Role of Mast Cells in Human Health and Disease: Controversies and Novel Therapies
Abstract
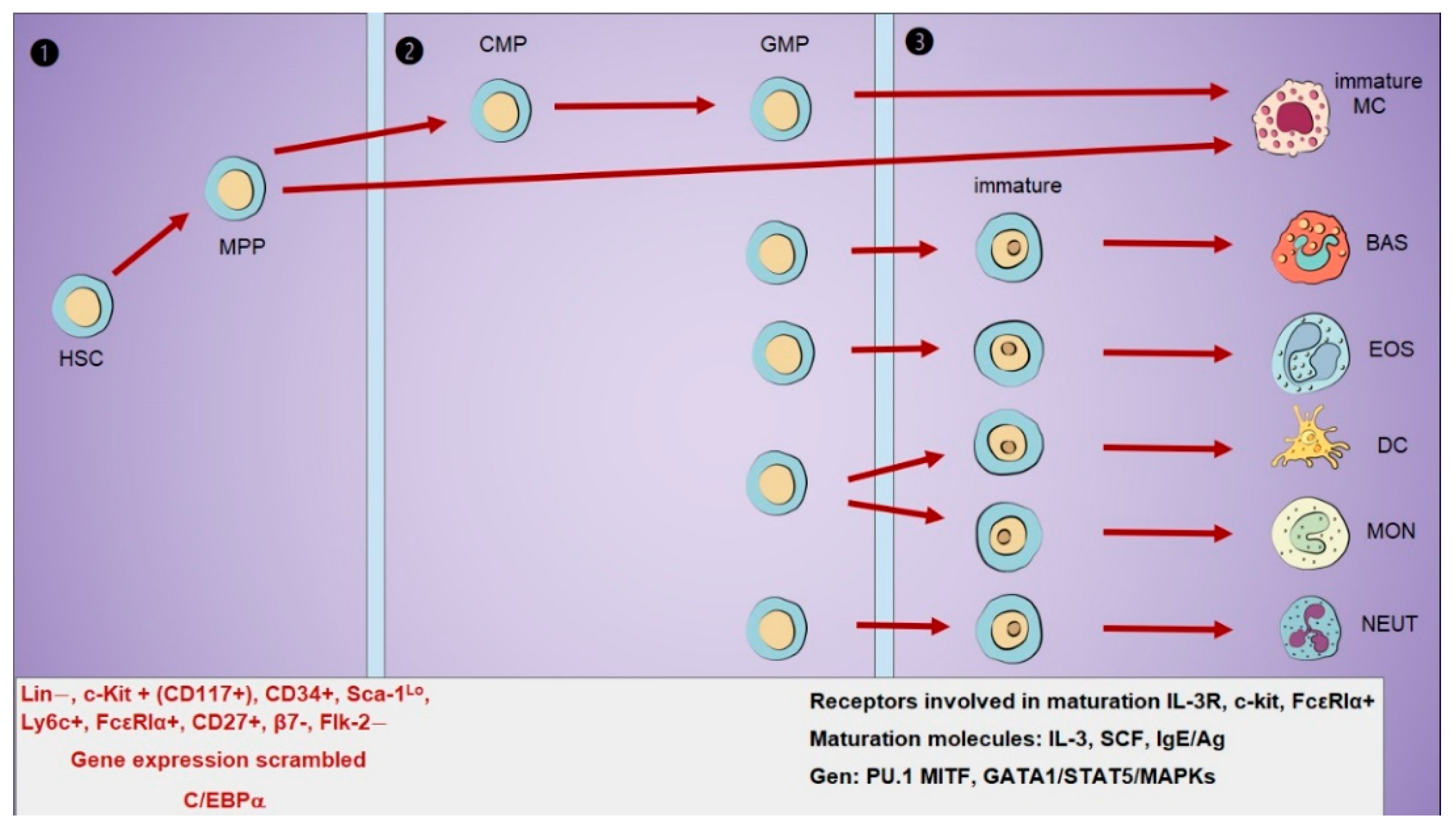
1. Origin and Development of Mast Cells
1.1. Mast Cell Precursors Identification
1.2. Receptor Expression During Differentiation
1.3. Receptors for Maturation and Tissue Migration
2. Activation Mechanisms of Mast Cells
2.1. IgE/Antigen-Dependent Activation
2.2. Inappropriate Mast Cell Reaction or Response
2.3. Non-IgE-Mediated Mast Cell Activation
3. Protective Roles in Homeostasis and Defense
3.1. Mast Cells as Mediators of Health
3.2. Proteases in Tissue Remodeling
3.3. Angiogenesis and Wound Repair
3.4. Reproductive System Regulation
3.5. UV Radiation Protection
3.6. Maintenance of Tissue Homeostasis
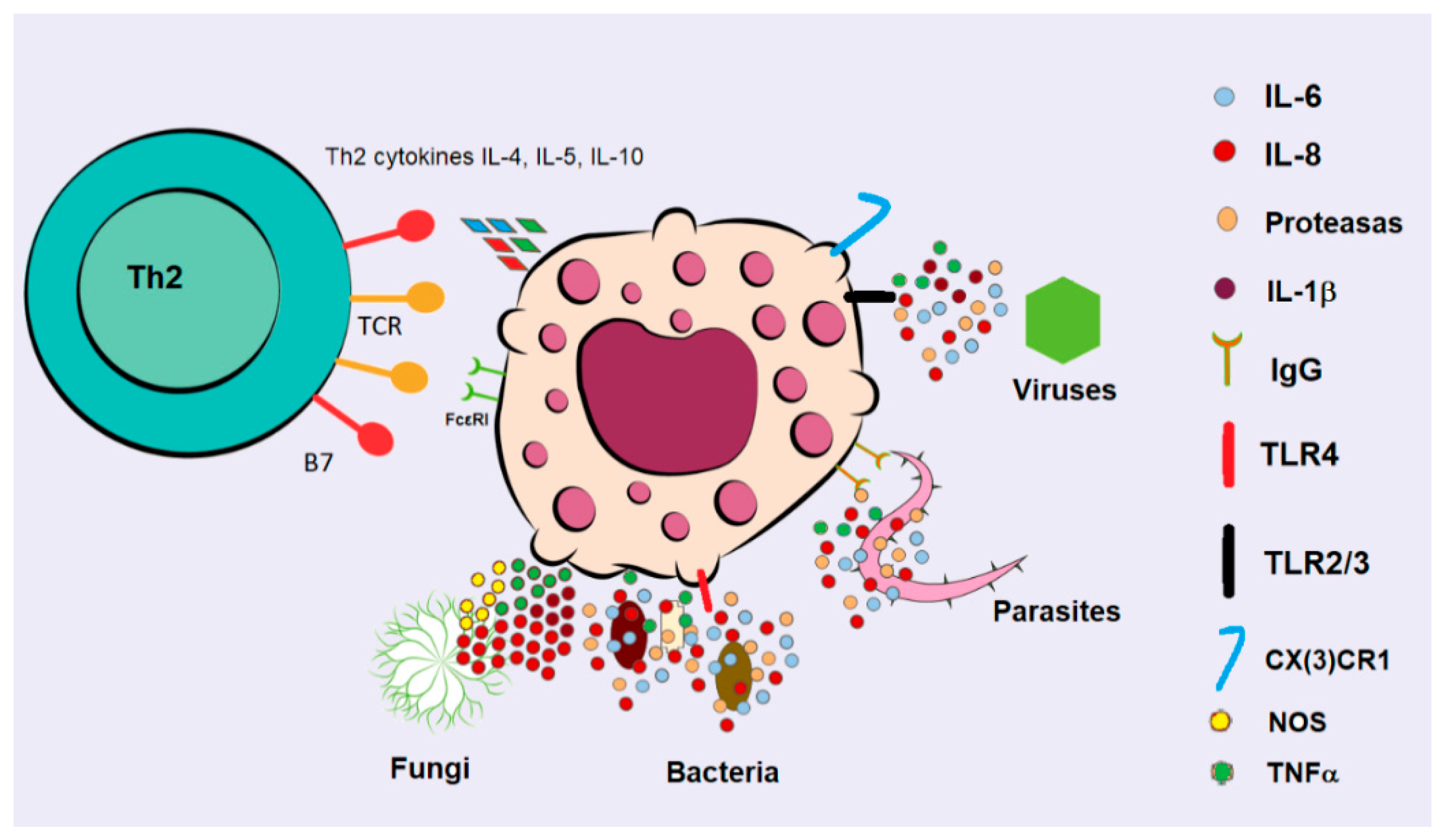
4. Mast Cells in Infections
Combating Infections
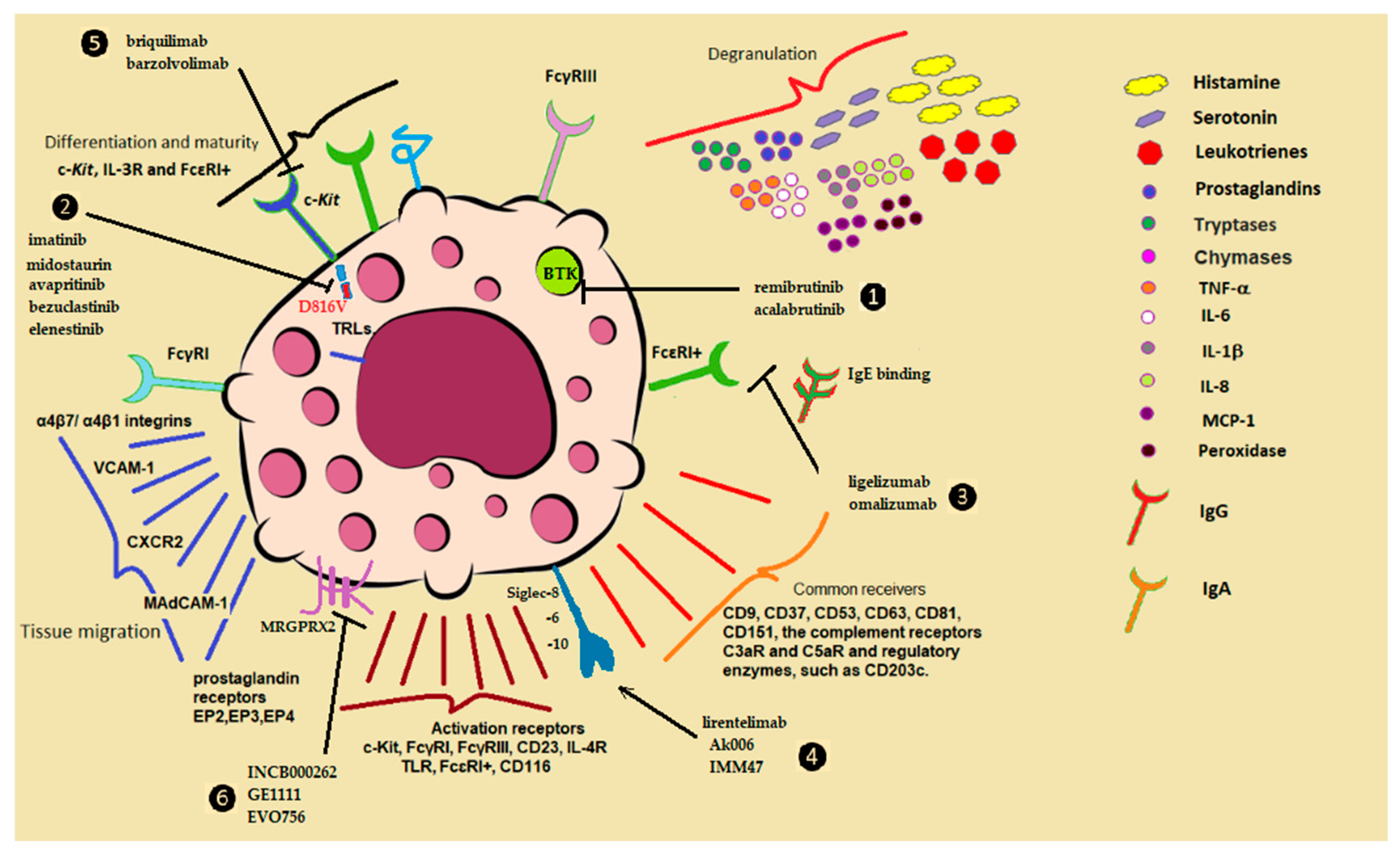
5. Mast Cell Dysfunction and Disease
5.1. Mastocytosis
5.2. Mast Cell Activation Syndrome (MCAS)
5.3. Allergic Diseases
5.4. Urticaria
5.5. Mast Cells in Cardiovascular Disease
6. Controversies
6.1. Heterogeneity in the Activity of MCs in Cancer
6.2. The Debated Role of MCs in Cardiovascular Pathologies
6.3. MCs in Inflammatory Diseases: Conflicting Inflammatory or Anti-Inflammatory Mechanisms
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribatti, D.; d’Amati, A. Hematopoiesis and Mast Cell Development. Int. J. Mol. Sci. 2023, 24, 10679. [Google Scholar] [CrossRef] [PubMed]
- Gentek, R.; Ghigo, C.; Hoeffel, G.; Bulle, M.J.; Msallam, R.; Gautier, G.; Launay, P.; Chen, J.; Ginhoux, F.; Bajénoff, M.C. Hemogenic Endothelial Fate Mapping Reveals Dual Developmental Origin of Mast Cells. Immunity 2018, 48, 1160–1171.e5. [Google Scholar] [CrossRef]
- Beaven, M.A. Our perception of the mast cell from Paul Ehrlich to now. Eur. J. Immunol. 2009, 39, 11–25. [Google Scholar] [CrossRef] [PubMed]
- Babina, M.; Schülke, Y.; Kirchhof, E.; Guhl, S.; Franke, R.; Böhm, S.; Zuberbier, T.; Henz, M.B.; Gombart, F.A. The transcription factor profile of human mast cells in comparison with monocytes and granulocytes. Cell. Mol. Life Sci. 2005, 62, 214–226. [Google Scholar] [CrossRef]
- Qi, X.; Hong, J.; Chaves, L.; Zhuang, Y.; Chen, Y.; Wang, D.; Chabon, J.; Graham, B.; Ohmori, K.; Li, Y.; et al. Antagonistic regulation by the transcription factors C/EBPα and MITF specifies basophil and mast cell fates. Immunity 2013, 39, 97–110. [Google Scholar] [CrossRef]
- Iketani, A.; Takano, M.; Kasakura, K.; Iwatsuki, M.; Tsuji, A.; Matsuda, K.; Minegishi, R.; Hosono, A.; Nakanishi, Y.; Takahashi, K. CCAAT/enhancer-binding protein α-dependent regulation of granule formation in mast cells by intestinal bacteria. Eur. J. Immunol. 2024, 54, e2451094. [Google Scholar]
- Sakata-Yanagimoto, M.; Nakagami-Yamaguchi, E.; Saito, T.; Kumano, K.; Yasutomo, K.; Ogawa, S.; Kurokawa, M.; Chiba, S. Coordinated regulation of transcription factors through Notch2 is an important mediator of mast cell fate. Proc. Natl. Acad. Sci. USA 2008, 105, 7839–7844. [Google Scholar]
- Huang, H.; Li, Y. Mechanisms controlling mast cell and basophil lineage decisions. Curr. Allergy Asthma Rep. 2014, 14, 457. [Google Scholar] [CrossRef]
- Chen, C.C.; Grimbaldeston, M.A.; Tsai, M.; Weissman, I.L.; Galli, S.J. Identification of mast cell progenitors in adult mice. Proc. Natl. Acad. Sci. USA 2005, 102, 11408–11413. [Google Scholar] [CrossRef]
- Hamey, F.K.; Lau, W.Y.; Kucinski, I.; Wang, X.; Diamanti, E.; Wilson, N.K.; Göttgens, B.; Dahlin, J.S. Single-cell molecular profiling provides a high-resolution map of basophil and mast cell development. Allergy 2021, 76, 1731–1742. [Google Scholar] [PubMed]
- Arinobu, Y.; Iwasaki, H.; Gurish, M.F.; Mizuno, S.; Shigematsu, H.; Ozawa, H.; Tenen, D.G.; Austen, K.F.; Akashi, K. Developmental checkpoints of the basophil/mast cell lineages in adult murine hematopoiesis. Proc. Natl. Acad. Sci. USA 2005, 102, 18105–18110. [Google Scholar] [CrossRef]
- Dahlin, J.S.; Hallgren, J. Mast cell progenitors: Origin, development and migration to tissues. Mol. Immunol. 2015, 63, 9–17. [Google Scholar] [CrossRef]
- Wölfler, A.; Danen-van Oorschot, A.A.; Haanstra, J.R.; Valkhof, M.; Bodner, C.; Vroegindeweij, E.; van Strien, P.; Novak, S.; Cupedo, T.; Touw, I.P. Lineage-instructive function of C/EBPα in multipotent hematopoietic cells and early thymic progenitors. Blood 2010, 116, 4116–4125. [Google Scholar] [CrossRef]
- Franco, C.B.; Chen, C.C.; Drukker, M.; Weissman, I.L.; Galli, S.J. Distinguishing mast cell and granulocyte differentiation at the single-cell level. Cell Stem Cell 2010, 6, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Valent, P.; Schernthaner, G.H.; Sperr, W.R.; Fritsch, G.; Agis, H.; Willheim, M.; Bühring, H.J.; Orfao, A.; Escribano, L. Variable expression of activation-linked surface antigens on human mast cells in health and disease. Immunol. Rev. 2001, 179, 74–81. [Google Scholar] [CrossRef]
- Pajulas, A.; Fu, Y.; Cheung, C.C.; Chu, M.; Cannon, A.; Alakhras, N.; Zhang, J.; Ulrich, B.J.; Nelson, A.S.; Zhou, B.; et al. Interleukin-9 promotes mast cell progenitor proliferation and CCR2-dependent mast cell migration in allergic airway inflammation. Mucosal Immunol. 2023, 16, 432–445. [Google Scholar] [CrossRef]
- Abonia, J.P.; Hallgren, J.; Jones, T.; Shi, T.; Xu, Y.; Koni, P.; Flavell, A.R.; Boyce, J.A.; Austen, K.F. Gurish, M.F. Alpha-4 integrins and VCAM-1, but not MAdCAM-1, are essential for recruitment of mast cell progenitors to the inflamed lung. Blood 2006, 108, 1588–1594. [Google Scholar] [CrossRef] [PubMed]
- Hallgren, J.; Jones, T.G.; Abonia, J.P.; Xing, W.; Humbles, A.; Austen, K.F.; Gurish, M.F. Pulmonary CXCR2 regulates VCAM-1 and antigen-induced recruitment of mast cell progenitors. Proc. Natl. Acad. Sci. USA 2007, 104, 20478–20483. [Google Scholar] [CrossRef]
- Hallgren, J.; Gurish, M.F. Mast cell progenitor trafficking and maturation. Adv. Exp. Med. Biol. 2011, 716, 14–28. [Google Scholar] [PubMed]
- Dahlin, J.S.; Feinstein, R.; Cui, Y.; Heyman, B.; Hallgren, J. CD11c+ cells are required for antigen-induced increase of mast cells in the lung. J. Immunol. 2012, 189, 3869–3877. [Google Scholar] [CrossRef]
- Berlanga, O.; Emambokus, N.; Frampton, J. GPIIb (CD41) integrin is expressed on mast cells and influences their adhesion properties. Hematol. Exp. 2005, 33, 403–412. [Google Scholar] [CrossRef]
- Oki, T.; Kitaura, J.; Eto, K.; Lu, Y.; Maeda-Yamamoto, M.; Inagaki, N.; Nagai, H.; Yamanishi, Y.; Nakajima, H.; Kumagai, H.; et al. Integrin alphaIIbbeta3 induces the adhesion and activation of mast cells through interaction with fibrinogen. J. Immunol. 2006, 176, 52–60. [Google Scholar] [CrossRef]
- Gruber, B.L.; Marchese, M.J.; Kew, R. Angiogenic factors stimulate mast-cell migration. Blood 1995, 86, 2488–2493. [Google Scholar] [CrossRef] [PubMed]
- Jamur, M.C.; Moreno, A.N.; Mello, L.F.; Souza Junior, D.A.; Campos, M.R.; Pastor, M.V.; Grodzki, A.C.; Silva, D.C.; Oliver, C. Mast cell repopulation of the peritoneal cavity: Contribution of mast cell progenitors versus bone marrow derived committed mast cell precursors. BMC Immunol. 2010, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Sakanaka, M.; Tanaka, S.; Sugimoto, Y.; Ichikawa, A. Essential role of EP3 sub-type in prostaglandin E2-induced adhesion of mouse cultured and peritonealmast cells to the Arg-Gly-Asp-enriched matrix. Am. J. Physiol. Cell Physiol. 2008, 295, C1427–C1433. [Google Scholar] [PubMed]
- Hatae, N.; Kita, A.; Tanaka, S.; Sugimoto, Y.; Ichikawa, A. Induction of adherent activity in mastocytoma P-815 cells by the cooperation of two prostaglandin E2 receptor subtypes, EP3 and EP4. J. Biol. Chem. 2003, 278, 17977–17981. [Google Scholar] [CrossRef]
- Collington, S.J.; Hallgren, J.; Pease, J.E.; Jones, T.G.; Rollins, B.J.; Westwick, J.; Austen, K.F.; Williams, T.J.; Gurish, M.F.; Weller, C.L. The role of the CCL2/CCR2 axis in mouse mast cell migration in vitro and in vivo. J. Immunol. 2010, 184, 6114–6123. [Google Scholar] [CrossRef]
- Sime, W.; Lunderius-Andersson, C.; Enoksson, M.; Rousselle, P.; Tryggvason, K.; Nilsson, G.; Harvima, I.; Patarroyo, M. Human Mast Cells Adhere to and Migrate on Epithelial and Vascular Basement Membrane Laminins LM-332 and LM-511 via 3 1 Integrin. J. Immunol. 2009, 183, 4657–4665. [Google Scholar] [CrossRef]
- Kushnir-Sukhov, N.M.; Gilfillan, A.M.; Coleman, J.W.; Brown, J.M.; Bruening, S.; Toth, M.; Metcalfe, D.D. 5-hydroxytryptamine induces mast cell adhesion and migration. J. Immunol. 2006, 177, 6422–6432. [Google Scholar] [CrossRef]
- Metcalfe, D.D. Mast cells and mastocytosis. Blood 2008, 112, 946–956. [Google Scholar] [CrossRef]
- Robuffo, I.; Toniato, E.; Tettamanti, L.; Mastrangelo, F.; Ronconi, G.; Frydas, I.; Caraffa, A.I.; Kritas, S.K.; Conti, P. Mast cell in innate immunity mediated by proinflammatory and antiinflammatory IL-1 family members. J. Biol. Regul. Homeost. Agents 2017, 31, 837–842. [Google Scholar] [PubMed]
- Tettamanti, L.; Kritas, S.K.; Gallenga, C.G.; D’Ovidio, C.; Mastrangelo, F.; Ronconi, G.; Caraffa, A.; Toniato, E.; Conti, P. IL-33 mediates allergy through mast cell activation: Potential inhibitory effect of certain cytokines. J. Biol. Regul. Homeost. Agents 2018, 32, 1061–1065. [Google Scholar]
- Gri, G.; Frossi, B.; D’Inca, F.; Danelli, L.; Betto, E.; Mion, F.; Sibilano, R.; Pucillo, C. Mast cell: An emerging partner in immune interaction. Front. Immunol. 2012, 25, 120. [Google Scholar] [CrossRef]
- Taylor, M.L.; Metcalfe, D.D. Mast cells in allergy and host defense. Allergy Asthma Proc. 2001, 22, 115–119. [Google Scholar] [CrossRef]
- Marcelino da Silva, E.Z.; Jamur, M.C.; Oliver, C. Mast cell function: A new vision of an old cell. J. Histochem. Cytochem. 2014, 62, 698–738. [Google Scholar] [CrossRef] [PubMed]
- Asai, K.; Kitaura, J.; Kawakami, Y.; Yamagata, N.; Tsai, M.; Carbone, D.P.; Liu, F.T.; Galli, S.J.; Kawakami, T. Regulation of mast cell survival by IgE. Immunity 2001, 14, 791–800. [Google Scholar] [CrossRef] [PubMed]
- Wedi, B. Ligelizumab for the treatment of chronic spontaneous urticaria. Expert Opin. Biol. Ther. 2020, 20, 853–861. [Google Scholar] [CrossRef]
- Borkowski, T.A.; Jouvin, M.H.; Lin, S.Y.; Kinet, J.P. Minimal requirements for IgE-mediated regulation of surface FcεRI. J. Immunol. 2001, 167, 1290–1296. [Google Scholar] [CrossRef]
- Galli, S.J.; Kalesnikoff, J.; Grimbaldeston, M.A.; Piliponsky, A.M.; Williams, M.C.; Tsai, M. Mast cells as ‘tunable’ effector and immunoregulatory cells: Recent advances. Annu. Rev. Immunol. 2005, 23, 749–786. [Google Scholar] [CrossRef]
- Fu, S.; Ni, S.; Wang, D.; Fu, M.; Hong, T. Berberine suppresses mast cell-mediated allergic responses via regulating FceRI-mediated and MAPK signaling. Int. Immunopharmacol. 2019, 71, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Yang, Y.; Sun, G.; Yang, D.; Yin, S.; Zhang, S.; Jin, W.; Zhao, D.; Sun, L. Jiang, R. Inhibitory effect of phellodendrine on C48/80-induced allergic reaction in vitro and in vivo. Int. Immunopharmacol. 2024, 134, 112256. [Google Scholar] [CrossRef]
- Kalesnikoff, J.; Huber, M.; Lam, V.; Damen, J.E.; Zhang, J.; Siraganian, R.P.; Krystal, G. Monomeric IgE stimulates signaling pathways in mast cells that lead to cytokine production and cell survival. Immunity 2001, 14, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Saggini, R.; Pellegrino, R. MAPK is implicated in sepsis, immunity, and inflammation. Int. J. Infect. 2024, 8, 100–104. [Google Scholar]
- Kraft, S.; Kinet, J.P. New developments in FcepsilonRI regulation, function and inhibition. Nat. Rev. Immunol. 2007, 7, 365–378. [Google Scholar] [CrossRef]
- Méndez-Enríquez, E.; Hallgren, J. Mast Cells and Their Progenitors in Allergic Asthma. Front. Immunol. 2019, 10, 821. [Google Scholar] [CrossRef]
- Rivera, J.; Gilfillan, A.M. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 2006, 117, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Kawakami, T.; Galli, S.J. Regulation of mast-cell and basophil function and survival by IgE. Nat. Rev. Immunol. 2002, 2, 773–786. [Google Scholar] [CrossRef] [PubMed]
- Sheikh, J. Autoantibodies to the high-affinity IgE receptor in chronic urticaria: How important are they? Curr. Opin. Allergy Clin. Immunol. 2005, 5, 403–407. [Google Scholar] [CrossRef] [PubMed]
- Abboud, G.; Staumont-Sallé, D.; Kanda, A.; Roumier, T.; Deruytter, N.; Lavogiez, C.; Fleury, S.; Rémy, P.; Papin, J.P.; Capron, M.; et al. Fc(epsilon)RI and FcgammaRIII/CD16 differentially regulate atopic dermatitis in mice. J. Immunol. 2009, 182, 6517–6526. [Google Scholar] [CrossRef]
- Holowka, D.; Sil, D.; Torigoe, C.; Baird, B. Insights into immunoglobulin E receptor signaling from structurally defined ligands. Immunol. Rev. 2007, 217, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Sayers, I.; Cain, S.A.; Swan, J.R.; Pickett, M.A.; Watt, P.J.; Holgate, S.T.; Padlan, E.A.; Schuck, P.; Helm, B.A. Amino acid residues that influence Fc epsilon RI-mediated effector functions of human immunoglobulin E. Biochemistry 1998, 37, 16152–16164. [Google Scholar] [CrossRef]
- Barni, S.; Mori, F.; Giovannini, M.; Liotti, L.; Mastrorilli, C.; Pecoraro, L.; Saretta, F.; Castagnoli, R.; Arasi, S.; Caminiti, L.; et al. Allergic Proctocolitis: Literature Review and Proposal of a Diagnostic-Therapeutic Algorithm. Life 2023, 13, 1824. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, Y.; Zhang, H.; Hu, L.; Liu, J.; Wang, L.; Wang, T.; Zhang, H.; Cong, L.; Wang, Q. Pathogenesis of allergic diseases and implications for therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 138. [Google Scholar] [CrossRef]
- Micherer, S.H.; Warren, C.M.; Dant, C.; Gupta, R.S.; Nadeau, K.C. Food Allergy from Infancy Through Adulthood. J. Allergy Clin. Immunol. Pract. 2020, 8, 1854–1864. [Google Scholar] [CrossRef]
- Gilfillan, A.M.; Tkaczyk, C. Integrated signalling pathways for mast-cell activation. Nat. Rev. Immunol. 2006, 6, 218–230. [Google Scholar] [CrossRef] [PubMed]
- Woolhiser, M.R.; Okayama, Y.; Gilfillan, A.M.; Metcalfe, D.D. IgG-dependent activation of human mast cells following up-regulation of FcγRI by IFN-γ. Eur. J. Immunol. 2001, 31, 3298–3307. [Google Scholar] [CrossRef] [PubMed]
- Price, M.M.; Oskeritzian, C.A.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate synthesis and functions in mast cells. Future Lipidol. 2008, 3, 665–674. [Google Scholar] [CrossRef][Green Version]
- Kormelink, T.G.; Askenase, P.W.; Redegeld, F.A. Immunobiology of antigen-specific immunoglobulin free light chains in chronic inflammatory diseases. Curr. Pharm. Des. 2012, 18, 2278–2289. [Google Scholar] [CrossRef]
- Galli, S.J.; Gaudenzio, N. Human mast cells as antigen-presenting cells: When is this role important in vivo? J. Allergy Clin. Immunol. 2018, 141, 92–93. [Google Scholar] [CrossRef]
- Thio, M.; Kormelink, T.G.; Fischer, J.M.; Blokhuis, B.R.; Nijkamp, F.P.; Redegeld, F.A. Antigen Binding Characteristics of Immunoglobulin Free Light Chains: Crosslinking by Antigen is Essential to Induce Allergic Inflammation. PLoS ONE 2012, 7, e40986. [Google Scholar] [CrossRef]
- Thapaliya, M.; Ayudhya, C.C.; Amponnawarat, A.; Roy, S.; Ali, H. Mast Cell-Specific MRGPRX2: A Key Modulator of Neuro-Immune Interaction in Allergic Diseases. Curr. Allergy Asthma Rep. 2021, 21, 3. [Google Scholar] [CrossRef]
- Nagamine, M.; Kaitani, A.; Izawa, K.; Ando, T.; Yoshikawa, A.; Nakamura, M.; Maehara, A.; Yamamoto, R.; Okamoto, Y.; Wang, H.; et al. Neuronal substance P-driven MRGPRX2-dependent mast cell degranulation products differentially promote vascular permeability. Front. Immunol. 2024, 15, 1477072. [Google Scholar] [CrossRef] [PubMed]
- Alvarado, D.; Maurer, M.; Gedrich, R.; Seibel, S.B.; Murphy, M.B.; Crew, L.; Goldstein, J.; Crocker, A.; Vitale, L.A.; Morani, P.A.; et al. Anti-KIT monoclonal antibody CDX-0159 induces profound and durable mast cell suppression in a healthy volunteer study. Allergy 2022, 77, 2393–2403. [Google Scholar]
- Lewis, A.; Wan, J.; Baothman, B.; Monk, P.N.; Suvarna, S.K.; Peachell, P.T. Heterogeneity in the responses of human lung mast cells to stem cell factor. Clin. Exp. Allergy 2013, 43, 50–59. [Google Scholar]
- Bischoff, S.C. Role of mast cells in allergic and non-allergic immune responses: Comparison of human and murine data. Nat. Rev. Immunol. 2007, 7, 93–104. [Google Scholar] [CrossRef] [PubMed]
- Jensen, B.M.; Frandsen, P.M.; Raaby, E.M.; Schiøtz, P.O.; Skov, P.S.; Poulsen, L.K. Molecular and stimulus-response profiles illustrate heterogeneity between peripheral and cord blood-derived human mast cells. J. Leukoc. Biol. 2014, 95, 893–901. [Google Scholar][Green Version]
- Gebhardt, T.; Sellge, G.; Lorentz, A.; Raab, R.; Manns, M.P.; Bischoff, S.C. Cultured human intestinal mast cells express functional IL-3 receptors and respond to IL-3 by enhancing growth and IgE receptor-dependent mediator release. Eur. J. Immunol. 2002, 32, 2308–2316. [Google Scholar] [CrossRef]
- Brown, M.A.; Hural, J. Functions of IL-4 and Control of Its Expression. Crit. Rev. Immunol. 2017, 37, 181–212. [Google Scholar] [CrossRef]
- LaPorte, S.L.; Juo, Z.S.; Vaclavikova, J.; Colf, L.A.; Qi, X.; Heller, N.M.; Keegan, A.D.; Garcia, K.C. Molecular and structural basis of cytokine receptor pleiotropy in the interleukin-4/13 system. Cell 2008, 132, 259–272. [Google Scholar] [CrossRef]
- Heeb, L.; Egholm, C.; Boyman, O. Evolution and function of interleukin-4 receptor signaling in adaptive immunity and neutrophils. Genes Immun. 2020, 21, 143–149. [Google Scholar] [CrossRef]
- Goswami, R.; Kaplan, M.H. A brief history of IL-9. J. Immunol. 2011, 186, 3283–3288. [Google Scholar] [CrossRef]
- Blom, L.; Poulsen, B.C.; Jensen, B.M.; Hansen, A.; Poulsen, L.K. IL-33 induces IL-9 production in human CD4+ T cells and basophils. PLoS ONE 2011, 6, e21695. [Google Scholar]
- Tomar, S.; Ganesan, V.; Sharma, A.; Zeng, C.; Waggoner, L.; Smith, A.; Kim, C.H.; Licona-Limón, P.; Reinhardt, R.L.; Flavell, R.A.; et al. IL-4–BATF signaling directly modulates IL-9 producing mucosal mast cell (MMC9) function in experimental food allergy. J. Allergy Clin. Immunol. 2021, 147, 280–295. [Google Scholar] [CrossRef] [PubMed]
- Pajulas, A.; Zhang, J.; Kaplan, M.H. The World according to IL-9. J. Immunol. 2023, 211, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Ho, L.H.; Ohno, T.; Oboki, K.; Kajiwara, N.; Suto, H.; Iikura, M.; Okayama, Y.; Akira, S.; Saito, H.; Galli, S.J.; et al. IL-33 induces IL-13 production by mouse mast cells independently of IgE-FcepsilonRI signals. J. Leukoc. Biol. 2007, 82, 1481–1490. [Google Scholar] [PubMed]
- Chiba, N.; Masuda, A.; Yoshikai, Y.; Matsuguchi, T. Ceramide inhibits LPS-induced production of IL-5, IL-10, and IL-13 from mast cells. J. Cell. Physiol. 2007, 213, 126–136. [Google Scholar] [CrossRef]
- Yu, Y.; Blokhuis, B.R.; Garssen, J.; Redegeld, F.A. Non-IgE mediated mast cell activation. Eur. J. Pharmacol. 2016, 778, 33–43. [Google Scholar]
- Juremalm, M.; Nilsson, G. Chemokine receptor expression by mast cells. Chem. Immunol. Allergy 2005, 87, 130–144. [Google Scholar]
- Agier, J.; Brzezińska-Błaszczyk, E.; Żelechowska, P.; Wiktorska, M.; Pietrzak, J.; Różalska, S. Cathelicidin LL-37 Affects Surface and Intracellular Toll-Like Receptor Expression in Tissue Mast Cells. J. Immunol. Res. 2018, 2018, 7357162. [Google Scholar] [CrossRef]
- Ali, H. Regulation of human mast cell and basophil function by anaphylatoxins C3a and C5a. Immunol. Lett. 2010, 128, 36–45. [Google Scholar] [CrossRef]
- Erdei, A.; Andrásfalvy, M.; Péterfy, H.; Tóth, G.; Pecht, I. Regulation of mast cell activation by complement-derived peptides. Immunol. Lett. 2004, 92, 39–42. [Google Scholar] [CrossRef]
- el-Lati, S.G.; Dahinden, C.A.; Church, M.K. Complement peptides C3a- and C5a-induced mediator release from dissociated human skin mast cells. J. Investig. Dermatol. 1994, 102, 803–806. [Google Scholar] [CrossRef]
- Alysandratos, K.; Asadi, S.; Angelidou, A.; Zhang, B.; Sismanopoulos, N.; Yang, H.; Theoharides, T.C. Neurotensin and CRH Interactions Augment Human Mast Cell Activation. PLoS ONE 2012, 7, e48934. [Google Scholar] [CrossRef]
- Alevizos, M.; Karagkouni, A.; Panagiotidou, S.; Vasiadi, M.; Theoharides, T.C. Stress triggers coronary mast cells leading to cardiac events. Ann. Allergy Asthma Immunol. 2014, 112, 309–316. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Alysandratos, K.D.; Angelidou, A.; Delivanis, D.A.; Sismanopoulos, N.; Zhang, B.; Asadi, S.; Vasiadi, M.; Weng, Z.; Miniati, A.; et al. Mast cells and inflammation. Biochim. Biophys. Acta 2012, 1822, 21–33. [Google Scholar] [CrossRef]
- Peng, W.M.; Maintz, L.; Allam, J.P.; Raap, U.; Gutgemann, I.; Kirfel, J.; Novak, N. Increased circulating levels of neurotrophins and elevated expression of their high-affinity receptors on skin and gut mast cells in mastocytosis. Blood 2013, 122, 1779–1788. [Google Scholar] [CrossRef]
- Metz, M.; Botchkarev, V.A.; Botchkareva, N.V.; Welker, P.; Tobin, D.J.; Knop, J.; Maurer, M.; Paus, R. Neurotrophin-3 regulates mast cell functions in neonatal mouse skin. Exp. Dermatol. 2004, 13, 273–281. [Google Scholar] [CrossRef]
- Lorentz, A.; Hoppe, J.; Worthmann, H.; Gebhardt, T.; Hesse, U.; Bienenstock, J.; Bischoff, S.C. Neurotrophin-3, but not nerve growth factor, promotes survival of human intestinal mast cells. Neurogastroenterol. Motil. 2007, 19, 301–308. [Google Scholar] [CrossRef]
- Kulka, M.; Sheen, C.H.; Tancowny, B.P.; Grammer, L.C.; Schleimer, R.P. Neuropeptides activate human mast cell degranulation and chemokine production. Immunology 2008, 123, 398–410. [Google Scholar] [CrossRef] [PubMed]
- Agier, J.; Pastwińska, J.; Brzezińska-Błaszczyk, E. An overview of mast cell pattern recognition receptors. Inflamm. Res. 2018, 67, 737–746. [Google Scholar] [CrossRef] [PubMed]
- Agier, J.; Żelechowska, P.; Kozłowska, E.; Brzezińska-Błaszczyk, E. Expression of surface and intracellular Toll-like receptors by mature mast cells. Cent. Eur. J. Immunol. 2016, 41, 333–338. [Google Scholar] [CrossRef]
- Abraham, S.N.; St John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef]
- Sandig, H.; Bulfone-Paus, S. TLR signaling in mast cells: Common and unique features. Front. Immunol. 2012, 4, 185. [Google Scholar] [CrossRef]
- Hill, P.B.; Martin, R.J.; Miller, H.R. Characterization of whole-cell currents in mucosal and connective tissue rat mast cells using amphotericin-B-perforated patches and temperature control. Pflug. Arch. 1996, 432, 986–994. [Google Scholar] [CrossRef] [PubMed]
- Makabe-Kobayashi, Y.; Hori, Y.; Adachi, T.; Ishigaki-Suzuki, S.; Kikuchi, S.; Kagaya, Y.; Shirato, K.; Nagy, A.; Ujike, A.; Takai, T. The control effect of histamine on body temperature and respiratory function in IgE-dependent systemic anaphylaxis. J. Allergy Clin. Immunol. 2002, 110, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Komi, D.E.; Khomtchouk, K.; Santa Maria, P.L. A Review of the Contribution of Mast Cells in Wound Healing: Involved Molecular and Cellular Mechanisms. Clin. Rev. Allergy Immunol. 2020, 58, 298–312. [Google Scholar]
- Voss, M.; Kotrba, J.; Gaffal, E.; Katsoulis-Dimitriou, K.; Dudeck, A. Mast Cells in the Skin: Defenders of Integrity or Offenders in Inflammation? Int. J. Mol. Sci. 2021, 22, 4589. [Google Scholar] [CrossRef] [PubMed]
- Dahlin, J.S.; Maurer, M.; Metcalfe, D.D.; Pejler, G.; Sagi-Eisenberg, R.; Nilsson, G. The ingenious mast cell: Contemporary insights into mast cell behavior and function. Allergy 2022, 77, 83–99. [Google Scholar] [CrossRef]
- Varricchi, G.; Rossi, F.W.; Galdiero, M.R.; Granata, F.; Criscuolo, G.; Spadaro, G.; de Paulis, A.; Marone, G. Physiological Roles of Mast Cells: Collegium Internationale Allergologicum Update 2019. Int. Arch. Allergy Immunol. 2019, 179, 247–261. [Google Scholar] [CrossRef]
- Klagsbrun, M.; Moses, M.A. Molecular angiogenesis. Chem. Biol. 1999, 6, R217–R224. [Google Scholar] [CrossRef]
- Betz, C.; Lenard, A.; Belting, H.G.; Affolter, M. Cell behaviors and dynamics during angiogenesis. Development 2016, 143, 2249–2260. [Google Scholar] [CrossRef]
- Norrby, K. Do mast cells contribute to the continued survival of vertebrates? APMIS 2022, 130, 618–624. [Google Scholar] [CrossRef]
- Hamouzova, P.; Cizek, P.; Bartoskova, A.; Vitasek, R.; Tichy, F. Changes in the mast cell distribution in the canine ovary and uterus throughout the estrus cycle. Reprod. Domest. Anim. 2020, 55, 479–485. [Google Scholar] [CrossRef] [PubMed]
- Sivridis, E.; Giatromanolaki, A.; Agnantis, N.; Anastasiadis, P. Mast cell distribution and density in the normal uterus-metachromatic staining using lectins. Eur. J. Obstet. Gynecol. Reprod. Biol. 2001, 98, 109–113. [Google Scholar] [CrossRef]
- Hampton, A.L.; Salamonsen, L.A. Expression of messenger ribonucleic acid encoding matrix metalloproteinases and their inhibitors is related to menstruation. J. Endocrinol. 1994, 141, R1–R3. [Google Scholar] [CrossRef]
- Jensen, F.; Woudwyk, M.; Teles, A.; Woidacki, K.; Taran, F.; Costa, S.; Malfertheiner, S.F.; Zenclussen, A.C. Estradiol and progesterone regulate the migration of mast cells from the periphery to the uterus and induce their maturation and degranulation. PLoS ONE 2010, 5, e14409. [Google Scholar] [CrossRef]
- Teles, A.; Zenclussen, A.C. How cells of the immune system prepare the endometrium for implantation. Semin. Reprod. Med. 2014, 32, 358–364. [Google Scholar] [CrossRef]
- Komi, D.E.; Hafaghat, F.; Haidl, G. Significance of mast cells in spermatogenesis, implantation, pregnancy, and abortion: Cross talk and molecular mechanisms. Am. J. Reprod. Immunol. 2020, 83, e13228. [Google Scholar] [CrossRef]
- Saito, H. Role of mast cell protease in tissue remodeling. Chem. Immunol. Allergy 2005, 87, 80. [Google Scholar] [PubMed]
- Hart, P.H.; Grimbaldeston, M.A.; Finlay-Jones, J.J. Sunlight, immunosuppression and skin cancer: Role of histamine and mast cells. Clin. Exp. Pharmacol. Physiol. 2001, 28, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Hart, P.H.; Grimbaldeston, M.A.; Swift, G.J.; Jaksic, A.; Noonan, F.P.; Finlay-Jones, J.J. Dermal mast cells determine susceptibility to ultraviolet B-induced systemic suppression of contact hypersensitivity responses in mice. J. Exp. Med. 1998, 187, 2045–2053. [Google Scholar] [CrossRef] [PubMed]
- Grimbaldeston, M.A.; Nakae, S.; Kalesnikoff, J.; Tsa, M.; Galli, S.J. Mast cell-derived interleukin 10 limits skin pathology in contact dermatitis and chronic irradiation with ultraviolet B. Nat. Immunol. 2007, 8, 1095–1104. [Google Scholar] [CrossRef]
- Speiran, K.; Bailey, D.P.; Fernando, J.; Macey, M.; Barnstein, B.; Kolawole, M.; Curley, D.; Watowich, S.S.; Murray, P.J.; Oskeritzian, C.; et al. Endogenous suppression of mast cell development and survival by IL-4 and IL-10. J. Leukoc. Biol. 2009, 85, 826–836. [Google Scholar] [CrossRef]
- Morales, J.K.; Falanga, Y.T.; Depcrynski, A.; Fernando, J.; Ryan, J.J. Mast cell homeostasis and the JAK-STAT pathway. Genes Immun. 2010, 11, 599–608. [Google Scholar] [CrossRef]
- Gehlhaar, P.; Schaper-Gerhardt, K.; Gutzmer, R.; Hasler, F.; Röhn, T.A.; Werfel, T.; Mommert, S. Histamine and TH2 cytokines regulate the biosynthesis of cysteinyl-leukotrienes and expression of their receptors in human mast cells. Inflamm. Res. 2025, 74, 32. [Google Scholar] [CrossRef]
- Margulis, A.; Nocka, K.H.; Brennan, A.M.; Deng, B.; Fleming, M.; Goldman, J.; Kasaian, M.T. Mast cell-dependent contraction of human airway smooth muscle cell-containing collagen gels: Influence of cytokines, matrix metalloproteases, and serine proteases. J. Immunol. 2009, 183, 1739–1750. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, S.C. Physiological and pathophysiological functions of intestinal mast cells. Semin. Immunopathol. 2009, 31, 185–205. [Google Scholar] [CrossRef]
- Boesiger, J.; Tsai, M.; Maurer, M.; Yamaguchi, M.; Brown, L.F.; Claffey, K.P.; Dvorak, H.F.; Galli, S.J. Mast cells can secrete vascular permeability factor/ vascular endothelial cell growth factor and exhibit enhanced release after immunoglobulin E-dependent upregulation of fc epsilon receptor I expression. J. Exp. Med. 1998, 188, 1135–1145. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Mascarenhas, N.; Eckmann, L.; Miyamoto, Y.; Sun, X.; Kawakami, T.; Di Nardo, A. Skin microbiome promotes mast cell maturation by triggering stem cell factor production in keratinocytes. J. Allergy Clin. Immunol. 2017, 139, 1205–1216.e6. [Google Scholar] [CrossRef]
- Gurung, P.; Moussa, K.; Adams-Huet, B.; Devaraj, S.; Jialal, I. Increased mast cell abundance in adipose tissue of metabolic syndrome: Relevance to the proinflammatory state and increased adipose tissue fibrosis. Am. J. Physiol. Endocrinol. Metab. 2019, 316, E504–E509. [Google Scholar] [CrossRef]
- Bao, C.; Abraham, S.N. Mast cell-sensory neuron crosstalk in allergic diseases. J. Allergy Clin. Immunol. 2024, 153, 939–953. [Google Scholar] [CrossRef]
- Bell, A.; Althaus, M.; Diener, M. Communication between mast cells and rat submucosal neurons. Pflug. Arch. 2015, 467, 1809–1823. [Google Scholar] [CrossRef]
- Huang, C.; Friend, D.S.; Qiu, W.T.; Wong, G.W.; Morales, G.; Hunt, J.; Stevens, R.L. Induction of a selective and persistent extravasation of neutrophils into the peritoneal cavity by tryptase mouse mast cell protease 6. J. Immunol. 1998, 160, 1910–1919. [Google Scholar] [CrossRef]
- Orinska, Z.; Bulanova, E.; Budagian, V.; Metz, M.; Maurer, M.; Bulfone-Paus, S. TLR3-induced activation of mast cells modulates CD8+ T-cell recruitment. Blood 2005, 106, 978–987. [Google Scholar] [CrossRef]
- De Filippo, K.; Dudeck, A.; Hasenberg, M.; Nye, E.; van Rooijen, N.; Hartmann, K.; Gunzer, M.; Roers, A.; Hogg, N. Mast cell and macrophage chemokines CXCL1/CXCL2 control the early stage of neutrophil recruitment during tissue inflammation. Blood 2013, 121, 4930–4937. [Google Scholar] [CrossRef]
- Lampiasi, N. Interactions between Macrophages and Mast Cells in the Female Reproductive System. Int. J. Mol. Sci. 2022, 23, 5414. [Google Scholar] [CrossRef] [PubMed]
- Mazzoni, A.; Siraganian, R.P.; Leifer, C.A.; Segal, D.M. Dendritic cell modulation by mast cells controls the Th1/Th2 balance in responding T cells. J. Immunol. 2006, 177, 3577–3581. [Google Scholar] [CrossRef] [PubMed]
- Migalovich-Sheikhet, H.; Friedman, S.; Mankuta, D.; Levi-Schaffer, F. Novel identified receptors on mast cells. Front. Immunol. 2012, 2, 238. [Google Scholar] [CrossRef]
- Piliponsky, A.M.; Acharya, M.; Shubin, N.J. Mast Cells in Viral, Bacterial, and Fungal Infection Immunity. Int. J. Mol. Sci. 2019, 20, 2851. [Google Scholar] [CrossRef]
- Katsoulis-Dimitriou, K.; Kotrba, J.; Voss, M.; Dudeck, J.; Dudeck, A. Mast Cell Functions Linking Innate Sensing to Adaptive in responding immunity. Cells 2020, 9, 2538. [Google Scholar] [CrossRef] [PubMed]
- Applequist, S.E.; Wallin, R.P.; Ljunggren, H.G. Variable expression of Toll-like receptor in murine innate and adaptive immune cell lines. Int. Immunol. 2002, 14, 1065–1074. [Google Scholar] [CrossRef]
- Galli, S.J.; Tsai, M. Mast cells: Versatile regulators of inflammation, tissue remodeling, host defense, and homeostasis. J. Dermatol. Sci. 2007, 49, 7–19. [Google Scholar] [CrossRef]
- Xie, G.; Wang, F.; Peng, X.; Liang, Y.; Yang, H.; Li, L. Modulation of Mast Cell Toll-Like Receptor 3 Expression and Cytokines Release by Histamine. Cell. Physiol. Biochem. 2018, 46, 2401–2411. [Google Scholar] [CrossRef] [PubMed]
- Fritscher, J.; Amberger, D.; Dyckhoff, S.; Bewersdorf, S.P.; Masouris, I.; Voelk, S.; Hammerschmidt, S.; Schmetzer, M.M.; Klein, M.; Pfister, H.W.; et al. Mast Cells Are Activated by Streptococcus pneumoniae In Vitro but Dispensable for the Host Defense Against Pneumococcal Central Nervous System Infection In Vivo. Front. Immunol. 2018, 9, 550. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, C.; Troeitzsch, D.; Gimenez-Riva, V.A.; Galli, S.J.; Metz, M.; Maurer, M.; Siebenhaar, F. Mast cells are critical for controlling the bacterial burden and the healing of infected wounds. Proc. Natl. Acad. Sci. USA 2019, 116, 20500–20504. [Google Scholar] [CrossRef]
- Dawicki, W.; Marshall, J.S. New and emerging roles for mast cells in host defence. Curr. Opin. Immunol. 2007, 19, 31–38. [Google Scholar] [CrossRef]
- Stassen, M.; Hartmann, A.K.; Delgado, S.J.; Dehmel, S.; Braun, A. Mast cells within cellular networks. J. Allergy Clin. Immunol. 2019, 144, S46–S54. [Google Scholar] [CrossRef]
- Johnzon, C.F.; Rönnberg, E.; Pejler, G. The Role of Mast Cells in Bacterial Infection. Am. J. Pathol. 2016, 186, 4–14. [Google Scholar] [CrossRef]
- Arifuzzaman, M.; Mobley, Y.R.; Choi, H.W.; Bist, P.; Salinas, C.A.; Brown, Z.D.; Chen, S.L.; Staats, H.F.; Abraham, S.N. MRGPR mediated activation of local mast cells clears cutaneous bacterial infection and protects against reinfection. Sci. Adv. 2019, 5, eaav0216. [Google Scholar] [CrossRef]
- Lei, Z.; Zhang, D.; Lu, B.; Zhou, W.; Wang, D. Activation of mast cells in skin abscess induced by Staphylococcus aureus (S. aureus) infection in mice. Res. Vet. Sci. 2018, 118, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Rönnberg, E.; Johnzon, C.F.; Calounova, G.; Faroldi, G.G.; Grujic, M.; Hartmann, K.; Roers, A.; Guss, B.; Lundequist, A.; Pejler, G. Mast cells are activated by Staphylococcus aureus in vitro but do not influence the outcome of intraperitoneal S. aureus infection in vivo. Immunology 2014, 143, 155–163. [Google Scholar] [CrossRef]
- Marshall, J.S.; Portales-Cervantes, L.; Leong, E. Mast Cell Responses to Viruses and Pathogen Products. Int. J. Mol. Sci. 2019, 20, 4241. [Google Scholar] [CrossRef] [PubMed]
- Jolly, S.; Detilleux, J.; Desmecht, D. Extensive mast cell degranulation in bovine respiratory syncytial virus-associated paroxystic respiratory distress syndrome. Vet. Immunol. Immunopathol. 2004, 97, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Taguchi, F. Mast cell degranulation is induced by A549 airway epithelial cell infected with respiratory syncytial virus. Virology 2009, 386, 88–93. [Google Scholar] [CrossRef]
- Kanokvalai, K.; Wongkamchai, S.; Triwongwaranat, D. Mosquito allergy: Clinical features and natural course. J. Dermatol. 2010, 37, 1025–1031. [Google Scholar] [CrossRef]
- King, C.A.; Anderson, R.; Marshall, J.S. Dengue virus selectively induces human mast cell chemokine production. J. Virol. 2002, 76, 8408–8419. [Google Scholar] [CrossRef]
- Londono-Renteria, B.; Marinez-Angarita, J.C.; Troupin, A.; Colpitts, T.M. Role of Mast Cells in Dengue Virus Pathogenesis. DNA Cell Biol. 2017, 36, 423–427. [Google Scholar] [CrossRef]
- Morrison, J.; Rathore, P.S.; Mantri, C.K.; Aman, A.B.; Nishida, A.; John, L.S. Transcriptional Profiling Confirms the Therapeutic Effects of Mast Cell Stabilization in a Dengue Disease Model. J. Virol. 2017, 91, e00617-17. [Google Scholar] [CrossRef]
- Pennock, J.L.; Grencis, R.K. The mast cell and gut nematodes: Damage and defence. Chem. Immunol. Allergy 2006, 90, 128–140. [Google Scholar]
- Martins, P.R.; Nascimento, R.D.; de Souza-Lisboa, A.; Martinelli, P.M.; Reis, D. Trypanosoma cruzi-induced megaoesophagus: Is there a role for mast cell proteases? Hum. Immunol. 2014, 75, 302–305. [Google Scholar] [CrossRef] [PubMed]
- Wilainam, P.; Nintasen, R.; Viriyavejakul, P. Mast cell activation in the skin of Plasmodium falciparum malaria patients. Malar. J. 2015, 7, 67. [Google Scholar] [CrossRef]
- Naqvi, N.; Ahuja, K.; Selvapandiyan, A.; Dey, R.; Nakhasi, H.; Puri, N. Role of Mast Cells in clearance of Leishmania through extracellular trap formation. Sci. Rep. 2017, 7, 13240. [Google Scholar] [CrossRef] [PubMed]
- Moss, R.B. The use of biological agents for the treatment of fungal asthma and allergic bronchopulmonary aspergillosis. Ann. N. Y. Acad. Sci. 2012, 1272, 49–57. [Google Scholar] [CrossRef]
- Kurup, V.P. Immunology of allergic bronchopulmonary aspergillosis. Indian J. Chest Dis. Allied Sci. 2000, 42, 225–237. [Google Scholar] [PubMed]
- Yu, M.; Song, X.; Liu, B.; Luan, T.; Liao, S.; Zhao, Z. The Emerging Role of Mast Cells in Response to Fungal Infection. Front. Immunol. 2021, 12, 688659. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, R.; Muthu, V.; Sehgal, I.S.; Dhooria, S.; Prasad, K.T.; Aggarwal, A.N. Allergic Bronchopulmonary Aspergillosis. Clin. Chest Med. 2022, 43, 99–125. [Google Scholar] [CrossRef]
- Żelechowska, P.; Pastwińska, J.; Brzezińska-Błaszczyk, E.; Justyna Agier, J. Do Mast Cells Contribute to the Antifungal Host Defense? Cells 2021, 10, 2510. [Google Scholar] [CrossRef]
- Yang, Z.; Marshall, J.S. Zymosan treatment of mouse mast cells enhances dectin-1 expression and induces dectin-1-dependent reactive oxygen species (ROS) generation. Immunobiology 2009, 214, 321–330. [Google Scholar] [CrossRef]
- Gulen, T. Using the Right Criteria for MCAS. Curr. Allergy Asthma Rep. 2024, 24, 39–51. [Google Scholar] [CrossRef]
- Ruiz-Peñaloza, M.A.; López-Tiro, J.J.; Ortíz-Monteón, Z.E.; García-Rosas, C. Control de asma grave predominantemente eosinofilica con el uso de anti IL-5. Rev. Alerg. Mex. 2023, 70, 199. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Alvarez-Twose, I.; Brockow, K.; Hermine, O.; Niedoszytko, M.; Schwaab, J.; Lyons, J.J.; Carter, M.C. Updated Diagnostic Criteria and Classification of Mast Cell Disorders: A Consensus Proposal. Hemasphere 2021, 5, e646. [Google Scholar] [CrossRef] [PubMed]
- Rydz, A.; Lange, M.; Lugowska-Umer, H.; Sikorska, M.; Nowicki, R.J.; Morales-Cabeza, C.; Alvarez-Twose, I. Diffuse Cutaneous Mastocytosis: A Current Understanding of a Rare Disease. Int. J. Mol. Sci. 2024, 25, 1401. [Google Scholar] [CrossRef]
- Schuch, A.; Brockow, K. Mastocytosis and Anaphylaxis. Immunol. Allergy Clin. N. Am. 2017, 37, 153–164. [Google Scholar] [CrossRef]
- Piris-Villaespesa, M.; Alvarez-Twose, I. Systemic Mastocytosis: Following the Tyrosine Kinase Inhibition Roadmap. Front. Pharmacol. 2020, 11, 43. [Google Scholar] [CrossRef]
- Pardanani, A. Systemic mastocytosis in adults: 2023 update on diagnosis, risk stratification and management. Am. J. Hematol. 2023, 98, 1097–1116. [Google Scholar] [CrossRef]
- Criscuolo, M.; Fianchi, L.; Maraglino, A.M.; Pagano, L. Mastocytosis: One Word for Different Diseases. Oncol. Ther. 2018, 6, 129–140. [Google Scholar] [CrossRef]
- Leguit, R.J.; Wang, S.A.; George, T.I.; Tzankov, A.; Orazi, A. The international consensus classification of mastocytosis and related entities. Virchows Arch. 2023, 482, 99–112. [Google Scholar] [CrossRef]
- Farmer, I.; Radia, D.H. Systemic Mastocytosis: State of the Art. Curr. Hematol. Malig. Rep. 2024, 19, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Worrall, W.P.; Reber, L.L. Current and future therapeutics targeting mast cells in disease. Pharmacol. Ther. 2025, 273, 108892. [Google Scholar] [CrossRef] [PubMed]
- Piao, X.; Bernstein, A. A point mutation in the catalytic domain of c-kit induces growth factor independence, tumorigenicity, and differentiation of mast cells. Blood 1996, 87, 3117–3123. [Google Scholar] [CrossRef]
- Furitsu, T.; Tsujimura, T.; Tono, T.; Ikeda, H.; Kitayama, H.; Koshimizu, U.; Sugahara, H.; Butterfield, J.H.; Ashman, L.K.; Kanayama, Y.; et al. Identification of mutations in the coding sequence of the proto-oncogene c-kit in a human mast cell leukemia cell line causing ligand-independent activation of c-kit product. J. Clin. Investig. 1993, 92, 1736–1744. [Google Scholar] [CrossRef] [PubMed]
- Longley, B.J.; Metcalfe, D.D. A proposed classification of mastocytosis incorporating molecular genetics. Hematol. Oncol. Clin. N. Am. 2000, 14, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Munugalavadla, V.; Sims, E.C.; Borneo, J.; Chan, R.J.; Kapur, R. Genetic and pharmacologic evidence implicating the p85 alpha, but not p85 beta, regulatory subunit of PI3K and Rac2 GTPase in regulating oncogenic KIT-induced transformation in acute myeloid leukemia and systemic mastocytosis. Blood 2007, 110, 1612–1620. [Google Scholar] [CrossRef]
- Kitayama, H.; Kanakura, Y.; Furitsu, T.; Tsujimura, T.; Oritani, K.; Ikeda, H.; Sugahara, H.; Mitsui, H.; Kanayama, Y.; Kitamura, Y.; et al. Constitutively activating mutations of c-kit receptor tyrosine kinase confer factor-independent growth and tumorigenicity of factor-dependent hematopoietic cell lines. Blood 1995, 85, 790–798. [Google Scholar] [CrossRef]
- Molderings, G.J.; Haenisch, B.; Bogdanow, M.; Fimmers, R.; Nöthen, M.M. Familial occurrence of systemic mast cell activation disease. PLoS ONE 2013, 8, e76241. [Google Scholar] [CrossRef]
- Navarro-Navarro, P.; Álvarez-Twose, I.; Pérez-Pons, A.; Henriques, A.; Mayado, A.; García-Montero, A.C.; Laura Sánchez-Muñoz, L.S.; González-López, O.; Matito, A.L.; Caldas, C.; et al. KITD816V mutation in blood for the diagnostic screening of systemic mastocytosis and mast cell activation syndromes. Allergy 2023, 78, 1347–1359. [Google Scholar] [CrossRef]
- Carter, M.C.; Desai, A.; Komarow, H.D.; Bai, Y.; Clayton, S.T.; Clark, A.S. A distinct biomolecular profile identifies monoclonal mast cell disorders in patients with idiopathic anaphylaxis. J. Allergy Clin. Immunol. 2018, 141, 180–188.e3. [Google Scholar] [CrossRef]
- Valent, P.; Akin, C.; Hartmann, K.; Nilsson, G.; Reiter, A.; Hermine, O.; Sotlar, K.; Sperr, W.R.; Escribano, L.; George, I.T.; et al. Advances in the Classification and Treatment of Mastocytosis: Current Status and Outlook toward the Future. Cancer Res. 2017, 77, 1261–1270. [Google Scholar] [CrossRef]
- Kuruvilla, M.E.; Lee, F.E.; Lee, G.B. Understanding asthma phenotypes, endotypes, and mechanisms of disease. Clin. Rev. Allergy Immunol. 2019, 56, 219–233. [Google Scholar] [CrossRef]
- Costanzo, G.; Marchetti, M.; Ledda, A.G.; Sambugaro, G.; Bullita, M.; Paoletti, G.; Heffler, E.; Firinu, D.; Costanzo, A.L. Mast Cells in Allergic and Non-Allergic Upper Airways Diseases: Sentinel in the Watchtower. Int. J. Mol. Sci. 2024, 25, 12615. [Google Scholar] [CrossRef] [PubMed]
- Braunstahl, G.J.; Fokkens, W.J.; Overbeek, S.E.; KleinJan, A.; Hoogsteden, H.C.; Prins, J.B. Mucosal and systemic inflammatory changes in allergic rhinitis and asthma: A comparison between upper and lower airways. Clin. Exp. Allergy 2003, 33, 579–587. [Google Scholar] [CrossRef]
- Velez, T.E.; Bryce, P.J.; Hulse, K.E. Mast Cell Interactions and Crosstalk in Regulating Allergic Inflammation. Curr. Allergy Asthma Rep. 2018, 18, 30. [Google Scholar] [CrossRef]
- Befus, A.D.; Kulka, M. Mast cell mediators: Their differential release and the secretory pathways involved. Front. Immunol. 2014, 5, 569. [Google Scholar] [CrossRef]
- Bannert, C.; Bidmon-Fliegenschnee, B.; Stary, G.; Hotzy, F.; Stift, J.; Nurko, S.; Szépfalusi, Z.; Fiebiger, E.; Dehlink, E. Fc-epsilon-RI, the high affinity IgE-receptor, is robustly expressed in the upper gastrointestinal tract and modulated by mucosal inflammation. PLoS ONE 2012, 7, e42066. [Google Scholar] [CrossRef]
- McNeil, B.D. MRGPRX2 and adverse drug reactions. Front. Immunol. 2021, 12, 676354. [Google Scholar] [CrossRef]
- Thapaliya, M.; Ali, H. GRK2 differentially regulates FcεRI and MRGPRB2-mediated responses in mast cells. Front. Immunol. 2023, 14, 1155777. [Google Scholar] [CrossRef] [PubMed]
- Gasser, P.; Tarchevskaya, S.S.; Guntern, P.; Brigger, D.; Ruppli, R.; Zbären, N.; Kleinboelting, S.; Heusser, C.; Jardetzky, T.S.; Eggel, A. The mechanistic and functional profile of the therapeutic anti-IgE antibody ligelizumab differs from omalizumab. Nat. Commun. 2020, 11, 165. [Google Scholar] [CrossRef] [PubMed]
- Kanani, A.; Betschel, S.D.; Warrington, R. Urticaria and angioedema. Allergy Asthma Clin. Immunol. 2018, 14 (Suppl. 2), 59. [Google Scholar] [CrossRef] [PubMed]
- Batyrbayeva, A.; Ispayeva, Z.; Pashimov, M.; Kaibullayeva, J.; Baidildayeva, M.; Kapalbekova, U.; Tokmurzayeva, E.; Plakhotina, O.; Maldybayeva, A.; Salmanova, A.; et al. Clinical phenotypes and biomarkers in chronic urticaria. Clin. Chim. Acta 2025, 571, 120233. [Google Scholar] [CrossRef]
- Saini, S.S.; Bindslev-Jensen, C.; Maurer, M.; Grob, J.J.; Bülbül, B.E.; Bradley, M.S.; Canvin, J.; Rahmaoui, A.; Georgiou, P.; Alpan, O.; et al. Efficacy and safety of omalizumab in patients with chronic idiopathic/spontaneous urticaria who remain symptomatic on H1 antihistamines: A randomized, placebo-controlled study. J. Investig. Dermatol. 2015, 135, 67–75, Erratum in J. Investig. Dermatol. 2015, 135, 925. [Google Scholar] [CrossRef]
- Azimi, E.; Xia, J.; Lerner, E.A. Peripheral Mechanisms of Itch. Curr. Probl. Dermatol. 2016, 50, 18–23. [Google Scholar]
- Reddy, V.B.; Iuga, A.O.; Shimada, S.G.; LaMotte, R.H.; Lerner, E.A. Cowhage-evoked itch is mediated by a novel cysteine protease: A ligand of protease-activated receptors. J. Neurosci. 2008, 28, 4331–4335. [Google Scholar] [CrossRef] [PubMed]
- Lin, E.V.; Suresh, R.V.; Dispenza, M.C. Bruton’s tyrosine kinase inhibition for the treatment of allergic disorders. Ann. Allergy Asthma Immunol. 2024, 133, 33–42. [Google Scholar] [CrossRef]
- m Steinhoff, M.; Neisius, U.; Ikoma, A.; Fartasch, M.; Heyer, G.; Skov, P.S.; Luger, T.A.; Schmelz, M. Proteinase-activated receptor-2 mediates itch: A novel pathway for pruritus in human skin. J. Neurosci. 2003, 23, 6176–6180. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Dong, X. Peripheral and Central Mechanisms of Itch. Neuron 2018, 98, 482–494. [Google Scholar] [CrossRef]
- Zuberbier, T.; Aberer, W.; Asero, R.; Abdul Latiff, A.H.; Baker, D.; Ballmer-Weber, B.; Bernstein, J.A.; Bindslev-Jensen, C.; Brzoza, Z.; Buense Bedrikow, R.; et al. The EAACI/GA(2)LEN/EDF/WAO guideline for the definition, classification, diagnosis and management of urticaria. Allergy 2018, 73, 1393–1414. [Google Scholar] [CrossRef]
- Yanase, Y.; Takahagi, S.; Ozawa, K.; Hide, M. The role of coagulation and complement factors for mast cell activation in the pathogenesis of chronic spontaneous urticaria. Cells 2021, 10, 1759. [Google Scholar] [CrossRef] [PubMed]
- Schmetzer, O.; Lakin, E.; Topal, F.A.; Preusse, P.; Freier, D.; Church, M.K.; Maurer, M. IL-24 is a common and specific autoantigen of IgE in patients with chronic spontaneous urticaria. J. Allergy Clin. Immunol. 2018, 142, 876–882. [Google Scholar] [CrossRef]
- Terhorst-Molawi, D.; Hawro, T.; Grekowitz, E.; Kiefer, L.; Merchant, K.; Alvarado, D.; Thomas, L.J.; Hawthorne, T.; Crowley, E.; Heath-Chiozzi, M.; et al. Anti-KIT antibody, barzolvolimab, reduces skin mast cells and disease activity in chronic inducible urticarial. Allergy 2023, 78, 1269–1279. [Google Scholar] [CrossRef]
- Tei, M. Role of IL-4 and IL-31 in mastocytosis. Int. J. Infect. 2024, 8, 18–19. [Google Scholar]
- Poto, R.; Marone, G.; Galli, S.J.; Varricchi, G. Mast cells: A novel therapeutic avenue for cardiovascular diseases? Cardiovasc. Res. 2024, 120, 681–698. [Google Scholar] [CrossRef] [PubMed]
- Marone, G.; de Crescenzo, G.; Florio, G.; Granata, F.; Dente, V.; Genovese, A. Immunological modulation of human cardiac mast cells. Neurochem. Res. 1999, 24, 1195–1202. [Google Scholar] [CrossRef]
- Kovanen, P.T. Mast Cells as Potential Accelerators of Human Atherosclerosis-From Early to Late Lesions. Int. J. Mol. Sci. 2019, 20, 4479. [Google Scholar] [CrossRef]
- Wezel, A.; Quax, P.H.; Kuiper, J.; Bot, I. The role of mast cells in atherosclerosis. Hamostaseologie 2015, 35, 113–120. [Google Scholar] [CrossRef]
- Varricchi, G.; Marone, G.; Kovanen, P.T. Cardiac Mast Cells: Underappreciated Immune Cells in Cardiovascular Homeostasis and Disease. Trends Immunol. 2020, 41, 734–746. [Google Scholar] [CrossRef]
- Ngkelo, A.; Richart, A.; Kirk, J.A.; Bonnin, P.; Vilar, J.; Lemitre, M.; Marck, P.; Branchereau, M.; Gall, S.L.; Renault, N. Mast cells regulate myofilament calcium sensitization and heart function after myocardial infarction. J. Exp. Med. 2016, 213, 1353–1374. [Google Scholar] [CrossRef]
- Somasundaram, P.; Ren, G.; Nagar, H.; Kraemer, D.; Mendoza, L.; Michael, L.H.; Caughey, G.H.; Entman, M.L.; Frangogiannis, N.G. Mast cell tryptase may modulate endothelial cell phenotype in healing myocardial infarcts. J. Pathol. 2005, 205, 102–111. [Google Scholar] [CrossRef]
- Martin-de-Lara, F.; Sánchez-Aparicio, P.; Arias de la Fuente, C.; Rey-Campos, J. Biological effects of FoxJ2 over-expression. Transgenic Res. 2008, 17, 1131–1141. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Paul, S.; Pickrell, A.M. Hidden phenotypes of PINK1/Parkin knockout mice. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 129871. [Google Scholar] [CrossRef] [PubMed]
- Patella, V.; Marinò, I.; Arbustini, E.; Lamparter-Schummert, B.; Verga, L.; Adt, M.; Marone, G. Stem cell factor in mast cells and increased mast cell density in idiopathic and ischemic cardiomyopathy. Circulation 1998, 97, 971–978. [Google Scholar] [CrossRef]
- Milutinovic, A.; Petrovič, D.; Zorc, M.; Vraspir, P.O.; Arko, M.; Pleskovič, A.; Alibegovic, A.; Zorc-Pleskovic, R. Mast Cells Might Have a Protective Role against the Development of Calcification and Hyalinisation in Severe Aortic Valve Stenosis. Folia Biol. 2016, 62, 160–166. [Google Scholar] [CrossRef]
- Wypasek, E.; Natorska, J.; Grudzień, G.; Filip, G.; Sadowski, J.; Undas, A. Mast cells in human stenotic aortic valves are associated with the severity of stenosis. Inflammation 2013, 36, 449–456. [Google Scholar] [CrossRef]
- Kovanen, P.T.; Bot, P. Mast cells in atherosclerotic cardiovascular disease—Activators and actions. Eur. J. Pharmacol. 2017, 816, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Lagraauw, H.M.; Westra, M.M.; Bot, M.; Wezel, A.; van Santbrink, P.J.; Pasterkamp, G.; Biessen, E.A.; Kuiper, J.; Bot, I. Vascular neuropeptide Y contributes to atherosclerotic plaque progression and perivascular mast cell activation. Atherosclerosis 2014, 235, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Bot, I.; Shi, G.P.; Kovanen, P.T. Mast cells as effectors in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Oldford, S.A.; Marshall, J.S. Mastocitos como dianas para la inmunoterapia de tumores sólidos. Mol. Immunol. 2015, 63, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Annese, T.; Tamma, R. Controversial role of mast cells in breast cancer tumor progression and angiogenesis. Clin. Breast Cancer 2021, 21, 486–491. [Google Scholar] [CrossRef]
- Miettinen, M.; Lasota, J. KIT (CD117): A review on expression in normal and neoplastic tissues, and mutations and their clinicopathologic correlation. Appl. Immunohistochem. Mol. Morphol. 2005, 13, 205–220. [Google Scholar] [CrossRef]
- Jachetti, E.; Rigoni, A.; Bongiovanni, L.; Arioli, I.; Botti, L.; Parenza, M.; Cancila, V.; Chiodoni, C.; Festinese, F.; Bellone, M.; et al. Imatinib Spares cKit-Expressing Prostate Neuroendocrine Tumors, whereas Kills Seminal Vesicle Epithelial-Stromal Tumors by Targeting PDGFR-beta. Mol. Cancer Ther. 2017, 16, 365–375. [Google Scholar] [CrossRef]
- Dyduch, G.; Kaczmarczyk, K.; Okoń, K. Mast cells and cancer: Enemies or allies? Pol. J. Pathol. 2012, 63, 1–7. [Google Scholar]
- Liu, J.; Zhang, Y.; Zhao, J.; Yang, Z.; Li, D.; Katirai, F.; Huang, B. Mast cell: Insight into remodeling a tumor microenvironment. Cancer Metastasis Rev. 2011, 30, 177–184. [Google Scholar] [CrossRef]
- Majorini, M.T.; Colombo, M.P.; Lecis, D. Few, but Efficient: The Role of Mast Cells in Breast Cancer and Other Solid Tumors. Cancer Res. 2022, 82, 1439–1447. [Google Scholar] [CrossRef] [PubMed]
- Longo, V.; Catino, A.; Montrone, M.; Galetta, D.; Ribatti, D. Controversial role of mast cells in NSCLC tumor progression and angiogenesis. Thorac. Cancer 2022, 13, 2929–2934. [Google Scholar] [CrossRef]
- Fucikova, J.; Palova-Jelinkova, L.; Bartunkova, J.; Spisek, R. Induction of tolerance and immunity by dendritic cells: Mechanisms and clinical applications. Front. Immunol. 2019, 10, 2393. [Google Scholar] [CrossRef] [PubMed]
- Levick, S.P.; Widiapradja, A. Mast Cells: Key Contributors to Cardiac Fibrosis. Int. J. Mol. Sci. 2018, 19, 231. [Google Scholar] [CrossRef]
- Shi, J.R.; Tian, C.J.; Zeng, Q.; Guo, X.J.; Lu, J.; Gao, C.R. Expressions of Mast Cell Tryptase and Brain Natriuretic Peptide in Myocardium of Sudden Death due to Hypersensitivity and Coronary Atherosclerotic Heart Disease. Fa Yi Xue Za Zhi 2016, 32, 161–164. [Google Scholar]
- Bradding, P.; Pejler, G. The controversial role of mast cells in fibrosis. Immunol. Rev. 2018, 282, 198–231. [Google Scholar] [CrossRef]
- Legere, S.A.; Ian D Haidl, I.D.; Légaré, J.F.; Marshall, J.S. Mast Cells in Cardiac Fibrosis: New Insights Suggest Opportunities for Intervention. Front. Immunol. 2019, 10, 580. [Google Scholar] [CrossRef]
- Rivellese, F.; Nerviani, A.; Rossi, F.W.; Marone, G.; Matucci-Cerinic, M.; Paulis, M.; Pitzalis, C. Mast cells in rheumatoid arthritis: Friends or foes? Autoimmun. Rev. 2017, 16, 557–563. [Google Scholar] [CrossRef]
- Melissa A Brown, M.A.; Hatfield, J.K. Mast Cells are Important Modifiers of Autoimmune Disease: With so Much Evidence, Why is There Still Controversy? Front. Immunol. 2012, 3, 147. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, G. Mast cell and autoimmune diseases. Mediat. Inflamm. 2015, 2015, 246126. [Google Scholar] [CrossRef]
- Hamilton, M.J. Addressing Controversies in Mast Cell Activation Syndrome: Analysis Using the Cluster Instrument. Dig. Dis. Sci. 2023, 68, 3208–3210. [Google Scholar] [CrossRef]
- Akin, C. Dilemma of Mast Cell Activation Syndrome: Overdiagnosed or Underdiagnosed? J. Allergy Clin. Immunol. Pract. 2024, 12, 762–763. [Google Scholar] [CrossRef]
- Jarido, V.; Kennedy, L.; Hargrove, L.; Demieville, J.; Thomson, J.; Stephenson, K.; Francis, H. The emerging role of mast cells in liver disease. Am. J. Physiol. Gastrointest. Liver Physiol. 2017, 313, G89–G101. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, J.; Akpang, N.; Ziemkiewicz, Z.; Zaborowska, L.; Ludwin, A. Molecular Insights into Elevated Autoantibodies in Polycystic Ovary Syndrome: Mechanisms and Clinical Implications. Int. J. Mol. Sci. 2025, 26, 8192. [Google Scholar] [CrossRef]
- Hamad, A.A.; Amer, B.E.; Hawas, Y.; Mabrouk, M.A.; Meshref, M. Masitinib as a neuroprotective agent: A scoping review of preclinical and clinical evidence. Neurol. Sci. 2024, 45, 1861–1873. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.; Li, J.; Zhang, K. Pathogenic role of S100 proteins in psoriasis. Front. Immunol. 2023, 14, 1191645. [Google Scholar] [CrossRef]
- Conti, P.; Pregliasco, F.E.; Bellomo, R.G.; Gallenga, C.E.; Caraffa, A.; Kritas, S.K.; Lauritano, D.; Ronconi, G.; Lauritano, D.; Ronconi, G. Mast Cell Cytokines IL-1, IL-33, and IL-36 Mediate Skin Inflammation in Psoriasis: A Novel Therapeutic Approach with the Anti-Inflammatory Cytokines IL-37, IL-38, and IL-1Ra. Int. J. Mol. Sci. 2021, 22, 8076. [Google Scholar] [CrossRef]
- Balbino, B.; Herviou, P.; Godon, O.; Stackowicz, J.; Richard-Le Goff, O.; Iannascoli, B.; Sterlin, D.; Sterlin, D.; Brûlé, S.; Millot, G.A.; et al. The anti-IgE mAb omalizumab induces adverse reactions by engaging Fcγ receptors. J. Clin. Investig. 2020, 130, 1330–1335. [Google Scholar] [CrossRef] [PubMed]
- Pirson, L.; Baron, F.; Meuris, N.; Giet, O.; Castermans, E.; Greimers, R.; Di Stefano, I.; Gothot, A.; Beguin, Y. Despite inhibition of hematopoietic progenitor cell growth in vitro, the tyrosine kinase inhibitor imatinib does not impair engraftment of human CD133+ cells into NOD/SCIDbeta2mNull mice. Stem Cells 2006, 24, 1814–1821. [Google Scholar] [CrossRef][Green Version]
| Mediator | Activation Mode | Response | References |
|---|---|---|---|
| IgG, adenosin. | β-hexosaminidase (β-hex) Lysosomal enzyme that degrades GM2 gangliosides, a group of nerve tissue chemicals. | IgG binds to high-affinity FcγRI in autoimmune diseases, neuropathies, and certain types of cancer. | [56] |
| Sphingosine-1-phosphate (S1P) Lysophosphatidic acid (LPA). | Lipid that, through its S1PR1-S1PR5 receptors, which are G protein-coupled receptors on MCs. | Contact hypersensitivity reaction for IgLC. | [57] |
| Free light chain immunoglobulins (IgLC). | IgLC and IgE bind specifically to neurons and mast cells at FcεRIα. | It rapidly releases 5-HT, H1, proteases, LT4, TNF-α, and MIP-2 (vasodilation and permeability). Promotes antigen-MHC to the late-phase response. Increases hypersensitivity, thereby exacerbating asthma, food allergies, and other conditions. | [58,59,60] |
| Substance P (SP) Neuropeptide. | Activates MCs when bound to G protein (MRGPRX2). | Its activation affects multiple sclerosis and psoriasis, neurodegenerative diseases, and infections. | [61,62] |
| Stem Cell Factor (SCF). | Receptor c-kit (CD117). | A potent stimulant for MC proliferation, differentiation, and survival. | [63,64] |
| Interleukin 3 (IL-3). | IL-3 and IL3Rα (CD123) receptor. | Together with SCF, FcεRI, crucial for MCs differentiation and maturation. | [12,65,66,67] |
| Interleukin 4 (IL-4). Interleukin 13 (IL-13). | Activated MC, Th2 cells, basophils, and eosinophils produce IL-4. IL-4 and IL-13 genes are closely related. | Inhibits production of IL-1, TNF-α, IL-6, and MIP-1β. Potently inhibits apoptosis, differentiation, and proliferation of B lymphocytes. Promotes asthma, dermatitis, and anaphylaxis. The allergic model involves IL-4 and IL-13 acting on the vasculature, sensitizing it to histamine, platelet-activating factor (PAF), or leukotriene C4 (LTC4). | [68,69,70] |
| Interleukin 9 (IL-9). | IL-4, IL-33, and TGFβ induce the production of IL-9. | IL-9 exhibits antiparasitic activity and exacerbates the IL-9- and MC-dependent allergic response. | [71,72,73,74] |
| Other cytokines. | IL-33, IL-5, IL-6, IL-10, TSLP, and GM-CSF. | Enhance human mast cell and basophil proliferation, both stem cell factor (SCF)-dependent and IgE/antigen-independent. | [75,76,77] |
| MC cytokines: IL-1, IL-1β, and IL-18. | Promote Th2 cytokine production and promote the expansion and differentiation of APCs, dendritic cells (DCs), macrophages, and MCs. | [78,79] | |
| Complement and anaphylatoxins C3a-C5a. | Through their receptors, C3aR and C5aR. C1q for pathogen opsonization. | Induces degranulation and chemotaxis in human MCs (C1q in cutaneous MCs and C3a for pulmonary MCs). | [80,81,82] |
| Neurotensin (NT) and the Corticotropin-releasing hormone (CRH). | Modulate MC activity. | It responds to stress, inflammation, and nervousness and is secreted locally at nerve endings. CRH also induces FcεRI receptor expression. | [83,84,85,86] |
| Neurotrophins: Nerve growth factor (NGF), Brain-derived neurotrophic factor (BDNF), Neurotrophin-3 (NT-3) and Neurotrophin-4/5 (NT-4/5). | MC-dependent regulation of neuronal survival and function. NTs are secreted mainly by neurons and glial cells. | Biological effects are mediated through two types of receptors: p75 and Trk. Highlights the significant role of neurotrophins in the development and survival of MCs. NT-3 promotes fetal MCs maturation. | [85,87,88,89] |
| Pattern recognition receptors (PRRs). Pathogen-Associated Molecular Patterns (PAMPs). Damage-Associated Molecular Patterns (DAMPs). | Viral particles, parasites, and various endogenous molecules activate toll-like receptors in MCs. TLRs: 2, 4, 5, 9. | Induces degranulation and chemotaxis of MCs. | [90,91,92,93] |
| Mast Cell Dysfunction and Disease (MCAD) Is a General Term for When MCs Release Mediators Inappropriately, Causing Disease. | ||
|---|---|---|
| Rankings of Mast Cell Activation Syndrome (MCAS) | ||
| MCAS: Mast Cell Activation Syndrome | ||
| Primary MCAS | Secondary MCAS | Idiopathic MCAS |
| (1) Low threshold for desgranulation. (2) Increase in MC population, with increased response. | (1) More common and of unclear etiology. (2) IgE-mediated mechanisms (an environmental allergen, such as food or medication) and non-IgE-mediated mechanisms (such as exercise). | (1) There are no positive medical, laboratory, or diagnostic data. (2) There are no allergic causes or clonal mast cell diseases. |
| Diagnostic Criteria | ||
| A: clinical criterion | B: laboratory criterion | C response criterion |
| Mastocytosis | ||
| Cutaneous mastocytosis | Systemic mastocytosis | Mast cell sarcoma and Leukemia |
| (1) Maculopapular cutaneous mastocytosis. (2) Diffuse cutaneous mastocytosis. | Systemic mastocytosis in bone marrow. Slow-progressing systemic mastocytosis. Systemic mastocytosis with neoplasia. Aggressive systemic mastocytosis. Mast cell leukemia. | (1) 30% of biopsies (+) with serum tryptase and/or D816V mutation of the Kit gene. (2) Myelodysplastic signs, hepatomegaly, acyrthosis, and others. |
| Hereditary Alpha-Tryptasemia Is a Genetic Condition | ||
| Genetic cause: Duplications or extra copies of the TPSAB1 gene, which is responsible for producing tryptase. | Autosomal dominant trait: Which means it can be inherited from one or both parents. | Elevated tryptase levels: Extra copies of the gene result in increased production of the tryptase protein, which is detectable in blood tests and can lead to anaphylaxis. |
| Symptoms: People with hereditary alpha-tryptasemia may experience a range of symptoms, including: Flushing. Itching (pruritus). Gastrointestinal problems, such as dysmotility. Autonomic nervous system dysfunction. Anaphylactic or anaphylactoid reactions. | ||
| WHO Categories | Subtypes | Diagnostic Criteria | Characteristics |
|---|---|---|---|
| Cutaneous Mastocytosis. | Urticaria pigmentosa. Diffuse cutaneous mastocytosis. | Immunohistochemistry. | Reddish papular lesions. Reddish diffuse thickening of skin. |
| Solitary mastocytoma of skin. | Brownish-yellow, minimally elevated plaque. | ||
| Systemic mastocytosis. | Indolent SM. | Diagnostic criteria of SM. | No C findings At least 2 B findings and no C findings. |
| Aggressive SM. | At least one C finding. | ||
| SM is associated with clonal hematological non-mast cell lineage disease. | WHO criteria for a clonal hematological neoplasm. | ||
| Mast cell leukemia. | Diagnostic criteria of SM. | No C findings. At least 2 B findings, and no C findings. Isolated BM mastocytosis. | |
| Mast cell sarcoma. | No criteria for SM. | PB smear > 10% MCs. Isolated MC tumor. Destructive growth pattern. | |
| Extracutaneous Mastocytoma. | No criteria for SM. | Isolated MC tumor. Non-destructive growth pattern. |
| Allergy Is Mediated by IgE and FcεRI Receptors. | Widely Recognized for the Recognition. | Specific Allergen. |
|---|---|---|
| IgE/FcεRI cross-linking molecules. | IgG, IgM, free IgG. | High allergen variability. |
| Autoallergy or autoreactive urticaria type I or autoimmune urticaria and autoimmune urticaria IIIb. | IgE vs. Auto-antigens. More than 200 different self-antigens were not detected in healthy controls. | The most frequent are IL-24, DNA, and Thyroid Peroxidase (TPO) IgG anti-TPO. |
| Complement. | Infections. | Autoantibodies activate C1 (classical). Alternative cascade, by C3a cleaving C5 to form C5a, and in the coagulation cascade, thrombin (FIIa) and factor Xa. |
| Coagulation. | Coagulation factors IIa (thrombin), VIIa and Xa are ligand-receptor 2 (PAR2) on the mast cell surface. | Activated by C5a, TLR-4 can be activated by fibrin. |
| Neuro-immune dysregulation. | Receptor-2 (PAR2) ligands interact with the Mas-related G protein-coupled X2 receptor (MRG). X2 (MRGPRX2) is expressed on MCs. | Susntacia P, Neurotrophins, Neurotensins, etc. |
| Leukotrienes. | Product of constitutively and de novo formed granules following mast cell activation (LTC4-LTD4). | Which activation product and LTB4 can be chemotactic for immature MCs. |
| Alarmins. | PRRs, PAMP, DAMP, Stem Cell Factor; ST2 (c-kit). | This IL-33 alarmin is released as an alarm signal in case of infection or epithelial damage. |
| T cells, CD4+ +. | Receptor; TLR ligand, CD40 ligand. | TPO, thyroid peroxidase, OX40 Receptor, TCR, Licos. |
| Infections. | PRRs. | Toll-like receptors. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Galván-Morales, M.Á.; Vizuet-de-Rueda, J.C.; Montero-Vargas, J.M.; Teran, L.M. Role of Mast Cells in Human Health and Disease: Controversies and Novel Therapies. Int. J. Mol. Sci. 2025, 26, 8895. https://doi.org/10.3390/ijms26188895
Galván-Morales MÁ, Vizuet-de-Rueda JC, Montero-Vargas JM, Teran LM. Role of Mast Cells in Human Health and Disease: Controversies and Novel Therapies. International Journal of Molecular Sciences. 2025; 26(18):8895. https://doi.org/10.3390/ijms26188895
Chicago/Turabian StyleGalván-Morales, Miguel Ángel, Juan Carlos Vizuet-de-Rueda, Josaphat Miguel Montero-Vargas, and Luis M. Teran. 2025. "Role of Mast Cells in Human Health and Disease: Controversies and Novel Therapies" International Journal of Molecular Sciences 26, no. 18: 8895. https://doi.org/10.3390/ijms26188895
APA StyleGalván-Morales, M. Á., Vizuet-de-Rueda, J. C., Montero-Vargas, J. M., & Teran, L. M. (2025). Role of Mast Cells in Human Health and Disease: Controversies and Novel Therapies. International Journal of Molecular Sciences, 26(18), 8895. https://doi.org/10.3390/ijms26188895






