Biological and Behavioral Responses of Drosophila melanogaster to Dietary Sugar and Sucralose
Abstract
1. Introduction
- -
- Shares a substantial proportion of its genome with humans, with approximately 60% of human disease-associated genes having identifiable homologs. Its fully sequenced and extensively annotated genome makes it a valuable model for assessing the impact of mutagens on specific genes and regulatory pathways that are conserved across species, including humans.
- -
- It has a short life cycle (approximately 10–12 days at 25 °C) and produces large numbers of offspring, making it particularly suitable for large-scale genetic screens and statistical analyses of mutagenic effects across generations. These features are essential for the efficient and cost-effective assessment of genotoxic risk.
- -
- There is an extensive collection of mutant lines, which enables the study of a wide range of biological conditions and responses. This genetic diversity allows researchers to investigate the effects of genotoxic agents under different genetic backgrounds, contributing to a more comprehensive understanding of mechanisms involved in DNA damage and repair.
- -
- Compared to mammalian models, the use of D. melanogaster raises fewer ethical concerns and is significantly more cost-effective, and its small size and ease of handling make it well suited for high-throughput screening of chemicals, radiation, and environmental pollutants for genotoxic potential.
- -
- Despite frequent application of Caenorhabditis elegans as a model for food additive safety genotoxicity tests, Drosophila is a much more versatile tool, capable of being applied to the genotoxicity test of sucralose as both a food additive and an environmental contaminant.
- -
- It has a simple and well-characterized gut microbiota that can be experimentally manipulated, allowing the investigation of how diet, environmental agents, or genetic factors influence microbial composition and host physiology.
2. Results
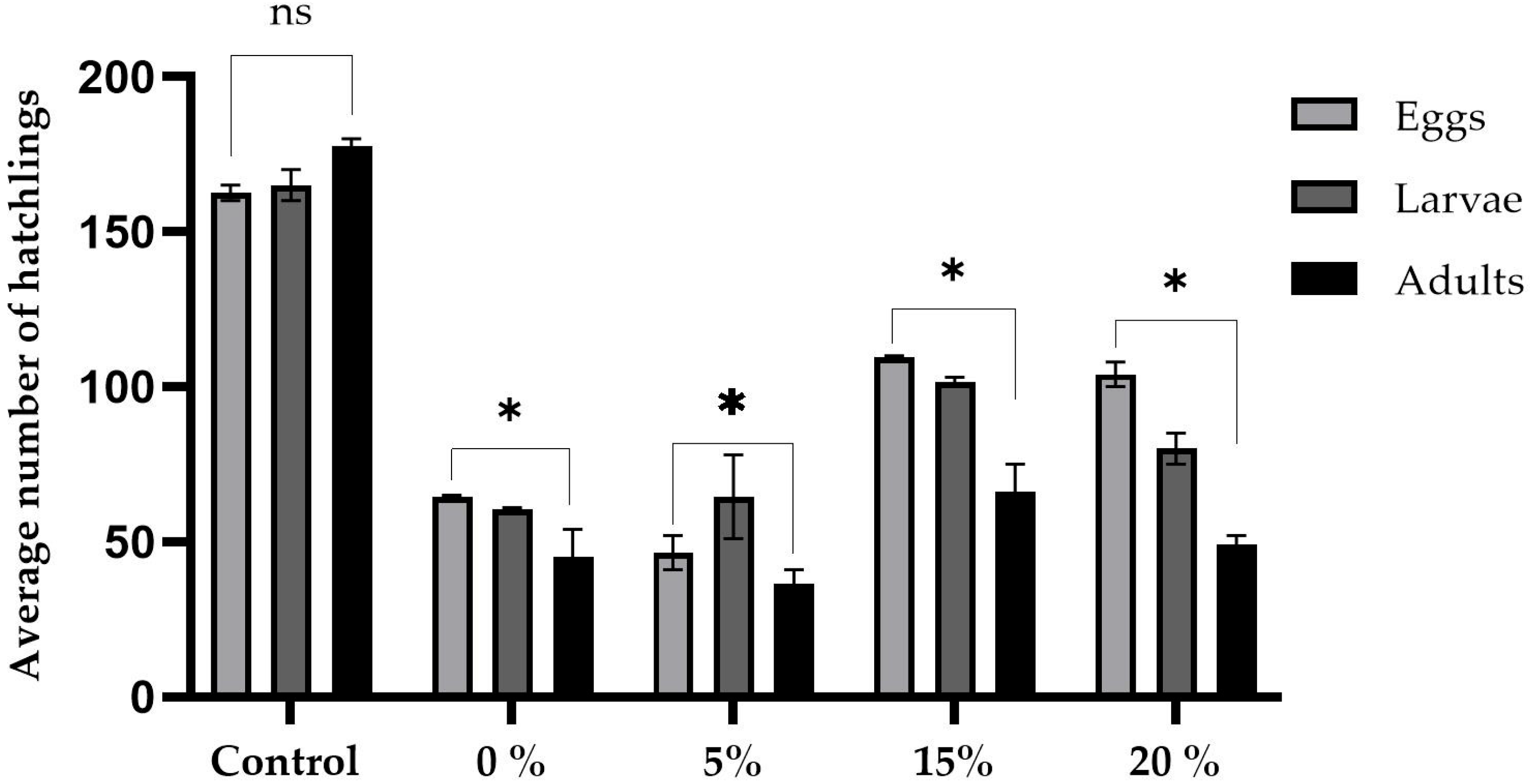
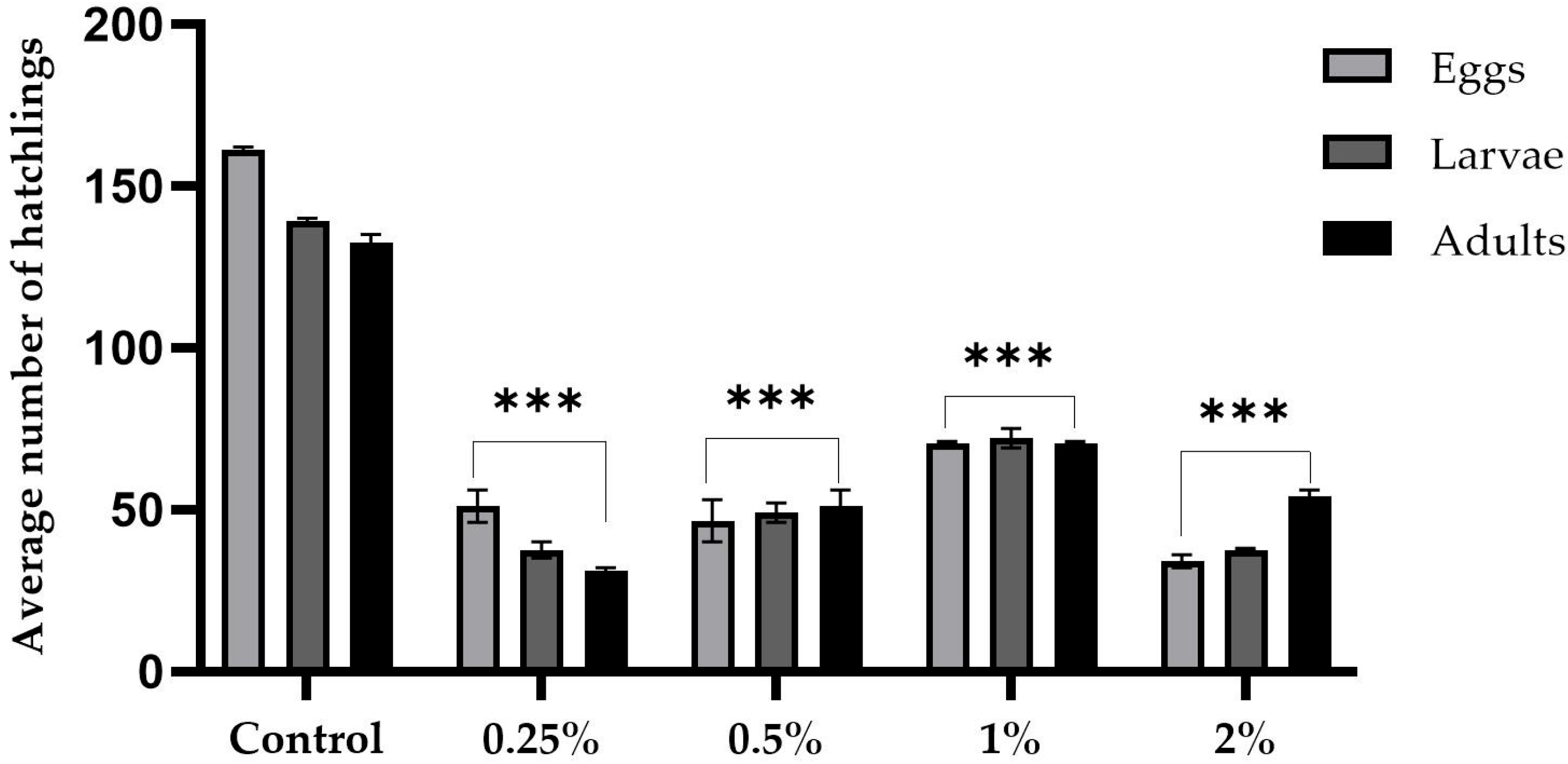
2.1. Proficiency
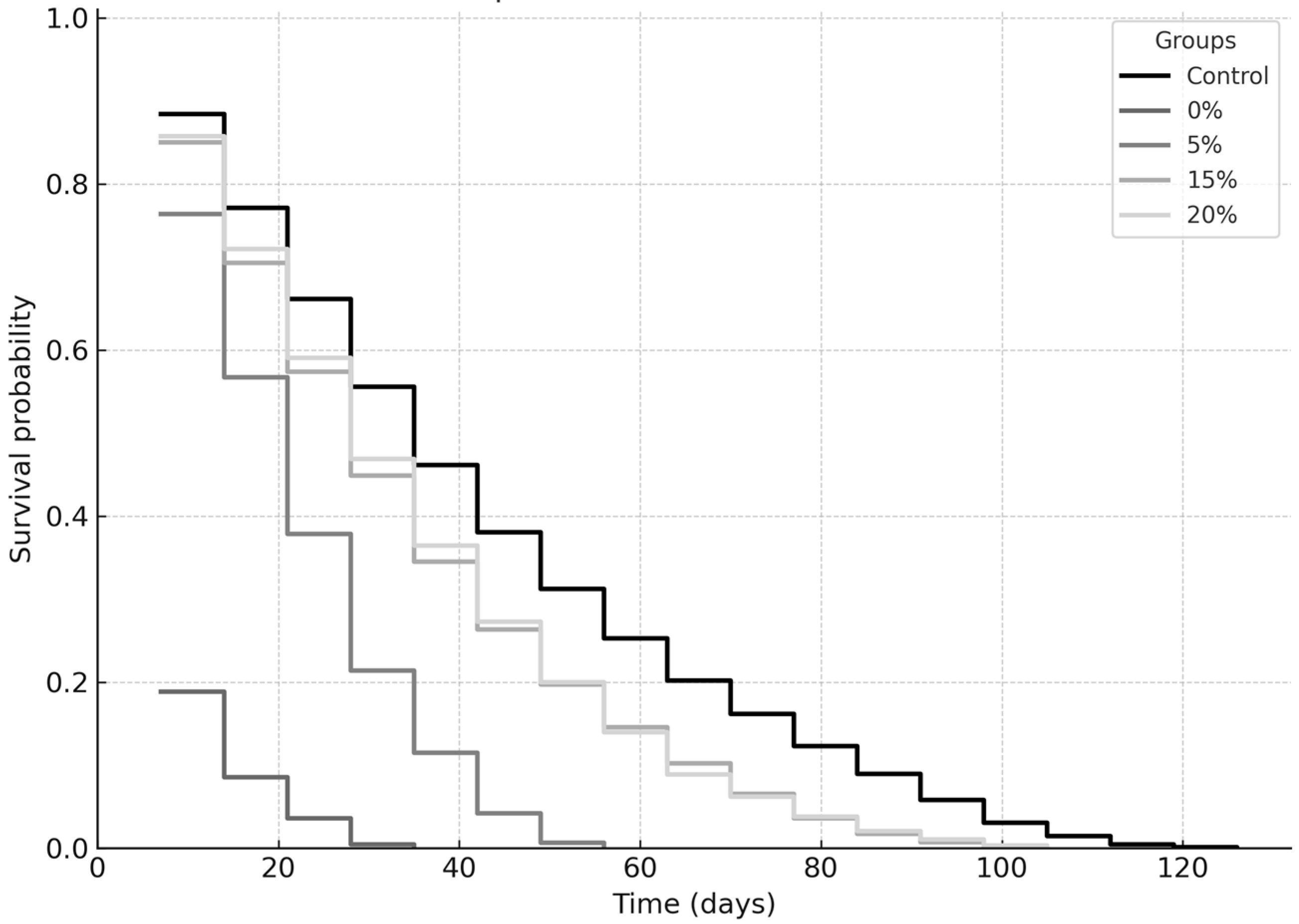
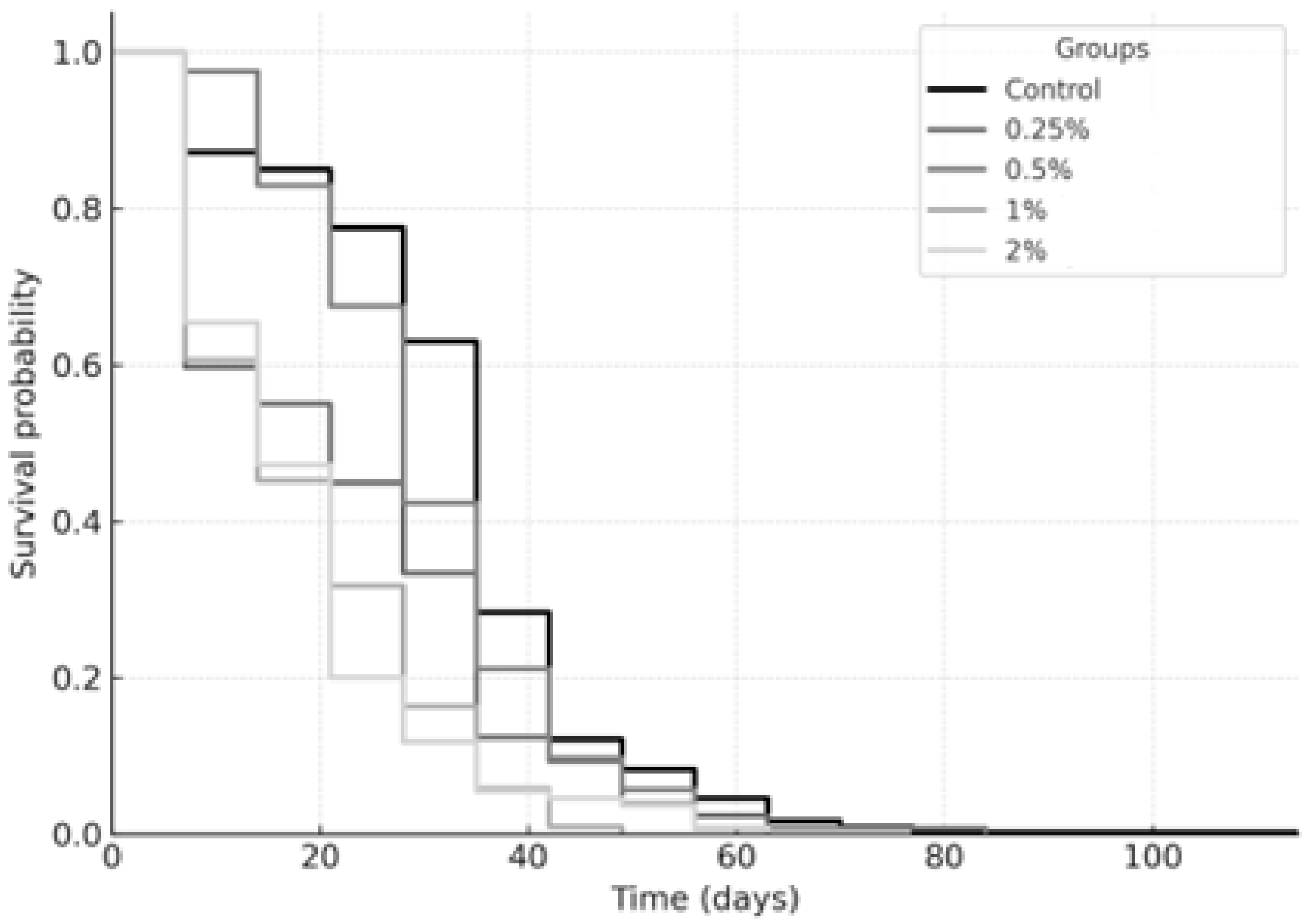
2.2. Longevity
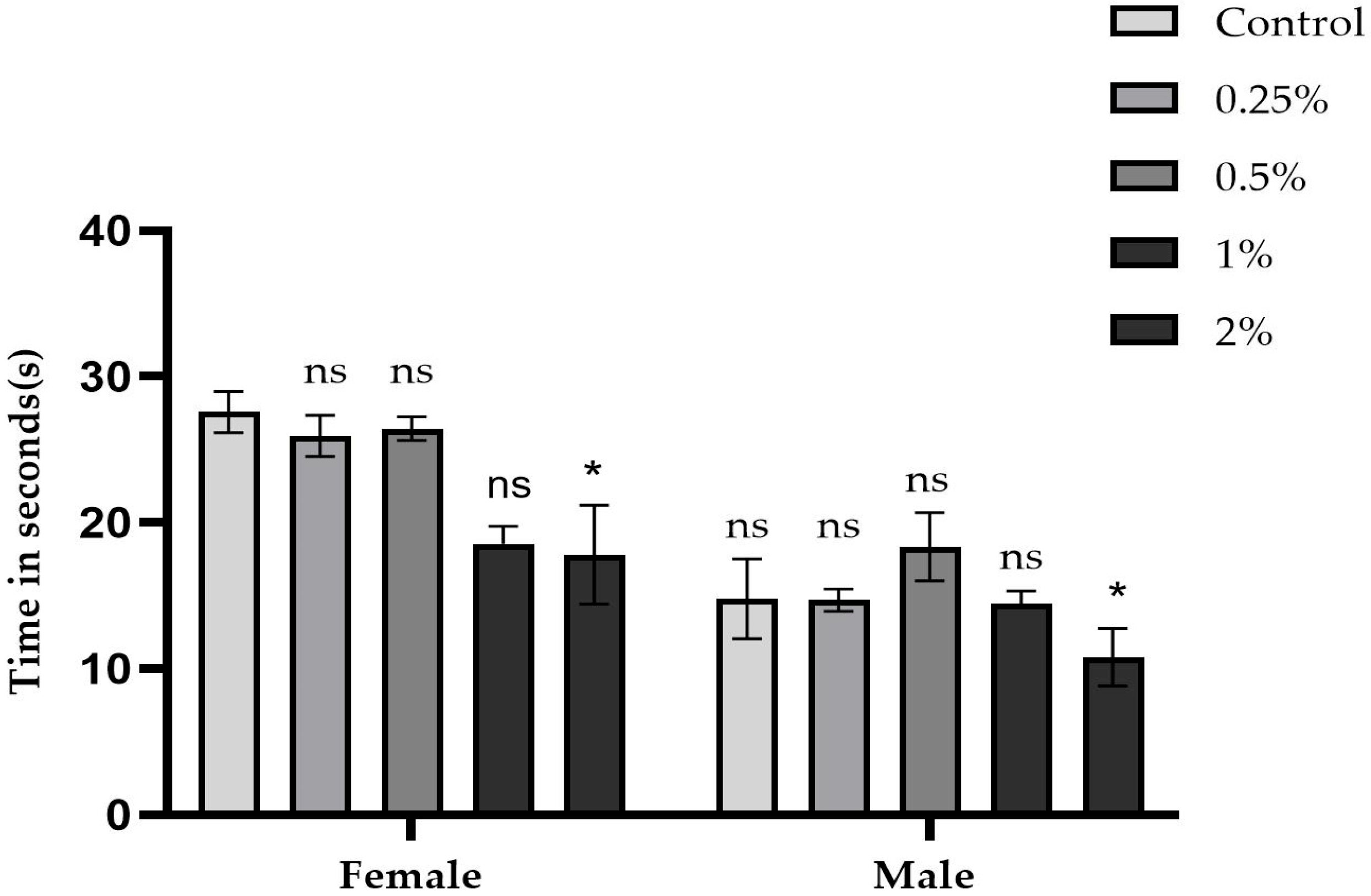
2.3. Negative Geotaxis Test
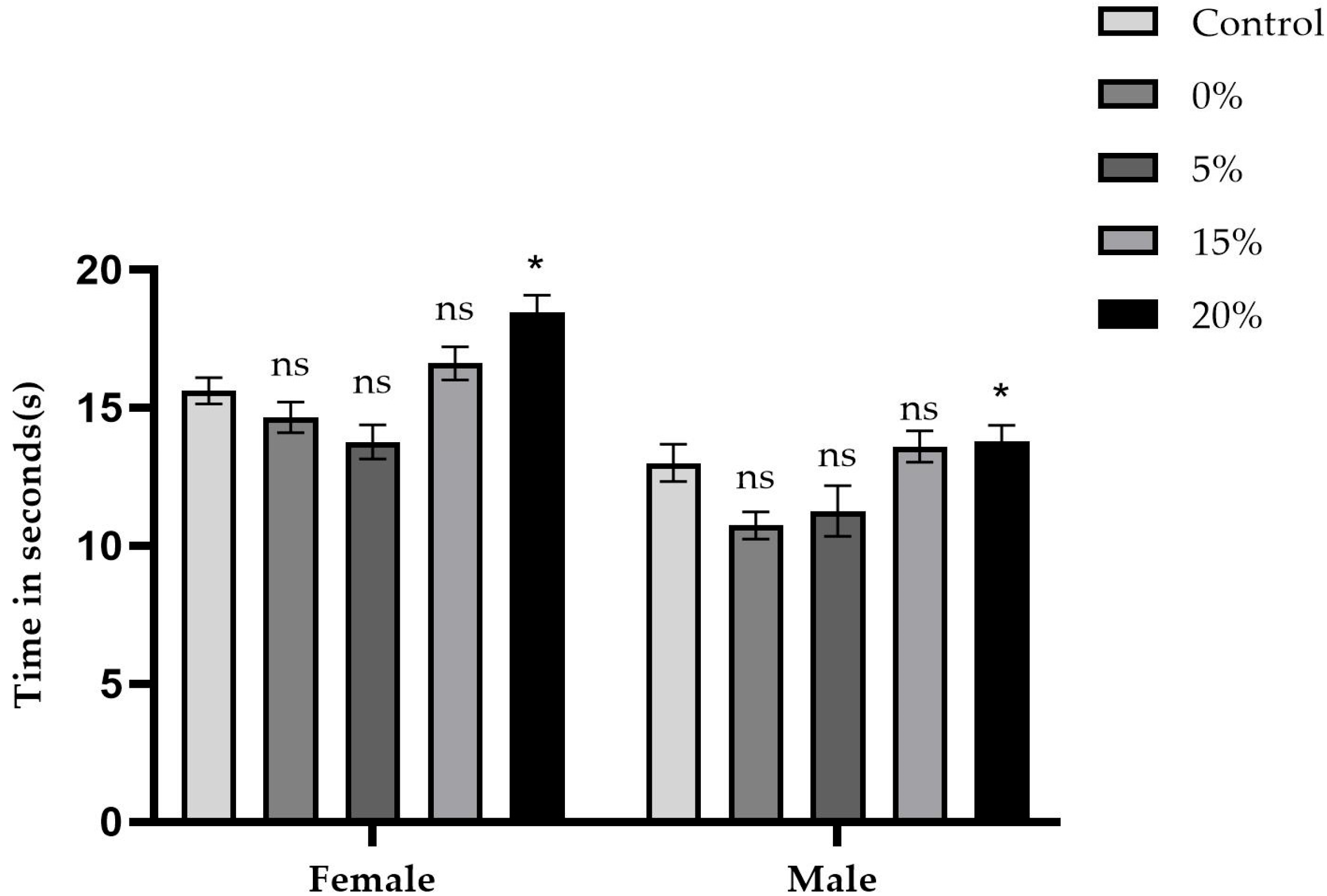
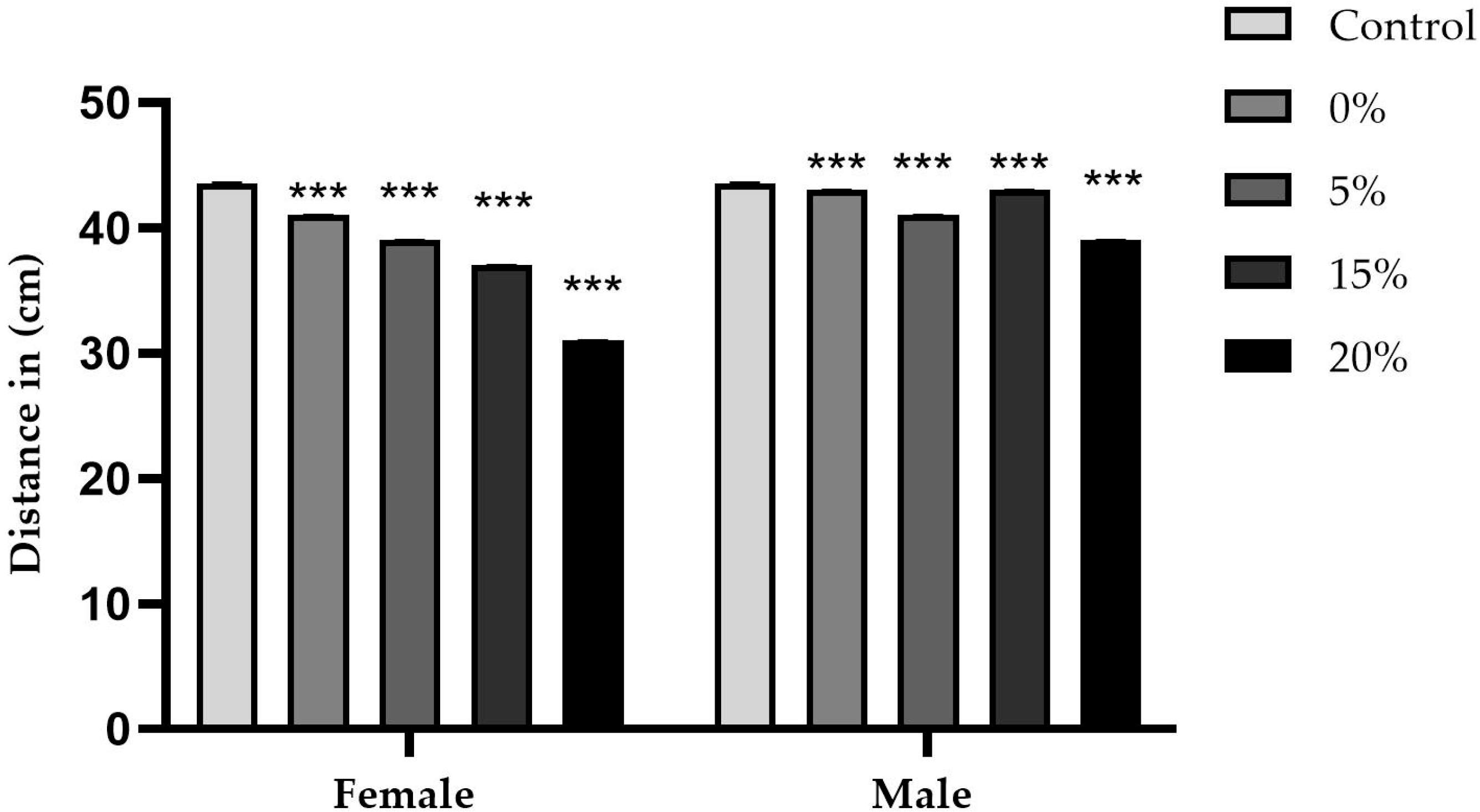
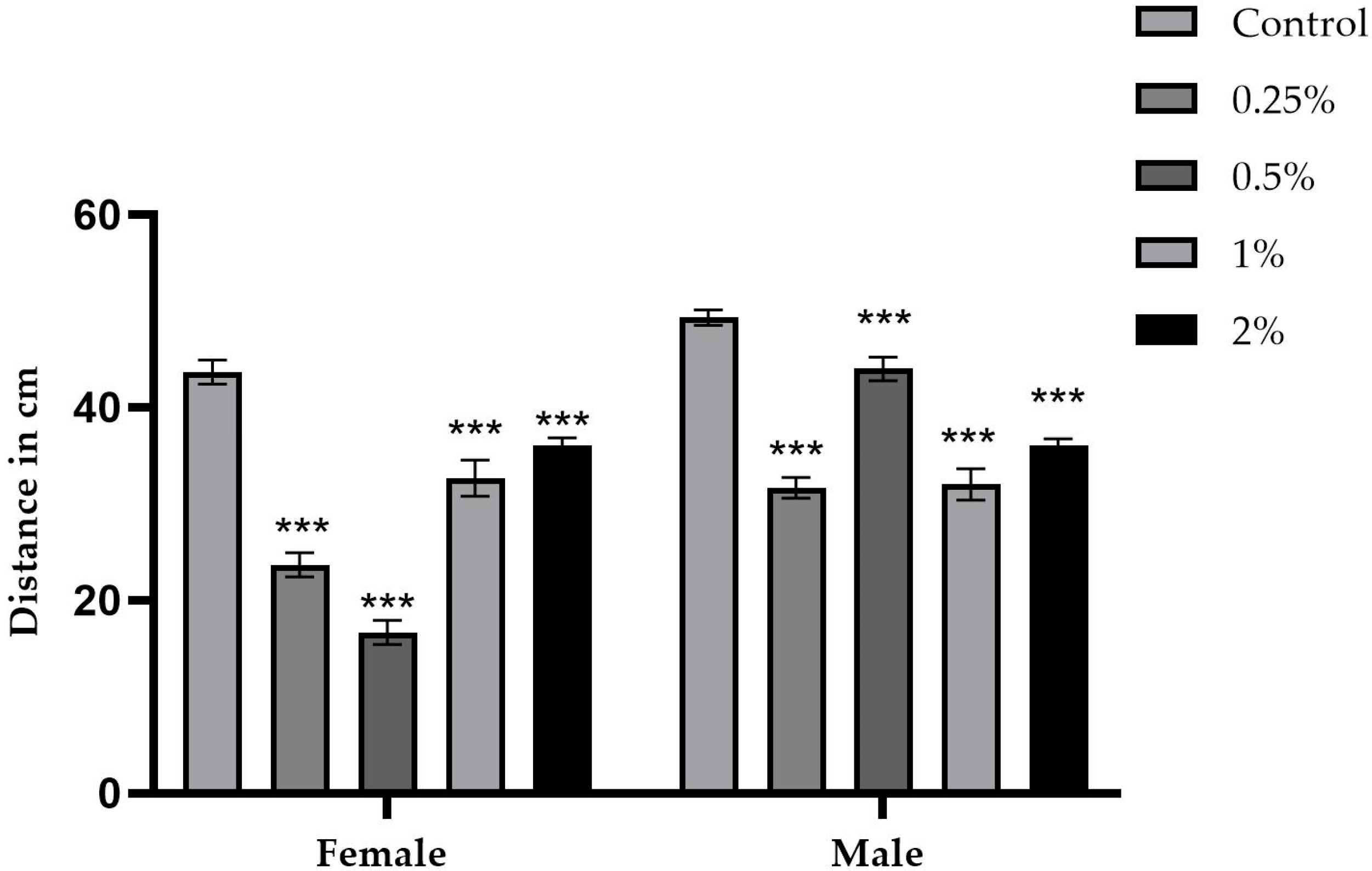
2.4. Exploration Test
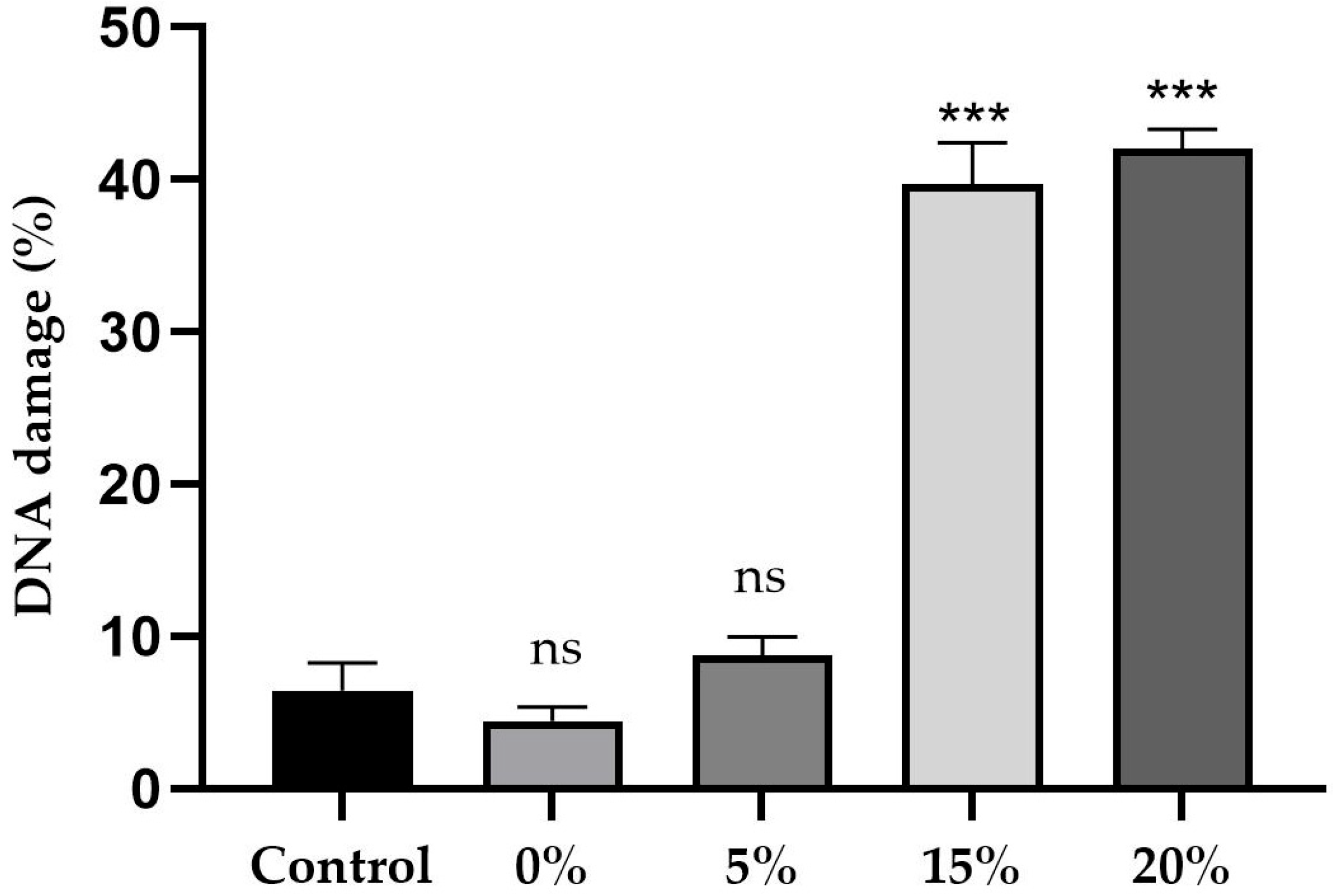
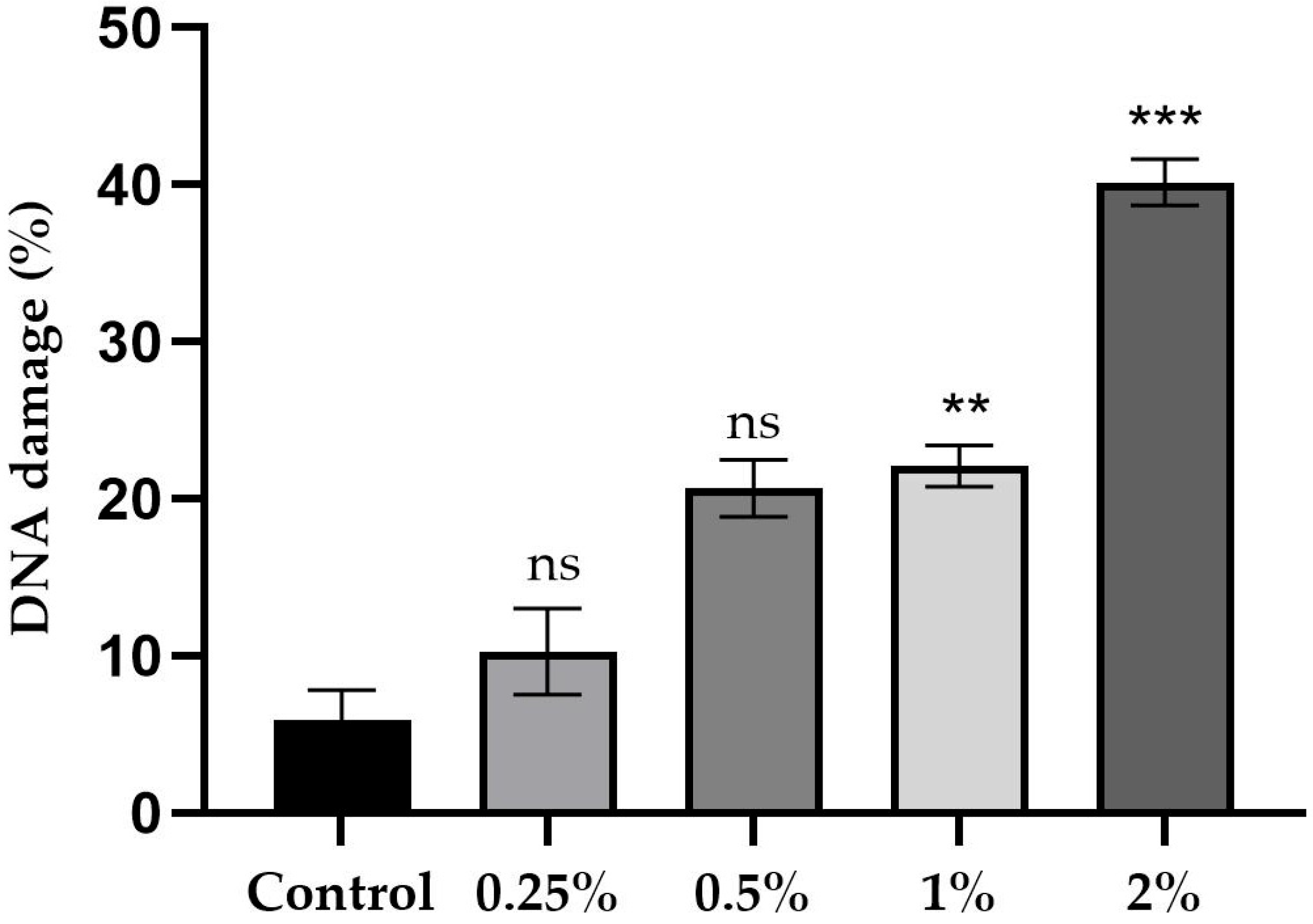
2.5. Comet Assay Results
3. Discussion
4. Materials and Methods
4.1. Strains
4.2. Culture Medium Conditions and Sample Preparation with Drosophila
4.3. Prolificacy Evaluation
4.4. Longevity Assay
4.5. Open Field Exploration Test (Evaluation of Exploratory)
4.6. Negative Geotaxis Test (Locomotion)
4.7. Comet Assay
4.8. Data Evaluation and Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Debras, C.; Chazelas, E.; Sellem, L.; Porcher, R.; Druesne-Pecollo, N.; Esseddik, Y.; de Edelenyi, F.S.; Agaësse, C.; De Sa, A.; Lutchia, R.; et al. Artificial Sweeteners and Risk of Cardiovascular Diseases: Result from the Prospective NutriNet-Sante Cohort. BMJ 2022, 378, e71204. [Google Scholar] [CrossRef]
- Juárez, G.; Sanz-Novo, M.; Alonso, J.L.; Alonso, E.R.; León, I. Rotational Spectrum and Conformational Analysis of Perillartine: Insights into the Structure—Sweetness Relationship. Molecules 2022, 27, 1924. [Google Scholar] [CrossRef]
- Xiao, Y.; Xiao, L.; Li, M.; Liu, S.; Wang, Y.; Huang, L.; Liu, S.; Jiang, T.; Zhou, L.; Li, Y. Perillartine Protects Against Metabolic Associated Fatty Liver in High Fat Diet Induced Obese Mice. Food Funct. 2023, 14, 961–977. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Jia, M.; Jiang, T.; Zhang, C.; Qi, X.; Sun, Y.; Gao, J.; Zhou, L.; Li, Y. Dietary Supplementation with Perillartine Ameliorates Lipid Metabolism Disorder Induced by a High-Fat Diet in Broiler Chickens. Biochem. Biophys. Res. Commun. 2022, 625, 66–74. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, S.; Gómez-Martínez, S.; Díaz, L.E.; Nova, E.; Urrialde, R.; Marcos, A. Potential Effects of Sucralose and Saccharin on Gut Microbiota: A Review. Nutrients 2022, 14, 1682. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://www.sciencesnail.com/science/the-synthesis-of-sucralose-from-sucrose (accessed on 10 March 2025).
- Méndez-García, L.A.; Bueno-Hernández, N.; Cid-Soto, M.A.; De León, K.L.; Mendoza-Martínez, V.M.; Espinosa-Flores, A.J.; Carrero-Aguirre, M.; Esquivel-Velázquez, M.; León-Hernández, M.; Viurcos-Sanabria, R.; et al. Ten-Week Sucralose Consumption Induces Gut Dysbiosis and Altered Glucose and Insulin Levels in Healthy Young Adults. Microorganisms 2022, 10, 434. [Google Scholar] [CrossRef]
- Huang, H.; Liu, S.; Peng, Z.; Wang, B.; Zhan, S.; Huang, S.; Li, W.; Liu, D.; Yang, X.; Zhu, Y.; et al. Comparative Effects of Different Sugar Substitutes: Mongroside V, Stevioside, Sucralose and Erythritol on Intestinal Health in a Type 2 Diabetes Mellitus Mouse. Food Funct. 2025, 16, 2108–2123. [Google Scholar] [CrossRef]
- Ashwell, M.; Gibson, S.; Bellisle, F.; Buttriss, J.; Drewnowski, A.; Fantino, M.; Gallagher, A.M.; de Graaf, K.; Goscinny, S.; Hardman, C.A.; et al. Expert consensus on low-calorie sweeteners: Facts, research gaps and suggested actions. Nutr. Res. Rev. 2020, 33, 145–154. [Google Scholar] [CrossRef]
- Plaza-Diaz, J.; Pastor-Villaescusa, B.; Rueda-Robles, A.; Abadia-Molina, F.; Ruiz-Ojeda, F.J. Plausible Biological Interactions of Low-and Non-Calorie Sweeteners with the Intestinal Microbiota: An Update of Recent Studies. Nutrients 2020, 12, 1153. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Begum, R.F.; Vijayan, S.; Vellapandian, C. Unveiling the Profound Influence of Sucralose on Metabolism and Its Role in Shaping Obesity Trends. Front. Nutr. 2024, 11, 1378646. [Google Scholar] [CrossRef]
- Aguayo-Guerrero, J.A.; Méndez-García, L.A.; Manjarrez-Reyna, A.N.; Esquivel-Velázquez, M.; León-Cabrera, S.; Meléndez, G.; Zambrano, E.; Ramos-Martínez, E.; Fragoso, J.M.; Briones-Garduño, J.C.; et al. Newborns from Mothers Who Intensely Consumed Sucralose during Pregnancy Are Heavier and Exhibit Markers of Metabolic Alteration and Low-Grade Systemic Inflammation: A Cross-Sectional, Prospective Study. Biomedicines 2023, 11, 650. [Google Scholar] [CrossRef]
- Stampe, S.; Leth-Møller, M.; Greibe, E.; Hoffmann-Lücke, E.; Pedersen, M.; Ovesen, P. Artificial Sweeteners in Breast Milk: A Clinical Investation with a Kinetical Perspective. Nutrients 2022, 14, 2635. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, Z.; Zheng, H.; Zhu, S.; Zhang, K.; Li, X.; Ma, X.; Dietrich, A.M. Sucralose, a persistent artificial sweetener in the urban water cycle: Insights into occurrence, chlorinated byproducts formation, and human exposure. J. Environ. Chem. Eng. 2021, 9, 105293. [Google Scholar] [CrossRef]
- Colín-García, K.; Elizalde-Velázquez, G.A.; Gómez-Oliván, L.M.; Islas-Flores, H.; García-Medina, S.; Galar-Martínez, M. Acute Exposure to Environmentally Relevant Concentrations of Sucralose Disrupts Embryonic Development and Leads to an Oxidative Stress in Danio Rerio. Sci. Total Environ. 2022, 829, 154689. [Google Scholar] [CrossRef]
- Mattoli, L.; Fodaroni, G.; Proietti, G.; Flamini, E.; Paoli, B.; Massa, L.; Ferrara, G.C.; Giovagnoni, E.; Gianni, M. Biodegradability of Dietary Supplements: Advanced Analytical Methods to Study the Environmental Fate of Artificial Sweeteners and Dyes. J. Pharm. Biomed. Anal. 2025, 255, 116575. [Google Scholar] [CrossRef]
- Tilg, H.; Adolph, T.E. Sucralose and Erythritol—Not Too Sweet. N. Engl. J. Med. 2023, 389, 859–861. [Google Scholar] [CrossRef]
- Schiffmann, S.; Scholl, E.; Furey, T.S.; Nagle, H.T. Toxicological and pharmacokinetic properties of sucralose-6-acetate and its parent sucralose: In vitro screening assays. J. Toxicol. Environ. Health 2023, 26, 307–341. [Google Scholar] [CrossRef]
- Blenkley, E.; Suckling, J.; Morse, S.; Murphy, R.; Raats, M.; Astley, S.; Halford, J.C.G.; Harrold, J.A.; Le-Bail, A.; Koukouna, E.; et al. Environmental life cycle assessment of production of the non-nutritive sweetener sucralose (E955) derived from cane sugar produced in the United States of America: The SWEET project. Int. J. Life Cycle Assess. 2023, 28, 1689–1704. [Google Scholar] [CrossRef]
- Bornemann, V.; Werness, S.; Buslinger, L.; Schiffman, S.S. Intestinal Metabolism and Bioaccumulation of Sucralose in Adipose Tissue in the Rat. J. Toxicol. Environ. Health Part A 2018, 81, 913–923. [Google Scholar] [CrossRef] [PubMed]
- Suez, J.; Cohen, Y.; Valdés-Mas, R.; Mor, U.; Dori-Bachash, M.; Federici, S.; Zmora, N.; Leshem, A.; Heinemann, M.; Linevsky, R.; et al. Personalized Microbiom-Driven Effects of Non-Nutritive Sweeteners on Human Glucose Tolerance. Cell 2022, 185, 3307–3328. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Barlow, G.M.; Leite, G.; Rashid, M.; Parodi, G.; Wang, J.; Morales, W.; Weitsman, S.; Rezaie, A.; Pimentel, M.; et al. Consuming Artificial Sweeteners May Alter the Structure and Function of Duodenal Microbial Communities. iScience 2023, 26, 108530. [Google Scholar] [CrossRef]
- Jarmakiewicz-Czaja, S.; Sokal-Dembowska, A.; Filip, R. Effects of Selected Food Additives on the Gut Microbiome and Metabolic Disfunction-Associated Steatotic Liver Disease (MASLD). Medicina 2025, 61, 192. [Google Scholar] [CrossRef] [PubMed]
- Harpaz, D.; Yeo, L.P.; Cecchini, F.; Koon, T.H.P.; Kushmaro, A.; Tok, A.I.Y.; Marks, R.S.; Eltzov, E. Measuring Artificial Sweeteners Toxicity Using a Bioluminescent Bacterial Panel. Molecules 2018, 23, 2454. [Google Scholar] [CrossRef]
- Ahmad, S.Y.; Friel, J.K.; Mackay, D.S. Effect of sucralose and aspartame on glucose metabolism and gut hormones. Nutr. Rev. 2020, 78, 725–746. [Google Scholar] [CrossRef]
- Zani, F.; Blagih, J.; Gruber, T.; Buck, M.D.; Jones, N.; Hennequart, M.; Newell, C.L.; Pilley, S.E.; Soro-Barrio, P.; Kelly, G.; et al. The Dietary Sweetener Sucralose is a Negative Modulator of T-Cell-Mediated Response. Nature 2023, 615, 705–711. [Google Scholar] [CrossRef]
- Risdon, S.; Battault, S.; Romo-Romo, A.; Roustit, M.; Briand, L.; Meyer, G.; Almeda-Valdes, P.; Walther, G. Sucralose and Cardiometabolic Health: Current Understanding from Receptors to Clinical Investigations. Adv. Nutr. 2021, 12, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Bai, D.; Yang, H.; Li, X.; Zhu, D.; Cao, X.; Ma, H.; Li, X.; Zheng, X. Hazardous Effects of Sucralose and its Disinfection Byproducts Identified From an E. coli Whole-Cell Array Analysis. Front. Environ. Sci. 2021, 9, 724685. [Google Scholar] [CrossRef]
- dos Santos, C.A.; Pedrosa, D.S.; Da Silva Santos, M. Adoçantes Sintéticos e Seus Riscos à Saúde. Rev. Ciên. Saúde 2023, 27, 122. (In Portuguese) [Google Scholar] [CrossRef]
- Aguayo-Guerrero, J.A.; Méndez-García, L.A.; Solleiro-Villavicencio, H.; Viurcos-Sanabria, R.; Escobedo, G. Sucralose: From Sweet Success to Metabolic Controversies—Unraveling the Global Health Implications of a Pervasive Non-Caloric Artificial Sweetener. Life 2024, 14, 323. [Google Scholar] [CrossRef]
- Hu, J.; Feng, J.; Bai, Y.; Yao, Z.-S.; Wu, X.-Y.; Hong, X.-Y.; Lu, G.D.; Xue, K. Sucralose Promotes Benzo(a)Pyrene-Induced Renal Toxicity in Mice by Regulating P-Glycoprotein. Antioxidants 2025, 14, 474. [Google Scholar] [CrossRef]
- Shil, A.; Olusanya, O.; Ghufoor, Z.; Forson, B.; Marks, J.; Chichger, H. Artificial Sweeteners Disrupt Tight Junctions and Barrier Function in the Intestinal Epithelium through Activation of the Sweet Taste Receptor, T1R3. Nutrients 2020, 12, 1862. [Google Scholar] [CrossRef]
- Yan, X.; Chen, Z.; Rong, X.; Chen, Z.; Wu, G.; Dong, Z.; Fu, Y.; Hai, T. The Impact of Sucralose and Neotame on the Safety of Metal Precipitation in Electronic Cigarettes. Front. Physiol. 2024, 15, 1437042. [Google Scholar] [CrossRef]
- Moser, D.; Leitner, P.; Filipek, P.; Hussain, S.; Rainer, M.; Jakschitz, T.; Rode, B.; Bonn, G.K. Quantification and Cytotoxicity of Degradation Products (Chloropropanols) in Sucralose Containing E-Liquids with Propylene Glycol and Glycerol as Base. Toxicol. Appl. Pharmacol. 2021, 430, 115727. [Google Scholar] [CrossRef] [PubMed]
- Haq, N.; Saqib, S.; Tafweez, R.; Ali, I.; Syami, A.F. Effects of Artificial Sweeteners Aspartame and Sucralose on the Size of Hepatocytes in Rat Liver. PJMHS 2022, 16, 359–362. [Google Scholar] [CrossRef]
- Wu, H.-T.; Lin, C.-H.; Pai, H.-L.; Chen, Y.-C.; Cheng, K.-P.; Kuo, H.-Y.; Li, C.-H.; Ou, H.-Y. Sucralose, a Non-nutritive Artificial Sweetener Exacerbates High Fat Diet-Induced Hepatic Steatosis Through Taste Receptor Type 1 Member 3. Front. Nutr. 2022, 9, 823723. [Google Scholar] [CrossRef]
- Borquez, J.C.; Hidalgo, M.; Rodriguez, J.M.; Montaña, A.; Porras, O.; Troncoso, R.; Bravo-Sagua, R. Sucralose Stimulates Mitochondrial Bioenergetics in Caco-2 Cells. Front. Nutr. 2021, 7, 585484. [Google Scholar] [CrossRef]
- Heredia-García, G.; Gómez-Oliván, L.M.; Orozco-Hernández, J.M.; Luja-Mondragón, M.; Islas-Flores, H.; SanJuan-Reyes, N.; Galar-Martínez, M.; García-Medina, S.; Dublán-García, O. Alterations to DNA, apoptosis and oxidative damage induced by sucralose in blood cells of Cyprinus carpio. Sci. Total Environ. 2019, 692, 411–421. [Google Scholar] [CrossRef] [PubMed]
- El-Tahan, H.M.; Elmasry, M.E.; Madian, H.A.; Alhimaidi, A.R.; Kim, I.H.; Park, J.H.; El-Tahan, H.M. Sucralose Influences the Productive Performance, Carcass Traits, Blood Components, and Gut Microflora Using 16S rRNA Sequencing of Growing APRI-Line Rabbits. Animals 2024, 14, 1925. [Google Scholar] [CrossRef]
- Stepanov, I.; Berman, M.; Brinkman, M.C.; Carll, A.; Exil, V.; Hansen, E.G.; El Hellani, A.; Jabba, S.V.; Kassem, N.O.F.; Rezk-Hanna, M.; et al. Sugars in Tobacco Products: Toxicity Research and Implications for Tobacco Product Regulation. Chem. Res. Toxicol. 2025, 38, 747–758. [Google Scholar] [CrossRef]
- Kaur, K.; Simon, A.F.; Chauhan, V.; Chauhan, A. Effect of bisphenol A on Drosophila melanogaster behavior—A new model for the studies on neurodevelopmental disorders. Behav. Brain Res. 2015, 284, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Wang, F.; Wen, D.; Mu, R. Exposure to Bisphenol A Induced Oxidative Stress, Cell Death and Impaired Epithelial Homeostasis in the Adult Drosophila melanogaster Midgut. Ecotoxicol. Environ. Safety 2022, 248, 114285. [Google Scholar] [CrossRef]
- Adesanoye, O.A.; Abolaji, A.O.; Faloye, T.R.; Olaoye, H.O.; Adedara, A.O. Luteolin/Supplemented Diets Ameliorates Bisphenol A-Induced Toxicity in Drosophila melanogaster. Food Chem. Toxicol. 2020, 142, 111478. [Google Scholar] [CrossRef]
- Sarkar, A.; Mahendran, T.S.; Meenakshisundaram, A.; Christopher, R.V.; Dan, P.; Sundararajan, V.; Jana, N.; Venkatasubbu, D.; Mohideen, S.S. Role of Cerium Oxide Nanoparticles in Improving Oxidative Stress and Developmental Delays in Drosophila melanogaster as an In Vivo Model for Bisphenol A Toxicity. Chemosphere 2021, 284, 131363. [Google Scholar] [CrossRef]
- Musachio, E.A.S.; de Freitas Couto, S.; Poetini, M.R.; Bortolotto, V.C.; Dahleh, M.M.M.; Janner, D.E.; Araujo, S.M.; Ramborger, B.P.; Rohers, R.; Guerra, G.P.; et al. Bisphenol A Exposure During the Embryonic Period: Insights into Dopamine Relationship and Behavioral Disorders in Drosophila melanogaster. Food Chem. Toxicol. 2021, 157, 112526. [Google Scholar] [CrossRef]
- Nguyen, U.; Tinsley, B.; Sen, Y.; Stein, J.; Palacios, Y.; Ceballos, A.; Welch, C.; Nzenkue, K.; Penn, A.; Murphy, L.; et al. Exposure to Bisphenol A Differentially Impacts Neurodevelopment and Behavior in Drosophila melanogaster from Distinct Genetic Backgrounds. Neurotoxicology 2021, 82, 146–157. [Google Scholar] [CrossRef]
- Begum, M.; Pallab, P.; Das, D.; Ghosh, S. Endocrine-Disrupting Plasticizer Bisphenol A Exposure Causes Changes in Behavioral Attributes in Drosophila melanogaster. Toxicol. Environ. Health Sci. 2020, 12, 237–246. [Google Scholar] [CrossRef]
- Ramadhan, M.J.; Kharomah, S.; Zahrah, N.A.; Maghfiroh, H.; Fahmi, M.I.N.; Zubaidah, S.; Fauzi, A. Negative Geotaxis Assay in Three Drosophila Strains Consuming Bisphenol A: Duration and Number of Climbing Successes. IOP Conf. Ser. Earth Environ. Sci. 2021, 1439, 012010. [Google Scholar] [CrossRef]
- Fishburn, J.L.; Larson, H.L.; Nguyen, A.; Welch, C.J.; Moore, T.; Penn, A.; Newman, J.; Mangino, A.; Widman, E.; Ghobashy, R.; et al. Bisphenol F Affects Neurodevelopmental Gene Expression, Mushroom Body Development and Behavior in Drosophila melanogaster. Neurotoxicol. Teratol. 2024, 102, 107331. [Google Scholar] [CrossRef]
- Rani, L.; Saini, S.; Thakur, R.S.; Patel, D.K.; Chowdhuri, D.K.; Gautam, N.K. Single and Combined Effect of Bisphenol A with High Sucrose Diet on the Diabetic and Renal Tubular Dysfunction Phenotypes in Drosophila melanogaster. Environ. Toxicol. Pharmacol. 2022, 96, 103977. [Google Scholar] [CrossRef] [PubMed]
- Skorupa, D.A.; Dervisefendic, A.; Zwiener, J.; Pletcher, S.D. Dietary composition specifies consumption, obesity, and lifespan in Drosophila melanogaster. Aging Cell 2008, 7, 478–490. [Google Scholar] [CrossRef]
- Piper, M.D.W.; Partridge, L. Dietary restriction in Drosophila: Delayed aging or experimental artefact? PLoS Genet. 2007, 3, e57. [Google Scholar] [CrossRef]
- Pepino, M.Y. Metabolic effects of non-nutritive sweeteners. Physiol. Behav. 2015, 152, 450–455. [Google Scholar] [CrossRef]
- Rogers, P.J.; Appleton, K.M. The effects of low-calorie sweeteners on energy intake and body weight: A systematic review and meta-analyses of sustained intervention studies. Int. J. Obes. 2021, 45, 464–478. [Google Scholar] [CrossRef]
- Partridge, L.; Gems, D.; Withers, D.J. Sex and death: What is the connection? Cell 2005, 120, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Katewa, S.D.; Kapahi, P. Dietary restriction and aging. Aging Cell 2010, 9, 105–112. [Google Scholar] [CrossRef]
- Abou-Donia, M.B.; El-Masry, E.M.; Abdel-Rahman, A.A.; McLendon, R.E.; Schiffman, S.S. Splenda alters gut microflora and increases intestinal p-glycoprotein and cytochrome P-450 in male rats. J. Toxicol. Environ. Health Part A 2008, 71, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Chen, S.; Dai, Y.; Duan, T.; Xu, Y.; Li, X.; Yang, J.; Zhu, X. Aspartame and sucralose extend the lifespan and improve the health status of C. elegans. Food Funct. 2021, 12, 9912–9921. [Google Scholar] [CrossRef] [PubMed]
- Gaivão, I.; Santos, R.A.; Morozova, T.V.; Tkach, V.V. Biological and Behavioural Effects of Bisphenol A (BPA) Exposure: An In Vivo Study in Drosophila Melanogaster. Appl. Sci. 2025, 15, 5588. [Google Scholar] [CrossRef]
- Musselman, L.P.; Fink, J.L.; Narzinski, K.; Ramachandran, P.V.; Hathiramani, S.S.; Cagan, R.L.; Baranski, T.J. A high-sugar diet produces obesity and insulin resistance in wild-type Drosophila. Dis. Models Mech. 2011, 4, 842–849. [Google Scholar] [CrossRef]
- Schiffman, S.S.; Rother, K.I. Sucralose, a synthetic organochlorine sweetener: Overview of biological issues. J. Toxicol. Environ. Health Part B Crit. Rev. 2013, 16, 399–451. [Google Scholar] [CrossRef]
- Bornemann, V.; Wörner, T.; Fiedler, H.; Weidauer, A.; Poetzsch, M.; Huetteroth, W. Chronic exposure to sucralose induces oxidative stress and DNA damage in human colon cells. Toxicol. Lett. 2023, 377, 39–50. [Google Scholar] [CrossRef]
- Atalay, E.Ç.; Demirhan, B.E.; Celep, A.G.S. Low-Calorie Sweeteners and Reproductive Health: Evidence and Debates. Curr. Nutr. Food Sci. 2025, 21, 309–332. [Google Scholar] [CrossRef]
- Pasqualli, T.; Chaves, P.E.E.; Pereira, L.V.; Serpa, E.A.; Souza de Oliveira, L.F.; Mansur Machado, M. Sucralose causes non-selective CD4 and CD8 lymphotoxicity via probable regulation of the MAPK8/APTX/EID1 genes: An in vitro/in silico study. Clin. Exp. Pharmacol. Physiol. 2020, 47, 1751–1757. [Google Scholar] [CrossRef]
- Rizk, R.M.; Soliman, M.I.; Rashed, A.A. Potential Mutagenicity of Some Artificial Sweeteners Using Allium Test. Asian J. Adv. Basic Sci. 2016, 4, 27–40. [Google Scholar]
- Samoilov, A.V.; Suraeva, N.M.; Zaitseva, M.V.; Kurbanova, M.N.; Stolbova, V.V. Comparative Assessment of Artificial Sweeteners Toxicity via Express Biotest. Health Risk Anal. 2019, 2019, 83–90. [Google Scholar] [CrossRef]
- Soffritti, M.; Padovani, M.; Tibaldi, E.; Falcioni, L.; Manservisi, F.; Lauriola, M.; Bua, L.; Manservigi, M.; Belpoggi, F. Sucralose administered in feed, beginning prenatally through lifespan, induces hematopoietic neoplasias in male swiss mice. Int. J. Occup. Environ. Health 2016, 22, 7–17. [Google Scholar] [CrossRef]
- Rodríguez, R.; Gaivão, I.; Aguado, L.; Espina, M.; Garcia, J.; Martínez-Camblor, P.; Sierra, L.M. The Comet Assay in Drosophila: A Tool to Study Interactions between DNA Repair Systems in DNA Damage Responses In Vivo and Ex Vivo. Cells 2023, 12, 1979. [Google Scholar] [CrossRef]
- Gaivão, I.; Comendador, M.A. The w/w+ somatic mutation and recombination test (SMART) of Drosophila melanogaster for detecting reactive oxygen species: Characterization of 6 strains. Mutat. Res. 1996, 360, 145–151. [Google Scholar] [CrossRef]
- Kacsoh, B.Z.; Lynch, Z.R.; Mortimer, N.T.; Schlenke, T.A. Fruit flies medicate offspring after seeing parasites. Science 2015, 347, 1036–1039. [Google Scholar] [CrossRef] [PubMed]
- Hevia, A.; Delgado, S.; Sánchez, B.; Margolles, A. Molecular players involved in the interaction between beneficial bacteria and the immune system. Front. Microbiol. 2015, 6, 1285. [Google Scholar] [CrossRef] [PubMed]
- Han, S.K.; Lee, D.; Lee, H.; Kim, D.; Son, H.G.; Yang, J.-S.; Lee, S.-J.V.; Kim, S. OASIS 2: Online application for survival analysis 2 with features for the analysis of maximal lifespan and healthspan in aging research. Oncotarget 2016, 7, 35. [Google Scholar] [CrossRef]
- Woods, J.K.; Kowalski, S.; Rogina, B. Determination of the Spontaneous Locomotor Activity in Drosophila melanogaster. J. Vis. Exp. 2014, 86, 51449. [Google Scholar] [CrossRef]
- Nichols, C.D.; Becnel, J.; Pandey, U.B. Methods to Assay Drosophila Behavior. JoVE Neurosci. 2012, 61, 3795. [Google Scholar] [CrossRef]
- Gargano, J.W.; Martin, I.; Bhandari, P.; Grotewiel, M.S. Rapid iterative negative geotaxis (RING): A new method for assessing age-related locomotor decline in Drosophila. Exp. Gerontol. 2005, 40, 386–395. [Google Scholar] [CrossRef] [PubMed]
- Collins, A.R.; Oscoz, A.A.; Brunborg, G.; Gaivao, I.; Giovannelli, L.; Kruszewski, M.; Smith, C.C.; Stetina, R. The comet assay: Topical issues. Mutagenesis 2008, 23, 143–151. [Google Scholar] [CrossRef]
- Azqueta, A.; Meier, S.; Priestley, C.; Gutzkow, K.B.; Brunborg, G.; Sallette, J.; Soussaline, F.; Collins, A. The influence of scoring method on variability in results obtained with the comet assay. Mutagenesis 2011, 26, 393–399. [Google Scholar] [CrossRef]
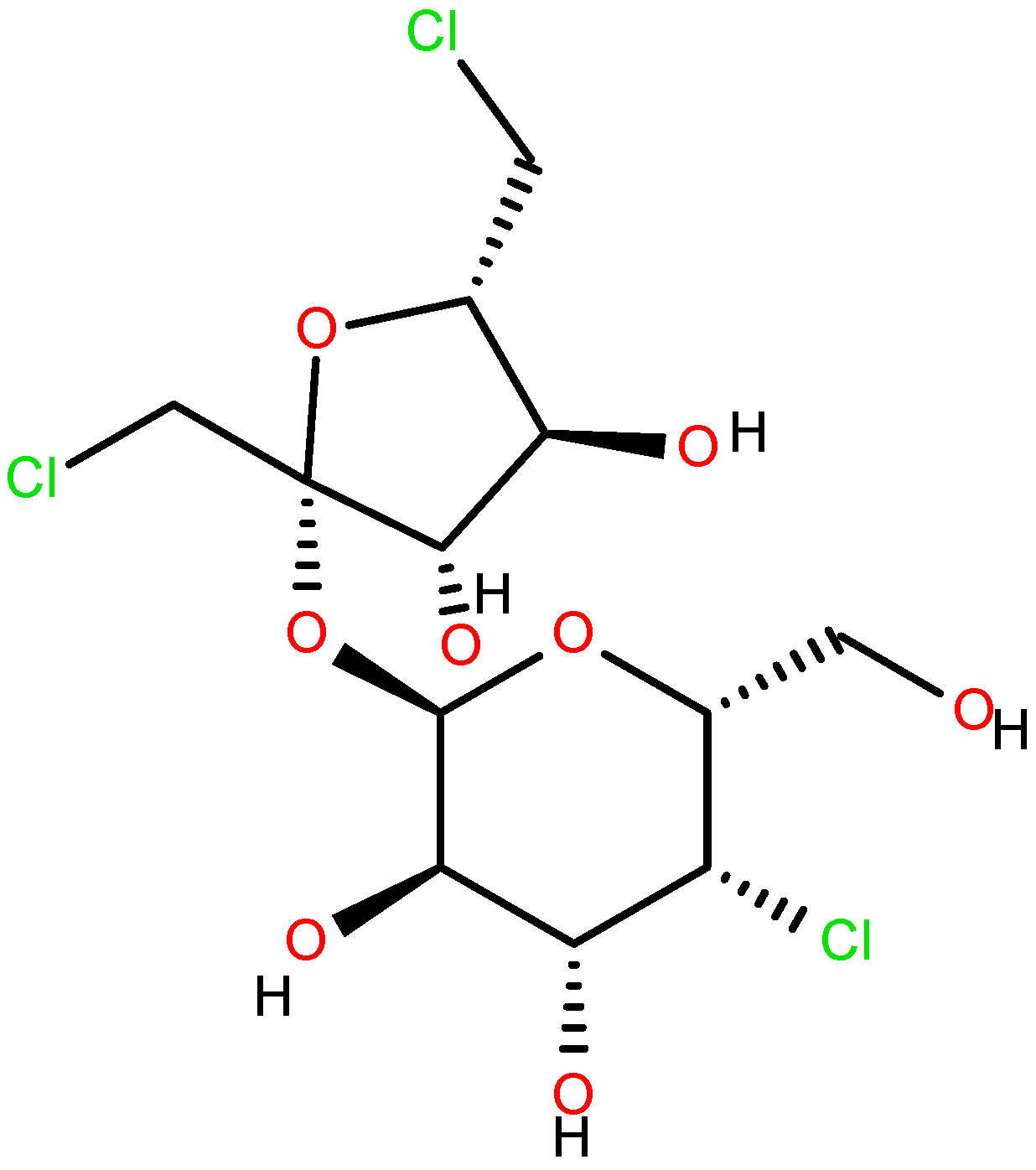
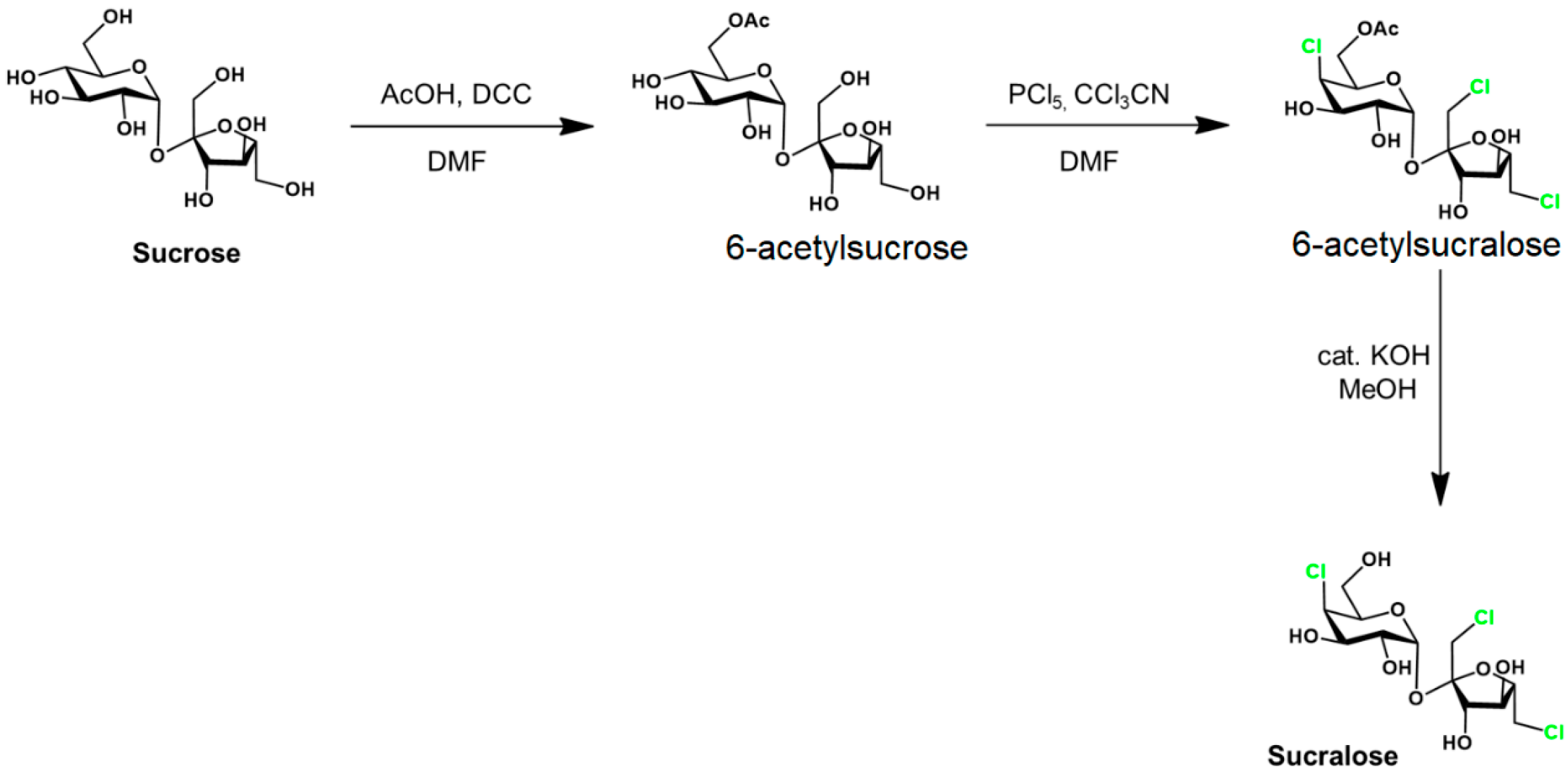
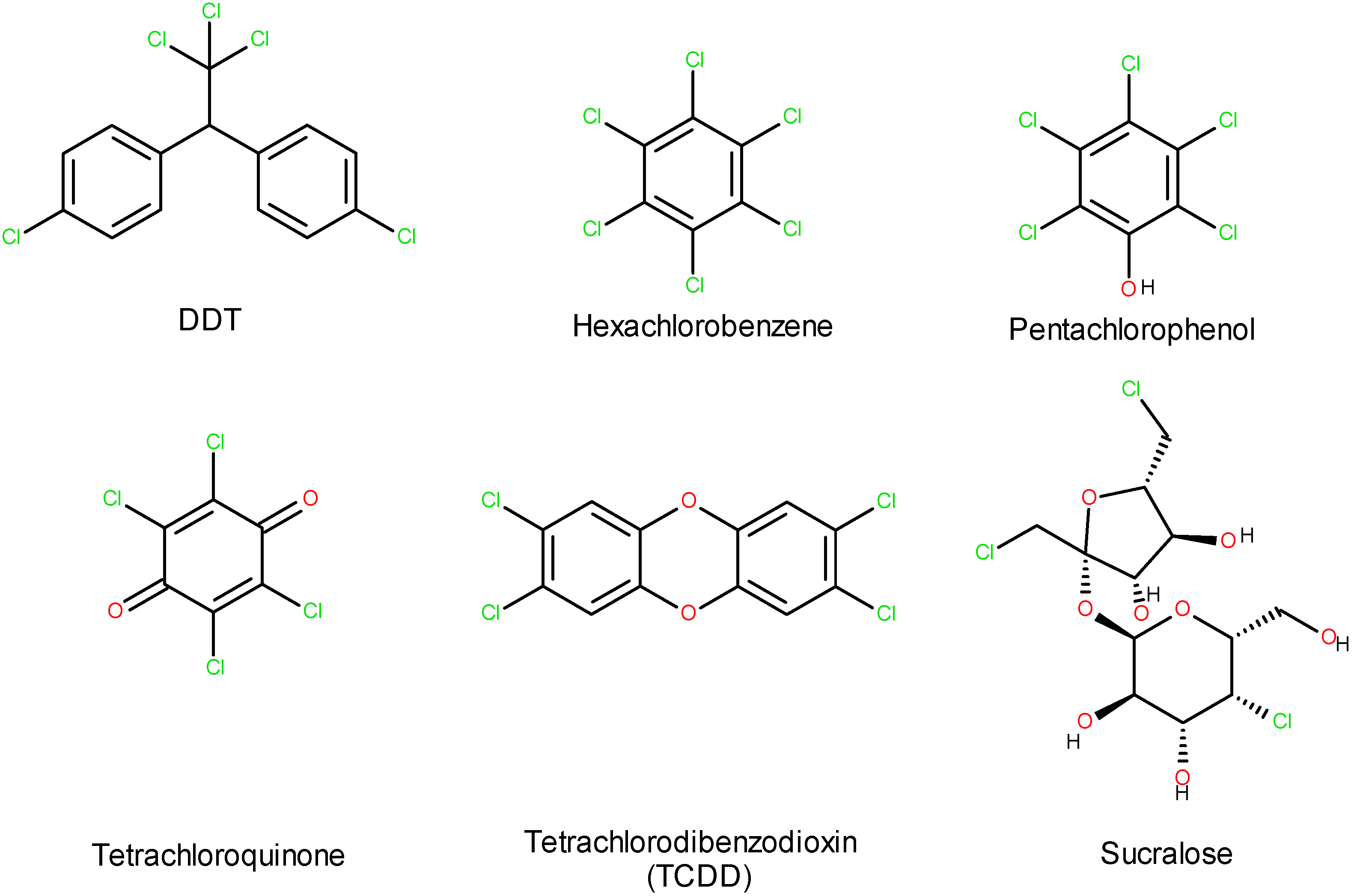
| Ingredients | Amount (Per Liter in Distilled Water) |
|---|---|
| Sucrose | 100 g |
| Agar-agar | 12 g |
| Inactive yeast | 100 g |
| Propionic acid | 5 mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miranda, N.; Tkach, V.V.; Barros, A.N.; Martins-Bessa, A.; Gaivão, I. Biological and Behavioral Responses of Drosophila melanogaster to Dietary Sugar and Sucralose. Int. J. Mol. Sci. 2025, 26, 8951. https://doi.org/10.3390/ijms26188951
Miranda N, Tkach VV, Barros AN, Martins-Bessa A, Gaivão I. Biological and Behavioral Responses of Drosophila melanogaster to Dietary Sugar and Sucralose. International Journal of Molecular Sciences. 2025; 26(18):8951. https://doi.org/10.3390/ijms26188951
Chicago/Turabian StyleMiranda, Natasha, Volodymyr V. Tkach, Ana Novo Barros, Ana Martins-Bessa, and Isabel Gaivão. 2025. "Biological and Behavioral Responses of Drosophila melanogaster to Dietary Sugar and Sucralose" International Journal of Molecular Sciences 26, no. 18: 8951. https://doi.org/10.3390/ijms26188951
APA StyleMiranda, N., Tkach, V. V., Barros, A. N., Martins-Bessa, A., & Gaivão, I. (2025). Biological and Behavioral Responses of Drosophila melanogaster to Dietary Sugar and Sucralose. International Journal of Molecular Sciences, 26(18), 8951. https://doi.org/10.3390/ijms26188951








