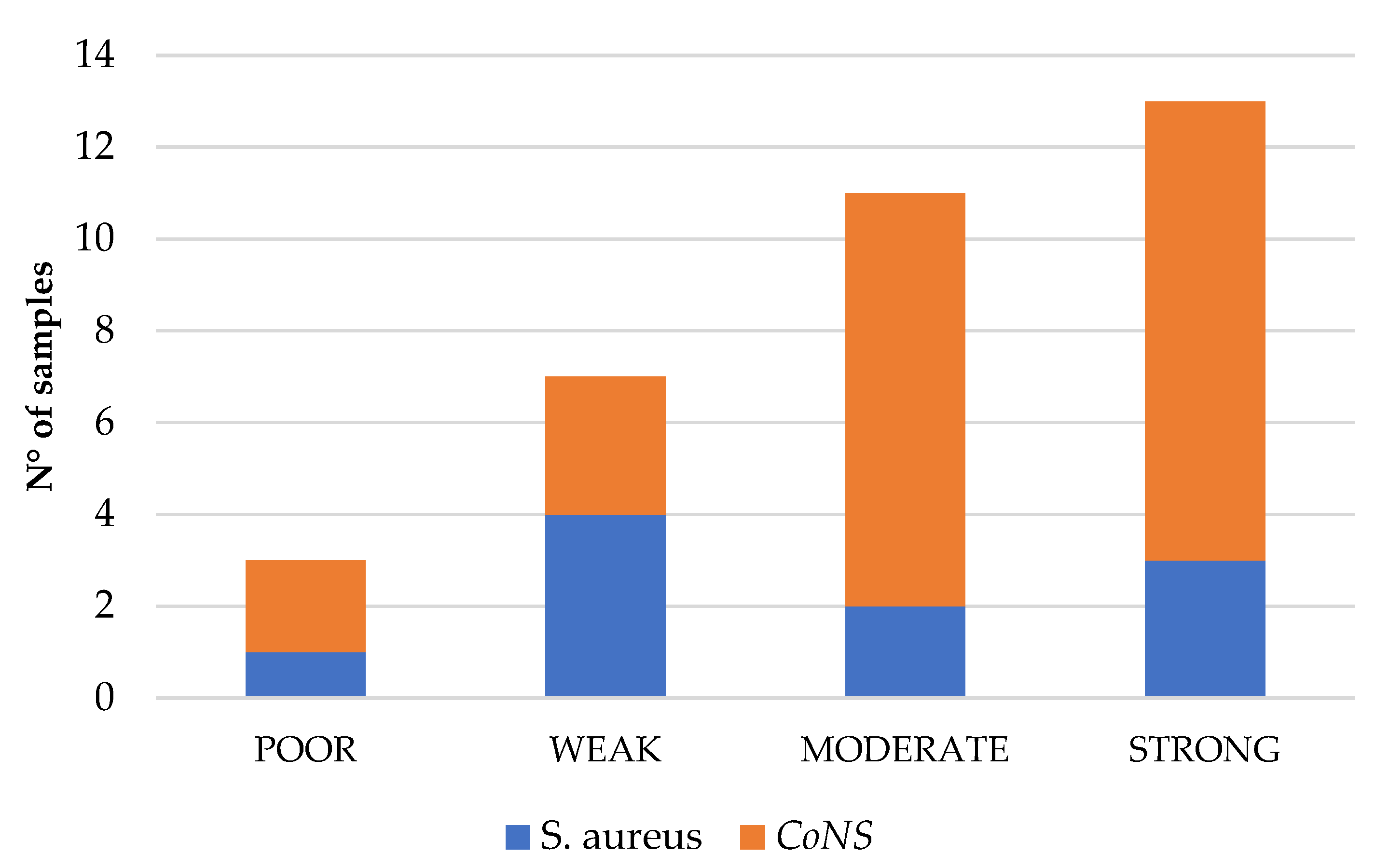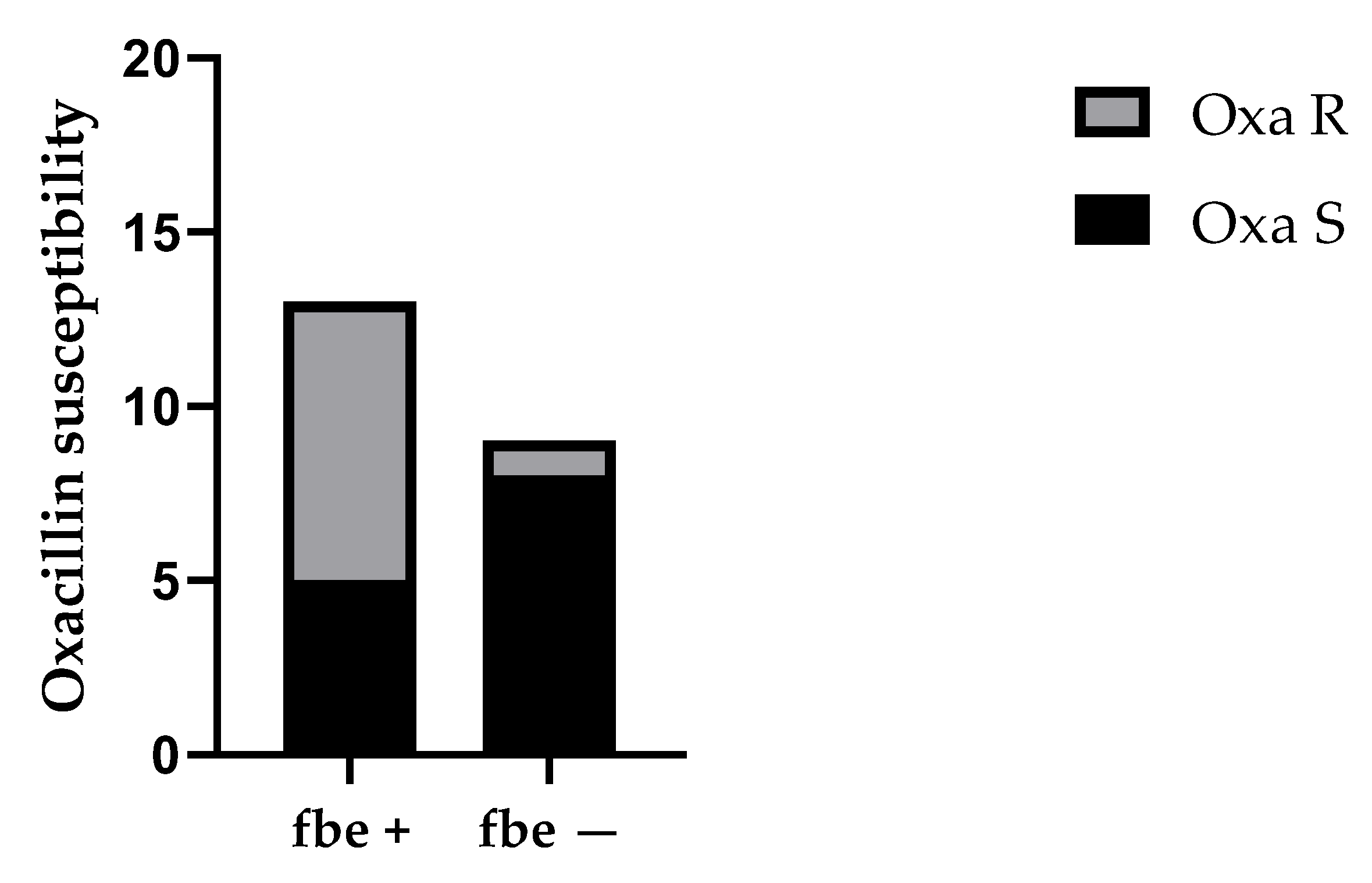1. Introduction
Total joint arthroplasty (TJA) and total joint replacement (TJR) are well-established and highly effective surgical treatments for end-stage osteoarthritis, significantly improving patients’ quality of life [
1,
2]. However, prosthetic joint infection (PJI)—an infection involving the prosthetic joint and surrounding soft tissues—remains one of the most serious complications in orthopedic surgery. Accurate microbiological diagnosis is essential for effective clinical management of such infections. Although the incidence of PJI is relatively low, ranging from 0.1% to 4.0% according to literature [
3,
4], its clinical impact is significant. Studies have reported a 1-year mortality rate of up to 8% following the index procedure and a twofold increase in in-hospital mortality with each surgical admission [
5,
6,
7].
In response to the COVID-19 pandemic, several countries reported a reduction in elective orthopedic surgeries in 2020 [
8]. In Italy, for example, hip replacements decreased by 20% and knee replacements by approximately 30% compared to 2019 [
8]. Despite this global decline in procedures, the incidence of periprosthetic infections has shown a rising trend and is now recognized as one of the most challenging complications in modern orthopedics. Several patient-related factors have been associated with an increased risk of PJI, including comorbidities such as rheumatoid arthritis, diabetes mellitus, malignancy, chronic kidney disease, and obesity [
9,
10]. Bloodstream infection (BSI) in the presence of a prosthetic joint is a well-established risk factor for PJI, particularly in cases involving
Staphylococcus spp., with studies indicating that 25% to 34% of staphylococcal BSIs can lead to PJI [
11,
12].
The most common microorganisms responsible for PJIs are
Staphylococcus spp., particularly
Staphylococcus aureus and coagulase-negative
Staphylococci (e.g.,
Staphylococcus epidermidis) [
13]. However, the microbial etiology can vary significantly based on factors such as time since implantation, joint site, and geographic location; for instance,
S. aureus predominates in the U.S., whereas
S. epidermidis is more common in Europe [
13]. Identification of the causative pathogen through culture is crucial for guiding antimicrobial therapy. Culture positivity ranges from 65% to 94% of PJIs, with acute infections often yielding positive cultures in up to 90% of cases [
14]. Early-onset PJIs require precise microbiological diagnosis due to their frequent management via surgical debridement [
15,
16,
17].
Treatment is further complicated when virulent and antimicrobial-resistant pathogens are involved, such as methicillin-resistant
S. aureus (MRSA), rifampin-resistant
Staphylococci, or ciprofloxacin-resistant Gram-negative bacilli, all of which are associated with poorer outcomes [
18,
19]. Culture-negative PJIs commonly result from prior antibiotic exposure or the presence of fastidious organisms such as fungi or mycobacteria. Advances in molecular diagnostics—including broad-range polymerase chain reaction (PCR) and metagenomic shotgun sequencing—are improving the detection of these difficult-to-identify pathogens [
20].
A major factor contributing to the persistence of PJIs is the formation of bacterial biofilms. Surgical implants and prosthetic materials serve as abiotic surfaces conducive to microbial adhesion and biofilm development. In biofilms, bacteria aggregate into a sessile community embedded within a self-produced extracellular polymeric substance (EPS) [
21,
22]. Biofilms confer significant resistance to antibiotics and host immune defenses [
23], in part due to altered gene expression, reduced antimicrobial penetration, and the protective properties of the EPS matrix [
24,
25,
26]. Classical culture techniques often fail to detect biofilm-associated pathogens, with success rates as low as 30% [
27]. Therefore, effective biofilm disruption is necessary prior to standard microbiological analysis [
28]. Gram-positive cocci—particularly
S. aureus, coagulase-negative
Staphylococci (CoNS), and enterococci—account for approximately 75% of biofilm-associated PJIs [
13,
29,
30].
Despite ongoing efforts, standardized guidelines for the diagnosis and management of biofilm-related infections remain incomplete and controversial. Given that many medical devices (e.g., catheters, implants) are prone to biofilm colonization [
31], there is an urgent need for reliable assays to detect biofilm-forming strains. In this context, the secondary messenger bis(3′,5′)-cyclic di-guanylic acid (c-di-GMP) plays a key regulatory role in bacterial physiology. It governs various cellular processes, including virulence and biofilm formation [
32]. Elevated intracellular levels of c-di-GMP promote a transition from a motile, planktonic lifestyle to a sessile, biofilm-forming state [
33], partly by inducing adhesin and exopolysaccharide biosynthesis while inhibiting flagellar motility [
34]. Furthermore, c-di-GMP can regulate gene expression post-transcriptionally by interacting with mRNA or small regulatory RNAs [
35].
Several genes have been implicated in staphylococcal biofilm formation. Among them, the
ica operon is well-characterized for encoding enzymes responsible for synthesizing polysaccharide intercellular adhesin (PIA) [
36]. More recently, proteinaceous components have also been shown to contribute significantly to biofilm development [
37]. Surface adhesins such as fibronectin-binding proteins (FnBPs) and clumping factor A (ClfA) are involved in host tissue colonization and immune evasion. Notably, ClfA binds complement factor I, facilitating neutrophil evasion [
38], while FnBPs and elastin bind plasminogen, enhancing biofilm formation [
39,
40]. In CoNS, biofilm formation involves surface proteins such as autolysin E (AtlE) and the fibrinogen-binding protein of
S. epidermidis (Fbe), in addition to PIA production via the
ica operon [
41].
The aim of this study was to characterize the microbial population identified in patients with PJI (≥90 days post-implantation) after TJA and TJR, focusing on c-di-GMP production and the activation of specific genes, like ica, as previously described, in order to assess the actual ability of microbial populations—particularly S. aureus and CoNS—to form biofilms. Biofilm formation represents a negative prognostic factor that significantly complicates both treatment and infection eradication. Therefore, accurate and timely identification of biofilms may represent a crucial additional tool for the appropriate management of therapy, not only guiding the selection of the most effective antibiotics but also modulating treatment duration and therapeutic strategies aimed at improving infection control and patient outcomes.
3. Discussion
Infection as a consequence of joint replacement is a rare event, but it can lead to severe complications due to the presence of pathogens capable of forming biofilms. However, with the increasing life expectancy, surgical procedures requiring total joint replacement are becoming more common, resulting in an alarming rise in difficult-to-treat (peri) PJIs [
42]. It is widely acknowledged that the presence of microbial biofilms is a major virulence factor associated with challenging infections. Bacteria embedded in biofilms are inherently more resistant to environmental and chemical agents, tolerating higher concentrations of antibiotics than their planktonic counterparts, allowing them to survive long-term in natural ecosystems and animal hosts [
43]. Moreover, biofilm formation can prevent bacterial clearance and hinder the host immune response, enabling bacteria to invade host tissues and increase morbidity and mortality [
36,
44]. Therefore, the aim of the present study was to better characterize biofilm-associated determinants of virulence in different bacterial strains isolated from synovial fluid derived from PJIs, with particular focus on factors influencing biofilm formation rather than antimicrobial resistance mechanisms.
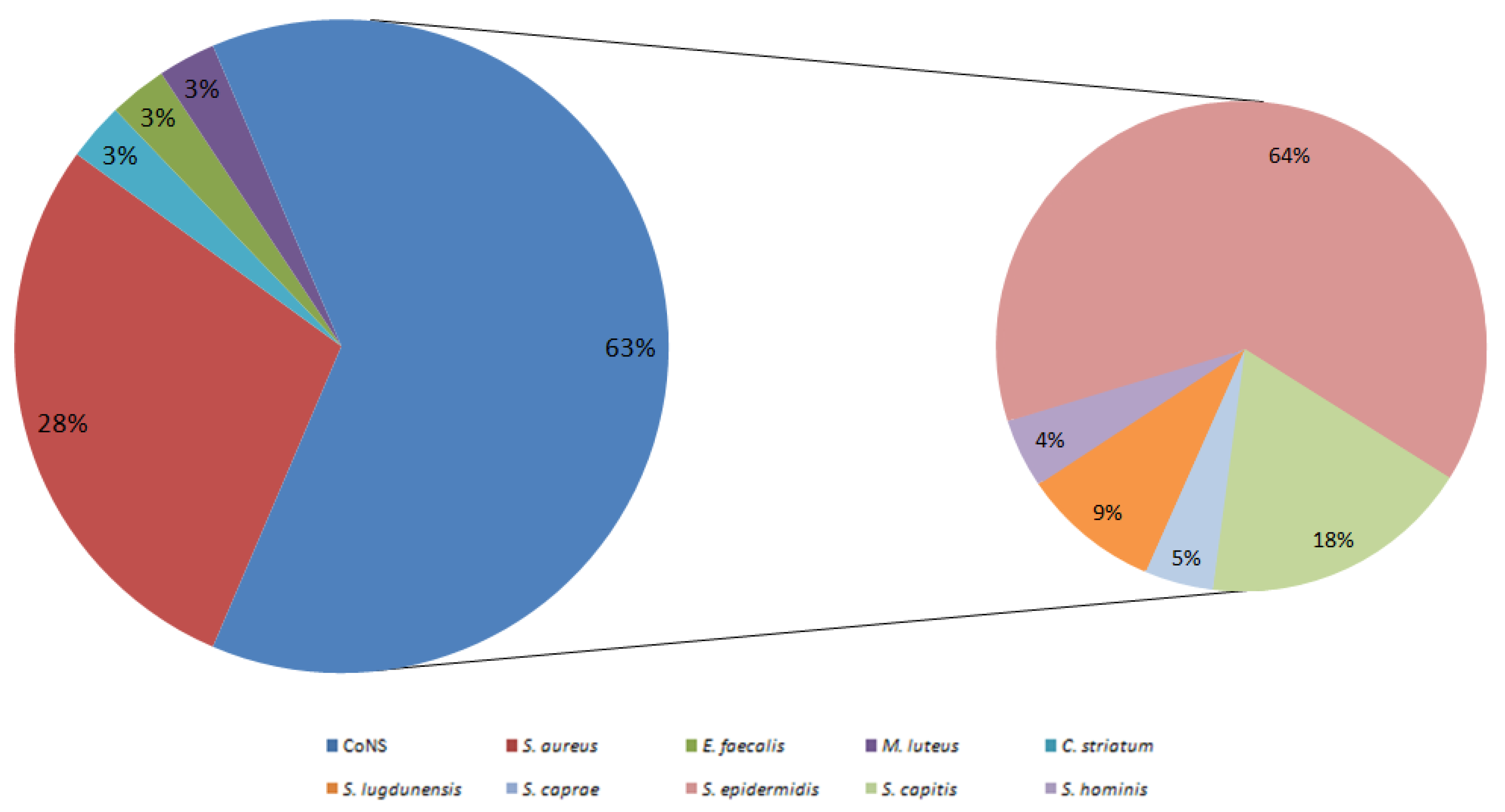
In this ongoing study, the prevalence of PJI from joint replacements was found to be 16.7% (33/198). Notably, in all synovial fluids that exhibited bacterial growth, only gram-positive bacteria were isolated (100%, 33/33). Incidence rates for PJIs can vary between 0.1% and 20%, depending on the specific orthopedic subspecialties. However, for hip and knee arthroplasty, the incidence of surgical site infections typically ranges from 0.1% to 4.0% [
45,
46,
47]. It is important to note that, when specifically considering
S. aureus, only 10 synovial fluids tested positive for this pathogen, yielding an incidence of 5.2% (10/193). These findings are consistent with a 17-year study conducted in Finland, which observed a similar range of
S. aureus infections following hip and knee arthroplasty, with an incidence varying from 1.6% to 4.0% [
46].
Antimicrobial susceptibility of the staphylococcal isolates was assessed by determining the MIC, and the methicillin resistance status was evaluated through oxacillin MIC testing. Overall, methicillin resistance was found in 37.5% (12/32) of bacterial isolates, while 100% (32/32) of the strains were vancomycin-susceptible. Although the number of
S. aureus isolates was limited, the MRSA incidence rate was 30% (3/10). This rate is consistent with a study by Pimentel de Araujo et al. (2022), which reported an incidence of MRSA in osteomyelitis cases of 30.1% [
48]. No correlation was found between the identification of methicillin-resistant isolates and their ability to produce biofilm or c-di-GMP. This lack of correlation is most likely due to the relatively small number of isolates analyzed, which limits the statistical power of our observations. Nevertheless, literature indicates that the presence of biofilm itself may offer effective protection from antibiotic action, as bacteria embedded in biofilms can survive antibiotic concentrations 10 to 10,000 times greater than their planktonic counterparts [
43,
49].
Subsequently, we aimed to further phenotypically characterize the isolated strains based on their ability to produce biofilm. The crystal violet assay, a widely used method for biofilm quantification, classifies bacterial strains as poor, weak, moderate, or strong biofilm producers [
50,
51]. Additionally, the production of c-di-GMP, a second messenger known to regulate bacterial physiology, including biofilm formation, was assessed [
52,
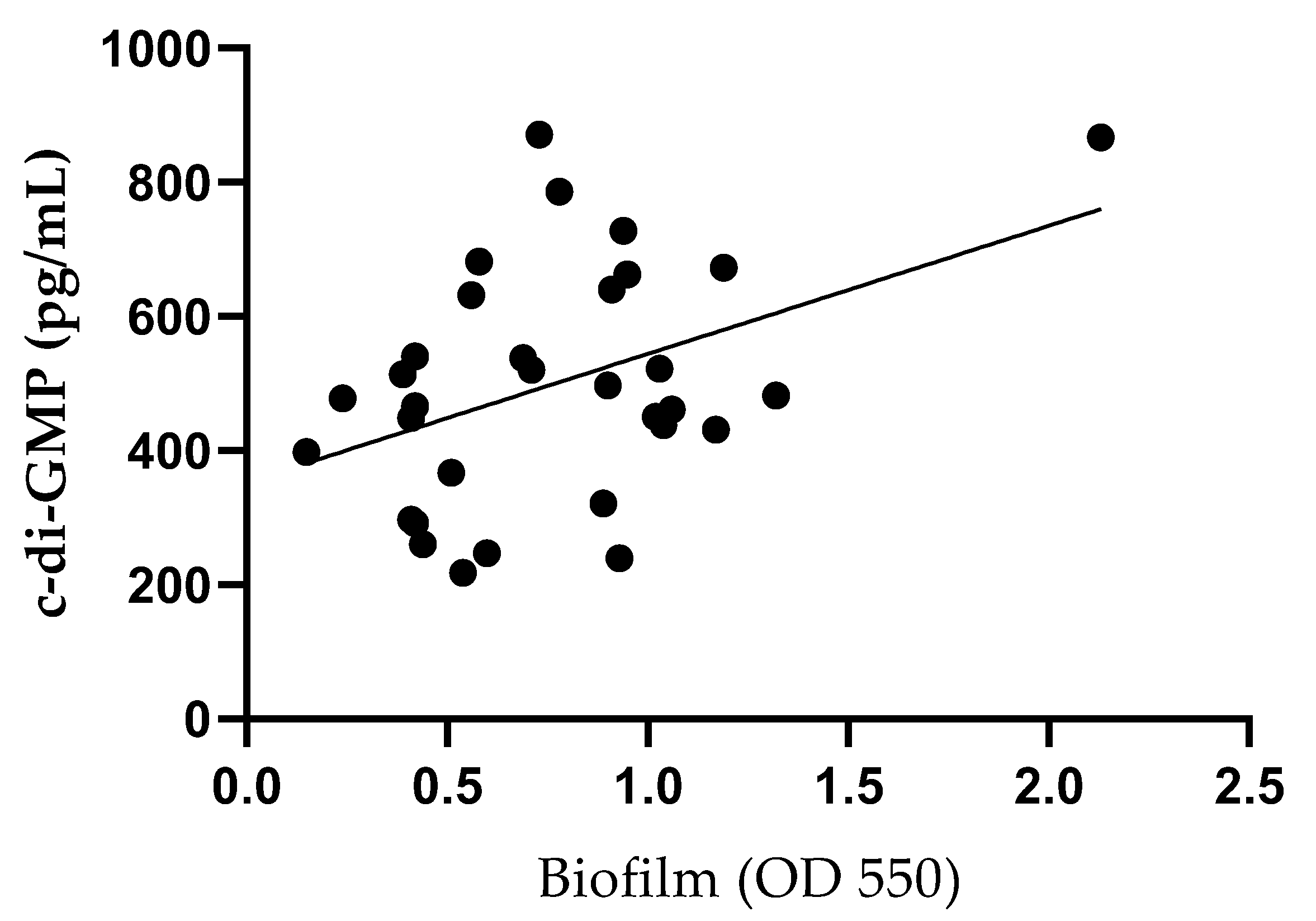
53]. Most of the isolated strains were classified as strong (34.4%, 11/32) or moderate (34.4%, 11/32) biofilm producers. Although a statistically significant linear regression (
p < 0.05) was observed between biofilm production and c-di-GMP levels, consistent with findings in other bacteria such as
Pseudomonas aeruginosa,
Pseudomonas resinovorans, and
Vibrio vulnificus [
54,
55,
56], the low R
2 value (0.20) indicates that c-di-GMP explains only a small proportion of the variability in biofilm formation. This suggests that, while the association is real, c-di-GMP alone is a poor predictive biomarker for biofilm production and likely requires consideration in combination with other regulatory factors.
Biofilm formation is a complex process that involves numerous genes, proteins, and signaling pathways. To better understand the genetic background of the isolated strains, we investigated the presence of several biofilm-associated genes. Specifically, all bacterial isolates were tested for the presence of the
icaA gene, which is responsible for the synthesis of the extracellular matrix and biofilm formation. The presence of the
icaA gene was detected in 53.1% (17/32) of bacterial isolates. Among CoNS, 31.8% (7/22) of isolates carried the
icaA gene, while in all
S. aureus isolates (100%, 10/10), the
icaA gene was present. A study by Pimentel de Araujo et al. (2022) reported that 95% of
S. aureus isolates responsible for osteomyelitis carried the
icaA gene [
48]. Moreover, a significant difference in intracellular c-di-GMP concentration was observed between
icaA-positive and
icaA-negative strains. While this finding suggests a link between gene presence and signaling activity, it also highlights the complexity of the regulatory mechanisms involved.
Furthermore, we observed a statistically significant difference in c-di-GMP intracellular concentration between strains harboring the icaA gene and strains without the icaA gene.
We also examined
S. aureus isolates for the presence of
fnbA and
clfA. Both genes are known to contribute to tissue colonization and biofilm formation [
57,
58]. All
S. aureus isolates tested positive for the
clfA gene (100%, 10/10), whereas 60% (6/10) carried the
fnbA gene. These findings are consistent with studies showing a high frequency of
clfA in
S. aureus isolates from hematogenous infections [
59]. However, Kouidhi et al. (2010) reported a much lower frequency of
clfA (9.1%) in
S. aureus strains isolated from dental caries, suggesting that the distribution of
clfA may vary depending on the infection site [
60].
For CoNS, we investigated the presence of the
atlE and
fbe genes. The
atlE gene codes for an autolysin that mediates cell wall lysis, leading to DNA release into the biofilm matrix and contributing to irreversible adhesion during biofilm development [
61,
62]. The
fbe gene encodes a fibrinogen-binding protein involved in attachment and biofilm production [
63,
64]. The
atlE gene was detected in 63.6% (14/22) of CoNS isolates, and the
fbe gene was found in 59.1% (13/22). Interestingly, strains with the
fbe gene were more frequently associated with oxacillin resistance compared to those without the
fbe gene (
p < 0.05). The involvement of fbe in biofilm formation could explain the statistically significant difference between the groups.
While few studies have addressed this specific aspect, our findings are in line with previous research suggesting that biofilm-associated genes are more frequently carried by methicillin-resistant CoNS strains compared to methicillin-sensitive strains [
65,
66].
Although biofilm formation was significantly associated with c-di-GMP production in this cohort, the low R
2 value (0.18) raises doubts about the consistency of the relationship, suggesting that further studies are needed to better characterize this pathway. Infact, as reported by Zhu et al., no correlation between biofilm formation and intracellular c-di-GMP levels was observed in
S. epidermidis [
67].