Initial Body Weight as an Important Factor for Improving the Reliability and Translational Relevance of the Preclinical Monocrotaline-Induced Rat Pulmonary Hypertension Model
Abstract
1. Introduction
2. Results
2.1. General
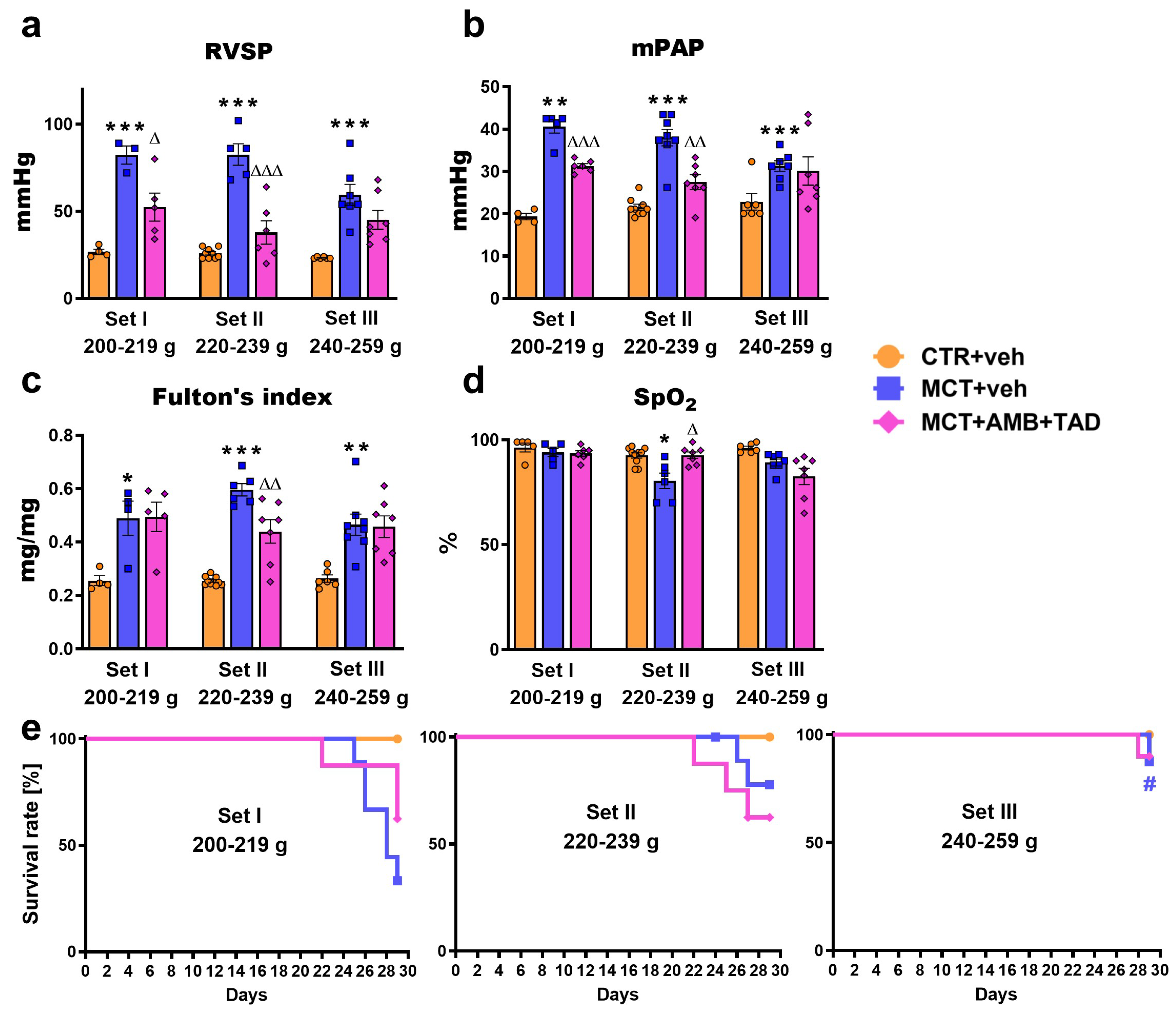
2.2. Influence of PH and Drug Therapy on RVSP, mPAP, Fulton’s Index, Blood Oxygen Saturation, and Survival Rates
2.3. Influence of PH and Drug Therapy on RV and RA Hypertrophy
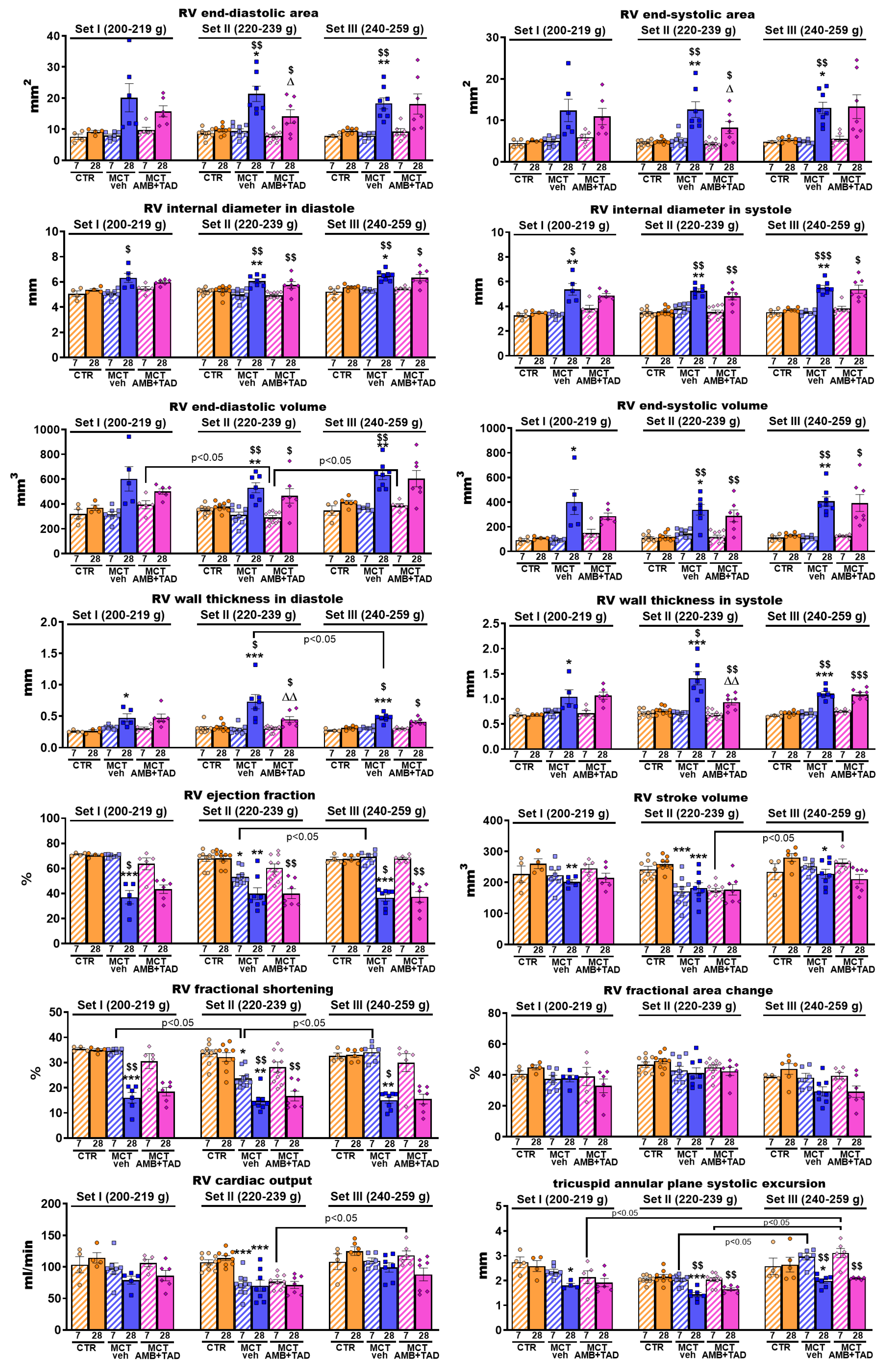
2.4. Influence of PH and Drug Therapy on RV Function
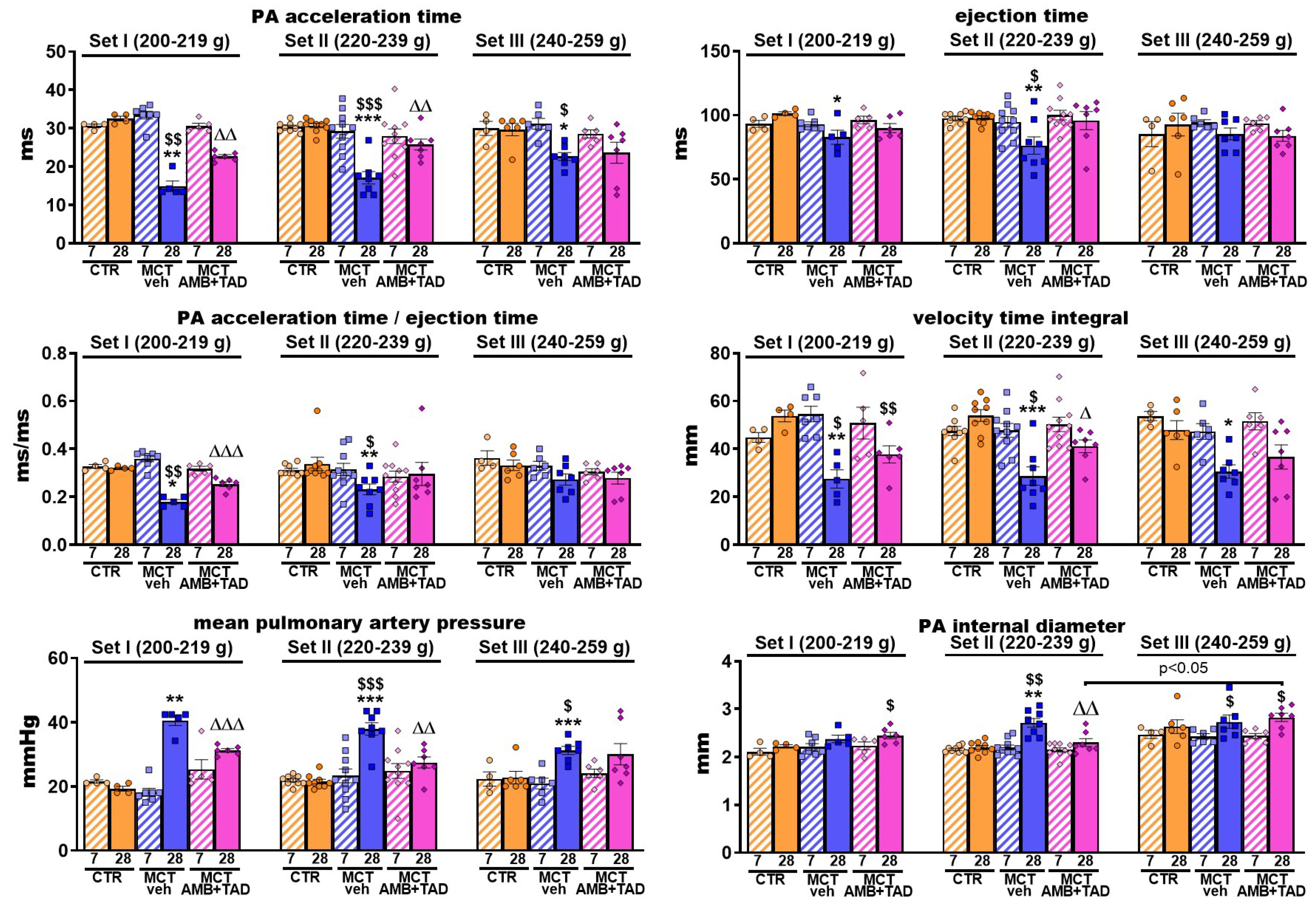
2.5. Influence of PH and Drug Therapy on PA-Related Echocardiographic Parameters
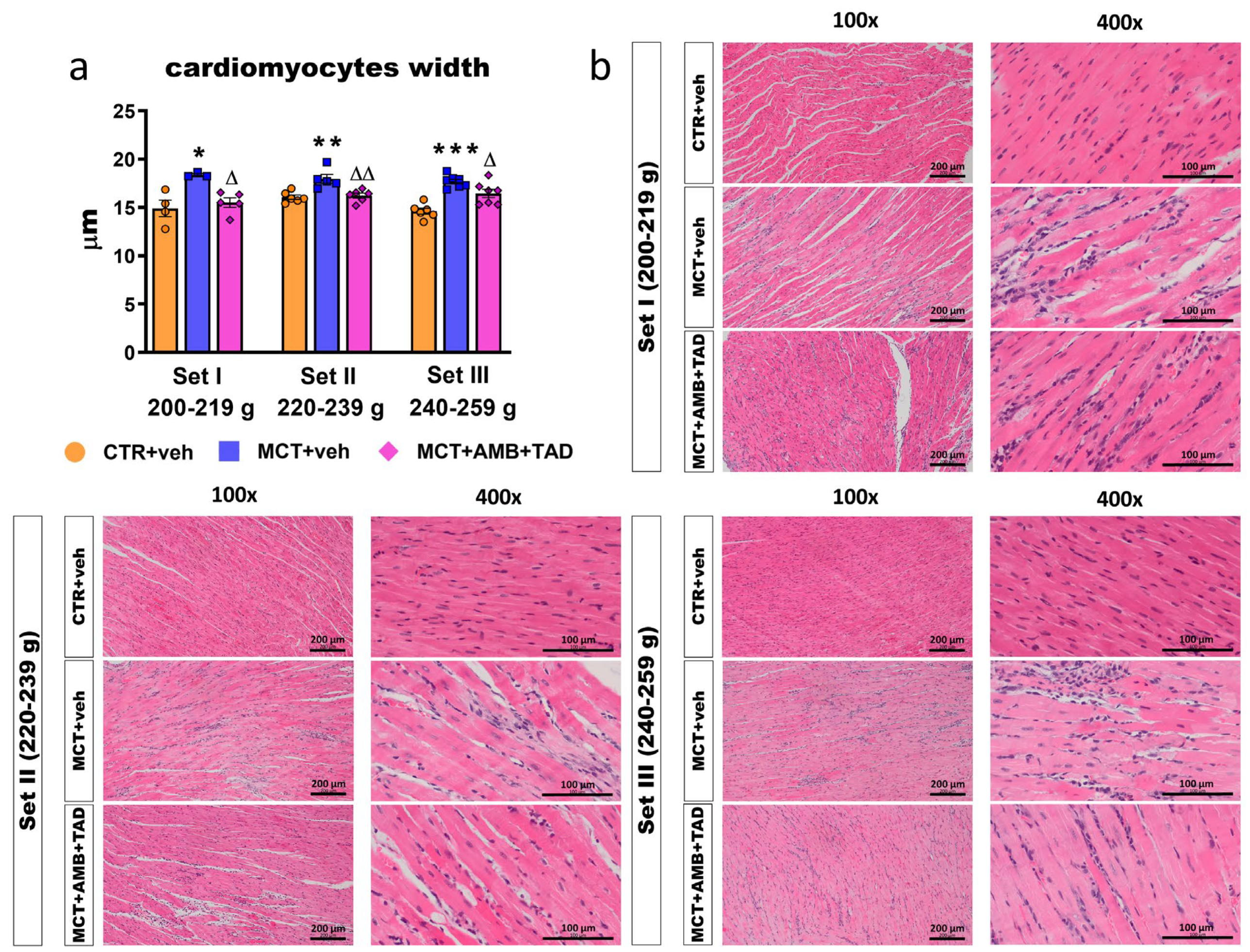
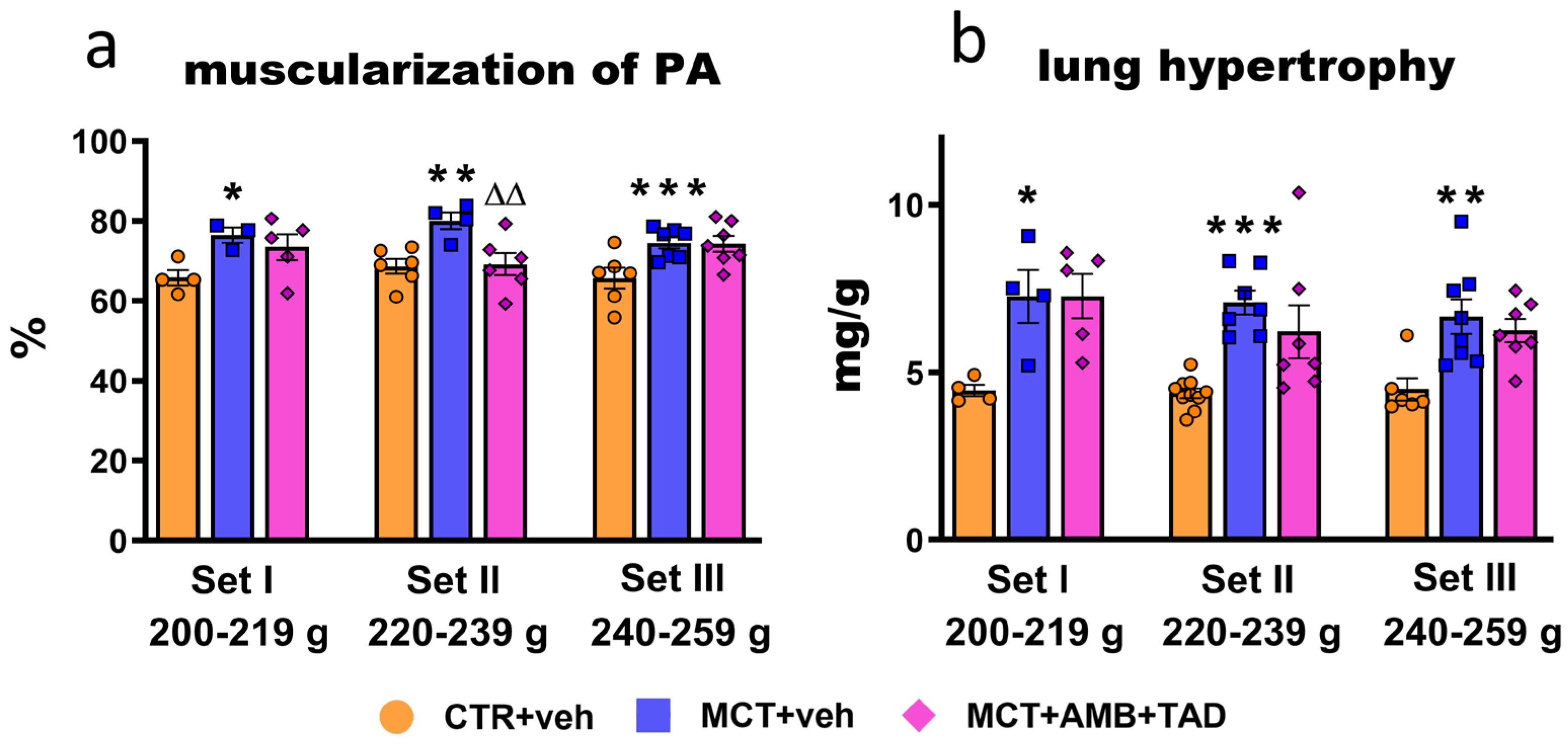
2.6. Influence of PH and Drug Therapy on Pulmonary Vascular Remodeling and Lung Hypertrophy
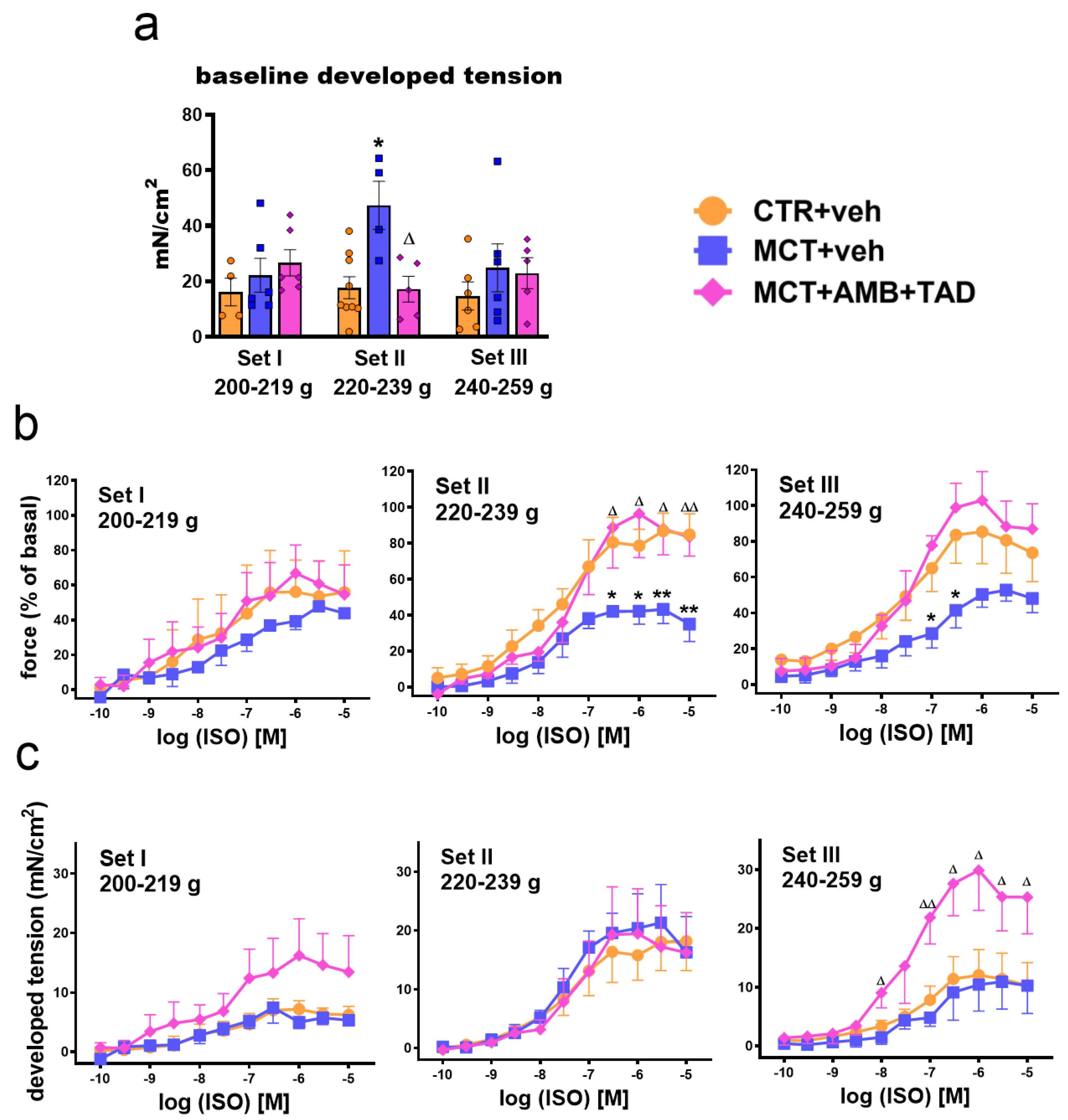
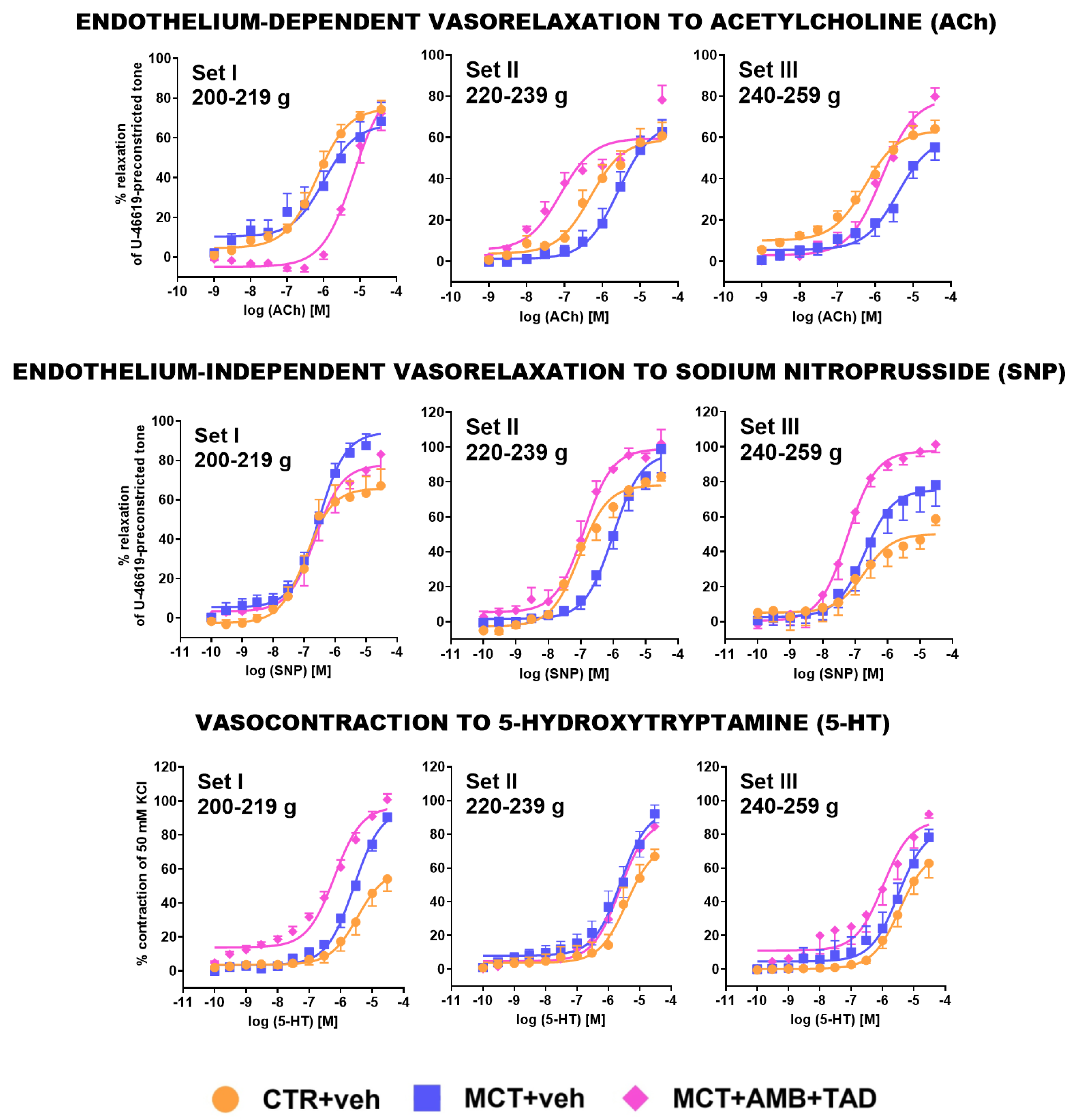
2.7. Influence of PH and Drug Therapy on Isolated PA Functional Studies
2.8. Influence of PH and Drug Therapy on Parameters Not Related Directly to PH
3. Discussion
3.1. General
3.2. Initial Rat BW Affects the Severity of MCT-Induced PH
3.3. Initial Rat BW Affects the Effectiveness of the Reference Therapy in MCT-Induced PH Model
3.4. Limitations of the Study
4. Materials and Methods
4.1. Animals
4.2. Protocol and Experimental Groups
4.3. Echocardiographic Measurements
4.4. Determination of Blood Oxygen Saturation
4.5. Determination of Parameters in Tail-Tip Blood Samples
4.6. Determination of Right Ventricular Systolic Pressure
4.7. Determination of Organ Weight and Hypertrophy Indices
4.8. Functional Studies on Isolated Papillary Muscles
4.9. Preparation of Pulmonary Arteries
4.10. Functional Studies on Isolated PAs
4.11. Histopathology
4.12. Statistical Analysis
4.13. Drugs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 5-HT | 5-Hydroxytryptamine |
| ACh | Acetylcholine |
| AMB | Ambrisentan |
| ANOVA | Analysis of variance |
| BW | Body weight |
| CO | Cardiac output |
| CRC | Concentration-response curve |
| CTR | Control |
| dP/dtmax | The rate of rise in right ventricular pressure |
| dP/dtmin | The rate of decrease in right ventricular pressure |
| EDA | End-diastolic area |
| EDV | End-diastolic volume |
| EF | Ejection fraction |
| Emax | Maximum effect |
| ESA | End-systolic area |
| ESV | End-systolic volume |
| ET | Ejection time |
| ET-1 | Endothelin 1 |
| FAC | Fractional area change |
| FS | Fractional shortening |
| HE | Hematoxylin and eosin |
| HR | Heart rate |
| IDD | Internal diameter in diastole |
| IDS | Internal diameter in systole |
| ISO | Isoprenaline |
| LA | Left atrium |
| LV | Left ventricle |
| LV + S | Left ventricle with septum |
| LVWTd | Left ventricular wall thickness in diastole |
| LVWTs | Left ventricular wall thickness in systole |
| MCT | Monocrotaline |
| mPAP | Mean aulmonary artery pressure |
| PA | Pulmonary artery |
| PAAT | Pulmonary artery acceleration time |
| PAH | Pulmonary arterial hypertension |
| pEC50 | Negative logarithm of the concentration causing the half-maximum effect |
| PH | Pulmonary hypertension |
| PDE-5 | Phosphodiesterase 5 |
| RA | Right atrium |
| RV | Right ventricle |
| RVOT | Right ventricular outflow tract |
| RVSP | Right ventricular systolic pressure |
| RVWTd | Right ventricular wall thickness in diastole |
| RVWTs | Right ventricular wall thickness in systole |
| SEM | Standard error of the mean |
| SNP | Sodium nitroprusside |
| SpO2 | Blood oxygen saturation |
| SV | Stroke volume |
| TAD | Tadalafil |
| TAPSE | Tricuspid annular plane systolic excursion |
| TL | Tibia length |
| veh | Vehicle |
| VTI | Velocity time integral |
References
- Mocumbi, A.; Humbert, M.; Saxena, A.; Jing, Z.C.; Sliwa, K.; Thienemann, F.; Archer, S.L.; Stewart, S. Pulmonary hypertension. Nat. Rev. Dis. Primers 2024, 10, 1. [Google Scholar] [CrossRef] [PubMed]
- Christou, H.; Khalil, R.A. Mechanisms of pulmonary vascular dysfunction in pulmonary hypertension and implications for novel therapies. Am. J. Physiol. Heart Circ. Physiol. 2022, 322, H702–H724. [Google Scholar] [CrossRef] [PubMed]
- Reinders, S.; Didden, E.M.; Ong, R. Survival, morbidity, and quality of life in pulmonary arterial hypertension patients: A systematic review of outcomes reported by population-based observational studies. Respir. Res. 2024, 25, 373. [Google Scholar] [CrossRef] [PubMed]
- Ghofrani, H.A.; Gomberg-Maitland, M.; Zhao, L.; Grimminger, F. Mechanisms and treatment of pulmonary arterial hypertension. Nat. Rev. Cardiol. 2025, 22, 105–120. [Google Scholar] [CrossRef]
- Chin, K.M.; Gaine, S.P.; Gerges, C.; Jing, Z.C.; Mathai, S.C.; Tamura, Y.; McLaughlin, V.V.; Sitbon, O. Treatment algorithm for pulmonary arterial hypertension. Eur. Respir. J. 2024, 64, 2401325. [Google Scholar] [CrossRef]
- Tello, K.; Seeger, W.; Naeije, R.; Vanderpool, R.; Ghofrani, H.A.; Richter, M.; Tedford, R.J.; Bogaard, H.J. Right heart failure in pulmonary hypertension: Diagnosis and new perspectives on vascular and direct right ventricular treatment. Br. J. Pharmacol. 2021, 178, 90–107. [Google Scholar] [CrossRef]
- Humbert, M.; Sitbon, O.; Guignabert, C.; Savale, L.; Boucly, A.; Gallant-Dewavrin, M.; McLaughlin, V.; Hoeper, M.M.; Weatherald, J. Treatment of pulmonary arterial hypertension: Recent progress and a look to the future. Lancet Respir. Med. 2023, 11, 804–819. [Google Scholar] [CrossRef]
- Boucherat, O.; Agrawal, V.; Lawrie, A.; Bonnet, S. The latest in animal models of pulmonary hypertension and right ventricular failure. Circ. Res. 2022, 130, 1466–1486. [Google Scholar] [CrossRef]
- Dignam, J.P.; Scott, T.E.; Kemp-Harper, B.K.; Hobbs, A.J. Animal models of pulmonary hypertension: Getting to the heart of the problem. Br. J. Pharmacol. 2022, 179, 811–837. [Google Scholar] [CrossRef]
- Wu, X.H.; Ma, J.L.; Ding, D.; Ma, Y.J.; Wei, Y.P.; Jing, Z.C. Experimental animal models of pulmonary hypertension: Development and challenges. Anim. Model. Exp. Med. 2022, 5, 207–216. [Google Scholar] [CrossRef]
- Gomez-Arroyo, J.G.; Farkas, L.; Alhussaini, A.A.; Farkas, D.; Kraskauskas, D.; Voelkel, N.F.; Bogaard, H.J. The monocrotaline model of pulmonary hypertension in perspective. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L363–L369. [Google Scholar] [CrossRef] [PubMed]
- Fröhlich, E. Animals in respiratory research. Int. J. Mol. Sci. 2024, 25, 2903. [Google Scholar] [CrossRef] [PubMed]
- Sztuka, K.; Jasińska-Stroschein, M. Animal models of pulmonary arterial hypertension: A systematic review and meta-analysis of data from 6126 animals. Pharmacol. Res. 2017, 125, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Jasińska-Stroschein, M. An updated review of experimental rodent models of pulmonary hypertension and left heart disease. Front. Pharmacol. 2024, 14, 1308095. [Google Scholar] [CrossRef]
- Stenmark, K.R.; Meyrick, B.; Galie, N.; Mooi, W.J.; McMurtry, I.F. Animal models of pulmonary arterial hypertension: The hope for etiological discovery and pharmacological cure. Am. J. Physiol. Lung Cell Mol. Physiol. 2009, 297, L1013–L1032. [Google Scholar] [CrossRef]
- Jasińska-Stroschein, M. Toward better reproducibility in experimental research on new agents for pulmonary hypertension. An analysis of data from four hundred animal studies. Cardiovasc. Drugs Ther. 2021, 35, 707–718. [Google Scholar] [CrossRef]
- Jasińska-Stroschein, M.; Orszulak-Michalak, D. Reporting experimental studies on animals—The problems with translating of outcomes to clinical benefits. Methodological and statistical considerations: The example of pulmonary hypertension. Eur. J. Pharmacol. 2021, 897, 173952. [Google Scholar] [CrossRef]
- Errington, T.M. Building reproducible bridges to cross the “valley of death”. J. Clin. Investig. 2024, 134, e177383. [Google Scholar] [CrossRef]
- Provencher, S.; Archer, S.L.; Ramirez, F.D.; Hibbert, B.; Paulin, R.; Boucherat, O.; Lacasse, Y.; Bonnet, S. Standards and methodological rigor in pulmonary arterial hypertension preclinical and translational research. Circ. Res. 2018, 122, 1021–1032. [Google Scholar] [CrossRef]
- Kwan, E.D.; Hardie, B.A.; Garcia, K.M.; Mu, H.; Wang, T.M.; Valdez-Jasso, D. Sex-dependent remodeling of right ventricular function in a rat model of pulmonary arterial hypertension. Am. J. Physiol. Heart Circ. Physiol. 2024, 327, H351–H363. [Google Scholar] [CrossRef]
- Dignam, J.P.; Sharma, S.; Stasinopoulos, I.; MacLean, M.R. Pulmonary arterial hypertension: Sex matters. Br. J. Pharmacol. 2024, 181, 938–966. [Google Scholar] [CrossRef]
- Hester, J.; Ventetuolo, C.; Lahm, T. Sex, gender, and sex hormones in pulmonary hypertension and right ventricular failure. Compr. Physiol. 2019, 10, 125–170. [Google Scholar] [CrossRef] [PubMed]
- Bueno-Beti, C.; Sassi, Y.; Hajjar, R.J.; Hadri, L. Pulmonary artery hypertension model in rats by monocrotaline administration. Methods Mol. Biol. 2018, 1816, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Hill, N.S.; Gillespie, M.N.; McMurtry, I.F. Fifty years of monocrotaline-induced pulmonary hypertension: What has it meant to the field? Chest 2017, 152, 1106–1108. [Google Scholar] [CrossRef]
- Krstic, A.M.; Jones, T.L.M.; Power, A.S.; Ward, M.L. The monocrotaline rat model of right heart disease induced by pulmonary artery hypertension. Biomedicines 2024, 12, 1944. [Google Scholar] [CrossRef] [PubMed]
- Toczek, M.; Baranowska-Kuczko, M.; Grzęda, E.; Pędzińska-Betiuk, A.; Weresa, J.; Malinowska, B. Age-specific influences of chronic administration of the fatty acid amide hydrolase inhibitor URB597 on cardiovascular parameters and organ hypertrophy in DOCA-salt hypertensive rats. Pharmacol. Rep. 2016, 68, 363–369. [Google Scholar] [CrossRef]
- Remiszewski, P.; Pędzińska-Betiuk, A.; Mińczuk, K.; Schlicker, E.; Klimek, J.; Dzięcioł, J.; Malinowska, B. Effects of the peripheral CB1 receptor antagonist JD5037 in mono- and polytherapy with the AMPK activator metformin in a monocrotaline-induced rat model of pulmonary hypertension. Front. Pharmacol. 2022, 13, 965613. [Google Scholar] [CrossRef]
- Sadowska, O.; Baranowska-Kuczko, M.; Gromotowicz-Popławska, A.; Biernacki, M.; Kicman, A.; Malinowska, B.; Kasacka, I.; Krzyżewska, A.; Kozłowska, H. Cannabidiol ameliorates monocrotaline-induced pulmonary hypertension in rats. Int. J. Mol. Sci. 2020, 21, 7077. [Google Scholar] [CrossRef]
- Foderaro, A.; Ventetuolo, C.E. Pulmonary arterial hypertension and the sex hormone paradox. Curr. Hypertens. Rep. 2016, 18, 84. [Google Scholar] [CrossRef]
- Seelemann, E.R.; Panchakshari, S.; Labana, P.K.; Wolverton, M.M.; Deng, Y.; Abdelwahab, H.; Consmueller, C.; Stewart, D.J.; Chaudhary, K.R. Sexual dimorphism in right ventricular adaptation to pressure overload involves differential angiogenic response. Am. J. Physiol. Heart Circ. Physiol. 2025, 328, H496–H508. [Google Scholar] [CrossRef]
- Martin, Y.N.; Pabelick, C.M. Sex differences in the pulmonary circulation: Implications for pulmonary hypertension. Am. J. Physiol. Heart Circ. Physiol. 2014, 306, H1253–H1264. [Google Scholar] [CrossRef] [PubMed]
- Frump, A.L.; Albrecht, M.; Yakubov, B.; Breuils-Bonnet, S.; Nadeau, V.; Tremblay, E.; Potus, F.; Omura, J.; Cook, T.; Fisher, A.; et al. 17β-Estradiol and estrogen receptor α protect right ventricular function in pulmonary hypertension via BMPR2 and apelin. J. Clin. Investig. 2021, 131, e129433. [Google Scholar] [CrossRef] [PubMed]
- Galiè, N.; Barbera, J.A.; Frost, A.E.; Ghofrani, H.A.; Hoeper, M.M.; McLaughlin, V.V.; Peacock, A.J.; Simonneau, G.; Vachiery, J.L.; Grünig, E.; et al. Initial use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N. Engl. J. Med. 2015, 373, 834–844. [Google Scholar] [CrossRef] [PubMed]
- Cavasin, M.A.; Demos-Davies, K.M.; Schuetze, K.B.; Blakeslee, W.W.; Stratton, M.S.; Tuder, R.M.; McKinsey, T.A. Reversal of severe angioproliferative pulmonary arterial hypertension and right ventricular hypertrophy by combined phosphodiesterase-5 and endothelin receptor inhibition. J. Transl. Med. 2014, 12, 314. [Google Scholar] [CrossRef]
- Aiello, R.J.; Bourassa, P.A.; Zhang, Q.; Dubins, J.; Goldberg, D.R.; De Lombaert, S.; Humbert, M.; Guignabert, C.; Cavasin, M.A.; McKinsey, T.A.; et al. Tryptophan hydroxylase 1 inhibition impacts pulmonary vascular remodeling in two rat models of pulmonary hypertension. J. Pharmacol. Exp. Ther. 2017, 360, 267–279. [Google Scholar] [CrossRef]
- Lemay, S.E.; Montesinos, M.S.; Grobs, Y.; Yokokawa, T.; Shimauchi, T.; Mougin, M.; Romanet, C.; Sauvaget, M.; Breuils-Bonnet, S.; Bourgeois, A.; et al. Exploring integrin α5β1 as a potential therapeutic target for pulmonary arterial hypertension: Insights from comprehensive multicenter preclinical studies. Circulation 2025, 151, 1162–1183. [Google Scholar] [CrossRef]
- Tsuboya, N.; Sawada, H.; Mitani, Y.; Oshita, H.; Ohya, K.; Takeoka, M.; Kabwe, J.C.; Miyasaka, Y.; Ito, H.; Yodoya, N.; et al. C-C Motif chemokine receptor-2 blockade ameliorates pulmonary hypertension in rats and synergizes with a pulmonary vasodilator. Cardiovasc. Res. 2025, 121, 1076–1090. [Google Scholar] [CrossRef]
- Kabwe, J.C.; Sawada, H.; Mitani, Y.; Oshita, H.; Tsuboya, N.; Zhang, E.; Maruyama, J.; Miyasaka, Y.; Ko, H.; Oya, K.; et al. CRISPR-mediated Bmpr2 point mutation exacerbates late pulmonary vasculopathy and reduces survival in rats with experimental pulmonary hypertension. Respir. Res. 2022, 23, 87. [Google Scholar] [CrossRef]
- Mamazhakypov, A.; Weiß, A.; Zukunft, S.; Sydykov, A.; Kojonazarov, B.; Wilhelm, J.; Vroom, C.; Petrovic, A.; Kosanovic, D.; Weissmann, N.; et al. Effects of macitentan and tadalafil monotherapy or their combination on the right ventricle and plasma metabolites in pulmonary hypertensive rats. Pulm. Circ. 2020, 10, 2045894020947283. [Google Scholar] [CrossRef]
- Souza, N.S.C.; Barenco-Marins, T.; Ferraz, A.P.; Barbosa, R.A.Q.; Maciel, L.; Ponte, C.G.; Seara, F.A.C.; Olivares, E.L.; Nascimento, J.H.M. Low thyroid hormones level attenuates mitochondrial dysfunction and right ventricular failure in pulmonary hypertensive rats. Cardiovasc. Drugs Ther. 2024. ahead of print. [Google Scholar] [CrossRef]
- Becker, C.U.; Sartório, C.L.; Campos-Carraro, C.; Siqueira, R.; Colombo, R.; Zimmer, A.; Belló-Klein, A. Exercise training decreases oxidative stress in skeletal muscle of rats with pulmonary arterial hypertension. Arch. Physiol. Biochem. 2022, 128, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Malikova, E.; Carlström, M.; Kmecova, Z.; Marusakova, M.; Zsigmondova, B.; Krenek, P.; Klimas, J.; Henrohn, D. Effects of inorganic nitrate in a rat model of monocrotaline-induced pulmonary arterial hypertension. Basic Clin. Pharmacol. Toxicol. 2020, 126, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Videja, M.; Vilskersts, R.; Korzh, S.; Cirule, H.; Sevostjanovs, E.; Dambrova, M.; Makrecka-Kuka, M. Microbiota-derived metabolite trimethylamine N-oxide protects mitochondrial energy metabolism and cardiac functionality in a rat model of right ventricle heart failure. Front. Cell Dev. Biol. 2021, 8, 622741. [Google Scholar] [CrossRef] [PubMed]
- Hołda, M.K.; Stachowicz, A.; Suski, M.; Wojtysiak, D.; Sowińska, N.; Arent, Z.; Palka, N.; Podolec, P.; Kopeć, G. Myocardial proteomic profile in pulmonary arterial hypertension. Sci. Rep. 2020, 10, 14351. [Google Scholar] [CrossRef]
- Hołda, M.K.; Szczepanek, E.; Bielawska, J.; Palka, N.; Wojtysiak, D.; Frączek, P.; Nowakowski, M.; Sowińska, N.; Arent, Z.; Podolec, P.; et al. Changes in heart morphometric parameters over the course of a monocrotaline-induced pulmonary arterial hypertension rat model. J. Transl. Med. 2020, 18, 262. [Google Scholar] [CrossRef]
- Hołda, M.K.; Raźny, U.; Sordyl, M.; Góralska, J.; Kapusta, M.; Słowińska-Solnica, K.; Wojtysiak, D.; Lis, G.; Solnica, B.; Kopeć, G.; et al. Autophagy and ubiquitin-dependent proteolysis processes in left ventricular mass loss in pulmonary arterial hypertension. Sci. Rep. 2024, 14, 15133. [Google Scholar] [CrossRef]
- Braga, C.L.; Santos, R.T.; da Silva, C.M.; de Novaes Rocha, N.; Felix, N.S.; Medeiros, M.; Melo, M.M.; Silva, J.D.; Teixeira, D.E.; Neves, C.C.; et al. Therapeutic effects of hypoxia-preconditioned bone marrow-derived mesenchymal stromal cells and their extracellular vesicles in experimental pulmonary arterial hypertension. Life Sci. 2023, 329, 121988. [Google Scholar] [CrossRef]
- Silva, A.F.; Faria-Costa, G.; Sousa-Nunes, F.; Santos, M.F.; Ferreira-Pinto, M.J.; Duarte, D.; Rodrigues, I.; Tiago Guimarães, J.; Leite-Moreira, A.; Moreira-Gonçalves, D.; et al. Anti-remodeling effects of xanthohumol-fortified beer in pulmonary arterial hypertension mediated by ERK and AKT inhibition. Nutrients 2019, 11, 583. [Google Scholar] [CrossRef]
- Kawade, A.; Yamamura, A.; Fujiwara, M.; Kobayashi, S.; Mori, S.; Horii, C.; Hiraku, A.; Suzumura, S.; Tsukamoto, K.; Ohara, N.; et al. Comparative analysis of age in monocrotaline-induced pulmonary hypertensive rats. J. Pharmacol. Sci. 2021, 147, 81–85. [Google Scholar] [CrossRef]
- Cuijpers, I.; Carai, P.; Mendes-Ferreira, P.; Simmonds, S.J.; Mulder, P.; Miranda-Silva, D.; De Giorgio, D.; Pokreisz, P.; Heymans, S.; Jones, E.A.V. The effect of different anaesthetics on echocardiographic evaluation of diastolic dysfunction in a heart failure with preserved ejection fraction model. Sci. Rep. 2020, 10, 15701. [Google Scholar] [CrossRef]
- Logantha, S.; Yamanushi, T.T.; Absi, M.; Temple, I.P.; Kabuto, H.; Hirakawa, E.; Quigley, G.; Zhang, X.; Gurney, A.M.; Hart, G.; et al. Remodelling and dysfunction of the sinus node in pulmonary arterial hypertension. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2023, 378, 20220178. [Google Scholar] [CrossRef] [PubMed]
- Han, J.C.; Guild, S.J.; Pham, T.; Nisbet, L.; Tran, K.; Taberner, A.J.; Loiselle, D.S. Left-ventricular energetics in pulmonary arterial hypertension-induced right-ventricular hypertrophic failure. Front. Physiol. 2017, 8, 1115. [Google Scholar] [CrossRef] [PubMed]
- Absi, M.; Eid, B.G.; Ashton, N.; Hart, G.; Gurney, A.M. Simvastatin causes pulmonary artery relaxation by blocking smooth muscle ROCK and calcium channels: Evidence for an endothelium-independent mechanism. PLoS ONE 2019, 14, e0220473. [Google Scholar] [CrossRef] [PubMed]
- Badagliacca, R.; Mercurio, V.; Romeo, E.; Correale, M.; Masarone, D.; Papa, S.; Tocchetti, C.G.; Agostoni, P.; Members of the Study Group on Right and Left Heart Failure of the Italian Society of Cardiology. Beta-blockers in pulmonary arterial hypertension: Time for a second thought? Vasc. Pharmacol. 2022, 144, 106974. [Google Scholar] [CrossRef]
- Frede, W.; Medert, R.; Poth, T.; Gorenflo, M.; Vennekens, R.; Freichel, M.; Uhl, S. TRPM4 modulates right ventricular remodeling under pressure load accompanied with decreased expression level. J. Card. Fail. 2020, 26, 599–609. [Google Scholar] [CrossRef]
- Kögler, H.; Hartmann, O.; Leineweber, K.; Nguyen van, P.; Schott, P.; Brodde, O.E.; Hasenfuss, G. Mechanical load-dependent regulation of gene expression in monocrotaline-induced right ventricular hypertrophy in the rat. Circ. Res. 2003, 93, 230–237. [Google Scholar] [CrossRef]
- Leineweber, K.; Seyfarth, T.; Abraham, G.; Gerbershagen, H.P.; Heinroth-Hoffmann, I.; Pönicke, K.; Brodde, O.E. Cardiac β-adrenoceptor changes in monocrotaline-treated rats: Differences between membrane preparations from whole ventricles and isolated ventricular cardiomyocytes. J. Cardiovasc. Pharmacol. 2003, 41, 333–342. [Google Scholar] [CrossRef]
- Zimmer, A.; Teixeira, R.B.; Constantin, R.L.; Campos-Carraro, C.; Aparicio Cordero, E.A.; Ortiz, V.D.; Donatti, L.; Gonzalez, E.; Bahr, A.C.; Visioli, F.; et al. The progression of pulmonary arterial hypertension induced by monocrotaline is characterized by lung nitrosative and oxidative stress, and impaired pulmonary artery reactivity. Eur. J. Pharmacol. 2021, 891, 173699. [Google Scholar] [CrossRef]
- Adão, R.; Mendes-Ferreira, P.; Santos-Ribeiro, D.; Maia-Rocha, C.; Pimentel, L.D.; Monteiro-Pinto, C.; Mulvaney, E.P.; Reid, H.M.; Kinsella, B.T.; Potus, F.; et al. Urocortin-2 improves right ventricular function and attenuates pulmonary arterial hypertension. Cardiovasc. Res. 2018, 114, 1165–1177. [Google Scholar] [CrossRef]
- Wanstall, J.C.; O’Donnell, S.R. Endothelin and 5-hydroxytryptamine on rat pulmonary artery in pulmonary hypertension. Eur. J. Pharmacol. 1990, 176, 159–168. [Google Scholar] [CrossRef]
- Sirmagul, B.; Ilgin, S.; Atli, O.; Usanmaz, S.E.; Demirel-Yilmaz, E. Assessment of the endothelial functions in monocrotaline-induced pulmonary hypertension. Clin. Exp. Hypertens. 2013, 35, 220–227. [Google Scholar] [CrossRef]
- Christou, H.; Reslan, O.M.; Mam, V.; Tanbe, A.F.; Vitali, S.H.; Touma, M.; Arons, E.; Mitsialis, S.A.; Kourembanas, S.; Khalil, R.A. Improved pulmonary vascular reactivity and decreased hypertrophic remodeling during nonhypercapnic acidosis in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 302, L875–L890. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, B.E.; McClelland, R.L.; Appleby, D.H.; Moutchia, J.S.; Minhas, J.K.; Min, J.; Mazurek, J.A.; Smith, K.A.; Fritz, J.S.; Pugliese, S.C.; et al. BMI and treatment response in patients with pulmonary arterial hypertension: A meta-analysis. Chest 2022, 162, 436–447. [Google Scholar] [CrossRef] [PubMed]
- Oppegard, L.J.; Barros, L.M.; Pi, H.; Kornfield, J.; Hough, C.L.; Rayner, S.G.; Robinson, J.C.; Leary, P.J. Premorbid weight in pulmonary arterial hypertension. Pulm. Circ. 2023, 13, e12308. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Osano, A.; Hayashi, H.; Itoh, K.; Okura, T.; Deguchi, Y.; Ito, Y.; Yamada, S. Endothelin-1 receptors in rat tissues: Characterization by bosentan, ambrisentan and CI-1020. Biol. Pharm. Bull. 2014, 37, 461–465. [Google Scholar] [CrossRef]
- Hutchings, D.C.; Anderson, S.G.; Caldwell, J.L.; Trafford, A.W. Phosphodiesterase-5 inhibitors and the heart: Compound cardioprotection? Heart 2018, 104, 1244–1250. [Google Scholar] [CrossRef]
- Lachant, D.J.; Meoli, D.F.; Haight, D.; Staicu, S.; Akers, S.; Glickman, S.; Ambrosini, R.; Champion, H.C.; White, R.J. Combination therapy improves vascular volume in female rats with pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 317, L445–L455. [Google Scholar] [CrossRef]
- Liang, F.; Yang, S.; Yao, L.; Belardinelli, L.; Shryock, J. Ambrisentan and tadalafil synergistically relax endothelin-induced contraction of rat pulmonary arteries. Hypertension 2012, 59, 705–711. [Google Scholar] [CrossRef]
- Iyinikkel, J.; Murray, F. GPCRs in pulmonary arterial hypertension: Tipping the balance. Br. J. Pharmacol. 2018, 175, 3063–3079. [Google Scholar] [CrossRef]
- Urboniene, D.; Haber, I.; Fang, Y.H.; Thenappan, T.; Archer, S.L. Validation of high-resolution echocardiography and magnetic resonance imaging vs. high-fidelity catheterization in experimental pulmonary hypertension. Am. J. Physiol. Lung Cell Mol. Physiol. 2010, 299, L401–L412. [Google Scholar] [CrossRef]
- Pędzińska-Betiuk, A.; Gergs, U.; Weresa, J.; Remiszewski, P.; Harasim-Symbor, E.; Malinowska, B. Comparison of cardioprotective potential of cannabidiol and β-adrenergic stimulation against hypoxia/reoxygenation injury in rat atria and ventricular papillary muscles. Pharmaceuticals 2024, 17, 1379. [Google Scholar] [CrossRef]
- Mellors, L.J.; Barclay, C.J. The energetics of rat papillary muscles undergoing realistic strain patterns. J. Exp. Biol. 2001, 204, 3765–3777. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Tracy, R.E.; Sander, G.E. Histologically measured cardiomyocyte hypertrophy correlates with body height as strongly as with body mass index. Cardiol. Res. Pract. 2011, 2011, 658958. [Google Scholar] [CrossRef]
- Curtis, M.J.; Alexander, S.; Cirino, G.; Docherty, J.R.; George, C.H.; Giembycz, M.A.; Hoyer, D.; Insel, P.A.; Izzo, A.A.; Ji, Y.; et al. Experimental design and analysis and their reporting II: Updated and simplified guidance for authors and peer reviewers. Br. J. Pharmacol. 2018, 175, 987–993. [Google Scholar] [CrossRef]
- De Castro, A.L.; Turck, P.; Tavares, A.M.V.; Gaspar, C.J.; Bahr, A.; Zimmer, A.; Fernandes, T.R.G.; Fernandes, R.O.; Ortiz, V.D.; Tasca, S.; et al. Evaluation of the use of the myocardial performance index as a parameter of cardiac function in two experimental models of heart disease: Myocardial infarction and pulmonary hypertension. Pflugers Arch. 2025. ahead of print. [Google Scholar] [CrossRef] [PubMed]
| Set I 200–219 g | Set II 220–239 g | Set III 240–259 g | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CTR + veh | MCT + veh | MCT + AMB + TAD | CTR + veh | MCT + veh | MCT + AMB + TAD | CTR + veh | MCT + veh | MCT + AMB + TAD | ||
| n | 4 | 3–9 | 5–8 | 9–10 | 5–9 | 6–10 | 6 | 7–8 | 7–8 | |
| Body weight (BW) (g) | day 0 | 216 ± 2 | 213 ± 1 | 211 ± 2 | 228 ± 2 | 229 ± 2 ### | 224 ± 1 ### | 250 ± 3 ### @ | 252 ± 2 ### @@@ | 250 ± 1 ### @@@ |
| day 29 | 335 ± 9 | 284 ± 17 * $ | 279 ± 6 $$$ | 319 ± 5 $$$ | 282 ± 10 ** $$ | 313 ± 10 Δ $$$ | 339 ± 3 $$$ | 296 ± 6 *** $$$ | 308 ± 10 $$ | |
| Tibia length (TL) (mm) | 37.3 ± 0.1 | 36.2 ± 0.4 | 36.2 ± 0.3 | 36.8 ± 0.2 | 36.8 ± 0.4 | 37.4 ± 0.2 ## | 37.1 ± 0.1 | 36.7 ± 0.2 | 37.2 ± 0.2 # | |
| Heart rate (beats/min) | by echo (day 28) | 439 ± 13 | 389 ± 24 | 398 ± 16 | 440 ± 6 | 377 ± 23 * | 410 ± 16 | 446 ± 10 | 429 ± 10 | 410 ± 24 |
| by catheter | 290 ± 17 °°° | 317 ± 38 | 300 ± 24 | 279 ± 10 °°° | 274 ± 20 ° | 282 ± 9 °°° | 307 ± 18 °°° | 271 ± 16 °°° | 255 ± 26 °°° | |
| dP/dtmax (mmHg/s) | 1734 ± 107 | 3427 ± 50 ** | 2803 ± 275 | 1682 ± 51 | 3365 ± 340 *** | 2244 ± 247 ΔΔ | 1490 ± 55 | 2733 ± 260 ** | 2183 ± 298 | |
| dP/dtmin (mmHg/s) | −1328 ± 117 | −2520 ± 50 *** | −1805 ± 120 ΔΔ | −1171 ± 43 | −2547 ± 231 *** | −1561 ± 189 ΔΔΔ | −1006 ± 56 # | −2052 ± 134 *** | −1579 ± 201 | |
| Heart weight (mg) | 1176 ± 44 | 1206 ± 80 | 1207 ± 65 | 1037 ± 41 | 1434 ± 42 *** | 1378 ± 93 | 1067 ± 48 | 1170 ± 54 | 1253 ± 37 | |
| Heart weight/BW (mg/g) | 3.5 ± 0.1 | 4.5 ± 0.4 | 4.3 ± 0.3 | 3.2 ± 0.1 | 5.1 ± 0.3 *** | 4.5 ± 0.4 | 3.1 ± 0.1 | 4.0 ± 0.2 | 4.1 ± 0.2 | |
| Heart weight/TL (mg/mm) | 32 ± 1 | 34 ± 2 | 33 ± 2 | 28 ± 1 | 39 ± 1 *** | 37 ± 2 | 29 ± 1 | 32 ± 1 | 34 ± 1 | |
| RV weight (mg) | 171 ± 14 | 324 ± 22 | 270 ± 30 | 151 ± 6 | 361 ± 13 *** | 277 ± 28 ΔΔ | 159 ± 6 | 272 ± 27 | 287 ± 24 | |
| RV weight/BW (mg/g) | 0.51 ± 0.03 | 1.05 ± 0.14 * | 1.02 ± 0.12 | 0.47 ± 0.01 | 1.32 ± 0.08 *** | 0.90 ± 0.10 | 0.47 ± 0.02 | 0.93 ± 0.10 ** @ | 0.95 ± 0.11 | |
| RV weight/TL (mg/mm) | 4.6 ± 0.4 | 8.1 ± 1.0 * | 7.8 ± 0.8 | 4.1 ± 0.1 | 9.8 ± 0.4 *** | 7.4 ± 0.7 ΔΔ | 4.3 ± 0.2 | 7.4 ± 0.7 ** | 7.7 ± 0.6 | |
| RA weight (mg) | 54 ± 6 | 66 ± 17 | 47 ± 3 | 52 ± 5 | 87 ± 15 | 69 ± 11 | 44 ± 4 | 52 ± 6 | 57 ± 7 | |
| RA weight/BW (mg/g) | 0.16 ± 0.02 | 0.23 ± 0.06 | 0.16 ± 0.02 | 0.16 ± 0.01 | 0.32 ± 0.07 | 0.22 ± 0.04 | 0.13 ± 0.01 | 0.18 ± 0.02 | 0.19 ± 0.03 | |
| RA weight/TL (mg/mm) | 1.4 ± 0.2 | 1.7 ± 0.3 | 1.3 ± 0.1 | 1.4 ± 0.1 | 2.4 ± 0.4 | 1.8 ± 0.3 | 1.2 ± 0.1 | 1.4 ± 0.2 | 1.5 ± 0.2 | |
| Influence of MCT in Comparison to Respective Control | Influence of AMB + TAD on the MCT-Induced PH | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Set I 200–219 g | Set II 220–239 g | Set III 240–259 g | Set I 200–219 g | Set II 220–239 g | Set III 240–259 g | |||||||||
| BW gain | ↓ | 40 | ↓ | 42 | ↓ | 51 | ↔ | ↑ | 68 | ↔ | ||||
| Mortality [%] | 67 | 22 | 13 | 38 | 38 | 10 | ||||||||
| RVSP | ↑ | 208 | ↑ | 219 | ↑ | 155 | ↓ | 36 | ↓ | 54 | ↔ | |||
| mPAP | ↑ | 110 | ↑ | 76 | ↑ | 37 | ↓ | 23 | ↓ | 28 | ↔ | |||
| SpO2 | ↔ | ↓ | 13 | ↔ | ↔ | ↑ | 15 | ↔ | ||||||
| Heart hypertrophy | Fulton’s index | ↑ | 92 | ↑ | 135 | ↑ | 77 | ↔ | ↓ | 26 | ↔ | |||
| Heart weight | ↔ | ↑ | 38 | ↔ | ↔ | ↔ | ↔ | |||||||
| Heart weight/BW | ↑ | ns | ↑ | 59 | ↑ | ns | ↔ | ↓ | ns | ↔ | ||||
| Heart weight/TL | ↔ | ↑ | 39 | ↔ | ↔ | ↔ | ↔ | |||||||
| RV weight | ↑ | ns | ↑ | 139 | ↑ | ns | ↓ | ns | ↓ | 23 | ↔ | |||
| RV weight/BW | ↑ | 106 | ↑ | 181 | ↑ | 98 | ↔ | ↓ | ns | ↔ | ||||
| RV weight/TL | ↑ | 76 | ↑ | 139 | ↑ | 72 | ↔ | ↓ | 24 | ↔ | ||||
| RV wall thickness in diastole | ↑ | 78 | ↑ | 124 | ↑ | 53 | ↔ | ↓ | 38 | ↔ | ||||
| RV wall thickness in systole | ↑ | 55 | ↑ | 85 | ↑ | 54 | ↔ | ↓ | 34 | ↔ | ||||
| RV cardiomyocytes width | ↑ | 23 | ↑ | 12 | ↑ | 21 | ↓ | 16 | ↓ | 10 | ↓ | 7 | ||
| RA weight | ↔ | ↑ | ns | ↔ | ↓ | ns | ↓ | ns | ↔ | |||||
| RA weight/BW | ↑ | ns | ↑ | ns | ↑ | ns | ↓ | ns | ↓ | ns | ↔ | |||
| RA weight/TL | ↔ | ↑ | ns | ↔ | ↓ | ns | ↓ | ns | ↔ | |||||
| Heart function | rise in dP/dtmax | ↑ | 97 | ↑ | 100 | ↑ | 83 | ↓ | ns | ↓ | 33 | ↓ | ns | |
| decrease in dP/dtmin | ↑ | 90 | ↑ | 118 | ↑ | 104 | ↓ | 28 | ↓ | 39 | ↓ | ns | ||
| HR (catheter) | ↔ | ↔ | ↓ | ns | ↔ | ↔ | ↔ | |||||||
| HR (echo) | ↓ | ns | ↓ | 14 | ↓ | ns | ↔ | ↔ | ↔ | |||||
| RV end-diastolic area | ↑ | ns | ↑ | 119 | ↑ | 92 | ↓ | ns | ↓ | 34 | ↔ | |||
| RV end-systolic area | ↑ | ns | ↑ | 155 | ↑ | 144 | ↔ | ↓ | 34 | ↔ | ||||
| RV fractional area change | ↓ | ns | ↓ | ns | ↓ | ns | ↔ | ↔ | ↔ | |||||
| RV internal diameter in diastole | ↑ | ns | ↑ | 15 | ↑ | 16 | ↔ | ↔ | ↔ | |||||
| RV internal diameter in systole | ↑ | 54 | ↑ | 47 | ↑ | 48 | ↔ | ↔ | ↔ | |||||
| RV fractional shortening | ↓ | 54 | ↓ | 54 | ↓ | 54 | ↔ | ↔ | ↔ | |||||
| RV end-diastolic volume | ↑ | ns | ↑ | 40 | ↑ | 53 | ↓ | ns | ↔ | ↔ | ||||
| RV end-systolic volume | ↑ | 268 | ↑ | 182 | ↑ | 204 | ↓ | ns | ↓ | ns | ↔ | |||
| RV stroke volume | ↓ | 22 | ↓ | 30 | ↓ | 19 | ↔ | ↔ | ↔ | |||||
| RV ejection fraction | ↓ | 47 | ↓ | 42 | ↓ | 46 | ↔ | ↔ | ↔ | |||||
| RV cardiac output | ↓ | ns | ↓ | 38 | ↓ | ns | ↔ | ↔ | ↔ | |||||
| TAPSE | ↓ | 30 | ↓ | 33 | ↓ | 25 | ↔ | ↔ | ↔ | |||||
| RV papillary muscles baseline developed tension | ↔ | ↑ | 167 | ↔ | ↔ | ↓ | 64 | ↔ | ||||||
| RV papillary muscles response to ISO | pEC50 (% basal and developed tension) | ↔ | ↔ | ↔ | ↔ | ↔ | ↔ | |||||||
| Emax (% basal) | ↓ | ns | ↓ | 50 | ↓ | ns | ↑ | ns | ↑ | 123 | ↑ | 87 | ||
| Emax (developed tension) | ↔ | ↔ | ↔ | ↑ | ns | ↔ | ↑ | 173 | ||||||
| Lung and PA hypertrophy and function | Lung hypertrophy | ↑ | 63 | ↑ | 62 | ↑ | 48 | ↔ | ↓ | ns | ↔ | |||
| Muscularization of PA | ↑ | 16 | ↑ | 17 | ↑ | 13 | ↔ | ↓ | 14 | ↔ | ||||
| PA acceleration time (AT) | ↓ | 54 | ↓ | 44 | ↓ | 24 | ↑ | 52 | ↑ | 51 | ↔ | |||
| PA ejection time (ET) | ↓ | 18 | ↓ | 22 | ↔ | ↔ | ↑ | ns | ↔ | |||||
| PA AT/ET | ↓ | 44 | ↓ | 32 | ↓ | ns | ↑ | 41 | ↑ | ns | ↔ | |||
| PA velocity time integral | ↓ | 49 | ↓ | 47 | ↓ | 36 | ↔ | ↑ | 43 | ↔ | ||||
| PA internal diameter | ↔ | ↑ | 23 | ↔ | ↔ | ↓ | 15 | ↔ | ||||||
| PA response to ACh | pEC50 | ↔ | ↓ | 11 | ↓ | 14 | ↓ | 15 | ↑ | 27 | ↑ | 7 | ||
| Emax | ↔ | ↔ | ↔ | ↔ | ↑ | ns | ↑ | 45 | ||||||
| PA response to SNP | pEC50 | ↓ | 6 | ↓ | 14 | ↔ | ↔ | ↑ | 15 | ↑ | 7 | |||
| Emax | ↑ | 49 | ↑ | ns | ↔ | ↓ | ns | ↔ | ↑ | 73 | ||||
| PA response to 5-HT | pEC50 | ↔ | ↔ | ↔ | ↑ | 11 | ↔ | ↑ | 11 | |||||
| Emax | ↑ | 67 | ↑ | 38 | ↑ | ns | ↑ | ns | ↔ | ↑ | ns | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Remiszewski, P.; Ryszkiewicz, P.; Baranowska-Kuczko, M.; Pędzińska-Betiuk, A.; Mińczuk, K.; Kloza, M.; Weresa, J.; Hutsch, T.; Malinowska, B. Initial Body Weight as an Important Factor for Improving the Reliability and Translational Relevance of the Preclinical Monocrotaline-Induced Rat Pulmonary Hypertension Model. Int. J. Mol. Sci. 2025, 26, 8916. https://doi.org/10.3390/ijms26188916
Remiszewski P, Ryszkiewicz P, Baranowska-Kuczko M, Pędzińska-Betiuk A, Mińczuk K, Kloza M, Weresa J, Hutsch T, Malinowska B. Initial Body Weight as an Important Factor for Improving the Reliability and Translational Relevance of the Preclinical Monocrotaline-Induced Rat Pulmonary Hypertension Model. International Journal of Molecular Sciences. 2025; 26(18):8916. https://doi.org/10.3390/ijms26188916
Chicago/Turabian StyleRemiszewski, Patryk, Piotr Ryszkiewicz, Marta Baranowska-Kuczko, Anna Pędzińska-Betiuk, Krzysztof Mińczuk, Monika Kloza, Jolanta Weresa, Tomasz Hutsch, and Barbara Malinowska. 2025. "Initial Body Weight as an Important Factor for Improving the Reliability and Translational Relevance of the Preclinical Monocrotaline-Induced Rat Pulmonary Hypertension Model" International Journal of Molecular Sciences 26, no. 18: 8916. https://doi.org/10.3390/ijms26188916
APA StyleRemiszewski, P., Ryszkiewicz, P., Baranowska-Kuczko, M., Pędzińska-Betiuk, A., Mińczuk, K., Kloza, M., Weresa, J., Hutsch, T., & Malinowska, B. (2025). Initial Body Weight as an Important Factor for Improving the Reliability and Translational Relevance of the Preclinical Monocrotaline-Induced Rat Pulmonary Hypertension Model. International Journal of Molecular Sciences, 26(18), 8916. https://doi.org/10.3390/ijms26188916






