Transcriptome Analysis of Different Chinese Cabbage Varieties Under Cd and Pb Stresses
Abstract
1. Introduction
2. Results
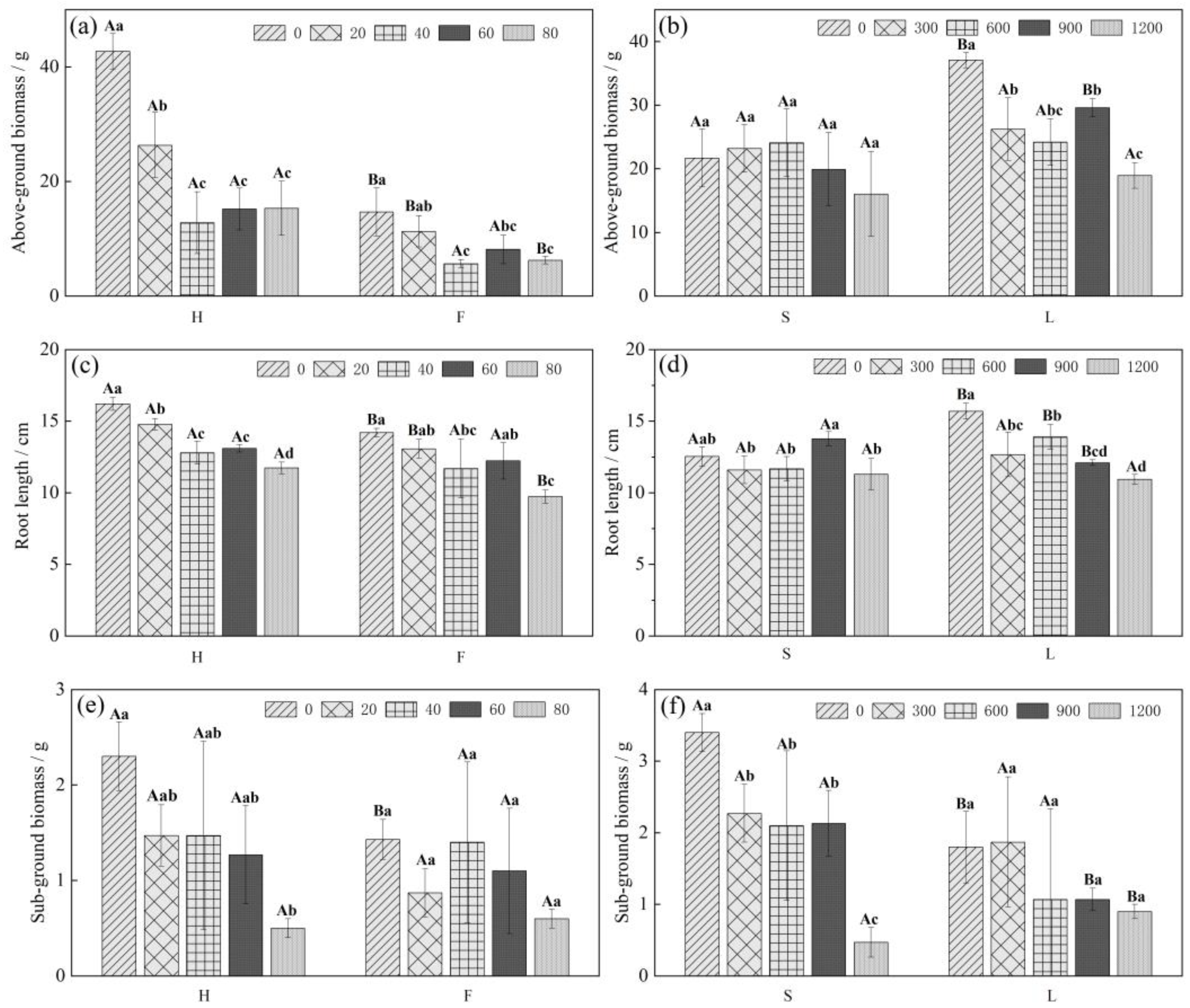
2.1. Effects of Cd and Pb Stress on the Growth Indices of Chinese Cabbage
2.2. Cd and Pb Accumulation in Different Chinese Cabbage Varieties
2.3. Cd and Pb Bioconcentration Factor (BCF) and Transfer Factor (TF) of Different Varieties of Chinese Cabbage
2.4. Quality Assessment of Sequencing Results and Statistics for DEGs
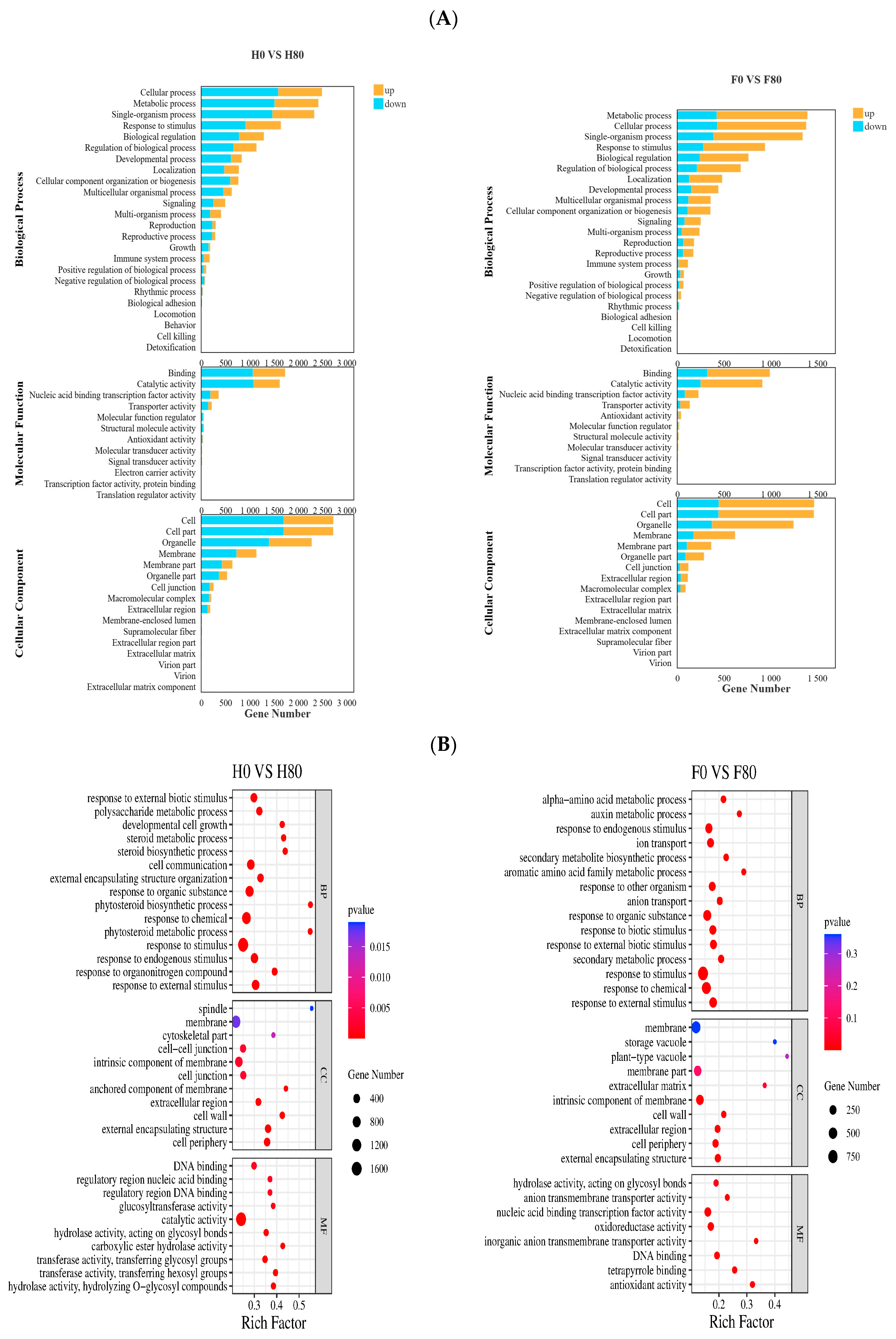
2.5. Gene Ontology (GO) Enrichment Analysis of DEGs
2.5.1. GO Enrichment Analysis of DEGs Under Cd Stress
2.5.2. GO Enrichment Analysis of DEGs Under Pb Stress
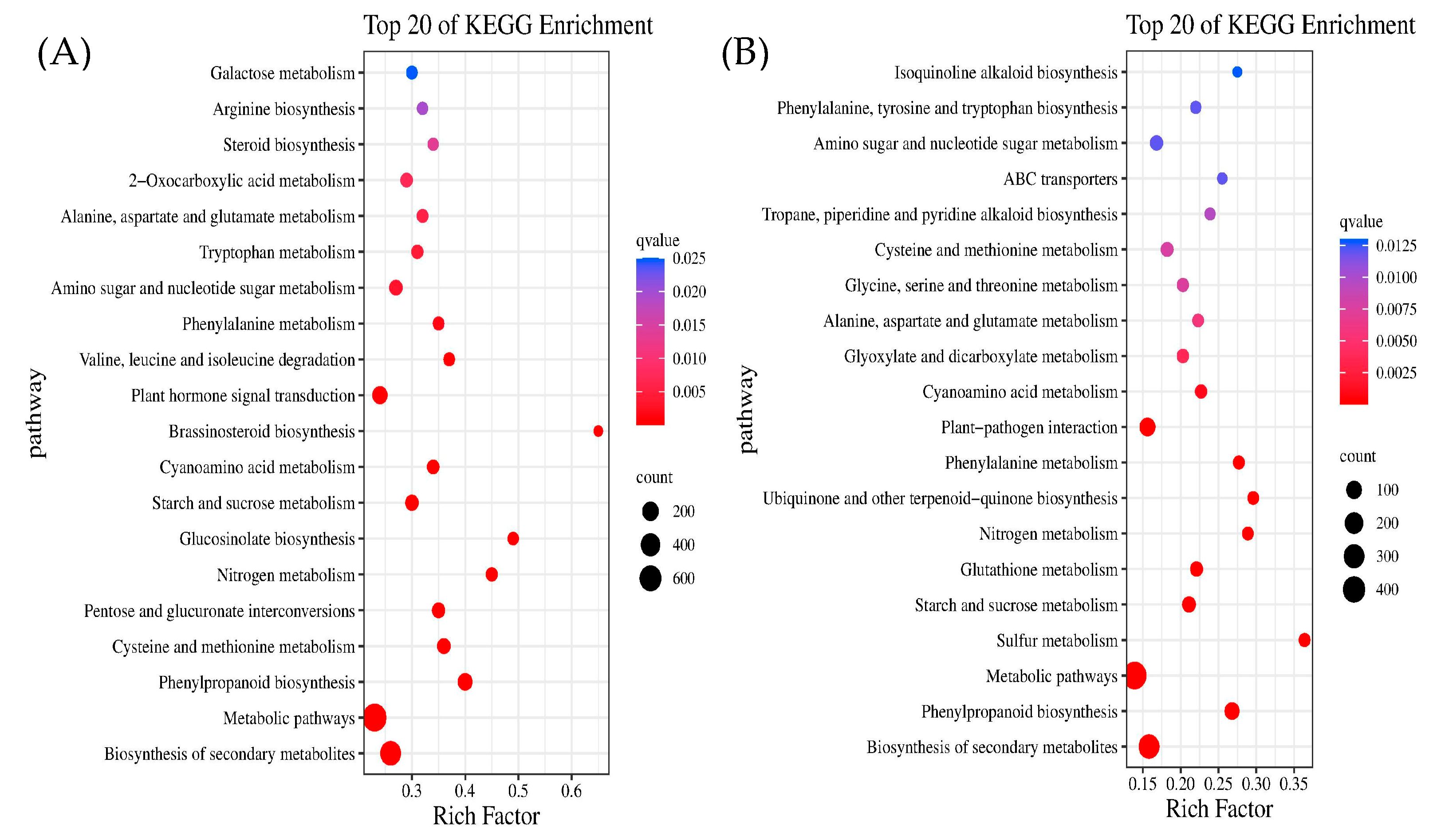
2.6. Kyoto Encyclopedia of Genes and Genomes (KEGG) Enrichment Analysis of DEGs
2.7. Analysis of Genes Related to Cd and Pb Stress Defense and Detoxification
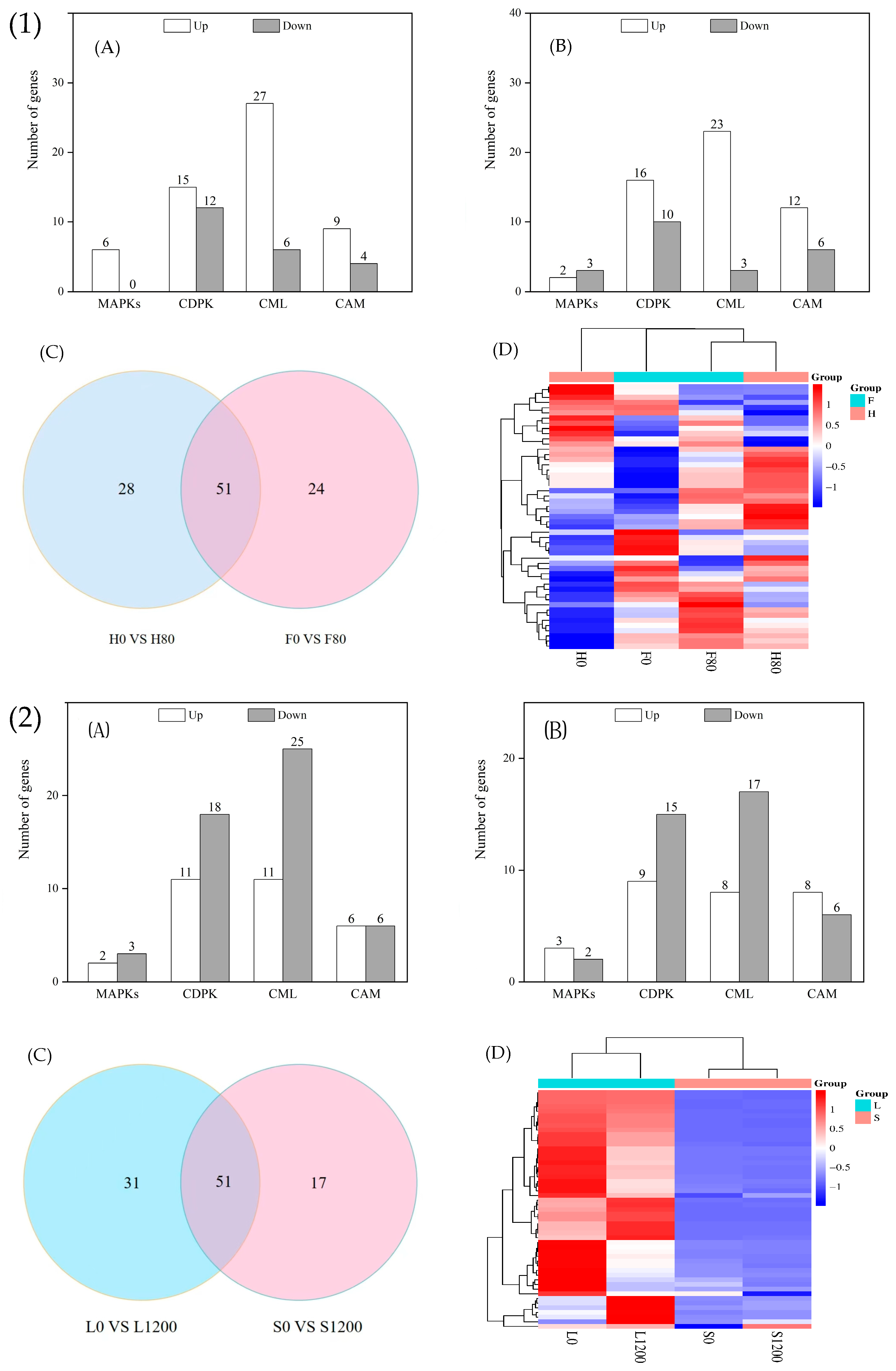
2.7.1. DEGs Related to Reactive Oxygen Species Scavenging System
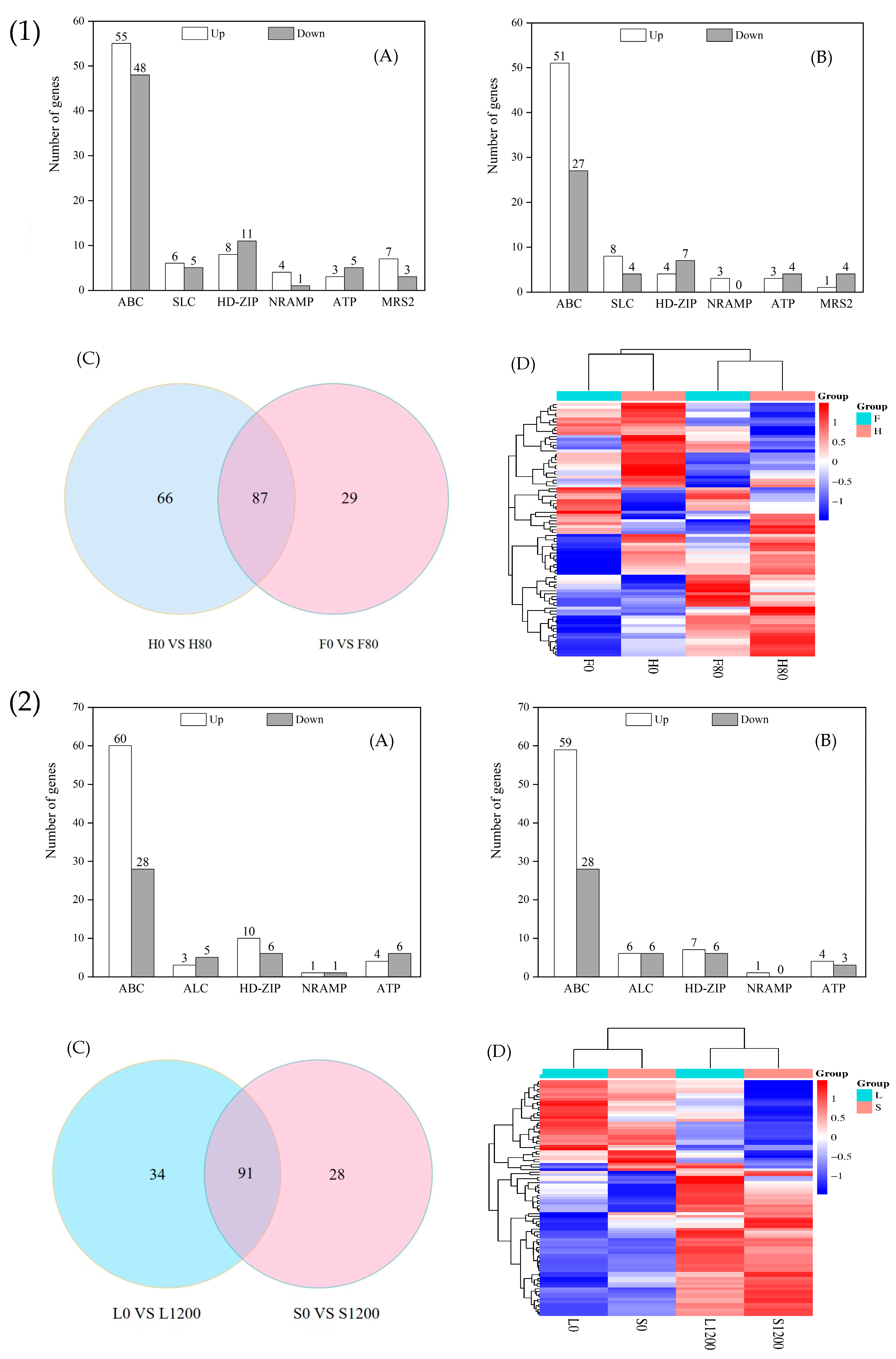
2.7.2. DEGs Related to Metal Transporter Proteins
2.7.3. DEGs Related to Signal Sensing and Transduction Proteins
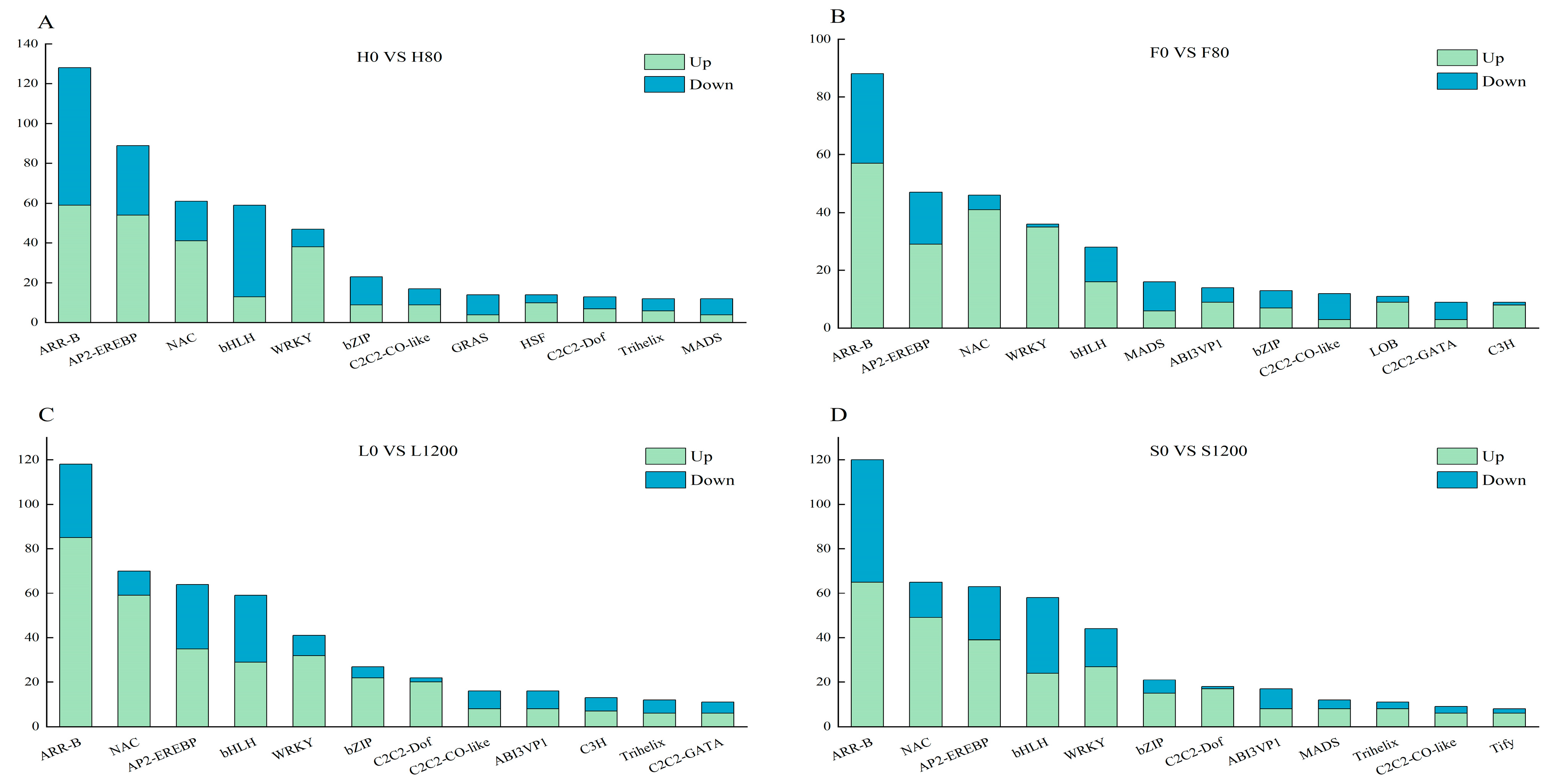
2.8. Transcription Factor Analysis of DEGs
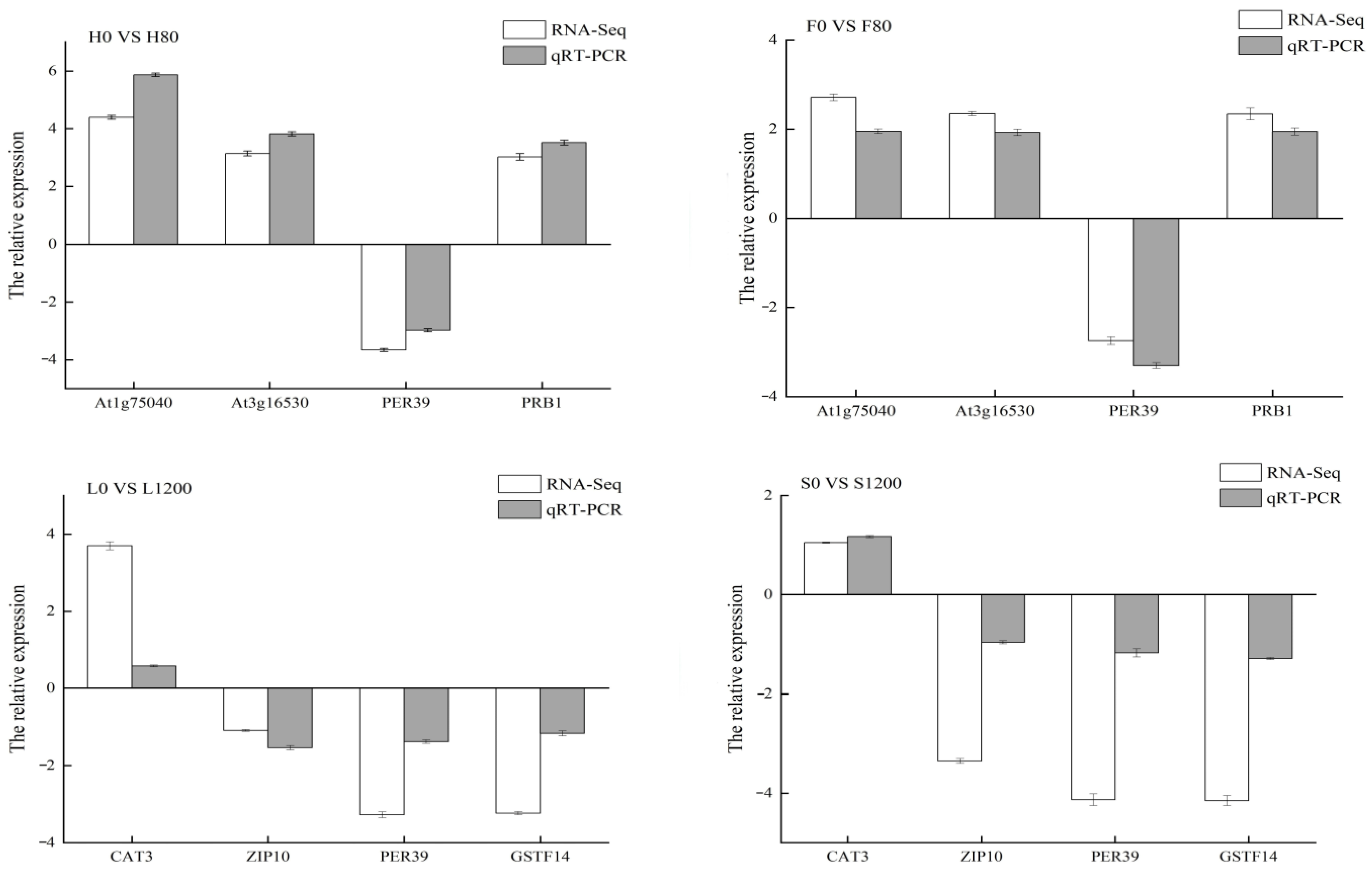
2.9. RT-qPCR Validation
3. Discussion
3.1. GO and KEGG Enrichment Analysis of DEGs
3.2. Analysis of Cd and Pb Stress Defense- and Detoxification-Related Genes
3.3. Investigation of Transcription Factor in DEGs
4. Materials and Methods
4.1. Experimental Materials and Design
4.2. Indicators and Measurement Methods
4.2.1. Determination of Cd and Pb Content in Plants
4.2.2. Transcriptome Sequencing Data and Data Analysis
Sample RNA Extraction and Quality Control
Construction of cDNA Library
Sequencing Data Quality Assessment and Filtering
Screening of DEGs
GO and KEGG Enrichment Analysis of DEGs
4.2.3. Fluorescence RT-qPCR Validation
4.3. Data Statistics and Analysis
5. Conclusions
- (a)
- Through transcriptome sequencing of the roots of different Chinese cabbage varieties under Cd and Pb treatments, GO enrichment analysis showed that Cd and Pb stress had a greater effect on the cell periphery, external encapsulation structure, cell wall, and response to external stimuli. KEGG enrichment analysis was mainly for secondary metabolite biosynthesis, metabolic pathways, and phenylpropanoid biosynthesis pathways.
- (b)
- An analysis of the DEGs related to defense and detoxification showed that in the reactive oxygen species scavenging system, the number of upregulated genes in the four species was greater than that of the downregulated genes, and the main difference was caused by the genes encoding CAT and GST; in the metal transporter system, there were more genes that were upregulated than downregulated after heavy metal stress, and the main difference was due to the differential expression of the genes encoding ABC transporter proteins; in the signal sensing and transducer system, the genes encoding MAPKs, CDPK, CML, and CAM were all more upregulated in the H and F varieties under Cd stress, whereas those encoding CDPK and CML were more downregulated in the L and S varieties under Pb stress.
- (c)
- Transcription factor family analysis of differentially expressed genes in Chinese cabbage roots under Cd and Pb stress revealed the presence of numerous transcription factors, such as ARR-B, AP2-EREBP, NAC, bHLH, WRKY, and bZIP, which are strongly associated with signaling regulation related to Cd and Pb tolerance.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DEGs | Differentially expressed genes |
| H | Harmony Express |
| F | Ziwei F1 |
| L | Green Crown |
| S | Suzhou Green |
Appendix A
| Sample | Raw Data/bp | Clean Reads/bp | Q20/% | GC | Alignment Rate/% | Unique Alignment Rate/% |
|---|---|---|---|---|---|---|
| F0-1 | 38,045,654 | 37,847,936 | 97.54 | 47.14 | 85.83 | 83.47 |
| F0-2 | 44,825,712 | 44,588,466 | 97.67 | 47.23 | 86.20 | 83.80 |
| F0-3 | 41,441,232 | 41,216,564 | 97.40 | 47.16 | 85.68 | 83.31 |
| F80-1 | 37,127,004 | 36,951,820 | 97.38 | 47.16 | 84.56 | 81.87 |
| F80-2 | 37,302,860 | 37,145,918 | 97.72 | 47.21 | 84.54 | 81.79 |
| F80-3 | 40,513,382 | 40,345,392 | 97.78 | 47.21 | 84.55 | 81.81 |
| H0-1 | 38,133,332 | 37,949,190 | 97.70 | 47.19 | 84.47 | 80.24 |
| H0-2 | 43,182,524 | 42,982,558 | 97.69 | 47.17 | 82.43 | 80.21 |
| H0-3 | 36,396,510 | 36,198,036 | 97.44 | 47.16 | 84.00 | 81.75 |
| H80-1 | 38,229,992 | 38,058,584 | 97.93 | 46.97 | 84.67 | 82.53 |
| H80-2 | 39,930,032 | 39,750,840 | 97.79 | 47.00 | 84.53 | 82.37 |
| H80-3 | 37,624,148 | 37,452,574 | 97.91 | 46.97 | 84.39 | 82.21 |
| Sample | Raw Data/bp | Clean Reads/bp | Q20/% | GC | Alignment Rate/% | Unique Alignment Rate/% |
|---|---|---|---|---|---|---|
| S0-1 | 45,794,082 | 45,590,598 | 97.80 | 46.80 | 83.35 | 81.04 |
| S0-2 | 46,843,230 | 46,624,614 | 97.40 | 46.84 | 82.14 | 79.89 |
| S0-3 | 44,147,364 | 43,947,908 | 97.87 | 46.83 | 83.48 | 81.23 |
| S1200-1 | 46,097,740 | 45,868,042 | 97.74 | 48.19 | 79.91 | 77.83 |
| S1200-2 | 45,465,400 | 45,218,548 | 97.24 | 48.27 | 79.12 | 77.03 |
| S1200-3 | 44,995,858 | 44,772,442 | 97.56 | 48.14 | 79.98 | 77.86 |
| L0-1 | 41,901,870 | 41,696,094 | 97.62 | 47.28 | 85.93 | 83.43 |
| L0-2 | 45,125,548 | 44,885,656 | 97.56 | 47.28 | 85.90 | 83.43 |
| L0-3 | 41,244,174 | 41,048,822 | 97.78 | 47.29 | 86.09 | 83.60 |
| L1200-1 | 42,175,062 | 41,973,480 | 97.69 | 46.97 | 84.54 | 82.43 |
| L1200-2 | 42,766,174 | 42,532,872 | 97.60 | 46.95 | 84.08 | 81.98 |
| L1200-3 | 38,192,598 | 38,028,248 | 97.87 | 46.97 | 84.37 | 82.26 |
References
- Xue, P.; Zhao, Q.; Sun, H. Characteristics of heavy metals in soils and grains of wheat and maize from farmland irrigated with sewage. Environ. Sci. Pollut. Res. 2019, 26, 5554–5563. [Google Scholar] [CrossRef]
- Ahado, S.K.; Nwaogu, C.; Sarkodie, V.Y.O. Modeling and Assessing the Spatial and Vertical Distributions of Potentially Toxic Elements in Soil and How the Concentrations Differ. Toxics 2021, 9, 181. [Google Scholar] [CrossRef]
- Chen, J.B.; Zheng, W.; Liu, Y.; Sun, R.Y.; Yuan, Y.; Meng, M.; Cai, H.M.; Liu, C.Q. Isotope Geochemistry and Earth System Circle Interactions and Global Change Research. Geol. Front. 2025, 32, 1–19. [Google Scholar]
- Guan, S.Y. Effects of different elements on photosynthetic characteristics and antioxidant system of wheat under cadmium (Cd) stress. Tianjin Agric. For. Sci. Technol. 2023, 6, 13–16. [Google Scholar]
- Wang, P.; Zhao, F.J. Thoughts on cadmium limits in major food crops in China. Sci. Bull. 2022, 67, 3252–3260. [Google Scholar]
- Xiao, W.D.; Ye, X.Z.; Zhang, Q.; Zhao, S.P.; Chen, D.; Huang, M.J.; Hu, J.; Gao, N. Source analysis of soil lead pollution based on stable isotope and multi-element. China Environ. Sci. 2021, 41, 2319–2328. [Google Scholar]
- Li, L.B.; Gao, Y.L.; Wang, X.D.; Cai, D.W.; Ding, H. Characteristics of heavy metal contamination in soil and crops around Dushan antimony mining area and health risk assessment. J. Guizhou Norm. Univ. (Nat. Sci. Ed.) 2024, 42, 58–65. [Google Scholar]
- Tu, C.Y.; Chen, T.T.; Liao, C.J.; Cao, W.S.; Zhang, C.L.; Zhou, Y.X.; Xie, T. Evaluation of heavy metal pollution and enrichment characteristics of vegetables in farmland in mining area. J. Agric. Environ. Sci. 2020, 39, 1713–1722. [Google Scholar]
- Tao, Y. Physicochemical Characterization and Transcriptome Analysis of Honeysuckle by Cadmium and Ozone Stress. Master’s Thesis, Shenyang Agricultural University, Shenyang, China, 2023. [Google Scholar]
- Gao, N. Determination of Lead Content in Agricultural Products Using Graphite Furnace Atomic Absorption Spectroscopy. Agric. Dev. Equip. 2025, 8, 103–105. [Google Scholar]
- Xie, K.C. Research on drought and cold tolerance of wheat and maize and analysis of resistance mechanisms. Seed Sci. Technol. 2024, 42, 145–147. [Google Scholar]
- Li, Q.; Li, C. Progress of research on the detoxification effect of sulfur on heavy metal hyper-enriched plants and its application. Mod. Agric. Sci. Technol. 2024, 21, 98–102. [Google Scholar]
- Wang, Y.; Xu, L.; Chen, Y.; Wu, K. Transcriptome Profiling of Radish (Raphanus sativus L.) Root and Identification of Genes Involved in Response to Lead (Pb) Stress with Next Generation Sequencing. PLoS ONE 2013, 8, e66539. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.Z.; Ren, C.; Xiao, J.H.; Li, J.T.; Du, Q.Q. Characteristics of Cd and Pb accumulation in different maize varieties and comparative study between Xianyu 335 and Dafeng 30. Jiangsu Agric. Sci. 2022, 50, 179–188. [Google Scholar]
- Xu, Y. Transcriptome Analysis and Screening of Key Genes in Polygonum Multiflorum Under Different Heavy Metal Stresses. Master’s Thesis, Northwest Normal University, Lanzhou, China, 2024. [Google Scholar]
- Zhu, L.; Liu, M.Y.; Xu, X.O.; Wang, Z.F.; Cao, X.F. Detection and response of MTs in Lonchocarpus indicus under soil cadmium stress: Effects of nanomaterials and microorganisms. J. Ecotoxicol. 2024, 19, 383–390. [Google Scholar]
- Li, C.P.; Fang, K.; Xiong, C.; Zhou, Y.; Xiang, Z.Q.; Dai, S.H.; He, C.Z. Effects of exogenous H2O2 combined with vermicompost initiation on physiology and transcriptome of seedless watermelon seeds at low temperature. Chin. Melon Veg. 2025, 38, 17–27. [Google Scholar]
- Zhang, H.K. Functional Study of Maize ZmG6PE Gene Responding to Low Phosphorus Stress and Affecting Seed Yield. Ph.D. Thesis, Sichuan Agricultural University, Chengdu, China, 2023. [Google Scholar]
- An, T.T. Mechanisms of Cadmium Stress Tolerance and Regulation by Exogenous Nitrogen and Silicon in Maize. Ph.D. Thesis, Northwest Agriculture and Forestry University, Yangling, China, 2024. [Google Scholar]
- Higashi, Y.; Saito, K. Lipidomic studies of membrane glycerolipids in plant leaves under heat stress. Prog. Lipid Res. 2019, 75, 100990. [Google Scholar] [CrossRef]
- Song, L.L.; Tang, K.; Peng, Z.; Mao, M.M.; Tu, D.H.; Zhu, W.C. Transcriptome analysis of Zhenning tree pepper seedlings under cadmium stress. Seed 2023, 42, 31–37. [Google Scholar]
- Yuan, Q.L.; Chen, Y.P.; Li, Q.; Sun, Y.; Cao, B.; Lu, Y.Z.; Zhang, X.L. Physiological, Biochemical, and Molecular Mechanisms of Cadmium Accumulation in Hyperaccumulator Plants. J. Eng. Sci. 2025, 47, 1753–1762. [Google Scholar]
- Zhang, M.Y.; Mo, Q.; Qi, X.S.; Tong, N.N.; Kong, F.; Liu, Z.A.; Lv, C.P.; Peng, L.P. Cloning and expression analysis of Peony PoLPAT2 gene. Zhejiang J. Agric. 2025, 37, 321–328. [Google Scholar]
- Yang, Y.N.; Li, W.T.; Huang, X.X. Effects of lead stress on the growth and physiological characteristics of Ivy vulgaris. West. For. Sci. 2020, 49, 126–131. [Google Scholar]
- Zhang, S.Q. Changes of Flavonoids Content in Maize Development and the Mechanism of Alleviating Lead Stress. Master’s Thesis, Tianjin University, Tianjin, China, 2020. [Google Scholar]
- Dong, C.L. Physiological Responses of Fengdan and Ornamental Peony Under Copper Stress and Transcriptome Analysis of Fengdan. Master’s Thesis, Nanjing Agricultural University, Nanjing, China, 2014. [Google Scholar]
- Zhang, D.J.; Wang, D.D.; Dong, W.; Ma, J.H.; Yang, S.F.; Zhang, Z.J.; Li, C.X. Transcriptomics and proteomics of young wheat roots under copper stress. Henan Agric. Sci. 2015, 44, 31–35. [Google Scholar]
- Ma, J.; Su, l.; Yuan, M.; Ji, H.F.; Li, A.Q.; Wang, X.J. Cloning and expression study of peanut C4H and ANR genes. J. Nucl. Agric. 2012, 26, 43–48. [Google Scholar]
- Sharma, A.; Shahzad, B.; Rehman, A. Response of Phenylpropanoid Pathway and the Role of Polyphenols in Plants Under Abiotic Stress. Molecules 2019, 24, 2452. [Google Scholar] [CrossRef]
- Jia, L.; Zhang, D.; Zhang, J.S. Effects of cadmium stress on antioxidant enzyme activities and endogenous hormone contents in Lonicera japonica. Earth Environ. 2024, 52, 21–28. [Google Scholar]
- Tang, T.X.; Kong, W.P.; Ren, K.L.; Cheng, H. Progress on the function of plant ABC transporter proteins. Northwest J. Agric. 2023, 32, 1–10. [Google Scholar]
- Dong, P.C.; Yuan, Z.; Liu, H.; Li, J.M.; Chen, Z.M.; Qin, C.; Shi, L.; Chen, Y.H. Comparative transcriptomics-based analysis of the molecular mechanism of the response of vegetable sweet potato to heavy metal cadmium. J. Jiangsu Norm. Univ. (Nat. Sci. Ed.) 2023, 4, 21–29+2. [Google Scholar]
- Zhang, C.M. Physiological and Molecular Mechanisms of Different Drought-Resistant Alfalfa in Response to Drought. Ph.D. Thesis, Gansu Agricultural University, Lanzhou, China, 2019. [Google Scholar]
- Xie, K.H.; Zhang, D.W.; Liu, Y.; He, D.; Zou, Z.X.; Yan, M.L.; Zhou, D.G. Identification of PaCDPK gene family and analysis of heavy metal stress response in American commercial land. Mol. Plant Breed. 2025, 23, 71–79. [Google Scholar]
- Xu, H.; Li, K.; Yang, F. Overexpression of CsNMAPK in tobacco enhanced seed germination under salt and osmotic stresses. Mol. Biol. Rep. 2009, 37, 3157–3163. [Google Scholar] [CrossRef]
- Chmielowska-Bąk, J.; Lefèvre, I.; Lutts, S. Short term signaling responses in roots of young soybean seedlings exposed to cadmium stress. J. Plant Physiol. 2013, 170, 1585–1594. [Google Scholar] [CrossRef]
- Li, C.; Du, C.Y.; Cui, X.F.; Li, Y.C.; Li, J.J.; Song, J.; Cui, B.; Gao, J.W. Research Progress on the Relationship Between Ca2⁺ and Calcium-Binding Proteins and Plant Salt Tolerance. Shandong Agric. Sci. 2025, 57, 163–170. [Google Scholar]
- Mu, Y.J.; Zhan, Y.J.; Xu, W.F. Arabidopsis root transcriptomics and network response under high pH stress. J. Soil. Sci. 2020, 57, 691–701. [Google Scholar]
- Hou, L.Y. Molecular Mechanism of Cytokinin B-Type Response Regulator in Regulating Frost Resistance in Arabidopsis. Ph.D. Thesis, China Agricultural University, Beijing, China, 2021. [Google Scholar]
- Wang, L.Y.; Chen, R.; Zhao, S.Q.; Yan, H.L.; Xu, W.X.; Liu, R.X.; Ma, M.; Yu, Y.J.; He, Z.Y. Overview of physiological and molecular mechanisms of cadmium accumulation in rice. J. Bot. 2022, 57, 236–249. [Google Scholar]
- Wang, B.X.; Tan, M.P.; Liu, Y. Application of Rice Transcription Factor OsNAC3 in Enhancing Cadmium Tolerance in Plants. CN111041035B, 23 August 2022. [Google Scholar]




| Variety | Aboveground | Underground | ||
|---|---|---|---|---|
| Cd (mg/kg) | Pb (mg/kg) | Cd (mg/kg) | Pb (mg/kg) | |
| Lvguan (L) | 9.87 ± 1.63 | 1.80 ± 1.71 | 15.96 ± 0.94 | 136.25 ± 1.70 |
| Hexiekuaicai (H) | 4.72 ± 2.59 | 9.90 ± 0.85 | 9.56 ± 1.39 | 154.78 ± 0.51 |
| Suzhouqing (S) | 10.00 ± 1.73 | 18.00 ± 1.00 | 13.21 ± 1.69 | 245.32 ± 0.71 |
| Ziwei F1 (F) | 10.60 ± 2.16 | 13.20 ± 2.71 | 17.69 ± 1.14 | 202.31 ± 1.49 |
| Variety | Cd | Pb | ||
|---|---|---|---|---|
| TF | BCF | TF | BCF | |
| Lvguan (L) | 0.62 ± 0.08 | 1.88 ± 1.12 | 0.013 ± 0.004 | 0.006 ± 0.002 |
| Hexiekuaicai (H) | 0.50 ± 0.20 | 0.90 ± 0.23 | 0.064 ± 0.008 | 0.032 ± 0.003 |
| Suzhouqing (S) | 0.78 ± 0.19 | 1.90 ± 1.26 | 0.073 ± 0.011 | 0.059 ± 0.003 |
| Ziwei F1 (F) | 0.60 ± 0.11 | 2.02 ± 1.32 | 0.065 ± 0.009 | 0.043 ± 0.002 |
| Variety Name | Manufacturer |
|---|---|
| Hexiekuaicai (H) | Cai Yun Seed Industry Co. (Yuxi, China) |
| Ziwei F1 (F) | Guangxi Hengxian Zilong Seed Industry Co. (Hengxian, China) |
| Lvguan (L) | Cai Yun Seed Industry Co. (Yuxi, China) |
| Suzhouqing (S) | Hefei Hefeng Seed Industry Co. (Hefei, China) |
| Gene ID | Forward Primer | Reverse Primer |
|---|---|---|
| CYP | GAACTTCCGTGCCCTCTG | GTCTTTGAACTTCATGCCGTA |
| Bra027257 | ATGACCCGTGACGAAACC | GTGGGGACGACGATGAAG |
| Bra035235 | AGCAACCAACAAGTGGAAAA | TCAAAGCCTCGGTAGCATT |
| Bra036984 | AGTTCCTCTGAAAGCCCAAG | CCCACATCCTCACTGCGT |
| Bra016511 | CCCCGTTTGGCCTGGAAT | CCCAGATTCTGCCGGACC |
| Bra035235 | AGCAACCAACAAGTGGAAAA | TCAAAGCCTCGGTAGCATT |
| Bra012238 | CCTCACTTGTGCTGATTTCC | ATGTTTGTTTTCGGGTTCG |
| Bra023171 | CCGCTTTTCAGCCCGAGA | CGCCAAGACAAGGAGACGA |
| Bra037204 | CGTTTAGGCGAGTCTTGTTATT | GCATACGGCGTTGTTTCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Peng, S.; Zhang, H.; Wang, J.; Mu, L.; Sun, S.; Li, A.; Zhang, N.; Bao, L. Transcriptome Analysis of Different Chinese Cabbage Varieties Under Cd and Pb Stresses. Int. J. Mol. Sci. 2025, 26, 8945. https://doi.org/10.3390/ijms26188945
Peng S, Zhang H, Wang J, Mu L, Sun S, Li A, Zhang N, Bao L. Transcriptome Analysis of Different Chinese Cabbage Varieties Under Cd and Pb Stresses. International Journal of Molecular Sciences. 2025; 26(18):8945. https://doi.org/10.3390/ijms26188945
Chicago/Turabian StylePeng, Shiqi, Hao Zhang, Junlei Wang, Liyuan Mu, Sijing Sun, Ao Li, Naiming Zhang, and Li Bao. 2025. "Transcriptome Analysis of Different Chinese Cabbage Varieties Under Cd and Pb Stresses" International Journal of Molecular Sciences 26, no. 18: 8945. https://doi.org/10.3390/ijms26188945
APA StylePeng, S., Zhang, H., Wang, J., Mu, L., Sun, S., Li, A., Zhang, N., & Bao, L. (2025). Transcriptome Analysis of Different Chinese Cabbage Varieties Under Cd and Pb Stresses. International Journal of Molecular Sciences, 26(18), 8945. https://doi.org/10.3390/ijms26188945




