Mitotic Disruption and Cytoskeletal Alterations Induced by Acorus calamus Essential Oil: Implications for Bioherbicidal Potential
Abstract
1. Introduction
2. Results
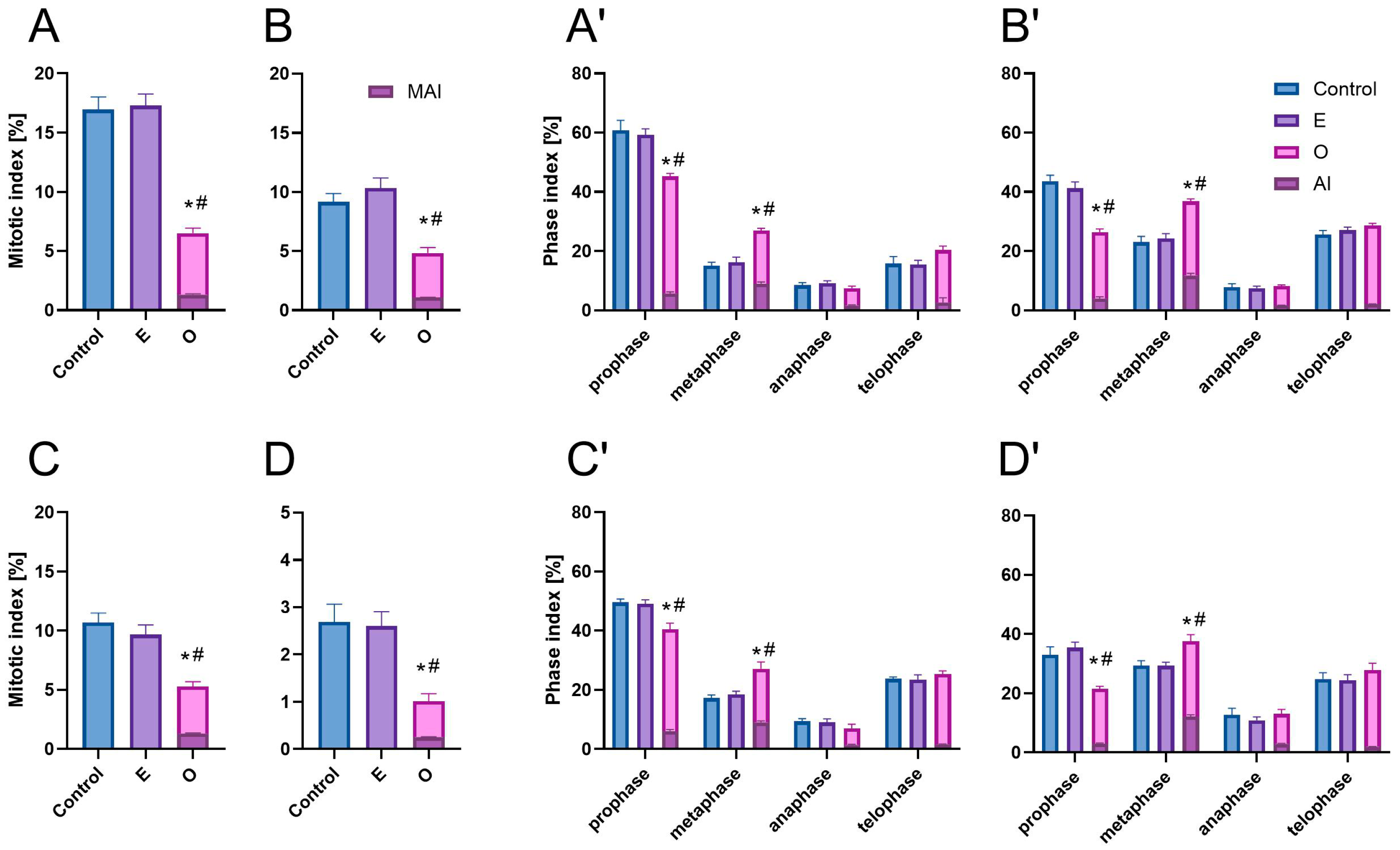
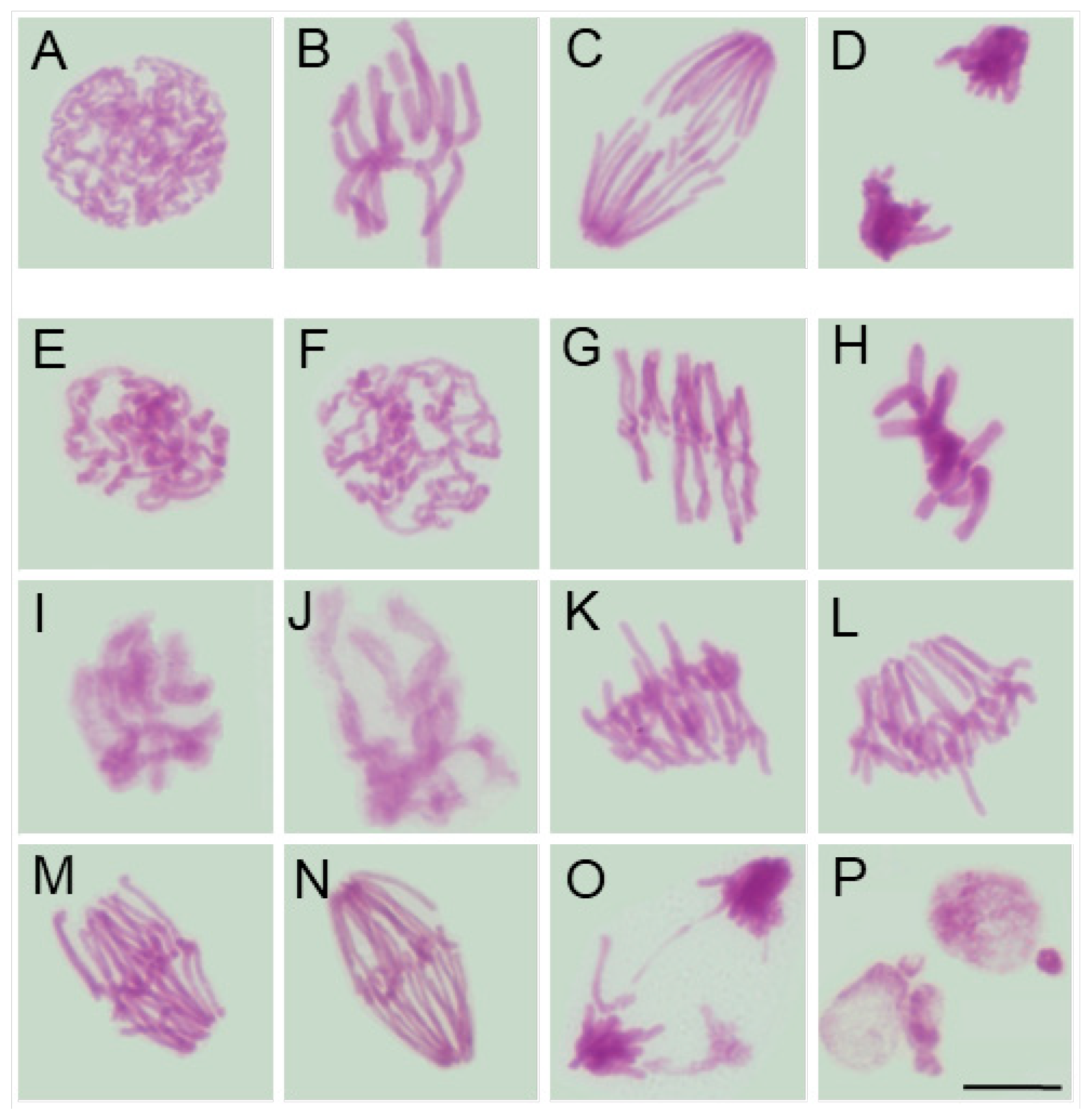
2.1. Effects of SEO on Mitotic Activity and Chromosomal Integrity in Root Meristematic Cells
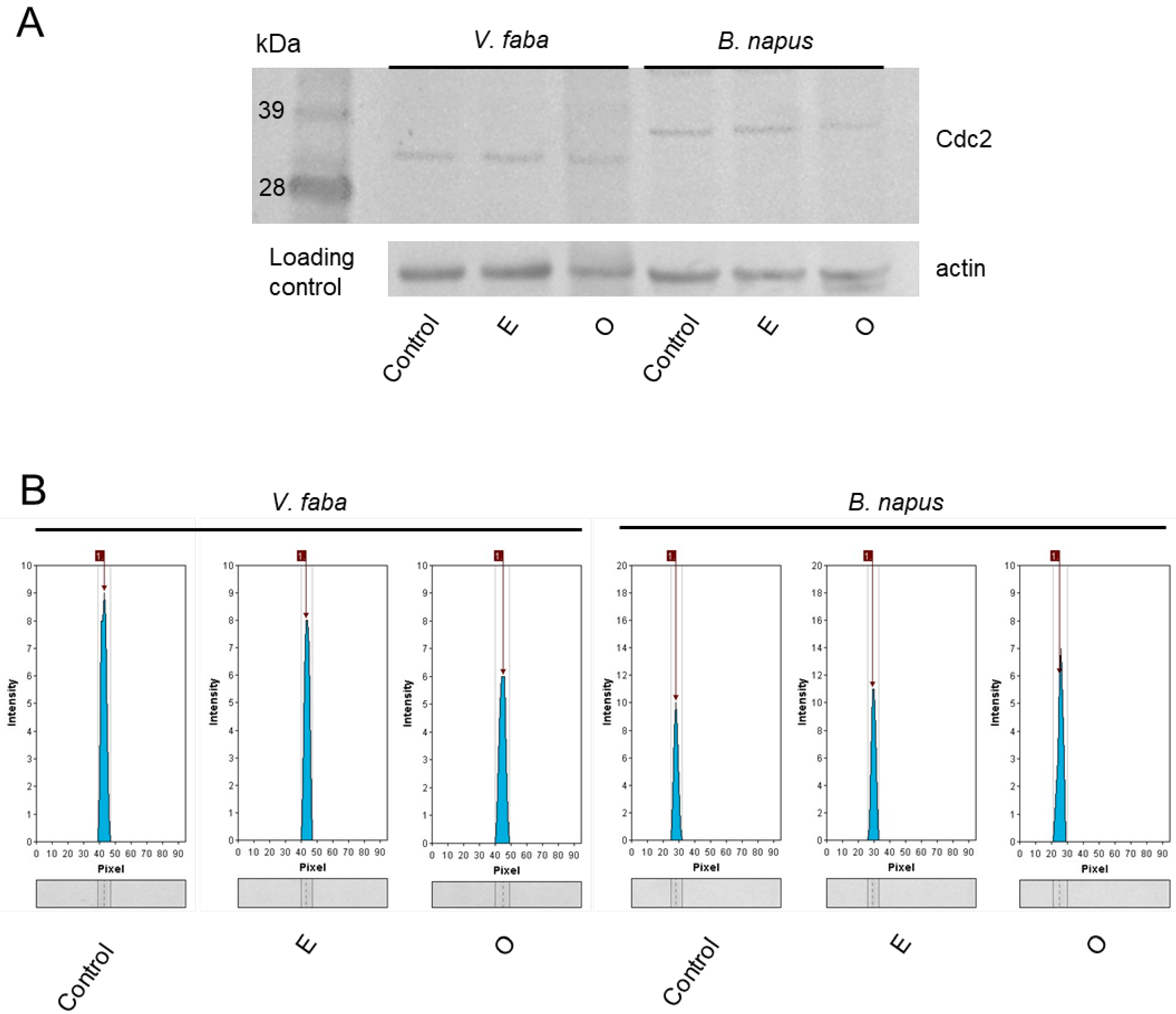
2.2. Assessment of the SEO Effects on Cell Cycle Regulator
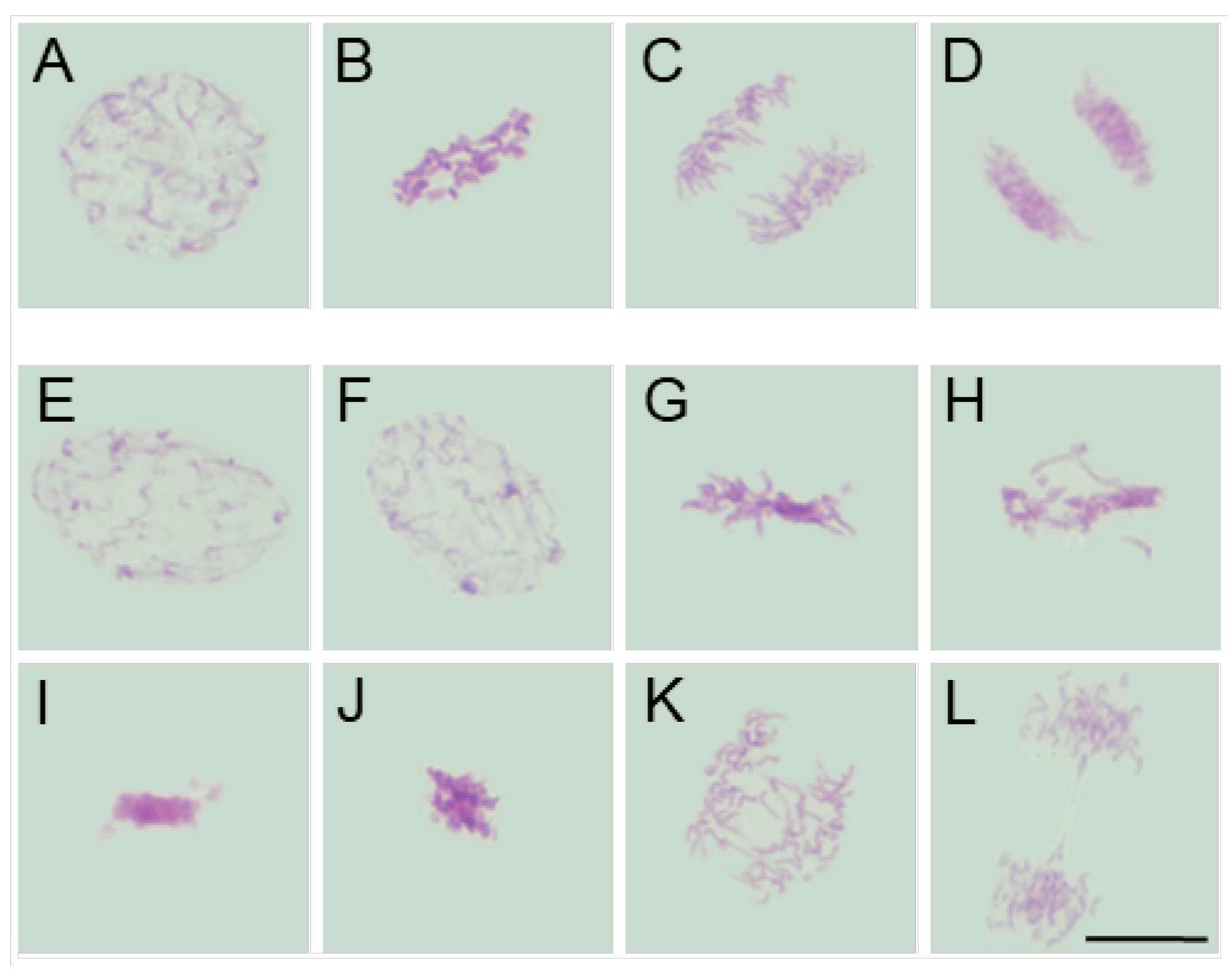
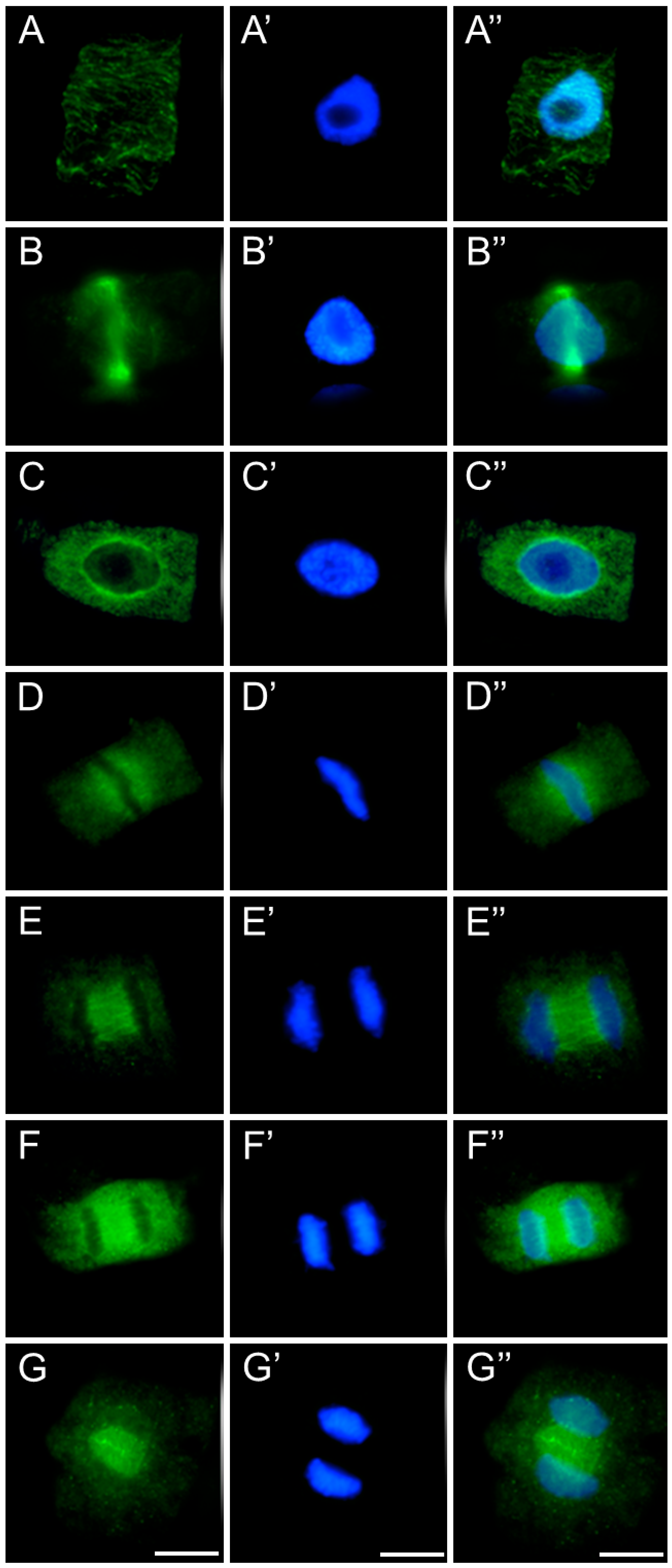
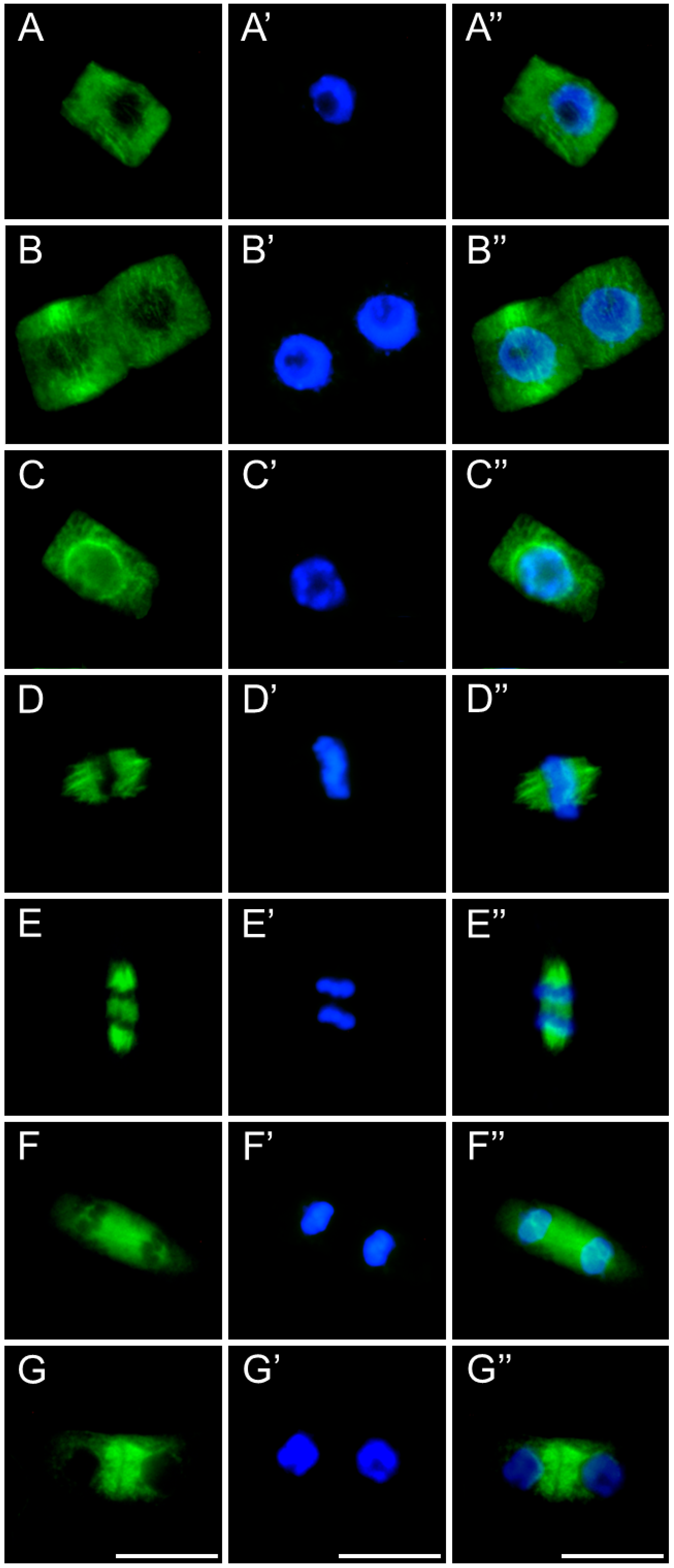
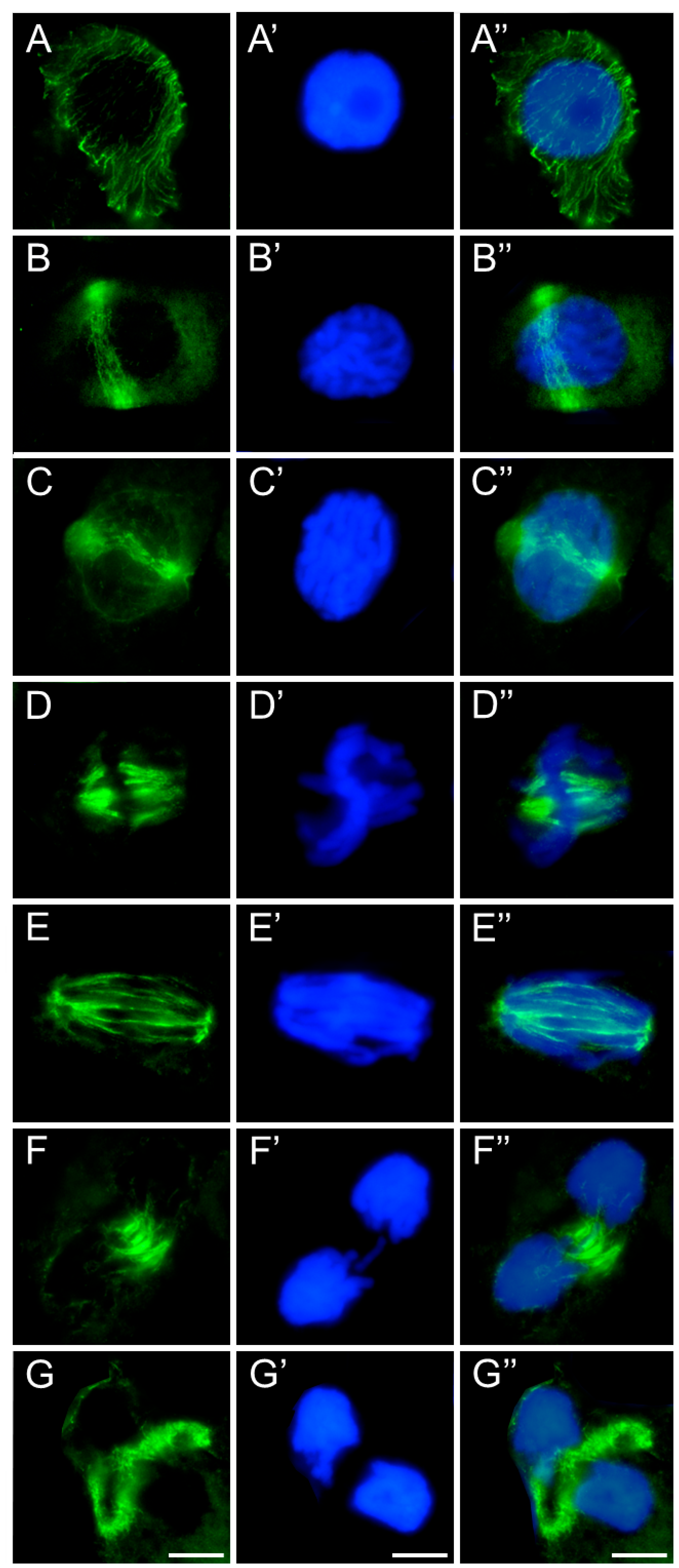
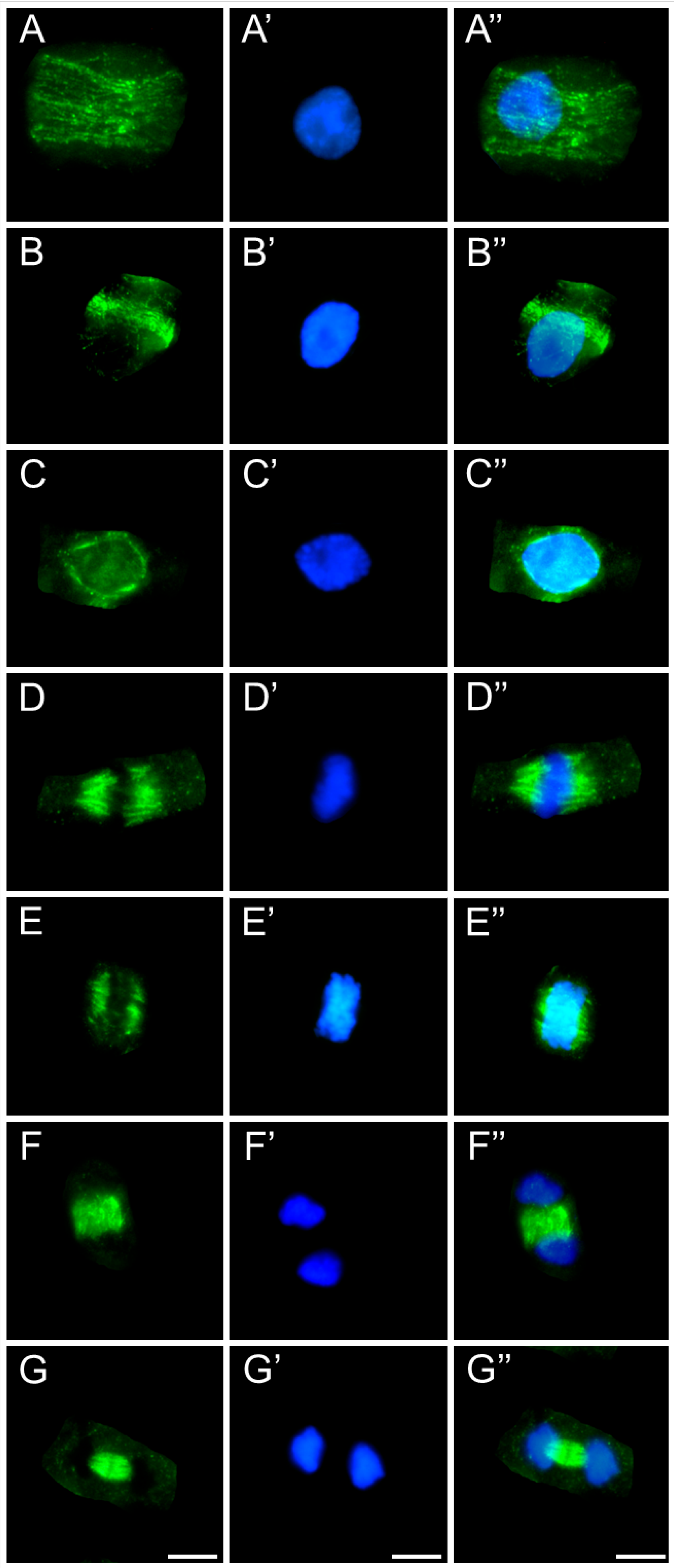
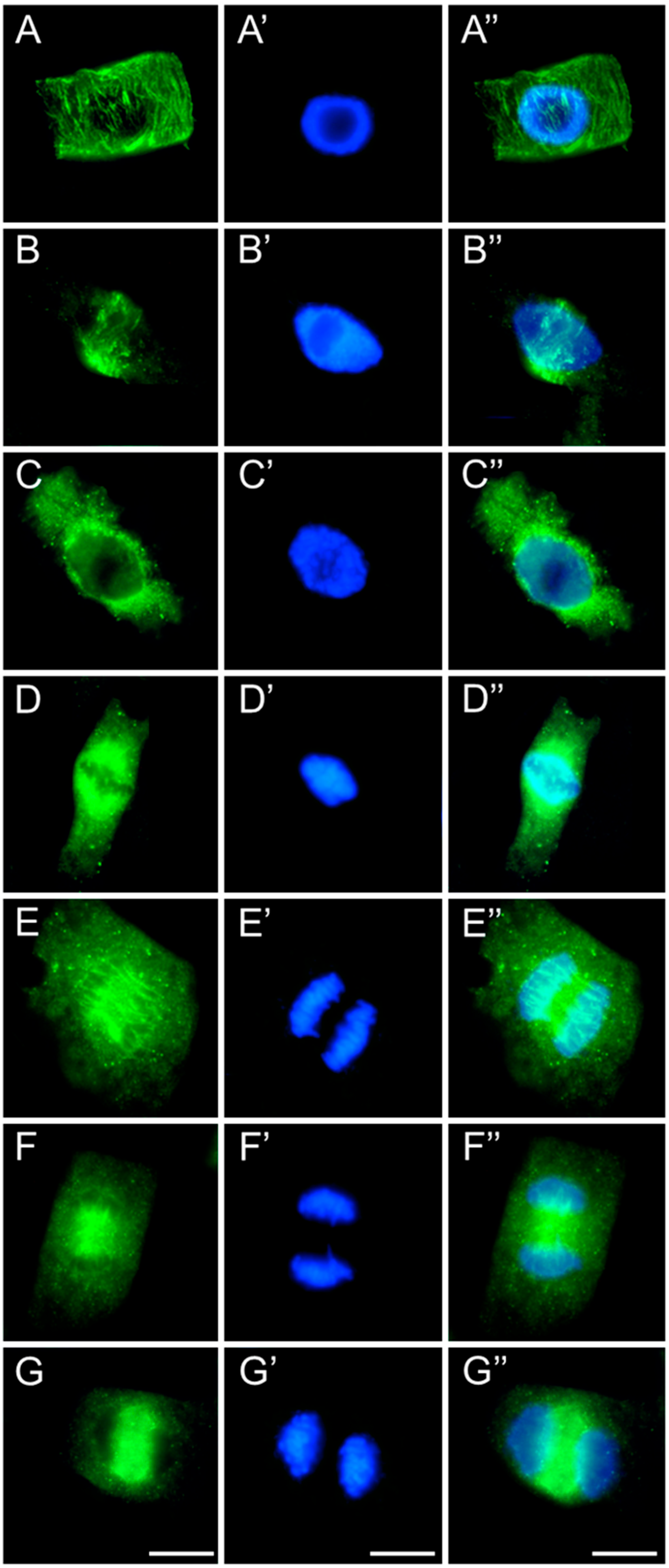
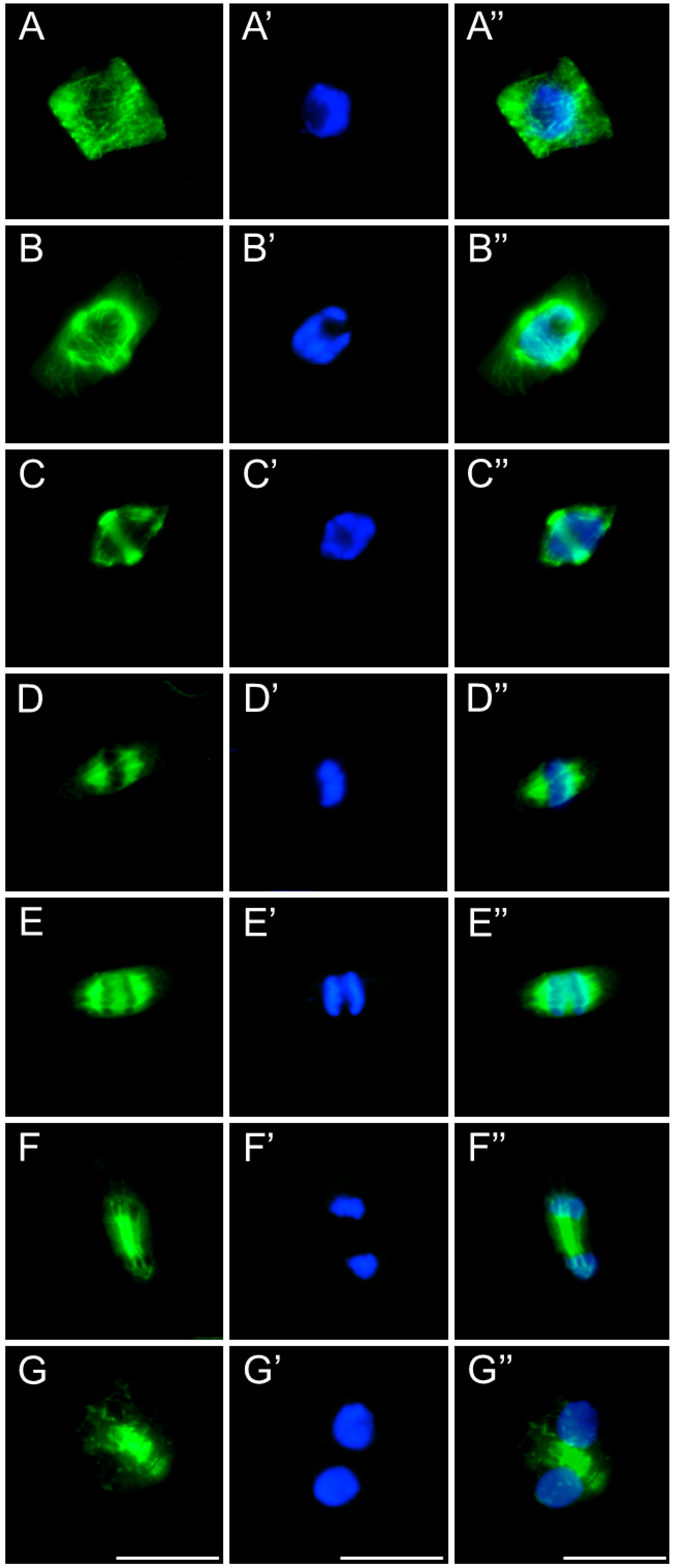
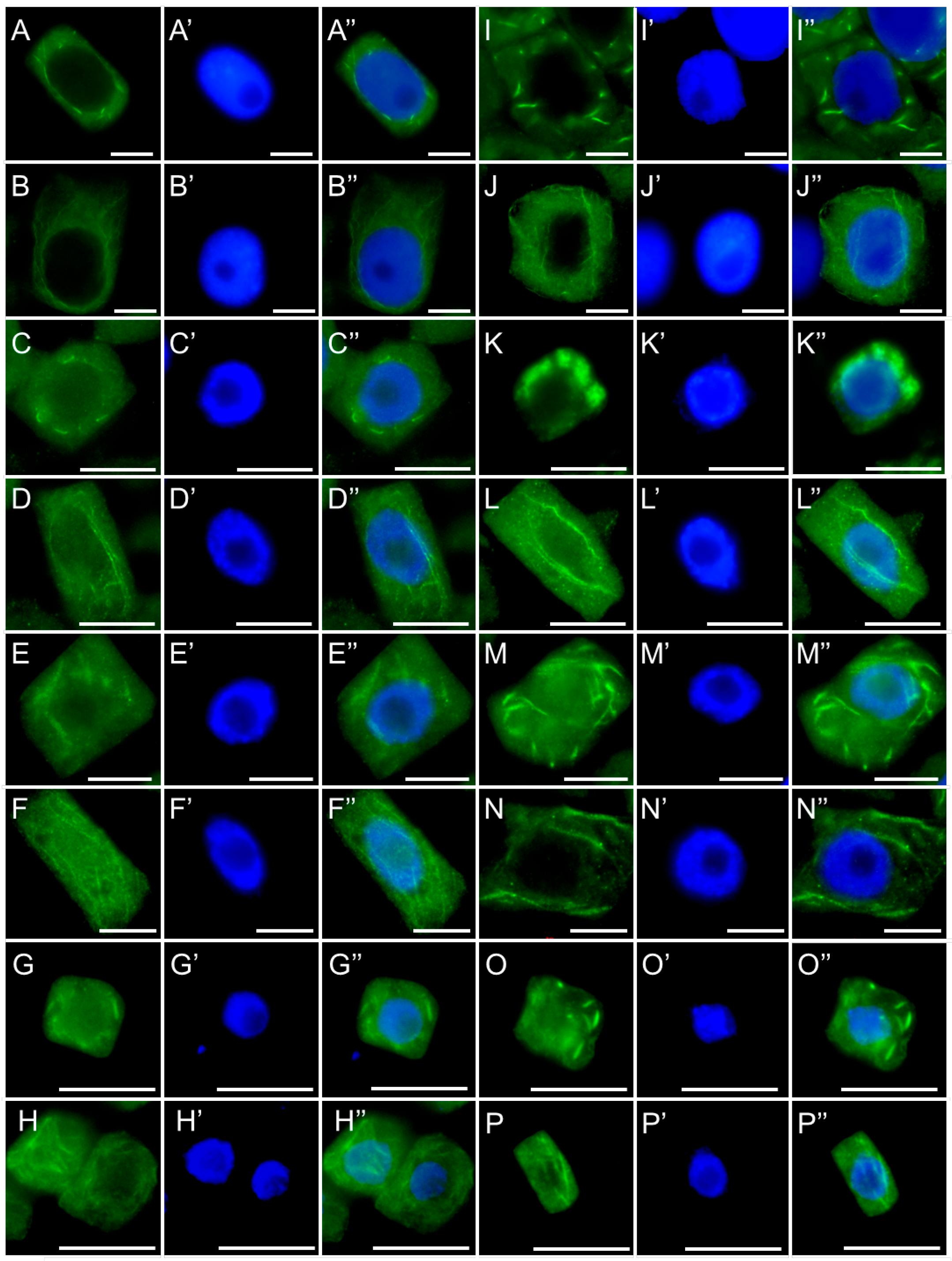
2.3. Assessment of the SEO Effects on Plant Cytoskeleton
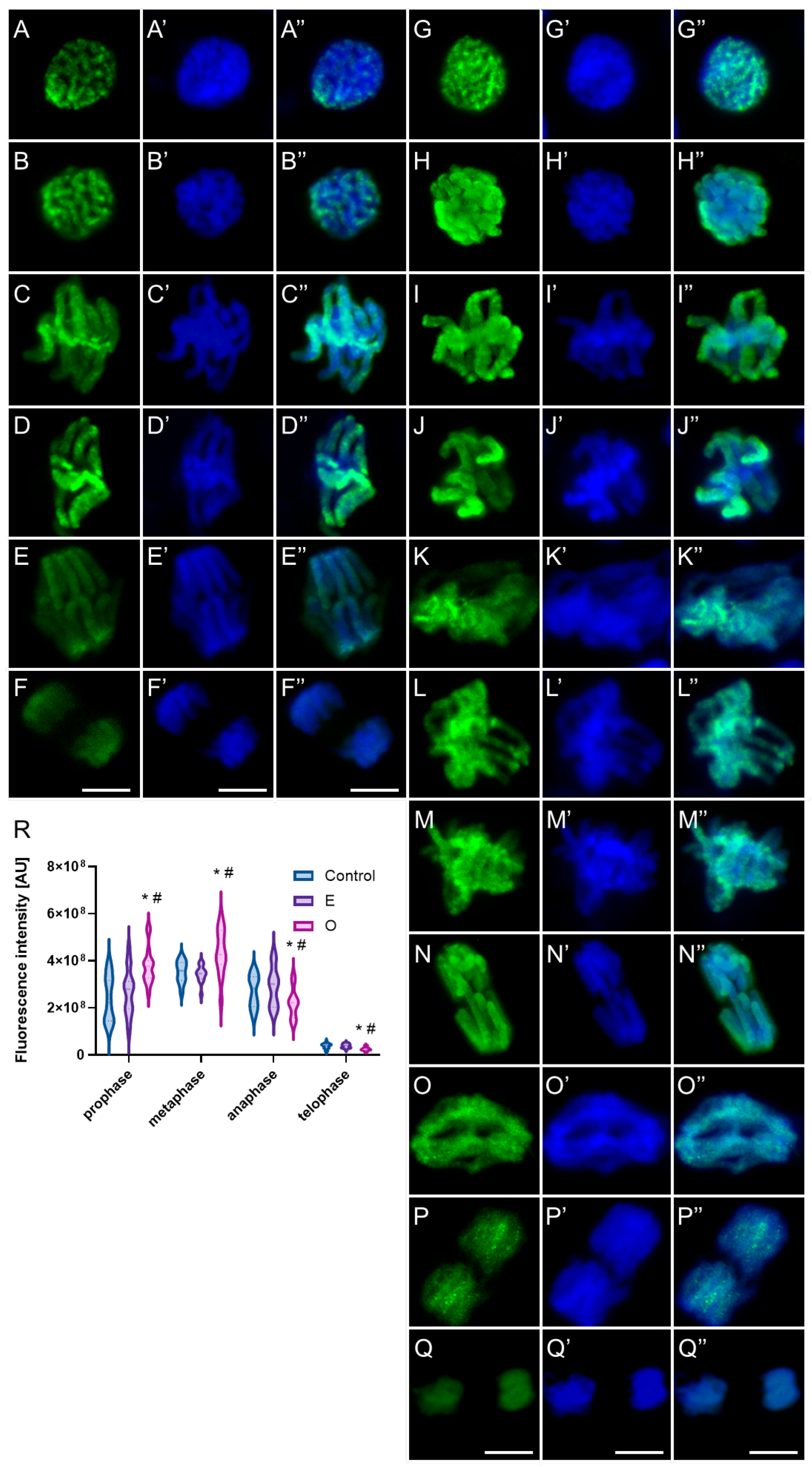
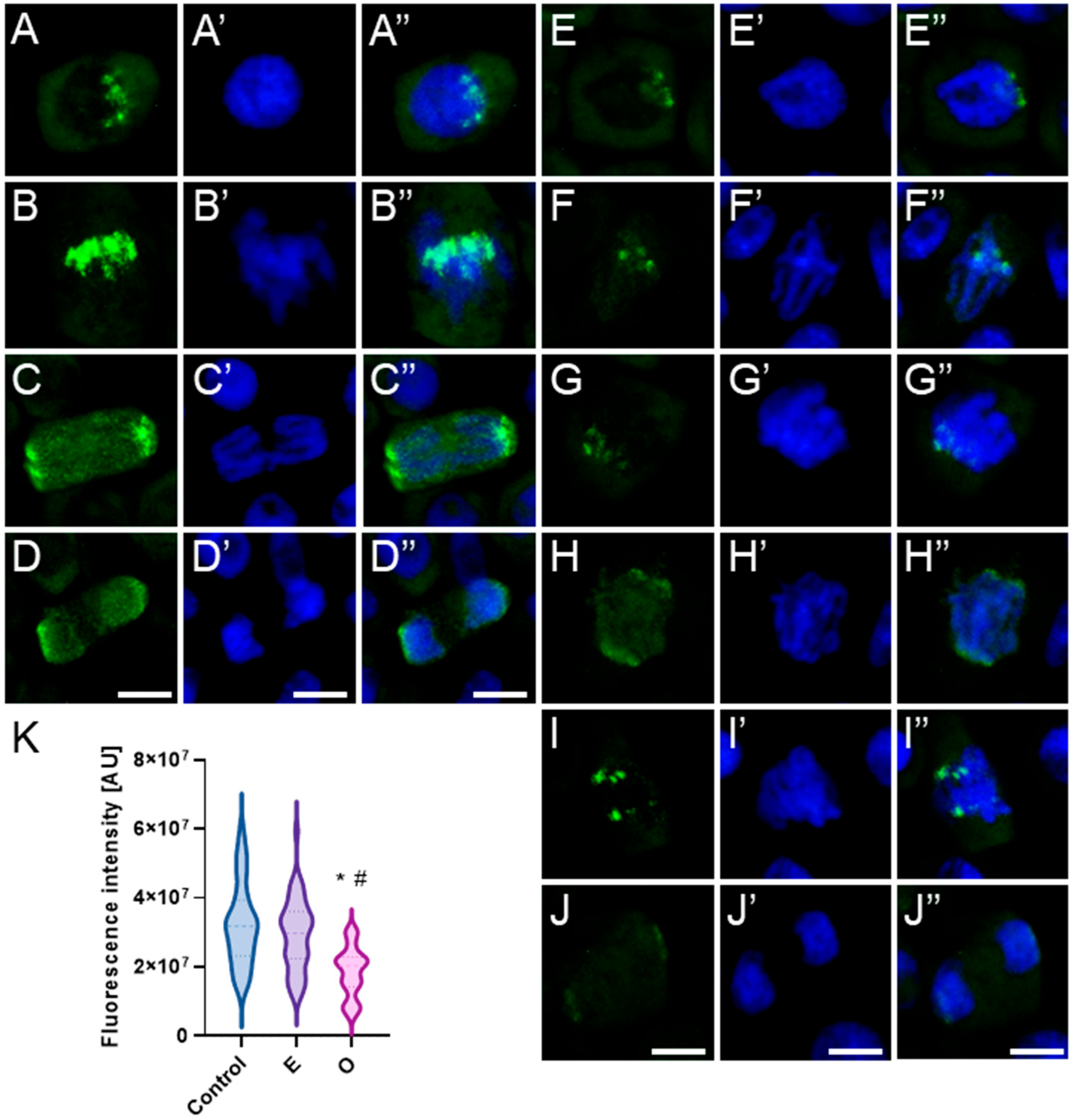
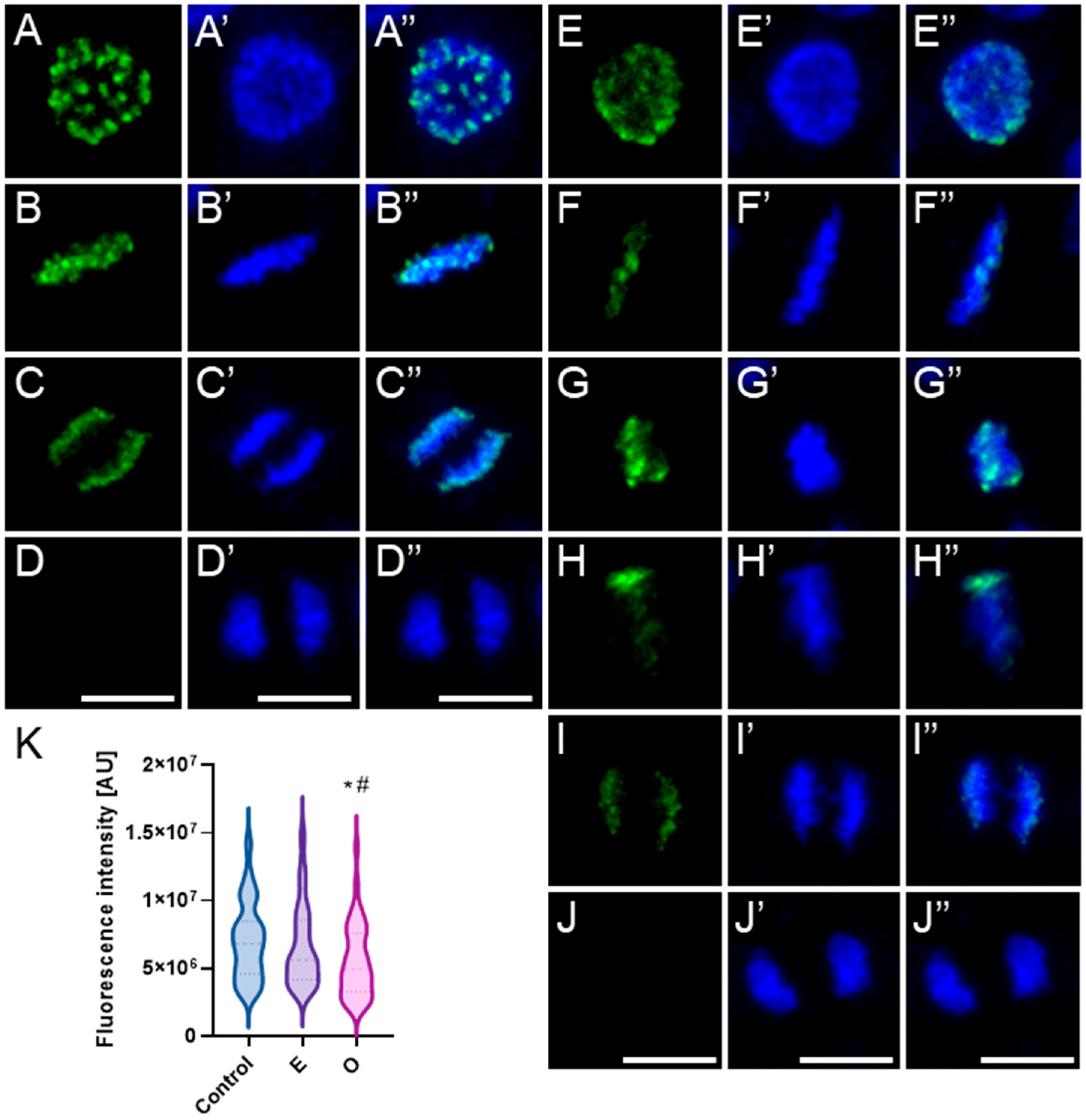
2.4. Assessment of the Alterations in Chromatin Structure by Modifying H3 Phosphorylation Patterns Followed by SEO Treatment
3. Discussion
3.1. Bioherbicidal Potential of SEO: Root Growth Inhibition via Cell Cycle Disruption
3.2. Underlying Mechanisms of Mitotic Index Reduction
3.3. Disruption of Mitotic Progression via Cytoskeletal Disorganization Induced by SEO
3.4. Disruption of Mitotic Progression via Epigenetic Modifications Induced by SEO
3.5. Toxicological Risks Associated with β-Asarone
4. Materials and Methods
4.1. Plant Material
4.2. Essential Oil Treatment
4.3. DNA Staining for Mitotic and Mitotic Phase Indices
4.4. Western Blot
4.5. Immunocytochemical Staining of β-Tubulin and Actin
4.6. Immunocytochemical Detection of Phosphorylated H3S10 and H3T3 Histones
4.7. Microscopic Observations, Measurements, and Analyses
4.8. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| APC/C | Anaphase-promoting complex/cyclosome |
| ATM | Ataxia-telangiectasia mutated kinase |
| ATR | Ataxia-telangiectasia and Rad3-related protein |
| BMF3 | BUB1/MAD3 family protein 3 |
| CAK | Cdk-activating kinase |
| Cdc2 | Cell division control 2 |
| CDC20 | Cell division cycle 20 protein |
| CDC25 | Cell division cycle 25 phosphatase |
| CDKs | Cyclin-dependent kinases |
| CKIs | Cyclin-dependent kinase inhibitors |
| CPC | Chromosomal passenger complex |
| Cyc | Cyclin |
| DP | Dimerization partner |
| MAD2 | Mitotic arrest deficient 2 |
| MAPs | Microtubule-associated proteins |
| MAPK | Mitogen-activated protein kinase |
| MCC | Mitotic checkpoint complex |
| MPF | Mitosis-promoting factor |
| NACK1 | NPK1-activating kinesin-like protein 1 |
| PP1-2A, B, C | Protein Phosphatase 1-2A, B, C |
| PPB | Preprophase band |
| Rb | Retinoblastoma protein |
| RBR | Retinoblastoma-related proteins |
| ROS | Reactive oxygen species |
| SAC | Spindle assembly checkpoint |
| SMC | Structural maintenance of chromosome protein |
| TPX2 | Targeting Protein of XKLP2 |
| γ-TuRC | γ-tubulin ring complex |
References
- Dehnert, G.K.; Karasov, W.H.; Wolman, M.A. 2,4-Dichlorophenoxyacetic Acid Containing Herbicide Impairs Essential Visually Guided Behaviors of Larval Fish. Aquat. Toxicol. 2019, 209, 1–12. [Google Scholar] [CrossRef]
- Nakka, S.; Jugulam, M.; Peterson, D.; Asif, M. Herbicide Resistance: Development of Wheat Production Systems and Current Status of Resistant Weeds in Wheat Cropping Systems. Crop J. 2019, 7, 750–760. [Google Scholar] [CrossRef]
- Perrin, L.; Spinosi, J.; Chaperon, L.; Kab, S.; Moisan, F.; Ebaz, A. Pesticides Expenditures by Farming Type and Incidence of Parkinson Disease in Farmers: A French Nationwide Study. Environ. Res. 2021, 197, 111161. [Google Scholar] [CrossRef]
- Schebesta, H.; Candel, J.J.L. Game-Changing Potential of the EU’s Farm to Fork Strategy. Nat. Food 2020, 1, 586–588. [Google Scholar] [CrossRef]
- Kocira, A.; Staniak, M.; Tomaszewska, M.; Kornas, R.; Cymerman, J.; Panasiewicz, K.; Lipińska, H. Legume Cover Crops as One of the Elements of Strategic Weed Management and Soil Quality Improvement. A Review. Agriculture 2020, 10, 394. [Google Scholar] [CrossRef]
- Sosnoskie, L.M.; Duke, S.O. Implications of Weakening of the United States Geological Survey Pesticide National Synthesis Project for Weed Scientists. Weed Sci. 2023, 71, 517–519. [Google Scholar] [CrossRef]
- Miller, Z.J.; Hubbel, K. Integrating Mechanical and Cultural Methods for Weed Control in Organic Chickpea. Weed Sci. 2024, 72, 774–781. [Google Scholar] [CrossRef]
- Abd-ElGawad, A.M.; Elshamy, A.I.; Al-Rowaily, S.L.; El-Amier, Y.A. Habitat Affects the Chemical Profile, Allelopathy, and Antioxidant Properties of Essential Oils and Phenolic Enriched Extracts of the Invasive Plant Heliotropium Curassavicum. Plants 2019, 8, 482. [Google Scholar] [CrossRef]
- Araniti, F.; Lupini, A.; Sorgonà, A.; Conforti, F.; Marrelli, M.; Statti, G.A.; Menichini, F.; Abenavoli, M.R. Allelopathic Potential of Artemisia arborescens: Isolation, Identification and Quantification of Phytotoxic Compounds through Fractionation-Guided Bioassays. Nat. Prod. Res. 2013, 27, 880–887. [Google Scholar] [CrossRef]
- Han, C.; Shao, H.; Zhou, S.; Mei, Y.; Cheng, Z.; Huang, L.; Lv, G. Chemical Composition and Phytotoxicity of Essential Oil from Invasive Plant, Ambrosia artemisiifolia L. Ecotoxicol. Environ. Saf. 2021, 211, 111879. [Google Scholar] [CrossRef]
- Li, J.; Chen, H.; Guo, C.; Chen, Q.; Zhao, T.; Chen, X.; Du, Y.; Du, H.; Miao, Y.; Liu, D. Artemisia argyi Essential Oil Exerts Herbicidal Activity by Inhibiting Photosynthesis and Causing Oxidative Damage. Ind. Crops Prod. 2023, 194, 116258. [Google Scholar] [CrossRef]
- Satyal, P.; Paudel, P.; Poudel, A.; Dosoky, N.S.; Moriarity, D.M.; Vogler, B.; Setzer, W.N. Chemical Compositions, Phytotoxicity, and Biological Activities of Acorus calamus Essential Oils from Nepal. Nat. Prod. Commun. 2013, 8, 1179–1181. [Google Scholar] [CrossRef]
- Zhou, S.; Han, C.; Zhang, C.; Kuchkarova, N.; Wei, C.; Zhang, C.; Shao, H. Allelopathic, Phytotoxic, and Insecticidal Effects of Thymus proximus Serg. Essential Oil and Its Major Constituents. Front. Plant Sci. 2021, 12, 689875. [Google Scholar] [CrossRef] [PubMed]
- Schandry, N.; Becker, C. Allelopathic Plants: Models for Studying Plant–Interkingdom Interactions. Trends Plant Sci. 2020, 25, 176–185. [Google Scholar] [CrossRef]
- Raveau, R.; Fontaine, J.; Lounès-Hadj Sahraoui, A. Essential Oils as Potential Alternative Biocontrol Products against Plant Pathogens and Weeds: A Review. Foods 2020, 9, 365. [Google Scholar] [CrossRef] [PubMed]
- Verdeguer, M.; Sánchez-Moreiras, A.M.; Araniti, F. Phytotoxic Effects and Mechanism of Action of Essential Oils and Terpenoids. Plants 2020, 9, 1571. [Google Scholar] [CrossRef] [PubMed]
- Pouresmaeil, M.; Movafeghi, A.; Sabzi-Nojadeh, M.; Kosari-Nasab, M.; Maggi, F. Exposure of Avena fatua L. Seedlings to Artemisia austriaca Jacq. Essential Oil, 1,8-Cineole, and Camphor Induces Oxidative Stress and Reduces Cell Viability. Ind. Crops Prod. 2024, 222, 119636. [Google Scholar] [CrossRef]
- Rienth, M.; Crovadore, J.; Ghaffari, S.; Lefort, F. Oregano Essential Oil Vapour Prevents Plasmopara viticola Infection in Grapevine (Vitis vinifera) and Primes Plant Immunity Mechanisms. PLoS ONE 2019, 14, e0222854. [Google Scholar] [CrossRef]
- Rys, M.; Miastkowska, M.; Łętocha, A.; Wajs-Bonikowska, A.; Lorenzo, P.; Synowiec, A. The Effect of Caraway Oil-Loaded Bio-Nanoemulsions on the Growth and Performance of Barnyard Grass and Maize. Sci. Rep. 2024, 14, 4313. [Google Scholar] [CrossRef]
- Sarheed, M.M.; Rajabi, F.; Kunert, M.; Boland, W.; Wetters, S.; Miadowitz, K.; Kaźmierczak, A.; Sahi, V.P.; Nick, P. Cellular Base of Mint Allelopathy: Menthone Affects Plant Microtubules. Front. Plant Sci. 2020, 11, 546345. [Google Scholar] [CrossRef]
- Synowiec, A.; Możdżeń, K.; Krajewska, A.; Landi, M.; Araniti, F. Carum carvi L. Essential Oil: A Promising Candidate for Botanical Herbicide against Echinochloa crus-galli (L.) P. Beauv. in Maize Cultivation. Ind. Crops Prod. 2019, 140, 111652. [Google Scholar] [CrossRef]
- Wróblewski, M.; Piotrowska-Niczyporuk, A.; Ciereszko, I.; Gocek, N.; Żabka, A.; Szczeblewski, P.; Sobiech, Ł.; Saja-Garbarz, D.; Polit, J.T. Phytotoxicity and Bioherbicidal Potential of Sweet Flag (Acorus calamus L.) Essential Oil on Fabaceae and Brassicaceae Species. Sci. Hortic. 2025, 347, 114180. [Google Scholar] [CrossRef]
- Araniti, F.; Landi, M.; Lupini, A.; Sunseri, F.; Guidi, L.; Abenavoli, M.R. Origanum Vulgare Essential Oils Inhibit Glutamate and Aspartate Metabolism Altering the Photorespiratory Pathway in Arabidopsis thaliana Seedlings. J. Plant Physiol. 2018, 231, 297–309. [Google Scholar] [CrossRef] [PubMed]
- Ben Kaab, S.; Fernández Pierna, J.A.; Foncoux, B.; Compère, P.; Baeten, V.; Jijakli, M.H. Biochemical and Physiological Responses of Weeds to the Application of a Botanical Herbicide Based on Cinnamon Essential Oil. Plants 2024, 13, 3432. [Google Scholar] [CrossRef]
- De Andrade Santiago, J.; Das Graças Cardoso, M.; Aparecida Da Cruz, F.; Palmieri, M.J.; Vieira De Souza, R.; Soares, L.I.; De Campos, J.M.S.; Andrade-Vieira, L.F. Cytogenotoxic Effect of Essential Oil from Backhousia citriodora L. (Myrtaceae) on Meristematic Cells of Lactuca sativa L. S. Afr. J. Bot. 2017, 112, 515–520. [Google Scholar] [CrossRef]
- Ghimire, B.K.; Hwang, M.H.; Sacks, E.J.; Yu, C.Y.; Kim, S.H.; Chung, I.M. Screening of Allelochemicals in Miscanthus Sacchariflorus Extracts and Assessment of Their Effects on Germination and Seedling Growth of Common Weeds. Plants 2020, 9, 1313. [Google Scholar] [CrossRef]
- López-González, D.; Graña, E.; Teijeira, M.; Verdeguer, M.; Reigosa, M.J.; Sánchez-Moreiras, A.M.; Araniti, F. Similarities on the Mode of Action of the Terpenoids Citral and Farnesene in Arabidopsis Seedlings Involve Interactions with DNA Binding Proteins. Plant Physiol. Biochem. 2023, 196, 507–519. [Google Scholar] [CrossRef]
- Fagodia, S.K.; Singh, H.P.; Batish, D.R.; Kohli, R.K. Phytotoxicity and Cytotoxicity of Citrus aurantiifolia Essential Oil and Its Major Constituents: Limonene and Citral. Ind. Crops Prod. 2017, 108, 708–715. [Google Scholar] [CrossRef]
- Singh, N.; Singh, H.P.; Batish, D.R.; Kohli, R.K.; Yadav, S.S. Chemical Characterization, Phytotoxic, and Cytotoxic Activities of Essential Oil of Mentha longifolia. Environ. Sci. Pollut. Res. 2020, 27, 13512–13523. [Google Scholar] [CrossRef]
- Li, J.; Zhao, T.; Chen, L.; Chen, H.; Luo, D.; Chen, C.; Miao, Y.; Liu, D. Artemisia Argyi Allelopathy: A Generalist Compromises Hormone Balance, Element Absorption, and Photosynthesis of Receptor Plants. BMC Plant Biol. 2022, 22, 368. [Google Scholar] [CrossRef]
- Araniti, F.; Graña, E.; Krasuska, U.; Bogatek, R.; Reigosa, M.J.; Abenavoli, M.R.; Sánchez-Moreiras, A.M. Loss of Gravitropism in Farnesene-Treated Arabidopsis Is Due to Microtubule Malformations Related to Hormonal and ROS Unbalance. PLoS ONE 2016, 11, e0160202. [Google Scholar] [CrossRef]
- Chaimovitsh, D.; Rogovoy (Stelmakh), O.; Altshuler, O.; Belausov, E.; Abu-Abied, M.; Rubin, B.; Sadot, E.; Dudai, N. The Relative Effect of Citral on Mitotic Microtubules in Wheat Roots and BY2 Cells. Plant Biol. 2012, 14, 354–364. [Google Scholar] [CrossRef]
- Pawlowski, Â.; Kaltchuk-Santos, E.; Zini, C.A.; Caramão, E.B.; Soares, G.L.G. Essential Oils of Schinus Terebinthifolius and S. Molle (Anacardiaceae): Mitodepressive and Aneugenic Inducers in Onion and Lettuce Root Meristems. S. Afr. J. Bot. 2012, 80, 96–103. [Google Scholar] [CrossRef]
- Bai, D.; Li, X.; Wang, S.; Zhang, T.; Wei, Y.; Wang, Q.; Dong, W.; Song, J.; Gao, P.; Li, Y.; et al. Advances in Extraction Methods, Chemical Constituents, Pharmacological Activities, Molecular Targets and Toxicology of Volatile Oil from Acorus calamus var. angustatus Besser. Front. Pharmacol. 2022, 13, 1004529. [Google Scholar] [CrossRef]
- Das, B.K.; Swamy, A.V.; Koti, B.C.; Gadad, P.C. Experimental Evidence for Use of Acorus calamus (Asarone) for Cancer Chemoprevention. Heliyon 2019, 5, e01585. [Google Scholar] [CrossRef] [PubMed]
- Rajput, S.B.; Tonge, M.B.; Karuppayil, S.M. An Overview on Traditional Uses and Pharmacological Profile of Acorus calamus Linn. (Sweet Flag) and Other Acorus Species. Phytomedicine 2014, 21, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, P.K.; Rai, S.; Kumar, V.; Mukherjee, K.; Hylands, P.; Hider, R. Plants of Indian Origin in Drug Discovery. Expert Opin. Drug Discov. 2007, 2, 633–657. [Google Scholar] [CrossRef] [PubMed]
- O’Neill, A.R.; Badola, H.K.; Dhyani, P.P.; Rana, S.K. Integrating Ethnobiological Knowledge into Biodiversity Conservation in the Eastern Himalayas. J. Ethnobiol. Ethnomedicine 2017, 13, 21. [Google Scholar] [CrossRef]
- Lam, K.Y.C.; Yao, P.; Wang, H.; Duan, R.; Dong, T.T.X.; Tsim, K.W.K. Asarone from Acori tatarinowii Rhizome Prevents Oxidative Stress-Induced Cell Injury in Cultured Astrocytes: A Signaling Triggered by Akt Activation. PLoS ONE 2017, 12, e0179077. [Google Scholar] [CrossRef]
- Uebel, T.; Hermes, L.; Haupenthal, S.; Müller, L.; Esselen, M. α-Asarone, Β-asarone, and Γ-asarone: Current Status of Toxicological Evaluation. J. Appl. Toxicol. 2021, 41, 1166–1179. [Google Scholar] [CrossRef]
- Wróblewski, M.; Krajewski, K.; Gocek, N.; Żabka, A.; Polit, J.T. Acorus Calamus L. Essential Oil Induces Oxidative Stress and DNA Replication Disruptions in Root Meristem Cells of Two Fabaceae and Two Brassicaceae Species. Int. J. Mol. Sci. 2025, 26, 4715. [Google Scholar] [CrossRef]
- Rajamanikkam, K.; Elaiya Raja, S.; Balaji, S.K.; Nadana Rajavadivu, G.; Sivasubramaniam, S.; Palanichelvam, K. Earthworm, a Novel In Vivo System to Validate Antimitotic Compounds. Turk. J. Zool. 2019, 43, 153–163. [Google Scholar] [CrossRef]
- Rajesh, C.; Palanimuthu, V.R.; Palanichelvam, K. Fatty Acids and Its Derivatives of Acorus calamus Linn. Rhizome Induce Stem Cell-Mediated Cell Division in Plants and Animals. Biocatal. Agric. Biotechnol. 2021, 36, 102153. [Google Scholar] [CrossRef]
- Polit, J.T. Protein Phosphorylation in Vicia faba Root Meristem Cells During the First Steps of Leaving Principal Control Points after Sucrose Application. Plant Cell Rep. 2009, 28, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Polit, J.T.; Ciereszko, I. Sucrose Synthase Activity and Carbohydrates Content in Relation to Phosphorylation Status of Vicia faba Root Meristems During Reactivation from Sugar Depletion. J. Plant Physiol. 2012, 169, 1597–1606. [Google Scholar] [CrossRef]
- Polit, J.T.; Ciereszko, I. In Situ Activities of Hexokinase and Fructokinase in Relation to Phosphorylation Status of Root Meristem Cells of Vicia faba during Reactivation from Sugar Starvation. Physiol. Plant. 2009, 135, 342–350. [Google Scholar] [CrossRef]
- Polit, J.; Maszewski, J. Protein Phosphorylation during the Transitions from G1 Arrest Point to S and G2 Arrest Point to M in Vicia faba Root Meristem. Biol. Plant. 2004, 48, 351–359. [Google Scholar] [CrossRef]
- Shimotohno, A.; Aki, S.S.; Takahashi, N.; Umeda, M. Regulation of the Plant Cell Cycle in Response to Hormones and the Environment. Annu. Rev. Plant Biol. 2021, 72, 273–296. [Google Scholar] [CrossRef]
- Carneiro, A.K.; Montessoro, P.D.F.; Fusaro, A.F.; Araújo, B.G.; Hemerly, A.S. Plant CDKs—Driving the Cell Cycle Through Climate Change. Plants 2021, 10, 1804. [Google Scholar] [CrossRef] [PubMed]
- Joubès, J.; Chevalier, C. Endoreduplication in Higher Plants. Plant Mol. Biol. 2000, 43, 735–745. [Google Scholar] [CrossRef]
- Mironov, V.; De Veylder, L.; Van Montagu, M.; Inzé, D. Cyclin-Dependent Kinases and Cell Division in Plants—The Nexus. Plant Cell 1999, 11, 509–521. [Google Scholar] [CrossRef]
- Vandepoele, K.; Raes, J.; De Veylder, L.; Rouzé, P.; Rombauts, S.; Inzé, D. Genome-Wide Analysis of Core Cell Cycle Genes in Arabidopsis. Plant Cell 2002, 14, 903–916. [Google Scholar] [CrossRef]
- Gutierrez, C. The Arabidopsis Cell Division Cycle. Arab. Book 2009, 7, e0120. [Google Scholar] [CrossRef]
- Pereira, C.; Lemischka, I.R.; Moore, K. Reprogramming Cell Fates: Insights from Combinatorial Approaches. Ann. N. Y. Acad. Sci. 2012, 1266, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Sablowski, R.; Carnier Dornelas, M. Interplay Between Cell Growth and Cell Cycle in Plants. J. Exp. Bot. 2014, 65, 2703–2714. [Google Scholar] [CrossRef] [PubMed]
- Boruc, J.; Van Den Daele, H.; Hollunder, J.; Rombauts, S.; Mylle, E.; Hilson, P.; Inzé, D.; De Veylder, L.; Russinova, E. Functional Modules in the Arabidopsis Core Cell Cycle Binary Protein–Protein Interaction Network. Plant Cell 2010, 22, 1264–1280. [Google Scholar] [CrossRef]
- Churchman, M.L.; Brown, M.L.; Kato, N.; Kirik, V.; Hülskamp, M.; Inzé, D.; De Veylder, L.; Walker, J.D.; Zheng, Z.; Oppenheimer, D.G.; et al. SIAMESE, a Plant-Specific Cell Cycle Regulator, Controls Endoreplication Onset in Arabidopsis thaliana. Plant Cell 2006, 18, 3145–3157. [Google Scholar] [CrossRef]
- Lipavská, H.; Mašková, P.; Vojvodová, P. Regulatory Dephosphorylation of CDK at G2/M in Plants: Yeast Mitotic Phosphatase Cdc25 Induces Cytokinin-like Effects in Transgenic Tobacco Morphogenesis. Ann. Bot. 2011, 107, 1071–1086. [Google Scholar] [CrossRef] [PubMed]
- Nakai, T.; Kato, K.; Shinmyo, A.; Sekine, M. Arabidopsis KRPs Have Distinct Inhibitory Activity toward Cyclin D2-associated Kinases, Including Plant-specific B-type Cyclin-dependent Kinase. FEBS Lett. 2006, 580, 336–340. [Google Scholar] [CrossRef]
- Peres, A.; Churchman, M.L.; Hariharan, S.; Himanen, K.; Verkest, A.; Vandepoele, K.; Magyar, Z.; Hatzfeld, Y.; Van Der Schueren, E.; Beemster, G.T.S.; et al. Novel Plant-Specific Cyclin-Dependent Kinase Inhibitors Induced by Biotic and Abiotic Stresses. J. Biol. Chem. 2007, 282, 25588–25596. [Google Scholar] [CrossRef]
- Verkest, A.; Weinl, C.; Inzé, D.; De Veylder, L.; Schnittger, A. Switching the Cell Cycle. Kip-Related Proteins in Plant Cell Cycle Control. Plant Physiol. 2005, 139, 1099–1106. [Google Scholar] [CrossRef]
- Wang, L.; Ruan, Y.-L. Regulation of Cell Division and Expansion by Sugar and Auxin Signaling. Front. Plant Sci. 2013, 4, 163. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Alvim Kamei, C.L.; Cools, T.; Vanderauwera, S.; Takahashi, N.; Okushima, Y.; Eekhout, T.; Yoshiyama, K.O.; Larkin, J.; Van Den Daele, H.; et al. The Arabidopsis SIAMESE-RELATED Cyclin-Dependent Kinase Inhibitors SMR5 and SMR7 Regulate the DNA Damage Checkpoint in Response to Reactive Oxygen Species. Plant Cell 2014, 26, 296–309. [Google Scholar] [CrossRef] [PubMed]
- Cook, G.S.; Grønlund, A.L.; Siciliano, I.; Spadafora, N.; Amini, M.; Herbert, R.J.; Bitonti, M.B.; Graumann, K.; Francis, D.; Rogers, H.J. Plant WEE1 Kinase Is Cell Cycle Regulated and Removed at Mitosis via the 26S Proteasome Machinery. J. Exp. Bot. 2013, 64, 2093–2106. [Google Scholar] [CrossRef] [PubMed]
- Cools, T.; De Veylder, L. DNA Stress Checkpoint Control and Plant Development. Curr. Opin. Plant Biol. 2009, 12, 23–28. [Google Scholar] [CrossRef]
- Perry, J.A.; Kornbluth, S. Cdc25 and Wee1: Analogous Opposites? Cell Div. 2007, 2, 12. [Google Scholar] [CrossRef]
- Abraham, E.; Miskolczi, P.; Ayaydin, F.; Yu, P.; Kotogany, E.; Bako, L.; Otvos, K.; Horvath, G.V.; Dudits, D. Immunodetection of Retinoblastoma-Related Protein and Its Phosphorylated Form in Interphase and Mitotic Alfalfa Cells. J. Exp. Bot. 2011, 62, 2155–2168. [Google Scholar] [CrossRef]
- Ramirez-Parra, E.; Gutierrez, C. The Many Faces of Chromatin Assembly Factor 1. Trends Plant Sci. 2007, 12, 570–576. [Google Scholar] [CrossRef]
- Spadafora, N.D.; Doonan, J.H.; Herbert, R.J.; Bitonti, M.B.; Wallace, E.; Rogers, H.J.; Francis, D. Arabidopsis T-DNA Insertional Lines for CDC25 Are Hypersensitive to Hydroxyurea but Not to Zeocin or Salt Stress. Ann. Bot. 2011, 107, 1183–1192. [Google Scholar] [CrossRef]
- Naouar, N.; Vandepoele, K.; Lammens, T.; Casneuf, T.; Zeller, G.; Van Hummelen, P.; Weigel, D.; Rätsch, G.; Inzé, D.; Kuiper, M.; et al. Quantitative RNA Expression Analysis with Affymetrix Tiling 1.0R Arrays Identifies New E2F Target Genes. Plant J. 2009, 57, 184–194, Erratum in Plant J. 2017, 91, 1129–1130.. [Google Scholar] [CrossRef]
- Rybaczek, D.; Maszewski, J. Induction of Foci of Phosphorylated H2AX Histones and Premature Chromosome Condensation after DNA Damage in Vicia faba Root Meristem. Biol. Plant. 2007, 51, 443–450. [Google Scholar] [CrossRef]
- Rybaczek, D.; Maszewski, J. Phosphorylation of H2AX Histones in Response to Double-Strand Breaks and Induction of Premature Chromatin Condensation in Hydroxyurea-Treated Root Meristem Cells of Raphanus sativus, Vicia faba, and Allium porrum. Protoplasma 2007, 230, 31–39. [Google Scholar] [CrossRef]
- Żabka, A.; Polit, J.T.; Maszewski, J. DNA Replication Stress Induces Deregulation of the Cell Cycle Events in Root Meristems of Allium cepa. Ann. Bot. 2012, 110, 1581–1591. [Google Scholar] [CrossRef]
- Dahan, J.; Wendehenne, D.; Ranjeva, R.; Pugin, A.; Bourque, S. Nuclear Protein Kinases: Still Enigmatic Components in Plant Cell Signalling. New Phytol. 2010, 185, 355–368. [Google Scholar] [CrossRef]
- Francis, D. A Commentary on the G2/M Transition of the Plant Cell Cycle. Ann. Bot. 2011, 107, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Shimotohno, A.; Umeda-Hara, C.; Bisova, K.; Uchimiya, H.; Umeda, M. The Plant-Specific Kinase CDKF;1 Is Involved in Activating Phosphorylation of Cyclin-Dependent Kinase-Activating Kinases in Arabidopsis. Plant Cell 2004, 16, 2954–2966. [Google Scholar] [CrossRef] [PubMed]
- Dissmeyer, N.; Weimer, A.K.; De Veylder, L.; Novak, B.; Schnittger, A. The Regulatory Network of Cell-Cycle Progression Is Fundamentally Different in Plants versus Yeast or Metazoans. Plant Signal. Behav. 2010, 5, 1613–1618. [Google Scholar] [CrossRef]
- Berckmans, B.; De Veylder, L. Transcriptional Control of the Cell Cycle. Curr. Opin. Plant Biol. 2009, 12, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Kiyokawa, H.; Ray, D. In Vivo Roles of CDC25 Phosphatases: Biological Insight into the Anti-Cancer Therapeutic Targets. Anti-Cancer Agents Med. Chem. 2008, 8, 832–836. [Google Scholar] [CrossRef]
- Nurse, P. Cyclin Dependent Kinases and Cell Cycle Control (Nobel Lecture) Copyright © the Nobel Foundation, 2002. We Thank the Nobel Foundation, Stockholm, for Permission to Print This Lecture. ChemBioChem 2002, 3, 596. [Google Scholar] [CrossRef]
- Tyson, J.J.; Novak, B. Temporal Organization of the Cell Cycle. Curr. Biol. 2008, 18, R759–R768. [Google Scholar] [CrossRef]
- Gutierrez, C.; Sequeira-Mendes, J.; Aragüez, I. Replication of the Plant Genome. In Molecular Biology; Howell, S.H., Ed.; Springer: New York, NY, USA, 2014; pp. 1–23. ISBN 978-1-4614-7569-9. [Google Scholar]
- Liu, B.; Lee, Y.-R.J. Spindle Assembly and Mitosis in Plants. Annu. Rev. Plant Biol. 2022, 73, 227–254. [Google Scholar] [CrossRef] [PubMed]
- Kozgunova, E.; Suzuki, T.; Ito, M.; Higashiyama, T.; Kurihara, D. Haspin Has Multiple Functions in the Plant Cell Division Regulatory Network. Plant Cell Physiol. 2016, 57, 848–861. [Google Scholar] [CrossRef] [PubMed]
- Sarheed, M.; Schärer, H.-J.; Wang-Müller, Q.; Flury, P.; Maes, C.; Genva, M.; Fauconnier, M.-L.; Nick, P. Signal, Not Poison—Screening Mint Essential Oils for Weed Control Leads to Horsemint. Agriculture 2023, 13, 712. [Google Scholar] [CrossRef]
- Li, S.; Sun, T.; Ren, H. The Functions of the Cytoskeleton and Associated Proteins During Mitosis and Cytokinesis in Plant Cells. Front. Plant Sci. 2015, 6, 282. [Google Scholar] [CrossRef]
- Kaduchová, K.; Marchetti, C.; Ovečka, M.; Galuszka, P.; Bergougnoux, V.; Šamaj, J.; Pecinka, A. Spatial Organization and Dynamics of Chromosomes and Microtubules During Barley Mitosis. Plant J. 2023, 115, 602–613. [Google Scholar] [CrossRef]
- Komaki, S.; Takeuchi, H.; Hamamura, Y.; Heese, M.; Hashimoto, T.; Schnittger, A. Functional Analysis of the Plant Chromosomal Passenger Complex. Plant Physiol. 2020, 183, 1586–1599. [Google Scholar] [CrossRef]
- Weimer, A.K.; Demidov, D.; Lermontova, I.; Beeckman, T.; Van Damme, D. Aurora Kinases Throughout Plant Development. Trends Plant Sci. 2016, 21, 69–79. [Google Scholar] [CrossRef]
- Houben, A.; Demidov, D.; Caperta, A.D.; Karimi, R.; Agueci, F.; Vlasenko, L. Phosphorylation of Histone H3 in Plants—A Dynamic Affair. Biochim. Et Biophys. Acta (BBA) Gene Struct. Expr. 2007, 1769, 308–315. [Google Scholar] [CrossRef]
- Saki, G.; Eidi, A.; Mortazavi, P.; Panahi, N.; Vahdati, A. Effect of β-Asarone in Normal and β-Amyloid-Induced Alzheimeric Rats. Arch. Med. Sci. 2020, 16, 699–706. [Google Scholar] [CrossRef]
- Pawłowski, K.P. Regulation of Weeds as a Factor Affecting the Profitability of Narrow-Leafed Lupine Production. Ann. PAAAE 2018, 20, 205–211. [Google Scholar] [CrossRef]
- Report from the Commission to the Council and the European Parliament on the Development of Plant Proteins in the European Union; European Commision: Brussels, Belgium, 2018; pp. 1–15.
- Weber, E.A.; Gruber, S.; Claupein, W. Emergence and Performance of Volunteer Oilseed Rape (Brassica napus) in Different Crops. Eur. J. Agron. 2014, 60, 33–40. [Google Scholar] [CrossRef]
- Aragão, F.; Palmieri, M.; Ferreira, A.; Costa, A.; Queiroz, V.; Pinheiro, P.; Andrade-Vieira, L. Phytotoxic and Cytotoxic Effects of Eucalyptus Essential Oil on Lettuce (Lactuca sativa L.). Allelopath. J. 2015, 35, 259–272. [Google Scholar]
- Sunohara, Y.; Nakano, K.; Matsuyama, S.; Oka, T.; Matsumoto, H. Cuminaldehyde, a Cumin Seed Volatile Component, Induces Growth Inhibition, Overproduction of Reactive Oxygen Species and Cell Cycle Arrest in Onion Roots. Sci. Hortic. 2021, 289, 110493. [Google Scholar] [CrossRef]
- Szczeblewski, P.; Wróblewski, M.; Borzyszkowska-Bukowska, J.; Bairamova, T.; Górska, J.; Laskowski, T.; Samulewicz, A.; Kosno, M.; Sobiech, Ł.; Polit, J.T.; et al. The Role of Centrifugal Partition Chromatography in the Removal of β-Asarone from Acorus Calamus Essential Oil. Sci. Rep. 2022, 12, 22217. [Google Scholar] [CrossRef]
- Dvořák Tomaštíková, E.; Rutten, T.; Dvořák, P.; Tugai, A.; Ptošková, K.; Petrovská, B.; Van Damme, D.; Houben, A.; Doležel, J.; Demidov, D. Functional Divergence of Microtubule-Associated TPX2 Family Members in Arabidopsis thaliana. Int. J. Mol. Sci. 2020, 21, 2183. [Google Scholar] [CrossRef]
- Guo, Y.; Liu, C.; Zhang, Y.; Zheng, S.; Cao, P.; Wang, X.; Tian, Z. Characterization Key Genes of Arabidopsis Seedlings in Response to β-Caryophyllene, Eugenol Using Combined Transcriptome and WGCN Analysis. Front. Plant Sci. 2024, 14, 1295779. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Gu, M.; Xia, X.; Shi, K.; Zhou, Y.; Yu, J. Effects of Phenylcarboxylic Acids on Mitosis, Endoreduplication and Expression of Cell Cycle-Related Genes in Roots of Cucumber (Cucumis sativus L.). J. Chem. Ecol. 2009, 35, 679–688. [Google Scholar] [CrossRef]
- Nagle, A.A.; Gan, F.-F.; Jones, G.; So, C.-L.; Wells, G.; Chew, E.-H. Induction of Tumor Cell Death through Targeting Tubulin and Evoking Dysregulation of Cell Cycle Regulatory Proteins by Multifunctional Cinnamaldehydes. PLoS ONE 2012, 7, e50125. [Google Scholar] [CrossRef]
- Li, L.; Wu, M.; Wang, C.; Yu, Z.; Wang, H.; Qi, H.; Xu, X. β-Asarone Inhibits Invasion and EMT in Human Glioma U251 Cells by Suppressing Splicing Factor HnRNP A2/B1. Molecules 2018, 23, 671. [Google Scholar] [CrossRef] [PubMed]
- Polit, J.; Maszewski, J. Effect of OA-Inhibitor of Protein Phosphatases PP1 and PP2A—on Initiation of DNA Replication and Mitosis in Vicia faba Root Meristems. Acta Physiol. Plant. 2005, 27, 303–311. [Google Scholar] [CrossRef]
- Planchais, S.; Glab, N.; Inzé, D.; Bergounioux, C. Chemical Inhibitors: A Tool for Plant Cell Cycle Studies. FEBS Lett. 2000, 476, 78–83. [Google Scholar] [CrossRef]
- Liu, P.; Qi, M.; Xue, X.; Ren, H. Dynamics and Functions of the Actin Cytoskeleton during the Plant Cell Cycle. Chin. Sci. Bull. 2011, 56, 3504–3510. [Google Scholar] [CrossRef][Green Version]
- Hermes, L.; Haupenthal, S.; Uebel, T.; Esselen, M. DNA Double Strand Break Repair as Cellular Response to Genotoxic Asarone Isomers Considering Phase I Metabolism. Food Chem. Toxicol. 2020, 142, 111484. [Google Scholar] [CrossRef]
- Deng, X.; Higaki, T.; Lin, H.; Lee, Y.-R.J.; Liu, B. Acentrosomal Spindle Morphogenesis Is Dependent on the Unconventional TPX2 Family Protein TPXL3 and a Aurora Kinase in Arabidopsis thaliana. Res. Sq. 2021, 10. [Google Scholar] [CrossRef]
- Krupnova, T.; Sasabe, M.; Ghebreghiorghis, L.; Gruber, C.W.; Hamada, T.; Dehmel, V.; Strompen, G.; Stierhof, Y.-D.; Lukowitz, W.; Kemmerling, B.; et al. Microtubule-Associated Kinase-like Protein RUNKEL Needed for Cell Plate Expansion in Arabidopsis Cytokinesis. Curr. Biol. 2009, 19, 518–523. [Google Scholar] [CrossRef]
- Smertenko, A.P.; Chang, H.-Y.; Sonobe, S.; Fenyk, S.I.; Weingartner, M.; Bögre, L.; Hussey, P.J. Control of the AtMAP65-1 Interaction with Microtubules Through the Cell Cycle. J. Cell Sci. 2006, 119, 3227–3237. [Google Scholar] [CrossRef]
- Romeiro Motta, M.; Nédélec, F.; Saville, H.; Woelken, E.; Jacquerie, C.; Pastuglia, M.; Stolze, S.C.; Van De Slijke, E.; Böttger, L.; Belcram, K.; et al. The Cell Cycle Controls Spindle Architecture in Arabidopsis by Activating the Augmin Pathway. Dev. Cell 2024, 59, 2947–2961.e9. [Google Scholar] [CrossRef] [PubMed]
- Ayaydin, F.; Vissi, E.; Mészáros, T.; Miskolczi, P.; Kovács, I.; Fehér, A.; Dombrádi, V.; Erdödi, F.; Gergely, P.; Dudits, D. Inhibition of Serine/Threonine-specific Protein Phosphatases Causes Premature Activation of cdc2MsF Kinase at G2/M Transition and Early Mitotic Microtubule Organisation in Alfalfa. Plant J. 2000, 23, 85–96. [Google Scholar] [CrossRef]
- Polit, J.T.; Kaźmierczak, A. Okadaic Acid (1 μM) Accelerates S Phase and Mitosis but Inhibits Heterochromatin Replication and Metaphase–Anaphase Transition in Vicia faba Meristem Cells. J. Exp. Bot. 2007, 58, 2785–2797. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Casas-Mollano, J.A.; Xu, J.; Riethoven, J.-J.M.; Zhang, C.; Cerutti, H. Osmotic Stress Induces Phosphorylation of Histone H3 at Threonine 3 in Pericentromeric Regions of Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2015, 112, 8487–8492. [Google Scholar] [CrossRef]
- de Cárcer, G.; Manning, G.; Malumbres, M. From Plk1 to Plk5: Functional Evolution of Polo-like Kinases. Cell Cycle (Georget. Tex.) 2011, 10, 2255–2262. [Google Scholar] [CrossRef]
- Wang, F.; Ulyanova, N.P.; van der Waal, M.S.; Patnaik, D.; Lens, S.M.A.; Higgins, J.M.G. A Positive Feedback Loop Involving Haspin and Aurora B Promotes CPC Accumulation at Centromeres in Mitosis. Curr. Biol. 2011, 21, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Choy, M.S.; Nguyen, H.T.; Kumar, G.S.; Peti, W.; Kettenbach, A.N.; Page, R. A Protein Phosphatase 1 Specific Phos Phatase Ta Rgeting p Eptide (PhosTAP) to Identify the PP1 Phosphatome. Proc. Natl. Acad. Sci. USA 2024, 121, e2415383121. [Google Scholar] [CrossRef] [PubMed]
- Saurin, A.T. Kinase and Phosphatase Cross-Talk at the Kinetochore. Front. Cell Dev. Biol. 2018, 6, 62. [Google Scholar] [CrossRef] [PubMed]
- Broad, A.J.; DeLuca, J.G. The Right Place at the Right Time: Aurora B Kinase Localization to Centromeres and Kinetochores. Essays Biochem. 2020, 64, 299–311. [Google Scholar] [CrossRef]
- Park, C.; Kim, S.-I.; Ahn, Y.-J. Insecticidal Activity of Asarones Identified in Acorus gramineus Rhizome against Three Coleopteran Stored-Product Insects. J. Stored Prod. Res. 2003, 39, 333–342. [Google Scholar] [CrossRef]
- Maszewski, J.; Kaźmierczak, A.; Polit, J. Cell Cycle Arrest in Antheridial Extract-Treated Root Meristems of Allium cepa and Melandrium noctiflorum. Folia Histochem. Cytobiol. 1998, 36, 35–43. [Google Scholar]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of Image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]






Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wróblewski, M.; Gocek, N.; Żabka, A.; Polit, J.T. Mitotic Disruption and Cytoskeletal Alterations Induced by Acorus calamus Essential Oil: Implications for Bioherbicidal Potential. Int. J. Mol. Sci. 2025, 26, 8933. https://doi.org/10.3390/ijms26188933
Wróblewski M, Gocek N, Żabka A, Polit JT. Mitotic Disruption and Cytoskeletal Alterations Induced by Acorus calamus Essential Oil: Implications for Bioherbicidal Potential. International Journal of Molecular Sciences. 2025; 26(18):8933. https://doi.org/10.3390/ijms26188933
Chicago/Turabian StyleWróblewski, Mateusz, Natalia Gocek, Aneta Żabka, and Justyna T. Polit. 2025. "Mitotic Disruption and Cytoskeletal Alterations Induced by Acorus calamus Essential Oil: Implications for Bioherbicidal Potential" International Journal of Molecular Sciences 26, no. 18: 8933. https://doi.org/10.3390/ijms26188933
APA StyleWróblewski, M., Gocek, N., Żabka, A., & Polit, J. T. (2025). Mitotic Disruption and Cytoskeletal Alterations Induced by Acorus calamus Essential Oil: Implications for Bioherbicidal Potential. International Journal of Molecular Sciences, 26(18), 8933. https://doi.org/10.3390/ijms26188933





