Altered Gut Microbial Diversity and Depletion of SCFA-Producing Taxa Associated with ASD-like Phenotypes in a Prenatal VPA Rat Model
Abstract
1. Introduction
1.1. Existing Studies on the GBA in ASD Suffer from Several Limitations
1.2. Our Approach to Addressing These Gaps
2. Results
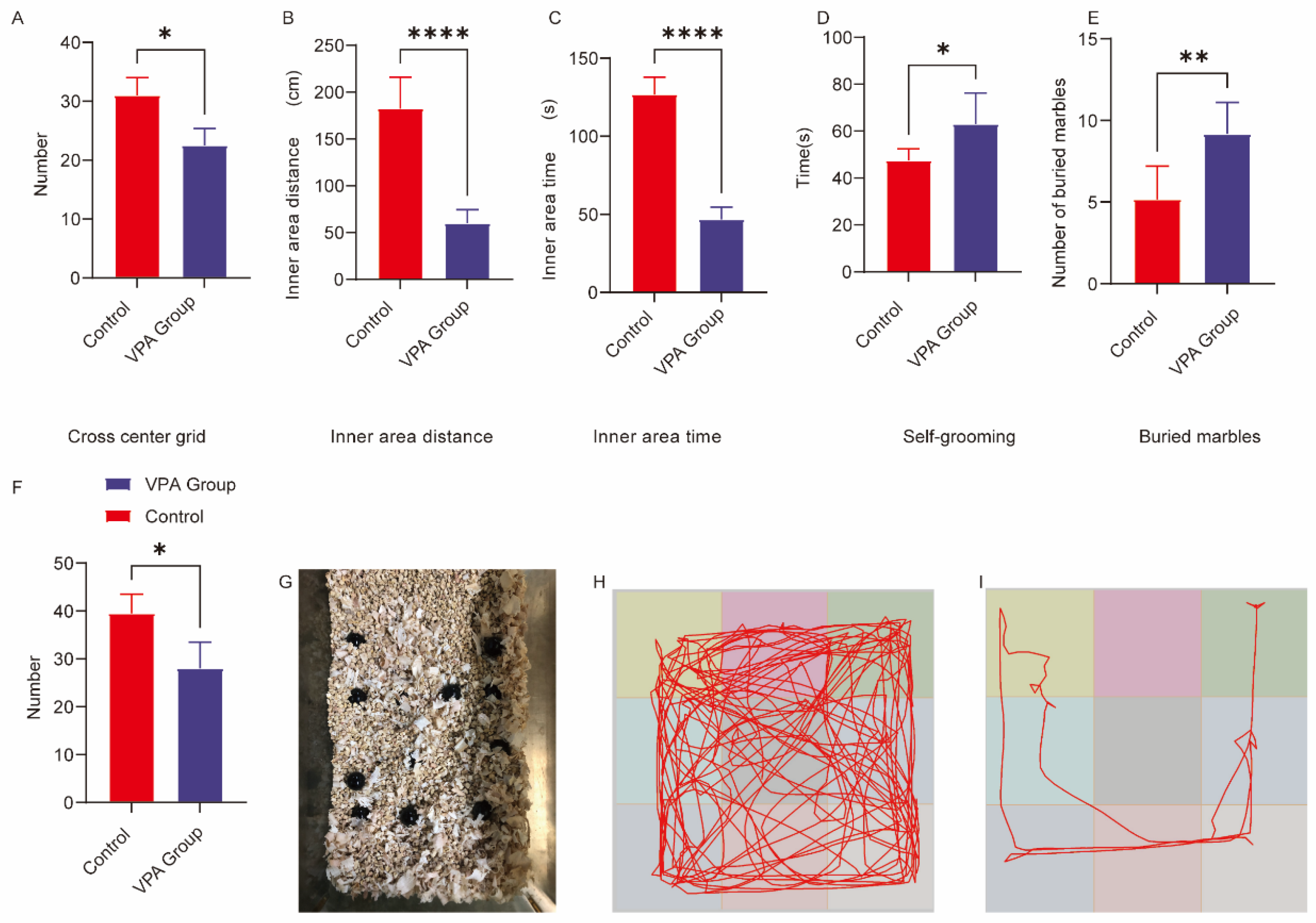
2.1. Open Field Test Behavioral Analysis
2.1.1. Exploratory Behavior Parameters
2.1.2. Anxiety-Related Behavioral Manifestations
2.2. Analysis of Repetitive Stereotypic Behaviors
- (1)
- Marble-burying Test
- (2)
- Self-grooming Behavior
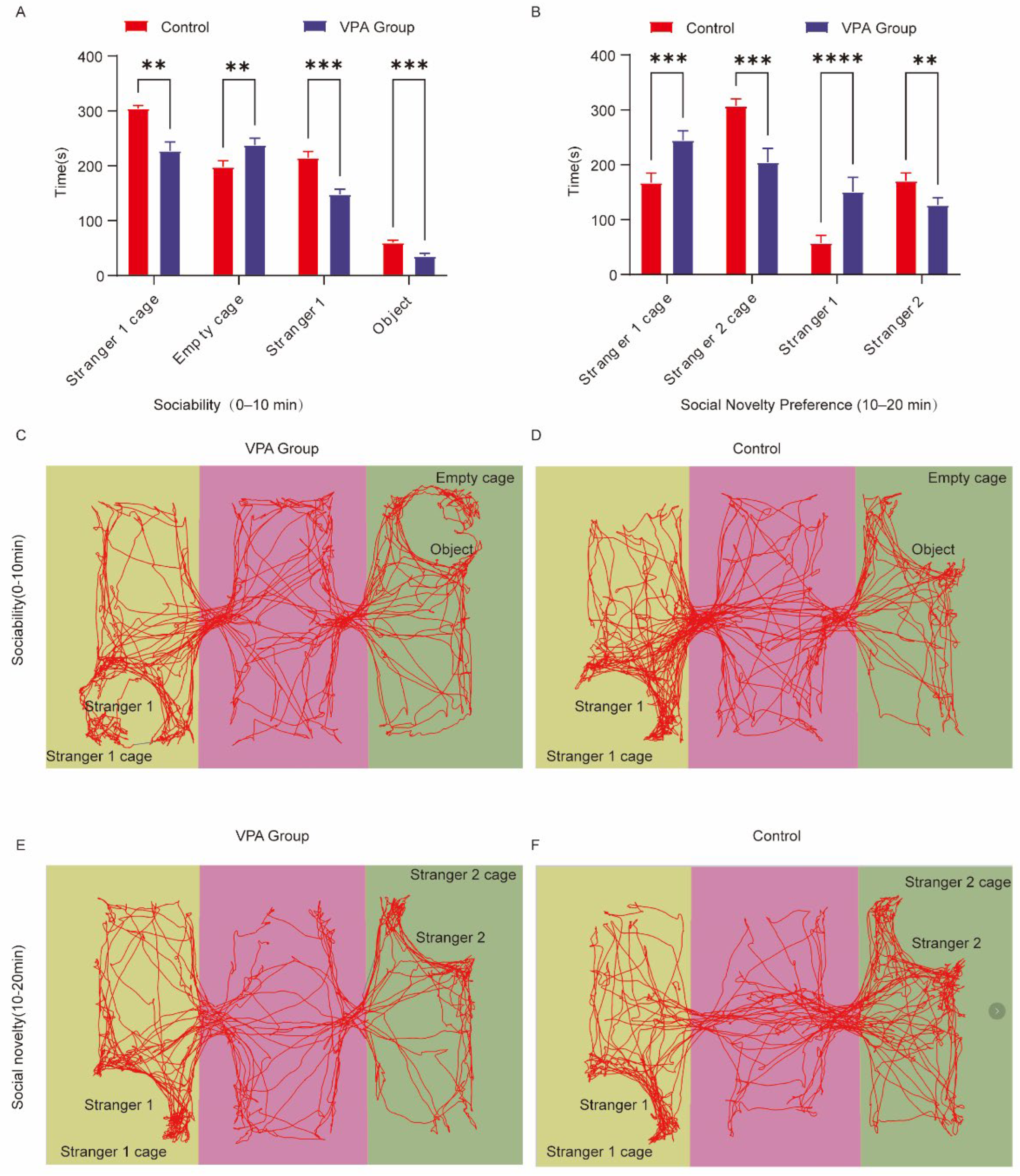
2.3. Three-Chamber Social Behavior Analysis
- Social Motivation Phase (Sociability, 0–10 min)
- (1)
- Spatial Exploration Preference
- (2)
- Social Interaction Patterns
- II.
- Social Novelty Preference Phase (10–20 min, Figure 2B)
- (1)
- Spatial Exploration Dynamics
- (2)
- Social Interaction Bias
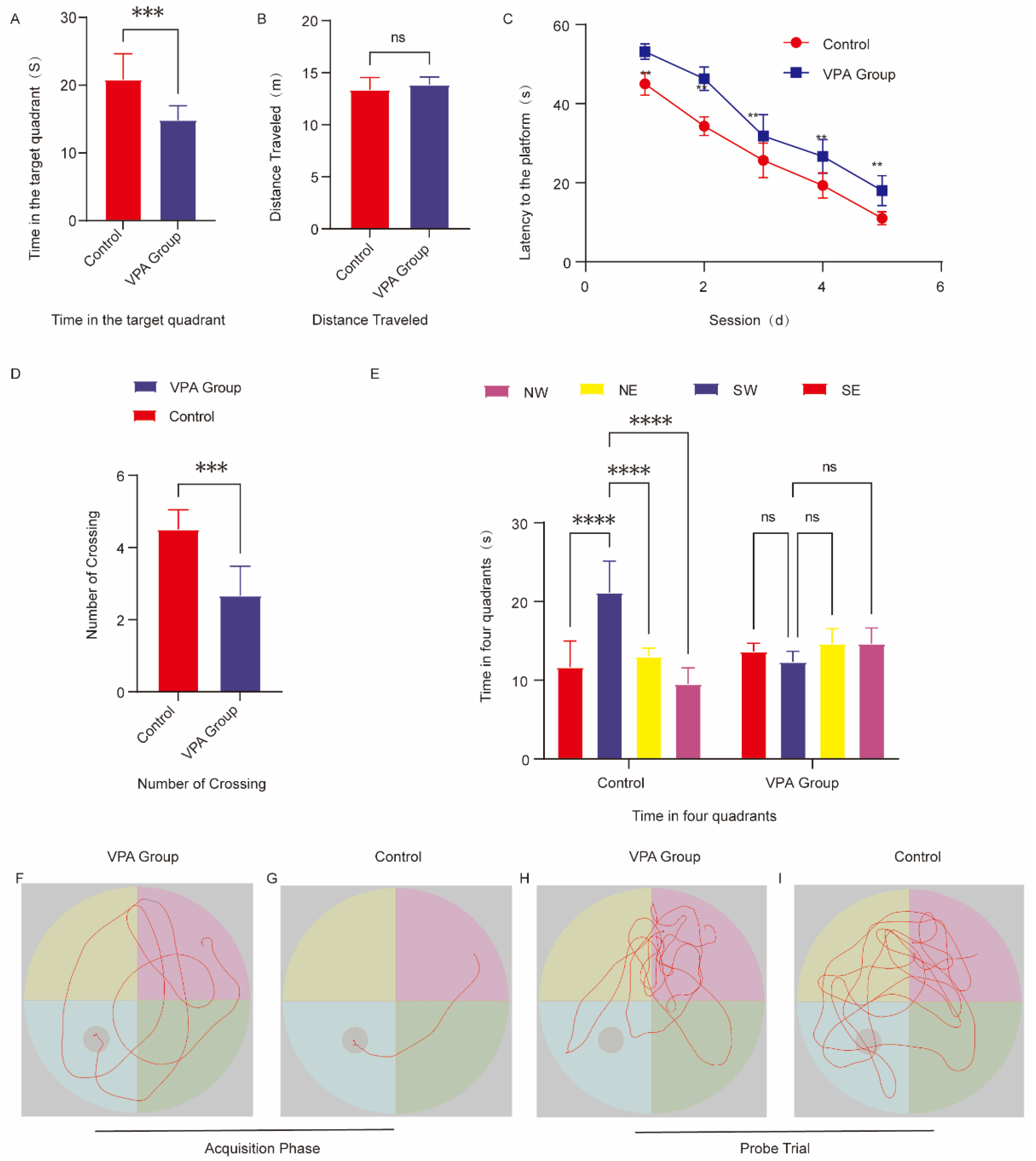
2.4. Assessment of Learning and Memory Capacity
2.4.1. Spatial Acquisition Trials
2.4.2. Spatial Probe Trials
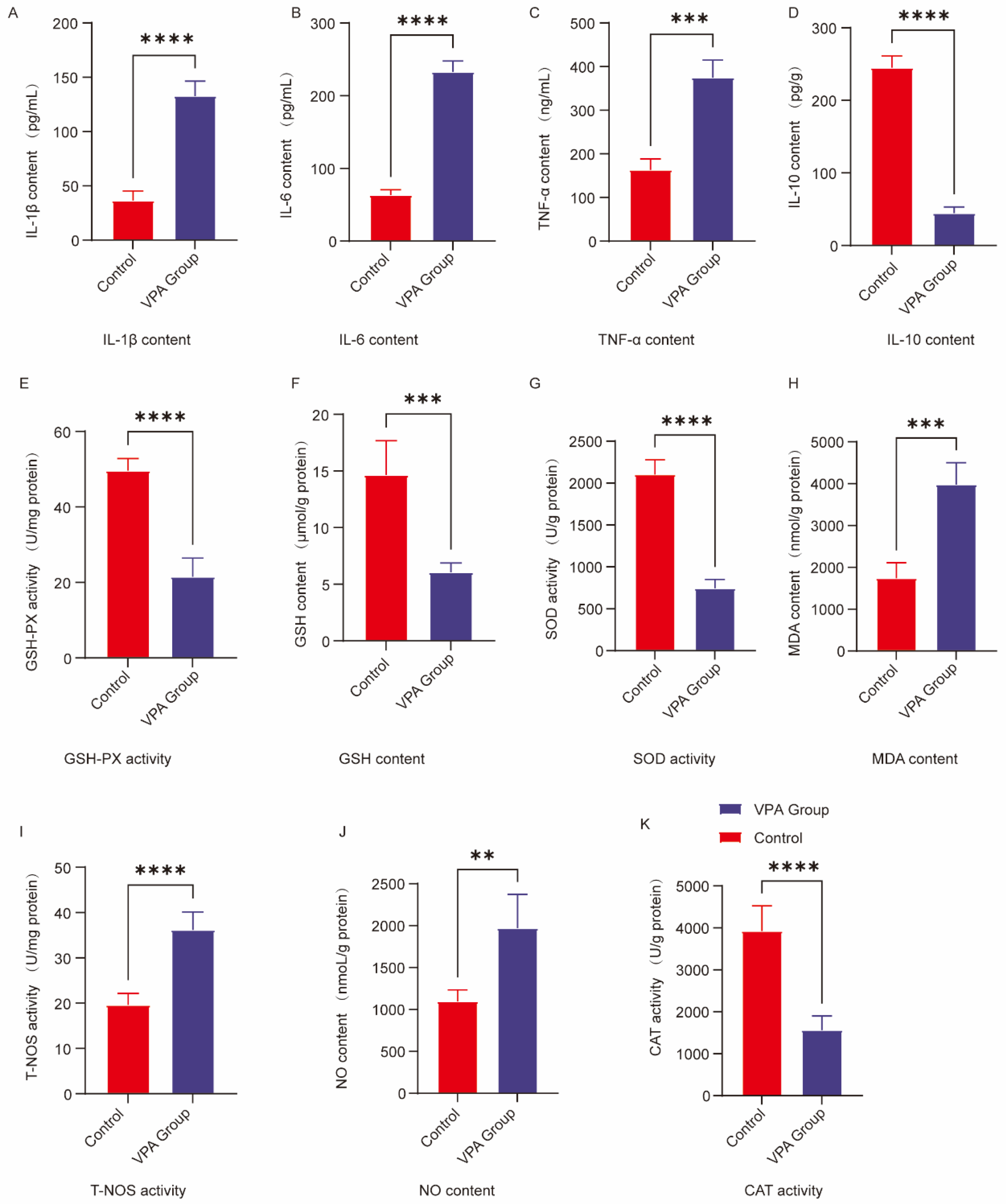
2.5. Analysis of Neuroinflammatory Markers in the Prefrontal Cortex
2.6. Analysis of Oxidative Stress Markers in the Prefrontal Cortex
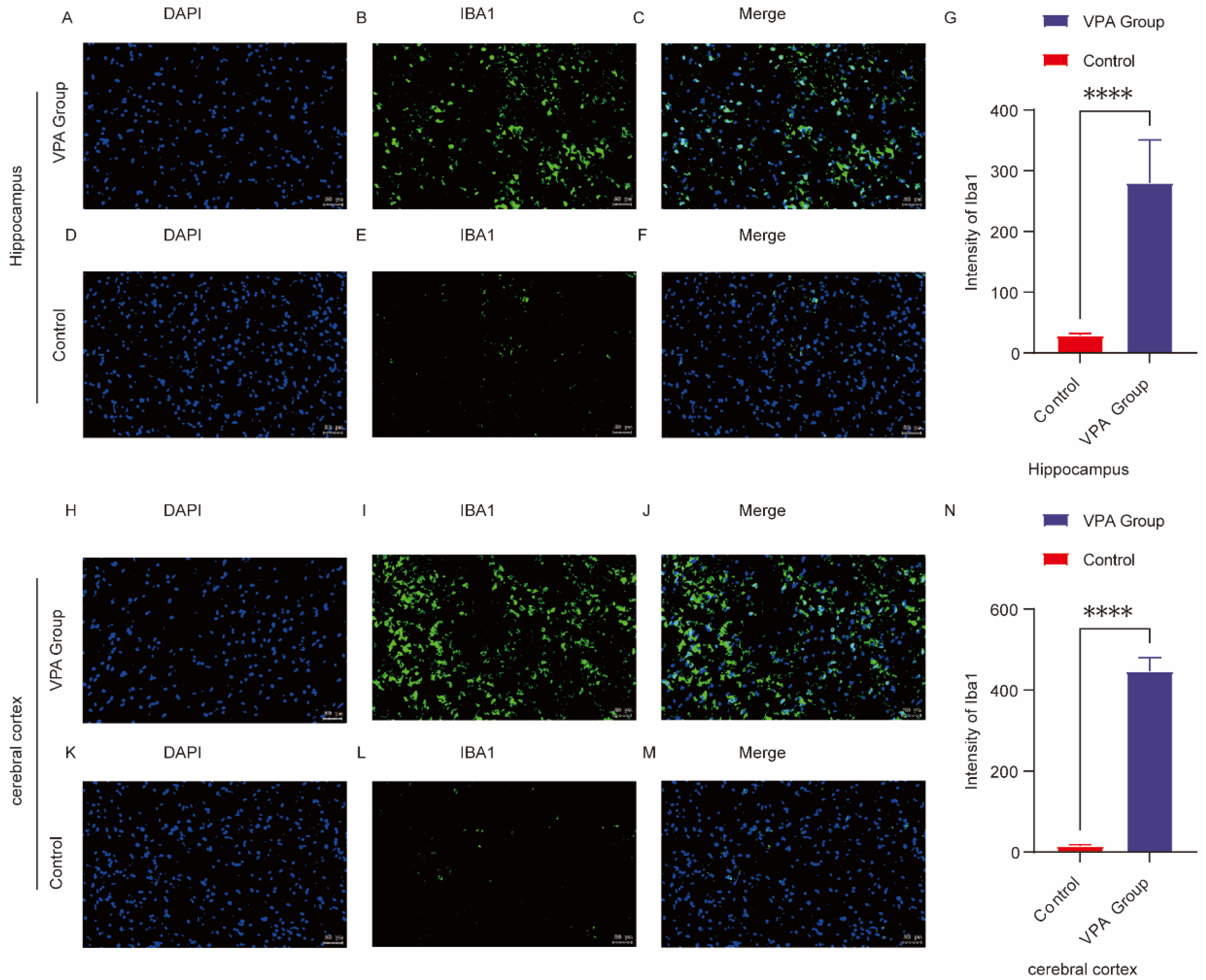
2.7. Microglia Activation and Neuroinflammation in ASD
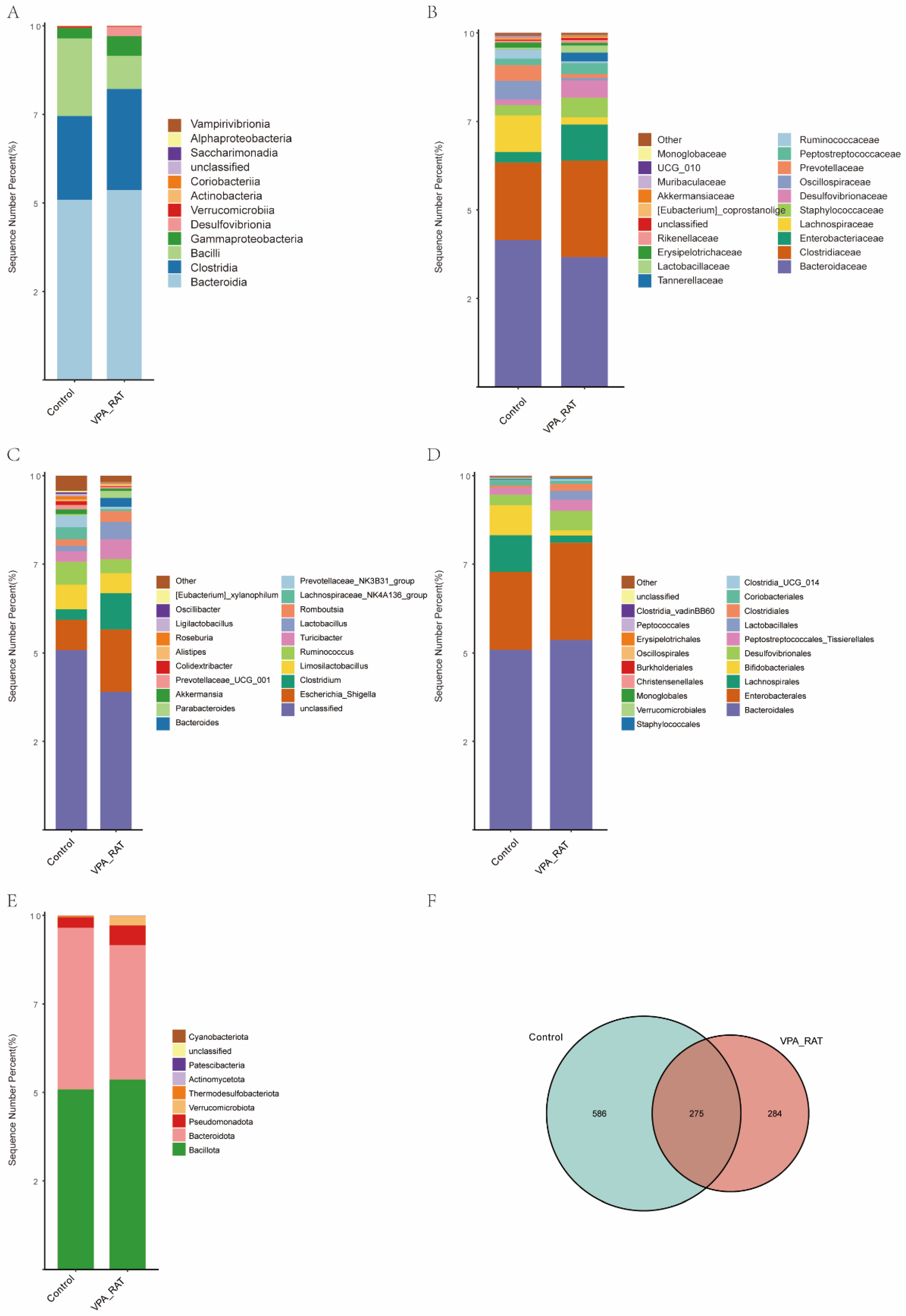
2.8. Taxonomic and Diversity Shifts in Gut Microbiota of VPA-Induced ASD Rat Models Versus Control Group
2.9. Gut Microbiota Alterations in VPA-Induced ASD Model Rats
2.10. Distinct Gut Microbiota Profiles in VPA-Induced ASD Rat Models Compared to the Control Group
2.11. Taxonomic Alterations in Gut Microbiota Composition Between Control and VPA-Induced ASD Model Rats
2.12. Gut Microbiota Alpha Diversity Analysis in VPA-Induced ASD Model
2.13. Beta Diversity Analysis of Gut Microbiota
2.14. Network Correlation Analysis
3. Discussion
4. Materials and Methods
4.1. Experimental Design and Animal Procedures
4.1.1. Animal Housing and Breeding
4.1.2. Prenatal VPA Exposure
4.1.3. Postnatal Group Allocation
4.2. Behavioral Tests
- (1)
- Three-chamber social interaction test
- (2)
- Self-grooming test
- (3)
- Marble-Burying Test
- (4)
- Open field test
- (5)
- Morris Water Maze Behavioral Assessment
4.3. Sacrificing Animals and Preparing Tissues
4.4. Biochemical Analysis
4.4.1. Sample Preparation
4.4.2. Cytokine Quantification (ELISA)
4.4.3. Oxidative Stress Marker Analysis
4.5. 16S rRNA Sequencing and Bioinformatics Analysis
- Sample Collection and Preservation
- (a)
- The rat tail was gently lifted, and sterile finger cot pressure was applied to the lower abdomen to promote defecation.
- (b)
- Fecal pellets were transferred into pre-labeled 2 mL sterile centrifuge tubes (Axygen, PCR-02-C, Corning Incorporated, Union City, CA, USA) using disposable sterile forceps.
- (c)
- Each tube contained 3–5 intact fecal pellets (approximately 100 mg).
- (d)
- Sampling was consistently performed between 09:00 and 11:00 daily to eliminate circadian rhythm effects on gut microbiota.
- (e)
- Samples were immediately snap-frozen in liquid nitrogen (<5 min), stored at −80 °C, and transported to the testing facility on dry ice.
- B.
- DNA Extraction and 16S rRNA Gene Amplification
- (1)
- Genomic DNA Extraction Using Modified CTAB Method
- (2)
- PCR Amplification of V3-V4 Regions
- C.
- Library Preparation and High-Throughput Sequencing
- (1)
- Product Purification
- (2)
- Library Construction
- (3)
- Illumina Sequencing
- D.
- Bioinformatics Analysis
- (1)
- Raw Data Processing
- (2)
- Diversity Analysis
- (3)
- Differential Species Identification
- (4)
- Statistical Analysis
4.6. Immunofluorescence Staining
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kong, Q.; Tian, P.; Zhao, J.; Zhang, H.; Wang, G.; Chen, W. The autistic-like behaviors development during weaning and sexual maturation in VPA-induced autistic-like rats is accompanied by gut microbiota dysbiosis. PeerJ 2021, 9, e11103. [Google Scholar] [CrossRef]
- Khodaverdi, M.; Rahdar, M.; Davoudi, S.; Hajisoltani, R.; Tavassoli, Z.; Ghasemi, Z.; Amini, A.E.; Hosseinmardi, N.; Behzadi, G.; Janahmadi, M. 5-HT7 receptor activation rescues impaired synaptic plasticity in an autistic-like rat model induced by prenatal VPA exposure. Neurobiol. Learn. Mem. 2021, 183, 107462. [Google Scholar] [CrossRef] [PubMed]
- Kong, Q.; Wang, B.; Tian, P.; Li, X.; Zhao, J.; Zhang, H.; Chen, W.; Wang, G. Daily intake of Lactobacillus alleviates autistic-like behaviors by ameliorating the 5-hydroxytryptamine metabolic disorder in VPA-treated rats during weaning and sexual maturation. Food Funct. 2021, 12, 2591–2604. [Google Scholar] [CrossRef]
- Gao, Q.; Sun, G.; Duan, J.; Luo, C.; Yangji, C.; Zhong, R.; Chen, L.; Zhu, Y.; Wangdui, B.; Zhang, H. Alterations in gut microbiota improve SCFA production and fiber utilization in Tibetan pigs fed alfalfa diet. Front. Microbiol. 2022, 13, 969524. [Google Scholar] [CrossRef] [PubMed]
- Vernocchi, P.; Ristori, M.V.; Guerrera, S.; Guarrasi, V.; Conte, F.; Russo, A.; Lupi, E.; Albitar-Nehme, S.; Gardini, S.; Paci, P.; et al. Gut Microbiota Ecology and Inferred Functions in Children With ASD Compared to Neurotypical Subjects. Front. Microbiol. 2022, 13, 871086. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.D.; Li, L.; Yu, F.; Ma, Z.S. A comprehensive diversity analysis on the gut microbiomes of ASD patients: From alpha, beta to gamma diversities. FEMS Microbiol. Lett. 2024, 371, fnae014. [Google Scholar] [CrossRef]
- Ziemons, J.; Aarnoutse, R.; Heuft, A.; Hillege, L.; Waelen, J.; de Vos-Geelen, J.; Valkenburg-van Iersel, L.; van Hellemond, I.E.G.; Creemers, G.M.; Baars, A.; et al. Fecal levels of SCFA and BCFA during capecitabine in patients with metastatic or unresectable colorectal cancer. Clin. Exp. Med. 2023, 23, 3919–3933. [Google Scholar] [CrossRef]
- Liu, F.; Horton-Sparks, K.; Hull, V.; Li, R.W.; Martinez-Cerdeno, V. The valproic acid rat model of autism presents with gut bacterial dysbiosis similar to that in human autism. Mol. Autism 2018, 9, 61. [Google Scholar] [CrossRef]
- Zhong, J.G.; Lan, W.T.; Feng, Y.Q.; Li, Y.H.; Shen, Y.Y.; Gong, J.H.; Zou, Z.; Hou, X. Associations between dysbiosis gut microbiota and changes of neurotransmitters and short-chain fatty acids in valproic acid model rats. Front. Physiol. 2023, 14, 1077821. [Google Scholar] [CrossRef]
- Zahedi, E.; Sadr, S.S.; Sanaeierad, A.; Roghani, M. Chronic acetyl-L-carnitine treatment alleviates behavioral deficits and neuroinflammation through enhancing microbiota derived-SCFA in valproate model of autism. Biomed. Pharmacother. 2023, 163, 114848. [Google Scholar] [CrossRef]
- Prince, N.; Peralta Marzal, L.N.; Markidi, A.; Ahmed, S.; Adolfs, Y.; Pasterkamp, R.J.; Kumar, H.; Roeselers, G.; Garssen, J.; Kraneveld, A.D.; et al. Prebiotic diet normalizes aberrant immune and behavioral phenotypes in a mouse model of autism spectrum disorder. Acta Pharmacol. Sin. 2024, 45, 1591–1603. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Yang, Y.; Lu, G.; Tan, J.S.; Mageswary, U.; Zhan, Y.; Ayad, M.E.; Lee, Y.Y.; Xie, D. Metabolomics Insights into Gut Microbiota and Functional Constipation. Metabolites 2025, 15, 269. [Google Scholar] [CrossRef]
- Chen, B.; Xu, X.; Wang, Y.; Yang, Z.; Liu, C.; Zhang, T. VPA-induced autism impairs memory ability through disturbing neural oscillatory patterns in offspring rats. Cogn. Neurodyn. 2024, 18, 1563–1574. [Google Scholar] [CrossRef] [PubMed]
- Chai, L.J.; Xu, P.X.; Qian, W.; Zhang, X.J.; Ma, J.; Lu, Z.M.; Wang, S.T.; Shen, C.H.; Shi, J.S.; Xu, Z.H. Profiling the Clostridia with butyrate-producing potential in the mud of Chinese liquor fermentation cellar. Int. J. Food Microbiol. 2019, 297, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Li, W.; Wang, L.; Gao, Y.; Ma, Y.; Zhou, M.; Yang, T.; Yue, C.; Yan, L.; Lyu, Y. Jiawei Qifuyin Enhances Immunity and Improves Cognitive Impairment in APP/PS1 Mice Through Modulation of Neuroinflammatory Pathways. J. Inflamm. Res. 2024, 17, 9021–9040. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, H.; Yin, S.; Xiao, F.; Gong, C.; Zhou, J.; Liu, K.; Cheng, Y. Changes in short-chain fatty acids affect brain development in mice with early life antibiotic-induced dysbacteriosis. Transl. Pediatr. 2024, 13, 1312–1326. [Google Scholar] [CrossRef]
- Tian, Y.; Xiao, X.; Liu, W.; Cheng, S.; Qian, N.; Wang, L.; Liu, Y.; Ai, R.; Zhu, X. TREM2 improves microglia function and synaptic development in autism spectrum disorders by regulating P38 MAPK signaling pathway. Mol. Brain 2024, 17, 12. [Google Scholar] [CrossRef]
- Manjeese, W.; Mvubu, N.E.; Steyn, A.J.C.; Mpofana, T. Mycobacterium tuberculosis-Induced Maternal Immune Activation Promotes Autism-Like Phenotype in Infected Mice Offspring. Int. J. Environ. Res. Public Health 2021, 18, 4513. [Google Scholar] [CrossRef]
- Li, B.; Xiong, Y.; Li, Y. The Impact of Valproic Acid on Microbiota in a Mouse Model of Autism Spectrum Disorder. Psychiatry Clin. Psychopharmacol. 2025, 35, 6–13. [Google Scholar] [CrossRef]
- Yang, X.; Li, H.; Yang, C.; Ge, J. Supplementation with stigma maydis polysaccharide attenuates autism-like behaviors and improves gut function in valproic acid-induced autism model male rats. Int. J. Dev. Neurosci. 2024, 84, 567–580. [Google Scholar] [CrossRef]
- Saadat, M.; Taherian, A.A.; Aldaghi, M.R.; Raise-Abdullahi, P.; Sameni, H.R.; Vafaei, A.A. Prangos ferulacea (L.) ameliorates behavioral alterations, hippocampal oxidative stress markers, and apoptotic deficits in a rat model of autism induced by valproic acid. Brain Behav. 2023, 13, e3224. [Google Scholar] [CrossRef]
- Lieberman, O.J.; Cartocci, V.; Pigulevskiy, I.; Molinari, M.; Carbonell, J.; Broseta, M.B.; Post, M.R.; Sulzer, D.; Borgkvist, A.; Santini, E. mTOR Suppresses Macroautophagy During Striatal Postnatal Development and Is Hyperactive in Mouse Models of Autism Spectrum Disorders. Front. Cell Neurosci. 2020, 14, 70. [Google Scholar] [CrossRef]
- Campolongo, M.; Kazlauskas, N.; Falasco, G.; Urrutia, L.; Salgueiro, N.; Hocht, C.; Depino, A.M. Sociability deficits after prenatal exposure to valproic acid are rescued by early social enrichment. Mol. Autism 2018, 9, 36. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhao, X.; Yang, Q.; Zhou, T.; Feng, Y.; Chen, Y.; Chen, Z.; Deng, C. A randomized controlled trial of the efficacy of music therapy on the social skills of children with autism spectrum disorder. Res. Dev. Disabil. 2025, 158, 104942. [Google Scholar] [CrossRef]
- Masoomi, M.; Saeidi, M.; Cedeno, R.; Shahrivar, Z.; Tehrani-Doost, M.; Ramirez, Z.; Gandi, D.A.; Gunturu, S. Emotion recognition deficits in children and adolescents with autism spectrum disorder: A comprehensive meta-analysis of accuracy and response time. Front. Child. Adolesc. Psychiatry 2024, 3, 1520854. [Google Scholar] [CrossRef] [PubMed]
- Volk, H.E.; Fortes, D.; Musci, R.; Kim, A.; Bastain, T.M.; Camargo, C.A., Jr.; Croen, L.A.; Dabelea, D.; Duarte, C.S.; Dunlop, A.L.; et al. Co-occurring Psychopathology in Children With and Without Autism Spectrum Disorder (ASD): Differences by Sex in the ECHO Cohorts. J. Autism Dev. Disord. 2025, 15, 101613. [Google Scholar] [CrossRef]
- Turpin, V.; Schaffhauser, M.; Thabault, M.; Aubert, A.; Joffre, C.; Balado, E.; Longueville, J.E.; Francheteau, M.; Burucoa, C.; Pichon, M.; et al. Mice prenatally exposed to valproic acid do not show autism-related disorders when fed with polyunsaturated fatty acid-enriched diets. Sci. Rep. 2023, 13, 11235. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Cui, Y.; Zhang, Y.; Xiang, G.; Yu, M.; Wang, X.; Qiu, B.; Li, X.G.; Liu, W.; Zhang, D. Rescue of social deficits by early-life melatonin supplementation through modulation of gut microbiota in a murine model of autism. Biomed. Pharmacother. 2022, 156, 113949. [Google Scholar] [CrossRef]
- de Theije, C.G.; Wopereis, H.; Ramadan, M.; van Eijndthoven, T.; Lambert, J.; Knol, J.; Garssen, J.; Kraneveld, A.D.; Oozeer, R. Altered gut microbiota and activity in a murine model of autism spectrum disorders. Brain Behav. Immun. 2014, 37, 197–206. [Google Scholar] [CrossRef]
- Wang, D.; Jiang, Y.; Jiang, J.; Pan, Y.; Yang, Y.; Fang, X.; Liang, L.; Li, H.; Dong, Z.; Fan, S.; et al. Gut microbial GABA imbalance emerges as a metabolic signature in mild autism spectrum disorder linked to overrepresented Escherichia. Cell Rep. Med. 2025, 6, 101919. [Google Scholar] [CrossRef] [PubMed]
- Moreno, R.J.; Ashwood, P. An Update on Microbial Interventions in Autism Spectrum Disorder with Gastrointestinal Symptoms. Int. J. Mol. Sci. 2024, 25, 13078. [Google Scholar] [CrossRef]
- Chamtouri, M.; Gaddour, N.; Merghni, A.; Mastouri, M.; Arboleya, S.; de Los Reyes-Gavilan, C.G. Age and severity-dependent gut microbiota alterations in Tunisian children with autism spectrum disorder. Sci. Rep. 2023, 13, 18218. [Google Scholar] [CrossRef]
- Wang, X.; Sun, Z.; Yang, T.; Lin, F.; Ye, S.; Yan, J.; Li, T.; Chen, J. Sodium butyrate facilitates CRHR2 expression to alleviate HPA axis hyperactivity in autism-like rats induced by prenatal lipopolysaccharides through histone deacetylase inhibition. mSystems 2023, 8, e0041523, Erratum in mSystems 2023, 8, e0091523. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Y.; Meng, F.; Chen, X.; Chang, T.; Huang, H.; He, F.; Zheng, Y. Altered Gut Microbiota as Potential Biomarkers for Autism Spectrum Disorder in Early Childhood. Neuroscience 2023, 523, 118–131. [Google Scholar] [CrossRef]
- Kasotakis, G.; Galvan, M.D.; Osathanugrah, P.; Dharia, N.; Bufe, L.; Breed, Z.; Mizgerd, J.P.; Remick, D.G. Timing of valproic acid in acute lung injury: Prevention is the best therapy? J. Surg. Res. 2017, 220, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Lv, D.; Jiang, S.; Jiang, J.; Liang, M.; Hou, F.; Chen, Y. Quantitative reduction in short-chain fatty acids, especially butyrate, contributes to the progression of chronic kidney disease. Clin. Sci. 2019, 133, 1857–1870. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.C.; Choi, C.S.; Gonzales, E.L.T.; Mabunga, D.F.N.; Lee, S.H.; Jeon, S.J.; Hwangbo, R.; Hong, M.; Ryu, J.H.; Han, S.H.; et al. Valproic Acid Induces Telomerase Reverse Transcriptase Expression during Cortical Development. Exp. Neurobiol. 2017, 26, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Ranger, P.; Ellenbroek, B.A. Perinatal Influences of Valproate on Brain and Behaviour: An Animal Model for Autism. Curr. Top. Behav. Neurosci. 2016, 29, 363–386. [Google Scholar] [CrossRef]
- Sen, P.; Sherwin, E.; Sandhu, K.; Bastiaanssen, T.F.S.; Moloney, G.M.; Golubeva, A.; Fitzgerald, P.; Paula Ventura Da Silva, A.; Chruscicka-Smaga, B.; Olavarria-Ramirez, L.; et al. The live biotherapeutic Blautia stercoris MRx0006 attenuates social deficits, repetitive behaviour, and anxiety-like behaviour in a mouse model relevant to autism. Brain Behav. Immun. 2022, 106, 115–126. [Google Scholar] [CrossRef]
- Dubois, T.; Zdanowicz, N.; Jacques, D.; Lepiece, B.; Jassogne, C. Microbiota Diversity and Inflammation as a New Target to Improve Mood: Probiotic Use in Depressive Disorder. Psychiatr. Danub. 2023, 35 (Suppl. S2), 72–76. [Google Scholar]
- He, X.; Yan, C.; Zhao, S.; Zhao, Y.; Huang, R.; Li, Y. The preventive effects of probiotic Akkermansia muciniphila on D-galactose/AlCl3 mediated Alzheimer’s disease-like rats. Exp. Gerontol. 2022, 170, 111959. [Google Scholar] [CrossRef]
- Palanivelu, L.; Chen, Y.Y.; Chang, C.J.; Liang, Y.W.; Tseng, H.Y.; Li, S.J.; Chang, C.W.; Lo, Y.C. Investigating brain-gut microbiota dynamics and inflammatory processes in an autistic-like rat model using MRI biomarkers during childhood and adolescence. Neuroimage 2024, 302, 120899. [Google Scholar] [CrossRef]
- Percie du Sert, N.; Hurst, V.; Ahluwalia, A.; Alam, S.; Avey, M.T.; Baker, M.; Browne, W.J.; Clark, A.; Cuthill, I.C.; Dirnagl, U.; et al. The ARRIVE guidelines 2.0: Updated guidelines for reporting animal research. J. Physiol. 2020, 598, 3793–3801. [Google Scholar] [CrossRef]
- Cass, J.S.; Campbell, I.R.; Lange, L. A guide to production, care and use of laboratory animals. An annotated bibliography. 1. Normal anatomy, physiology, psychology, including the role of factors in the environment. Fed. Proc. 1963, 3, 1–55. [Google Scholar]
- Sabatini, G.; Boccadoro, I.; Prete, R.; Battista, N.; Corsetti, A. Autism Spectrum Disorder: From Experimental Models to Probiotic Application with a Special Focus on Lactiplantibacillus plantarum. Nutrients 2025, 17, 2470. [Google Scholar] [CrossRef] [PubMed]
- Soleimanpour, S.; Abavisani, M.; Khoshrou, A.; Sahebkar, A. Probiotics for autism spectrum disorder: An updated systematic review and meta-analysis of effects on symptoms. J. Psychiatr. Res. 2024, 179, 92–104. [Google Scholar] [CrossRef] [PubMed]
- Watanangura, A.; Meller, S.; Farhat, N.; Suchodolski, J.S.; Pilla, R.; Khattab, M.R.; Lopes, B.C.; Bathen-Nothen, A.; Fischer, A.; Busch-Hahn, K.; et al. Behavioral comorbidities treatment by fecal microbiota transplantation in canine epilepsy: A pilot study of a novel therapeutic approach. Front. Vet. Sci. 2024, 11, 1385469. [Google Scholar] [CrossRef]
- Goo, N.; Bae, H.J.; Park, K.; Kim, J.; Jeong, Y.; Cai, M.; Cho, K.; Jung, S.Y.; Kim, D.H.; Ryu, J.H. The effect of fecal microbiota transplantation on autistic-like behaviors in Fmr1 KO mice. Life Sci. 2020, 262, 118497. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Li, X.; Wang, H.; Liu, Z. Altered Gut Microbial Diversity and Depletion of SCFA-Producing Taxa Associated with ASD-like Phenotypes in a Prenatal VPA Rat Model. Int. J. Mol. Sci. 2025, 26, 8931. https://doi.org/10.3390/ijms26188931
Wu C, Li X, Wang H, Liu Z. Altered Gut Microbial Diversity and Depletion of SCFA-Producing Taxa Associated with ASD-like Phenotypes in a Prenatal VPA Rat Model. International Journal of Molecular Sciences. 2025; 26(18):8931. https://doi.org/10.3390/ijms26188931
Chicago/Turabian StyleWu, Caixia, Xianjie Li, Han Wang, and Zhaoming Liu. 2025. "Altered Gut Microbial Diversity and Depletion of SCFA-Producing Taxa Associated with ASD-like Phenotypes in a Prenatal VPA Rat Model" International Journal of Molecular Sciences 26, no. 18: 8931. https://doi.org/10.3390/ijms26188931
APA StyleWu, C., Li, X., Wang, H., & Liu, Z. (2025). Altered Gut Microbial Diversity and Depletion of SCFA-Producing Taxa Associated with ASD-like Phenotypes in a Prenatal VPA Rat Model. International Journal of Molecular Sciences, 26(18), 8931. https://doi.org/10.3390/ijms26188931





