight Growth, and Continuous Growth from Birth to 6 Years of Age in Very-Preterm-Born Children. Neonatology 2018, 114, 285–293. [Google Scholar] [CrossRef]
- Kim, I.S.; Jo, E.K. Inosine: A Bioactive Metabolite with Multimodal Actions in Human Diseases. Front. Pharmacol. 2022, 13, 1043970. [Google Scholar] [CrossRef]
- Duerden, E.G.; Taylor, M.J.; Miller, S.P. Brain Development in Infants Born Preterm: Looking Beyond Injury. Semin. Pediatr. Neurol. 2013, 20, 65–74. [Google Scholar] [CrossRef]
- Horner, D.; Jepsen, J.R.M.; Chawes, B.; Aagaard, K.; Rosenberg, J.B.; Mohammadzadeh, P.; Sevelsted, A.; Vahman, N.; Vinding, R.; Fagerlund, B.; et al. A Western Dietary Pattern during Pregnancy Is Associated with Neurodevelopmental Disorders in Childhood and Adolescence. Nat. Metab. 2025, 7, 586–601. [Google Scholar] [CrossRef]
- Beghetti, I.; Barone, M.; Brigidi, P.; Sansavini, A.; Corvaglia, L.; Aceti, A.; Turroni, S. Early-Life Gut Microbiota and Neurodevelopment in Preterm Infants: A Narrative Review. Front. Nutr. 2023, 10, 1241303. [Google Scholar] [CrossRef] [PubMed]
- Georgakopoulou, I.; Chasapi, S.A.; Bariamis, S.E.; Varvarigou, A.; Spraul, M.; Spyroulias, G.A. Metabolic Changes in Early Neonatal Life: NMR Analysis of the Neonatal Metabolic Profile to Monitor Postnatal Metabolic Adaptations. Metabolomics 2020, 16, 58. [Google Scholar] [CrossRef]
- Picaud, J.-C.; De Magistris, A.; Mussap, M.; Corbu, S.; Dessì, A.; Noto, A.; Fanos, V.; Marincola, F.C. Urine NMR Metabolomics Profile of Preterm Infants With Necrotizing Enterocolitis Over the First Two Months of Life: A Pilot Longitudinal Case-Control Study. Front. Mol. Biosci. 2021, 8, 1. [Google Scholar] [CrossRef]
- Sakata, H.; Yoshioka, H.; Fujita, K. Development of the Intestinal Flora in Very Low Birth Weight Infants Compared to Normal Full-Term Newborns. Eur. J. Pediatr. 1985, 144, 186–190. [Google Scholar] [CrossRef]
- Schwiertz, A.; Gruhl, B.; Löbnitz, M.; Michel, P.; Radke, M.; Blaut, M. Development of the Intestinal Bacterial Composition in Hospitalized Preterm Infants in Comparison with Breast-Fed, Full-Term Infants. Pediatr. Res. 2003, 54, 393–399. [Google Scholar] [CrossRef] [PubMed]
- Dibartolomeo, M.E.; Claud, E.C. The Developing Microbiome of the Preterm Infant. Clin. Ther. 2016, 38, 733–739. [Google Scholar] [CrossRef] [PubMed]
- Jiang, P.; Trimigno, A.; Stanstrup, J.; Khakimov, B.; Viereck, N.; Engelsen, S.B.; Sangild, P.T.; Dragsted, L.O. Antibiotic Treatment Preventing Necrotising Enterocolitis Alters Urinary and Plasma Metabolomes in Preterm Pigs. J. Proteome Res. 2017, 16, 3547–3557. [Google Scholar] [CrossRef]
- Biagi, E.; Aceti, A.; Quercia, S.; Beghetti, I.; Rampelli, S.; Turroni, S.; Soverini, M.; Zambrini, A.V.; Faldella, G.; Candela, M.; et al. Microbial Community Dynamics in Mother’s Milk and Infant’s Mouth and Gut in Moderately Preterm Infants. Front. Microbiol. 2018, 9, 2512. [Google Scholar] [CrossRef]
- Xu, W.; Judge, M.P.; Maas, K.; Hussain, N.; McGrath, J.M.; Henderson, W.A.; Cong, X. Systematic Review of the Effect of Enteral Feeding on Gut Microbiota in Preterm Infants. J. Obstet. Gynecol. Neonatal Nurs. 2018, 47, 451–463. [Google Scholar] [CrossRef]
- Blaser, M.J.; Dominguez-Bello, M.G. The Human Microbiome before Birth. Cell Host Microbe 2016, 20, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Dello, S.A.W.G.; Neis, E.P.J.G.; de Jong, M.C.; van Eijk, H.M.H.; Kicken, C.H.; Olde Damink, S.W.M.; Dejong, C.H.C. Systematic Review of Ophthalmate as a Novel Biomarker of Hepatic Glutathione Depletion. Clin. Nutr. 2013, 32, 325–330. [Google Scholar] [CrossRef] [PubMed]
- Daniels, R.C.; Tiba, M.H.; Cummings, B.C.; Yap, Y.R.; Ansari, S.; McCracken, B.M.; Sun, Y.; Jennaro, T.S.; Ward, K.R.; Stringer, K.A. Redox Potential Correlates with Changes in Metabolite Concentrations Attributable to Pathways Active in Oxidative Stress Response in Swine Traumatic Shock. Shock 2022, 57, 282. [Google Scholar] [CrossRef] [PubMed]
- Romero, R.; Mazaki-Tovi, S.; Vaisbuch, E.; Kusanovic, J.P.; Chaiworapongsa, T.; Gomez, R.; Nien, J.K.; Yoon, B.H.; Mazor, M.; Luo, J.; et al. Metabolomics in Premature Labor: A Novel Approach to Identify Patients at Risk for Preterm Delivery. J. Matern. Fetal Neonatal Med. 2010, 23, 1344–1359. [Google Scholar] [CrossRef]
- Perrone, S.; Tataranno, M.L.; Stazzoni, G.; Buonocore, G. Oxidative Stress and Free Radicals Related Diseases of the Newborn. Adv. Biosci. Biotechnol. 2012, 2012, 1043–1050. [Google Scholar] [CrossRef]
- Melville, J.M.; Moss, T.J.M. The Immune Consequences of Preterm Birth. Front. Neurosci. 2013, 7, 79. [Google Scholar] [CrossRef]
- Bavineni, M.; Wassenaar, T.M.; Agnihotri, K.; Ussery, D.W.; Lüscher, T.F.; Mehta, J.L. Mechanisms Linking Preterm Birth to Onset of Cardiovascular Disease Later in Adulthood. Eur. Heart J. 2019, 40, 1107. [Google Scholar] [CrossRef]
- Li, H.; Miao, C.; Xu, L.; Gao, H.; Bai, M.; Liu, W.; Li, W.; Wu, Z.; Zhu, Y. Maternal Pre-Pregnancy Body Mass Index, Gestational Weight Gain Trajectory, and Risk of Adverse Perinatal Outcomes. Int. J. Gynecol. Obstet. 2022, 157, 723–732. [Google Scholar] [CrossRef]
- Cho, K.; Moon, J.S.; Kang, J.H.; Jang, H.B.; Lee, H.J.; Park, S.I.; Yu, K.S.; Cho, J.Y. Combined Untargeted and Targeted Metabolomic Profiling Reveals Urinary Biomarkers for Discriminating Obese from Normal-Weight Adolescents. Pediatr. Obes. 2017, 12, 93–101. [Google Scholar] [CrossRef] [PubMed]
- Alexandre-Gouabau, M.C.; Courant, F.; Moyon, T.; Küster, A.; Le Gall, G.; Tea, I.; Antignac, J.P.; Darmaun, D. Maternal and Cord Blood LC-HRMS Metabolomics Reveal Alterations in Energy and Polyamine Metabolism, and Oxidative Stress in Very-Low Birth Weight Infants. J. Proteome Res. 2013, 12, 2764–2778. [Google Scholar] [CrossRef]
- Tanaka, H.; Sirich, T.L.; Plummer, N.S.; Weaver, D.S.; Meyer, T.W. An Enlarged Profile of Uremic Solutes. PLoS ONE 2015, 10, e0135657. [Google Scholar] [CrossRef]
- Post, A.; Groothof, D.; Kremer, D.; Knobbe, T.J.; Abma, W.; Koops, C.A.; Tsikas, D.; Wallimann, T.; Dullaart, R.P.F.; Franssen, C.F.M.; et al. Creatine Homeostasis and the Kidney: Comparison between Kidney Transplant Recipients and Healthy Controls. Amino Acids 2024, 56, 42. [Google Scholar] [CrossRef]
- Sass, J.O.; Mohr, V.; Olbrich, H.; Engelke, U.; Horvath, J.; Fliegauf, M.; Loges, N.T.; Schweitzer-Krantz, S.; Moebus, R.; Weiler, P.; et al. Mutations in ACY1, the Gene Encoding Aminoacylase 1, Cause a Novel Inborn Error of Metabolism. Am. J. Hum. Genet. 2006, 78, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.E.; Perez, L.; Ryan, R.O. 3-Methylglutaric Acid in Energy Metabolism. Clin. Chim. Acta 2020, 502, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Abitbol, C.L.; DeFreitas, M.J.; Strauss, J. Assessment of Kidney Function in Preterm Infants: Lifelong Implications. Pediatr. Nephrol. 2016, 31, 2213–2222. [Google Scholar] [CrossRef]
- Carrascosa, A.; Fernández, J.M.; Ferrández, Á.; López-Siguero, J.P.; López, D.; Sánchez, E.; Grupo Colaborador. Estudios Españoles de Crecimiento 2010. Rev. Española Endocrinol. Pediátrica 2011, 2, 59–62. [Google Scholar] [CrossRef]
- Carrascosa Lezcano, A.; Delgado Beltrán, P.; Ferrández-Longás, A.; García-Dihinx, J.; Hernández-Rodríguez, M.; Romo, A.; Sobradillo, B. Patrones de Crecimiento y Desarrollo En España. Atlas de Gráficas y Tablas; Ergón; Pfizer: Madrid, Spain, 2004; ISBN 84-8473-292-4. [Google Scholar]
- Schmid, R.; Heuckeroth, S.; Korf, A.; Smirnov, A.; Myers, O.; Dyrlund, T.S.; Bushuiev, R.; Murray, K.J.; Hoffmann, N.; Lu, M.; et al. Integrative Analysis of Multimodal Mass Spectrometry Data in MZmine 3. Nat. Biotechnol. 2023, 41, 447–449. [Google Scholar] [CrossRef]
- Guitton, Y.; Tremblay-Franco, M.; Le Corguillé, G.; Martin, J.F.; Pétéra, M.; Roger-Mele, P.; Delabrière, A.; Goulitquer, S.; Monsoor, M.; Duperier, C.; et al. Create, Run, Share, Publish, and Reference Your LC–MS, FIA–MS, GC–MS, and NMR Data Analysis Workflows with the Workflow4Metabolomics 3.0 Galaxy Online Infrastructure for Metabolomics. Int. J. Biochem. Cell Biol. 2017, 93, 89–101. [Google Scholar] [CrossRef]
- Cañueto, D.; Gómez, J.; Salek, R.M.; Correig, X.; Cañellas, N. RDolphin: A GUI R Package for Proficient Automatic Profiling of 1D 1H-NMR Spectra of Study Datasets. Metabolomics 2018, 14, 24. [Google Scholar] [CrossRef]
- Wishart, D.S.; Guo, A.C.; Oler, E.; Wang, F.; Anjum, A.; Peters, H.; Dizon, R.; Sayeeda, Z.; Tian, S.; Lee, B.L.; et al. HMDB 5.0: The Human Metabolome Database for 2022. Nucleic Acids Res. 2022, 50, D622–D631. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.r-project.org/ (accessed on 13 July 2025).
- Dieterle, F.; Ross, A.; Schlotterbeck, G.; Senn, H. Probabilistic Quotient Normalization as Robust Method to Account for Dilution of Complex Biological Mixtures. Application in 1H NMR Metabonomics. Anal. Chem. 2006, 78, 4281–4290. [Google Scholar] [CrossRef]
- Pang, Z.; Chong, J.; Li, S.; Xia, J. MetaboAnalystR 3.0: Toward an Optimized Workflow for Global Metabolomics. Metabolites 2020, 10, 186. [Google Scholar] [CrossRef]
- Bennouna, D.; Tourniaire, F.; Durand, T.; Galano, J.M.; Fine, F.; Fraser, K.; Benatia, S.; Rosique, C.; Pau, C.; Couturier, C.; et al. The Brassica Napus (Oilseed Rape) Seeds Bioactive Health Effects Are Modulated by Agronomical Traits as Assessed by a Multi-Scale Omics Approach in the Metabolically Impaired Ob-Mouse. Food Chem. Mol. Sci. 2021, 2, 100011. [Google Scholar] [CrossRef] [PubMed]
- Wold, S.; Kettaneh, N.; Tjessem, K. Hierarchical Multiblock PLS and PC Models for Easier Model Interpretation and as an Alternative to Variable Selection. J. Chemom. 1996, 10, 463–482. [Google Scholar] [CrossRef]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2/3, 18–22. [Google Scholar]
- Schäfer, J.; Opgen-Rhein, R.; Strimmer, K. Reverse Engineering Genetic Networks Using the GeneNet Package. R News 2006, 6, 50–53. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bibiloni, P.; Martin, J.-C.; Cobo, P.; Jiménez-Cabanillas, M.V.; DeLucas, M.; Tardivel, C.; Picó, C.; Serra, F.; Sánchez, J. Neonatal Urine Metabolic Signature Reflects Multisystemic Adaptations Linked to Preterm Birth. Int. J. Mol. Sci. 2025, 26, 8953. https://doi.org/10.3390/ijms26188953
Bibiloni P, Martin J-C, Cobo P, Jiménez-Cabanillas MV, DeLucas M, Tardivel C, Picó C, Serra F, Sánchez J. Neonatal Urine Metabolic Signature Reflects Multisystemic Adaptations Linked to Preterm Birth. International Journal of Molecular Sciences. 2025; 26(18):8953. https://doi.org/10.3390/ijms26188953
Chicago/Turabian StyleBibiloni, Pere, Jean-Charles Martin, Pilar Cobo, María Victoria Jiménez-Cabanillas, María DeLucas, Catherine Tardivel, Catalina Picó, Francisca Serra, and Juana Sánchez. 2025. "Neonatal Urine Metabolic Signature Reflects Multisystemic Adaptations Linked to Preterm Birth" International Journal of Molecular Sciences 26, no. 18: 8953. https://doi.org/10.3390/ijms26188953
APA StyleBibiloni, P., Martin, J.-C., Cobo, P., Jiménez-Cabanillas, M. V., DeLucas, M., Tardivel, C., Picó, C., Serra, F., & Sánchez, J. (2025). Neonatal Urine Metabolic Signature Reflects Multisystemic Adaptations Linked to Preterm Birth. International Journal of Molecular Sciences, 26(18), 8953. https://doi.org/10.3390/ijms26188953









