Recent Developments in Osteoarthritis Research: Innovative Therapeutic Approaches and the Role of Polyphenols and Nanotechnology
Abstract
1. Introduction
2. Structural Homeostasis of Cartilage and Chondrocyte Activation in OA
3. Factors Contributing to the Pathophysiology of OA: Aging, Mechanics, Angiogenesis, and Genetic and Epigenetic Aspects
4. Importance of Interleukin-1β in OA: Therapeutic Implications
5. Molecular Pathways and Molecules Involved in OA
5.1. NF-κB Pathway in OA
5.2. Nrf2 and Its Role in Antioxidant Response and Cellular Protection
5.3. ADAMTS and ADAMs in the Degradation of ECM in OA
5.4. MMPs: Regulators of Cartilage Remodeling and Degradation in OA
5.5. FOXO1 in Cellular Homeostasis and Cartilage Metabolism
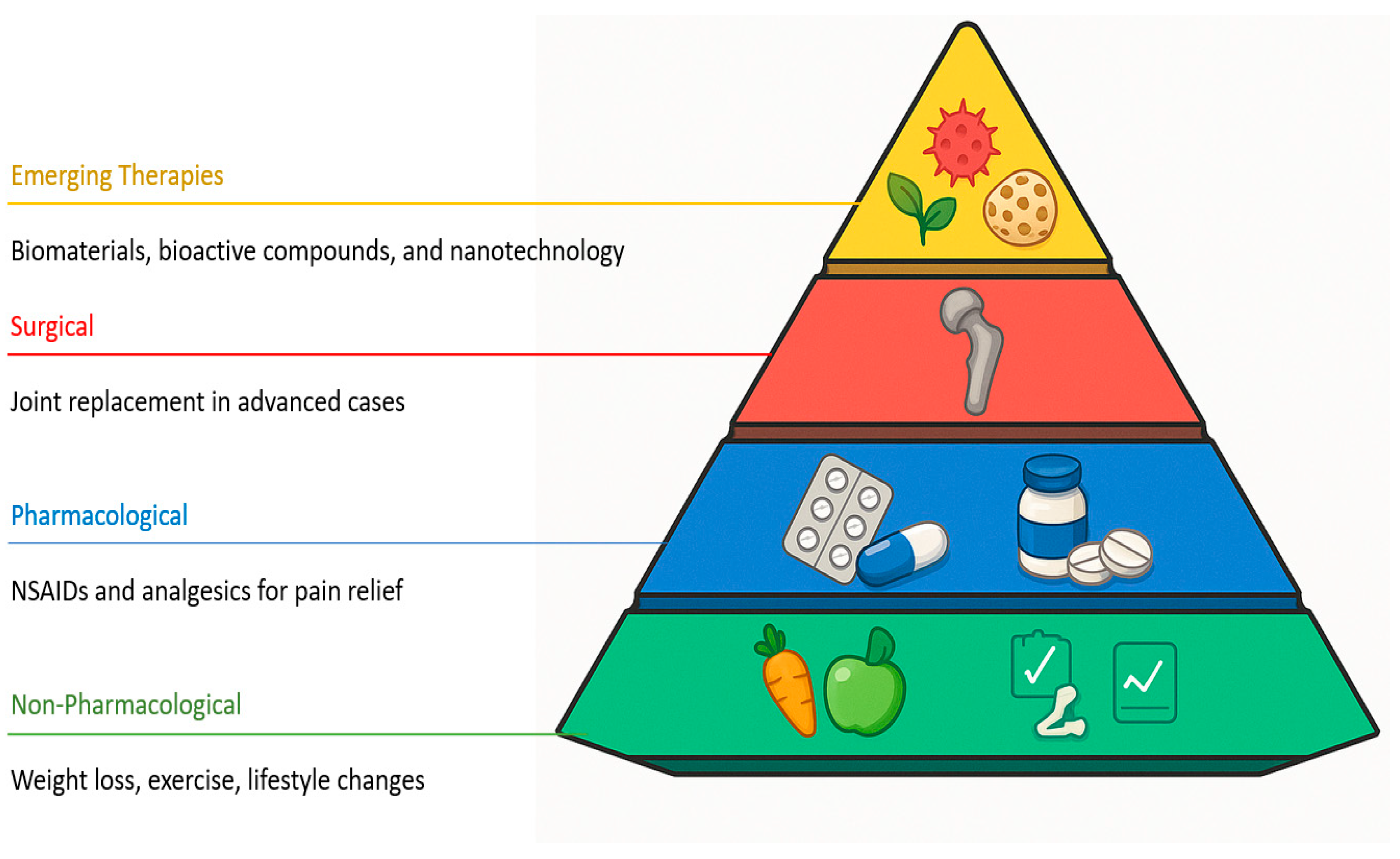
6. Therapeutic Strategies and Emerging Trends in OA and Cartilage Regeneration
6.1. Therapies Based on Natural Products
6.1.1. Propolis: Composition, Properties, and Applications in OA
6.1.2. The Role of Polyphenols in Osteoarthritis
6.1.3. Main Bioactive Compounds: Pinocembrin and CAPE
6.2. Nanotechnology to Improve the Bioavailability of Polyphenols and Bioactive Compounds: Applications in OA Treatments
6.3. Nanotechnology-Enhanced Delivery Systems for OA
6.3.1. Lipid-Based Nanocarriers
6.3.2. Polymeric Nanocarriers and Nanostructured Systems for Osteoarthritis Treatment
6.3.3. Hybrid and Nanocomposite Strategies
6.3.4. Metal-Based Nanoparticles
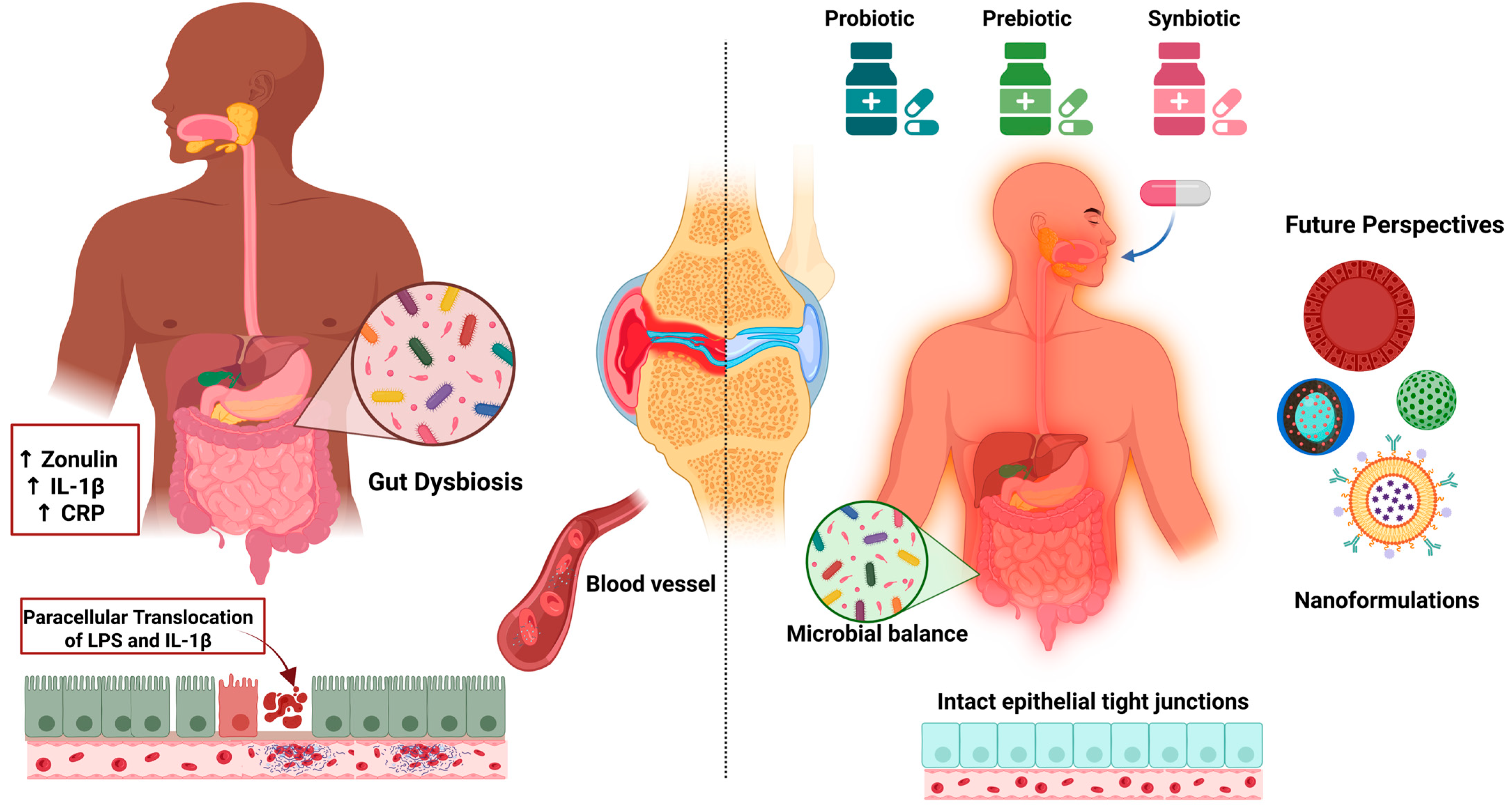
6.3.5. Probiotics, Prebiotics, and Synbiotics
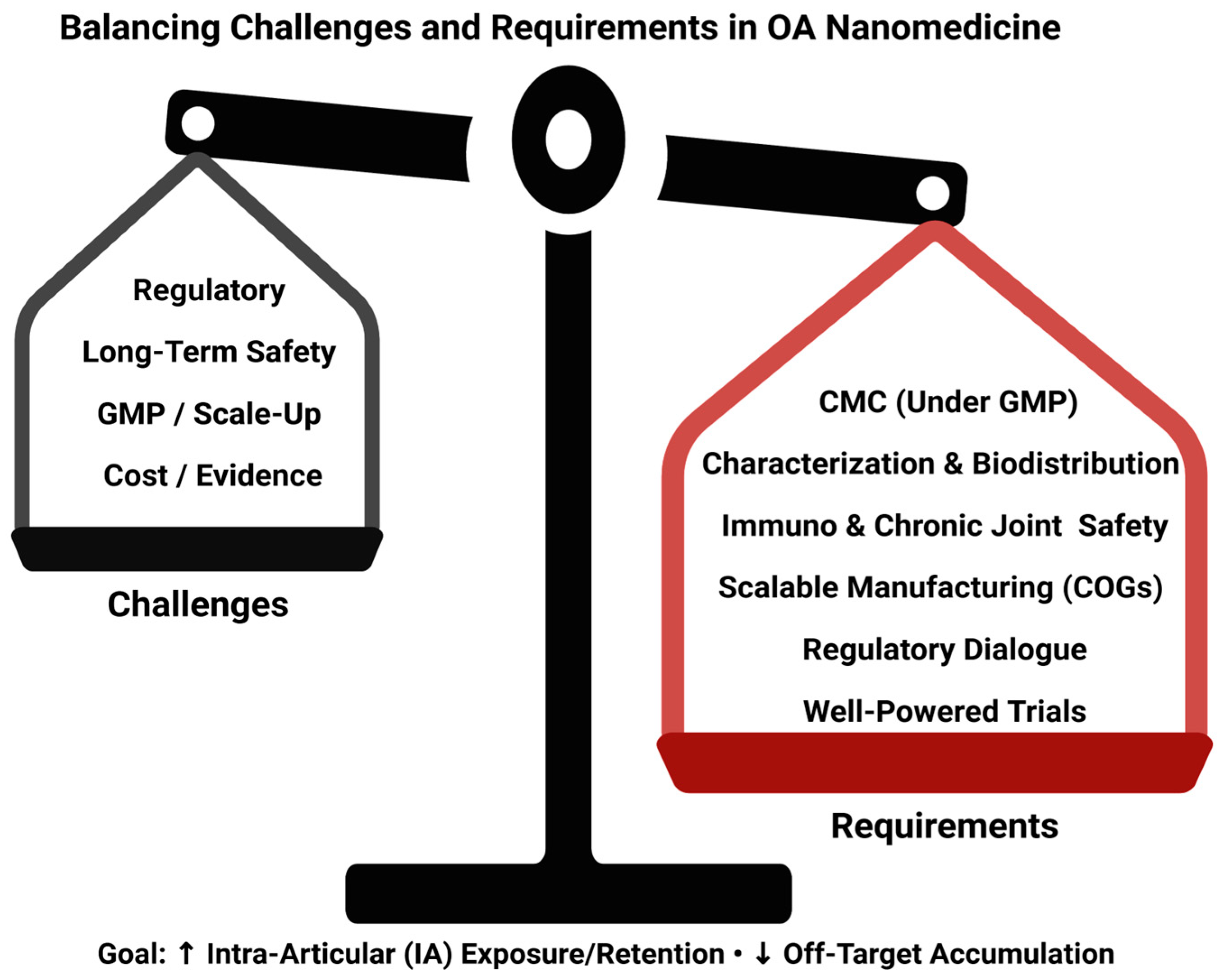
6.3.6. Translational Considerations (Regulation, CMC/GMP, Safety, Cost)
7. Perspective and Research Priorities
8. Approach to Literature Selection
9. Critical Appraisal: Why Preclinical Signals Fade in Clinical Trials
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADAMTS | A Disintegrin and Metalloproteinase with Thrombospondin Motifs |
| ARE | Antioxidant Response Element |
| CAPE | Caffeic Acid Phenethyl Ester |
| CCL | Calcified Cartilage |
| CD | Cyclodextrin |
| COX-2 | Cyclooxygenase-2 |
| ECM | Extracellular Matrix |
| GAG | Glycosaminoglycans |
| GST | Glutathione S-transferase |
| HO-1 | Heme Oxygenase-1 |
| IL-1β | Interleukin-1 beta |
| IL-6 | Interleukin-6 |
| MMP | Matrix Metalloproteinase |
| NF-κB | Nuclear Factor kappa B |
| NO | Nitric Oxide |
| NSAIDs | Nonsteroidal Anti-inflammatory Drugs |
| OA | Osteoarthritis |
| PGE2 | Prostaglandin E2 |
| RER | Rough Endoplasmic Reticulum |
| ROS | Reactive Oxygen Species |
| TNF-α | Tumor Necrosis Factor-alpha |
| CMC | Chemistry, Manufacturing, and Controls |
| GMP | Good Manufacturing Practice |
| COGs | Cost of Goods |
| IA | Intra-Articular |
| BD | Biodistribution |
References
- Gavín, C.; Sebastián, V.; Gimeno, M.; Coronel, P. Beyond Boundaries of a Trial: Post-Market Clinical Follow-Up of SOYA Patients. J. Clin. Med. 2024, 13, 6308. [Google Scholar] [CrossRef] [PubMed]
- Kolasinski, S.L.; Neogi, T.; Hochberg, M.C.; Oatis, C.; Guyatt, G.; Block, J.; Callahan, L.; Copenhaver, C.; Dodge, C.; Felson, D.; et al. 2019 American College of Rheumatology/Arthritis Foundation Guideline for the Management of Osteoarthritis of the Hand, Hip, and Knee. Arthritis Care Res. 2020, 72, 149–162, Erratum in Arthritis Care Res. 2021, 73, 764. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.K.; Zhu, Y.; Murray, P.; Madden, L. Future Proofing of Chondroitin Sulphate Production: Importance of Sustainability and Quality for the End-Applications. Int. J. Biol. Macromol. 2024, 267, 131577. [Google Scholar] [CrossRef]
- Abramoff, B.; Caldera, F.E. Osteoarthritis: Pathology, Diagnosis, and Treatment Options. Med. Clin. N. Am. 2020, 104, 293–311. [Google Scholar] [CrossRef] [PubMed]
- Holden, M.A.; Nicolson, P.J.A.; Thomas, M.J.; Corp, N.; Hinman, R.S.; Bennell, K.L. Osteoarthritis Year in Review 2022: Rehabilitation. Osteoarthr. Cartil. 2023, 31, 177–186. [Google Scholar] [CrossRef]
- Hunter, D.J.; Bierma-Zeinstra, S. Osteoarthritis. Lancet 2019, 393, 1745–1759. [Google Scholar] [CrossRef]
- McAlindon, T.E.; LaValley, M.P.; Harvey, W.F.; Price, L.L.; Driban, J.B.; Zhang, M.; Ward, R.J. Effect of Intra-Articular Triamcinolone vs Saline on Knee Cartilage Volume and Pain in Patients With Knee Osteoarthritis. JAMA 2017, 317, 1967–1975. [Google Scholar] [CrossRef]
- Bannuru, R.R.; Osani, M.C.; Vaysbrot, E.E.; Arden, N.K.; Bennell, K.; Bierma-Zeinstra, S.M.A.; Kraus, V.B.; Lohmander, L.S.; Abbott, J.H.; Bhandari, M.; et al. OARSI Guidelines for the Non-Surgical Management of Knee, Hip, and Polyarticular Osteoarthritis. Osteoarthr. Cartil. 2019, 27, 1578–1589. [Google Scholar] [CrossRef]
- Brophy, R.H.; Fillingham, Y.A. AAOS Clinical Practice Guideline Summary: Management of Osteoarthritis of the Knee (Nonarthroplasty), Third Edition. J. Am. Acad. Orthop. Surg. 2022, 30, e721–e729. [Google Scholar] [CrossRef]
- Kloppenburg, M.; Namane, M.; Cicuttini, F. Osteoarthritis. Lancet 2025, 405, 71–85, Erratum in Lancet 2025, 405, 2278. [Google Scholar] [CrossRef]
- Tang, S.; Zhang, C.; Oo, W.M.; Fu, K.; Risberg, M.A.; Bierma-Zeinstra, S.M.; Neogi, T.; Atukorala, I.; Malfait, A.-M.; Ding, C.; et al. Osteoarthritis. Nat. Rev. Dis. Prim. 2025, 11, 10. [Google Scholar] [CrossRef]
- de Toledo, T.M.; Valerio, H.P.; de Melo, A.T.; Gomes, R.N.; de Melo, T.C.; Buri, M.V.; de Souza, M.M.; Santos, D.M.; Vigerelli, H.; Alvarez-Flores, M.P.; et al. Proteomic Analysis of Hydrogen Peroxide-Treated Human Chondrocytes Shows Endoplasmic Reticulum Stress, Cytoskeleton Remodeling, and Altered Secretome Composition. Cell Commun. Signal. 2025, 23, 282. [Google Scholar] [CrossRef]
- Grässel, S.; Muschter, D. Recent Advances in the Treatment of Osteoarthritis. F1000Res 2020, 9, 325. [Google Scholar] [CrossRef] [PubMed]
- Bliddal, H. Definition, pathology and pathogenesis of osteoarthritis. Ugeskr. Laeger 2020, 182, V06200477. [Google Scholar] [PubMed]
- Moon, J.; Cho, K.-H.; Jhun, J.; Choi, J.; Na, H.-S.; Lee, J.S.; Lee, S.Y.; Min, J.-K.; Shetty, A.; Park, S.-H.; et al. Small Heterodimer Partner-Interacting Leucine Zipper Protein Suppresses Pain and Cartilage Destruction in an Osteoarthritis Model by Modulating the AMPK/STAT3 Signaling Pathway. Arthritis Res. Ther. 2024, 26, 199. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Wang, M.; Cao, S. Inhibition of Toll-Like Receptor 3 Relieves Osteoarthritis by Suppression of Cartilage Degradation, Nuclear Factor Kappa B-Mediated Inflammation, and Activation of Autophagy. Cartilage 2025, 19476035251317713. [Google Scholar] [CrossRef]
- Zou, Z.; Pan, S.; Sun, C.; Wei, J.; Xu, Y.; Xiao, K.; Zhao, J.; Gu, R. AM1241 Inhibits Chondrocyte Inflammation and ECM Degradation through the Nrf2/HO-1 and NF-κB Pathways and Alleviates Osteoarthritis in Mice. Mol. Med. 2025, 31, 9. [Google Scholar] [CrossRef]
- Yu, Y.; Wang, Y.; Li, J.; Xue, C.; Zhang, J.; Gao, P.; Tao, Z.; Li, Z.; Chen, X.; Ding, Z. P-Synephrine Loaded by Injectable Gelma Hydrogel Ameliorates Cartilage Degeneration in Osteoarthritis by Inhibiting the MAPK and NF-κB Signaling Pathways. Biol. Pharm. Bull. 2025, 48, 882–894. [Google Scholar] [CrossRef]
- Li, W.; Shi, Z.; Jing, H.; Dou, Y.; Liu, X.; Zhang, M.; Qiu, Z.; Heger, Z.; Li, N. Streamlined Metal-Based Hydrogel Facilitates Stem Cell Differentiation, Extracellular Matrix Homeostasis and Cartilage Repair in Male Rats. Nat. Commun. 2025, 16, 4344. [Google Scholar] [CrossRef]
- Ng, N.; Parkinson, L.; Brown, W.J.; Moorin, R.; Peeters, G.M.E.E.G. Lifestyle Behaviour Changes Associated with Osteoarthritis: A Prospective Cohort Study. Sci. Rep. 2024, 14, 6242. [Google Scholar] [CrossRef]
- Santolini, M.; Rios, J.L.; Custers, R.J.H.; Creemers, L.B.; Korpershoek, J.V. Mesenchymal Stromal Cell Injections for Osteoarthritis: In Vitro Mechanisms of Action and Clinical Evidence. Knee 2025, 56, 267–275. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, B.; Xiong, C.; Yue, Z. Pinocembrin Inhibits Matrix Metalloproteinase Expression in Chondrocytes. IUBMB Life 2015, 67, 36–41. [Google Scholar] [CrossRef]
- Sirše, M. Effect of Dietary Polyphenols on Osteoarthritis—Molecular Mechanisms. Life 2022, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Zhou, J. The Protective Activity of Natural Flavonoids against Osteoarthritis by Targeting NF-κB Signaling Pathway. Front. Endocrinol. 2023, 14, 1117489. [Google Scholar] [CrossRef] [PubMed]
- Williamson, G. Bioavailability of Food Polyphenols: Current State of Knowledge. Annu. Rev. Food Sci. Technol. 2025, 16, 315–332. [Google Scholar] [CrossRef] [PubMed]
- Nicolaescu, O.E.; Ionescu, C.; Samide, A.; Tigae, C.; Spînu, C.I.; Oprea, B. Advancements in Cyclodextrin Complexes with Bioactive Secondary Metabolites and Their Pharmaceutical Applications. Pharmaceutics 2025, 17, 506. [Google Scholar] [CrossRef]
- Wen, J.; Li, H.; Dai, H.; Hua, S.; Long, X.; Li, H.; Ivanovski, S.; Xu, C. Intra-Articular Nanoparticles Based Therapies for Osteoarthritis and Rheumatoid Arthritis Management. Mater. Today Bio 2023, 19, 100597. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, H. Intra-Articular Injection of Nanomaterials for the Treatment of Osteoarthritis: From Lubrication Function Restoration to Cell and Gene Therapy. Adv. Funct. Mater. 2024, 34, 2401547. [Google Scholar] [CrossRef]
- Trendafilova, I.; Popova, M. Porous Silica Nanomaterials as Carriers of Biologically Active Natural Polyphenols: Effect of Structure and Surface Modification. Pharmaceutics 2024, 16, 1004. [Google Scholar] [CrossRef]
- Spiridon, I.; Anghel, N. Cyclodextrins as Multifunctional Platforms in Drug Delivery and Beyond: Structural Features, Functional Applications, and Future Trends. Molecules 2025, 30, 3044. [Google Scholar] [CrossRef]
- Doyle, S.E.; Snow, F.; Duchi, S.; O’Connell, C.D.; Onofrillo, C.; Di Bella, C.; Pirogova, E. 3D Printed Multiphasic Scaffolds for Osteochondral Repair: Challenges and Opportunities. Int. J. Mol. Sci. 2021, 22, 12420. [Google Scholar] [CrossRef] [PubMed]
- Bačenková, D.; Trebuňová, M.; Demeterová, J.; Živčák, J. Human Chondrocytes, Metabolism of Articular Cartilage, and Strategies for Application to Tissue Engineering. Int. J. Mol. Sci. 2023, 24, 17096. [Google Scholar] [CrossRef]
- Kurenkova, A.D.; Romanova, I.A.; Kibirskiy, P.D.; Timashev, P.; Medvedeva, E.V. Strategies to Convert Cells into Hyaline Cartilage: Magic Spells for Adult Stem Cells. Int. J. Mol. Sci. 2022, 23, 11169. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, B.P.; Urban, J.P.; Maroudas, A. Influence of Cyclic Loading on the Nutrition of Articular Cartilage. Ann. Rheum. Dis. 1990, 49, 536–539. [Google Scholar] [CrossRef]
- Buckwalter, J.A.; Mankin, H.J. Articular Cartilage Repair and Transplantation. Arthritis Rheum. 1998, 41, 1331–1342. [Google Scholar] [CrossRef]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The Basic Science of Articular Cartilage. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Charlier, E.; Deroyer, C.; Ciregia, F.; Malaise, O.; Neuville, S.; Plener, Z.; Malaise, M.; de Seny, D. Chondrocyte Dedifferentiation and Osteoarthritis (OA). Biochem. Pharmacol. 2019, 165, 49–65. [Google Scholar] [CrossRef]
- Lin, X.; Zhang, Y.; Li, J.; Oliver, B.G.; Wang, B.; Li, H.; Yong, K.-T.; Li, J.J. Biomimetic Multizonal Scaffolds for the Reconstruction of Zonal Articular Cartilage in Chondral and Osteochondral Defects. Bioact. Mater. 2024, 43, 510–549. [Google Scholar] [CrossRef]
- Wang, W.; Ye, R.; Xie, W.; Zhang, Y.; An, S.; Li, Y.; Zhou, Y. Roles of the Calcified Cartilage Layer and Its Tissue Engineering Reconstruction in Osteoarthritis Treatment. Front. Bioeng. Biotechnol. 2022, 10, 911281. [Google Scholar] [CrossRef]
- Kongdang, P.; Chokchaitaweesuk, C.; Tangyuenyong, S.; Ongchai, S. Proinflammatory Effects of IL-1β Combined with IL-17A Promoted Cartilage Degradation and Suppressed Genes Associated with Cartilage Matrix Synthesis In Vitro. Molecules 2019, 24, 3682. [Google Scholar] [CrossRef]
- Fu, W.; Vasylyev, D.; Bi, Y.; Zhang, M.; Sun, G.; Khleborodova, A.; Huang, G.; Zhao, L.; Zhou, R.; Li, Y.; et al. Nav1.7 as a Chondrocyte Regulator and Therapeutic Target for Osteoarthritis. Nature 2024, 625, 557–565. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, R.; Huang, K.; Fu, W.; Wang, A.; Du, G.; Tang, H.; Yin, L.; Yin, Z.S. CHMP5 Attenuates Osteoarthritis via Inhibiting Chondrocyte Apoptosis and Extracellular Matrix Degradation: Involvement of NF-κB Pathway. Mol. Med. 2024, 30, 55. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, J.; Li, J.-J.; Zhou, H.; Zhu, X.-W.; Zhang, P.-H.; Huang, B.; Zhao, W.-T.; Zhao, X.-F.; Chen, E.-S. Chondrocyte Autophagy Mechanism and Therapeutic Prospects in Osteoarthritis. Front. Cell Dev. Biol. 2024, 12, 1472613. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Pang, Y.; Cao, R.; Zheng, Z.; Zheng, K.; Tian, Y.; Peng, X.; Liu, D.; Du, D.; Du, L.; et al. Targeting Parkin-Regulated Metabolomic Change in Cartilage in the Treatment of Osteoarthritis. iScience 2024, 27, 110597. [Google Scholar] [CrossRef]
- Court, A.C.; Vega-Letter, A.M.; Parra-Crisóstomo, E.; Velarde, F.; García, C.; Ortloff, A.; Vernal, R.; Pradenas, C.; Luz-Crawford, P.; Khoury, M.; et al. Mitochondrial Transfer Balances Cell Redox, Energy and Metabolic Homeostasis in the Osteoarthritic Chondrocyte Preserving Cartilage Integrity. Theranostics 2024, 14, 6471–6486. [Google Scholar] [CrossRef]
- Little-Letsinger, S.E.; Rubin, J.; Diekman, B.; Rubin, C.T.; McGrath, C.; Pagnotti, G.M.; Klett, E.L.; Styner, M. Exercise to Mend Aged-Tissue Crosstalk in Bone Targeting Osteoporosis & Osteoarthritis. Semin. Cell Dev. Biol. 2022, 123, 22–35. [Google Scholar] [CrossRef]
- Tang, X.; He, J.; Hao, Y. Histone Demethylase PHF8 Protected against Chondrocyte Injury and Alleviated Posttraumatic Osteoarthritis by Epigenetically Enhancing WWP2 Expression. Hum. Exp. Toxicol. 2024, 43, 9603271241292165. [Google Scholar] [CrossRef]
- Trengove, A.; Caballero Aguilar, L.M.; Di Bella, C.; Onofrillo, C.; Duchi, S.; O’Connor, A.J. A Dynamically Loaded Ex Vivo Model to Study Neocartilage and Integration in Human Cartilage Repair. Front. Cell Dev. Biol. 2024, 12, 1449015. [Google Scholar] [CrossRef]
- Lu, S.; Fang, C. Isosakuranetin Inhibits Subchondral Osteoclastogenesis for Attenuating Osteoarthritis via Suppressing NF-κB/CXCL2 Axis. Int. Immunopharmacol. 2024, 143, 113321. [Google Scholar] [CrossRef]
- Boer, C.G. Osteoarthritis Year in Review 2024: Genetics, Genomics, and Epigenetics. Osteoarthr. Cartil. 2025, 33, 50–57. [Google Scholar] [CrossRef]
- Sofat, N.; Howe, F.A. Bone Marrow Lesions in Osteoarthritis: Characterising Genetic and Histological Changes to Understand Disease Pathophysiology. Osteoarthr. Cart. Open 2024, 6, 100531. [Google Scholar] [CrossRef]
- Yau, M.S.; Okoro, P.C.; Haugen, I.K.; Lynch, J.A.; Nevitt, M.C.; Lewis, C.E.; Torner, J.C.; Felson, D.T. Assessing the Association of Epigenetic Age Acceleration with Osteoarthritis in the Multicenter Osteoarthritis Study (MOST). Osteoarthr. Cartil. 2024, 32, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-X.; He, S.-H.; Liang, X.; Li, W.; Li, T.-F.; Li, D.-F. Aging, Cell Senescence, the Pathogenesis and Targeted Therapies of Osteoarthritis. Front. Pharmacol. 2021, 12, 728100. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Wang, K.; Ding, S.; Zhang, M. Cross-Talk of Inflammation and Cellular Senescence: A New Insight into the Occurrence and Progression of Osteoarthritis. Bone Res. 2024, 12, 69. [Google Scholar] [CrossRef]
- Wang, N.; Lu, Y.; Rothrauff, B.B.; Zheng, A.; Lamb, A.; Yan, Y.; Lipa, K.E.; Lei, G.; Lin, H. Mechanotransduction Pathways in Articular Chondrocytes and the Emerging Role of Estrogen Receptor-α. Bone Res. 2023, 11, 13. [Google Scholar] [CrossRef]
- Zhao, T.; Huang, Y.; Zhu, J.; Qin, Y.; Wu, H.; Yu, J.; Zhai, Q.; Li, S.; Qin, X.; Wang, D.; et al. Extracellular Matrix Signaling Cues: Biological Functions, Diseases, and Therapeutic Targets. MedComm (2020) 2025, 6, e70281. [Google Scholar] [CrossRef]
- Mapp, P.I.; Walsh, D.A. Mechanisms and Targets of Angiogenesis and Nerve Growth in Osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 390–398. [Google Scholar] [CrossRef]
- Kiełbowski, K.; Herian, M.; Bakinowska, E.; Banach, B.; Sroczyński, T.; Pawlik, A. The Role of Genetics and Epigenetic Regulation in the Pathogenesis of Osteoarthritis. Int. J. Mol. Sci. 2023, 24, 11655. [Google Scholar] [CrossRef]
- Waheed, A.; Rai, M.F. Osteoarthritis Year in Review 2023: Genetics, Genomics, and Epigenetics. Osteoarthr. Cartil. 2024, 32, 128–137. [Google Scholar] [CrossRef]
- Liu, W.; Guo, N.; Wang, J.; Xu, B. Osteoarthritis: Mechanisms and Therapeutic Advances. MedComm (2020) 2025, 6, e70290. [Google Scholar] [CrossRef]
- Hu, B.; Du, G. OSTF1 Knockdown Mitigates IL-1β-Induced Chondrocyte Injury via Inhibiting the NF-κB Signaling Pathway. Heliyon 2024, 10, e30110. [Google Scholar] [CrossRef]
- Lee, W.; Nims, R.J.; Savadipour, A.; Zhang, Q.; Leddy, H.A.; Liu, F.; McNulty, A.L.; Chen, Y.; Guilak, F.; Liedtke, W.B. Inflammatory Signaling Sensitizes Piezo1 Mechanotransduction in Articular Chondrocytes as a Pathogenic Feed-Forward Mechanism in Osteoarthritis. Proc. Natl. Acad. Sci. USA 2021, 118, e2001611118. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, D.; Wei, G.; Liao, Z.; Liang, R.; Huang, X.; Lu, W.W.; Chen, Y. Pyroptosis in Osteoarthritis: Molecular Mechanisms and Therapeutic Implications. J. Inflamm. Res. 2024, 17, 791–803. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Kim, J.; Ryu, J.-H.; Oh, H.; Chun, C.-H.; Kim, B.J.; Min, B.H.; Chun, J.-S. Hypoxia-Inducible Factor-2alpha Is a Catabolic Regulator of Osteoarthritic Cartilage Destruction. Nat. Med. 2010, 16, 687–693. [Google Scholar] [CrossRef]
- Fleischmann, R.M.; Bliddal, H.; Blanco, F.J.; Schnitzer, T.J.; Peterfy, C.; Chen, S.; Wang, L.; Feng, S.; Conaghan, P.G.; Berenbaum, F.; et al. A Phase II Trial of Lutikizumab, an Anti-Interleukin-1α/β Dual Variable Domain Immunoglobulin, in Knee Osteoarthritis Patients With Synovitis. Arthritis Rheumatol. 2019, 71, 1056–1069. [Google Scholar] [CrossRef] [PubMed]
- Kloppenburg, M.; Peterfy, C.; Haugen, I.K.; Kroon, F.; Chen, S.; Wang, L.; Liu, W.; Levy, G.; Fleischmann, R.M.; Berenbaum, F.; et al. Phase IIa, Placebo-Controlled, Randomised Study of Lutikizumab, an Anti-Interleukin-1α and Anti-Interleukin-1β Dual Variable Domain Immunoglobulin, in Patients with Erosive Hand Osteoarthritis. Ann. Rheum. Dis. 2019, 78, 413–420. [Google Scholar] [CrossRef]
- Lian, C.; Tao, T.; Su, P.; Liao, Z.; Wang, X.; Lei, Y.; Zhao, P.; Liu, L. Targeting miR-18a Sensitizes Chondrocytes to Anticytokine Therapy to Prevent Osteoarthritis Progression. Cell Death Dis. 2020, 11, 947. [Google Scholar] [CrossRef]
- Zhou, H.; Zou, L.; Ren, H.; Shen, Z.; Lin, Y.; Cai, H.; Zhang, J. Cathelicidin-BF Regulates the AMPK/SIRT1/NF-κB Pathway to Ameliorate Murine Osteoarthritis: In Vitro and In Vivo Studie. Int. Immunopharmacol. 2024, 134, 112201. [Google Scholar] [CrossRef]
- van den Bosch, M.H.J. Osteoarthritis Year in Review 2020: Biology. Osteoarthr. Cartil. 2021, 29, 143–150. [Google Scholar] [CrossRef]
- Li, Z.; Lu, J. CircRNAs in Osteoarthritis: Research Status and Prospect. Front. Genet. 2023, 14, 1173812. [Google Scholar] [CrossRef]
- Zou, X.; Xu, H.; Qian, W. The Role and Current Research Status of Resveratrol in the Treatment of Osteoarthritis and Its Mechanisms: A Narrative Review. Drug Metab. Rev. 2024, 56, 399–412. [Google Scholar] [CrossRef] [PubMed]
- Yao, Q.; Wu, X.; Tao, C.; Gong, W.; Chen, M.; Qu, M.; Zhong, Y.; He, T.; Chen, S.; Xiao, G. Osteoarthritis: Pathogenic Signaling Pathways and Therapeutic Targets. Signal Transduct. Target. Ther. 2023, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.Y.; Ahmad, N.; Haqqi, T.M. Oxidative Stress and Inflammation in Osteoarthritis Pathogenesis: Role of Polyphenols. Biomed. Pharmacother. 2020, 129, 110452. [Google Scholar] [CrossRef] [PubMed]
- Guo, Q.; Chen, X.; Chen, J.; Zheng, G.; Xie, C.; Wu, H.; Miao, Z.; Lin, Y.; Wang, X.; Gao, W.; et al. STING Promotes Senescence, Apoptosis, and Extracellular Matrix Degradation in Osteoarthritis via the NF-κB Signaling Pathway. Cell Death Dis. 2021, 12, 13. [Google Scholar] [CrossRef]
- Zeng, R.-M.; Lu, X.-H.; Lin, J.; Hu, J.; Rong, Z.-J.; Xu, W.-C.; Liu, Z.-W.; Zeng, W.-T. Knockdown of FOXM1 Attenuates Inflammatory Response in Human Osteoarthritis Chondrocytes. Int. Immunopharmacol. 2019, 68, 74–80. [Google Scholar] [CrossRef]
- Mu, Y.; Wang, L.; Fu, L.; Li, Q. Knockdown of LMX1B Suppressed Cell Apoptosis and Inflammatory Response in IL-1β-Induced Human Osteoarthritis Chondrocytes through NF-κB and NLRP3 Signal Pathway. Mediat. Inflamm. 2022, 2022, 1870579. [Google Scholar] [CrossRef]
- da Cunha, A.L.; Aguiar, J.A.K.; Correa da Silva, F.S.; Michelacci, Y.M. Do Chondroitin Sulfates with Different Structures Have Different Activities on Chondrocytes and Macrophages? Int. J. Biol. Macromol. 2017, 103, 1019–1031. [Google Scholar] [CrossRef]
- Arra, M.; Swarnkar, G.; Ke, K.; Otero, J.E.; Ying, J.; Duan, X.; Maruyama, T.; Rai, M.F.; O’Keefe, R.J.; Mbalaviele, G.; et al. LDHA-Mediated ROS Generation in Chondrocytes Is a Potential Therapeutic Target for Osteoarthritis. Nat. Commun. 2020, 11, 3427. [Google Scholar] [CrossRef]
- Saha, S.; Rebouh, N.Y. Anti-Osteoarthritis Mechanism of the Nrf2 Signaling Pathway. Biomedicines 2023, 11, 3176. [Google Scholar] [CrossRef]
- Zhao, X.; Duan, B.; Wu, J.; Huang, L.; Dai, S.; Ding, J.; Sun, M.; Lin, X.; Jiang, Y.; Sun, T.; et al. Bilirubin Ameliorates Osteoarthritis via Activating Nrf2/HO-1 Pathway and Suppressing NF-κB Signalling. J. Cell. Mol. Med. 2024, 28, e18173. [Google Scholar] [CrossRef]
- Sun, J.; Song, X.; Wang, C.; Ruan, Q. Geniposidic Acid Alleviates Osteoarthritis Progression through Inhibiting Inflammation and Chondrocytes Ferroptosis. J. Cell. Mol. Med. 2024, 28, e18228. [Google Scholar] [CrossRef] [PubMed]
- Robledinos-Antón, N.; Fernández-Ginés, R.; Manda, G.; Cuadrado, A. Activators and Inhibitors of NRF2: A Review of Their Potential for Clinical Development. Oxid. Med. Cell. Longev. 2019, 2019, 9372182. [Google Scholar] [CrossRef] [PubMed]
- Malemud, C.J. Inhibition of MMPs and ADAM/ADAMTS. Biochem. Pharmacol. 2019, 165, 33–40. [Google Scholar] [CrossRef]
- Yang, C.-Y.; Chanalaris, A.; Troeberg, L. ADAMTS and ADAM Metalloproteinases in Osteoarthritis—Looking beyond the “Usual Suspects”. Osteoarthr. Cartil. 2017, 25, 1000–1009. [Google Scholar] [CrossRef]
- Rim, Y.A.; Nam, Y.; Ju, J.H. The Role of Chondrocyte Hypertrophy and Senescence in Osteoarthritis Initiation and Progression. Int. J. Mol. Sci. 2020, 21, 2358. [Google Scholar] [CrossRef]
- Jiang, Y. Osteoarthritis Year in Review 2021: Biology. Osteoarthr. Cartil. 2022, 30, 207–215. [Google Scholar] [CrossRef]
- Wang, C.; Shen, J.; Ying, J.; Xiao, D.; O’Keefe, R.J. FoxO1 Is a Crucial Mediator of TGF-β/TAK1 Signaling and Protects against Osteoarthritis by Maintaining Articular Cartilage Homeostasis. Proc. Natl. Acad. Sci. USA 2020, 117, 30488–30497. [Google Scholar] [CrossRef]
- Lee, K.I.; Choi, S.; Matsuzaki, T.; Alvarez-Garcia, O.; Olmer, M.; Grogan, S.P.; D’Lima, D.D.; Lotz, M.K. FOXO1 and FOXO3 Transcription Factors Have Unique Functions in Meniscus Development and Homeostasis during Aging and Osteoarthritis. Proc. Natl. Acad. Sci. USA 2020, 117, 3135–3143. [Google Scholar] [CrossRef]
- Webb, A.E.; Brunet, A. FOXO Transcription Factors: Key Regulators of Cellular Quality Control. Trends Biochem. Sci. 2014, 39, 159–169. [Google Scholar] [CrossRef]
- Alvarez-Garcia, O.; Matsuzaki, T.; Olmer, M.; Miyata, K.; Mokuda, S.; Sakai, D.; Masuda, K.; Asahara, H.; Lotz, M.K. FOXO Are Required for Intervertebral Disk Homeostasis during Aging and Their Deficiency Promotes Disk Degeneration. Aging Cell 2018, 17, e12800. [Google Scholar] [CrossRef]
- Matsuzaki, T.; Alvarez-Garcia, O.; Mokuda, S.; Nagira, K.; Olmer, M.; Gamini, R.; Miyata, K.; Akasaki, Y.; Su, A.I.; Asahara, H.; et al. FoxO Transcription Factors Modulate Autophagy and Proteoglycan 4 in Cartilage Homeostasis and Osteoarthritis. Sci. Transl. Med. 2018, 10, eaan0746. [Google Scholar] [CrossRef]
- Yue, J.; Aobulikasimu, A.; Sun, W.; Liu, S.; Xie, W.; Sun, W. Targeted Regulation of FoxO1 in Chondrocytes Prevents Age-Related Osteoarthritis via Autophagy Mechanism. J. Cell. Mol. Med. 2022, 26, 3075–3082. [Google Scholar] [CrossRef]
- Mergen Duymaz, G.; Duz, G.; Ozkan, K.; Karadag, A.; Yilmaz, O.; Karakus, A.; Cengiz, O.; Akyildiz, I.E.; Basdogan, G.; Damarlı, E.; et al. The Evaluation of L-Arginine Solution as a Solvent for Propolis Extraction: The Phenolic Profile, Antioxidant, Antibacterial Activity, and In Vitro Bioaccessibility. Food Sci. Nutr. 2024, 12, 2724–2735. [Google Scholar] [CrossRef]
- Saroglu, O.; Karadag, A. Propolis-Loaded Liposomes: Characterization and Evaluation of the In Vitro Bioaccessibility of Phenolic Compounds. ADMET DMPK 2024, 12, 209–224. [Google Scholar] [CrossRef]
- Guzmán-Oyarzo, D.; Plaza, T.; Recio-Sánchez, G.; Abdalla, D.S.P.; Salazar, L.A.; Hernández-Montelongo, J. Use of nPSi-βCD Composite Microparticles for the Controlled Release of Caffeic Acid and Pinocembrin, Two Main Polyphenolic Compounds Found in a Chilean Propolis. Pharmaceutics 2019, 11, 289. [Google Scholar] [CrossRef] [PubMed]
- Alvear, M.; Santos, E.; Cabezas, F.; Pérez-SanMartín, A.; Lespinasse, M.; Veloz, J. Geographic Area of Collection Determines the Chemical Composition and Antimicrobial Potential of Three Extracts of Chilean Propolis. Plants 2021, 10, 1543. [Google Scholar] [CrossRef] [PubMed]
- Veloz, J.J.; Alvear, M.; Salazar, L.A. Antimicrobial and Antibiofilm Activity against Streptococcus Mutans of Individual and Mixtures of the Main Polyphenolic Compounds Found in Chilean Propolis. BioMed Res. Int. 2019, 2019, 7602343. [Google Scholar] [CrossRef] [PubMed]
- Arias, C.; Vásquez, B.; Salazar, L.A. Propolis as a Potential Therapeutic Agent to Counteract Age-Related Changes in Cartilage: An In Vivo Study. Int. J. Mol. Sci. 2023, 24, 14272. [Google Scholar] [CrossRef]
- Henrotin, Y.; Clutterbuck, A.L.; Allaway, D.; Lodwig, E.M.; Harris, P.; Mathy-Hartert, M.; Shakibaei, M.; Mobasheri, A. Biological Actions of Curcumin on Articular Chondrocytes. Osteoarthr. Cartil. 2010, 18, 141–149. [Google Scholar] [CrossRef]
- Ma, X.; Zhao, W.; Yang, F.; Chin, K.-Y. Efficacy and Mechanisms of Curcumin in the Treatment of Osteoarthritis: A Scoping Review. Biomol. Biomed 2025, 25, 761–785. [Google Scholar] [CrossRef]
- Moon, M.-H.; Jeong, J.-K.; Lee, Y.-J.; Seol, J.-W.; Jackson, C.J.; Park, S.-Y. SIRT1, a Class III Histone Deacetylase, Regulates TNF-α-Induced Inflammation in Human Chondrocytes. Osteoarthr. Cartil. 2013, 21, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Sun, M.; Zhang, X. Protective Effect of Resveratrol on Knee Osteoarthritis and Its Molecular Mechanisms: A Recent Review in Preclinical and Clinical Trials. Front. Pharmacol. 2022, 13, 921003. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, Z.; Anbazhagan, A.N.; Akhtar, N.; Ramamurthy, S.; Voss, F.R.; Haqqi, T.M. Green Tea Polyphenol Epigallocatechin-3-Gallate Inhibits Advanced Glycation End Product-Induced Expression of Tumor Necrosis Factor-α and Matrix Metalloproteinase-13 in Human Chondrocytes. Arthritis Res. Ther. 2009, 11, R71. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Li, X.; Xu, G.; Chen, L.; Zhao, J. Quercetin Attenuates the Symptoms of Osteoarthritis in Vitro and in Vivo by Suppressing Ferroptosis via Activation of AMPK/Nrf2/Gpx4 Signaling. Mol. Med. Rep. 2024, 31, 60. [Google Scholar] [CrossRef]
- Sun, W.; Xie, W.; Huang, D.; Cui, Y.; Yue, J.; He, Q.; Jiang, L.; Xiong, J.; Sun, W.; Yi, Q. Caffeic Acid Phenethyl Ester Attenuates Osteoarthritis Progression by Activating NRF2/HO-1 and Inhibiting the NF-κB Signaling Pathway. Int. J. Mol. Med. 2022, 50, 1–14. [Google Scholar] [CrossRef]
- Aatif, M. Current Understanding of Polyphenols to Enhance Bioavailability for Better Therapies. Biomedicines 2023, 11, 2078. [Google Scholar] [CrossRef]
- Kuptniratsaikul, V.; Dajpratham, P.; Taechaarpornkul, W.; Buntragulpoontawee, M.; Lukkanapichonchut, P.; Chootip, C.; Saengsuwan, J.; Tantayakom, K.; Laongpech, S. Efficacy and Safety of Curcuma Domestica Extracts Compared with Ibuprofen in Patients with Knee Osteoarthritis: A Multicenter Study. Clin. Interv. Aging 2014, 9, 451–458. [Google Scholar] [CrossRef]
- Panahi, Y.; Rahimnia, A.-R.; Sharafi, M.; Alishiri, G.; Saburi, A.; Sahebkar, A. Curcuminoid Treatment for Knee Osteoarthritis: A Randomized Double-Blind Placebo-Controlled Trial. Phytother. Res. 2014, 28, 1625–1631. [Google Scholar] [CrossRef]
- Hashemzadeh, K.; Davoudian, N.; Jaafari, M.R.; Mirfeizi, Z. The Effect of Nanocurcumin in Improvement of Knee Osteoarthritis: A Randomized Clinical Trial. Curr. Rheumatol. Rev. 2020, 16, 158–164. [Google Scholar] [CrossRef]
- Nguyen, C.; Coudeyre, E.; Boutron, I.; Baron, G.; Daste, C.; Lefèvre-Colau, M.-M.; Sellam, J.; Zauderer, J.; Berenbaum, F.; Rannou, F. Oral Resveratrol in Adults with Knee Osteoarthritis: A Randomized Placebo-Controlled Trial (ARTHROL). PLoS Med. 2024, 21, e1004440. [Google Scholar] [CrossRef]
- Conaghan, P.G.; Hunter, D.J.; Cohen, S.B.; Kraus, V.B.; Berenbaum, F.; Lieberman, J.R.; Jones, D.G.; Spitzer, A.I.; Jevsevar, D.S.; Katz, N.P.; et al. Effects of a Single Intra-Articular Injection of a Microsphere Formulation of Triamcinolone Acetonide on Knee Osteoarthritis Pain. J. Bone Jt. Surg. Am. 2018, 100, 666–677. [Google Scholar] [CrossRef] [PubMed]
- Nishida, Y.; Kano, K.; Nobuoka, Y.; Seo, T. Efficacy and Safety of Diclofenac–Hyaluronate Conjugate (Diclofenac Etalhyaluronate) for Knee Osteoarthritis: A Randomized Phase III Trial in Japan. Arthritis Rheumatol. 2021, 73, 1646–1655. [Google Scholar] [CrossRef] [PubMed]
- Bolandnazar, N.S.; Raeissadat, S.A.; Haghighatkhah, H.; Rayegani, S.M.; Oshnari, R.S.; Keshel, S.H.; Zahraei, M.; Aalipour, K.; Babaee, M.; Zamani, A.; et al. Safety and Efficacy of Placental Mesenchymal Stromal Cells-Derived Extracellular Vesicles in Knee Osteoarthritis: A Randomized, Triple-Blind, Placebo-Controlled Clinical Trial. BMC Musculoskelet. Disord. 2024, 25, 856. [Google Scholar] [CrossRef]
- Singla, R.K.; Dubey, A.K.; Garg, A.; Sharma, R.K.; Fiorino, M.; Ameen, S.M.; Haddad, M.A.; Al-Hiary, M. Natural Polyphenols: Chemical Classification, Definition of Classes, Subcategories, and Structures. J. AOAC Int. 2019, 102, 1397–1400. [Google Scholar] [CrossRef] [PubMed]
- Bojarczuk, A.; Dzitkowska-Zabielska, M. Polyphenol Supplementation and Antioxidant Status in Athletes: A Narrative Review. Nutrients 2022, 15, 158. [Google Scholar] [CrossRef]
- Kim, Y.S.; Young, M.R.; Bobe, G.; Colburn, N.H.; Milner, J.A. Bioactive Food Components, Inflammatory Targets, and Cancer Prevention. Cancer Prev. Res. 2009, 2, 200–208. [Google Scholar] [CrossRef]
- Hussain, T.; Tan, B.; Yin, Y.; Blachier, F.; Tossou, M.C.B.; Rahu, N. Oxidative Stress and Inflammation: What Polyphenols Can Do for Us? Oxid. Med. Cell. Longev. 2016, 2016, 7432797. [Google Scholar] [CrossRef]
- Cao, G.; Zuo, J.; Wu, B.; Wu, Y. Polyphenol Supplementation Boosts Aerobic Endurance in Athletes: Systematic Review. Front. Physiol. 2024, 15, 1369174. [Google Scholar] [CrossRef]
- Leong, D.J.; Choudhury, M.; Hanstein, R.; Hirsh, D.M.; Kim, S.J.; Majeska, R.J.; Schaffler, M.B.; Hardin, J.A.; Spray, D.C.; Goldring, M.B.; et al. Green Tea Polyphenol Treatment Is Chondroprotective, Anti-Inflammatory and Palliative in a Mouse Post-Traumatic Osteoarthritis Model. Arthritis Res. Ther. 2014, 16, 508. [Google Scholar] [CrossRef]
- Aini, H.; Ochi, H.; Iwata, M.; Okawa, A.; Koga, D.; Okazaki, M.; Sano, A.; Asou, Y. Procyanidin B3 Prevents Articular Cartilage Degeneration and Heterotopic Cartilage Formation in a Mouse Surgical Osteoarthritis Model. PLoS ONE 2012, 7, e37728. [Google Scholar] [CrossRef]
- Masuda, I.; Koike, M.; Nakashima, S.; Mizutani, Y.; Ozawa, Y.; Watanabe, K.; Sawada, Y.; Sugiyama, H.; Sugimoto, A.; Nojiri, H.; et al. Apple Procyanidins Promote Mitochondrial Biogenesis and Proteoglycan Biosynthesis in Chondrocytes. Sci. Rep. 2018, 8, 7229. [Google Scholar] [CrossRef]
- Bustamante, A.; García-Díaz, D.; Jiménez, P.; Valenzuela, R.; Pando, M.E.; Echeverría, F. Potencial efecto terapéutico de los polifenoles obtenidos de la cáscara de granada en la esteatosis hepática. Rev. Chil. Nutr. 2022, 49, 89–99. [Google Scholar] [CrossRef]
- Krisna, A.I.; Widyaningrum, I.; Wahyuningsih, D. Systematic Literature Rreview: Pengaruh Polifenol Delima (Punica granatum L.) Terhadap Kadar Interleukin-6 Pada Penyakit Yang Melibatkan Patofisiologi Inflamasi. J. Kedokt. Komunitas J. Community Med. 2021, 9. [Google Scholar]
- Elbatreek, M.H.; Mahdi, I.; Ouchari, W.; Mahmoud, M.F.; Sobeh, M. Current Advances on the Therapeutic Potential of Pinocembrin: An Updated Review. Biomed Pharmacother. 2023, 157, 114032. [Google Scholar] [CrossRef] [PubMed]
- Zhu, M.; Sun, Y.; Su, Y.; Guan, W.; Wang, Y.; Han, J.; Wang, S.; Yang, B.; Wang, Q.; Kuang, H. Luteolin: A Promising Multifunctional Natural Flavonoid for Human Diseases. Phytother. Res. 2024, 38, 3417–3443. [Google Scholar] [CrossRef]
- Wahnou, H.; Limami, Y.; Oudghiri, M. Flavonoids and Flavonoid-Based Nanoparticles for Osteoarthritis and Rheumatoid Arthritis Management. BioChem 2024, 4, 38–61. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Yang, H.; Jing, X.; Wang, W.; Liu, X.; Zhang, B.; Liu, X.; Shao, Y.; Cui, X. Activation of the Nrf-2 Pathway by Pinocembrin Safeguards Vertebral Endplate Chondrocytes against Apoptosis and Degeneration Caused by Oxidative Stress. Life Sci. 2023, 333, 122162. [Google Scholar] [CrossRef]
- Saavedra, N.; Cuevas, A.; Cavalcante, M.F.; Dörr, F.A.; Saavedra, K.; Zambrano, T.; Abdalla, D.S.P.; Salazar, L.A. Polyphenols from Chilean Propolis and Pinocembrin Reduce MMP-9 Gene Expression and Activity in Activated Macrophages. Biomed. Res. Int. 2016, 2016, 6505383. [Google Scholar] [CrossRef]
- Ganguly, R.; Singh, S.V.; Jaiswal, K.; Kumar, R.; Pandey, A.K. Modulatory Effect of Caffeic Acid in Alleviating Diabetes and Associated Complications. World J. Diabetes 2023, 14, 62–75. [Google Scholar] [CrossRef]
- Bhargava, P.; Kumari, A.; Putri, J.F.; Ishida, Y.; Terao, K.; Kaul, S.C.; Sundar, D.; Wadhwa, R. Caffeic Acid Phenethyl Ester (CAPE) Possesses pro-Hypoxia and Anti-Stress Activities: Bioinformatics and Experimental Evidences. Cell Stress Chaperones 2018, 23, 1055–1068. [Google Scholar] [CrossRef]
- Armutcu, F.; Akyol, S.; Ustunsoy, S.; Turan, F.F. Therapeutic Potential of Caffeic Acid Phenethyl Ester and Its Anti-Inflammatory and Immunomodulatory Effects (Review). Exp. Ther. Med. 2015, 9, 1582–1588. [Google Scholar] [CrossRef]
- Olgierd, B.; Kamila, Ż.; Anna, B.; Emilia, M. The Pluripotent Activities of Caffeic Acid Phenethyl Ester. Molecules 2021, 26, 1335. [Google Scholar] [CrossRef]
- Wang, L.-C.; Lin, Y.-L.; Liang, Y.-C.; Yang, Y.-H.; Lee, J.-H.; Yu, H.-H.; Wu, W.-M.; Chiang, B.-L. The Effect of Caffeic Acid Phenethyl Ester on the Functions of Human Monocyte-Derived Dendritic Cells. BMC Immunol. 2009, 10, 39. [Google Scholar] [CrossRef]
- Chang, H.; Wang, Y.; Yin, X.; Liu, X.; Xuan, H. Ethanol Extract of Propolis and Its Constituent Caffeic Acid Phenethyl Ester Inhibit Breast Cancer Cells Proliferation in Inflammatory Microenvironment by Inhibiting TLR4 Signal Pathway and Inducing Apoptosis and Autophagy. BMC Complement. Altern. Med. 2017, 17, 471. [Google Scholar] [CrossRef]
- Sahoo, D.K.; Heilmann, R.M.; Paital, B.; Patel, A.; Yadav, V.K.; Wong, D.; Jergens, A.E. Oxidative Stress, Hormones, and Effects of Natural Antioxidants on Intestinal Inflammation in Inflammatory Bowel Disease. Front. Endocrinol. 2023, 14, 1217165. [Google Scholar] [CrossRef]
- Tambuwala, M.M.; Khan, M.N.; Thompson, P.; McCarron, P.A. Albumin Nano-Encapsulation of Caffeic Acid Phenethyl Ester and Piceatannol Potentiated Its Ability to Modulate HIF and NF-kB Pathways and Improves Therapeutic Outcome in Experimental Colitis. Drug Deliv. Transl. Res. 2019, 9, 14–24. [Google Scholar] [CrossRef] [PubMed]
- Acar, T.; Arayici, P.P.; Ucar, B.; Coksu, I.; Tasdurmazli, S.; Ozbek, T.; Acar, S. Host–Guest Interactions of Caffeic Acid Phenethyl Ester with β-Cyclodextrins: Preparation, Characterization, and In Vitro Antioxidant and Antibacterial Activity. ACS Omega 2024, 9, 3625–3634. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Ren, S.; Wang, X.; Hu, Y.; Xu, M.; Zhang, H.; Cao, H.; Huang, K.; Wang, C.; Guan, X. Encapsulation of Caffeic Acid Phenethyl Ester by Self-Assembled Sorghum Peptide Nanoparticles: Fabrication, Storage Stability and Interaction Mechanisms. Food Chem. 2024, 453, 139642. [Google Scholar] [CrossRef]
- Fraga, C.G.; Galleano, M.; Verstraeten, S.V.; Oteiza, P.I. Basic Biochemical Mechanisms behind the Health Benefits of Polyphenols. Mol. Asp. Med. 2010, 31, 435–445. [Google Scholar] [CrossRef] [PubMed]
- Krook, M.A.; Hagerman, A.E. Stability of Polyphenols Epigallocatechin Gallate and Pentagalloyl Glucose in a Simulated Digestive System. Food Res. Int. 2012, 49, 112–116. [Google Scholar] [CrossRef]
- Milbury, P.E.; Vita, J.A.; Blumberg, J.B. Anthocyanins Are Bioavailable in Humans Following an Acute Dose of Cranberry Juice. J. Nutr. 2010, 140, 1099–1104. [Google Scholar] [CrossRef]
- Manach, C.; Morand, C.; Gil-Izquierdo, A.; Bouteloup-Demange, C.; Rémésy, C. Bioavailability in Humans of the Flavanones Hesperidin and Narirutin after the Ingestion of Two Doses of Orange Juice. Eur. J. Clin. Nutr. 2003, 57, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Simonetti, P.; Gardana, C.; Pietta, P. Caffeic Acid as Biomarker of Red Wine Intake. Methods Enzymol. 2001, 335, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Ed Nignpense, B.; Francis, N.; Blanchard, C.; Santhakumar, A.B. Effect of Gastrointestinal Digestion on the Stability, Antioxidant Activity, and Caco-2 Cellular Transport of Pigmented Grain Polyphenols. J. Food Sci. 2024, 89, 2701–2715. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Xu, K.; Zhao, Z.; Dong, C.; Zhang, Y. In Vitro Investigation of Anti-Inflammatory Activity of Propolis/Saffron Extract/Curcumin-Loaded ZIF8 Nanoparticles and Their Potential Application for Treating Osteoarthritis. Mater. Sci. Pol. 2024, 42, 41–51. [Google Scholar] [CrossRef]
- Čolić, M.; Kraljević Pavelić, S.; Peršurić, Ž.; Agaj, A.; Bulog, A.; Pavelić, K. Enhancing the Bioavailability and Activity of Natural Antioxidants with Nanobubbles and Nanoparticles. Redox Rep. 2024, 29, 2333619. [Google Scholar] [CrossRef]
- Szymczak, J.; Cielecka-Piontek, J. Fisetin-In Search of Better Bioavailability-From Macro to Nano Modifications: A Review. Int. J. Mol. Sci. 2023, 24, 14158. [Google Scholar] [CrossRef]
- Deng, R.; Zhao, R.; Zhang, Z.; Chen, Y.; Yang, M.; Lin, Y.; Ye, J.; Li, N.; Qin, H.; Yan, X.; et al. Chondrocyte Membrane-Coated Nanoparticles Promote Drug Retention and Halt Cartilage Damage in Rat and Canine Osteoarthritis. Sci. Transl. Med. 2024, 16, eadh9751. [Google Scholar] [CrossRef]
- Yi, Z.; Chen, X.; Chen, G.; Deng, Z.; Tong, Q.; Sun, Z.; Ma, X.; Su, W.; Ma, L.; Ran, Y.; et al. General Nanomedicine Platform by Solvent-Mediated Disassembly/Reassembly of Scalable Natural Polyphenol Colloidal Spheres. ACS Appl. Mater. Interfaces 2020, 12, 37914–37928. [Google Scholar] [CrossRef]
- Center for Drug Evaluation and Research. Drug Products, Including Biological Products, That Contain Nanomaterials—Guidance for Industry. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/drug-products-including-biological-products-contain-nanomaterials-guidance-industry (accessed on 28 August 2025).
- Musazzi, U.M.; Franzè, S.; Condorelli, F.; Minghetti, P.; Caliceti, P. Feeding Next-Generation Nanomedicines to Europe: Regulatory and Quality Challenges. Adv. Healthc. Mater. 2023, 12, 2301956. [Google Scholar] [CrossRef]
- Clogston, J.D.; Foss, W.; Harris, D.; Oberoi, H.; Pan, J.; Pu, E.; Guzmán, E.A.T.; Walter, K.; Brown, S.; Soo, P.L. Current State of Nanomedicine Drug Products: An Industry Perspective. J. Pharm. Sci. 2024, 113, 3395–3405. [Google Scholar] [CrossRef]
- Liao, S.; Jia, S.; Yue, Y.; Zeng, H.; Lin, J.; Liu, P. Advancements in pH-Responsive Nanoparticles for Osteoarthritis Treatment: Opportunities and Challenges. Front. Bioeng. Biotechnol. 2024, 12, 1426794. [Google Scholar] [CrossRef]
- Liang, Q.; Cheng, Z.; Qin, L. Advanced Nanoparticles in Osteoarthritis Treatment. Biomater. Transl. 2024, 5, 95–113. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Lin, W.; Kluzek, M.; Miotla-Zarebska, J.; Batchelor, V.; Gardiner, M.; Chan, C.; Culmer, P.; Chanalaris, A.; Goldberg, R.; et al. Liposomic Lubricants Suppress Acute Inflammatory Gene Regulation in the Joint In Vivo. Acta Biomater. 2025, 198, 366–376. [Google Scholar] [CrossRef] [PubMed]
- Mitsou, E.; Klein, J. Liposome-Based Interventions in Knee Osteoarthritis. Small 2025, 21, 2410060. [Google Scholar] [CrossRef] [PubMed]
- Del Castillo-Santaella, T.; Peula-García, J.M.; Maldonado-Valderrama, J.; Jódar-Reyes, A.B. Interaction of Surfactant and Protein at the O/W Interface and Its Effect on Colloidal and Biological Properties of Polymeric Nanocarriers. Colloid Surf. B 2019, 173, 295–302. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, M.; Chen, Y.; Ding, S.; Qin, M.; Wu, T.; Li, J.; Xie, J. Zwitterionic-Based Cyclic Brush Polymer Nanomicelles with Improved Lubrication and Antioxidation Properties. J. Mater. Chem. B 2025, 13, 8026–8037. [Google Scholar] [CrossRef]
- Çitoğlu, S.; Duran, H. Recent Advances in Porous Nanomaterials-Based Drug Delivery Systems for Osteoarthritis. Nano Sel. 2024, 5, 2300099. [Google Scholar] [CrossRef]
- Johnsen, H.M.; Filtvedt, W.; Klaveness, J.; Hiorth, M. Nano-Strategies for Advancing Oral Drug Delivery: Porous Silicon Particles and Cyclodextrin Encapsulation for Enhanced Dissolution of Poorly Soluble Drugs. Int. J. Pharm. 2024, 666, 124809. [Google Scholar] [CrossRef]
- Deng, Z.; Yang, C.; Xiang, T.; Dou, C.; Sun, D.; Dai, Q.; Ling, Z.; Xu, J.; Luo, F.; Chen, Y. Gold Nanoparticles Exhibit Anti-Osteoarthritic Effects via Modulating Interaction of the “Microbiota-Gut-Joint” Axis. J. Nanobiotechnol. 2024, 22, 157. [Google Scholar] [CrossRef]
- Tian, M.; Zhu, Y.; Lu, S.; Qin, Y.; Li, X.; Wang, T.; Guo, Y.; Shi, H.; Qin, D. Clinical Efficacy of Probiotic Supplementation in the Treatment of Knee Osteoarthritis: A Meta-Analysis. Front. Microbiol. 2025, 16, 1526690. [Google Scholar] [CrossRef]
- Moyseos, M.; Michael, J.; Ferreira, N.; Sophocleous, A. The Effect of Probiotics on the Management of Pain and Inflammation in Osteoarthritis: A Systematic Review and Meta-Analysis of Clinical Studies. Nutrients 2024, 16, 2243. [Google Scholar] [CrossRef] [PubMed]
- Karim, A. Unveiling the Potential of Probiotics in Osteoarthritis Management. Curr. Rheumatol. Rep. 2024, 27, 2. [Google Scholar] [CrossRef] [PubMed]
- Karim, A.; Khan, H.A.; Iqbal, M.S.; Ahmad, F.; Qaisar, R. Probiotics’ Supplementation Alleviates Disease Severity and Improves Postural Balance by Repairing Intestinal Leak in Patients Suffering from Osteoarthritis: A Double-Blinded Clinical Trial. Br. J. Nutr. 2024, 132, 1602–1610. [Google Scholar] [CrossRef] [PubMed]
- Shine, B.-K.; Li, Q.; Song, M.; Song, K.; Shim, J.; Han, S.-H. Efficacy and Safety of Latilactobacillus Sakei LB-P12 in Patients with Knee Osteoarthritis: An Exploratory Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Sci. Rep. 2025, 15, 25980. [Google Scholar] [CrossRef]
- Bhardwaj, A.; Sapra, L.; Tiwari, A.; Mishra, P.K.; Sharma, S.; Srivastava, R.K. “Osteomicrobiology”: The Nexus Between Bone and Bugs. Front. Microbiol. 2021, 12, 812466. [Google Scholar] [CrossRef]
- Dahshan, D.; Gallagher, N.; Workman, A.; Perdue, J.; Aikens, J.; Schmicker, T.; Shuler, F.D. Targeting the Gut Microbiome for Inflammation and Pain Management in Orthopedic Conditions. Orthopedics 2022, 45, e226–e234. [Google Scholar] [CrossRef]
- Arora, V.; Singh, G.; O-Sullivan, I.; Ma, K.; Natarajan Anbazhagan, A.; Votta-Velis, E.G.; Bruce, B.; Richard, R.; van Wijnen, A.J.; Im, H.-J. Gut-Microbiota Modulation: The Impact of Thegut-Microbiotaon Osteoarthritis. Gene 2021, 785, 145619. [Google Scholar] [CrossRef]
- Liao, J.; Gu, Q.; Liu, Z.; Wang, H.; Yang, X.; Yan, R.; Zhang, X.; Song, S.; Wen, L.; Wang, Y. Edge Advances in Nanodrug Therapies for Osteoarthritis Treatment. Front. Pharmacol. 2024, 15, 1402825. [Google Scholar] [CrossRef]
- European Medicines Agency (EMA); Heads of Medicines Agencies (HMA). Nanotechnology-Based Medicinal Products for Human Use—EU-IN Horizon Scanning Report; EMA: Amsterdam, The Netherlands, 2025. [Google Scholar]
- Ghodasara, A.; Raza, A.; Wolfram, J.; Salomon, C.; Popat, A. Clinical Translation of Extracellular Vesicles. Adv. Healthc. Mater. 2023, 12, 2301010. [Google Scholar] [CrossRef]

| Signaling Pathway | Description | References |
|---|---|---|
| IkBα | It regulates the stability of NF-κB, related to joint inflammation. | [51,52,53,54,55,62] |
| MMP-13 | Key enzyme in the degradation of type II collagen. | [62,63,64] |
| ADAMTS-5 | An enzyme involved in the degradation of aggrecan in cartilage. | [61,62] |
| Nrf2 | Regulates cellular antioxidant response. | [57,58] |
| HO-1 | Protects cartilage against oxidative stress. | [57,58] |
| FoxO1 | It regulates chondrocyte homeostasis and autophagic response. | [69,70] |
| Polyphenols | Key Pathways/Targets | OA-Relevant Outcomes | Ref. |
|---|---|---|---|
| Curcumin | NF-κB ↓; MMPs ↓ | Suppresses IL-1β/OSM-induced MMPs in chondrocytes; chondroprotection | [95,96] |
| Resveratrol | SIRT1 ↑; NF-κB ↓ | Anti-inflammatory; protects cartilage | [97,98] |
| EGCG | NF-κB/TNF-α ↓; MMP-13 ↓ | Lowers TNF-α and MMP-13; slows OA progression (in vivo) | [99] |
| Quercetin | AMPK/Nrf2/GPX4 ↑ | Inhibits chondrocyte ferroptosis; in vitro/in vivo benefit | [100] |
| Pinocembrin | NF-κB (p65) nuclear translocation ↓; MMP-1/-3/-13 ↓ | Anti-catabolic; chondroprotection | [101] |
| CAPE | Nrf2/HO-1 ↑; NF-κB ↓; iNOS/COX-2 ↓ | Reduces NO and PGE2; in vitro + in vivo improvement | [102] |
| Category | Study (Year) | Design/N | Intervention (Dose; Duration) | Comparator | Primary Outcome | Main Limitations | Evidence Tier |
|---|---|---|---|---|---|---|---|
| Polyphenol | Curcuma domestica extract (2014) [107] | RCT, non-inferiority; n = 367 | 1500 mg/day; 4 weeks | Ibuprofen 1200 mg/day | Non-inferior pain/function; fewer GI adverse events | Short duration; single country; product heterogeneity | Early clinical |
| Polyphenol | Curcuminoids (2014) [108] | RCT, double-blind; n ≈ 40 | 1500 mg/day; 6 weeks | Placebo | ↓ WOMAC pain/stiffness/function | Small sample; short follow-up; product variability | Early clinical |
| Polyphenol (nano) | Nanocurcumin (2020) [109] | RCT, double-blind; n ≈ 70 | 40 mg q12h; 6 weeks | Placebo | ↓ WOMAC total and subscales | Short duration; single center | Early clinical |
| Polyphenol | Resveratrol (2024) [110] | Phase 3 RCT; n ≈ 140 | 40 mg bid → 20 mg bid; 6 months | Placebo | No reduction in knee pain (primary endpoint) | Underpowered; concomitant medications | Late clinical (negative) |
| Nanomedicine | TA-ER (PLGA microspheres) (2018) [111] | Phase 3 RCT/analyses | IA 32 mg single dose; follow-up to 24 weeks | Saline placebo/TA crystalline | Greater, durable pain relief vs. comparators | Symptomatic steroid; generalizability; post hoc data | Late clinical |
| Nanomedicine | Diclofenac etalhyaluronate (2021) [112] | Phase 2/3 RCTs | IA 30 mg every 4 weeks; 12–24 weeks | Placebo | ↓ WOMAC pain vs. placebo | Regional development; long-term safety pending | Late clinical |
| Nanomedicine | MSC-derived extracellular vesicles (2024) [113] | RCT, triple-blind | IA EVs (protocol-defined); short-term follow-up | Saline placebo | Improved pain/function; acceptable short-term safety | Early phase: manufacturing/standardization challenges | Early clinical |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vélez-Slimani, H.; Hernández-Montelongo, J.; Salazar, L.A. Recent Developments in Osteoarthritis Research: Innovative Therapeutic Approaches and the Role of Polyphenols and Nanotechnology. Int. J. Mol. Sci. 2025, 26, 8925. https://doi.org/10.3390/ijms26188925
Vélez-Slimani H, Hernández-Montelongo J, Salazar LA. Recent Developments in Osteoarthritis Research: Innovative Therapeutic Approaches and the Role of Polyphenols and Nanotechnology. International Journal of Molecular Sciences. 2025; 26(18):8925. https://doi.org/10.3390/ijms26188925
Chicago/Turabian StyleVélez-Slimani, Humberto, Jacobo Hernández-Montelongo, and Luis A. Salazar. 2025. "Recent Developments in Osteoarthritis Research: Innovative Therapeutic Approaches and the Role of Polyphenols and Nanotechnology" International Journal of Molecular Sciences 26, no. 18: 8925. https://doi.org/10.3390/ijms26188925
APA StyleVélez-Slimani, H., Hernández-Montelongo, J., & Salazar, L. A. (2025). Recent Developments in Osteoarthritis Research: Innovative Therapeutic Approaches and the Role of Polyphenols and Nanotechnology. International Journal of Molecular Sciences, 26(18), 8925. https://doi.org/10.3390/ijms26188925







