The Interplay of Inflammation and Gut-Microbiota Dysbiosis in Alzheimer’s Disease: Mechanisms and Therapeutic Potential
Abstract
1. Introduction
2. Alzheimer’s Disease
2.1. Main Clinical, Anatomical and Histological Features
2.2. Demographic Features
2.3. Heterogeneity of Alzheimer’s Disease
3. Alzheimer’s Disease and Inflammation
3.1. Alzheimer’s Disease and Neuroinflammation
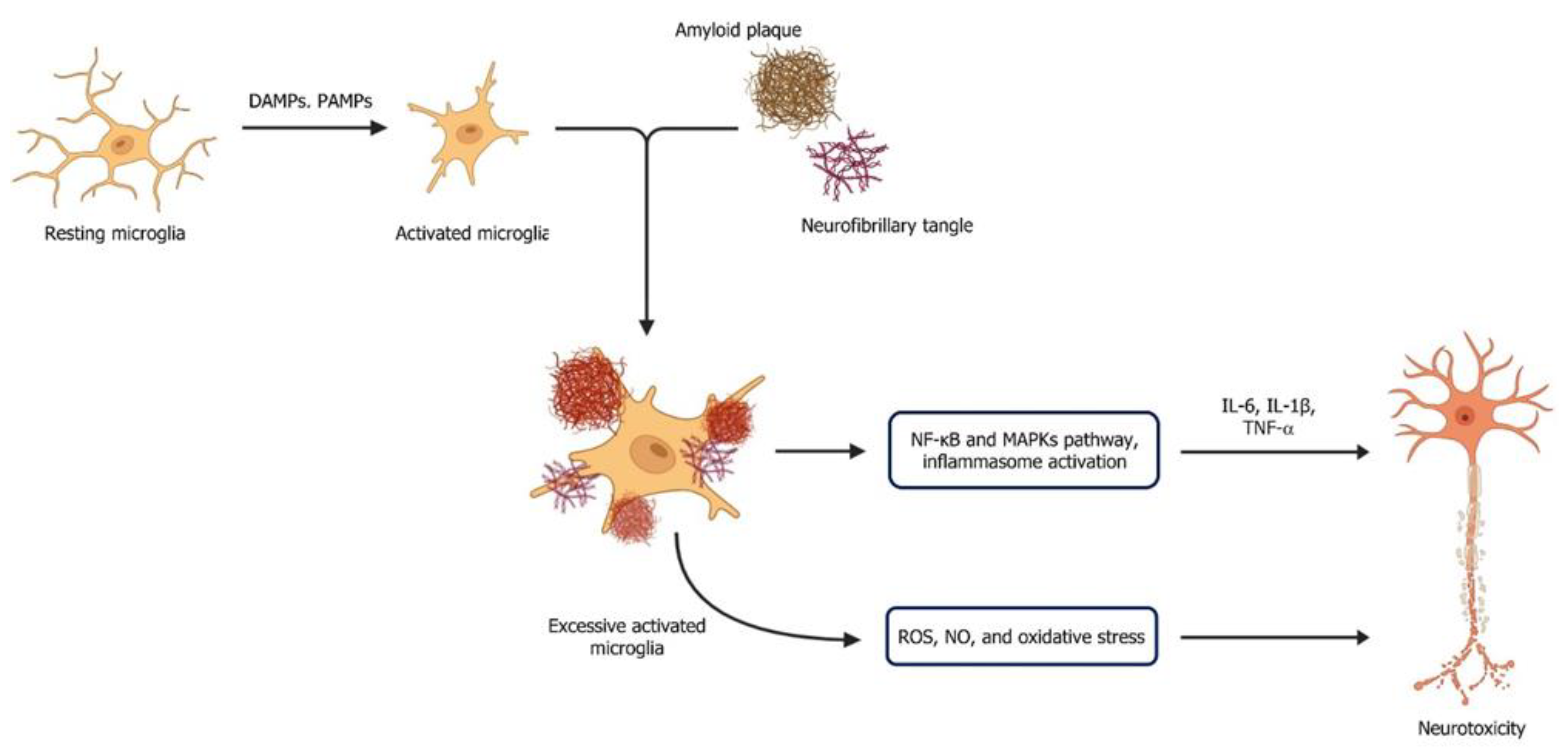
3.2. Microglial Activation
3.3. Astrocytes
3.4. Alzheimer’s Disease and Systemic Inflammation
4. Association of Gut-Microbiota and Alzheimer’s Disease
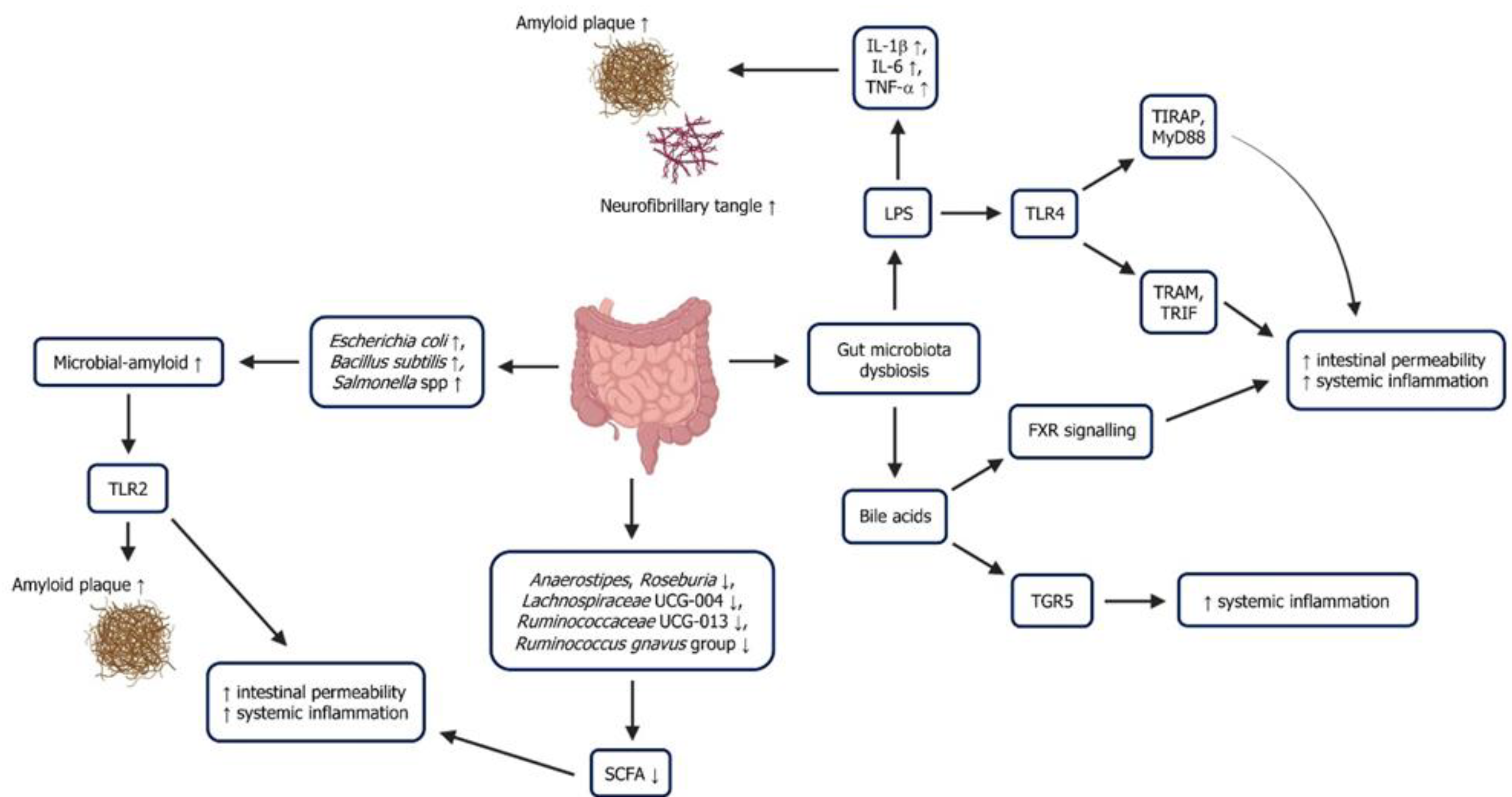
5. Gut-Microbiota Dysbiosis, Inflammation and Alzheimer’s Disease
5.1. Lipopolysaccharide
5.2. Short-Chain Fatty Acid
5.3. Microbial-Derived Amyloid
5.4. Bile Acids
5.5. GABA Neurotransmitter
5.6. Tryptophan
6. Potential Therapeutic Approaches Targeting the Gut–Brain-Axis in Alzheimer’s Disease
6.1. Microbiota-Based Therapies
6.2. Faecal Microbiota Transplantation
6.3. Small-Molecule Therapies
6.4. Protein-Peptide Drug Therapies
6.5. Dietary Interventions
| Therapeutic Approach | Key Effects/Mechanisms | References |
|---|---|---|
| Probiotics | Improved cognitive function; Reduced neuroinflammation; Modulated microbial composition | [159,160,161] |
| Faecal Microbiota Transplantation | Restored microbial diversity; Reduced Aβ/tau pathology; Decreased neuroinflammation | [163] |
| Small molecules | ||
| Enhanced cholinergic transmission; Symptomatic cognitive improvement | [164,165,166,167,168] |
| Reduced Aβ production; Selective APP processing | [173,174] |
| Inhibited Aβ oligomerization/fibrillization | [175,176,177] |
| HDAC inhibition (butyrate); Antioxidant/anti-inflammatory effects (indoles) | [122,178,179] |
Protein-peptide therapies | ||
| Targeted Aβ clearance; Reduced plaque burden | [180] |
| Modulated neuroinflammation; Protected BBB integrity | [181] |
| Inhibited Aβ/tau aggregation; β-hairpin scaffolds | [182] |
Dietary Interventions | ||
| Improved cognitive function; Reduced AD risk | [187,188] |
| Enhanced ketone metabolism; Reduced amyloid burden | [189,190] |
| Anti-inflammatory effects; Preserved neuronal function | [191] |
7. Current and Emerging Therapeutic Strategies for Alzheimer’s Disease: From Small Molecules to the Microbiome
8. Future Direction
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| Aβ | Amyloid-β |
| AD | Alzheimer disease |
| ADRD | Alzheimer disease and related dementias |
| AhR | Aryl hydrocarbon receptor |
| aMCI | Amnestic mild cognitive impairment |
| Amy+ | Amyloidosis |
| APP | Amyloid precursor protein |
| ARIA | Myloid-related imaging abnormalities |
| BBB | Blood–brain barrier |
| BDNF | Brain-derived neurotrophic factor |
| CA | Cholic acid |
| CD14 | Cluster of differentiations 14 |
| CDR-SB | Clinical Dementia Rating–Sum of Boxes |
| ChEI | Cholinesterase inhibitors |
| CNS | Central nervous system |
| CPP | Cell-penetrating peptide |
| CRP | C-reactive protein |
| CSF | Cerebrospinal fluid |
| CypA-MMP9 | Cyclophilin A-matrix metalloproteinase-9 |
| DAMPs | Danger-associated molecular patterns |
| DCA | Deoxycholic acid |
| DLB | Dementia with Lewy bodies |
| EAOD | Early-onset Alzheimer disease |
| EGCG | Epigallocatechin gallate |
| EVOO | Extra virgin live oil |
| FMT | Faecal microbiota transplantation |
| FXR | Farnesoid X receptor |
| GABA | γ-aminobutyric acid |
| GCA | Glycocholic acid |
| GDCA | Glycodeoxycholic acid |
| GCDCA | Glycochenodeoxycholic acid |
| GUDCA | Glycoursodeoxycholic acid |
| HCs | Healthy controls |
| HDAC | Histone deacetylase |
| HOMA-IR | Homeostasis model assessment–insulin resistance |
| I3A | Indole-3-aldehyde |
| IBD | Inflammatory bowel disease |
| ICAM-1 | Intercellular adhesion molecule-1 |
| IDO1 | Indoleamine-2,3-dioxygenase 1 |
| IgG | Immunoglobulin G |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| IPA | Indole-3-propionic acid |
| KD | Ketogenic diet |
| KYNA | Kynurenic acid |
| LCA | Lithocholic acid |
| LPS | Lipopolysaccharides |
| MAB | Monoclonal antibodies |
| MAD | Modified Atkins diet |
| MAPKs | Mitogen-activated protein kinases |
| MCI | Mild cognitive impairment |
| MCT | Medium-chain triglyceride |
| MeDi | Mediterranean diet |
| MMPs | Matrix metalloproteinases |
| MMSE | Mini-mental state examination |
| MRP14 | Myeloid-related protein 14 |
| NaB | Sodium butyrate |
| NACC | National Alzheimer’s Coordinating Center |
| nAChR | α7 nicotinic acetylcholine receptor |
| naMCI | Non-amnestic mild cognitive impairment |
| NIA | National Institute on Ageing |
| NF-κB | Nuclear factor kappa B |
| NFT | Neurofibrillary tangles |
| NO | Nitric oxide |
| PAMPs | Pathogen-associated molecular patterns |
| PET | Positron emission tomography |
| PGF | Pseudo-germ-free |
| Poly I:C | Polyinosinic-polycytidylic acid |
| PRRs | Pattern recognition receptors |
| QUIN | Quinolinic acid |
| RBC | Red blood cell |
| RCTs | Randomised controlled trials |
| ROS | Reactive oxygen species |
| SCFA | Short chain fatty acid |
| TCA | Taurocholic acid |
| TDO | Tryptophan 2,3-dioxygenase |
| TDP-43 | TAR-DNA-binding protein 43 |
| Tg | TgCRND8 |
| TGR5 | G protein-coupled bile acid receptor 1 |
| TLRs | Toll-like receptors |
| TMCA | Tauromuricholic acid |
| TNF-α | Tumour necrosis factor-alpha |
| TREM2 | Triggering receptor expressed on myeloid cells 2 |
| Trp | Tryptophan |
| TSPO | Translocator protein |
| SCFAs | Short-chain fatty acids |
| SRs | Scavenger receptors |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| VNS | Vagus nerve stimulation |
References
- Kumar, A.; Sidhu, J.; Lui, F.; Tsao, J.W. Alzheimer disease. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2024. [Google Scholar]
- Nichols, E.; Szoeke, C.E.I.; Vollset, S.E.; Abbasi, N.; Abd-Allah, F.; Abdela, J.; Aichour, M.T.E.; Akinyemi, R.O.; Alahdab, F.; Asgedom, S.W.; et al. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, P88–P106. [Google Scholar] [CrossRef]
- Lai, W.-H.; Hon, Y.-K.; Pang, G.M.-H.; Chong, E.M.-G.; Nordin, N.; Tiong, L.-L.; Tan, S.H.; Abu Sapian, R.; Lee, Y.-F.; Rosli, N. Dementia of the ageing population in Malaysia: A scoping review of published research. Aging Health Res. 2022, 2, 100077. [Google Scholar] [CrossRef]
- Naheeda, A.; Hakim, M.; Islam, M.S.; Islam, M.B.; Tang, E.Y.H.; Prodhane, A.A.; Amine, M.R.; Stephang, B.C.M.; Mohammad, Q.D. Prevalence of dementia among older age people and variation across different sociodemographic characteristics: A cross-sectional study in Bangladesh. Lancet 2023, 17, 100257. [Google Scholar] [CrossRef] [PubMed]
- Duara, R.; Barker, W. Heterogeneity in Alzheimer’s disease diagnosis and progression rates: Implications for therapeutic trials. Neurotherapeutics 2022, 19, 8–25. [Google Scholar] [CrossRef] [PubMed]
- Murphy, M.P.; LeVine, H., III. Alzheimer’s disease and the amyloid-β peptide. J. Alzheimer’s Dis. 2010, 19, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Rawat, P.; Sehar, U.; Bisht, J.; Selman, A.; Culberson, J.; Reddy, P.H. Phosphorylated tau in Alzheimer’s disease and other tauopathies. Int. J. Mol. Sci. 2022, 23, 12841. [Google Scholar] [CrossRef]
- Lue, L.-F.; Brachova, L.; Civin, W.H.; Rogers, J. Inflammation, Aβ deposition, and neurofibrillary tangle formation as correlates of Alzheimer’s disease neurodegeneration. J. Neuropathol. Exp. Neurol. 1996, 55, 1083–1088. [Google Scholar] [CrossRef]
- Guzman-Martinez, L.; Maccioni, R.B.; Andrade, V.; Navarrete, L.P.; Pastor, M.G.; Ramos-Escobar, N. Neuroinflammation as a common feature of neurodegenerative disorders. Front. Pharmacol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Lou, I.X.; Ali, K.; Chen, Q. Effect of nutrition in Alzheimer’s disease: A systematic review. Front. Neurosci. 2023, 17, 1147177. [Google Scholar] [CrossRef]
- Dhana, K.; Evans, D.A.; Rajan, K.B.; Bennett, D.A.; Morris, M.C. Healthy lifestyle and the risk of Alzheimer dementia: Findings from 2 longitudinal studies. Neurology 2020, 95, e374–e383. [Google Scholar] [CrossRef]
- Troutwine, B.R.; Hamid, L.; Lysaker, C.R.; Strope, T.A.; Wilkins, H.M. Apolipoprotein E and Alzheimer’s disease. Acta Pharm. Sin. B 2022, 12, 496–510. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Li, G.; Yang, H. Microbe-host interactions: Structure and functions of Gram-negative bacterial membrane vesicles. Front. Microbiol. 2023, 14, 1225513. [Google Scholar] [CrossRef] [PubMed]
- Ashique, S.; Mohanto, S.; Ahmed, M.G.; Mishra, N.; Garg, A.; Chellappan, D.K.; Omara, T.; Iqbal, S.; Kahwa, I. Gut-brain axis: A cutting-edge approach to target neurological disorders and potential synbiotic application. Heliyon 2024, 10, e34092. [Google Scholar] [CrossRef] [PubMed]
- Di-Nicolantonio, J.J.; Mehta, V.; Onkaramurthy, N.; O’Keefe, J.H. Fructose-induced inflammation and increased cortisol: A new mechanism for how sugar induces visceral adiposity. Prog. Cardiovasc. Dis. 2018, 61, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Chang, H.W.; Yan, D.; Lee, K.M.; Ucmak, D.; Wong, K.; Abrouk, M.; Farahnik, B.; Nakamura, M.; Zhu, T.H.; et al. Influence of diet on the gut microbiome and implications for human health. J. Transl. Med. 2017, 15, 73. [Google Scholar] [CrossRef]
- Rinninella, E.; Tohumcu, E.; Raoul, P.; Fiorani, M.; Cintoni, M.; Mele, M.C.; Cammarota, G.; Gasbarrini, A.; Ianiro, G. The role of diet in shaping human gut microbiota. Best Pract. Res. Clin. Gastroenterol. 2023, 62–63, 101828. [Google Scholar] [CrossRef]
- Boyle, P.A.; Yu, L.; Leurgans, S.E.; Wilson, R.S.; Brookmeyer, R.; Schneider, J.A.; Bennett, D.A. Attributable risk of Alzheimer’s dementia attributed to age-related neuropathologies. Ann. Neurol. 2019, 85, 114–124. [Google Scholar] [CrossRef]
- Kawas, C.H.; Kim, R.C.; Sonnen, J.A.; Bullain, S.S.; Trieu, T.; Corrada, M.M. Multiple pathologies are common and related to dementia in the oldest-old: The 90+ Study. Neurology 2015, 85, 535–542. [Google Scholar] [CrossRef]
- Schneider, J.A.; Arvanitakis, Z.; Bang, W.; Bennett, D.A. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007, 69, 2197–2204. [Google Scholar] [CrossRef]
- Rahimi, J.; Kovacs, G.G. Prevalence of mixed pathologies in the aging brain. Alzheimer’s Res. Ther. 2014, 6, 82. [Google Scholar] [CrossRef]
- Beach, T.G.; Monsell, S.E.; Phillips, L.E.; Kukull, W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005-2010. J. Neuropathol. Exp. Neurol. 2012, 71, 266–273. [Google Scholar] [CrossRef]
- Bennett, D.A.; Schneider, J.A.; Arvanitakis, Z.; Kelly, J.F.; Aggarwal, N.T.; Shah, R.C.; Wilson, R.S. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology 2006, 66, 1837–1844. [Google Scholar] [CrossRef]
- Boyle, P.A.; Yu, L.; Wilson, R.S.; Schneider, J.A.; Bennett, D.A. Relation of neuropathology with cognitive decline among older persons without dementia. Front. Aging Neurosci. 2013, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Karanth, S.; Nelson, P.T.; Katsumata, Y.; Kryscio, R.J.; Schmitt, F.A.; Cykowski, M.D.; Jicha, G.A.; Van Eldik, L.J.; Abner, E.L. Prevalence and clinical phenotype of quadruple misfolded proteins in older adults. JAMA Neurol. 2020, 77, 1299–1307. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.L.; Kim, C.; Haldiman, T.; Elhag, M.; Mehndiratta, P.; Pichet, T.; Lissemore, F.; Shea, M.; Cohen, Y.; Chen, W.; et al. Rapidly progressive Alzheimer’s disease features distinct structures of amyloid-β. Brain 2015, 138 Pt 4, 1009–1022. [Google Scholar] [CrossRef] [PubMed]
- Condello, C.; Lemmin, T.; Stöhr, J.; Nick, M.; Wu, Y.; Maxwell, A.M.; Watts, J.C.; Caro, C.D.; Oehler, A.; Keene, C.D.; et al. Structural heterogeneity and intersubject variability of Aβ in familial and sporadic Alzheimer’s disease. Proc. Natl. Acad. Sci. USA 2018, 115, E782–E791. [Google Scholar] [CrossRef]
- Murray, M.E.; Graff-Radford, N.R.; Ross, O.A.; Petersen, R.C.; Duara, R.; Dickson, D.W. Neuropathologically defined subtypes of Alzheimer’s disease with distinct clinical characteristics: A retrospective study. Lancet Neurol. 2011, 10, 785–796. [Google Scholar] [CrossRef]
- 2023 Alzheimer’s disease facts and figures. Alzheimer’s Dement. J. 2023, 19, 1598–1695. [CrossRef]
- Chêne, G.; Beiser, A.; Au, R.; Preis, S.R.; Wolf, P.A.; Dufouil, C.; Seshadri, S. Gender and incidence of dementia in the Framingham Heart Study from mid-adult life. Alzheimer’s Dement. J. 2015, 11, 310–320. [Google Scholar] [CrossRef]
- Levine, D.A.; Gross, A.L.; Briceño, E.M.; Tilton, N.; Giordani, B.J.; Sussman, J.B.; Hayward, R.A.; Burke, J.F.; Hingtgen, S.; Elkind, M.S.V.; et al. Sex differences in cognitive decline among US adults. JAMA Netw. Open 2021, 4, e210169. [Google Scholar] [CrossRef]
- Gurland, B.J.; Wilder, D.E.; Lantigua, R.; Stern, Y.; Chen, J.; Killeffer, E.H.; Mayeux, R. Rates of dementia in three ethnoracial groups. Int. J. Geriatr. Psychiatry 1999, 14, 481–493. [Google Scholar] [CrossRef]
- Froehlich, T.E.; Bogardus, S.T., Jr.; Inouye, S.K. Dementia and race: Are there differences between African Americans and Caucasians? J. Am. Geriatr. Soc. 2001, 49, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Glymour, M.M.; Manly, J.J. Lifecourse social conditions and racial and ethnic patterns of cognitive aging. Neuropsychol. Rev. 2008, 18, 223–254. [Google Scholar] [CrossRef] [PubMed]
- Weuve, J.; Barnes, L.L.; de Leon, C.F.M.; Rajan, K.B.; Beck, T.; Aggarwal, N.T.; Hebert, L.E.; Bennett, D.A.; Wilson, R.S.; Evans, D.A. Cognitive aging in Black and White Americans: Cognition, cognitive decline, and incidence of Alzheimer disease dementia. Epidemiology 2018, 29, 151–159. [Google Scholar] [CrossRef]
- Petersen, R.C. Mild cognitive impairment as a diagnostic entity. J. Intern. Med. 2004, 256, 183–194. [Google Scholar] [CrossRef]
- Guan, H.; Tao, L.; Jiyang, J.; Tao, D.; Zhang, J.; Niu, H.; Zhu, W.; Wang, Y.; Cheng, J.; Kochan, N.A.; et al. Classifying MCI subtypes in community-dwelling elderly using cross-sectional and longitudinal MRI-based biomarkers. Front. Aging Neurosci. 2017, 9, 309–322. [Google Scholar] [CrossRef]
- Phillips, M.L.; Stage, E.C., Jr.; Lane, K.A.; Gao, S.; Risacher, S.L.; Goukasian, N.; Saykin, A.J.; Carrillo, M.C.; Dickerson, B.C.; Rabinovici, G.D.; et al. Neurodegenerative patterns of cognitive clusters of early-onset Alzheimer’s disease subjects: Evidence for disease heterogeneity. Dement. Geriatr. Cogn. Disord. 2019, 48, 131–142. [Google Scholar] [CrossRef]
- Qian, J.; Betensky, R.A.; Hyman, B.T.; Serrano-Pozo, A. Association of APOE genotype with heterogeneity of cognitive decline rate in Alzheimer disease. Neurology 2021, 96, e2414–e2428. [Google Scholar] [CrossRef]
- Lam, B.; Masellis, M.; Freedman, M.; Stuss, D.T.; Black, S.E. Clinical, imaging, and pathological heterogeneity of the Alzheimer’s disease syndrome. Alzheimer’s Res. Ther. 2013, 5, 1. [Google Scholar] [CrossRef]
- Peter, J.; Abdulkadir, A.; Kaller, C.; Kümmerer, D.; Hüll, M.; Vach, W.; Klöppel, S. Subgroups of Alzheimer’s disease: Stability of empirical clusters over time. J. Alzheimer’s Disease 2014, 42, 651–661. [Google Scholar] [CrossRef]
- Bradburn, S.; Murgatroyd, C.; Ray, N. Neuroinflammation in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Ageing Res. Rev. 2019, 50, 1–8. [Google Scholar] [CrossRef]
- Fan, Z.; Brooks, D.J.; Okello, A.; Edison, P. An early and late peak in microglial activation in Alzheimer’s disease trajectory. Brain 2017, 140, 792–803. [Google Scholar] [CrossRef]
- Parbo, P.; Ismail, R.; Hansen, K.V.; Amidi, A.; Mårup, F.H.; Gottrup, H.; Brændgaard, H.; Eriksson, B.O.; Eskildsen, S.F.; Lund, T.E.; et al. Brain inflammation accompanies amyloid in the majority of mild cognitive impairment cases due to Alzheimer’s disease. Brain 2017, 140, 2002–2011. [Google Scholar] [CrossRef]
- Dani, M.; Wood, M.; Mizoguchi, R.; Fan, Z.; Walker, Z.; Morgan, R.; Hinz, R.; Biju, M.; Kuruvilla, T.; Brooks, D.J.; et al. Microglial activation correlates in vivo with both tau and amyloid in Alzheimer’s disease. Brain 2018, 141, 2740–2754. [Google Scholar] [CrossRef]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef]
- Domingues, C.; da Cruz e Silva, O.A.B.; Henriques, A. Impact of cytokines and chemokines on Alzheimer’s disease neuropathological hallmarks. Curr. Alzheimer Res. 2017, 14, 870–882. [Google Scholar] [CrossRef]
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Jiang, J.; Tan, Y.; Chen, S. Microglia in neurodegenerative diseases: Mechanism and potential therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 359. [Google Scholar] [CrossRef] [PubMed]
- Chiarini, A.; Armato, U.; Hu, P.; Dal Prà, I. Danger-sensing/patten recognition receptors and neuroinflammation in Alzheimer’s disease. Int. J. Mol. Sci. 2020, 21, 9036. [Google Scholar] [CrossRef] [PubMed]
- Salminen, A.; Ojala, J.; Kauppinen, A.; Kaarniranta, K.; Suuronen, T. Inflammation in Alzheimer’s disease: Amyloid-β oligomers trigger innate immunity defence via pattern recognition receptors. Prog. Neurobiol. 2009, 87, 181–194. [Google Scholar] [CrossRef]
- Qin, J.; Ma, Z.; Chen, X.; Shu, S. Microglia activation in central nervous system disorders: A review of recent mechanistic investigations and development efforts. Front. Neurol. 2023, 14, 1103416. [Google Scholar] [CrossRef] [PubMed]
- Simpson, D.S.A.; Oliver, P.L. ROS Generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef]
- Wennström, M.; Hall, S.; Nägga, K.; Londos, E.; Minthon, L.; Hansson, O. Cerebrospinal fluid levels of IL-6 are decreased and correlate with cognitive status in DLB patients. Alz. Res. Therapy 2015, 7, 63. [Google Scholar] [CrossRef]
- Lyra e Silva, N.M.; Gonçalves, R.A.; Pascoal, T.A.; Lima-Filho, R.A.S.; de Paula França Resende, E.; Vieira, E.L.M.; Teixeira, A.L.; de Souza, L.C.; Peny, J.A.; Fortuna, J.T.S.; et al. Pro-inflammatory interleukin-6 signaling links cognitive impairments and peripheral metabolic alterations in Alzheimer’s disease. Transl. Psychiatry 2021, 11, 251. [Google Scholar] [CrossRef] [PubMed]
- Babcock, A.A.; Ilkjær, L.; Clausen, B.H.; Villadsen, B.; Dissing-Olesen, L.; Bendixen, A.T.M.; Lyck, L.; Lambertsen, K.L.; Finsen, B. Cytokine-producing microglia have an altered beta-amyloid load in aged APP/PS1 Tg mice. Brain Behav. Immun. 2015, 48, 86–101. [Google Scholar] [CrossRef] [PubMed]
- Taupenot, L.; Ciesielski-Treska, J.; Ulrich, G.; Chasserot-Golaz, S.; Aunis, D.; Bader, M.-F. Chromogranin a triggers a phenotypic transformation and the generation of nitric oxide in brain microglial cells. Neuroscience 1996, 72, 377–389. [Google Scholar] [CrossRef]
- Kummer, M.P.; Vogl, T.; Axt, D.; Griep, A.; Vieira-Saecker, A.; Jessen, F.; Gelpi, E.; Roth, J.; Heneka, M.T. Mrp14 deficiency ameliorates amyloid β burden by increasing microglial phagocytosis and modulation of amyloid precursor protein processing. J. Neurosci. 2012, 32, 17824–17829. [Google Scholar] [CrossRef]
- González-Reyes, R.E.; Nava-Mesa, M.O.; Vargas-Sánchez, K.; Ariza-Salamanca, D.; Mora-Muñoz, L. Involvement of astrocytes in Alzheimer’s disease from a neuroinflammatory and oxidative stress perspective. Front. Mol. Neurosci. 2017, 10, 427. [Google Scholar] [CrossRef]
- Zhang, Y.; Qi, Y.; Gao, Y.; Chen, W.; Zhou, T.; Zang, Y.; Li, J. Astrocyte metabolism and signaling pathways in the CNS. Front. Neurosci. 2023, 17, 1217451. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol.-Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Jayaraman, A.; Htike, T.T.; James, R.; Picon, C.; Reynolds, R. TNF-mediated neuroinflammation is linked to neuronal necroptosis in Alzheimer’s disease hippocampus. Acta Neuropathol. Commun. 2021, 9, 159. [Google Scholar] [CrossRef] [PubMed]
- Decourt, B.; Lahiri, D.K.; Sabbagh, M.N. Targeting tumor necrosis factor alpha for Alzheimer’s disease. Curr. Alzheimer Res. 2017, 14, 412–425. [Google Scholar] [CrossRef] [PubMed]
- Clark, I.A.; Vissel, B. Excess cerebral TNF causing glutamate excitotoxicity rationalizes treatment of neurodegenerative diseases and neurogenic pain by anti-TNF agents. J. Neuroinflamm. 2016, 13, 236. [Google Scholar] [CrossRef] [PubMed]
- Azizi, G.; Khannazer, N.; Mirshafiey, A. The potential role of chemokines in Alzheimer’s disease pathogenesis. Am. J. Alzheimers Dis. Other Demen. 2014, 29, 415–425. [Google Scholar] [CrossRef]
- Wyss-Coray, T.; Loike, J.D.; Brionne, T.C.; Lu, E.; Anankov, R.; Yan, F.; Silverstein, S.C.; Husemann, J. Adult mouse astrocytes degrade amyloid-β in vitro and in situ. Nat. Med. 2003, 9, 453–457. [Google Scholar] [CrossRef]
- Rodríguez-Giraldo, M.; González-Reyes, R.E.; Ramírez-Guerrero, S.; Bonilla-Trilleras, C.E.; Guardo-Maya, S.; Nava-Mesa, M.O. Astrocytes as a therapeutic target in Alzheimer’s disease–comprehensive review and recent developments. Int. J. Mol. Sci. 2022, 23, 13630. [Google Scholar] [CrossRef]
- Wen, L.; Bi, D.; Shen, Y. Complement-mediated synapse loss in Alzheimer’s disease: Mechanisms and involvement of risk factors. Trends Neurosci. 2024, 47, 135–149. [Google Scholar] [CrossRef]
- Shah, A.; Kishore, U.; Shastri, A. Complement system in Alzheimer’s disease. Int. J. Mol. Sci. 2021, 22, 13647. [Google Scholar] [CrossRef]
- Soteros, M.B.; Sia, G.M. Complement and microglia dependent synapse elimination in brain development. WIREs Mech. Dis. 2023, 14, e1545. [Google Scholar] [CrossRef]
- Millán Solano, M.V.; Salinas Lara, C.; Sánchez-Garibay, C.; Soto-Rojas, L.O.; Escobedo-Ávila, I.; Tena-Suck, M.L.; Ortíz-Butrón, R.; Choreño-Parra, J.A.; Romero-López, J.P.; Meléndez Camargo, M.E. Effect of systemic inflammation in the CNS: A silent history of neuronal damage. Int. J. Mol. Sci. 2023, 24, 11902. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Erickson, M.A.; Banks, W.A. Blood–brain barrier dysfunction as a cause and consequence of Alzheimer’s disease. J. Cereb. Blood Flow Metab. 2013, 33, 1500–1513. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Zhang, J.; Wu, Y.; Xie, M.; Tao, S.; Lv, Q.; Wang, Q. Plasma IL-6 levels and their association with brain health and dementia risk: A population-based cohort study. Brain Behav. Immun. 2024, 120, 430–438. [Google Scholar] [CrossRef] [PubMed]
- Griseta, C.; Battista, P.; Castellana, F.; Colonna, I.; Sciarra, S.; Zupo, R.; Bortone, I.; Lampignano, L.; Tirelli, S.; Berardino, G.; et al. Serum levels of IL-6 are associated with cognitive impairment in the Salus in Apulia population-based study. Heliyon 2023, 9, e13972. [Google Scholar] [CrossRef] [PubMed]
- Singh-Manoux, A.; Dugravot, A.; Brunner, E.; Kumari, M.; Shipley, M.; Elbaz, A.; Kivimaki, M. Interleukin-6 and C-reactive protein as predictors of cognitive decline in late midlife. Neurology 2014, 83, 486–493. [Google Scholar] [CrossRef]
- Akiyama, H.; Barger, S.; Barnum, S.; Bradt, B.; Bauer, J.; Cole, G.M.; Cooper, N.R.; Eikelenboom, P.; Emmerling, M.; Fiebich, B.L.; et al. Inflammation and Alzheimer’s disease. Neurobiol. Aging 2000, 21, 383–421. [Google Scholar] [CrossRef]
- Fischer, R.; Maier, O. Interrelation of oxidative stress and inflammation in neurodegenerative disease: Role of TNF. Oxidative Med. Cell. Longev. 2015, 2015, 610813. [Google Scholar] [CrossRef]
- Song, K.; Li, Y.; Zhang, H.; An, N.; Wei, Y.; Wang, L.; Tian, C.; Yuan, M.; Sun, Y.; Xing, Y.; et al. Oxidative stress-mediated blood-brain barrier (BBB) disruption in neurological diseases. Oxidative Med. Cell. Longev. 2020, 2020, 4356386. [Google Scholar] [CrossRef]
- Tang, W.; Zhu, H.; Feng, Y.; Guo, R.; Wan, D. The impact of gut microbiota disorders on the blood–brain barrier. Infect. Drug Resist. 2020, 13, 3351–3363. [Google Scholar] [CrossRef]
- Dietrich, J.-B. The adhesion molecule ICAM-1 and its regulation in relation with the blood–brain barrier. J. Neuroimmunol. 2002, 128, 58–68. [Google Scholar] [CrossRef]
- Hannocks, M.-J.; Zhang, X.; Gerwien, H.; Chashchina, A.; Burmeister, M.; Korpos, E.; Song, J.; Sorokin, L. The gelatinases, MMP-2 and MMP-9, as fine tuners of neuroinflammatory processes. Matrix Biol. 2019, 75–76, 102–113. [Google Scholar] [CrossRef] [PubMed]
- Alkhalifa, A.E.; Al-Ghraiybah, N.F.; Odum, J.; Shunnarah, J.G.; Austin, N.; Kaddoumi, A. Blood-brain barrier breakdown in Alzheimer’s disease: Mechanisms and targeted strategies. Int. J. Mol. Sci. 2023, 24, 16288. [Google Scholar] [CrossRef] [PubMed]
- Candelario-Jalil, E.; Thompson, J.; Taheri, S.; Grossetete, M.; Adair, J.C.; Edmonds, E.; Prestopnik, J.; Wills, J.; Rosenberg, G.A. Matrix metalloproteinases are associated with increased blood–brain barrier opening in vascular cognitive impairment. Stroke 2011, 42, 1345–1350. [Google Scholar] [CrossRef] [PubMed]
- Bell, R.D.; Winkler, E.A.; Singh, I.; Sagare, A.P.; Deane, R.; Wu, Z.; Holtzman, D.M.; Betsholtz, C.; Armulik, A.; Sallstrom, J. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature 2012, 485, 512–516, Correction in Nature 2023, 617, E12. [Google Scholar] [CrossRef]
- Erdő, F.; Denes, L.; de Lange, E. Age-associated physiological and pathological changes at the blood-brain barrier: A review. J. Cereb. Blood Flow Metab. 2017, 37, 4–24. [Google Scholar] [CrossRef]
- Surendranathan, A.; Su, L.; Mak, E.; Passamonti, L.; Hong, Y.T.; Arnold, R.; Vázquez Rodríguez, P.; Bevan-Jones, W.R.; Brain, S.A.E.; Fryer, T.D.; et al. Early microglial activation and peripheral inflammation in dementia with Lewy bodies. Brain 2018, 141, 3415–3427. [Google Scholar] [CrossRef]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef]
- Almutairi, M.M.A.; Gong, C.; Xu, Y.G.; Chang, Y.; Shi, H. Factors controlling permeability of the blood–brain barrier. Cell. Mol. Life Sci. 2016, 73, 57–77. [Google Scholar] [CrossRef]
- Li, H.; Cui, X.; Yuxiu Lin, Y.; Huang, F.; Tian, A.; Zhang, R. Gut microbiota changes in patients with Alzheimer’s disease spectrum based on 16S rRNA sequencing: A systematic review and meta-analysis. Front. Aging Neurosci. 2024, 16, 1422350. [Google Scholar] [CrossRef]
- Sheng, C.; Lin, L.; Lin, H.; Wang, X.; Han, Y.; Liu, S. Altered gut microbiota in adults with subjective cognitive decline: The SILCODE study. J. Alzheimer’s Dis. 2021, 82, 513–526. [Google Scholar] [CrossRef]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Ling, Z.; Zhu, M.; Yan, X.; Cheng, Y.; Shao, L.; Liu, X.; Jiang, R.; Wu, S. Structural and functional dysbiosis of fecal microbiota in Chinese patients with Alzheimer’s disease. Front. Cell Dev. Biol. 2020, 8, 634069. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Y.; Liu, W.; Wang, T.; Wang, L.; Hao, L.; Ju, M.; Xiao, R. Diet quality, gut microbiota, and microRNAs associated with mild cognitive impairment in middle-aged and elderly Chinese population. Am. J. Clin. Nutr. 2021, 114, 429–440. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, Z.; Shen, L.; Li, W.; Fu, X.; Zeng, F.; Gui, L.; Lü, Y.; Cai, M.; Zhu, C.; Tan, Y.-L.; et al. Gut microbiota is altered in patients with Alzheimer’s disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s disease microbiome is associated with dysregulation of the anti-inflammatory P-glycoprotein pathway. mBio 2019, 10, e00632-19. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; He, Y.; Ma, J.; Huang, P.; Du, J.; Cao, L.; Wang, Y.; Xiao, Q.; Tang, H.; Chen, S. Mild cognitive impairment has similar alterations as Alzheimer’s disease in gut microbiota. Alzheimer’s Dement. 2019, 15, 1357–1366. [Google Scholar] [CrossRef]
- Guo, M.; Peng, J.; Huang, X.; Xiao, L.; Huang, F.; Zuo, Z. Gut microbiome features of Chinese patients newly diagnosed with Alzheimer’s disease or mild cognitive impairment. J. Alzheimer’s Dis. 2021, 80, 299–310. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, Y.; Quan, M.; Zhao, H.; Jia, J. Gut microbiota changes and their correlation with cognitive and neuropsychiatric symptoms in Alzheimer’s disease. J. Alzheimer’s Dis. 2021, 81, 583–595. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef]
- Kaiyrlykyzy, A.; Kozhakhmetov, S.; Babenko, D.; Zholdasbekova, G.; Alzhanova, D.; Olzhayev, F.; Baibulatova, A.; Kushugulova, A.R.; Askarova, S. Study of gut microbiota alterations in Alzheimer’s dementia patients from Kazakhstan. Sci. Rep. 2022, 12, 15115. [Google Scholar] [CrossRef]
- Nagpal, R.; Neth, B.J.; Wang, S.; Mishra, S.P.; Craft, S.; Yadav, H. Gut mycobiome and its interaction with diet, gut bacteria and Alzheimer’s disease markers in subjects with mild cognitive impairment: A pilot study. eBioMedicine 2020, 59, 102950. [Google Scholar] [CrossRef]
- Heumann, D.; Roger, T. Initial responses to endotoxins and Gram-negative bacteria. Clin. Chim. Acta 2002, 323, 59–72. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.S.; Wang, J.; Yannie, P.J.; Ghosh, S. Intestinal barrier dysfunction, LPS translocation, and disease development. J. Endocr. Soc. 2020, 4, bvz039. [Google Scholar] [CrossRef] [PubMed]
- Ciesielska, A.; Matyjek, M.; Kwiatkowska, K. TLR4 and CD14 trafficking and its influence on LPS-induced pro-inflammatory signaling. Cell. Mol. Life Sci. 2021, 78, 1233–1261. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-M.; Wu, Q.; Kirk, R.A.; Horn, K.P.; Salem, A.H.E.; Hoffman, J.M.; Yap, J.T.; Sonnen, J.A.; Towner, R.A.; Bozza, F.A.; et al. Lipopolysaccharide endotoxemia induces amyloid-β and p-tau formation in the rat brain. Am. J. Nucl. Med. Mol. Imaging 2018, 8, 86–99. [Google Scholar]
- Kahn, M.S.; Kranjac, D.; Alonzo, C.A.; Haase, J.H.; Cedillos, R.O.; McLinden, K.A.; Boehm, G.W.; Chumley, M.J. Prolonged elevation in hippocampal Aβ and cognitive deficits following repeated endotoxin exposure in the mouse. Behav. Brain Res. 2012, 229, 176–184. [Google Scholar] [CrossRef]
- Tejera, D.; Mercan, D.; Sanchez-Caro, J.M.; Hanan, M.; Greenberg, D.; Soreq, H.; Latz, E.; Golenbock, D.; Heneka, M.T. Systemic inflammation impairs microglial Aβ clearance through NLRP3 inflammasome. EMBO J. 2019, 38, e101064. [Google Scholar] [CrossRef]
- Wang, F.; Zhang, Z.-Z.; Cao, L.; Yang, Q.-G.; Lu, Q.-F.; Chen, G.-H. Lipopolysaccharide exposure during late embryogenesis triggers and drives Alzheimer-like behavioral and neuropathological changes in CD-1 mice. Brain Behav. 2020, 10, e01546. [Google Scholar] [CrossRef]
- Sly, L.M.; Krzesicki, R.F.; Brashler, J.R.; Buhl, A.E.; McKinley, D.D.; Carter, D.B.; Chin, J.E. Endogenous brain cytokine mRNA and inflammatory responses to lipopolysaccharide are elevated in the Tg2576 transgenic mouse model of Alzheimer’s disease. Brain Res. Bull. 2001, 56, 581–588. [Google Scholar] [CrossRef]
- Qiao, X.; Cummins, D.J.; Paul, S.M. Neuroinflammation-induced acceleration of amyloid deposition in the APPV717F transgenic mouse. Eur. J. Neurosci. 2001, 14, 474–482. [Google Scholar] [CrossRef]
- Sheng, J.G.; Bora, S.H.; Xu, G.; Borchelt, D.R.; Price, D.L.; Koliatsos, V.E. Lipopolysaccharide-induced-neuroinflammation increases intracellular accumulation of amyloid precursor protein and amyloid β peptide in APPswe transgenic mice. Neurobiol. Dis. 2003, 14, 133–145. [Google Scholar] [CrossRef]
- Xie, J.; Gorlé, N.; Vandendriessche, C.; Van Imschoot, G.; Van Wonterghem, E.; Van Cauwenberghe, C.; Parthoens, E.; Van Hamme, E.; Lippens, S.; Van Hoecke, L.; et al. Low-grade peripheral inflammation affects brain pathology in the AppNL-G-F mouse model of Alzheimer’s disease. Acta Neuropathol. Commun. 2021, 9, 163. [Google Scholar] [CrossRef] [PubMed]
- Krstic, D.; Madhusudan, A.; Doehner, J.; Vogel, P.; Notter, T.; Imhof, C.; Manalastas, A.; Hilfiker, M.; Pfister, S.; Schwerdel, C.; et al. Systemic immune challenges trigger and drive Alzheimer-like neuropathology in mice. J. Neuroinflamm. 2012, 9, 151. [Google Scholar] [CrossRef]
- Liu, P.; Wang, Y.; Yang, G.; Zhang, Q.; Meng, L.; Xin, Y.; Jiang, X. The role of short-chain fatty acids in intestinal barrier function, inflammation, oxidative stress, and colonic carcinogenesis. Pharmacol. Res. 2021, 165, 105420. [Google Scholar] [CrossRef] [PubMed]
- Koh, A.; Vadder, F.D.; Kovatcheva-Datchary, P.; Bäckhed, F. From dietary fiber to host physiology: Short-chain fatty acids as key bacterial metabolites. Cell 2016, 165, 1332–1345. [Google Scholar] [CrossRef] [PubMed]
- Louis, P.; Flint, H.J. Formation of propionate and butyrate by the human colonic microbiota. Environ. Microbiol. 2017, 19, 29–41. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Engertsberger, L.; Komarova, I.; Feldbacher, N.; Leber, B.; Pichler, G.; Fink, N.; Scarpatetti, M.; Schippinger, W.; Schmidt, R.; et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: A pilot study. BMC Geriatr. 2020, 20, 248. [Google Scholar] [CrossRef]
- Inaba, T.; Yamashiro, K.; Kurita, N.; Ueno, Y.; Miyamoto, N.; Hira, K.; Nakajima, S.; Kijima, C.; Nakaguro, R.; Urabe, T.; et al. Microbial lipopolysaccharide-induced inflammation contributes to cognitive impairment and white matter lesion progression in diet-induced obese mice with chronic cerebral hypoperfusion. CNS Neurosci. Ther. 2023, 29, 200–212. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Yang, B.; Chen, K.; Kong, Y.; Fang, N.; Gong, T.; Wang, F.; Ling, Z.; Liu, J. Effect of Clostridium Butyricum against Microglia-Mediated Neuroinflammation in Alzheimer’s Disease via Regulating Gut Microbiota and Metabolites Butyrate. Mol. Nutr. Food Res. 2020, 64, 1900636. [Google Scholar] [CrossRef]
- Fernando, W.M.A.D.B.; Martins, I.J.; Morici, M.; Bharadwaj, P.; Rainey-Smith, S.R.; Lim, W.L.F.; Martins, R.N. Sodium butyrate reduces brain amyloid-β levels and improves cognitive memory performance in an Alzheimer’s disease transgenic mouse model at an early disease stage. J. Alzheimer’s Dis. 2020, 74, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Boles, B.R. Microbial amyloids-functions and interactions within the host. Curr. Opin. Microbiol. 2013, 16, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.G.; Stribinskis, V.; Rane, M.J.; Demuth, D.R.; Gozal, E.; Roberts, A.M.; Jagadapillai, R.; Liu, R.; Choe, K.; Shivakumar, B.; et al. Exposure to the functional bacterial amyloid protein curli enhances alpha-synuclein aggregation in aged Fischer 344 rats and Caenorhabditis elegans. Sci. Rep. 2016, 6, 34477. [Google Scholar] [CrossRef] [PubMed]
- Honarpisheh, P.; Reynolds, C.R.; Blasco Conesa, M.P.; Moruno Manchon, J.F.; Putluri, N.; Bhattacharjee, M.B.; Urayama, A.; McCullough, L.D.; Ganesh, B.P. Dysregulated gut homeostasis observed prior to the accumulation of the brain amyloid-β in Tg2576 mice. Int. J. Mol. Sci. 2020, 21, 1711. [Google Scholar] [CrossRef]
- Das, T.K.; Blasco-Conesa, M.P.; Korf, J.; Honarpisheh, P.; Chapman, M.R.; Ganesh, B.P. Bacterial amyloid curli associated gut epithelial neuroendocrine activation predominantly observed in Alzheimer’s disease mice with central amyloid-β pathology. J. Alzheimer’s Dis. 2022, 88, 191–205. [Google Scholar] [CrossRef]
- Romero, D.; Aguilar, C.; Losick, R.; Kolter, R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc. Natl. Acad. Sci. USA 2010, 107, 2230–2234. [Google Scholar] [CrossRef]
- Erskine, E.; MacPhee, C.E.; Stanley-Wall, N.R. Functional amyloid and other protein fibers in the biofilm matrix. J. Mol. Biol. 2018, 430, 3642–3656. [Google Scholar] [CrossRef]
- Tursi, S.A.; Tükel, Ç. Curli-containing enteric biofilms inside and out: Matrix composition, immune recognition, and disease implications. Microbiol. Mol. Biol. Rev. 2018, 82, e00028-18. [Google Scholar] [CrossRef]
- Tükel, Ç.; Wilson, R.P.; Nishimori, J.H.; Pezeshki, M.; Chromy, B.A.; Bäumler, A.J. Responses to amyloids of microbial and host origin are mediated through toll-like receptor 2. Cell Host Microbe 2009, 6, 45–53. [Google Scholar] [CrossRef]
- Zhou, C.; Sun, X.; Hu, Y.; Song, J.; Dong, S.; Kong, D.; Wang, Y.; Hua, X.; Han, J.; Zhou, Y.; et al. Genomic deletion of TLR2 induces aggravated white matter damage and deteriorated neurobehavioral functions in mouse models of Alzheimer’s disease. Aging 2019, 11, 7257–7273. [Google Scholar] [CrossRef]
- Liu, S.; Liu, Y.; Hao, W.; Wolf, L.; Kiliaan, A.J.; Penke, B.; Rübe, C.E.; Walter, J.; Heneka, M.T.; Hartmann, T.; et al. TLR2 is a primary receptor for Alzheimer’s amyloid β peptide to trigger neuroinflammatory activation. J. Immunol. 2012, 188, 1098–1107. [Google Scholar] [CrossRef]
- McDonald, C.L.; Hennessy, E.; Rubio-Araiz, A.; Keogh, B.; McCormack, W.; McGuirk, P.; Reilly, M.; Lynch, M.A. Inhibiting TLR2 activation attenuates amyloid accumulation and glial activation in a mouse model of Alzheimer’s disease. Brain Behav. Immun. 2016, 58, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Donkin, J.; Wellington, C. Greasing the wheels of Aβ clearance in Alzheimer’s disease: The role of lipids and apolipoprotein E. BioFactors 2009, 35, 239–248. [Google Scholar] [CrossRef]
- Ogundare, M.; Theofilopoulos, S.; Lockhart, A.; Hall, L.J.; Arenas, E.; Sjövall, J.; Brenton, A.G.; Wang, Y.; Griffiths, W.J. Cerebrospinal fluid steroidomics: Are bioactive bile acids present in brain? J. Biol. Chem. 2010, 285, 4666–4679. [Google Scholar] [CrossRef] [PubMed]
- Greenberg, N.; Grassano, A.; Thambisetty, M.; Lovestone, S.; Legido-Quigley, C. A Proposed metabolic strategy for monitoring disease progression in Alzheimer’s disease. Electrophoresis 2009, 30, 1235–1239. [Google Scholar] [CrossRef] [PubMed]
- Olazarán, J.; Gil-De-Gómez, L.; Rodríguez-Martín, A.; Valentí-Soler, M.; Frades-Payo, B.; Marín-Muñoz, J.; Antúnez, C.; Frank-García, A.; Acedo-Jiménez, C.; Morlán-Gracia, L.; et al. A blood-based, 7-metabolite signature for the early diagnosis of Alzheimer’s disease. J. Alzheimer’s Dis. 2015, 45, 1157–1173. [Google Scholar] [CrossRef]
- Mapstone, M.; Cheema, A.K.; Fiandaca, M.S.; Zhong, X.; Mhyre, T.R.; MacArthur, L.H.; Hall, W.J.; Fisher, S.G.; Peterson, D.R.; Haley, J.M.; et al. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med. 2014, 20, 415–418. [Google Scholar] [CrossRef]
- Pan, X.; Elliott, C.T.; McGuinness, B.; Passmore, P.; Kehoe, P.G.; Hölscher, C.; McClean, P.L.; Graham, S.F.; Green, B.D. Metabolomic profiling of bile acids in clinical and experimental samples of Alzheimer’s disease. Metabolites 2017, 7, 28. [Google Scholar] [CrossRef]
- Gadaleta, R.M.; van Erpecum, K.J.; Oldenburg, B.; Willemsen, E.C.L.; Renooij, W.; Murzilli, S.; Klomp, L.W.J.; Siersema, P.D.; Schipper, M.E.I.; Danese, S.; et al. Farnesoid X receptor activation inhibits inflammation and preserves the intestinal barrier in inflammatory bowel disease. Gut 2011, 60, 463–472. [Google Scholar] [CrossRef]
- Fiorucci, S.; Biagioli, M.; Zampella, A.; Distrutti, E. Bile acids activated receptors regulate innate immunity. Front. Immunol. 2018, 9, 1853. [Google Scholar] [CrossRef]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, E.Y.; McBride, S.W.; Hsien, S.; Sharon, G.; Hyde, E.R.; McCue, T.; Codelli, J.A.; Chow, J.; Reisman, S.E.; Petrosino, J.F.; et al. Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell 2013, 155, 1451–1463. [Google Scholar] [CrossRef] [PubMed]
- Conn, K.A.; Borsom, E.M.; Cope, E.K. Implications of microbe-derived ɣ-aminobutyric acid (GABA) in gut and brain barrier integrity and GABAergic signaling in Alzheimer’s disease. Gut Microbes 2024, 16, 2371950. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Halabisky, B.; Zhou, Y.; Palop, J.J.; Yu, G.; Mucke, L.; Gan, L. Imbalance between GABAergic and glutamatergic transmission impairs adult neurogenesis in an animal model of Alzheimer’s disease. Cell Stem Cell 2009, 5, 624–633. [Google Scholar] [CrossRef]
- Otaru, N.; Ye, K.; Mujezinovic, D.; Berchtold, L.; Constancias, F.; Cornejo, F.A.; Krzystek, A.; de Wouters, T.; Braegger, C.; Lacroix, C.; et al. GABA production by human intestinal Bacteroides Spp.: Prevalence, regulation, and role in acid stress tolerance. Front. Microbiol. 2021, 12, 656895. [Google Scholar] [CrossRef]
- Bravo, J.A.; Forsythe, P.; Chew, M.V.; Escaravage, E.; Savignac, H.M.; Dinan, T.G.; Bienenstock, J.; Cryan, J.F. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc. Natl. Acad. Sci. USA 2011, 108, 16050–16055. [Google Scholar] [CrossRef]
- Zhu, N.; Wei, M.; Yuan, L.; He, X.; Chen, C.; Ji, A.; Zhang, G. Claudin-5 relieves cognitive decline in Alzheimer’s disease mice through suppression of inhibitory GABAergic neurotransmission. Aging 2022, 14, 3554–3568. [Google Scholar] [CrossRef]
- Braun, H.-S.; Sponder, G.; Pieper, R.; Aschenbach, J.R.; Deiner, C. GABA selectively increases mucin-1 expression in isolated pig jejunum. Genes Nutr. 2015, 10, 47. [Google Scholar] [CrossRef]
- Ma, X.; Sun, Q.; Sun, X.; Chen, D.; Wei, C.; Yu, X.; Liu, C.; Li, Y.; Li, J. Activation of GABAA receptors in colon epithelium exacerbates acute colitis. Front. Immunol. 2018, 9, 987. [Google Scholar] [CrossRef]
- Marrosu, F.; Serra, A.; Maleci, A.; Puligheddu, M.; Biggio, G.; Piga, M. Correlation between GABAA receptor density and vagus nerve stimulation in individuals with drug-resistant partial epilepsy. Epilepsy Res. 2003, 55, 59–70. [Google Scholar] [CrossRef]
- Ben-Menachem, E.; Hamberger, A.; Hedner, T.; Hammond, E.J.; Uthman, B.M.; Slater, J.; Treig, T.; Stefan, H.; Ramsay, R.E.; Wernicke, J.F.; et al. Effects of vagus nerve stimulation on amino acids and other metabolites in the CSF of patients with partial seizures. Epilepsy Res. 1995, 20, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zou, Q.; Han, S.; Liang, J.; Yan, G.; Wang, Q.; Wang, Y.; Zhang, Z.; Hu, J.; Li, J.; Yuan, T.; et al. Alleviating effect of vagus nerve cutting in salmonella-induced gut infections and anxiety-like behavior via enhancing microbiota-derived GABA. Brain Behav. Immun. 2024, 119, 607–620. [Google Scholar] [CrossRef] [PubMed]
- Auteri, M.; Zizzo, M.G.; Serio, R. GABA and GABA receptors in the gastrointestinal tract: From motility to inflammation. Pharmacol. Res. 2015, 93, 11–21. [Google Scholar] [CrossRef] [PubMed]
- Gheorghe, C.E.; Martin, J.A.; Manriquez, F.V.; Dinan, T.G.; Cryan, J.F.; Clarke, G. Focus on the essentials: Tryptophan metabolism and the microbiome-gut-brain axis. Curr. Opin. Pharmacol. 2019, 48, 137–145. [Google Scholar] [CrossRef]
- Argolo, D.S.; de Oliveira, L.M.G.; Guillemin, G.J.; Barreto, G.E.; Butt, A.M.; Costa, S.L.; Costa, M.d.F.D. Tryptophan metabolism through the kynurenine pathway in glial cells. Neuroglia 2025, 6, 14. [Google Scholar] [CrossRef]
- Xie, L.; Wu, Q.; Li, K.; Khan, M.A.S.; Zhang, A.; Sinha, B.; Li, S.; Chang, S.L.; Brody, D.L.; Grinstaff, M.W.; et al. Tryptophan metabolism in Alzheimer’s disease with the involvement of microglia and astrocyte crosstalk and gut-brain axis. Aging Dis. 2024, 15, 2168–2190. [Google Scholar] [CrossRef]
- Yusufu, I.; Ding, K.; Smith, K.; Wankhade, U.D.; Sahay, B.; Patterson, G.T.; Pacholczyk, R.; Adusumilli, S.; Hamrick, M.W.; Hill, W.D.; et al. A tryptophan-deficient diet induces gut microbiota dysbiosis and increases systemic inflammation in aged mice. Int. J. Mol. Sci. 2021, 22, 5005. [Google Scholar] [CrossRef]
- Akhgarjand, C.; Vahabi, Z.; Shab-Bidar, S.; Etesam, F.; Djafarian, K. Effects of probiotic supplements on cognition, anxiety, and physical activity in subjects with mild and moderate Alzheimer’s disease: A randomized, double-blind, and placebo-controlled study. Front. Aging Neurosci. 2022, 14, 1032494. [Google Scholar] [CrossRef]
- Medeiros, D.; McMurry, K.; Pfeiffer, M.; Newsome, K.; Testerman, T.; Graf, J.; Silver, A.C.; Sacchetti, P. Slowing Alzheimer’s disease progression through probiotic supplementation. Front. Neurosci. 2024, 18, 1309075. [Google Scholar] [CrossRef]
- Webberley, T.S.; Bevan, R.J.; Kerry-Smith, J.; Dally, J.; Michael, D.R.; Thomas, S.; Rees, M.; Morgan, J.E.; Marchesi, J.R.; Good, M.A.; et al. Assessment of Lab4P probiotic effects on cognition in 3xTg-AD Alzheimer’s disease model mice and the SH-SY5Y neuronal cell line. Int. J. Mol. Sci. 2023, 24, 4683. [Google Scholar] [CrossRef]
- Li, X.; Lv, C.; Song, J.; Li, J. Effect of probiotic supplementation on cognitive function and metabolic status in mild cognitive impairment and Alzheimer’s disease: A meta-analysis. Front. Nutr. 2021, 8, 757673. [Google Scholar] [CrossRef]
- Qu, C.; Xu, Q.-Q.; Yang, W.; Zhong, M.; Yuan, Q.; Xian, Y.-F.; Lin, Z.-X. Gut dysbiosis aggravates cognitive deficits, amyloid pathology and lipid metabolism dysregulation in a transgenic mouse model of Alzheimer’s disease. J. Pharm. Anal. 2023, 13, 1526–1547. [Google Scholar] [CrossRef]
- Relman, A.S. Tacrine as a treatment for Alzheimer’s dementia. N. Engl. J. Med. 1991, 324, 349. [Google Scholar] [CrossRef] [PubMed]
- Becker, R.E.; Greig, N.H.; Giacobini, E. Why do so many drugs for Alzheimer’s disease fail in development? Time for new methods and new practices? J. Alzheimer’s Dis. 2008, 15, 303–325. [Google Scholar] [CrossRef]
- Weinreb, O.; Bar-Am, O.; Amit, T.; Drigues, N.; Sagi, Y.; Youdim, M.B.H. The neuroprotective effect of ladostigil against hydrogen peroxide-mediated cytotoxicity. Chem.-Biol. Interact. 2008, 175, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Adebambo, K.; Ojoh, O. (Claudia). In silico investigation of novel compounds as inhibitors of acetylcholinesterase enzyme for the treatment of Alzheimer’s diseases. Int. J. Alzheimer’s Dis. 2024, 2988685. [Google Scholar] [CrossRef] [PubMed]
- Damar, U.; Gersner, R.; Johnstone, J.T.; Schachter, S.; Rotenberg, A. Huperzine a as a neuroprotective and antiepileptic drug: A review of preclinical research. Expert Rev. Neurother. 2016, 16, 671–680. [Google Scholar] [CrossRef]
- Reisberg, B.; Doody, R.; Stöffler, A.; Schmitt, F.; Ferris, S.; Möbius, H.J. Memantine in moderate-to-severe Alzheimer’s disease. N. Engl. J. Med. 2003, 348, 1333–1341. [Google Scholar] [CrossRef]
- Califf, T. Safety and effectiveness of physostigmine: A 10-year retrospective review. J. Emerg. Med. 2018, 54, 577–578. [Google Scholar] [CrossRef]
- Maelicke, A.; Hoeffle-Maas, A.; Ludwig, J.; Maus, A.; Samochocki, M.; Jordis, U.; Koepke, A.K.E. Memogain is a galantamine pro-drug having dramatically reduced adverse effects and enhanced efficacy. J. Mol. Neurosci. 2010, 40, 135–137. [Google Scholar] [CrossRef]
- Lendvai, B.; Kassai, F.; Szájli, Á.; Némethy, Z. A7 nicotinic acetylcholine receptors and their role in cognition. Brain Res. Bull. 2013, 93, 86–96. [Google Scholar] [CrossRef]
- Forman, M.; Palcza, J.; Tseng, J.; Leempoels, J.; Ramael, S.; Han, D.; Jhee, S.; Ereshefsky, L.; Tanen, M.; Laterza, O.; et al. The novel BACE inhibitor MK-8931 dramatically lowers cerebrospinal fluid Aβ peptides in healthy subjects following single- and multiple-dose administration. Alzheimer’s Dement. 2012, 8, P704. [Google Scholar] [CrossRef]
- Gillman, K.W.; Starrett, J.E., Jr.; Parker, M.F.; Xie, K.; Bronson, J.J.; Marcin, L.R.; McElhone, K.E.; Bergstrom, C.P.; Mate, R.A.; Williams, R.; et al. Discovery and evaluation of BMS-708163, a potent, selective and orally bioavailable γ-secretase inhibitor. ACS Med. Chem. Lett. 2010, 1, 120–124. [Google Scholar] [CrossRef]
- Aisen, P.S.; Gauthier, S.; Vellas, B.; Briand, R.; Saumier, D.; Laurin, J.; Garceau, D. Alzhemed: A potential treatment for Alzheimer’s disease. Curr. Alzheimer Res. 2007, 4, 473–478. [Google Scholar] [CrossRef]
- Mandel, S.A.; Amit, T.; Kalfon, L.; Reznichenko, L.; Weinreb, O.; Youdim, M.B.H. Cell signaling pathways and iron chelation in the neurorestorative activity of green tea polyphenols: Special reference to epigallocatechin gallate (EGCG). J. Alzheimer’s Dis. 2008, 15, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.-L.; Li, C.; Yin, H.-H.; He, Y.; Guan, Y.-X. In silico screening of small molecule inhibitors for amyloid-β aggregation. J. Chem. Inf. Model. 2025, 65, 6238–6248. [Google Scholar] [CrossRef]
- Ciaglia, T.; Miranda, M.R.; Di Micco, S.; Vietri, M.; Smaldone, G.; Musella, S.; Di Sarno, V.; Auriemma, G.; Sardo, C.; Moltedo, O.; et al. Neuroprotective potential of indole-based compounds: A biochemical study on antioxidant properties and amyloid disaggregation in neuroblastoma cells. Antioxidants 2024, 13, 1585. [Google Scholar] [CrossRef]
- Barresi, E.; Baglini, E.; Poggetti, V.; Castagnoli, J.; Giorgini, D.; Salerno, S.; Taliani, S.; Da Settimo, F. Indole-Based Compounds in the Development of Anti-Neurodegenerative Agents. Molecules 2024, 29, 2127. [Google Scholar] [CrossRef]
- Cummings, J. Anti-amyloid monoclonal antibodies are transformative treatments that redefine Alzheimer’s disease therapeutics. Drugs 2023, 83, 569–576. [Google Scholar] [CrossRef]
- Ayan, E.; DeMirci, H.; Serdar, M.A.; Palermo, F.; Baykal, A.T. Bridging the gap between gut microbiota and Alzheimer’s disease: A metaproteomic approach for biomarker discovery in transgenic mice. Int. J. Mol. Sci. 2023, 24, 12819. [Google Scholar] [CrossRef] [PubMed]
- Hoyer, W.; Grönwall, C.; Jonsson, A.; Ståhl, S.; Härd, T. Stabilization of a β-hairpin in monomeric Alzheimer’s amyloid-β peptide inhibits amyloid formation. Proc. Natl. Acad. Sci. USA 2008, 105, 5099–5104. [Google Scholar] [CrossRef]
- Allen, S.G.; Meade, R.M.; White Stenner, L.L.; Mason, J.M. Peptide-based approaches to directly target alpha-synuclein in Parkinson’s disease. Mol. Neurodegener. 2023, 18, 80. [Google Scholar] [CrossRef] [PubMed]
- Oller-Salvia, B.; Sánchez-Navarro, M.; Giralt, E.; Teixidó, M. Blood–brain barrier shuttle peptides: An emerging paradigm for brain delivery. Chem. Soc. Rev. 2016, 45, 4690–4707. [Google Scholar] [CrossRef] [PubMed]
- Parthsarathy, V.; McClean, P.L.; Hölscher, C.; Taylor, M.; Tinker, C.; Jones, G.; Kolosov, O.; Salvati, E.; Gregori, M.; Masserini, M.; et al. A novel retro-inverso peptide inhibitor reduces amyloid deposition, oxidation and inflammation and stimulates neurogenesis in the APPswe/PS1ΔE9 mouse model of Alzheimer’s disease. PLoS ONE 2013, 8, e54769. [Google Scholar] [CrossRef]
- Wu, Y.; Angelova, A. Recent uses of lipid nanoparticles, cell-penetrating and bioactive peptides for the development of brain-targeted nanomedicines against neurodegenerative disorders. Nanomaterials 2023, 13, 3004. [Google Scholar] [CrossRef]
- Scarmeas, N.; Stern, Y.; Mayeux, R.; Manly, J.J.; Schupf, N.; Luchsinger, J.A. Mediterranean diet and mild cognitive impairment. Arch. Neurol. 2009, 66, 216–225. [Google Scholar] [CrossRef]
- Petersson, S.D.; Philippou, E. Mediterranean diet, cognitive function, and dementia: A systematic review of the evidence. Adv. Nutr. 2016, 7, 889–904. [Google Scholar] [CrossRef]
- Brandt, J.; Buchholz, A.; Henry-Barron, B.; Vizthum, D.; Avramopoulos, D.; Cervenka, M.C. Preliminary report on the feasibility and efficacy of the modified Atkins diet for treatment of mild cognitive impairment and early Alzheimer’s disease. J. Alzheimer’s Dis. 2019, 68, 969–981. [Google Scholar] [CrossRef]
- Taylor, M.K.; Sullivan, D.K.; Mahnken, J.D.; Burns, J.M.; Swerdlow, R.H. Feasibility and efficacy data from a ketogenic diet intervention in Alzheimer’s disease. Alzheimer’s Dement. Transl. Res. Clin. Interv. 2018, 4, 28–36. [Google Scholar] [CrossRef]
- Freund-Levi, Y.; Eriksdotter-Jönhagen, M.; Cederholm, T.; Basun, H.; Faxén-Irving, G.; Garlind, A.; Vedin, I.; Vessby, B.; Wahlund, L.-O.; Palmblad, J. ω-3 fatty acid treatment in 174 patients with mild to moderate Alzheimer disease: OmegAD study: A randomized double-blind trial. Arch. Neurol. 2006, 63, 1402–1408. [Google Scholar] [CrossRef]
- Rosenberg, A.; Ngandu, T.; Rusanen, M.; Antikainen, R.; Bäckman, L.; Havulinna, S.; Hänninen, T.; Laatikainen, T.; Lehtisalo, J.; Levälahti, E.; et al. Multidomain lifestyle intervention benefits a large elderly population at risk for cognitive decline and dementia regardless of baseline characteristics: The FINGER trial. Alzheimers Dement. 2018, 14, 263–270. [Google Scholar] [CrossRef]
- Hershey, M.S.; Sotos-Prieto, M.; Andrieu, S.; Hofman, A.; Magiatis, P.; Martinez-Gonzalez, M.A.; Yannakoulia, M.; Kales, S.N.; Scarmeas, N. Prevention of Alzheimer’s disease and cognitive decline with diet & lifestyle: Proceedings of the A. G. Leventis Foundation Conference. J. Prev. Alzheimer’s Dis. 2023, 10, 137–143. [Google Scholar] [CrossRef]
- Alzheimer’s disease: Research summaries—How effective are cholinesterase inhibitors? In InformedHealth.org; IQWiG: Cologne, Germany, 2022.
- Su, C.H.; Chang, Y.T.; Tseng, H.S.; Kuo, C.Y.; Chen, J.H.; Chien, P.Y.; Chang, Y.J.; Hung, C.C. Comparisons of efficacy and safety of immunotherapies for Alzheimer’s disease treatment: A network meta-analysis of randomised controlled trials. Clin. Med. 2025, 25, 100336. [Google Scholar] [CrossRef]
- Honig, L.S.; Sabbagh, M.N.; van Dyck, C.H.; Sperling, R.A.; Hersch, S.; Matta, A.; Giorgi, L.; Gee, M.; Kanekiyo, M.; Li, D.; et al. Updated safety results from phase 3 lecanemab study in early Alzheimer’s disease. Alzheimers Res. Ther. 2024, 10, 105. [Google Scholar] [CrossRef]
- Das, B.; Yan, R. A close look at BACE1 Inhibitors for Alzheimer’s disease treatment. CNS Drugs 2019, 33, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Henley, D.B.; Sundell, K.L.; Sethuraman, G.; Dowsett, S.A.; May, P.C. Safety profile of semagacestat, a gamma-secretase inhibitor: IDENTITY trial findings. Curr. Med. Res. Opin. 2014, 30, 2021–2032. [Google Scholar] [CrossRef] [PubMed]
- Lanzillotta, A.; Sarnico, I.; Benarese, M.; Branca, C.; Baiguera, C.; Hutter-Paier, B.; Windisch, M.; Spano, P.; Imbimbo, B.P.; Pizzi, M. The γ-secretase modulator CHF5074 reduces the accumulation of native hyperphosphorylated tau in a transgenic mouse model of Alzheimer’s disease. J. Mol. Neurosci. 2011, 45, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Yao, W.; Yang, H.; Yang, J. Small-molecule drugs development for Alzheimer’s disease. Front. Aging Neurosci. 2022, 14, 1019412. [Google Scholar] [CrossRef]
- Morris, M.C.; Tangney, C.C.; Wang, Y.; Sacks, F.M.; Bennett, D.A.; Aggarwal, N.T. MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1007–1014. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abdol Samat, H.N.; Razali, N.N.; Mahadzir, H.; Tengku Muhammad, T.S.; Ling, K.-H.; Mansor, N.I.; Abidin, S.Z. The Interplay of Inflammation and Gut-Microbiota Dysbiosis in Alzheimer’s Disease: Mechanisms and Therapeutic Potential. Int. J. Mol. Sci. 2025, 26, 8905. https://doi.org/10.3390/ijms26188905
Abdol Samat HN, Razali NN, Mahadzir H, Tengku Muhammad TS, Ling K-H, Mansor NI, Abidin SZ. The Interplay of Inflammation and Gut-Microbiota Dysbiosis in Alzheimer’s Disease: Mechanisms and Therapeutic Potential. International Journal of Molecular Sciences. 2025; 26(18):8905. https://doi.org/10.3390/ijms26188905
Chicago/Turabian StyleAbdol Samat, Hanis Nabilah, Nurul Nadirah Razali, Hazlina Mahadzir, Tengku Sifzizul Tengku Muhammad, King-Hwa Ling, Nur Izzati Mansor, and Shahidee Zainal Abidin. 2025. "The Interplay of Inflammation and Gut-Microbiota Dysbiosis in Alzheimer’s Disease: Mechanisms and Therapeutic Potential" International Journal of Molecular Sciences 26, no. 18: 8905. https://doi.org/10.3390/ijms26188905
APA StyleAbdol Samat, H. N., Razali, N. N., Mahadzir, H., Tengku Muhammad, T. S., Ling, K.-H., Mansor, N. I., & Abidin, S. Z. (2025). The Interplay of Inflammation and Gut-Microbiota Dysbiosis in Alzheimer’s Disease: Mechanisms and Therapeutic Potential. International Journal of Molecular Sciences, 26(18), 8905. https://doi.org/10.3390/ijms26188905






