A Pharmacological Dose of Liraglutide Improves Mitochondrial Performance in Mouse Leydig Cells
Abstract
1. Introduction
2. Results
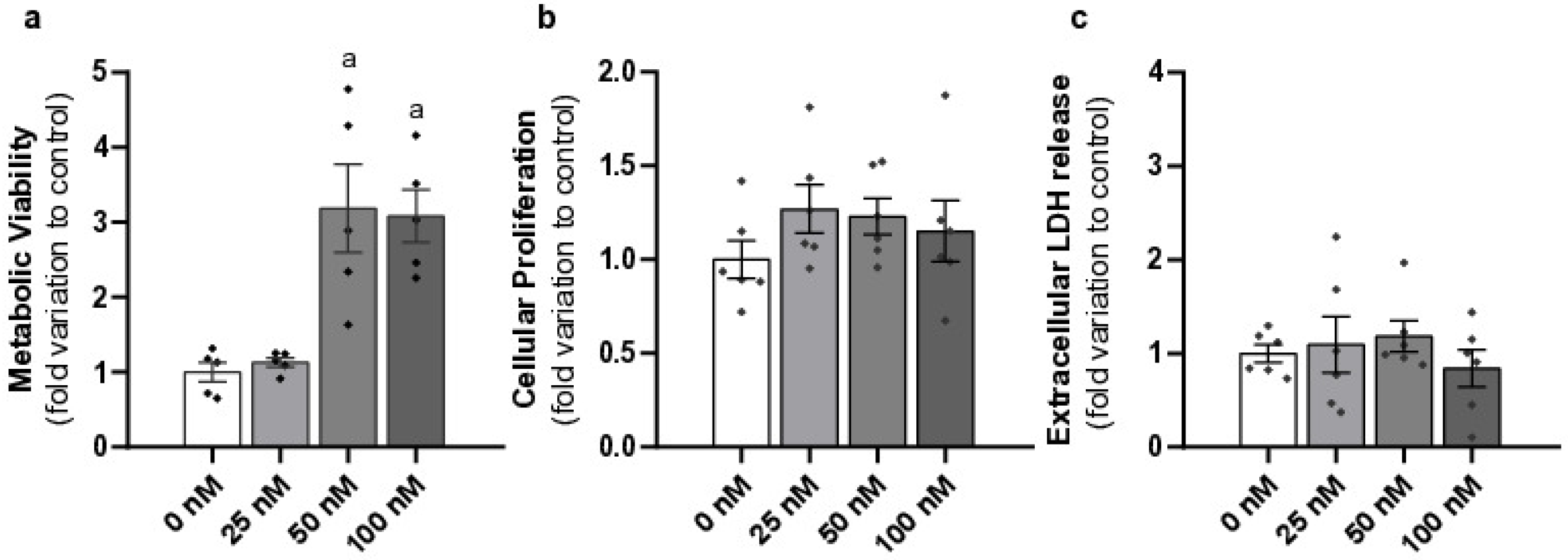
2.1. Liraglutide Increased Metabolic Viability Without Causing Cytotoxicity in Mouse Leydig Cells
2.2. Liraglutide Decreased ROS Production and Did Not Alter the Mitochondrial Membrane Potential of Mouse Leydig Cells
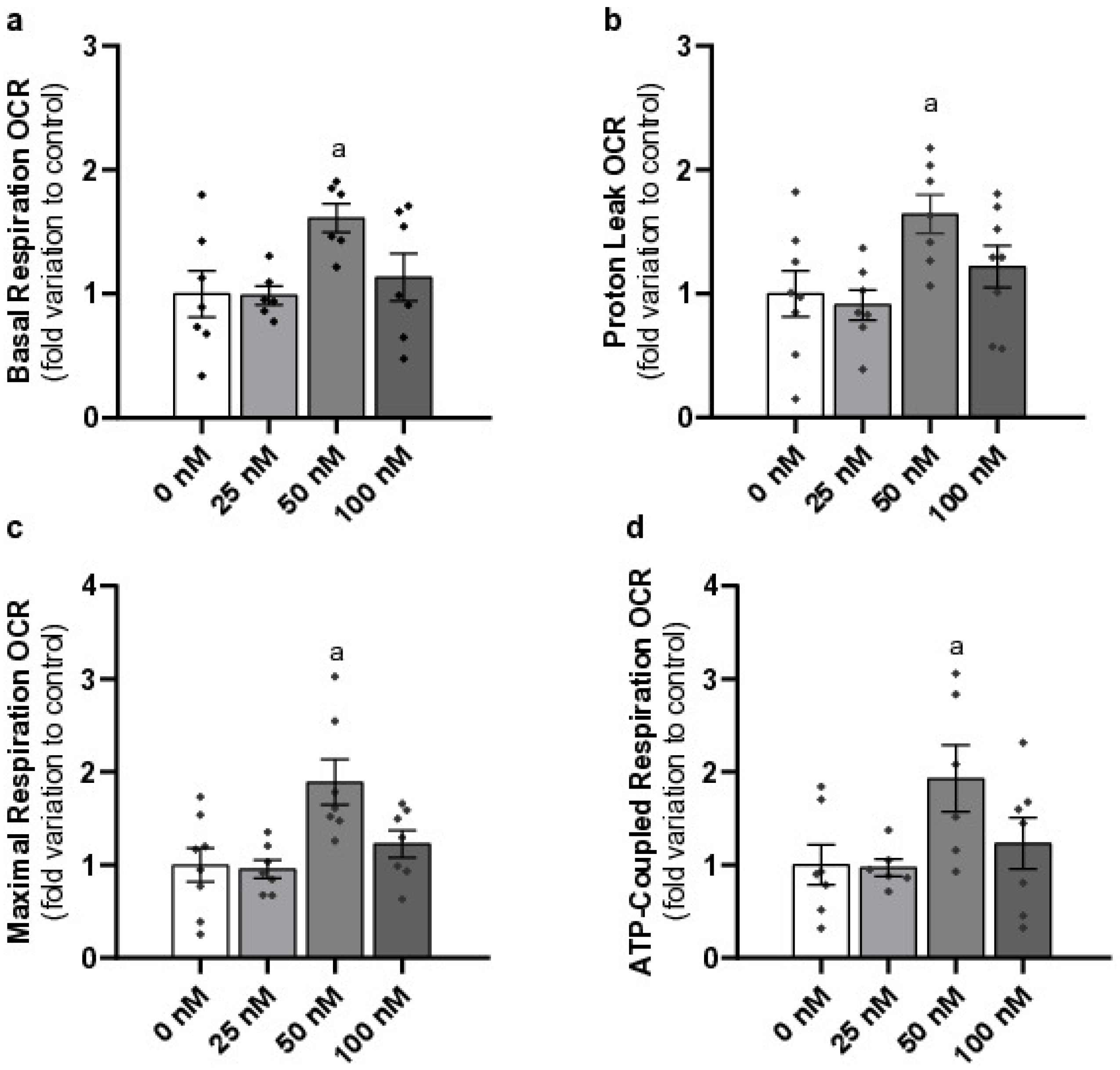
2.3. Pharmacological Concentration of Liraglutide Increases Basal Respiration, Proton Leak, Maximal Respiration, and ATP-Coupled Respiration of Mouse Leydig Cells
2.4. Liraglutide Does Not Affect Mitochondrial DNA Copy Number or Androstenedione Production in Mouse Leydig Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Experimental Groups
4.2. Viability Assays
4.2.1. Sulforhodamine B (SRB) Cytotoxicity Assay
4.2.2. Lactate Dehydrogenase (LDH) Release Assay
4.2.3. MTT Viability Assay
4.3. General Oxidative Stress Indicator Assay
4.4. Gene Expression
4.4.1. DNA Extraction
4.4.2. Quantitative Real-Time PCR (qPCR)
4.5. Mitochondrial Membrane Potential Assay
4.6. Cellular Oxygen Consumption Analysis
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agbaje, I.M.; Rogers, D.A.; McVicar, C.M.; McClure, N.; Atkinson, A.B.; Mallidis, C.; Lewis, S.E. Insulin dependant diabetes mellitus: Implications for male reproductive function. Hum. Reprod. 2007, 22, 1871–1877. [Google Scholar] [CrossRef]
- Hamilton, B.E.; Hoyert, D.L.; Martin, J.A.; Strobino, D.M.; Guyer, B. Annual summary of vital statistics: 2010–2011. Pediatrics 2013, 131, 548–558. [Google Scholar] [CrossRef]
- Drucker, D.J.; Dritselis, A.; Kirkpatrick, P. Liraglutide. Nat. Rev. Drug Discov. 2010, 9, 267–268. [Google Scholar] [CrossRef] [PubMed]
- Rato, L.; Oliveira, P.F.; Sousa, M.; Silva, B.M.; Alves, M.G. Role of reactive oxygen species in diabetes-induced male reproductive dysfunction. In Oxidants, Antioxidants and Impact of the Oxidative Status in Male Reproduction; Elsevier: Amsterdam, The Netherlands, 2019; pp. 135–147. [Google Scholar]
- Bode, B. An overview of the pharmacokinetics, efficacy and safety of liraglutide. Diabetes Res. Clin. Pract. 2012, 97, 27–42. [Google Scholar] [CrossRef]
- Ladenheim, E.E. Liraglutide and obesity: A review of the data so far. Drug Des. Dev. Ther. 2015, 9, 1867–1875. [Google Scholar] [CrossRef]
- Gorboulev, V.; Schürmann, A.; Vallon, V.; Kipp, H.; Jaschke, A.; Klessen, D.; Friedrich, A.; Scherneck, S.; Rieg, T.; Cunard, R. Na+-D-glucose cotransporter SGLT1 is pivotal for intestinal glucose absorption and glucose-dependent incretin secretion. Diabetes 2012, 61, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.; Bloom, S.R.; Buenaventura, T.; Tomas, A.; Rutter, G.A. Control of insulin secretion by GLP-1. Peptides 2018, 100, 75–84. [Google Scholar] [CrossRef]
- Chaudhury, A.; Duvoor, C.; Reddy Dendi, V.S.; Kraleti, S.; Chada, A.; Ravilla, R.; Marco, A.; Shekhawat, N.S.; Montales, M.T.; Kuriakose, K.; et al. Clinical Review of Antidiabetic Drugs: Implications for Type 2 Diabetes Mellitus Management. Front. Endocrinol. 2017, 8, 6. [Google Scholar] [CrossRef]
- El-Kaissi, S.; Sherbeeni, S. Pharmacological management of type 2 diabetes mellitus: An update. Curr. Diabetes Rev. 2011, 7, 392–405. [Google Scholar] [CrossRef]
- Pelusi, C. The Effects of the New Therapeutic Treatments for Diabetes Mellitus on the Male Reproductive Axis. Front. Endocrinol. 2022, 13, 821113. [Google Scholar] [CrossRef]
- Oride, A.; Kanasaki, H.; Mijiddorj, T.; Sukhbaatar, U.; Hara, T.; Tumurbaatar, T.; Kyo, S. GLP-1 increases Kiss-1 mRNA expression in kisspeptin-expressing neuronal cells. Biol. Reprod. 2017, 97, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Caltabiano, R.; Condorelli, D.; Panza, S.; Boitani, C.; Musso, N.; Ježek, D.; Memeo, L.; Colarossi, L.; Rago, V.; Mularoni, V. Glucagon-like peptide-1 receptor is expressed in human and rodent testis. Andrology 2020, 8, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Cannarella, R.; Calogero, A.E.; Condorelli, R.A.; Greco, E.A.; Aversa, A.; La Vignera, S. Is there a role for glucagon-like peptide-1 receptor agonists in the treatment of male infertility? Andrology 2021, 9, 1499–1503. [Google Scholar] [CrossRef] [PubMed]
- Martins, A.D.; Monteiro, M.P.; Silva, B.M.; Barros, A.; Sousa, M.; Carvalho, R.A.; Oliveira, P.F.; Alves, M.G. Metabolic dynamics of human Sertoli cells are differentially modulated by physiological and pharmacological concentrations of GLP-1. Toxicol. Appl. Pharmacol. 2019, 362, 1–8. [Google Scholar] [CrossRef]
- MacLusky, N.J.; Cook, S.; Scrocchi, L.; Shin, J.; Kim, J.; Vaccarino, F.; Asa, S.L.; Drucker, D.J. Neuroendocrine function and response to stress in mice with complete disruption of glucagon-like peptide-1 receptor signaling. Endocrinology 2000, 141, 752–762. [Google Scholar] [CrossRef][Green Version]
- Zhang, E.; Xu, F.; Liang, H.; Yan, J.; Xu, H.; Li, Z.; Wen, X.; Weng, J. GLP-1 Receptor Agonist Exenatide Attenuates the Detrimental Effects of Obesity on Inflammatory Profile in Testis and Sperm Quality in Mice. Am. J. Reprod. Immunol. 2015, 74, 457–466. [Google Scholar] [CrossRef]
- Giagulli, V.A.; Carbone, M.D.; Ramunni, M.I.; Licchelli, B.; De Pergola, G.; Sabba, C.; Guastamacchia, E.; Triggiani, V. Adding liraglutide to lifestyle changes, metformin and testosterone therapy boosts erectile function in diabetic obese men with overt hypogonadism. Andrology 2015, 3, 1094–1103. [Google Scholar] [CrossRef]
- La Vignera, S.; Condorelli, R.A.; Calogero, A.E.; Cannarella, R.; Aversa, A. Sexual and Reproductive Outcomes in Obese Fertile Men with Functional Hypogonadism After Treatment with Liraglutide: Preliminary Results. J. Clin. Med. 2023, 12, 672. [Google Scholar] [CrossRef]
- Fontoura, P.; Cardoso, M.C.; Erthal-Martins, M.C.; Werneck, C.; Sartorio, C.; Ramos, C.F. The effects of liraglutide on male fertility: A case report. Reprod. Biomed. Online 2014, 29, 644–646. [Google Scholar] [CrossRef]
- Pourheydar, M.; Hasanzadeh, S.; Razi, M.; Pourheydar, B.; Najafi, G. Effects of liraglutide on sperm characteristics and fertilization potential following experimentally induced diabetes in mice. Vet. Res. Forum 2021, 12, 109–116. [Google Scholar] [CrossRef]
- Chew, N.W.S.; Ng, C.H.; Tan, D.J.H.; Kong, G.; Lin, C.; Chin, Y.H.; Lim, W.H.; Huang, D.Q.; Quek, J.; Fu, C.E.; et al. The global burden of metabolic disease: Data from 2000 to 2019. Cell Metab. 2023, 35, 414–428.e3. [Google Scholar] [CrossRef]
- Wronka, M.; Krzemińska, J.; Młynarska, E.; Rysz, J.; Franczyk, B. New Insights into the Use of Liraglutide—Impact on Cardiovascular Risk and Microvascular Outcomes. Biomedicines 2023, 11, 1159. [Google Scholar] [CrossRef]
- Rafiullah, M.; Benabdelkamel, H.; Masood, A.; Ekhzaimy, A.A.; Musambil, M.; Joy, S.S.; Alfadda, A.A. Urinary Proteome Differences in Patients with Type 2 Diabetes Pre and Post Liraglutide Treatment. Curr. Issues Mol. Biol. 2023, 45, 1407–1421. [Google Scholar] [CrossRef] [PubMed]
- Ray, S.; Pramanik, S. Reproductive dysfunctions in males with type 2 diabetes mellitus: An updated review. Diabetes 2020, 8, 79–89. [Google Scholar] [CrossRef]
- Ingwersen, S.H.; Khurana, M.; Madabushi, R.; Watson, E.; Jonker, D.M.; Le Thi, T.D.; Jacobsen, L.V.; Tornoe, C.W. Dosing rationale for liraglutide in type 2 diabetes mellitus: A pharmacometric assessment. J. Clin. Pharmacol. 2012, 52, 1815–1823. [Google Scholar] [CrossRef] [PubMed]
- Nuffer, W.A.; Trujillo, J.M. Liraglutide: A New Option for the Treatment of Obesity. Pharmacotherapy 2015, 35, 926–934. [Google Scholar] [CrossRef]
- Salvio, G.; Ciarloni, A.; Ambo, N.; Bordoni, M.; Perrone, M.; Rossi, S.; Balercia, G. Effects of glucagon-like peptide 1 receptor agonists on testicular dysfunction: A systematic review and meta-analysis. Andrology 2025. [Google Scholar] [CrossRef]
- Fang, D.; Huang, Z.; Guan, H.; Liu, J.; Yao, B.; Xiao, H.; Li, Y. The Akt/FoxO1/p27 pathway mediates the proliferative action of liraglutide in beta cells. Mol. Med. Rep. 2012, 5, 233–238. [Google Scholar] [CrossRef]
- Bao, Y.; Jiang, L.; Chen, H.; Zou, J.; Liu, Z.; Shi, Y. The neuroprotective effect of liraglutide is mediated by glucagon-like peptide 1 receptor-mediated activation of cAMP/PKA/CREB pathway. Cell. Physiol. Biochem. 2015, 36, 2366–2378. [Google Scholar] [CrossRef]
- Zhang, L.; Tian, J.; Diao, S.; Zhang, G.; Xiao, M.; Chang, D. GLP-1 receptor agonist liraglutide protects cardiomyocytes from IL-1beta-induced metabolic disturbance and mitochondrial dysfunction. Chem. Biol. Interact. 2020, 332, 109252. [Google Scholar] [CrossRef]
- Allen, J.A.; Shankara, T.; Janus, P.; Buck, S.; Diemer, T.; Hales, K.H.; Hales, D.B. Energized, polarized, and actively respiring mitochondria are required for acute Leydig cell steroidogenesis. Endocrinology 2006, 147, 3924–3935. [Google Scholar] [CrossRef] [PubMed]
- Ardalan, A.; Smith, M.D.; Jelokhani-Niaraki, M. Uncoupling Proteins and Regulated Proton Leak in Mitochondria. Int. J. Mol. Sci. 2022, 23, 1528. [Google Scholar] [CrossRef] [PubMed]
- Brookes, P.S. Mitochondrial H+ leak and ROS generation: An odd couple. Free Radic. Biol. Med. 2005, 38, 12–23. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.; Nanayakkara, G.; Shao, Y.; Cueto, R.; Wang, L.; Yang, W.Y.; Tian, Y.; Wang, H.; Yang, X. Mitochondrial Proton Leak Plays a Critical Role in Pathogenesis of Cardiovascular Diseases. Adv. Exp. Med. Biol. 2017, 982, 359–370. [Google Scholar] [CrossRef]
- Chen, A.; Chen, Z.; Xia, Y.; Lu, D.; Yang, X.; Sun, A.; Zou, Y.; Qian, J.; Ge, J. Liraglutide attenuates NLRP3 inflammasome-dependent pyroptosis via regulating SIRT1/NOX4/ROS pathway in H9c2 cells. Biochem. Biophys. Res. Commun. 2018, 499, 267–272. [Google Scholar] [CrossRef]
- Zarzuelo, M.J.; Lopez-Sepulveda, R.; Sanchez, M.; Romero, M.; Gomez-Guzman, M.; Ungvary, Z.; Perez-Vizcaino, F.; Jimenez, R.; Duarte, J. SIRT1 inhibits NADPH oxidase activation and protects endothelial function in the rat aorta: Implications for vascular aging. Biochem. Pharmacol. 2013, 85, 1288–1296. [Google Scholar] [CrossRef]
- Jeng, J.Y.; Yeh, T.S.; Lee, J.W.; Lin, S.H.; Fong, T.H.; Hsieh, R.H. Maintenance of mitochondrial DNA copy number and expression are essential for preservation of mitochondrial function and cell growth. J. Cell. Biochem. 2008, 103, 347–357. [Google Scholar] [CrossRef]
- Engeli, R.T.; Fürstenberger, C.; Kratschmar, D.V.; Odermatt, A. Currently available murine Leydig cell lines can be applied to study early steps of steroidogenesis but not testosterone synthesis. Heliyon 2018, 4, e00527. [Google Scholar] [CrossRef]
- Pabreja, K.; Mohd, M.A.; Koole, C.; Wootten, D.; Furness, S.G. Molecular mechanisms underlying physiological and receptor pleiotropic effects mediated by GLP-1R activation. Br. J. Pharmacol. 2014, 171, 1114–1128. [Google Scholar] [CrossRef]
- McLean, B.A.; Wong, C.K.; Campbell, J.E.; Hodson, D.J.; Trapp, S.; Drucker, D.J. Revisiting the Complexity of GLP-1 Action from Sites of Synthesis to Receptor Activation. Endocr. Rev. 2021, 42, 101–132. [Google Scholar] [CrossRef]
- Puddu, A.; Maggi, D. Emerging Role of Caveolin-1 in GLP-1 Action. Front. Endocrinol. 2021, 12, 668012. [Google Scholar] [CrossRef] [PubMed]
- Forgacs, A.L.; Ding, Q.; Jaremba, R.G.; Huhtaniemi, I.T.; Rahman, N.A.; Zacharewski, T.R. BLTK1 murine Leydig cells: A novel steroidogenic model for evaluating the effects of reproductive and developmental toxicants. Toxicol. Sci. 2012, 127, 391–402. [Google Scholar] [CrossRef] [PubMed]
- Tichopad, A.; Dilger, M.; Schwarz, G.; Pfaffl, M.W. Liraglutide in Type 2 Diabetes Mellitus: Clinical Pharmacokinetics and Pharmacodynamics. Clin. Pharmacokinet. 2016, 55, 657–672. [Google Scholar] [CrossRef]
- Li, Q.; Xue, A.Y.; Li, Z.L.; Yin, Z. Liraglutide promotes apoptosis of HepG2 cells by activating JNK signaling pathway. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 3520–3526. [Google Scholar] [CrossRef]
- Martins, A.D.; Oliveira, P.F.; Alves, M.G. Assessment of Sertoli Cell Proliferation by 3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide and Sulforhodamine B Assays. Curr. Protoc. Toxicol. 2019, 81, e85. [Google Scholar] [CrossRef]
- Tichopad, A.; Dilger, M.; Schwarz, G.; Pfaffl, M.W. Standardized determination of real-time PCR efficiency from a single reaction set-up. Nucleic Acids Res. 2003, 31, e122. [Google Scholar] [CrossRef]


| Gene | Sequence 5′-3′ | Annealing T° | Cycles |
|---|---|---|---|
| ND1 | FWD: CATCTTATCCACGCTTCCG RVS: GTGGTACTCCCGCTGTAA | 60 °C | 35 |
| β-2-M | FWD: GTAACACAGTTCCACCCG RVS: TCGATCCCAGTAGACGGT | 58 °C | 30 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira-Lopes, B.; Braga, P.C.; Oliveira, P.F.; Alves, M.G.; Bernardino, R.L. A Pharmacological Dose of Liraglutide Improves Mitochondrial Performance in Mouse Leydig Cells. Int. J. Mol. Sci. 2025, 26, 8903. https://doi.org/10.3390/ijms26188903
Oliveira-Lopes B, Braga PC, Oliveira PF, Alves MG, Bernardino RL. A Pharmacological Dose of Liraglutide Improves Mitochondrial Performance in Mouse Leydig Cells. International Journal of Molecular Sciences. 2025; 26(18):8903. https://doi.org/10.3390/ijms26188903
Chicago/Turabian StyleOliveira-Lopes, Bruno, Patrícia C. Braga, Pedro F. Oliveira, Marco G. Alves, and Raquel L. Bernardino. 2025. "A Pharmacological Dose of Liraglutide Improves Mitochondrial Performance in Mouse Leydig Cells" International Journal of Molecular Sciences 26, no. 18: 8903. https://doi.org/10.3390/ijms26188903
APA StyleOliveira-Lopes, B., Braga, P. C., Oliveira, P. F., Alves, M. G., & Bernardino, R. L. (2025). A Pharmacological Dose of Liraglutide Improves Mitochondrial Performance in Mouse Leydig Cells. International Journal of Molecular Sciences, 26(18), 8903. https://doi.org/10.3390/ijms26188903








