Druggability of Sodium Calcium Exchanger (NCX): Challenges and Recent Development
Abstract
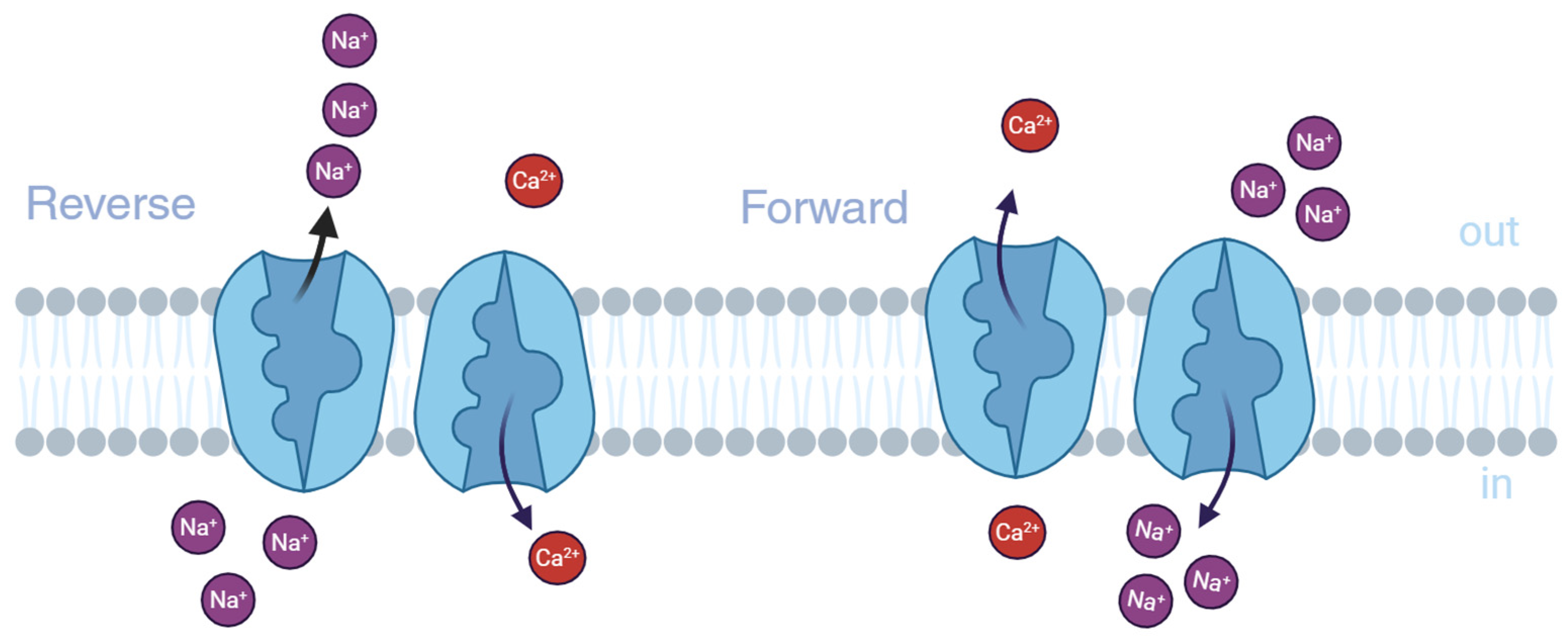
1. Introduction
2. Functions and Pathogenetic Role of NCX Isoforms
2.1. NCX Genes and Splice Variants
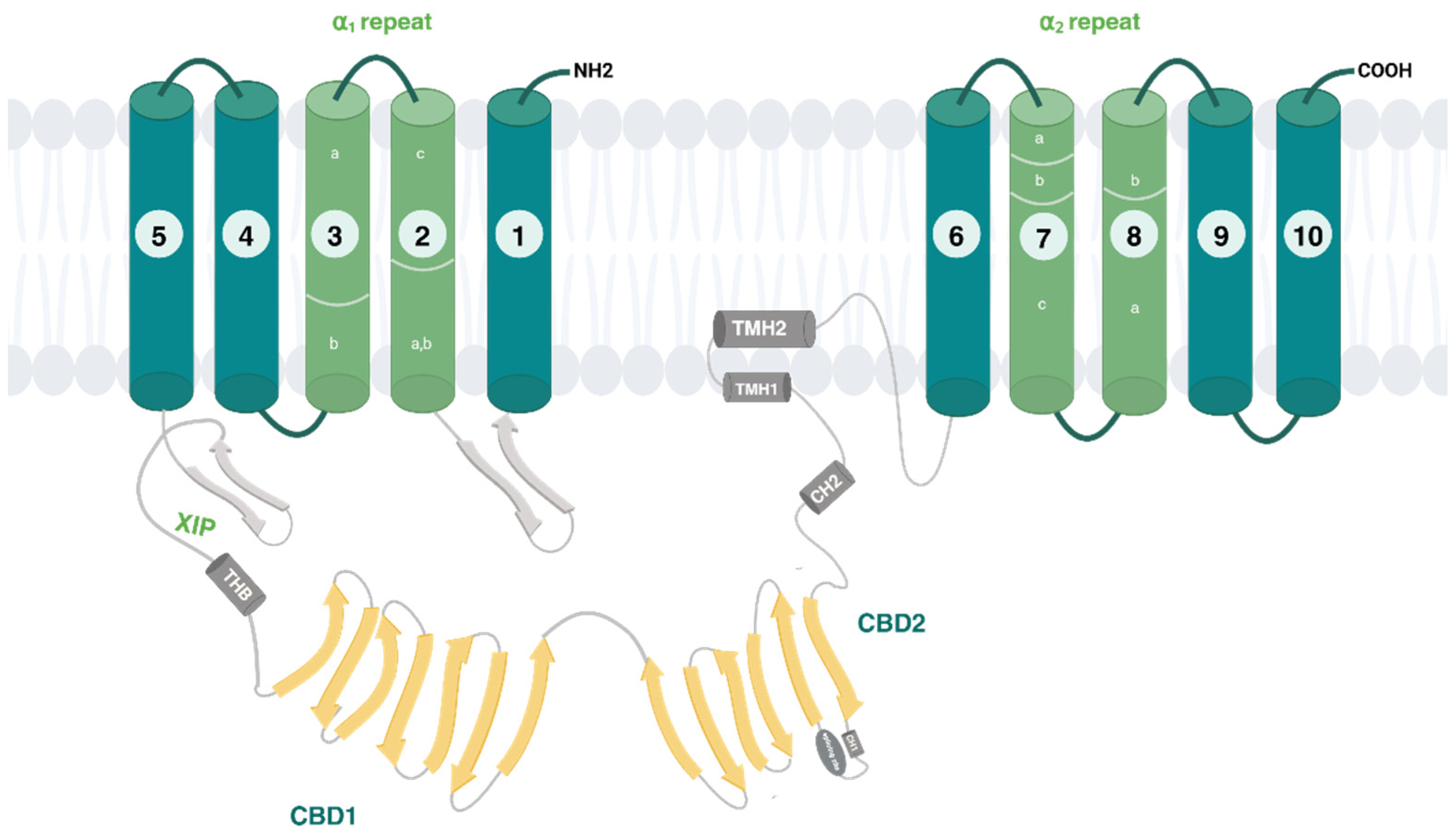
2.2. Regulation of NCX Isoforms and Their Splice Variants
- -
- The auto-inhibitory sequence (XIP);
- -
- A two-helix bundle (THB) module;
- -
- Two regulatory Ca2+ binding domains 1 (CBD1) and 2 (CBD2);
- -
- A short palmitoylation helix (TMH2).
2.3. NCX Isoforms in Neurological Diseases
2.3.1. Cerebral Ischemia—Inadequate Blood Flow to the Brain
2.3.2. Alzheimer’s Disease (AD)
2.3.3. Multiple Sclerosis
2.3.4. Parkinson’s Disease (PD)
2.3.5. Amyotrophic Lateral Sclerosis (ALS)
2.3.6. NCX in Neuroplastic Diseases of CNS: Glioblastoma
| Cerebral Ischemia | Study Type | Ref |
| NCX3 is minimally affected by ATP depletion and helps maintain Ca2+ homeostasis in ischemia-like conditions. BHK cells transfected with NCX3 showed greater resistance to hypoxia/reoxygenation than those with NCX1 or NCX2. | In vitro | [26] |
| NCX1/NCX3 knockout worsens infarct size and neurological outcome. Upregulation of NCX3 and NCX1 mRNA in peri-infarcted regions interpreted as a protective mechanism. | In vivo (rat model) | [27] |
| Ischemic preconditioning upregulates NCX3 and NCX1 via HIF1α, enhancing ischemic tolerance. | In vivo (preconditioning) | [27] |
| Postconditioning (IPoC) induces upregulation of NCX3 protein and mRNA in protected brain regions via p-AKT; NCX3 silencing reduces this effect | In vivo (postconditioning in rats) | [28] |
| Alzheimer’s Disease | Study Type | Ref |
| Calcium hypothesis suggests that ionic imbalance precedes symptoms and promotes Aβ and Tau pathology. | Experimental models | [31] |
| Aβ oligomers enhance NMDAR activity, increasing intracellular Ca2+ levels and excitotoxicity. | Experimental models | [32] |
| NCX3 is significantly downregulated in AD parietal cortex and synaptosomes; NCX2 is upregulated. All three NCX isoforms colocalize with Aβ oligomers at nerve terminals, suggesting a protective mechanism. | Human post-mortem tissue study | [33] |
| NCX3 may help refill ER Ca2+ stores, thus alleviating Aβ-induced ER stress. | Hypothesis/literature evidence | [4] |
| Multiple Sclerosis | Study Type | Ref |
| NCX3 expression and activity are significantly increased in mature OPCs (e.g., MO3.13 human oligodendrocyte cells). NCX3 overexpression in MO3.13 cells correlates with myelin markers and may promote OPC differentiation and myelin synthesis. | In vitro (MO3.13 cells) | [36] |
| NCX3 pharmacological inhibition followed by washout causes NCX3 upregulation and enhanced reverse mode activity. | In vitro (MO3.13 cells) | [37] |
| Parkinson’s Disease | Study Type | Ref |
| In SH-SY5Y cells treated with α-synuclein and rotenone, Ca2+ dysregulation occurs via VGCCs; CGP37157 (mNCX/VGCC blocker) prevents this. | In vitro (SH-SY5Y cells) | [39] |
| NCX3 is located on the outer mitochondrial membrane and regulates mitochondrial Ca2+ under normoxic/hypoxic conditions. | Literature review/Experimental | [23,24] |
| NCX2 at the plasma membrane helps maintain mitochondrial ionic balance and prevent neurodegeneration. | In vitro (human dopaminergic neurons) | [40] |
| NCX3 downregulation in midbrain of A53T mice causes mitochondrial dysfunction and calcium imbalance. | In vivo (A53T transgenic mice) | [41] |
| Disrupted calcium homeostasis induces neuroinflammation and progressive neurodegeneration. NCX1 upregulation in glia may be a compensatory response to neuroinflammation; potential therapeutic target. | In vivo (A53T mice) | [42] |
| Amyotrophic Lateral Sclerosis (ALS) | Study Type | Ref |
| In SOD1G93A mice, wild-type SOD1 exposure counteracts L-BMAA-induced ER stress. | In vivo (transgenic mice) | [45] |
| NCX3 is involved in neuroprotection in ALS; its expression is preserved via L-BMAA preconditioning in SOD1G93A mice. | In vivo (SOD1G93A mice) | [47] |
| NCX1 mediates SOD1-induced neuroprotection via reverse mode Ca2+ influx, ER refilling, Akt activation. NCX1 stimulation (e.g., CN-PYB2) reduces L-BMAA toxicity, suggesting therapeutic relevance. | In vitro (ALS model) | [49] |
| Glioblastoma | Study Type | Ref |
| NCX is highly expressed in lamellipodia of GBM cells; NCX inhibition disrupts lamellipodia and cell migration, highlighting its role in motility. | In vitro (U251, U87, GSCs) | [54] |
| SKF 96365 induces reverse NCX mode, increasing intracellular Ca2+ and reducing GBM cell proliferation. NCX1 is upregulated in GBM cells vs. astrocytes; NCX1 knockout reduces SKF 96365 efficacy. | In vitro | [55] |
| Bepridil, CB-DMB, KB-R7943 (NCX forward mode blockers) raise [Ca2+]i and induce GBM cell death. These effects are calcium-dependent and selective for GBM cells (not astrocytes), suggesting therapeutic window. | In vitro | [56] |
3. Targeting NCXs with Heterocyclic Compounds
3.1. Inhibitors
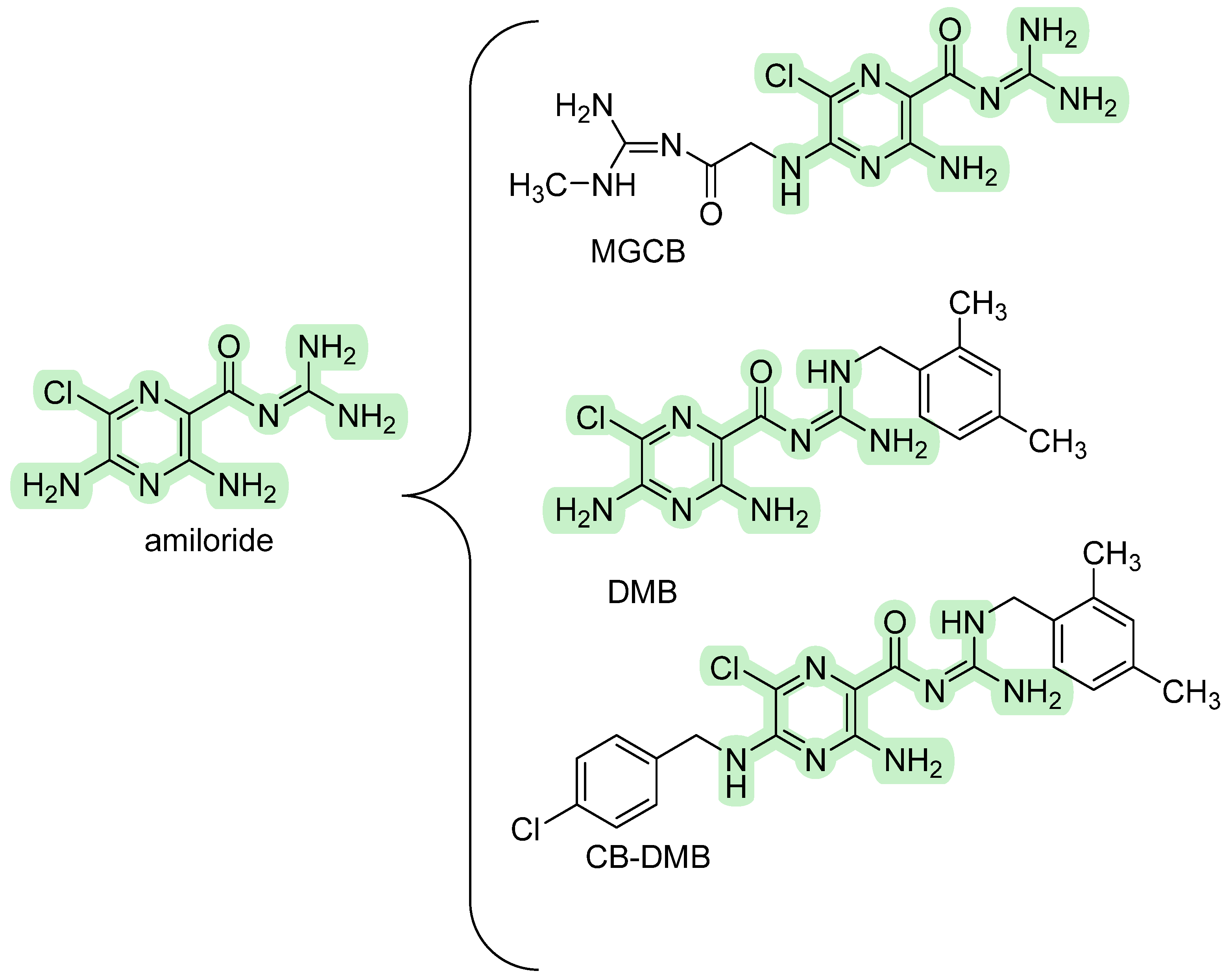
3.1.1. Amiloride Derivatives
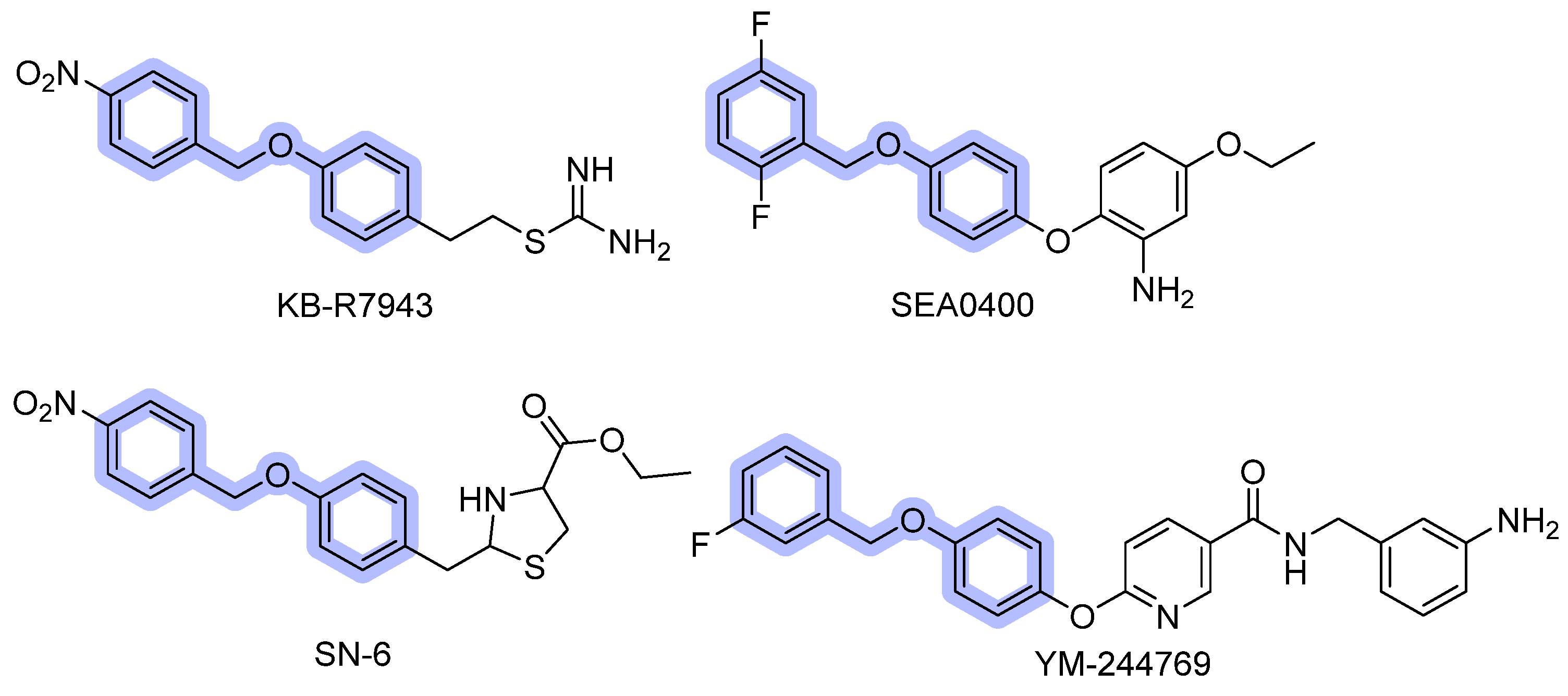
3.1.2. Benzyloxyphenyl Inhibitors
3.1.3. Quinazolinone Derivatives
3.1.4. Flavan and Furan Derivatives
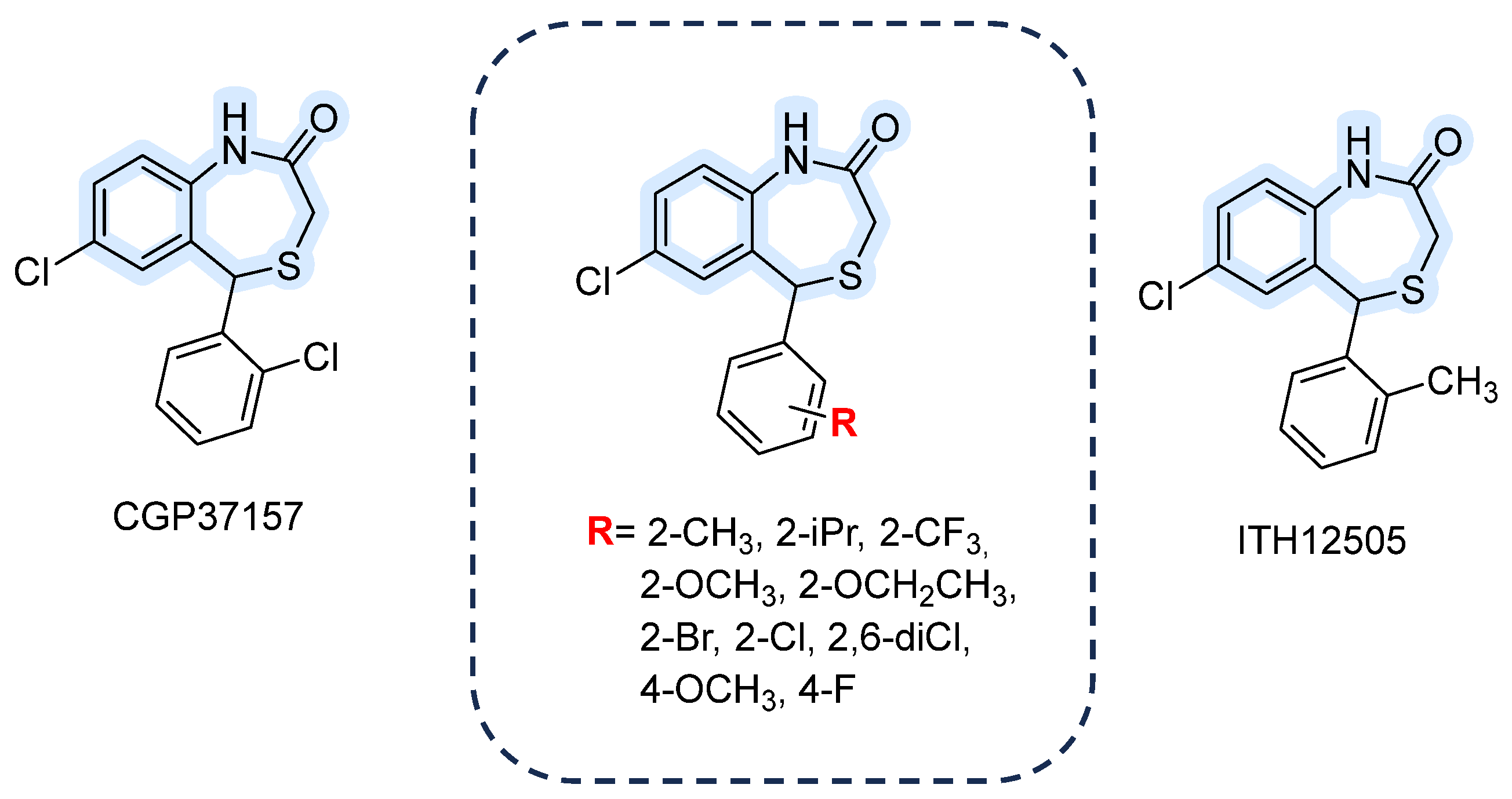
3.1.5. 1,4-Benzothiazepines
3.2. Activators
The 1,4-Benzodiazepine-2-One Analogs
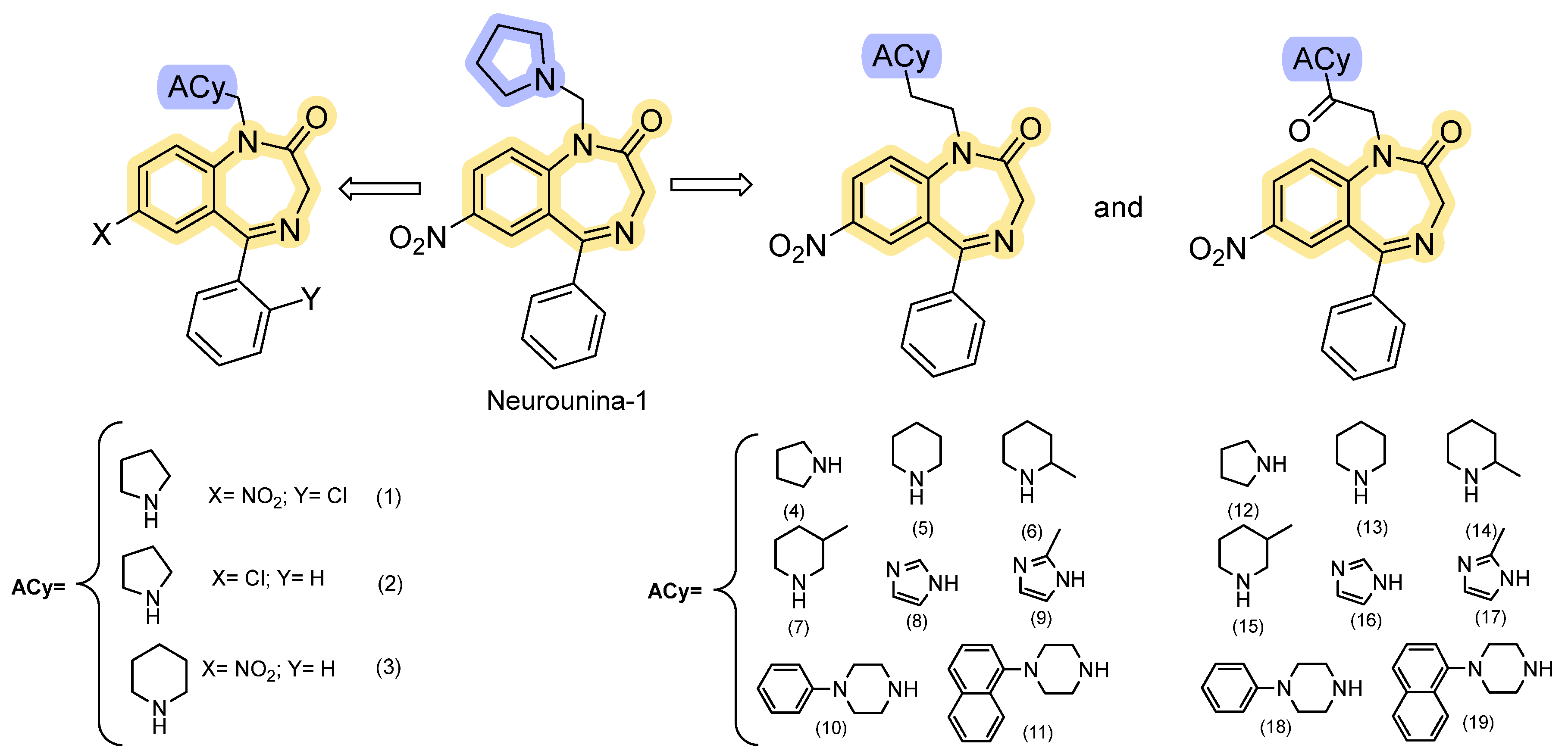
| Cmp | Isoform Selectivity | Representative IC50/EC50 | Reversibility | Ref. |
|---|---|---|---|---|
| Amiloride | Weak, non-selective NCX blocker | ≈100 µM–1 mM (reverse mode; highly assay-dependent) | Reversible; many off-targets (NHE1, ENaC, ASICs). | [2] |
| CB-DMB | Pan-NCX inhibitor (blocks NCX1/2/3 bidirectionally) | Nanomolar–low µM for outward/inward components reported (assay-dependent). | Reversible; mutation mapping suggests interaction near the f-loop. Useful pan-NCX tool. | [60] |
| KB-R7943 | NCX1 reverse-mode | 1.2–2.4 µM (Nai+-dependent 45Ca2+ uptake and Nai+-dependent [Ca2+]i increase in cardiomyocytes, smooth muscle cells, and NCX1-transfected fibroblasts) 4.3 µM (Na+ independent 45Ca2+ uptake in fibroblasts) | Reversible; promiscuous off-target profile (NMDA, mitochondrial complex I, other channels). | [64,65,66,67,92] |
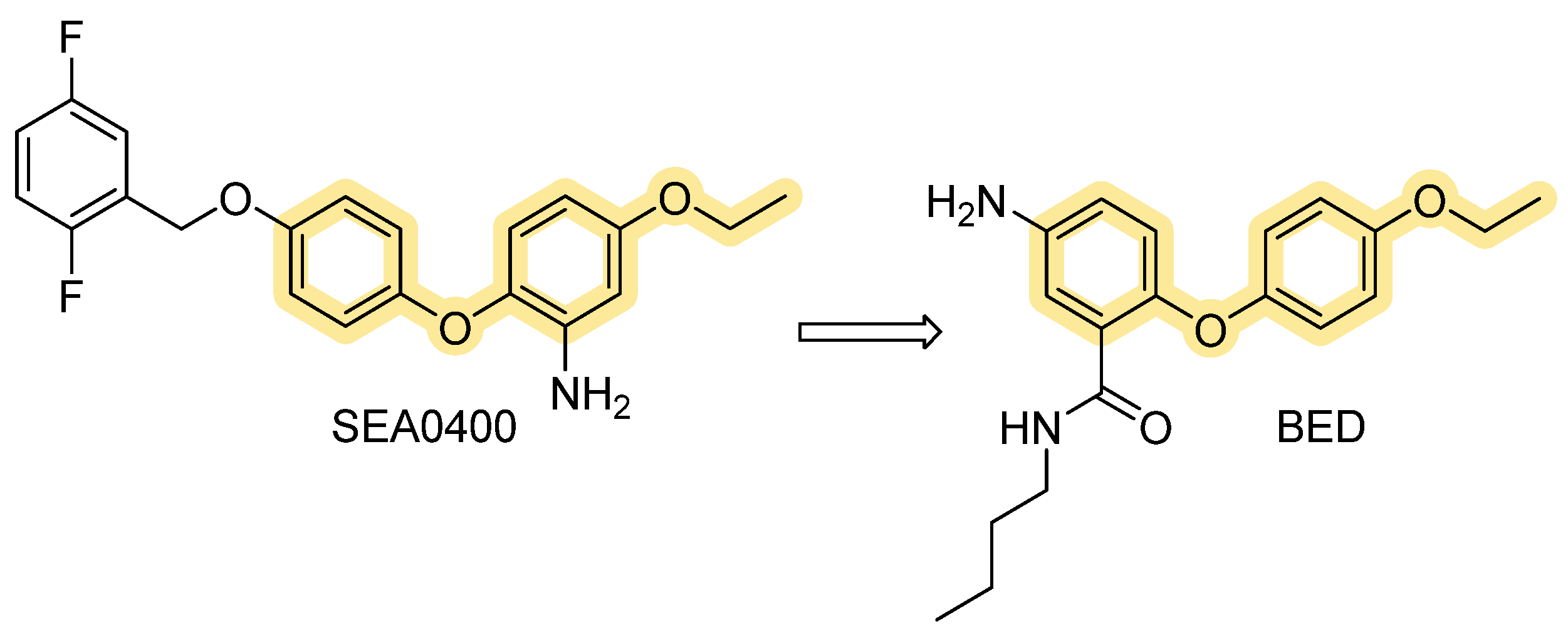
| SEA0400 | Strong preference for NCX1 > NCX2/3; inhibits both modes but reverse stronger | NCX1 ≈ 53 nM; NCX2 ≈ 0.98 µM (Nai+-dependent 45Ca2+ uptake into stable transfected CCL39 cells); IC50 of 33 nM, 5.0 nM and 8.3 nM (Na+-dependent Ca2+ uptake in cultured neurons, astrocytes, and microglia) | Reversible; more selective and potent than KB-R7943 but assay-dependent. | [69,70,71] |
| SM-15811 | Potent NCX1 inhibition; structure modified to obtain Neurounina-1 activator | IC30 = 0.46 µM (Na+-free-induced Fura 2 microfluorimetry) | Reversible | [80] |
| SN-6 | Selective for NCX1 reverse mode; negligible on NCX2/3 | NCX1 ≈ 2.3–2.9 µM; NCX2 ≈ 16 µM; NCX3 ≈ 8.6 µM. (Nai+-dependent 45Ca2+ uptake into stable transfected CCL39 cells) | Reversible, improved selectivity over SEA0400 | [72,93] |
| YM-244769 | Preferential potency for NCX3 (NCX3 > NCX1 > NCX2) | NCX3 ≈ 18 nM; NCX1 ≈ 68 nM; NCX2 ≈ 96 nM (Nai+-dependent 45 Ca2+ uptake into stable transfected CCL39 cells) | Reversible; used to probe NCX3 contribution in neuronal models. | [73] |
| BED | Potent NCX3 inhibitor (used to interrogate NCX3 roles) | NCX3: ~ 1.9 nM; NCX2 ~ 3.5 nM (Nai+-dependent 45Ca2+ uptake and Na0+-dependent 45Ca2+ efflux in stably transfected BHK cells). | Reversible; reported to worsen anoxic injury in cortical neurons when NCX3 inhibited. | [74] |
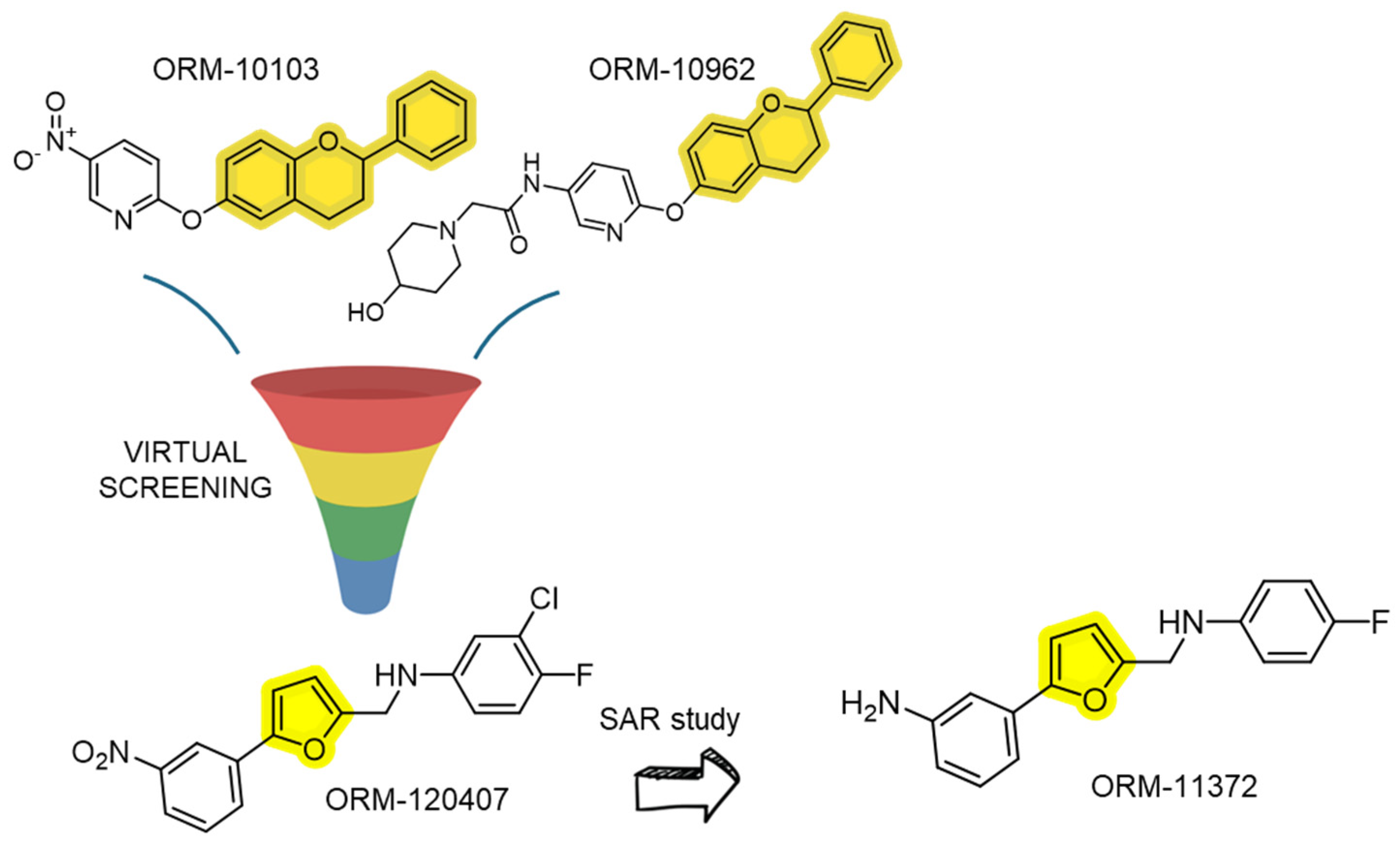
| ORM-10103/ORM-10962 | Designed as selective NCX inhibitors | Sub-µM to nM range in whole-cell cardiac NCX assays. | Reversible; improved selectivity vs. earlier scaffolds; abolishes triggered arrhythmias in models. | [94,95] |
| ORM-11372 | Very potent on human NCX1.1 | EC50 (human NCX1.1): reverse ≈ 5 nM; forward ≈ 6 nM (on human-induced pluripotent stem cell (iPSc)-derived cardiomyocytes). | Reversible; optimized drug-like profile, profiled for cardiac safety. | [82] |
| CGP-37157/ITH12505 | Inhibit mitochondrial Na+/Ca2+ exchange; also affect plasma membrane NCX at higher conc. | Active concentrations low-µM depending on system (mitochondrial vs. plasma assays). | Reversible; not fully selective—affect VGCCs and other Ca2+ handling pathways at similar concentrations. | [87,88,96] |
| Neurounina-1 and derivatives | Functional activation of NCX1 and NCX2 | Allosteric activation of NCX1 and NCX2 isoforms, with EC50 values in the low nanomolar range (0.03–2.7 nM). It does not affect NCX3 activity. | Reversible; potential therapeutic applications in stroke-related pathologies. | [89,90,91] |
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Pignataro, G.; Sirabella, R.; Anzilotti, S.; Di Renzo, G.; Annunziato, L. Does Na+/Ca2+ Exchanger, NCX, Represent a New Druggable Target in Stroke Intervention? Transl. Stroke Res. 2014, 5, 145–155. [Google Scholar] [CrossRef]
- Annunziato, L.; Pignataro, G.; Di Renzo, G.F. Pharmacology of Brain Na+/Ca2+ Exchanger: From Molecular Biology to Therapeutic Perspectives. Pharmacol. Rev. 2004, 56, 633–654. [Google Scholar] [CrossRef]
- Annunziato, L. Sodium Calcium Exchange: A Growing Spectrum of Pathophysiological Implications. In Proceedings of the 6th International Conference on Sodium Calcium Exchange, Lacco Ameno, Italy, 1–5 October 2011, 1st ed.; Springer: Berlin/Heidelberg, Germany, 2013. [Google Scholar] [CrossRef]
- Pannaccione, A.; Piccialli, I.; Secondo, A.; Ciccone, R.; Molinaro, P.; Boscia, F.; Annunziato, L. The Na+/Ca2+ exchanger in Alzheimer’s Disease. Cell Calcium 2020, 87, 102190. [Google Scholar] [CrossRef]
- Khananshvili, D. Sodium-Calcium Exchangers (NCX): Molecular Hallmarks Underlying the Tissue-Specific and Systemic Functions. Pflugers Arch. 2014, 466, 43–60. [Google Scholar] [CrossRef]
- Lytton, J. Na+/Ca2+ Exchangers: Three Mammalian Gene Families Control Ca2+ Transport. Biochem. J. 2007, 406, 365–382. [Google Scholar] [CrossRef]
- Morales, A.; Lachuer, J.; Bilbaut, A.; Georges, B.; Andrieu, J.-L.; Diez, J.; Ojeda, C. Characterization of a Na+-Ca2+ Exchanger NCX1 Isoform in Bovine Fasciculata Cells of Adrenal Gland. Mol. Cell. Biochem. 2001, 218, 41–45. [Google Scholar] [CrossRef]
- Sosnoski, D.M.; Gay, C.V. NCX3 Is a Major Functional Isoform of the Sodium–Calcium Exchanger in Osteoblasts. J. Cell. Biochem. 2008, 103, 1101–1110. [Google Scholar] [CrossRef] [PubMed]
- Szewczyk, M.M.; Davis, K.A.; Samson, S.E.; Simpson, F.; Rangachari, P.K.; Grover, A.K. Ca2+-pumps and Na+-Ca2+-exchangers in coronary artery endothelium versus smooth muscle. J. Cell. Mol. Med. 2007, 11, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Hilgemann, D.W.; Collins, A.; Matsuoka, S. Steady-State and Dynamic Properties of Cardiac Sodium-Calcium Exchange. Secondary Modulation by Cytoplasmic Calcium and ATP. J. Gen. Physiol. 1992, 100, 933–961. [Google Scholar] [CrossRef] [PubMed]
- Hilgemann, D.W.; Matsuoka, S.; Nagel, G.A.; Collins, A. Steady-State and Dynamic Properties of Cardiac Sodium-Calcium Exchange. Sodium-Dependent Inactivation. J. Gen. Physiol. 1992, 100, 905–932. [Google Scholar] [CrossRef]
- Giladi, M.; Lee, S.Y.; Ariely, Y.; Teldan, Y.; Granit, R.; Strulovich, R.; Haitin, Y.; Chung, K.Y.; Khananshvili, D. Structure-Based Dynamic Arrays in Regulatory Domains of Sodium-Calcium Exchanger (NCX) Isoforms. Sci. Rep. 2017, 7, 993. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zeng, W.; Han, Y.; John, S.; Ottolia, M.; Jiang, Y. Structural Mechanisms of the Human Cardiac Sodium-Calcium Exchanger NCX1. Nat. Commun. 2023, 14, 6181. [Google Scholar] [CrossRef]
- Giladi, M.; Fojtík, L.; Strauss, T.; Da’adoosh, B.; Hiller, R.; Man, P.; Khananshvili, D. Structural Dynamics of Na+ and Ca2+ Interactions with Full-Size Mammalian NCX. Commun. Biol. 2024, 7, 463. [Google Scholar] [CrossRef] [PubMed]
- Boyman, L.; Hagen, B.M.; Giladi, M.; Hiller, R.; Lederer, W.J.; Khananshvili, D. Proton-Sensing Ca2+ Binding Domains Regulate the Cardiac Na+/Ca2+ Exchanger. J. Biol. Chem. 2011, 286, 28811–28820. [Google Scholar] [CrossRef]
- Besserer, G.M.; Ottolia, M.; Nicoll, D.A.; Chaptal, V.; Cascio, D.; Philipson, K.D.; Abramson, J. The Second Ca2+-Binding Domain of the Na+-Ca2+ Exchanger Is Essential for Regulation: Crystal Structures and Mutational Analysis. Proc. Natl. Acad. Sci. USA 2007, 104, 18467–18472. [Google Scholar] [CrossRef]
- Nicoll, D.A.; Sawaya, M.R.; Kwon, S.; Cascio, D.; Philipson, K.D.; Abramson, J. The Crystal Structure of the Primary Ca2+ Sensor of the Na+/Ca2+ Exchanger Reveals a Novel Ca2+ Binding Motif. J. Biol. Chem. 2006, 281, 21577–21581. [Google Scholar] [CrossRef]
- Matsuoka, S.; Nicoll, D.A.; He, Z.; Philipson, K.D. Regulation of the Cardiac Na+-Ca2+ Exchanger by the Endogenous XIP Region. J. Gen. Physiol. 1997, 109, 273–286. [Google Scholar] [CrossRef]
- Molinaro, P.; Pannaccione, A.; Sisalli, M.J.; Secondo, A.; Cuomo, O.; Sirabella, R.; Cantile, M.; Ciccone, R.; Scorziello, A.; di Renzo, G.; et al. A New Cell-Penetrating Peptide That Blocks the Autoinhibitory XIP Domain of NCX1 and Enhances Antiporter Activity. Mol. Ther. 2015, 23, 465–476. [Google Scholar] [CrossRef]
- Gök, C.; Fuller, W. Regulation of NCX1 by Palmitoylation. Cell Calcium 2020, 86, 102158. [Google Scholar] [CrossRef]
- Gök, C.; Plain, F.; Robertson, A.D.; Howie, J.; Baillie, G.S.; Fraser, N.J.; Fuller, W. Dynamic Palmitoylation of the Sodium-Calcium Exchanger Modulates Its Structure, Affinity for Lipid-Ordered Domains, and Inhibition by XIP. Cell Rep. 2020, 31, 107697. [Google Scholar] [CrossRef] [PubMed]
- Gök, C.; Fuller, W. Rise of Palmitoylation: A New Trick to Tune NCX1 Activity. Biochim. Biophys. Acta Mol. Cell Res. 2024, 1871, 119719. [Google Scholar] [CrossRef]
- Scorziello, A.; Savoia, C.; Sisalli, M.J.; Adornetto, A.; Secondo, A.; Boscia, F.; Esposito, A.; Polishchuk, E.V.; Polishchuk, R.S.; Molinaro, P.; et al. NCX3 Regulates Mitochondrial Ca2+ Handling through the AKAP121-Anchored Signaling Complex and Prevents Hypoxia-Induced Neuronal Death. J. Cell Sci. 2013, 126, 5566–5577. [Google Scholar] [CrossRef]
- Sisalli, M.J.; Feliciello, A.; Della Notte, S.; Di Martino, R.; Borzacchiello, D.; Annunziato, L.; Scorziello, A. Nuclear-Encoded NCX3 and AKAP121: Two Novel Modulators of Mitochondrial Calcium Efflux in Normoxic and Hypoxic Neurons. Cell Calcium 2020, 87, 102193. [Google Scholar] [CrossRef]
- Canitano, A.; Papa, M.; Boscia, F.; Castaldo, P.; Sellitti, S.; Taglialatela, M.; Annunziato, L. Brain Distribution of the Na+/Ca2+ Exchanger-Encoding Genes NCX1, NCX2, and NCX3 and Their Related Proteins in the Central Nervous System. Ann. N. Y. Acad. Sci. 2002, 976, 394–404. [Google Scholar] [CrossRef]
- Secondo, A.; Staiano, R.I.; Scorziello, A.; Sirabella, R.; Boscia, F.; Adornetto, A.; Valsecchi, V.; Molinaro, P.; Canzoniero, L.M.T.; Di Renzo, G.; et al. BHK Cells Transfected with NCX3 Are More Resistant to Hypoxia Followed by Reoxygenation than Those Transfected with NCX1 and NCX2: Possible Relationship with Mitochondrial Mem-brane Potential. Cell Calcium 2007, 42, 521–535. [Google Scholar] [CrossRef] [PubMed]
- Boscia, F.; Gala, R.; Pignataro, G.; de Bartolomeis, A.; Cicale, M.; Ambesi-Impiombato, A.; Di Renzo, G.; Annunziato, L. Permanent Focal Brain Ischemia Induces Isoform-Dependent Changes in the Pattern of Na+/Ca2+ Exchanger Gene Expression in the Ischemic Core, Periinfarct Area, and Intact Brain Regions. J. Cereb. Blood Flow Metab. 2006, 26, 502–517. [Google Scholar] [CrossRef] [PubMed]
- Pignataro, G.; Esposito, E.; Cuomo, O.; Sirabella, R.; Boscia, F.; Guida, N.; Di Renzo, G.; Annunziato, L. The NCX3 Isoform of the Na+/Ca2+ Exchanger Contributes to Neuroprotection Elicited by Ischemic Postconditioning. J. Cereb. Blood Flow Metab. 2011, 31, 362–370. [Google Scholar] [CrossRef]
- Querfurth, H.W.; LaFerla, F.M. Alzheimer’s disease. N. Engl. J. Med. 2010, 362, 329–344. [Google Scholar] [CrossRef] [PubMed]
- Piccialli, I.; Ciccone, R.; Secondo, A.; Boscia, F.; Tedeschi, V.; de Rosa, V.; Cepparulo, P.; Annunziato, L.; Pannaccione, A. The Na+/Ca2+ Exchanger 3 Is Functionally Coupled with the NaV1.6 Voltage-Gated Channel and Promotes an Endoplasmic Reticulum Ca2+ Refilling in a Transgenic Model of Alzheimer’s Disease. Front. Pharmacol. 2021, 12, 775271. [Google Scholar] [CrossRef]
- Berridge, M.J. Calcium hypothesis of Alzheimer’s disease. Pflugers Arch. 2010, 459, 441–449. [Google Scholar] [CrossRef]
- De Felice, F.G.; Velasco, P.T.; Lambert, M.P.; Viola, K.; Fernandez, S.J.; Ferreira, S.T.; Klein, W.L. Aβ Oligomers Induce Neuronal Oxidative Stress through an N-Methyl-D-Aspartate Receptor-Dependent Mechanism That Is Blocked by the Alzheimer Drug Memantine. J. Biol. Chem. 2007, 282, 11590–11601. [Google Scholar] [CrossRef]
- Sokolow, S.; Luu, S.H.; Headley, A.J.; Hanson, A.Y.; Kim, T.; Miller, C.A.; Vinters, H.V.; Gylys, K.H. High Levels of Synaptosomal Na+-Ca2+ Exchangers (NCX1, NCX2, NCX3) Co-Localized with Amyloid-Beta in Human Cerebral Cortex Affected by Alzheimer’s Disease. Cell Calcium 2011, 49, 208–216. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Natale, S.; Anzilotti, S.; Petrozziello, T.; Ciccone, R.; Serani, A.; Calabrese, L.; Severino, B.; Frecentese, F.; Secondo, A.; Pannaccione, A.; et al. Genetic Up-Regulation or Pharmacological Activation of the Na+/Ca2+ Exchanger 1 (NCX1) Enhances Hippocampal-Dependent Contextual and Spatial Learning and Memory. Mol. Neurobiol. 2020, 57, 2358–2376. [Google Scholar] [CrossRef] [PubMed]
- Boscia, F.; de Rosa, V.; Cammarota, M.; Secondo, A.; Pannaccione, A.; Annunziato, L. The Na+/Ca2+ Exchangers in Demyelinating Diseases. Cell Calcium 2020, 85, 102130. [Google Scholar] [CrossRef] [PubMed]
- Boscia, F.; D’Avanzo, C.; Pannaccione, A.; Secondo, A.; Casamassa, A.; Formisano, L.; Guida, N.; Annunziato, L. Silencing or Knocking out the Na+/Ca2+ Exchanger-3 (NCX3) Impairs Oligodendrocyte Differentiation. Cell Death Differ. 2012, 19, 562–572. [Google Scholar] [CrossRef]
- Cammarota, M.; de Rosa, V.; Pannaccione, A.; Secondo, A.; Tedeschi, V.; Piccialli, I.; Fiorino, F.; Severino, B.; Annunziato, L.; Boscia, F. Rebound Effects of NCX3 Pharmacological Inhibition: A Novel Strategy to Accelerate Myelin Formation in Oligodendrocytes. Biomed. Pharmacother. 2021, 143, 112111. [Google Scholar] [CrossRef]
- Spillantini, M.G.; Crowther, R.A.; Jakes, R.; Hasegawa, M.; Goedert, M. α-Synuclein in Filamentous Inclusions of Lewy Bodies from Parkinson’s Disease and Dementia with Lewy Bodies. Proc. Natl. Acad. Sci. USA 1998, 95, 6469–6473. [Google Scholar] [CrossRef]
- Bastioli, G.; Piccirillo, S.; Castaldo, P.; Magi, S.; Tozzi, A.; Amoroso, S.; Calabresi, P. Selective Inhibition of Mitochondrial Sodium-Calcium Exchanger Protects Striatal Neurons from α-Synuclein plus Rotenone Induced Toxicity. Cell Death Dis. 2019, 10, 80. [Google Scholar] [CrossRef]
- Wood-Kaczmar, A.; Deas, E.; Wood, N.W.; Abramov, A.Y. The Role of the Mitochondrial NCX in the Mechanism of Neurodegeneration in Parkinson’s Disease. Adv. Exp. Med. Biol. 2013, 961, 241–249. [Google Scholar] [CrossRef]
- Sirabella, R.; Sisalli, M.J.; Costa, G.; Omura, K.; Ianniello, G.; Pinna, A.; Morelli, M.; Di Renzo, G.M.; Annunziato, L.; Scorziello, A. NCX1 and NCX3 as Potential Factors Contributing to Neurodegeneration and Neuroinflammation in the A53T Transgenic Mouse Model of Parkinson’s Disease. Cell Death Dis. 2018, 9, 725. [Google Scholar] [CrossRef]
- Golovko, M.Y.; Barceló-Coblijn, G.; Castagnet, P.I.; Austin, S.; Combs, C.K.; Murphy, E.J. The Role of α-Synuclein in Brain Lipid Metabolism: A Downstream Impact on Brain Inflammatory Response. Mol. Cell. Biochem. 2009, 326, 55–66. [Google Scholar] [CrossRef]
- Appel, S.H. Is ALS a Systemic Disorder? Evidence from Muscle Mitochondria. Exp. Neurol. 2006, 198, 1–3. [Google Scholar] [CrossRef]
- Rosen, D.R.; Siddique, T.; Patterson, D.; Figlewicz, D.A.; Sapp, P.; Hentati, A.; Donaldson, D.; Goto, J.; O’Regan, J.P.; Deng, H.-X.; et al. Mutations in Cu/Zn Superoxide Dismutase Gene Are Associated with Familial Amyotrophic Lateral Sclerosis. Nature 1993, 362, 59–62. [Google Scholar] [CrossRef] [PubMed]
- Petrozziello, T.; Secondo, A.; Tedeschi, V.; Esposito, A.; Sisalli, M.; Scorziello, A.; Di Renzo, G.; Annunziato, L. ApoSOD1 Lacking Dismutase Activity Neuroprotects Motor Neurons Exposed to Beta-Methylamino-L-Alanine through the Ca2+/Akt/ERK1/2 Prosurvival Pathway. Cell Death Differ. 2017, 24, 511–522. [Google Scholar] [CrossRef] [PubMed]
- Cozzolino, M.; Pesaresi, M.G.; Gerbino, V.; Grosskreutz, J.; Carrì, M.T. Amyotrophic Lateral Sclerosis: New Insights into Underlying Molecular Mechanisms and Opportunities for Therapeutic Intervention. Antioxid. Redox Signal. 2012, 17, 1277–1330. [Google Scholar] [CrossRef] [PubMed]
- Anzilotti, S.; Brancaccio, P.; Simeone, G.; Valsecchi, V.; Vinciguerra, A.; Secondo, A.; Petrozziello, T.; Guida, N.; Sirabella, R.; Cuomo, O.; et al. Preconditioning, Induced by Sub-Toxic Dose of the Neurotoxin L-BMAA, Delays ALS Progression in Mice and Prevents Na+/Ca2+ Exchanger 3 Downregulation. Cell Death Dis. 2018, 9, 206. [Google Scholar] [CrossRef]
- Anzilotti, S.; Valsecchi, V.; Brancaccio, P.; Guida, N.; Laudati, G.; Tedeschi, V.; Petrozziello, T.; Frecentese, F.; Magli, E.; Hassler, B.; et al. Prolonged NCX Activation Prevents SOD1 Accumulation, Reduces Neuroinflammation, Ameliorates Motor Behavior and Prolongs Survival in a ALS Mouse Model. Neurobiol. Dis. 2021, 159, 105480. [Google Scholar] [CrossRef]
- Petrozziello, T.; Boscia, F.; Tedeschi, V.; Pannaccione, A.; de Rosa, V.; Corvino, A.; Severino, B.; Annunziato, L.; Secondo, A. Na+/Ca2+ Exchanger Isoform 1 Takes Part to the Ca2+-Related Prosurvival Pathway of SOD1 in Primary Motor Neurons Exposed to Beta-Methylamino-l-Alanine. Cell Commun. Signal. 2022, 20, 8. [Google Scholar] [CrossRef] [PubMed]
- Newton, H.B. Glioblastoma Multiforme. Curr. Treat. Options Neurol. 2008, 10, 285–294. [Google Scholar] [CrossRef]
- Brandalise, F.; Ratto, D.; Leone, R.; Olivero, F.; Roda, E.; Locatelli, C.A.; Grazia Bottone, M.; Rossi, P. Deeper and Deeper on the Role of BK and Kir4.1 Channels in Glioblastoma Invasiveness: A Novel Summative Mechanism? Front. Neurosci. 2020, 14, 595664. [Google Scholar] [CrossRef]
- Ratto, D.; Ferrari, B.; Roda, E.; Brandalise, F.; Siciliani, S.; De Luca, F.; Priori, E.C.; Di Iorio, C.; Cobelli, F.; Veneroni, P.; et al. Squaring the Circle: A New Study of Inward and Outward-Rectifying Potassium Currents in U251 GBM Cells. Cell. Mol. Neurobiol. 2020, 40, 813–828. [Google Scholar] [CrossRef]
- Turner, K.L.; Sontheimer, H. Cl− and K+ Channels and Their Role in Primary Brain Tumour Biology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2014, 369, 20130095. [Google Scholar] [CrossRef]
- Brandalise, F.; Ramieri, M.; Pastorelli, E.; Priori, E.C.; Ratto, D.; Venuti, M.T.; Roda, E.; Talpo, F.; Rossi, P. Role of Na+/Ca2+ Exchanger (NCX) in Glioblastoma Cell Migration (In Vitro). Int. J. Mol. Sci. 2023, 24, 12673. [Google Scholar] [CrossRef]
- Song, M.; Chen, D.; Yu, S.P. The TRPC Channel Blocker SKF96365 Inhibits Glioblastoma Cell Growth by Enhancing Reverse Mode of the Na+/Ca2+ Exchanger and Increasing Intracellular Ca2+. Br. J. Pharmacol. 2014, 171, 3432–3447. [Google Scholar] [CrossRef]
- Hu, H.; Wang, S.; Wang, Y.; Liu, Y.; Feng, X.; Shen, Y.; Zhu, L.; Chen, H.; Song, M. Blockade of the Forward Na+/Ca 2+ Exchanger Suppresses the Growth of Glioblastoma Cells through Ca2+-mediated Cell Death. Br. J. Pharmacol. 2019, 176, 2691–2707. [Google Scholar] [CrossRef] [PubMed]
- Amoroso, S.; Taglialatela, M.; Canzoniero, L.M.T.; Cragoe, E.J.; di Renzo, G.; Annunziato, L. Possible Involvement of Ca++ Ions, Protein Kinase C and Na+-H+ Antiporter in Insulin-Induced Endogenous Dopamine Release from Tuberoinfundibular Neurons. Life Sci. 1990, 46, 885–894. [Google Scholar] [CrossRef]
- Liu, Z.; Cheng, Q.; Ma, X.; Song, M. Suppressing Effect of Na+/Ca2+ Exchanger (NCX) Inhibitors on the Growth of Melanoma Cells. Int. J. Mol. Sci. 2022, 23, 901. [Google Scholar] [CrossRef] [PubMed]
- Rogister, F.; Laeckmann, D.; Plasman, P.O.; Van Eylen, F.; Ghyoot, M.; Maggetto, C.; Liégeois, J.-F.; Géczy, J.; Herchuelz, A.; Delarge, J. Novel Inhibitors of the Sodium–Calcium Exchanger: Benzene Ring Analogues of N-Guanidino Substituted Amiloride Derivatives. Eur. J. Med. Chem. 2001, 36, 597–614, Erratum in Eur. J. Med. Chem. 2002, 37, 441. [Google Scholar] [CrossRef]
- Secondo, A.; Pannaccione, A.; Molinaro, P.; Ambrosino, P.; Lippiello, P.; Esposito, A.; Cantile, M.; Khatri, P.R.; Melisi, D.; Di Renzo, G.; et al. Molecular Pharmacology of the Amiloride Analog 3-Amino-6-Chloro-5-[(4-Chloro-Benzyl)Amino]-N-[[(2,4-Dimethylbenzyl)-Amino]Iminomethyl]-Pyrazinecarboxamide (CB-DMB) as a Pan Inhibitor of the Na+-Ca2+ Exchanger Isoforms NCX1, NCX2, and NCX3 in Stably Transfected Cells. J. Pharmacol. Exp. Ther. 2009, 33, 212–221. [Google Scholar] [CrossRef]
- Shigekawa, M.; Iwamoto, T. Cardiac Na+-Ca2+ Exchange. Circ. Res. 2001, 88, 864–876. [Google Scholar] [CrossRef]
- Watano, T.; Kimura, J.; Morita, T.; Nakanishi, H. A Novel Antagonist, No. 7943, of the Na+/Ca2+ Exchange Current in Guinea-Pig Cardiac Ventricular Cells. Br. J. Pharmacol. 1996, 119, 555–563. [Google Scholar] [CrossRef]
- Iwamoto, T.; Watano, T.; Shigekawa, M. A Novel Isothiourea Derivative Selectively Inhibits the Reverse Mode of Na+/Ca2+ Exchange in Cells Expressing NCX1. J. Biol. Chem. 1996, 271, 22391–22397. [Google Scholar] [CrossRef]
- Amran, M.S.; Homma, N.; Hashimoto, K. Pharmacology of KB-R7943: A Na+-Ca2+ Exchange Inhibitor. Cardiovasc. Drug Rev. 2003, 21, 255–276. [Google Scholar] [CrossRef]
- Sobolevsky, A.I.; Khodorov, B.I. Blockade of NMDA Channels in Acutely Isolated Rat Hippocampal Neurons by the Na+/Ca2+ Exchange Inhibitor KB-R7943. Neuropharmacology 1999, 38, 1235–1242. [Google Scholar] [CrossRef] [PubMed]
- Pintado, A.J.; Herrero, C.J.; García, A.G.; Montiel, C. The Novel Na+/Ca2+ Exchange Inhibitor KB-R7943 Also Blocks Native and Expressed Neuronal Nicotinic Receptors. Br. J. Pharmacol. 2000, 130, 1893–1902. [Google Scholar] [CrossRef]
- MacDonald, A.C.; Howlett, S.E. Differential Effects of the Sodium Calcium Exchange Inhibitor, KB-R7943, on Ischemia and Reperfusion Injury in Isolated Guinea Pig Ventricular Myocytes. Eur. J. Pharmacol. 2008, 580, 214–223. [Google Scholar] [CrossRef]
- Andreeva-Gateva, P.; Hristov, M.; Strokova-Stoilova, M.; Ivanova, N.; Sabit, Z.; Surcheva, S.; Beliakov, M.; Karakashev, G.; Sukhov, I.; Belinskaya, D.; et al. Therapeutic potential of orally applied KB-R7943 in streptozotocin-induced neuropathy in rats. Heliyon 2024, 10, e27367. [Google Scholar] [CrossRef]
- Matsuda, T.; Arakawa, N.; Takuma, K.; Kishida, Y.; Kawasaki, Y.; Sakaue, M.; Takahashi, K.; Takahashi, T.; Suzuki, T.; Ota, T.; et al. SEA0400, a novel and selective inhibitor of the Na+-Ca2+ exchanger, attenuates reperfusion injury in the in vitro and in vivo cerebral ischemic models. J. Pharmacol. Exp. Ther. 2001, 298, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, H.; Nishimaru, K.; Aikawa, T.; Hirayama, W.; Tanaka, Y.; Shigenobu, K. Effect of SEA0400, a Novel In-hibitor of Sodium-calcium Exchanger, on Myocardial Ionic Currents. Br. J. Pharmacol. 2002, 135, 1096–1100. [Google Scholar] [CrossRef] [PubMed]
- Matsuda, T.; Koyama, Y.; Baba, A. Functional Proteins Involved in Regulation of Intracellular Ca2+ for Drug Development: Pharmacology of SEA0400, a Specific Inhibitor of the Na+-Ca2+ Exchanger. J. Pharmacol. Sci. 2005, 97, 339–343. [Google Scholar] [CrossRef]
- Kita, S.; Iwamoto, T. Inhibitory Mechanism of SN-6, A Novel Benzyloxyphenyl Na+/Ca2+ Exchange Inhibitor. Ann. N. Y. Acad. Sci. 2007, 1099, 529–533. [Google Scholar] [CrossRef]
- Iwamoto, T.; Inoue, Y.; Ito, K.; Sakaue, T.; Kita, S.; Katsuragi, T. The Exchanger Inhibitory Peptide Region-Dependent Inhibition of Na+ /Ca2+ Exchange by SN-6 [2-[4-(4-Nitrobenzyloxy)Benzyl]Thiazolidine-4-Carboxylic Acid Ethyl Ester], a Novel Benzyloxyphenyl Derivative. Mol. Pharmacol. 2004, 66, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Kuramochi, T.; Kakefuda, A.; Yamada, H.; Ogiyama, T.; Taguchi, T.; Sakamoto, S. Synthesis and Structure–Activity Relationships of Benzyloxyphenyl Derivatives as a Novel Class of NCX Inhibitors: Effects on Heart Failure. Bioorg. Med. Chem. 2005, 13, 725–734. [Google Scholar] [CrossRef]
- Kuramochi, T.; Kakefuda, A.; Sato, I.; Tsukamoto, I.; Taguchi, T.; Sakamoto, S. Synthesis and Structure–Activity Relationships of 6-{4-[(3-Fluorobenzyl)oxy]Phenoxy}nicotinamide Derivatives as a Novel Class of NCX Inhibitors: A QSAR Study. Bioorg. Med. Chem. 2005, 13, 717–724. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, T.; Kita, S. YM-244769, a Novel Na+/Ca2+ Exchange Inhibitor That Preferentially Inhibits NCX3, Effi-ciently Protects against Hypoxia/Reoxygenation-Induced SH-SY5Y Neuronal Cell Damage. Mol. Pharmacol. 2006, 70, 2075–2083. [Google Scholar] [CrossRef]
- Secondo, A.; Pignataro, G.; Ambrosino, P.; Pannaccione, A.; Molinaro, P.; Boscia, F.; Cantile, M.; Cuomo, O.; Esposito, A.; Sisalli, M.J.; et al. Pharmacological Characterization of the Newly Synthesized 5-Amino-N-Butyl-2-(4-Ethoxyphenoxy)-Benzamide Hydrochloride (BED) as a Potent NCX3 Inhibitor That Worsens Anoxic Injury in Cortical Neurons, Organotypic Hippocampal Cultures, and Ischemic Brain. ACS Chem. Neurosci. 2015, 6, 1361–1370. [Google Scholar] [CrossRef]
- Annunziato, L.; Secondo, A.; Pignataro, G.; Scorziello, A.; Molinaro, P. New Perspectives for Selective NCX Activators in Neurodegenerative Diseases. Cell Calcium 2020, 87, 102170. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, Z.; Li, Y.; Huang, B.; Bai, Q.; Gao, Y.; Chen, Q.; Li, N.; He, L.; Zhao, Y. Structural Insight into the Allosteric Inhibition of Human Sodium-Calcium Exchanger NCX1 by XIP and SEA0400. EMBO J. 2024, 43, 14–31. [Google Scholar] [CrossRef]
- Hasegawa, H.; Muraoka, M.; Matsui, K.; Kojima, A. Discovery of a Novel Potent Na+/Ca2+ Exchanger Inhibitor: Design, Synthesis and Structure–Activity Relationships of 3,4-Dihydro-2(1 H)-Quinazolinone Derivatives. Bioorg. Med. Chem. Lett. 2003, 13, 3471–3475. [Google Scholar] [CrossRef]
- Hasegawa, H.; Muraoka, M.; Ohmori, M.; Matsui, K.; Kojima, A. A Novel Class of Sodium/Calcium Exchanger Inhibitor: Design, Synthesis, and Structure–Activity Relationships of 3,4-Dihydro-2(1H)-Quinazolinone Derivatives. Bioorg. Med. Chem. 2005, 13, 3721–3735. [Google Scholar] [CrossRef] [PubMed]
- Otsomaa, L.; Levijoki, J.; Wohlfahrt, G.; Chapman, H.; Koivisto, A.P.; Syrjänen, K.; Koskelainen, T.; Peltokorpi, S.E.; Finckenberg, P.; Heikkilä, A.; et al. Discovery and Characterization of ORM-11372, a Novel Inhibitor of the Sodium-Calcium Exchanger with Positive Inotropic Activity. Br. J. Pharmacol. 2020, 177, 5534–5554. [Google Scholar] [CrossRef]
- Giacomello, M.; Drago, I.; Pizzo, P.; Pozzan, T. Mitochondrial Ca2+ as a Key Regulator of Cell Life and Death. Cell Death Differ. 2007, 14, 1267–1274. [Google Scholar] [CrossRef]
- Szabadkai, G.; Simoni, A.M.; Bianchi, K.; De Stefani, D.; Leo, S.; Wieckowski, M.R.; Rizzuto, R. Mitochondrial Dynamics and Ca2+ Signaling. Biochim. Biophys. Acta 2006, 1763, 442–449. [Google Scholar] [CrossRef]
- Castaldo, P.; Cataldi, M.; Magi, S.; Lariccia, V.; Arcangeli, S.; Amoroso, S. Role of the Mitochondrial Sodium/Calcium Exchanger in Neuronal Physiology and in the Pathogenesis of Neurological Diseases. Prog. Neurobiol. 2009, 87, 58–79. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Nguyen, T.T.; Choi, S.-K.; Xu, S.; Das, R.; Cha, S.-K.; Kim, N.; Han, J.; Wiederkehr, A.; Wollheim, C.B.; et al. Essential Role of Mitochondrial Ca2+ Uniporter in the Generation of Mitochondrial PH Gradient and Metabolism-Secretion Coupling in Insulin-Releasing Cells. J. Biol. Chem. 2015, 290, 4086–4096. [Google Scholar] [CrossRef]
- Martínez-Sanz, F.J.; Lajarín-Cuesta, R.; Moreno-Ortega, A.J.; González-Lafuente, L.; Fernández-Morales, J.C.; López-Arribas, R.; Cano-Abad, M.F.; De Los Ríos, C. Benzothiazepine CGP37157 Analogues Exert Cytoprotection in Various in Vitro Models of Neurodegeneration. ACS Chem. Neurosci. 2015, 6, 1626–1636. [Google Scholar] [CrossRef]
- González-Lafuente, L.; Egea, J.; León, R.; Martínez-Sanz, F.J.; Monjas, L.; Perez, C.; Merino, C.; García-De Diego, A.M.; Rodríguez-Franco, M.I.; García, A.G.; et al. Benzothiazepine CGP37157 and Its Isosteric 2′-Methyl Analogue Provide Neuroprotection and Block Cell Calcium Entry. ACS Chem. Neurosci. 2012, 3, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Molinaro, P.; Cantile, M.; Cuomo, O.; Secondo, A.; Pannaccione, A.; Ambrosino, P.; Pignataro, G.; Fiorino, F.; Severino, B.; Gatta, E.; et al. Neurounina-1, a Novel Compound That Increases Na+ /Ca2+ Exchanger Activity, Effectively Protects against Stroke Damage. Mol. Pharmacol. 2013, 83, 142–156. [Google Scholar] [CrossRef] [PubMed]
- Severino, B.; Corvino, A.; Fiorino, F.; Frecentese, F.; Perissutti, E.; Caliendo, G.; Santagada, V.; Magli, E.; Molinaro, P.; Pignataro, G.; et al. Development, Validation of LC-MS/MS Method and Determination of Pharmacokinetic Parameters of the Stroke Neuroprotectant Neurounina-1 in Beagle Dog Plasma After Intravenous Administration. Front. Pharmacol. 2019, 10, 432. [Google Scholar] [CrossRef]
- Magli, E.; Fattorusso, C.; Persico, M.; Corvino, A.; Esposito, G.; Fiorino, F.; Luciano, P.; Perissutti, E.; Santagada, V.; Severino, B.; et al. New Insights into the Structure–Activity Relationship and Neuroprotective Profile of Benzodiazepinone Derivatives of Neurounina-1 as Modulators of the Na+/Ca2+ Exchanger Isoforms. J. Med. Chem. 2021, 64, 17901–17919. [Google Scholar] [CrossRef]
- Iwamoto, T. Forefront of Na+/Ca2+ exchanger studies: Molecular pharmacology of Na+/Ca2+ exchange inhibitors. J. Pharmacol. Sci. 2004, 96, 27–32. [Google Scholar] [CrossRef]
- Niu, C.F.; Watanabe, Y.; Ono, K.; Iwamoto, T.; Yamashita, K.; Satoh, H.; Urushida, T.; Hayashi, H.; Kimura, J. Characterization of SN-6, a novel Na+/Ca2+ exchange inhibitor in guinea pig cardiac ventricular myocytes. Eur. J. Pharmacol. 2007, 573, 161–169. [Google Scholar] [CrossRef]
- Jost, N.; Nagy, N.; Corici, C.; Kohajda, Z.; Horváth, A.; Acsai, K.; Biliczki, P.; Levijoki, J.; Pollesello, P.; Koskelainen, T.; et al. ORM-10103, a novel specific inhibitor of the Na+/Ca2+ exchanger, decreases early and delayed afterdepolarizations in the canine heart. Br. J. Pharmacol. 2013, 170, 768–778. [Google Scholar] [CrossRef] [PubMed]
- Kohajda, Z.; Farkas-Morvay, N.; Jost, N.; Nagy, N.; Geramipour, A.; Horváth, A.; Varga, R.S.; Hornyik, T.; Corici, C.; Acsai, K.; et al. The Effect of a Novel Highly Selective Inhibitor of the Sodium/Calcium Exchanger (NCX) on Cardiac Arrhythmias in In Vitro and In Vivo Experiments. PLoS ONE 2016, 1, e0166041. [Google Scholar] [CrossRef] [PubMed]
- Cox, D.A.; Conforti, L.; Sperelakis, N.; Matlib, M.A. Selectivity of inhibition of Na+-Ca2+ exchange of heart mitochondria by benzothiazepine CGP-37157. J. Cardiovasc. Pharmacol. 1993, 21, 595–599. [Google Scholar] [CrossRef] [PubMed]
- Giladi, M.; Tal, I.; Khananshvili, D. Structural Features of Ion Transport and Allosteric Regulation in Sodium-Calcium Exchanger (NCX) Proteins. Front. Physiol. 2016, 7, 30. [Google Scholar] [CrossRef]
- Xue, J.; Zeng, W.; John, S.; Attiq, N.; Ottolia, M.; Jiang, Y. Structural Mechanisms of PIP2 Activation and SEA0400 Inhibition in Human Cardiac Sodium-Calcium Exchanger NCX1. eLife 2025, 14, RP105396. [Google Scholar] [CrossRef]
- Galvankova, K.; Rezuchova, I.; Klena, L.; Grman, M.; Gazova, S.; Liskova, V.; Kozovska, Z.; Roller, L.; Babula, P.; Krizanova, O. Role of the sodium/calcium exchanger type 3 in cancer cells. Eur. J. Cell Biol. 2025, 104, 151493. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scognamiglio, A.; Corvino, A.; Caliendo, G.; Fiorino, F.; Perissutti, E.; Santagada, V.; Severino, B. Druggability of Sodium Calcium Exchanger (NCX): Challenges and Recent Development. Int. J. Mol. Sci. 2025, 26, 8888. https://doi.org/10.3390/ijms26188888
Scognamiglio A, Corvino A, Caliendo G, Fiorino F, Perissutti E, Santagada V, Severino B. Druggability of Sodium Calcium Exchanger (NCX): Challenges and Recent Development. International Journal of Molecular Sciences. 2025; 26(18):8888. https://doi.org/10.3390/ijms26188888
Chicago/Turabian StyleScognamiglio, Antonia, Angela Corvino, Giuseppe Caliendo, Ferdinando Fiorino, Elisa Perissutti, Vincenzo Santagada, and Beatrice Severino. 2025. "Druggability of Sodium Calcium Exchanger (NCX): Challenges and Recent Development" International Journal of Molecular Sciences 26, no. 18: 8888. https://doi.org/10.3390/ijms26188888
APA StyleScognamiglio, A., Corvino, A., Caliendo, G., Fiorino, F., Perissutti, E., Santagada, V., & Severino, B. (2025). Druggability of Sodium Calcium Exchanger (NCX): Challenges and Recent Development. International Journal of Molecular Sciences, 26(18), 8888. https://doi.org/10.3390/ijms26188888












