Generating a Cell Model to Study ER Stress in iPSC-Derived Medium Spiny Neurons from a Patient with Huntington’s Disease
Abstract
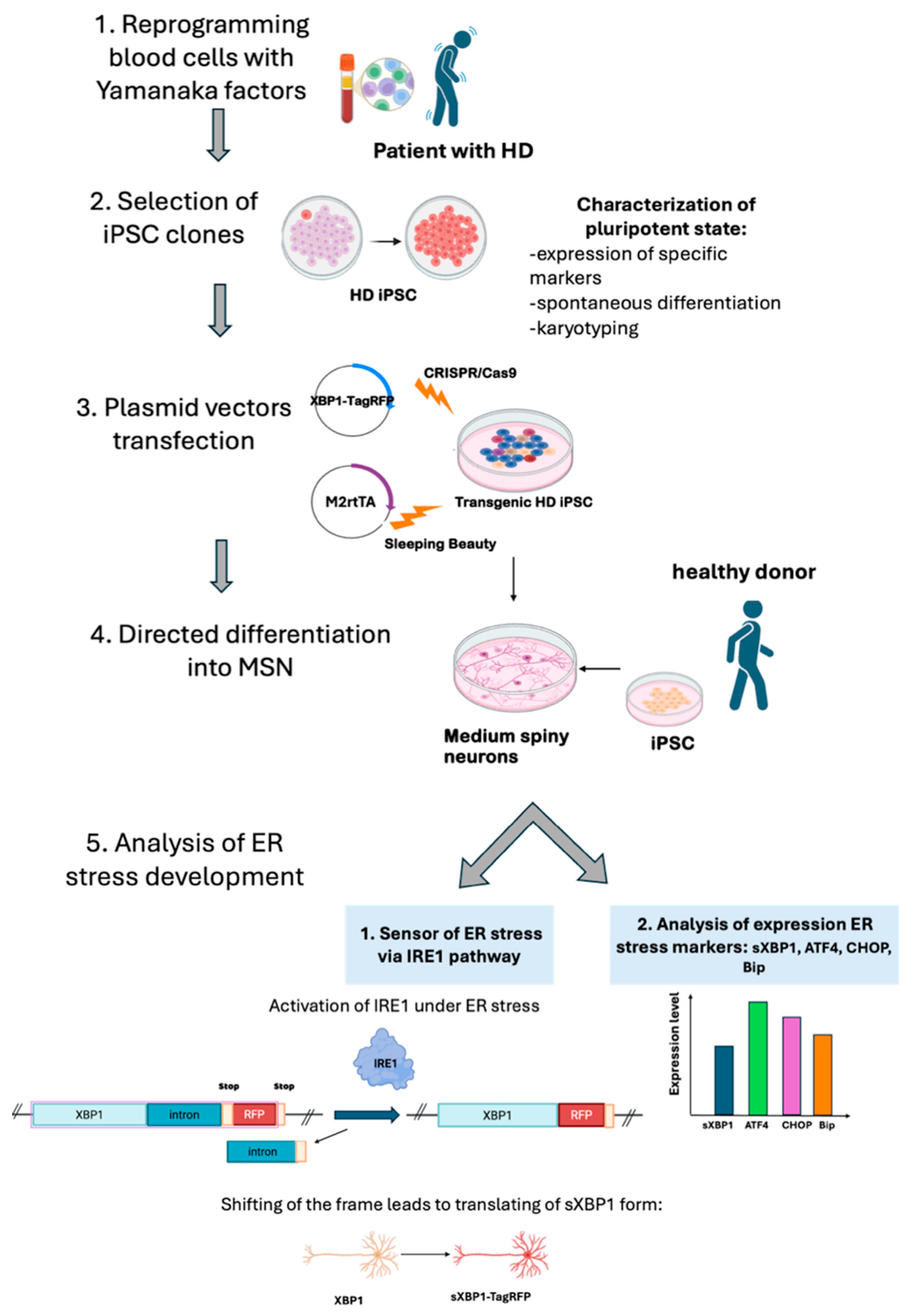
1. Introduction
2. Results
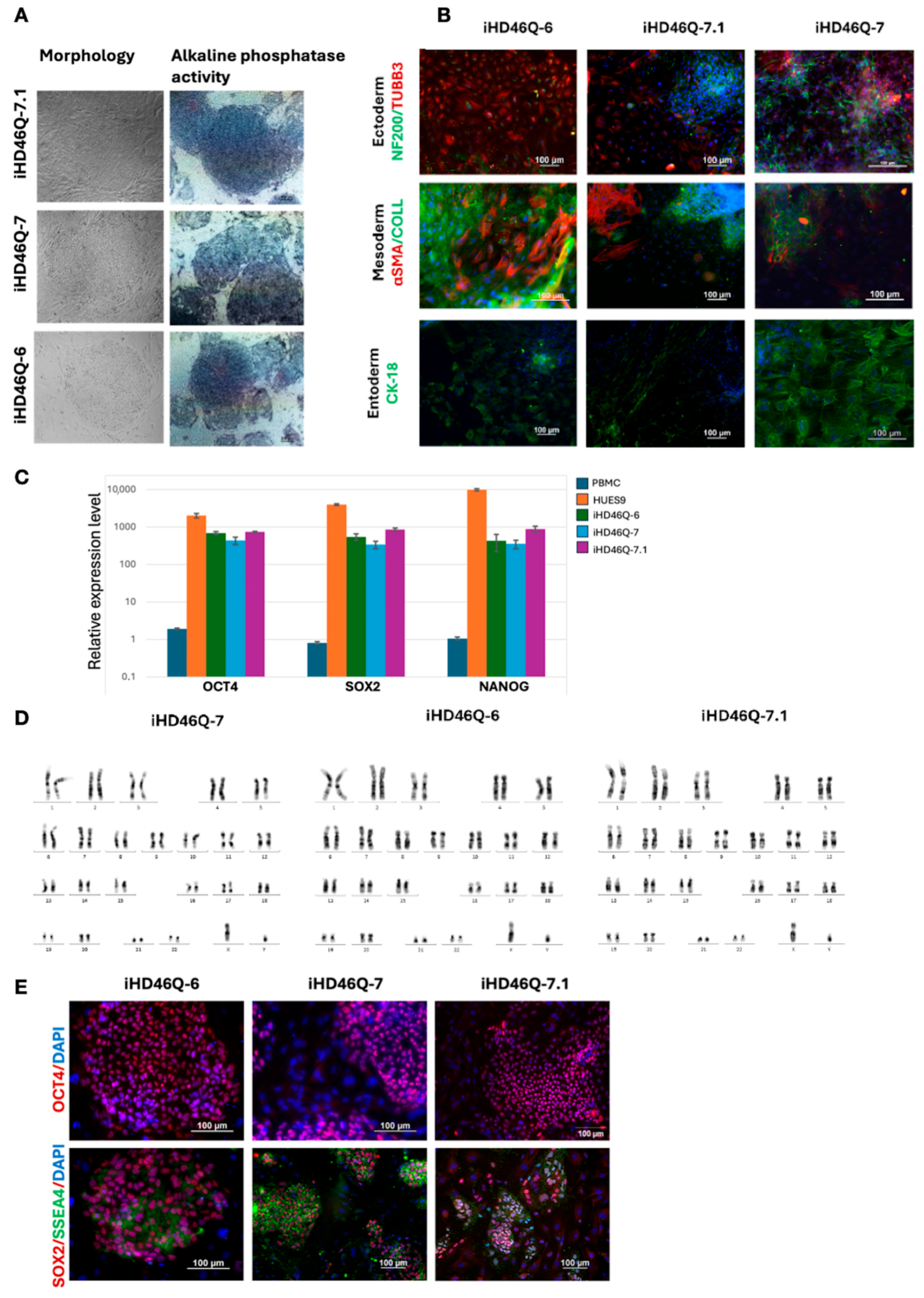
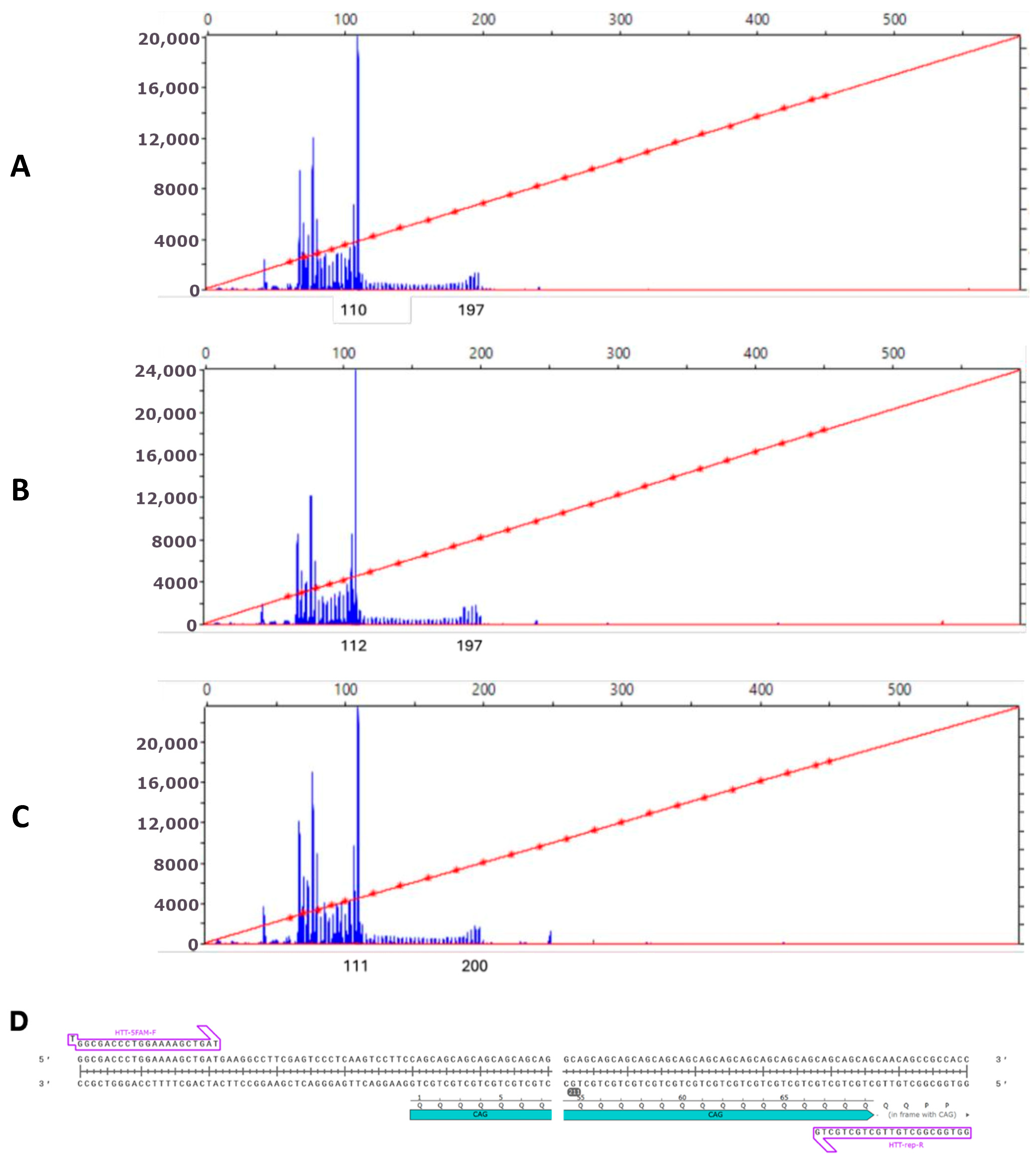
2.1. Generation and Characterization of iPSC Lines of a Patient with HD
2.2. Generation of Transgenic iPSC Lines Expressing XBP1-TagRFP Biosensor
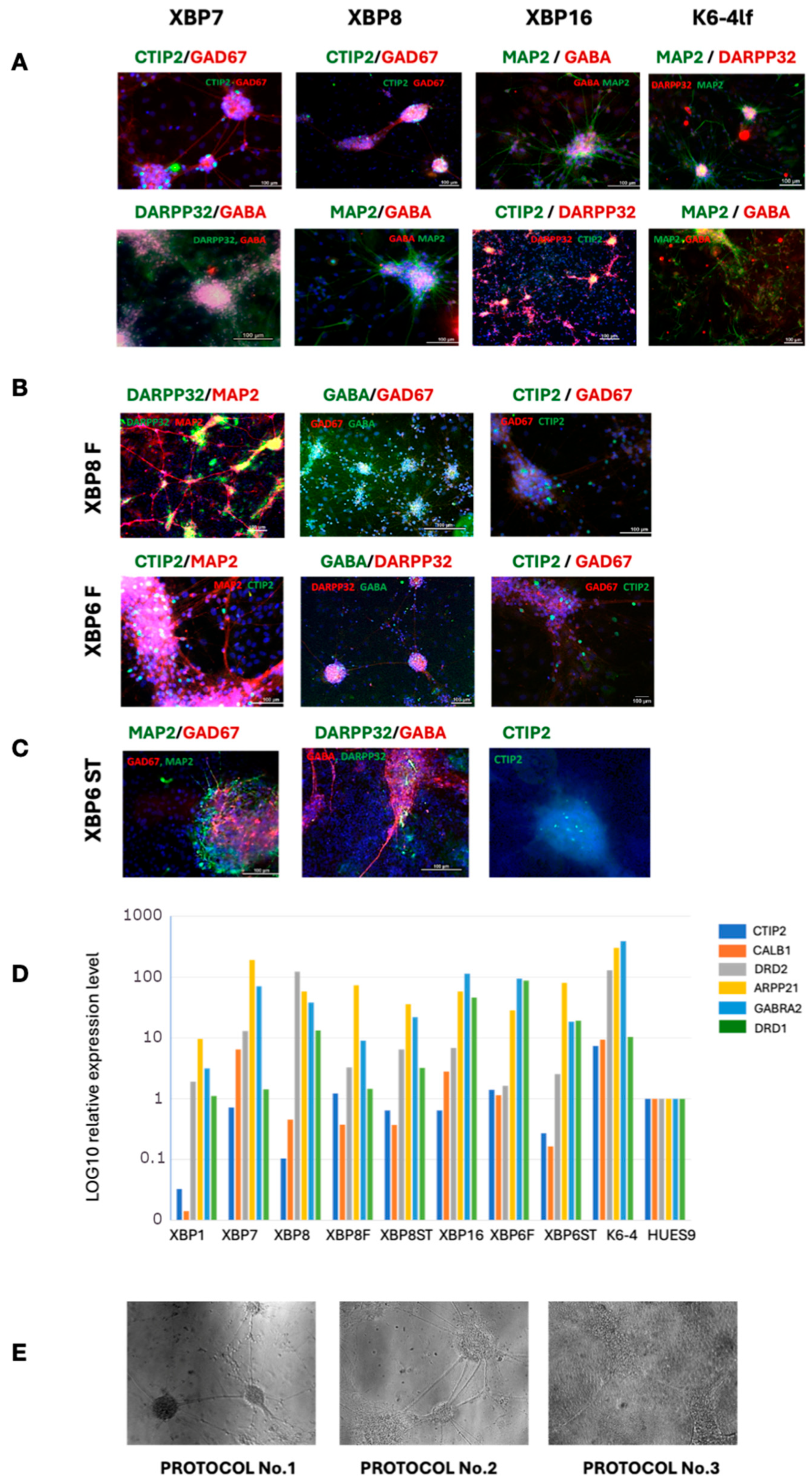
2.3. Testing Capability of Obtained iPSC Lines to Differentiate into Neural Derivatives
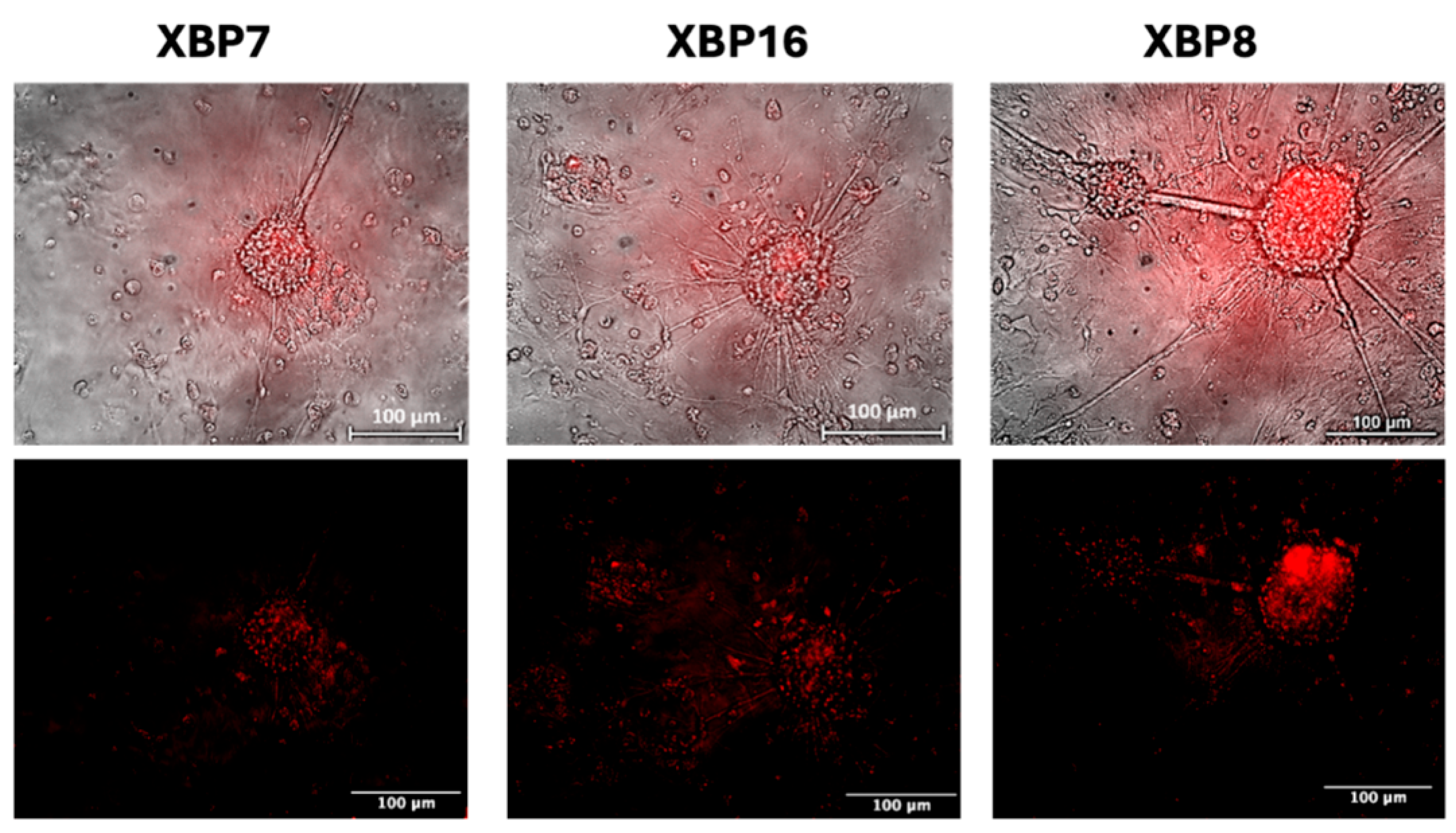
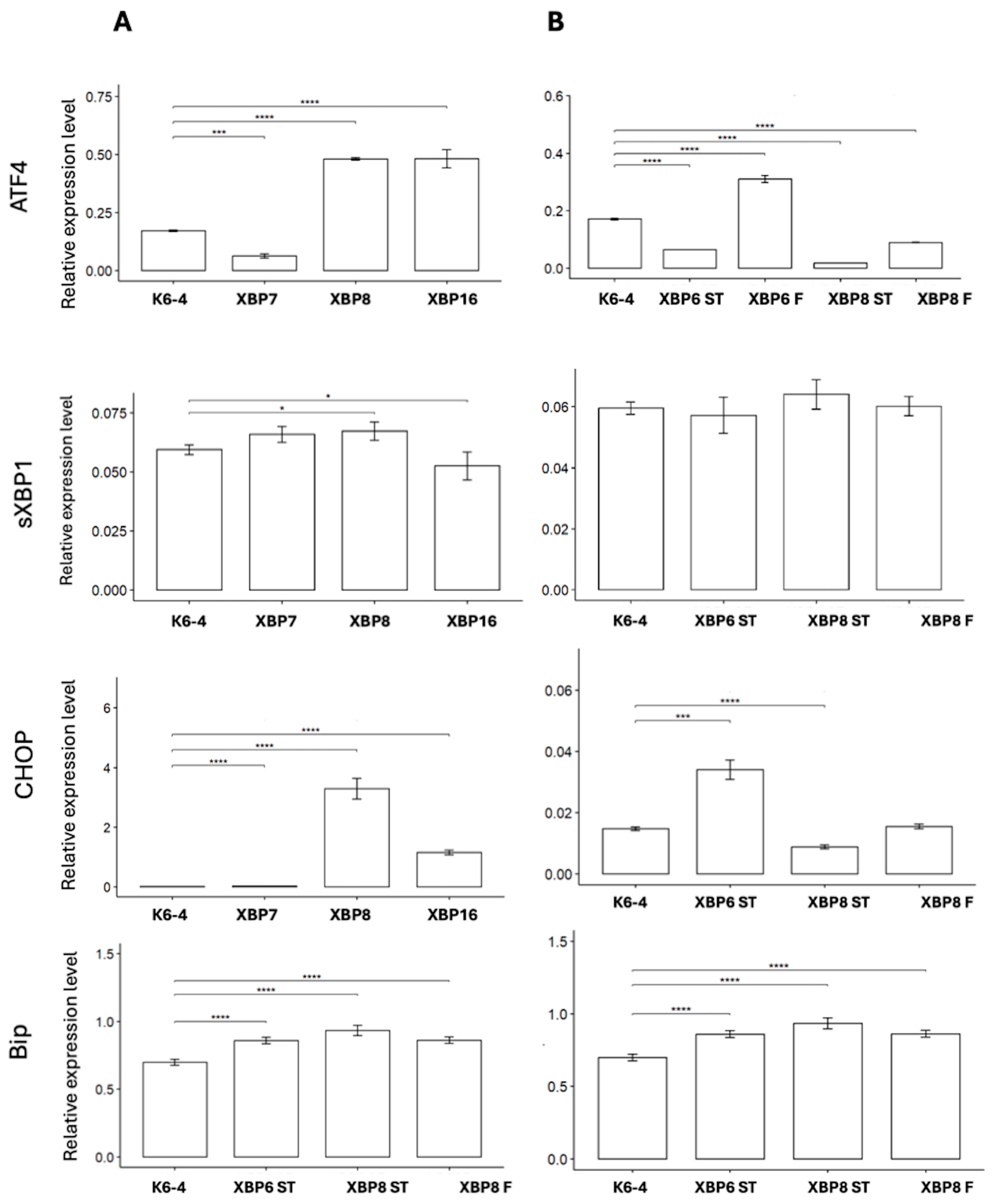
2.4. Detection of XBP1-Mediated ER Stress in MSNs
3. Discussion
4. Materials and Methods
4.1. Ethical Statement
4.2. Generation of iPSC Lines by Reprogramming PBMCs of an HD Patient
4.3. Assessment of CAG Repeat Tract Length in HTT Gene
4.4. Generation of Transgenic iPSC Lines with XBP1-TagRFP Biosensor
4.5. Spontaneous Differentiation
4.6. Immunofluorescence Staining
4.7. Directed Differentiation iPSC into Medium Spiny Neurons
4.7.1. Protocol No. 1 [27]
4.7.2. Protocol No. 2 [26]
4.7.3. Protocol No. 3 [25]
4.8. Analysis of MSN and ER Stress Marker Genes Expression by RT-qPCR
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| iPSC | Induced pluripotent stem cell |
| HD | Huntington’s disease |
| ER | Endoplasmic reticulum |
| ERAD | Endoplasmic reticulum-associated protein degradation system |
| UPR | Unfolded protein response |
References
- Saudou, F.; Humbert, S. The Biology of Huntingtin. Neuron 2016, 89, 910–926. [Google Scholar] [CrossRef]
- Makeeva, V.S.; Dyrkheeva, N.S.; Lavrik, O.I.; Zakian, S.M.; Malakhova, A.A. Mutant-Huntingtin Molecular Pathways Elucidate New Targets for Drug Repurposing. Int. J. Mol. Sci. 2023, 24, 16798. [Google Scholar] [CrossRef] [PubMed]
- Caron, N.S.; Dorsey, E.R.; Hayden, M.R. Therapeutic Approaches to Huntington Disease: From the Bench to the Clinic. Nat. Rev. Drug Discov. 2018, 17, 729–750. [Google Scholar] [CrossRef]
- Ferguson, M.W.; Kennedy, C.J.; Palpagama, T.H.; Waldvogel, H.J.; Faull, R.L.M.; Kwakowsky, A. Current and Possible Future Therapeutic Options for Huntington’s Disease. J. Cent. Nerv. Syst. Dis. 2022, 14, 11795735221092517. [Google Scholar] [CrossRef] [PubMed]
- Kaye, J.; Reisine, T.; Finkbeiner, S. Huntington’s Disease IPSC Models—Using Human Patient Cells to Understand the Pathology Caused by Expanded CAG Repeats. Fac. Rev. 2022, 11, 16. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Oikonomopoulos, A.; Sayed, N.; Wu, J.C. Modeling Human Diseases with Induced Pluripotent Stem Cells: From 2D to 3D and Beyond. Development 2018, 145, dev156166. [Google Scholar] [CrossRef]
- Kaye, J.A.; Finkbeiner, S. Modeling Huntington’s Disease with Induced Pluripotent Stem Cells. Mol. Cell. Neurosci. 2013, 56, 50–64. [Google Scholar] [CrossRef]
- Galimberti, M.; Nucera, M.R.; Bocchi, V.D.; Conforti, P.; Vezzoli, E.; Cereda, M.; Maffezzini, C.; Iennaco, R.; Scolz, A.; Falqui, A.; et al. Huntington’s Disease Cellular Phenotypes Are Rescued Non-Cell Autonomously by Healthy Cells in Mosaic Telencephalic Organoids. Nat. Commun. 2024, 15, 6534. [Google Scholar] [CrossRef]
- Marei, H.E. Stem Cell and Synthetic Embryo Models: Advances, Applications, and Ethical Considerations. Stem Cell Rev. Rep. 2025, 21, 1648–1668. [Google Scholar] [CrossRef]
- Liu, W.; Kennington, L.A.; Rosas, H.D.; Hersch, S.; Cha, J.H.; Zamore, P.D.; Aronin, N. Linking SNPs to CAG Repeat Length in Huntington’s Disease Patients. Nat. Methods 2008, 5, 951–953. [Google Scholar] [CrossRef][Green Version]
- Martin, D.D.O.; Kay, C.; Collins, J.A.; Nguyen, Y.T.; Slama, R.A.; Hayden, M.R. A Human Huntingtin SNP Alters Post-Translational Modification and Pathogenic Proteolysis of the Protein Causing Huntington Disease. Sci. Rep. 2018, 8, 8096. [Google Scholar] [CrossRef]
- Claassen, D.O.; Corey-Bloom, J.; Dorsey, E.R.; Edmondson, M.; Kostyk, S.K.; Ledoux, M.S.; Reilmann, R.; Rosas, H.D.; Walker, F.; Wheelock, V.; et al. Genotyping Single Nucleotide Polymorphisms for Allele-Selective Therapy in Huntington Disease. Neurol. Genet. 2020, 6, e430. [Google Scholar] [CrossRef]
- Handsaker, R.E.; Kashin, S.; Reed, N.M.; Tan, S.; Lee, W.S.; McDonald, T.M.; Morris, K.; Kamitaki, N.; Mullally, C.D.; Morakabati, N.R.; et al. Long Somatic DNA-Repeat Expansion Drives Neurodegeneration in Huntington’s Disease. Cell 2025, 188, 623–639.e19. [Google Scholar] [CrossRef]
- Duennwald, M.L.; Lindquist, S. Impaired ERAD and ER Stress Are Early and Specific Events in Polyglutamine Toxicity. Genes. Dev. 2008, 22, 3308–3319. [Google Scholar] [CrossRef] [PubMed]
- Leitman, J.; Ulrich Hartl, F.; Lederkremer, G.Z. Soluble Forms of PolyQ-Expanded Huntingtin Rather than Large Aggregates Cause Endoplasmic Reticulum Stress. Nat. Commun. 2013, 4, 2753. [Google Scholar] [CrossRef] [PubMed]
- Shenkman, M.; Eiger, H.; Lederkremer, G.Z. Genesis of ER Stress in Huntington’s Disease. Endoplasmic Reticulum Stress Dis. 2015, 2, 94–106. [Google Scholar] [CrossRef]
- Chen, Y.; Brandizzi, F. IRE1: ER Stress Sensor and Cell Fate Executor. Trends Cell Biol. 2013, 23, 547–555. [Google Scholar] [CrossRef]
- Vidal, R.L.; Figueroa, A.; Court, F.A.; Thielen, P.; Molina, C.; Wirth, C.; Caballero, B.; Kiffin, R.; Segura-Aguilar, J.; Cuervo, A.M.; et al. Targeting the UPR Transcription Factor XBP1 Protects against Huntington’s Disease through the Regulation of FoxO1 and Autophagy. Hum. Mol. Genet. 2012, 21, 2245–2262. [Google Scholar] [CrossRef] [PubMed]
- Le, Q.G.; Kimata, Y. Multiple Ways for Stress Sensing and Regulation of the Endoplasmic Reticulum-Stress Sensors. Cell Struct. Funct. 2021, 46, 37–49. [Google Scholar] [CrossRef]
- Iwawaki, T.; Akai, R.; Kohno, K.; Miura, M. A Transgenic Mouse Model for Monitoring Endoplasmic Reticulum Stress. Nat. Med. 2004, 10, 98–102. [Google Scholar] [CrossRef]
- Yarkova, E.S.; Grigor’eva, E.V.; Medvedev, S.P.; Tarasevich, D.A.; Pavlova, S.V.; Valetdinova, K.R.; Minina, J.M.; Zakian, S.M.; Malakhova, A.A. Detection of ER Stress in IPSC-Derived Neurons Carrying the p.N370S Mutation in the GBA1 Gene. Biomedicines 2024, 12, 744. [Google Scholar] [CrossRef]
- Ustyantseva, E.I.; Medvedev, S.P.; Vetchinova, A.S.; Minina, J.M.; Illarioshkin, S.N.; Zakian, S.M. A Platform for Studying Neurodegeneration Mechanisms Using Genetically Encoded Biosensors. Biochemistry 2019, 84, 299–309. [Google Scholar] [CrossRef]
- Arber, C.; Precious, S.V.; Cambray, S.; Risner-Janiczek, J.R.; Kelly, C.; Noakes, Z.; Fjodorova, M.; Heuer, A.; Ungless, M.A.; Rodríguez, T.A.; et al. Activin A Directs Striatal Projection Neuron Differentiation of Human Pluripotent Stem Cells. Development 2015, 142, 1375–1386. [Google Scholar] [CrossRef]
- Telezhkin, V.; Schnell, C.; Yarova, P.; Yung, S.; Cope, E.; Hughes, A.; Thompson, B.A.; Sanders, P.; Geater, C.; Hancock, J.M.; et al. Forced Cell Cycle Exit and Modulation of GABAA, CREB, and GSK3β Signaling Promote Functional Maturation of Induced Pluripotent Stem Cell-Derived Neurons. Am. J. Physiol. Cell Physiol. 2016, 310, C520–C541. [Google Scholar] [CrossRef]
- Stanslowsky, N.; Reinhardt, P.; Glass, H.; Kalmbach, N.; Naujock, M.; Hensel, N.; Lübben, V.; Pal, A.; Venneri, A.; Lupo, F.; et al. Neuronal Dysfunction in IPSC-Derived Medium Spiny Neurons from Chorea-Acanthocytosis Patients Is Reversed by Src Kinase Inhibition and F-Actin Stabilization. J. Neurosci. 2016, 36, 12027–12043. [Google Scholar] [CrossRef]
- Fjodorova, M.; Li, M. Robust Induction of DARPP32-Expressing GABAergic Striatal Neurons from Human Pluripotent Stem Cells. Methods Mol. Biol. 2018, 1780, 585–605. [Google Scholar] [CrossRef] [PubMed]
- Grigor’eva, E.V.; Malankhanova, T.B.; Surumbayeva, A.; Pavlova, S.V.; Minina, J.M.; Kizilova, E.A.; Suldina, L.A.; Morozova, K.N.; Kiseleva, E.; Sorokoumov, E.D.; et al. Generation of GABAergic Striatal Neurons by a Novel IPSC Differentiation Protocol Enabling Scalability and Cryopreservation of Progenitor Cells. Cytotechnology 2020, 72, 649–663. [Google Scholar] [CrossRef]
- Le Cann, K.; Foerster, A.; Rösseler, C.; Erickson, A.; Hautvast, P.; Giesselmann, S.; Pensold, D.; Kurth, I.; Rothermel, M.; Mattis, V.B.; et al. The Difficulty to Model Huntington’s Disease in Vitro Using Striatal Medium Spiny Neurons Differentiated from Human Induced Pluripotent Stem Cells. Sci. Rep. 2021, 11, 6934. [Google Scholar] [CrossRef]
- Golas, M.M. Human Cellular Models of Medium Spiny Neuron Development and Huntington Disease. Life Sci. 2018, 209, 179–196. [Google Scholar] [CrossRef] [PubMed]
- Telias, M. Neural Differentiation Protocols: How to Choose the Correct Approach. Neural Regen. Res. 2023, 18, 1273–1274. [Google Scholar] [CrossRef] [PubMed]
- Okita, K.; Yamakawa, T.; Matsumura, Y.; Sato, Y.; Amano, N.; Watanabe, A.; Goshima, N.; Yamanaka, S. An Efficient Nonviral Method to Generate Integration-Free Human-Induced Pluripotent Stem Cells from Cord Blood and Peripheral Blood Cells. Stem Cells 2013, 31, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Malakhova, A.A.; Grigor’eva, E.V.; Pavlova, S.V.; Malankhanova, T.B.; Valetdinova, K.R.; Vyatkin, Y.V.; Khabarova, E.A.; Rzaev, J.A.; Zakian, S.M.; Medvedev, S.P. Generation of Induced Pluripotent Stem Cell Lines ICGi021-A and ICGi022-A from Peripheral Blood Mononuclear Cells of Two Healthy Individuals from Siberian Population. Stem Cell Res. 2020, 48, 101952. [Google Scholar] [CrossRef] [PubMed]
- Straccia, M.; Barriga, G.G.D.; Sanders, P.; Bombau, G.; Carrere, J.; Mairal, P.B.; Vinh, N.N.; Yung, S.; Kelly, C.M.; Svendsen, C.N.; et al. Quantitative High-Throughput Gene Expression Profiling of Human Striatal Development to Screen Stem Cell-Derived Medium Spiny Neurons. Mol. Ther. Methods Clin. Dev. 2015, 2, 15030. [Google Scholar] [CrossRef] [PubMed]
- Maity, S.; Komal, P.; Kumar, V.; Saxena, A.; Tungekar, A.; Chandrasekar, V. Impact of ER Stress and ER-Mitochondrial Crosstalk in Huntington’s Disease. Int. J. Mol. Sci. 2022, 23, 780. [Google Scholar] [CrossRef]
- Lee, H.; Noh, J.Y.; Oh, Y.; Kim, Y.; Chang, J.W.; Chung, C.W.; Lee, S.T.; Kim, M.; Ryu, H.; Jung, Y.K. IRE1 Plays an Essential Role in ER Stress-Mediated Aggregation of Mutant Huntingtin via the Inhibition of Autophagy Flux. Hum. Mol. Genet. 2012, 21, 101–114. [Google Scholar] [CrossRef]
- Bae, D.; Jones, R.E.; Piscopo, K.M.; Tyagi, M.; Shepherd, J.D.; Hollien, J. Regulation of Blos1 by IRE1 Prevents the Accumulation of Huntingtin Protein Aggregates. Mol. Biol. Cell 2022, 33, ar125. [Google Scholar] [CrossRef]
- Brandstaetter, H.; Kruppa, A.J.; Buss, F. Huntingtin Is Required for ER-to-Golgi Transport and for Secretory Vesicle Fusion at the Plasma Membrane. DMM Dis. Models Mech. 2014, 7, 1335–1340. [Google Scholar] [CrossRef]
- Jin, H.; Komita, M.; Aoe, T. Decreased Protein Quality Control Promotes the Cognitive Dysfunction Associated with Aging and Environmental Insults. Front. Neurosci. 2018, 12, 386941. [Google Scholar] [CrossRef]
- Valdés, P.; Mercado, G.; Vidal, R.L.; Molina, C.; Parsons, G.; Court, F.A.; Martinez, A.; Galleguillos, D.; Armentano, D.; Schneider, B.L.; et al. Control of dopaminergic neuron survival by the unfolded protein response transcription factor XBP1. Proc. Natl. Acad. Sci. USA 2014, 111, 6804–6809. [Google Scholar] [CrossRef]
- Keene, C.D.; Rodrigues, C.M.; Eich, T.; Chhabra, M.S.; Steer, C.J.; Low, W.C. Tauroursodeoxycholic acid, a bile acid, is neuroprotective in a transgenic animal model of Huntington’s disease. Proc. Natl. Acad. Sci. USA 2002, 99, 10671–10676. [Google Scholar] [CrossRef]
- Ong, G.; Ragetli, R.; Mnich, K.; Doble, B.W.; Kammouni, W.; Logue, S.E. IRE1 signaling increases PERK expression during chronic ER stress. Cell Death Dis. 2024, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Cowan, C.A.; Klimanskaya, I.; McMahon, J.; Atienza, J.; Witmyer, J.; Zucker, J.P.; Wang, S.; Morton, C.C.; McMahon, A.P.; Powers, D.; et al. Derivation of Embryonic Stem-Cell Lines from Human Blastocysts. N. Engl. J. Med. 2004, 350, 1353–1356. [Google Scholar] [CrossRef] [PubMed]

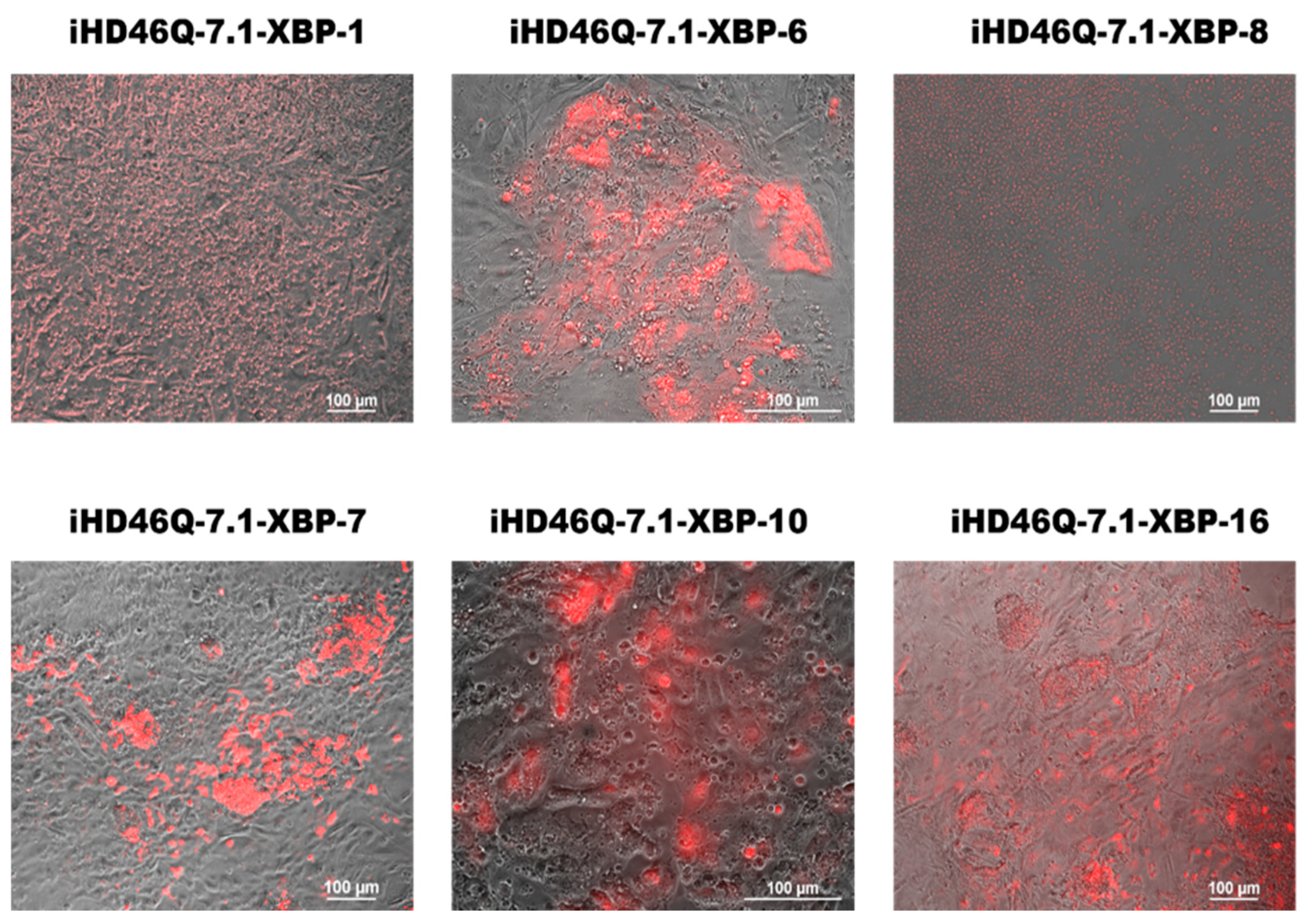

| Protocol | Transgenic iPSC Line | MSN Culture Name |
|---|---|---|
| No. 1 | iHD46Q-7.1-XBP-1 | XBP-1 |
| No. 2 | iHD46Q-7.1-XBP-6 | XBP-6F |
| No. 3 | XBP-6ST | |
| No. 1 | iHD46Q-7.1-XBP-7 | XBP-7 |
| No. 1 | iHD46Q-7.1-XBP-8 | XBP-8 |
| No. 2 | XBP-8F | |
| No. 3 | XBP-8ST | |
| No. 1 | iHD46Q-7.1-XBP-16 | XBP-16 |
| No. 1 | K6-4f (healthy donor) | K6-4 |
| Neuronal Culture | ATF4 | sXBP1 | CHOP | BIP |
|---|---|---|---|---|
| XBP-7 | up | up | up | |
| XBP-8 | up | up | up | up |
| XBP-16 | up | up | up | up |
| XBP-6F | up | up | ||
| XBP-8F | down | up | up | up |
| XBP-6ST | down | up | up | up |
| XBP-8ST | down | up |
| Gene | Sequence | |
|---|---|---|
| Markers of ER stress | ATF4 | TTCTCCAGCGACAAGGCTAAGG/CTCCAACATCCAATCTGTCCCG |
| CHOP | GGTATGAGGACCTGCAAGAGGT/CTTGTGACCTCTGCTGGTTCTG | |
| Bip (GRP78) | CTGTCCAGGCTGGTGTGCTCT/CTTGGTAGGCACCACTGTGTTC | |
| sXBP1 | TCTGCTGAGTCCGCAGCAG/GAAAAGGGAGGCTGGTAAGGAAC | |
| Markers of pluripotency | NANOG | CAGCCCCGATTCTTCCACCAGTCCC/CGGAAGATTCCCAGTCGGGTTCACC |
| SOX2 | GCTTAGCCTCGTCGATGAAC/AACCCCAAGATGCACAACTC | |
| OCT4 | CTTCTGCTTCAGGAGCTTGG/GAAGGAGAAGCTGGAGCAAA | |
| Markers of MSN | GABRA2 | CCCAATGCACTTGGAGGATTTCC/AGAGCCATCAGGAGCAACCTGT |
| ARPP21 | CCTACCTCAACCACGCAACAGT/CCTGTTGACCAGACAAGACTGG | |
| CTIP2 (BCL11B) | CTCCCTTTGGATGCCAGTGTCA/GGCTCCAGGTAGATGCGGAAG | |
| DRD1 | TGGTCTGTGCTGCCGTTATCAG/CAATCTCAGCCACTGCCTTCCA | |
| DRD2 | CAATACGCGCTACAGCTCCAAG/GGCAATGATGCACTCGTTCTGG | |
| CALB1 | TTTCCTGCTGCTCTTCCGATGC/GCTCCTCAGTTTCTATGAAGCCA | |
| Detection of transgene insertions | XBP1-TagRFP | CCGGACCACTTTGAGCTCTAC/AGGCGCACCGTGGGCTTGTAC |
| M2rtTA | CAGCCGGAACACGGCGGCATC/ACACCGTGCGTTTTATTCTGTC | |
| Reference | B2M | TAGCTGTGCTCGCGCTACT/ TCTCTGCTGGATGACGTGAG |
| sgRNA for AAVS1 | AAVS1 | CACCGGTCACCAATCCTGTCCCTAG/ AAACCTAGGGACAGGATTGGTGACC |
| Marker | Antibody | Dilution | Company |
|---|---|---|---|
| Markers of ectoderm | Rabbit IgG anti-NF200 | 1:1000 | Sigma-Aldrich Cat. # N4142 |
| Mouse IgG2a anti-Tubulin β 3 (TUBB3)/Clone: TUJ1 | 1:1000 | BioLegend Cat. # 801201 | |
| Markers of mesoderm | Mouse IgG2a anti-αSMA | 1:100 | Dako Cat. # M0851 |
| Mouse IgG1 anti-Collagen IV | 1:100 | LifeSpan Biosciences Cat # LS-C79603 | |
| Markers of endoderm | Mouse IgG1 anti-CK18 | 1:200 | Abcam, ab668 |
| Markers of pluripotency | Mouse IgGγ3 anti-SSEA4 | 1:200 | Abcam, ab16287 |
| Rabbit IgG anti-SOX2 | 1:500 | Cell Signaling, 3579 | |
| Mouse IgGγ2b anti-OCT4 | 1:50 | Santa Cruz, sc-5279 | |
| Mouse IgGγ1 anti-NANOG | 1:50 | Santa Cruz, sc-293121 | |
| Rabbit IgG anti-NANOG | 1:500 | REPROCELL, RCAB004P-F | |
| Secondary antibodies | Goat anti-Mouse IgG (H + L) Secondary Antibody, Alexa Fluor 568 | 1:400 | Thermo Fisher Scientific, A11031 |
| Goat anti-Rabbit IgG (H + L) Highly Cross-Adsorbed Secondary Antibody, Alexa Fluor 488 | 1:400 | Thermo Fisher Scientific, A11008 | |
| Goat anti-Mouse IgG2b CrossAdsorbed Secondary Antibody, Alexa Fluor 568 | 1:400 | Thermo Fisher Scientific, A-21141 | |
| Goat anti-Mouse IgG1 Cross-Adsorbed Secondary Antibody, Alexa Fluor™ 488 | 1:400 | Thermo Fisher Scientific, A-21121 | |
| Goat anti-Mouse IgG3 CrossAdsorbed Secondary Antibody, Alexa Fluor 488 | 1:400 | Thermo Fisher Scientific, A21151 |
| Name of Primer | Sequence |
|---|---|
| Forward (FAM-labeled) | [FAM-AH]TGGCGACCCTGGAAAAGCTGAT |
| Reverse | GGTGGCGGCTGTTGCTGCTGCTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Makeeva, V.S.; Sivkov, A.Y.; Zakian, S.M.; Malakhova, A.A. Generating a Cell Model to Study ER Stress in iPSC-Derived Medium Spiny Neurons from a Patient with Huntington’s Disease. Int. J. Mol. Sci. 2025, 26, 8930. https://doi.org/10.3390/ijms26188930
Makeeva VS, Sivkov AY, Zakian SM, Malakhova AA. Generating a Cell Model to Study ER Stress in iPSC-Derived Medium Spiny Neurons from a Patient with Huntington’s Disease. International Journal of Molecular Sciences. 2025; 26(18):8930. https://doi.org/10.3390/ijms26188930
Chicago/Turabian StyleMakeeva, Vladlena S., Anton Yu. Sivkov, Suren M. Zakian, and Anastasia A. Malakhova. 2025. "Generating a Cell Model to Study ER Stress in iPSC-Derived Medium Spiny Neurons from a Patient with Huntington’s Disease" International Journal of Molecular Sciences 26, no. 18: 8930. https://doi.org/10.3390/ijms26188930
APA StyleMakeeva, V. S., Sivkov, A. Y., Zakian, S. M., & Malakhova, A. A. (2025). Generating a Cell Model to Study ER Stress in iPSC-Derived Medium Spiny Neurons from a Patient with Huntington’s Disease. International Journal of Molecular Sciences, 26(18), 8930. https://doi.org/10.3390/ijms26188930





