Boosting Neurogenesis as a Strategy in Treating Alzheimer’s Disease
Abstract
1. Introduction
2. Overview of Adult Neurogenesis
3. Regulation of Adult Neurogenesis
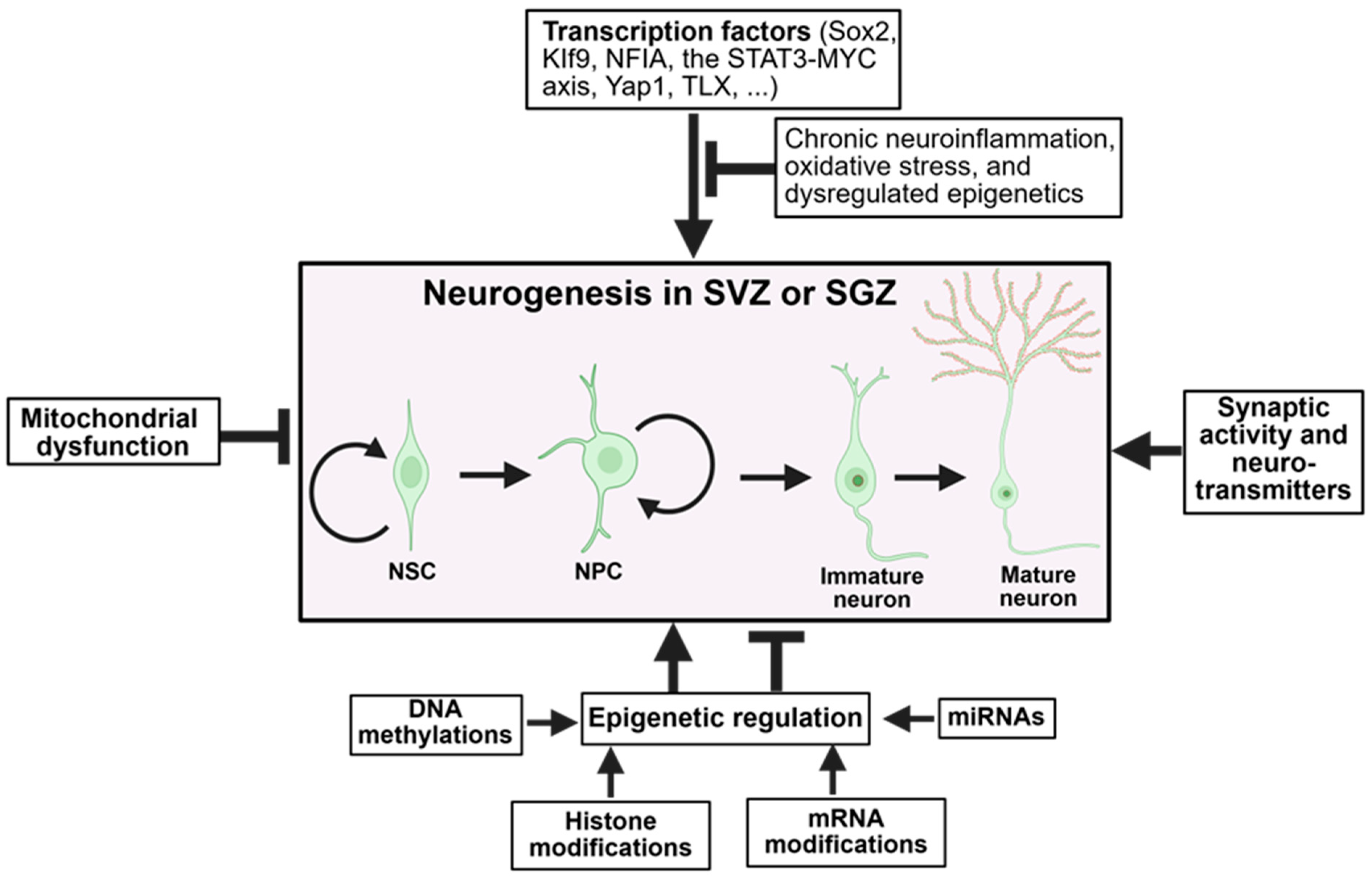
3.1. Transcription Factors and Intrinsic Regulators
3.2. Epigenetic Regulation
3.3. Mitochondrial Dysfunction
3.4. Synaptic Activity and Neurotransmitters
4. Role of Neurogenesis in Cognition and Memory
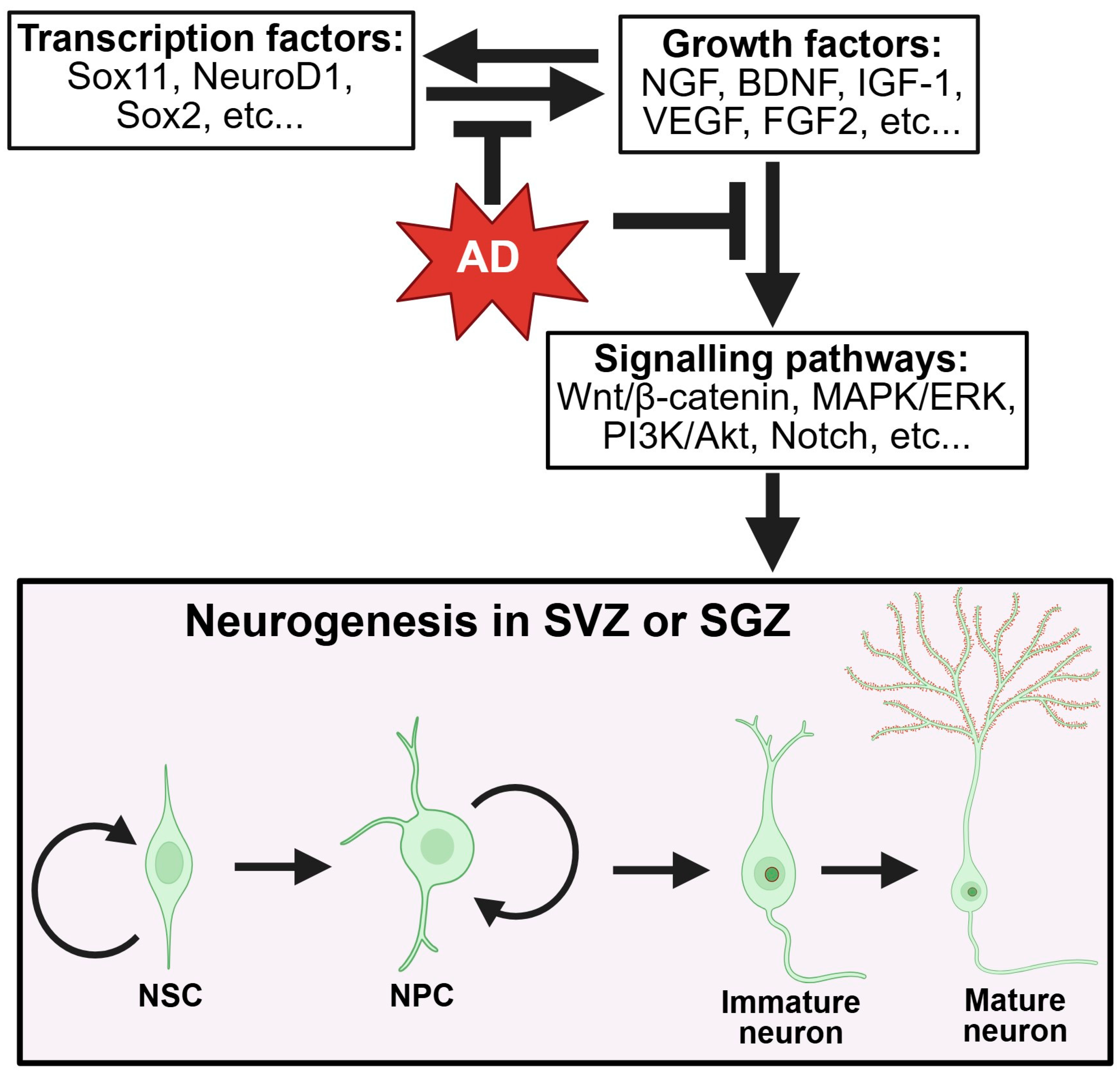
5. Impairment of Neurogenesis in AD
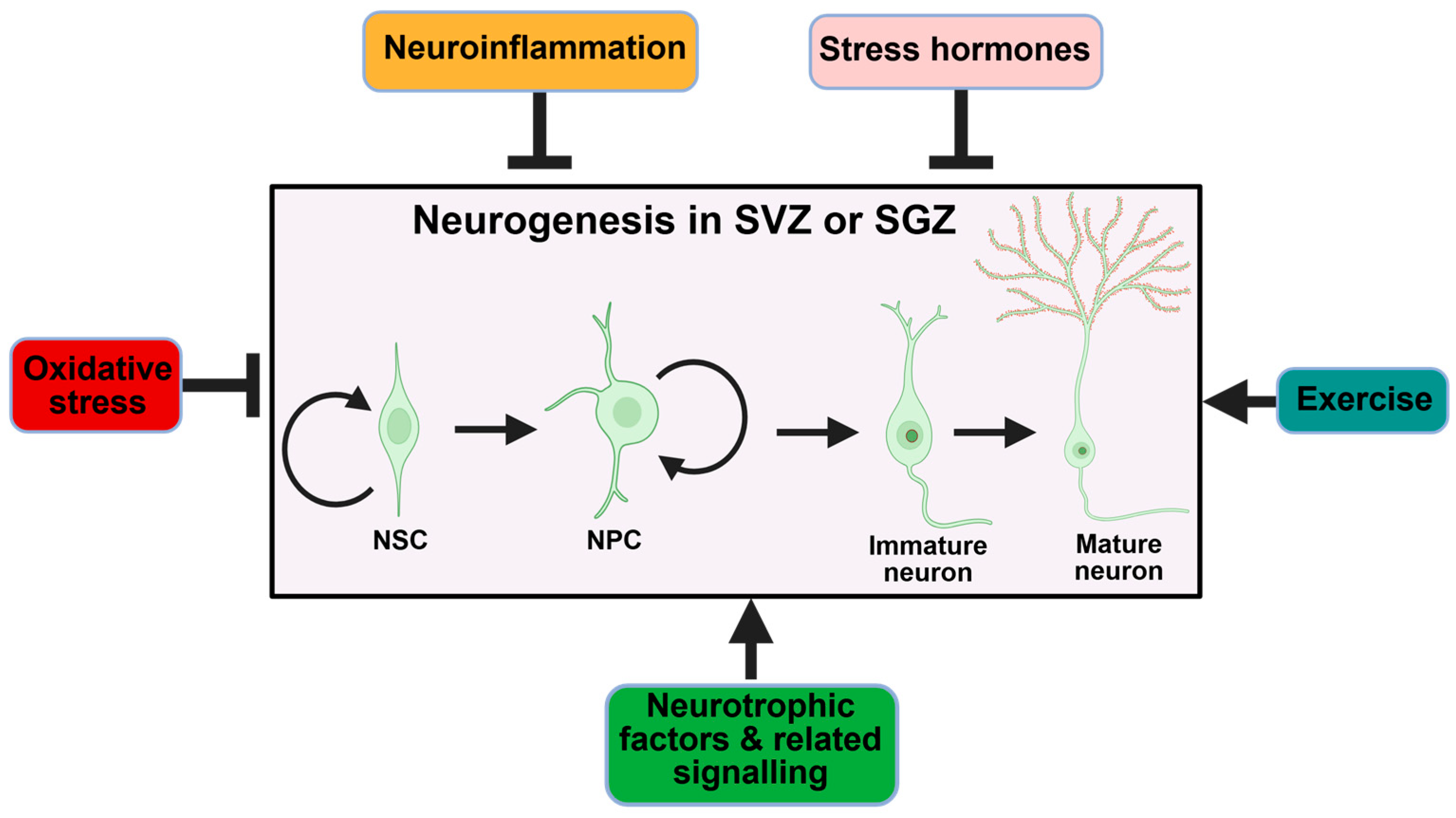
6. Physiological and Pathological Modulators of Neurogenesis
6.1. Neuroinflammation
6.2. Oxidative Stress
6.3. Dysregulated Signaling and Growth Factors
7. Therapeutic Strategies to Enhance Neurogenesis
7.1. Pharmacotherapy
7.1.1. Acetylcholinesterase Inhibitors
7.1.2. Proteostasis Enhancers
7.1.3. Mitogen-Pathway Inhibitors
7.1.4. Phosphodiesterase Inhibitors
7.1.5. Antidepressants (SSRIs)
7.1.6. Glucagon-like Peptide-1 (GLP-1) Agonists
7.2. Stem Cell Therapies
7.3. Genetic and Epigenetic Manipulation
7.3.1. Genetic Manipulation
7.3.2. Epigenetic Modulation
7.4. Exosome-Based Therapies
8. Translational and Clinical Implications
8.1. Complementary Therapy
8.2. Identifying Biomarkers
8.3. Early Intervention
8.4. Potential Risks and Safety Considerations
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Busche, M.A.; Chen, X.; Henning, H.A.; Reichwald, J.; Staufenbiel, M.; Sakmann, B.; Konnerth, A. Critical role of soluble amyloid-β for early hippocampal hyperactivity in a mouse model of Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2012, 109, 8740–8745. [Google Scholar] [CrossRef]
- Kadamangudi, S.; Marcatti, M.; Zhang, W.R.; Fracassi, A.; Kayed, R.; Limon, A.; Taglialatela, G. Amyloid-β oligomers increase the binding and internalization of tau oligomers in human synapses. Acta Neuropathol. 2024, 149, 2. [Google Scholar] [CrossRef]
- Walsh, D.M.; Klyubin, I.; Fadeeva, J.V.; Cullen, W.K.; Anwyl, R.; Wolfe, M.S.; Rowan, M.J.; Selkoe, D.J. Naturally secreted oligomers of amyloid β protein potently inhibit hippocampal long-term potentiation in vivo. Nature 2002, 416, 535–539. [Google Scholar] [CrossRef]
- Jin, S.X.; Bellier, J.P.; Wells, A.; PM, L.I.; Anekal, P.V.; Tresback, J.S.; Caldarone, B.J.; Liu, L.; Li, S.; Dettmer, U.; et al. Inhibition of hippocampal mossy fiber plasticity and episodic memory by human Aβ oligomers is prevented by enhancing cAMP signaling in Alzheimer’s mice. Alzheimers Dement. 2025, 21, e70194. [Google Scholar] [CrossRef] [PubMed]
- de la Cueva, M.; Antequera, D.; Ordoñez-Gutierrez, L.; Wandosell, F.; Camins, A.; Carro, E.; Bartolome, F. Amyloid-β impairs mitochondrial dynamics and autophagy in Alzheimer’s disease experimental models. Sci. Rep. 2022, 12, 10092, Erratum in Sci. Rep. 2023, 13, 19303. https://doi.org/10.1038/s41598-023-46106-y. [Google Scholar] [CrossRef] [PubMed]
- WHO. Dementia. 2023 September. 2023. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia (accessed on 2 August 2025).
- Altman, J.; Das, G.D. Post-Natal Origin of Microneurones in the Rat Brain. Nature 1965, 207, 953–956. [Google Scholar] [CrossRef]
- Kaplan, M.S.; Hinds, J.W. Neurogenesis in the Adult Rat: Electron Microscopic Analysis of Light Radioautographs. Science 1977, 197, 1092–1094. [Google Scholar] [CrossRef]
- Mu, Y.; Gage, F.H. Adult hippocampal neurogenesis and its role in Alzheimer’s disease. Mol. Neurodegener. 2011, 6, 85. [Google Scholar] [CrossRef]
- Sorrells, S.F.; Paredes, M.F.; Cebrian-Silla, A.; Sandoval, K.; Qi, D.; Kelley, K.W.; James, D.; Mayer, S.; Chang, J.; Auguste, K.I.; et al. Human hippocampal neurogenesis drops sharply in children to undetectable levels in adults. Nature 2018, 555, 377–381. [Google Scholar] [CrossRef]
- Moreno-Jiménez, E.P.; Flor-García, M.; Terreros-Roncal, J.; Rábano, A.; Cafini, F.; Pallas-Bazarra, N.; Ávila, J.; Llorens-Martín, M. Adult hippocampal neurogenesis is abundant in neurologically healthy subjects and drops sharply in patients with Alzheimer’s disease. Nat. Med. 2019, 25, 554–560. [Google Scholar] [CrossRef]
- Tobin, M.K.; Musaraca, K.; Disouky, A.; Shetti, A.; Bheri, A.; Honer, W.G.; Kim, N.; Dawe, R.J.; Bennett, D.A.; Arfanakis, K.; et al. Human Hippocampal Neurogenesis Persists in Aged Adults and Alzheimer’s Disease Patients. Cell Stem Cell 2019, 24, 974–982.e973. [Google Scholar] [CrossRef] [PubMed]
- Hollands, C.; Tobin, M.K.; Hsu, M.; Musaraca, K.; Yu, T.S.; Mishra, R.; Kernie, S.G.; Lazarov, O. Depletion of adult neurogenesis exacerbates cognitive deficits in Alzheimer’s disease by compromising hippocampal inhibition. Mol. Neurodegener. 2017, 12, 64. [Google Scholar] [CrossRef]
- Doody, R.S.; Raman, R.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; He, F.; Sun, X.; Thomas, R.G.; et al. A Phase 3 Trial of Semagacestat for Treatment of Alzheimer’s Disease. N. Engl. J. Med. 2013, 369, 341–350. [Google Scholar] [CrossRef]
- Ivanoiu, A.; Pariente, J.; Booth, K.; Lobello, K.; Luscan, G.; Hua, L.; Lucas, P.; Styren, S.; Yang, L.; Li, D.; et al. Long-term safety and tolerability of bapineuzumab in patients with Alzheimer’s disease in two phase 3 extension studies. Alzheimer’s Res. Ther. 2016, 8, 24. [Google Scholar] [CrossRef]
- National Institutes of Health. A Study to Evaluate the Efficacy and Safety of Semorinemab in Patients with Prodromal to Mild Alzheimer’s Disease. 2022. Available online: https://clinicaltrials.gov/study/NCT03289143 (accessed on 16 March 2022).
- National Institutes of Health. A Study of Semorinemab in Patients with Moderate Alzheimer’s Disease. 2024. Available online: https://www.clinicaltrials.gov/study/NCT03828747 (accessed on 1 September 2025).
- Alvarez-Buylla, A.; Garcia-Verdugo, J.M. Neurogenesis in adult subventricular zone. J. Neurosci. 2002, 22, 629–634. [Google Scholar] [CrossRef]
- Cebrian-Silla, A.; Nascimento, M.A.; Redmond, S.A.; Mansky, B.; Wu, D.; Obernier, K.; Romero Rodriguez, R.; Gonzalez-Granero, S.; García-Verdugo, J.M.; Lim, D.A.; et al. Single-cell analysis of the ventricular-subventricular zone reveals signatures of dorsal and ventral adult neurogenesis. eLife 2021, 10, e67436. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef]
- Doetsch, F.; Caillé, I.; Lim, D.A.; García-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Deng, W.; Gage, F.H. Mechanisms and Functional Implications of Adult Neurogenesis. Cell 2008, 132, 645–660. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, J. Orchestrating transcriptional control of adult neurogenesis. Genes Dev. 2012, 26, 1010–1021. [Google Scholar] [CrossRef]
- Kempermann, G.; Fabel, K.; Ehninger, D.; Babu, H.; Leal-Galicia, P.; Garthe, A.; Wolf, S.A. Why and how physical activity promotes experience-induced brain plasticity. Front. Neurosci. 2010, 4, 189. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Jang, S.E.; Zeng, L. The Effects of Extrinsic and Intrinsic Factors on Neurogenesis. Cells 2023, 12, 1285. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chichung Lie, D.; Taupin, P.; Nakashima, K.; Ray, J.; Yu, R.T.; Gage, F.H.; Evans, R.M. Expression and function of orphan nuclear receptor TLX in adult neural stem cells. Nature 2004, 427, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Fan, W.; Jurado-Arjona, J.; Alanis-Lobato, G.; Péron, S.; Berger, C.; Andrade-Navarro, M.A.; Falk, S.; Berninger, B. The transcriptional co-activator Yap1 promotes adult hippocampal neural stem cell activation. EMBO J. 2023, 42, e110384. [Google Scholar] [CrossRef]
- Favaro, R.; Valotta, M.; Ferri, A.L.; Latorre, E.; Mariani, J.; Giachino, C.; Lancini, C.; Tosetti, V.; Ottolenghi, S.; Taylor, V.; et al. Hippocampal development and neural stem cell maintenance require Sox2-dependent regulation of Shh. Nat. Neurosci. 2009, 12, 1248–1256. [Google Scholar] [CrossRef]
- Li, Y.; Ma, W.; Ma, R.; Wang, S.; Liu, X.; Guo, X.; Li, W.; Chen, X.; Cui, Y.-L.; Song, H. FMRP regulation of STAT3-MYC signaling is critical for adult hippocampal neurogenesis and cognitive flexibility. Cell Death Differ. 2025; in press. [Google Scholar] [CrossRef]
- Wang, M.; van Bruggen, R.; Mohammed, L.; Egor, K.; Tan, Q. Loss of NFIA Impairs Adult Hippocampal Neurogenesis. Hippocampus 2025, 35, e70016. [Google Scholar] [CrossRef]
- Mir, S.; Cai, W.; Carlson, S.W.; Saatman, K.E.; Andres, D.A. IGF-1 mediated Neurogenesis Involves a Novel RIT1/Akt/Sox2 Cascade. Sci. Rep. 2017, 7, 3283. [Google Scholar] [CrossRef]
- Guo, N.; McDermott, K.D.; Shih, Y.-T.; Zanga, H.; Ghosh, D.; Herber, C.; Meara, W.R.; Coleman, J.; Zagouras, A.; Wong, L.P.; et al. Transcriptional regulation of neural stem cell expansion in the adult hippocampus. eLife 2022, 11, e72195. [Google Scholar] [CrossRef]
- Sun, G.; Yu, R.T.; Evans, R.M.; Shi, Y. Orphan nuclear receptor TLX recruits histone deacetylases to repress transcription and regulate neural stem cell proliferation. Proc. Natl. Acad. Sci. USA 2007, 104, 15282–15287. [Google Scholar] [CrossRef]
- Sleiman, S.F.; Henry, J.; Al-Haddad, R.; El Hayek, L.; Abou Haidar, E.; Stringer, T.; Ulja, D.; Karuppagounder, S.S.; Holson, E.B.; Ratan, R.R.; et al. Exercise promotes the expression of brain derived neurotrophic factor (BDNF) through the action of the ketone body β-hydroxybutyrate. eLife 2016, 5, e15092. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Zhang, Y.; Zhang, M.; Chang, J.; Zeng, Z.; Kou, X.; Chen, N. Exercise Attenuates Brain Aging by Rescuing Down-Regulated Wnt/β-Catenin Signaling in Aged Rats. Front. Aging Neurosci. 2020, 12, 105. [Google Scholar] [CrossRef]
- Wrann, C.D.; White, J.P.; Salogiannnis, J.; Laznik-Bogoslavski, D.; Wu, J.; Ma, D.; Lin, J.D.; Greenberg, M.E.; Spiegelman, B.M. Exercise induces hippocampal BDNF through a PGC-1α/FNDC5 pathway. Cell Metab. 2013, 18, 649–659. [Google Scholar] [CrossRef]
- Speisman, R.B.; Kumar, A.; Rani, A.; Pastoriza, J.M.; Severance, J.E.; Foster, T.C.; Ormerod, B.K. Environmental enrichment restores neurogenesis and rapid acquisition in aged rats. Neurobiol. Aging 2013, 34, 263–274. [Google Scholar] [CrossRef]
- Anacker, C.; Zunszain, P.A.; Cattaneo, A.; Carvalho, L.A.; Garabedian, M.J.; Thuret, S.; Price, J.; Pariante, C.M. Antidepressants increase human hippocampal neurogenesis by activating the glucocorticoid receptor. Mol. Psychiatry 2011, 16, 738–750. [Google Scholar] [CrossRef]
- Rafa-Zabłocka, K.; Kreiner, G.; Bagińska, M.; Nalepa, I. Selective Depletion of CREB in Serotonergic Neurons Affects the Upregulation of Brain-Derived Neurotrophic Factor Evoked by Chronic Fluoxetine Treatment. Front. Neurosci. 2018, 12, 637. [Google Scholar] [CrossRef]
- Leschik, J.; Gentile, A.; Cicek, C.; Péron, S.; Tevosian, M.; Beer, A.; Radyushkin, K.; Bludau, A.; Ebner, K.; Neumann, I.; et al. Brain-derived neurotrophic factor expression in serotonergic neurons improves stress resilience and promotes adult hippocampal neurogenesis. Prog. Neurobiol. 2022, 217, 102333. [Google Scholar] [CrossRef]
- Blanco-Luquin, I.; Acha, B.; Urdánoz-Casado, A.; Gómez-Orte, E.; Roldan, M.; Pérez-Rodríguez, D.R.; Cabello, J.; Mendioroz, M. NXN Gene Epigenetic Changes in an Adult Neurogenesis Model of Alzheimer’s Disease. Cells 2022, 11, 1069. [Google Scholar] [CrossRef]
- Zocher, S.; Overall, R.W.; Berdugo-Vega, G.; Rund, N.; Karasinsky, A.; Adusumilli, V.S.; Steinhauer, C.; Scheibenstock, S.; Händler, K.; Schultze, J.L.; et al. De novo DNA methylation controls neuronal maturation during adult hippocampal neurogenesis. EMBO J. 2021, 40, e107100. [Google Scholar] [CrossRef] [PubMed]
- Garone, C.; De Giorgio, F.; Carli, S. Mitochondrial metabolism in neural stem cells and implications for neurodevelopmental and neurodegenerative diseases. J. Transl. Med. 2024, 22, 238. [Google Scholar] [CrossRef] [PubMed]
- Hansson Petersen, C.A.; Alikhani, N.; Behbahani, H.; Wiehager, B.; Pavlov, P.F.; Alafuzoff, I.; Leinonen, V.; Ito, A.; Winblad, B.; Glaser, E.; et al. The amyloid β-peptide is imported into mitochondria via the TOM import machinery and localized to mitochondrial cristae. Proc. Natl. Acad. Sci. USA 2008, 105, 13145–13150. [Google Scholar] [CrossRef]
- Atlante, A.; Valenti, D. Mitochondrial Complex I and β-Amyloid Peptide Interplay in Alzheimer’s Disease: A Critical Review of New and Old Little Regarded Findings. Int. J. Mol. Sci. 2023, 24, 15951. [Google Scholar] [CrossRef]
- Moawad, M.H.E.D.; Serag, I.; Alkhawaldeh, I.M.; Abbas, A.; Sharaf, A.; Alsalah, S.; Sadeq, M.A.; Shalaby, M.M.M.; Hefnawy, M.T.; Abouzid, M.; et al. Exploring the Mechanisms and Therapeutic Approaches of Mitochondrial Dysfunction in Alzheimer’s Disease: An Educational Literature Review. Mol. Neurobiol. 2025, 62, 6785–6810. [Google Scholar] [CrossRef]
- Li, X.; Wu, Z.; Si, X.; Li, J.; Wu, G.; Wang, M. The role of mitochondrial dysfunction in the pathogenesis of Alzheimer’s disease and future strategies for targeted therapy. Eur. J. Med. Res. 2025, 30, 434. [Google Scholar] [CrossRef]
- Han, A.R.; Moon, T.K.; Kang, I.K.; Yu, D.B.; Kim, Y.; Byon, C.; Park, S.; Kim, H.L.; Kim, H.L.; Lee, K.J.; et al. Integrative analysis of microRNA-mediated mitochondrial dysfunction in hippocampal neural progenitor cell death in relation with Alzheimer’s disease. BMB Rep. 2024, 57, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Spinelli, M.; Fusco, S.; Mainardi, M.; Scala, F.; Natale, F.; Lapenta, R.; Mattera, A.; Rinaudo, M.; Li Puma, D.D.; Ripoli, C.; et al. Brain insulin resistance impairs hippocampal synaptic plasticity and memory by increasing GluA1 palmitoylation through FoxO3a. Nat. Commun. 2017, 8, 2009. [Google Scholar] [CrossRef] [PubMed]
- Fishel, M.A.; Watson, G.S.; Montine, T.J.; Wang, Q.; Green, P.S.; Kulstad, J.J.; Cook, D.G.; Peskind, E.R.; Baker, L.D.; Goldgaber, D.; et al. Hyperinsulinemia provokes synchronous increases in central inflammation and beta-amyloid in normal adults. Arch. Neurol. 2005, 62, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Stranahan, A.M.; Arumugam, T.V.; Cutler, R.G.; Lee, K.; Egan, J.M.; Mattson, M.P. Diabetes impairs hippocampal function through glucocorticoid-mediated effects on new and mature neurons. Nat. Neurosci. 2008, 11, 309–317. [Google Scholar] [CrossRef]
- Guo, Z.; Chen, Y.; Mao, Y.-F.; Zheng, T.; Jiang, Y.; Yan, Y.; Yin, X.; Zhang, B. Long-term treatment with intranasal insulin ameliorates cognitive impairment, tau hyperphosphorylation, and microglial activation in a streptozotocin-induced Alzheimer’s rat model. Sci. Rep. 2017, 7, 45971. [Google Scholar] [CrossRef]
- AboEl-Azm, Y.H.; El-Samahy, M.; Hendi, N.I.; Arar, A.; Yasen, N.S.; Ramadan, S.; Zedan, E.M.; Al-Dardery, N.M.; Khaity, A. Safety and efficacy of intranasal insulin in patients with Alzheimer’s disease: A systematic review and meta-analysis. J. Clin. Transl. Res. 2023, 9, 222–235. [Google Scholar]
- Yang, Y.; Kim, J.; Kim, H.Y.; Ryoo, N.; Lee, S.; Kim, Y.; Rhim, H.; Shin, Y.K. Amyloid-β Oligomers May Impair SNARE-Mediated Exocytosis by Direct Binding to Syntaxin 1a. Cell Rep. 2015, 12, 1244–1251. [Google Scholar] [CrossRef]
- Dickerson, B.C.; Salat, D.H.; Greve, D.N.; Chua, E.F.; Rand-Giovannetti, E.; Rentz, D.M.; Bertram, L.; Mullin, K.; Tanzi, R.E.; Blacker, D.; et al. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology 2005, 65, 404–411. [Google Scholar] [CrossRef]
- Mishra, R.; Phan, T.; Kumar, P.; Morrissey, Z.; Gupta, M.; Hollands, C.; Shetti, A.; Lopez, K.L.; Maienschein-Cline, M.; Suh, H.; et al. Augmenting neurogenesis rescues memory impairments in Alzheimer’s disease by restoring the memory-storing neurons. J. Exp. Med. 2022, 219, e20220391. [Google Scholar] [CrossRef]
- Chen, Z.R.; Huang, J.B.; Yang, S.L.; Hong, F.F. Role of Cholinergic Signaling in Alzheimer’s Disease. Molecules 2022, 27, 1816. [Google Scholar] [CrossRef]
- Schilit Nitenson, A.; Manzano Nieves, G.; Poeta, D.L.; Bahar, R.; Rachofsky, C.; Mandairon, N.; Bath, K.G. Acetylcholine Regulates Olfactory Perceptual Learning through Effects on Adult Neurogenesis. iScience 2019, 22, 544–556. [Google Scholar] [CrossRef]
- Stazi, M.; Wirths, O. Chronic Memantine Treatment Ameliorates Behavioral Deficits, Neuron Loss, and Impaired Neurogenesis in a Model of Alzheimer’s Disease. Mol. Neurobiol. 2021, 58, 204–216. [Google Scholar] [CrossRef] [PubMed]
- McHugh, S.B.; Lopes-dos-Santos, V.; Gava, G.P.; Hartwich, K.; Tam, S.K.E.; Bannerman, D.M.; Dupret, D. Adult-born dentate granule cells promote hippocampal population sparsity. Nat. Neurosci. 2022, 25, 1481–1491. [Google Scholar] [CrossRef] [PubMed]
- Clelland, C.D.; Choi, M.; Romberg, C.; Clemenson, G.D., Jr.; Fragniere, A.; Tyers, P.; Jessberger, S.; Saksida, L.M.; Barker, R.A.; Gage, F.H.; et al. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science 2009, 325, 210–213. [Google Scholar] [CrossRef]
- Sahay, A.; Scobie, K.N.; Hill, A.S.; O’Carroll, C.M.; Kheirbek, M.A.; Burghardt, N.S.; Fenton, A.A.; Dranovsky, A.; Hen, R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature 2011, 472, 466–470. [Google Scholar] [CrossRef]
- Mathews, K.J.; Allen, K.M.; Boerrigter, D.; Ball, H.; Shannon Weickert, C.; Double, K.L. Evidence for reduced neurogenesis in the aging human hippocampus despite stable stem cell markers. Aging Cell 2017, 16, 1195–1199. [Google Scholar] [CrossRef] [PubMed]
- Geigenmüller, J.N.; Tari, A.R.; Wisloff, U.; Walker, T.L. The relationship between adult hippocampal neurogenesis and cognitive impairment in Alzheimer’s disease. Alzheimers Dement. 2024, 20, 7369–7383. [Google Scholar] [CrossRef] [PubMed]
- Kang, E.; Wen, Z.; Song, H.; Christian, K.M.; Ming, G.L. Adult Neurogenesis and Psychiatric Disorders. Cold Spring Harb. Perspect. Biol. 2016, 8, a019026. [Google Scholar] [CrossRef]
- Anacker, C.; Luna, V.M.; Stevens, G.S.; Millette, A.; Shores, R.; Jimenez, J.C.; Chen, B.; Hen, R. Hippocampal neurogenesis confers stress resilience by inhibiting the ventral dentate gyrus. Nature 2018, 559, 98–102. [Google Scholar] [CrossRef]
- Kuhn, H.G.; Dickinson-Anson, H.; Gage, F.H. Neurogenesis in the dentate gyrus of the adult rat: Age-related decrease of neuronal progenitor proliferation. J. Neurosci. 1996, 16, 2027–2033. [Google Scholar] [CrossRef]
- Zhou, Y.; Su, Y.; Li, S.; Kennedy, B.C.; Zhang, D.Y.; Bond, A.M.; Sun, Y.; Jacob, F.; Lu, L.; Hu, P.; et al. Molecular landscapes of human hippocampal immature neurons across lifespan. Nature 2022, 607, 527–533. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic reactive astrocytes are induced by activated microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of Astrocytes in Alzheimer’s Disease from a Neuroinflammatory and Oxidative Stress Perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Kohman, R.A.; Bhattacharya, T.K.; Kilby, C.; Bucko, P.; Rhodes, J.S. Effects of minocycline on spatial learning, hippocampal neurogenesis and microglia in aged and adult mice. Behav. Brain Res. 2013, 242, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Biscaro, B.; Lindvall, O.; Tesco, G.; Ekdahl, C.T.; Nitsch, R.M. Inhibition of microglial activation protects hippocampal neurogenesis and improves cognitive deficits in a transgenic mouse model for Alzheimer’s disease. Neurodegener. Dis. 2012, 9, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Ghosal, K.; Stathopoulos, A.; Pimplikar, S.W. APP intracellular domain impairs adult neurogenesis in transgenic mice by inducing neuroinflammation. PLoS ONE 2010, 5, e11866. [Google Scholar] [CrossRef] [PubMed]
- Kitazawa, M.; Cheng, D.; Tsukamoto, M.R.; Koike, M.A.; Wes, P.D.; Vasilevko, V.; Cribbs, D.H.; LaFerla, F.M. Blocking IL-1 signaling rescues cognition, attenuates tau pathology, and restores neuronal β-catenin pathway function in an Alzheimer’s disease model. J. Immunol. 2011, 187, 6539–6549. [Google Scholar] [CrossRef] [PubMed]
- Deng, Q.; Wu, C.; Parker, E.; Liu, T.C.; Duan, R.; Yang, L. Microglia and Astrocytes in Alzheimer’s Disease: Significance and Summary of Recent Advances. Aging Dis. 2024, 15, 1537–1564. [Google Scholar] [CrossRef]
- Parajuli, B.; Sonobe, Y.; Horiuchi, H.; Takeuchi, H.; Mizuno, T.; Suzumura, A. Oligomeric amyloid β induces IL-1β processing via production of ROS: Implication in Alzheimer’s disease. Cell Death Dis. 2013, 4, e975. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Kummer, M.P.; Stutz, A.; Delekate, A.; Schwartz, S.; Vieira-Saecker, A.; Griep, A.; Axt, D.; Remus, A.; Tzeng, T.-C.; et al. NLRP3 is activated in Alzheimer’s disease and contributes to pathology in APP/PS1 mice. Nature 2013, 493, 674–678. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef]
- Li, C.; Wu, J.; Dong, Q.; Ma, J.; Gao, H.; Liu, G.; Chen, Y.; Ning, J.; Lv, X.; Zhang, M.; et al. The crosstalk between oxidative stress and DNA damage induces neural stem cell senescence by HO-1/PARP1 non-canonical pathway. Free Radic. Biol. Med. 2024, 223, 443–457. [Google Scholar] [CrossRef]
- Le Belle, J.E.; Orozco, N.M.; Paucar, A.A.; Saxe, J.P.; Mottahedeh, J.; Pyle, A.D.; Wu, H.; Kornblum, H.I. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 2011, 8, 59–71. [Google Scholar] [CrossRef]
- Oguchi, T.; Ono, R.; Tsuji, M.; Shozawa, H.; Somei, M.; Inagaki, M.; Mori, Y.; Yasumoto, T.; Ono, K.; Kiuchi, Y. Cilostazol Suppresses Aβ-induced Neurotoxicity in SH-SY5Y Cells through Inhibition of Oxidative Stress and MAPK Signaling Pathway. Front. Aging Neurosci. 2017, 9, 337. [Google Scholar] [CrossRef]
- Colié, S.; Sarroca, S.; Palenzuela, R.; Garcia, I.; Matheu, A.; Corpas, R.; Dotti, C.G.; Esteban, J.A.; Sanfeliu, C.; Nebreda, A.R. Neuronal p38α mediates synaptic and cognitive dysfunction in an Alzheimer’s mouse model by controlling β-amyloid production. Sci. Rep. 2017, 7, 45306. [Google Scholar] [CrossRef]
- Moreno-Cugnon, L.; Arrizabalaga, O.; Llarena, I.; Matheu, A. Elevated p38MAPK activity promotes neural stem cell aging. Aging 2020, 12, 6030–6036. [Google Scholar] [CrossRef]
- Amoroso, R.; Maccallini, C.; Bellezza, I. Activators of Nrf2 to Counteract Neurodegenerative Diseases. Antioxidants 2023, 12, 778. [Google Scholar] [CrossRef]
- Gao, L.; Zhang, Y.; Sterling, K.; Song, W. Brain-derived neurotrophic factor in Alzheimer’s disease and its pharmaceutical potential. Transl. Neurodegener. 2022, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Luo, W.; Cheng, C.; Shen, L.; Wu, X.; Xiao, X. BDNF gene therapy rescues neuronal function via unique and common transcriptional responses in Aβ and tau-driven Alzheimer’s disease mouse models. Biochem. Biophys. Rep. 2025, 43, 102089. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, S.; Dohm-Hansen, S.; Lavelle, A.; Bastiaanssen, T.F.S.; English, J.A.; Cryan, J.F.; Nolan, Y.M. Exercise mitigates a gut microbiota-mediated reduction in adult hippocampal neurogenesis and associated behaviours in rats. Transl. Psychiatry 2024, 14, 195. [Google Scholar] [CrossRef]
- Codd, L.N.; Blackmore, D.G.; Vukovic, J.; Bartlett, P.F. Exercise reverses learning deficits induced by hippocampal injury by promoting neurogenesis. Sci. Rep. 2020, 10, 19269. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Sun, W.; Gao, F.; Ma, H.; Yuan, T.; Liu, Z.; Liu, H.; Hu, J.; Bai, J.; Zhang, X.; et al. Brain-Derived Estrogen Regulates Neurogenesis, Learning and Memory with Aging in Female Rats. Biology 2023, 12, 760. [Google Scholar] [CrossRef]
- Shin, H.S.; Lee, S.H.; Moon, H.J.; So, Y.H.; Jang, H.J.; Lee, K.-H.; Ahn, C.; Jung, E.-M. Prolonged stress response induced by chronic stress and corticosterone exposure causes adult neurogenesis inhibition and astrocyte loss in mouse hippocampus. Brain Res. Bull. 2024, 208, 110903. [Google Scholar] [CrossRef]
- Macht, V.; Vetreno, R.; Elchert, N.; Crews, F. Galantamine prevents and reverses neuroimmune induction and loss of adult hippocampal neurogenesis following adolescent alcohol exposure. J. Neuroinflamm. 2021, 18, 212. [Google Scholar] [CrossRef]
- Kotani, S.; Yamauchi, T.; Teramoto, T.; Ogura, H. Donepezil, an acetylcholinesterase inhibitor, enhances adult hippocampal neurogenesis. Chem. Biol. Interact. 2008, 175, 227–230. [Google Scholar] [CrossRef]
- Kwon, K.J.; Kim, M.K.; Lee, E.J.; Kim, J.N.; Choi, B.R.; Kim, S.Y.; Cho, K.S.; Han, J.S.; Kim, H.Y.; Shin, C.Y.; et al. Effects of donepezil, an acetylcholinesterase inhibitor, on neurogenesis in a rat model of vascular dementia. J. Neurol. Sci. 2014, 347, 66–77. [Google Scholar] [CrossRef]
- Sahin, F.; Gunel, A.; Atasoy, B.T.; Guler, U.; Salih, B.; Kuzu, I.; Taspinar, M.; Cinar, O.; Kahveci, S. Enhancing proteasome activity by NMDAR antagonists explains their therapeutic effect in neurodegenerative and mental diseases. Sci. Rep. 2025, 15, 1165. [Google Scholar] [CrossRef]
- Ma, X.; Xiao, W.; Li, H.; Pang, P.; Xue, F.; Wan, L.; Pei, L.; Yan, H. Metformin restores hippocampal neurogenesis and learning and memory via regulating gut microbiota in the obese mouse model. Brain Behav. Immun. 2021, 95, 68–83. [Google Scholar] [CrossRef]
- Kim, M.-Y.; Kim, M.J.; Lee, C.; Lee, J.; Kim, S.S.; Hong, S.; Kim, H.T.; Seo, J.; Yoon, K.-J.; Han, S. Trametinib activates endogenous neurogenesis and recovers neuropathology in a model of Alzheimer’s disease. Exp. Mol. Med. 2023, 55, 2177–2189. [Google Scholar] [CrossRef]
- Shi, Y.; Lv, J.; Chen, L.; Luo, G.; Tao, M.; Pan, J.; Hu, X.; Sheng, J.; Zhang, S.; Zhou, M.; et al. Phosphodiesterase-4D Knockdown in the Prefrontal Cortex Alleviates Memory Deficits and Synaptic Failure in Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 722580. [Google Scholar] [CrossRef]
- Kumar, A.; Kim, F.; Song, D.K.; Choung, J.J. Polypharmacological Potential of Phosphodiesterase 5 Inhibitors for the Treatment of Neurocognitive Disorders. Aging Dis. 2024, 15, 2008–2014. [Google Scholar] [CrossRef]
- Wang, C.; Yang, X.-M.; Zhuo, Y.-Y.; Zhou, H.; Lin, H.-B.; Cheng, Y.-F.; Xu, J.-P.; Zhang, H.-T. The phosphodiesterase-4 inhibitor rolipram reverses Aβ-induced cognitive impairment and neuroinflammatory and apoptotic responses in rats. Int. J. Neuropsychopharmacol. 2012, 15, 749–766. [Google Scholar] [CrossRef] [PubMed]
- Hutson, P.; Finger, E.N.; Magliaro, B.; Smith, S.; Converso, A.; Sanderson, P.E.; Mullins, D.; Hyde, L.; Eschle, B.K.; Turnbull, Z.; et al. The selective phosphodiesterase 9 (PDE9) inhibitor PF-04447943 (6-[(3S,4S)-4-methyl-1-(pyrimidin-2-ylmethyl)pyrrolidin-3-yl]-1-(tetrahydro-2H-pyran-4-yl)-1,5-dihydro-4H-pyrazolo[3,4-d]pyrimidin-4-one) enhances synaptic plasticity and cognitive function in rodents. Neuropharmacology 2011, 61, 665–676. [Google Scholar] [CrossRef]
- Bonato, J.; Meyer, E.; Mendonça, P.; Milani, H.; Prickaerts, J.; Oliveira, R. Roflumilast protects against spatial memory impairments and exerts anti-inflammatory effects after transient global cerebral ischemia. Eur. J. Neurosci. 2021, 53, 1171–1188. [Google Scholar] [CrossRef] [PubMed]
- Gohel, D.; Zhang, P.; Gupta, A.K.; Li, Y.; Chiang, C.W.; Li, L.; Hou, Y.; Pieper, A.A.; Cummings, J.; Cheng, F. Sildenafil as a Candidate Drug for Alzheimer’s Disease: Real-World Patient Data Observation and Mechanistic Observations from Patient-Induced Pluripotent Stem Cell-Derived Neurons. J. Alzheimers Dis. 2024, 98, 643–657. [Google Scholar] [CrossRef] [PubMed]
- Chua, W.Y.; Lim, L.K.E.; Wang, J.J.D.; Mai, A.S.; Chan, L.L.; Tan, E.K. Sildenafil and risk of Alzheimer disease: A systematic review and meta-analysis. Aging 2025, 17, 726–739. [Google Scholar] [CrossRef]
- Olivas-Cano, I.; Rodriguez-Andreu, J.M.; Blasco-Ibañez, J.M.; Crespo, C.; Nácher, J.; Varea, E. Fluoxetine increased adult neurogenesis is mediated by 5-HT3 receptor. Neurosci. Lett. 2023, 795, 137027. [Google Scholar] [CrossRef] [PubMed]
- Bougea, A.; Angelopoulou, E.; Vasilopoulos, E.; Gourzis, P.; Papageorgiou, S. Emerging Therapeutic Potential of Fluoxetine on Cognitive Decline in Alzheimer’s Disease: Systematic Review. Int. J. Mol. Sci. 2024, 25, 6542. [Google Scholar] [CrossRef] [PubMed]
- McClean, P.L.; Parthsarathy, V.; Faivre, E.; Hölscher, C. The diabetes drug liraglutide prevents degenerative processes in a mouse model of Alzheimer’s disease. J. Neurosci. 2011, 31, 6587–6594. [Google Scholar] [CrossRef]
- Cummings, J.L.; Atri, A.; Feldman, H.H.; Hansson, O.; Sano, M.; Knop, F.K.; Johannsen, P.; León, T.; Scheltens, P. evoke and evoke+: Design of two large-scale, double-blind, placebo-controlled, phase 3 studies evaluating efficacy, safety, and tolerability of semaglutide in early-stage symptomatic Alzheimer’s disease. Alzheimers Res. Ther. 2025, 17, 14. [Google Scholar] [CrossRef]
- Femminella, G.D.; Frangou, E.; Love, S.B.; Busza, G.; Holmes, C.; Ritchie, C.; Lawrence, R.; McFarlane, B.; Tadros, G.; Ridha, B.H.; et al. Evaluating the effects of the novel GLP-1 analogue liraglutide in Alzheimer’s disease: Study protocol for a randomised controlled trial (ELAD study). Trials 2019, 20, 191, Erratum in Trials 2020, 21, 660. https://doi.org/10.1186/s13063-020-04608-4. [Google Scholar] [CrossRef]
- Edison, P. Evaluation of Novel GLP-1 analogue in the treatment of Alzheimer’s disease. Alzheimer’s Dement. 2024, 20, e089799. [Google Scholar] [CrossRef]
- Liu, Y.; Weick, J.P.; Liu, H.; Krencik, R.; Zhang, X.; Ma, L.; Zhou, G.M.; Ayala, M.; Zhang, S.C. Medial ganglionic eminence-like cells derived from human embryonic stem cells correct learning and memory deficits. Nat. Biotechnol. 2013, 31, 440–447. [Google Scholar] [CrossRef] [PubMed]
- Ji, Q.; Lv, Y.; Hu, B.; Su, Y.; Shaikh, I.I.; Zhu, X. Study on the therapeutic potential of induced neural stem cells for Alzheimer’s disease in mice. Biol. Res. 2024, 57, 89. [Google Scholar] [CrossRef]
- Brody, M.; Agronin, M.; Herskowitz, B.J.; Bookheimer, S.Y.; Small, G.W.; Hitchinson, B.; Ramdas, K.; Wishard, T.; McInerney, K.F.; Vellas, B.; et al. Results and insights from a phase I clinical trial of Lomecel-B for Alzheimer’s disease. Alzheimer’s Dement. 2023, 19, 261–273. [Google Scholar] [CrossRef]
- Nato, G.; Corti, A.; Parmigiani, E.; Jachetti, E.; Lecis, D.; Colombo, M.P.; Delia, D.; Buffo, A.; Magrassi, L. Immune-tolerance to human iPS-derived neural progenitors xenografted into the immature cerebellum is overridden by species-specific differences in differentiation timing. Sci. Rep. 2021, 11, 651. [Google Scholar] [CrossRef]
- Bedel, A.; Beliveau, F.; Lamrissi-Garcia, I.; Rousseau, B.; Moranvillier, I.; Rucheton, B.; Guyonnet-Dupérat, V.; Cardinaud, B.; de Verneuil, H.; Moreau-Gaudry, F.; et al. Preventing Pluripotent Cell Teratoma in Regenerative Medicine Applied to Hematology Disorders. Stem Cells Transl. Med. 2017, 6, 382–393. [Google Scholar] [CrossRef]
- Xiao, L.; Yang, X.; Sharma, V.K.; Abebe, D.; Loh, Y.P. Hippocampal delivery of neurotrophic factor-α1/carboxypeptidase E gene prevents neurodegeneration, amyloidosis, memory loss in Alzheimer’s Disease male mice. Mol. Psychiatry 2023, 28, 3332–3342, Erratum in Mol. Psychiatry 2024, 29, 3971–3972. https://doi.org/10.1038/s41380-024-02633-2. [Google Scholar] [CrossRef] [PubMed]
- A Phase I Study to Assess the Safety, Tolerability and Preliminary Efficacy of AAV2-BDNF [Adeno-Associated Virus (AAV)-Based, Vector-Mediated Delivery of Human Brain Derived Neurotrophic Factor] in Subjects with Early Alzheimer’s Disease and Mild Cognitive Impairment; The Ohio State University: Columbus, OH, USA, 2021.
- Nagahara, A.H.; Tuszynski, M.H. Potential therapeutic uses of BDNF in neurological and psychiatric disorders. Nat. Rev. Drug Discov. 2011, 10, 209–219. [Google Scholar] [CrossRef] [PubMed]
- Roussel-Gervais, A.; Sgroi, S.; Cambet, Y.; Lemeille, S.; Seredenina, T.; Krause, K.H.; Jaquet, V. Genetic knockout of NTRK2 by CRISPR/Cas9 decreases neurogenesis and favors glial progenitors during differentiation of neural progenitor stem cells. Front. Cell Neurosci. 2023, 17, 1289966. [Google Scholar] [CrossRef] [PubMed]
- Ruetz, T.J.; Pogson, A.N.; Kashiwagi, C.M.; Gagnon, S.D.; Morton, B.; Sun, E.D.; Na, J.; Yeo, R.W.; Leeman, D.S.; Morgens, D.W.; et al. CRISPR–Cas9 screens reveal regulators of ageing in neural stem cells. Nature 2024, 634, 1150–1159. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Q.; Long, Z.; Feng, M.; Zhao, Y.; Luo, S.; Wang, K.; Wang, Y.; Yang, G.; He, G. Valproic Acid Stimulates Hippocampal Neurogenesis via Activating the Wnt/β-Catenin Signaling Pathway in the APP/PS1/Nestin-GFP Triple Transgenic Mouse Model of Alzheimer’s Disease. Front. Aging Neurosci. 2019, 11, 62. [Google Scholar] [CrossRef]
- Nakatsuka, D.; Izumi, T.; Tsukamoto, T.; Oyama, M.; Nishitomi, K.; Deguchi, Y.; Niidome, K.; Yamakawa, H.; Ito, H.; Ogawa, K. Histone Deacetylase 2 Knockdown Ameliorates Morphological Abnormalities of Dendritic Branches and Spines to Improve Synaptic Plasticity in an APP/PS1 Transgenic Mouse Model. Front. Mol. Neurosci. 2021, 14, 782375. [Google Scholar] [CrossRef]
- Chang, Z.; Xu, W.; Jiang, S.; Liu, X.; Zhu, H.; Wang, P.; Gao, B.; Gong, K.; Guo, G.; Sun, K.; et al. Effects of 5-Aza on neurogenesis contribute to learning and memory in the mouse hippocampus. Biomed. Pharmacother. 2022, 154, 113623. [Google Scholar] [CrossRef]
- Chatterjee, S.; Cassel, R.; Schneider-Anthony, A.; Merienne, K.; Cosquer, B.; Tzeplaeff, L.; Halder Sinha, S.; Kumar, M.; Chaturbedy, P.; Eswaramoorthy, M.; et al. Reinstating plasticity and memory in a tauopathy mouse model with an acetyltransferase activator. EMBO Mol. Med. 2018, 10, e8587. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Thomas, C.M.; Nge, G.G.; Zaya, A.; Dasari, R.; Chongtham, N.; Manandhar, B.; Kortagere, S.; Elefant, F. Tip60 HAT activators as therapeutic modulators for Alzheimer’s disease. Nat. Commun. 2025, 16, 3347. [Google Scholar] [CrossRef]
- Chen, Y.A.; Lu, C.H.; Ke, C.C.; Chiu, S.J.; Jeng, F.S.; Chang, C.W.; Yang, B.H.; Liu, R.S. Mesenchymal Stem Cell-Derived Exosomes Ameliorate Alzheimer’s Disease Pathology and Improve Cognitive Deficits. Biomedicines 2021, 9, 594. [Google Scholar] [CrossRef]
- Liu, S.; Fan, M.; Xu, J.-X.; Yang, L.-J.; Qi, C.-C.; Xia, Q.-R.; Ge, J.-F. Exosomes derived from bone-marrow mesenchymal stem cells alleviate cognitive decline in AD-like mice by improving BDNF-related neuropathology. J. Neuroinflamm. 2022, 19, 35. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Song, Q.; Dai, C.; Cui, S.; Tang, R.; Li, S.; Chang, J.; Li, P.; Wang, J.; Li, J.; et al. Clinical safety and efficacy of allogenic human adipose mesenchymal stromal cells-derived exosomes in patients with mild to moderate Alzheimer’s disease: A phase I/II clinical trial. Gen. Psychiatr. 2023, 36, e101143. [Google Scholar] [CrossRef]
- Rai, A.; Claridge, B.; Lozano, J.; Greening, D.W. The Discovery of Extracellular Vesicles and Their Emergence as a Next-Generation Therapy. Circ. Res. 2024, 135, 198–221. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.; Zhang, J.; Liu, G.; Wolfram, J. Immunogenicity of Extracellular Vesicles. Adv. Mater. 2024, 36, 2403199. [Google Scholar] [CrossRef] [PubMed]
- Van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Kokkali, M.; Karali, K.; Thanou, E.; Papadopoulou, M.A.; Zota, I.; Tsimpolis, A.; Efstathopoulos, P.; Calogeropoulou, T.; Li, K.W.; Sidiropoulou, K.; et al. Multimodal beneficial effects of BNN27, a nerve growth factor synthetic mimetic, in the 5xFAD mouse model of Alzheimer’s disease. Mol. Psychiatry 2025, 30, 2265–2283. [Google Scholar] [CrossRef]
- Martí-Clúa, J. 5-Bromo-2′-deoxyuridine labeling: Historical perspectives, factors influencing the detection, toxicity, and its implications in the neurogenesis. Neural Regen. Res. 2024, 19, 302–308. [Google Scholar] [CrossRef]
- Cha, D.J.; Mengel, D.; Mustapic, M.; Liu, W.; Selkoe, D.J.; Kapogiannis, D.; Galasko, D.; Rissman, R.A.; Bennett, D.A.; Walsh, D.M. miR-212 and miR-132 Are Downregulated in Neurally Derived Plasma Exosomes of Alzheimer’s Patients. Front. Neurosci. 2019, 13, 1208. [Google Scholar] [CrossRef]
- Walgrave, H.; Balusu, S.; Snoeck, S.; Vanden Eynden, E.; Craessaerts, K.; Thrupp, N.; Wolfs, L.; Horré, K.; Fourne, Y.; Ronisz, A.; et al. Restoring miR-132 expression rescues adult hippocampal neurogenesis and memory deficits in Alzheimer’s disease. Cell Stem Cell 2021, 28, 1805–1821.e1808. [Google Scholar] [CrossRef]
- Scopa, C.; Marrocco, F.; Latina, V.; Ruggeri, F.; Corvaglia, V.; La Regina, F.; Ammassari-Teule, M.; Middei, S.; Amadoro, G.; Meli, G.; et al. Impaired adult neurogenesis is an early event in Alzheimer’s disease neurodegeneration, mediated by intracellular Aβ oligomers. Cell Death Differ. 2020, 27, 934–948, Erratum in Cell Death Differ. 2020, 27, 2035. https://doi.org/10.1038/s41418-019-0478-3. [Google Scholar] [CrossRef]
- Lybrand, Z.R.; Goswami, S.; Zhu, J.; Jarzabek, V.; Merlock, N.; Aktar, M.; Smith, C.; Zhang, L.; Varma, P.; Cho, K.-O.; et al. A critical period of neuronal activity results in aberrant neurogenesis rewiring hippocampal circuitry in a mouse model of epilepsy. Nat. Commun. 2021, 12, 1423. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, Y.; Cheng, H.; Li, Z.; Lai, N.; Li, M.; Ruan, Y.; Zheng, Y.; Fei, F.; Xu, C.; et al. Adult-born neurons in critical period maintain hippocampal seizures via local aberrant excitatory circuits. Signal Transduct. Target. Ther. 2023, 8, 225. [Google Scholar] [CrossRef] [PubMed]
- Maddaloni, G.; Migliarini, S.; Napolitano, F.; Giorgi, A.; Nazzi, S.; Biasci, D.; De Felice, A.; Gritti, M.; Cavaccini, A.; Galbusera, A.; et al. Serotonin depletion causes valproate-responsive manic-like condition and increased hippocampal neuroplasticity that are reversed by stress. Sci. Rep. 2018, 8, 11847. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Yao, H.; Dong, Q.; Zhang, H.; Yang, Z.; Yang, Y.; Zhu, J.; Xu, M.; Xu, R. Tumourigenicity and Immunogenicity of Induced Neural Stem Cell Grafts Versus Induced Pluripotent Stem Cell Grafts in Syngeneic Mouse Brain. Sci. Rep. 2016, 6, 29955. [Google Scholar] [CrossRef]
- Xiao, R.; Xu, D.; Zhang, M.; Chen, Z.; Cheng, L.; Du, S.; Lu, M.; Zhou, T.; Li, R.; Bai, F.; et al. Aneuploid embryonic stem cells drive teratoma metastasis. Nat. Commun. 2024, 15, 1087, Erratum in Nat. Commun. 2024, 15, 8883. https://doi.org/10.1038/s41467-024-53288-0. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dwamena, A.; Beragama-Arachchi, R.; Wang, H. Boosting Neurogenesis as a Strategy in Treating Alzheimer’s Disease. Int. J. Mol. Sci. 2025, 26, 8926. https://doi.org/10.3390/ijms26188926
Dwamena A, Beragama-Arachchi R, Wang H. Boosting Neurogenesis as a Strategy in Treating Alzheimer’s Disease. International Journal of Molecular Sciences. 2025; 26(18):8926. https://doi.org/10.3390/ijms26188926
Chicago/Turabian StyleDwamena, Abena, Rashini Beragama-Arachchi, and Hongmin Wang. 2025. "Boosting Neurogenesis as a Strategy in Treating Alzheimer’s Disease" International Journal of Molecular Sciences 26, no. 18: 8926. https://doi.org/10.3390/ijms26188926
APA StyleDwamena, A., Beragama-Arachchi, R., & Wang, H. (2025). Boosting Neurogenesis as a Strategy in Treating Alzheimer’s Disease. International Journal of Molecular Sciences, 26(18), 8926. https://doi.org/10.3390/ijms26188926






