Characterization of the Binding Modes of Cu2+ Ions with Tyrosine and Ado, AMP, ADP, and ATP: A Comprehensive Potentiometric, Spectroscopic, and Computational Approach
Abstract
1. Introduction
2. Results and Discussion
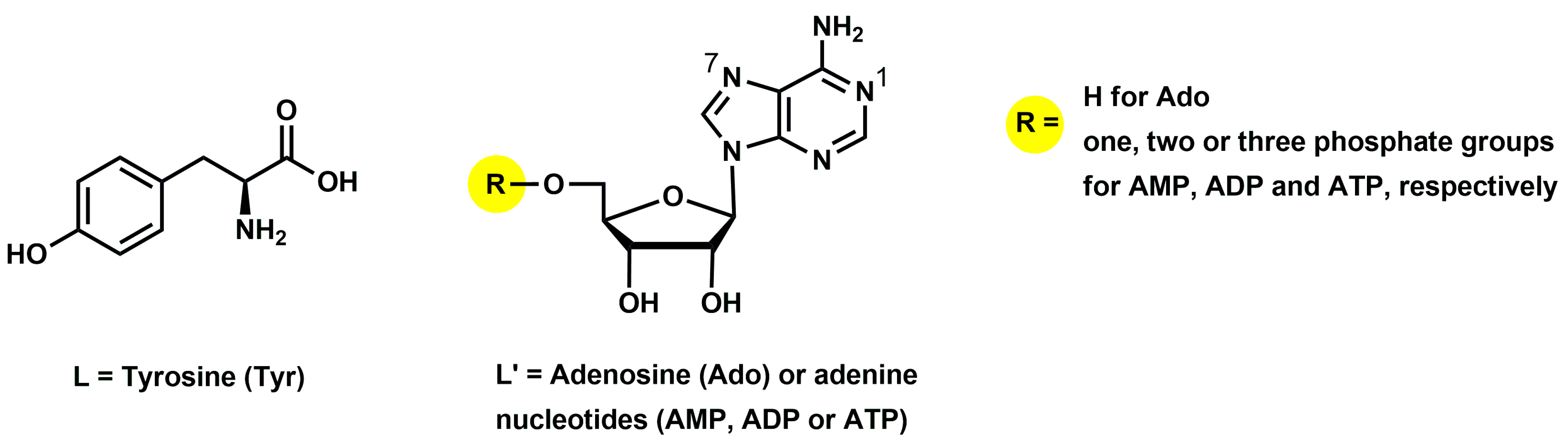
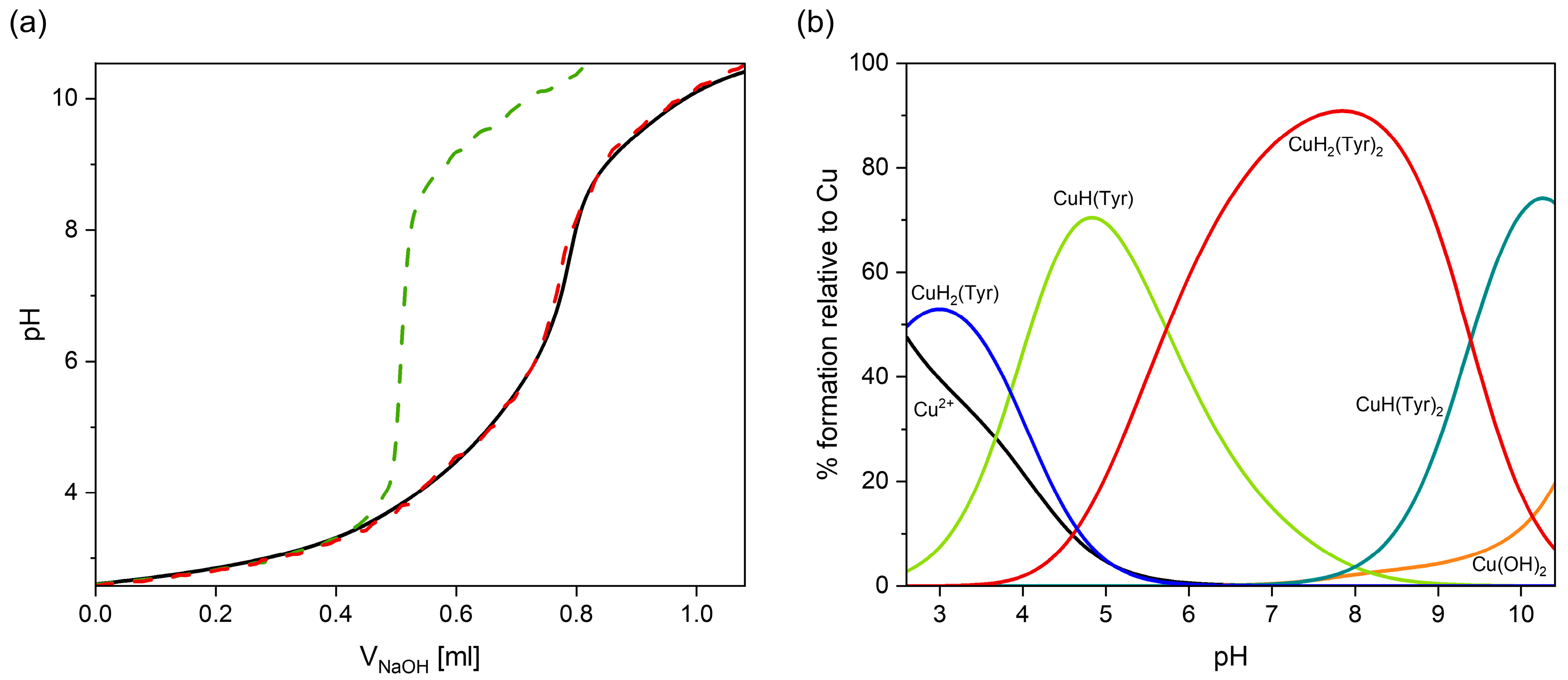
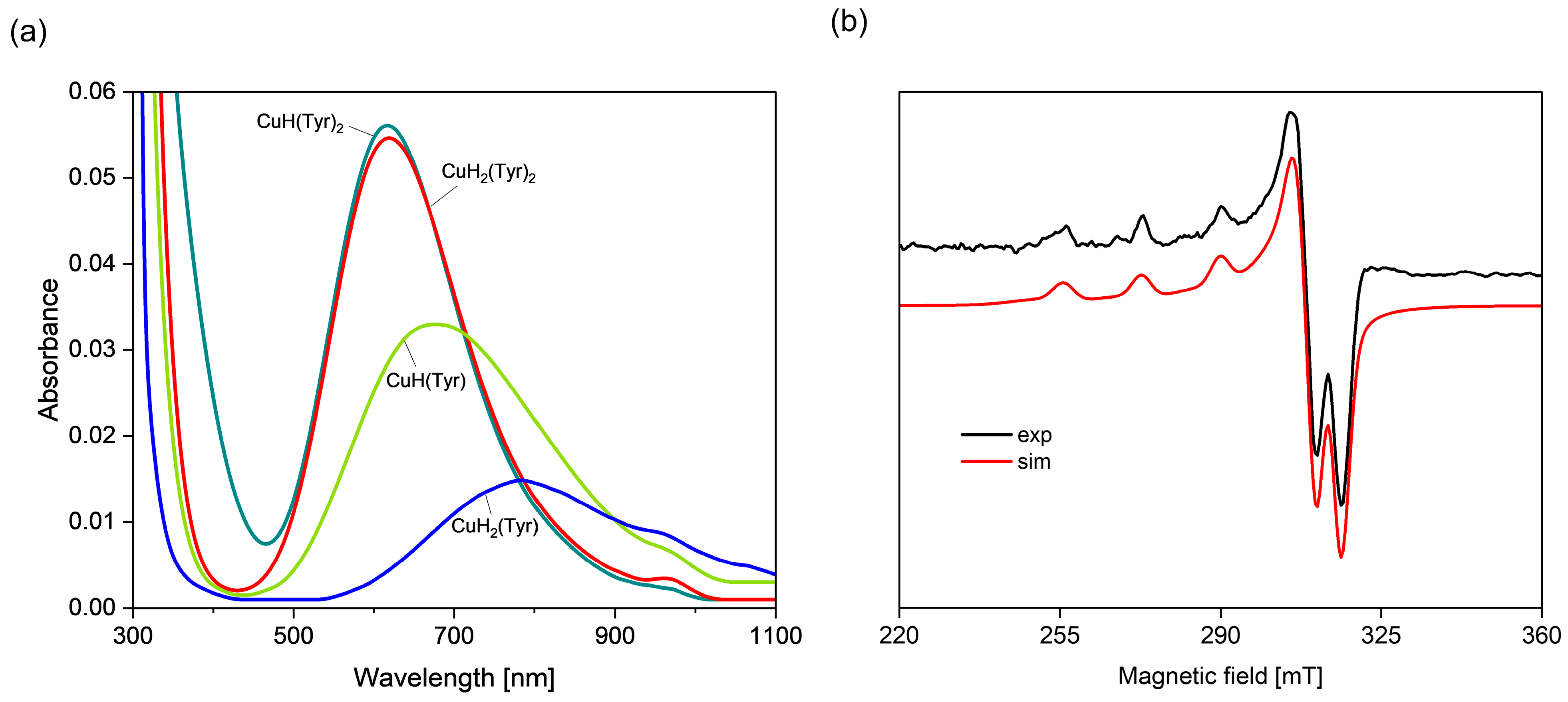
2.1. Interactions in the Binary Cu(II)/Tyrosine System
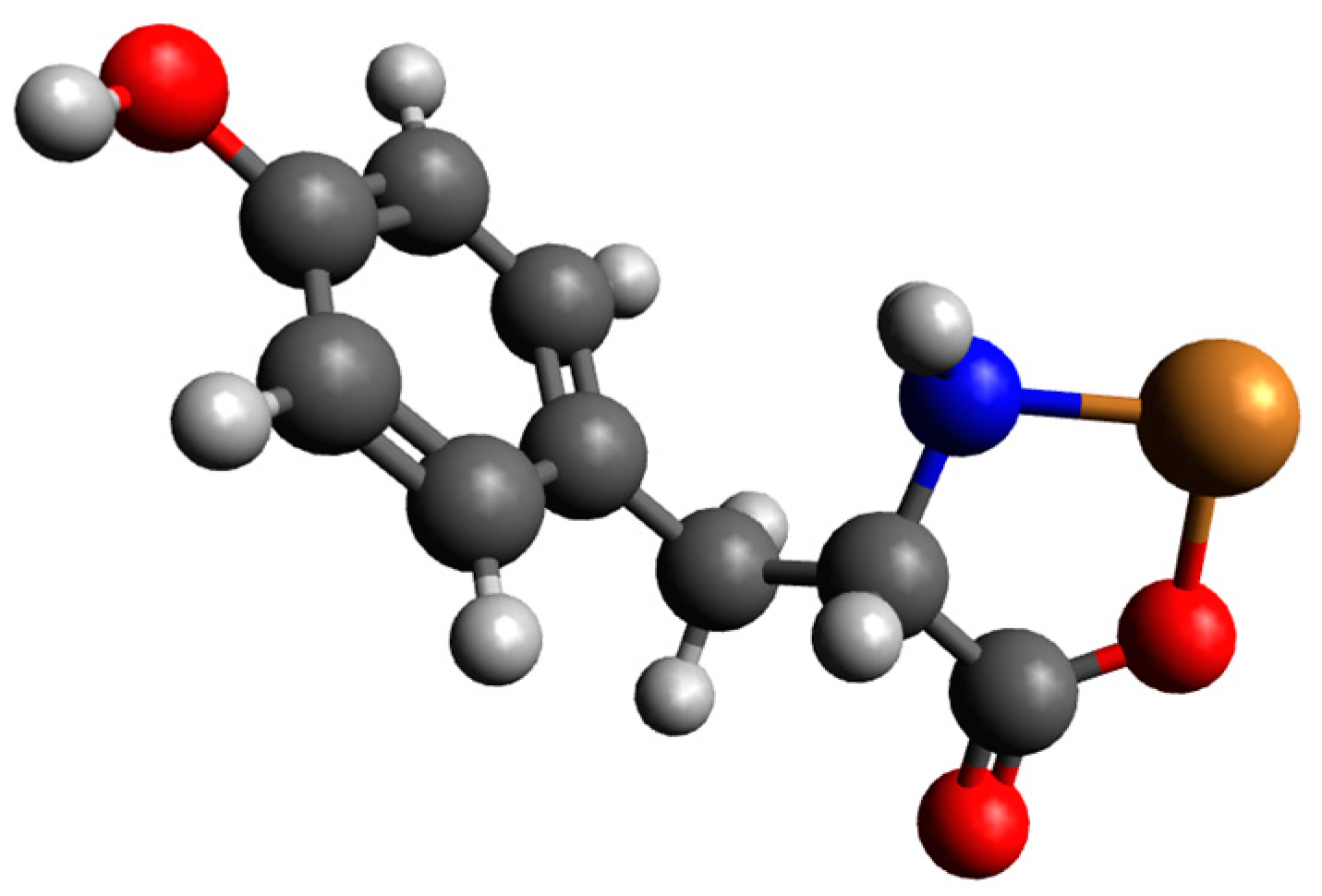
Quantum-Chemical Calculations in the Binary Cu(II)/Tyr System
2.2. Interactions in Cu(II)/Tyrosine/Adenosine (or AMP, ADP, ATP) Ternary Systems
2.2.1. Cu(II)–Tyrosine–Adenosine Complexes
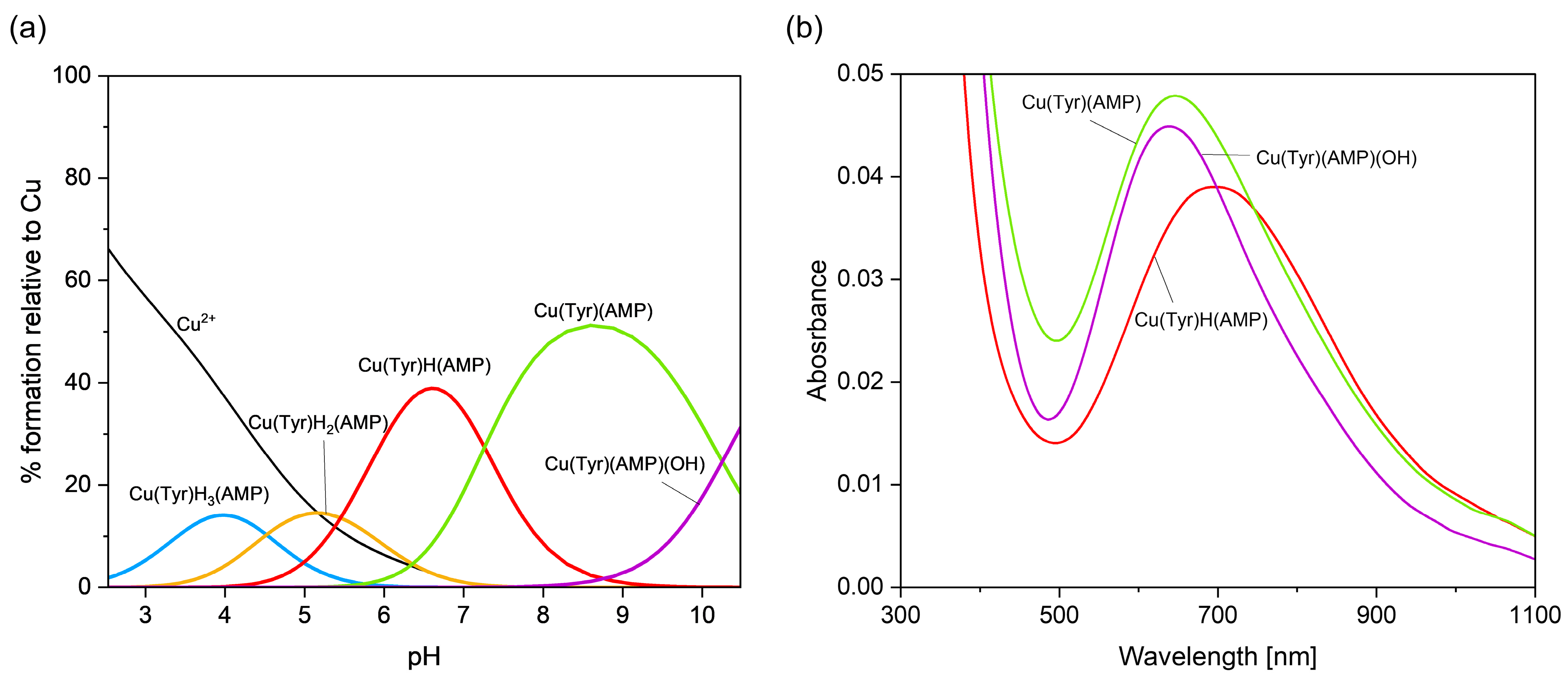
2.2.2. Cu(II)–Tyrosine–Adenosine-5’-Monophosphate Complexes
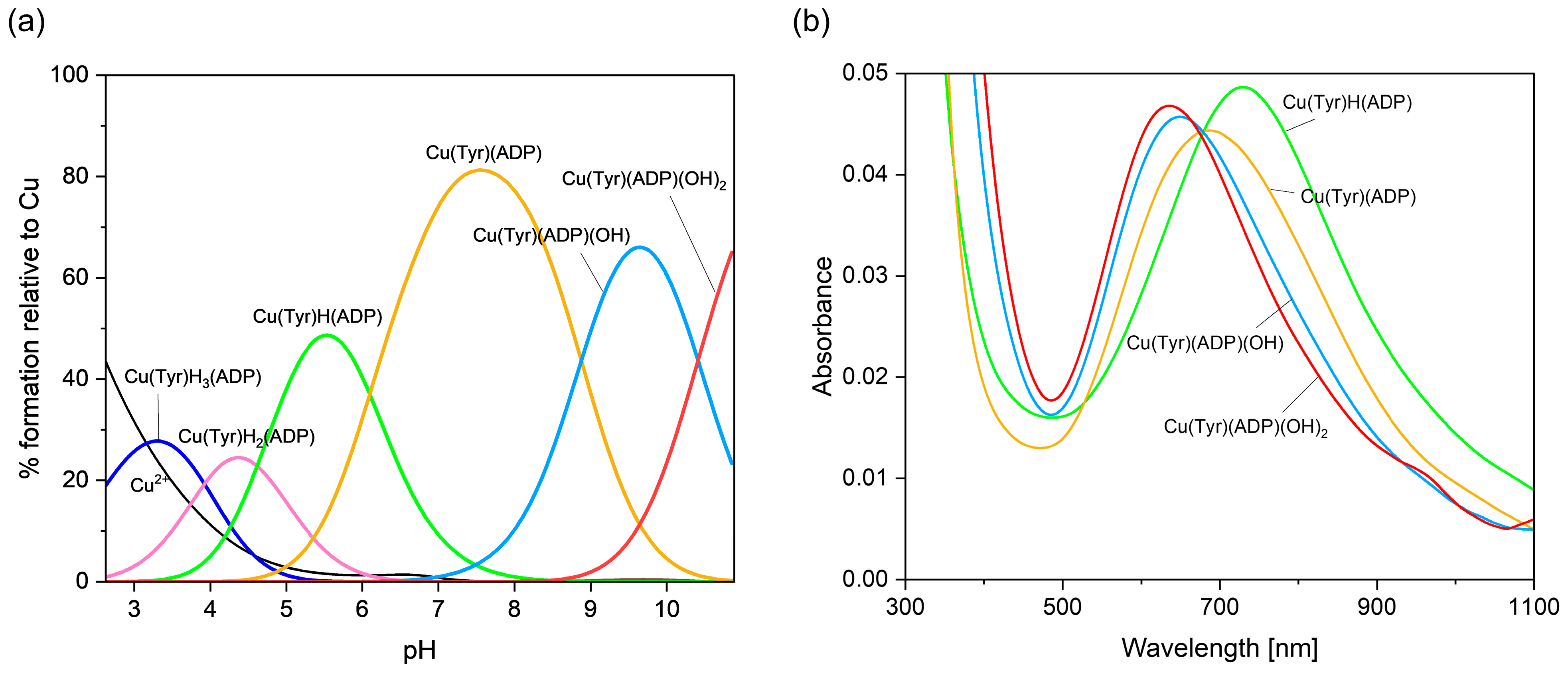
2.2.3. Cu(II)–Tyrosine–Adenosine-5’-Diphosphate Complexes
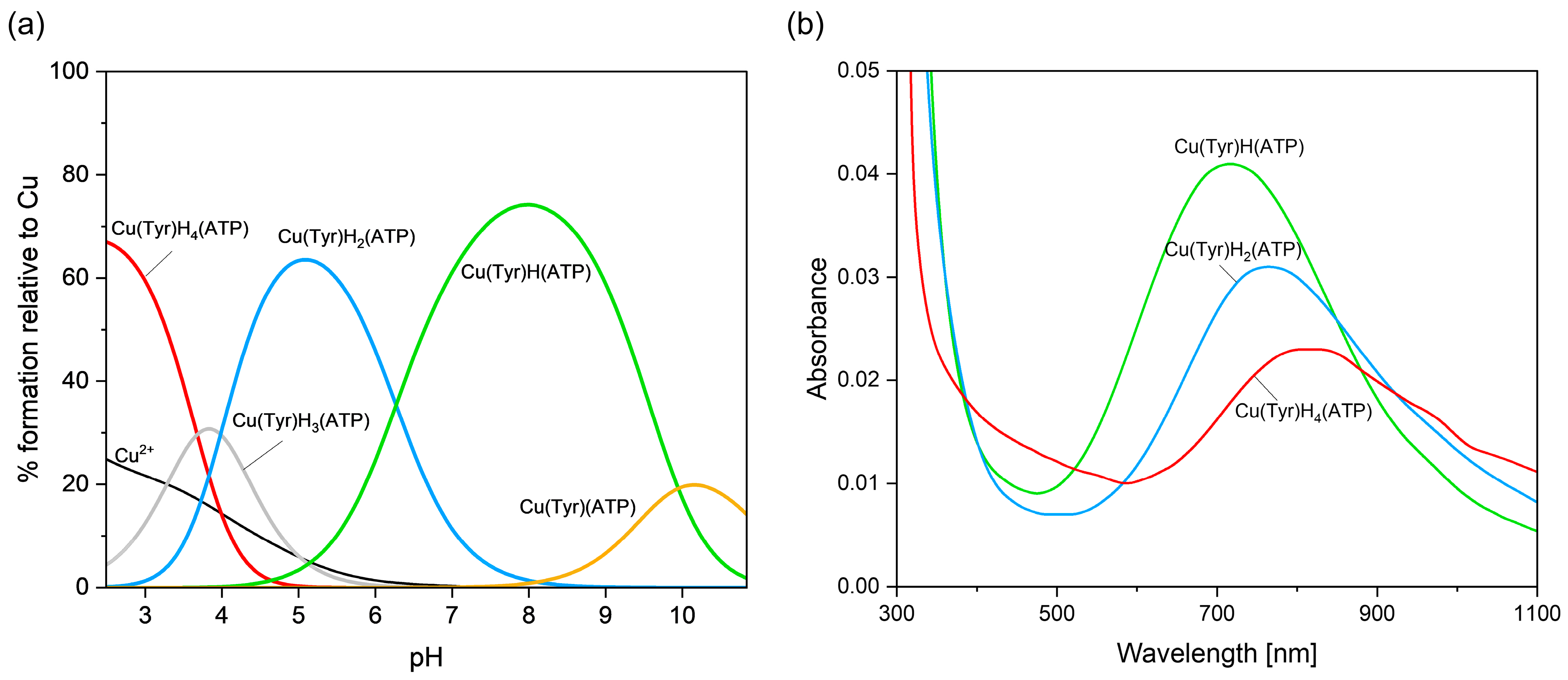
2.2.4. Cu(II)–Tyrosine–Adenosine-5’-Triphosphate Complexes
2.3. Quantum-Chemical Calculations in Ternary Systems
2.3.1. The Cu(II)/Tyr/Ado System
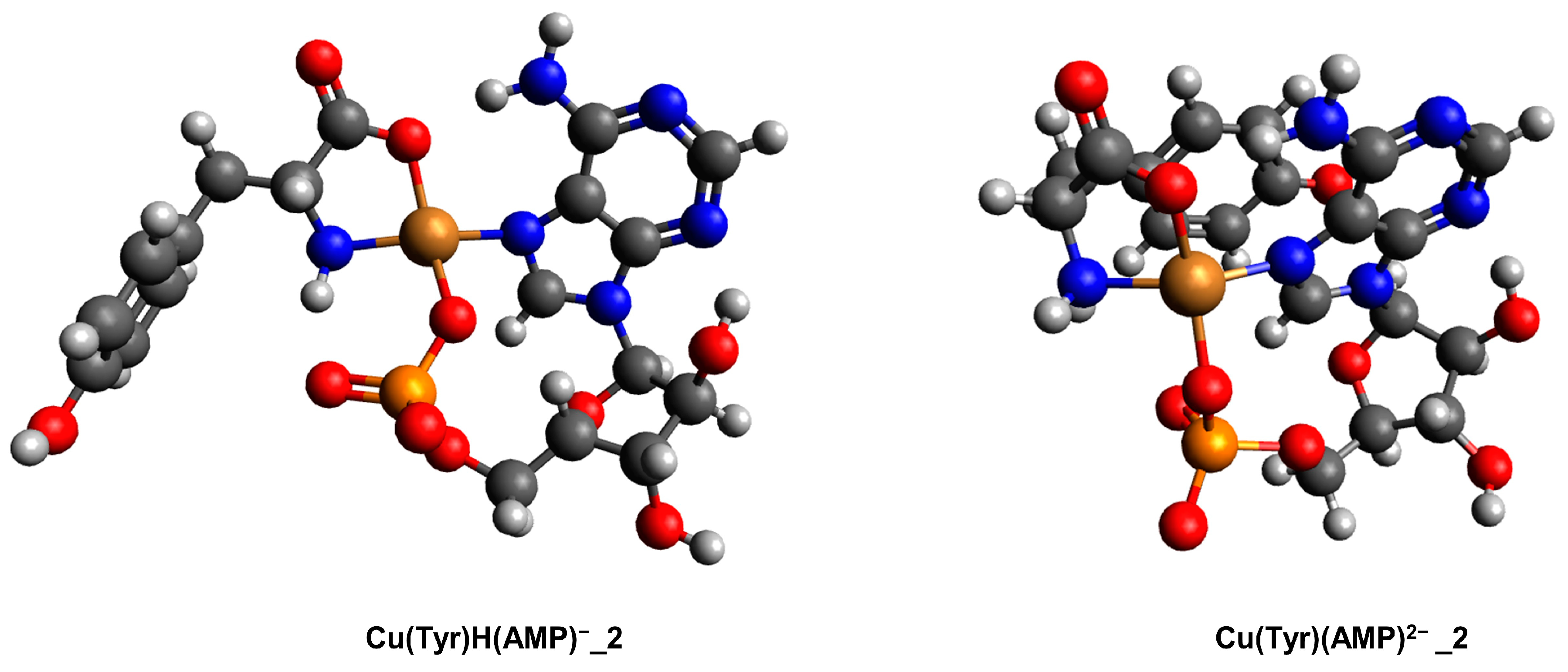
2.3.2. The Cu(II)/Tyr/AMP System
2.3.3. The Cu(II)/Tyr/ADP System
2.3.4. The Cu(II)/Tyr/ATP System
3. Materials and Methods
3.1. Chemicals
3.2. Potentiometric Measurements
3.3. Spectroscopic Measurements
3.3.1. VIS Spectroscopy
3.3.2. EPR Spectroscopy
3.3.3. Infrared Spectroscopy (IR)
3.4. The Crystal Structure
3.5. The Quantum-Chemical Calculations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Tyr | Tyrosine |
| Ado | Adenosine |
| AMP | Adenosine-5’-monophosphate |
| ADP | Adenosine-5’-diphosphate |
| ATP | Adenosine-5’-triphosphate |
| VIS | Visible light spectroscopy |
| ICP OES | Inductively coupled plasma optical emission spectrometry |
| EPR | Electron paramagnetic resonance |
| IR | Infrared spectroscopy |
| D2O | Deuterium oxide |
| NaOD | Sodium deuteroxide |
| DCl | Deuterium chloride |
References
- Yoo, J.; Han, J.; Lim, M.H. Transition Metal Ions and Neurotransmitters: Coordination Chemistry and Implications for Neurodegeneration. RSC Chem. Biol. 2023, 4, 548–563. [Google Scholar] [CrossRef]
- Seo, H.; Prosser, K.E.; Kalaj, M.; Karges, J.; Dick, B.L.; Cohen, S.M. Evaluating Metal–Ligand Interactions of Metal-Binding Isosteres Using Model Complexes. Inorg. Chem. 2021, 60, 17161–17172. [Google Scholar] [CrossRef]
- Skrobanska, M.; Zabiszak, M.; Grześkiewicz, A.M.; Kaczmarek, M.T.; Jastrzab, R. Investigation of Gallium(III) Complexes with Thiouracil Derivatives: Effects of pH on Coordination and Stability. Int. J. Mol. Sci. 2024, 25, 12869. [Google Scholar] [CrossRef]
- İnci, D.; Aydın, R.; Yılmaz, D.; Gençkal, H.M.; Vatan, Ö.; Çinkılıç, N.; Zorlu, Y. New Water-Soluble Copper (II) Complexes Including 4,7-Dimethyl-1,10-Phenanthroline and l-Tyrosine: Synthesis, Characterization, DNA Interactions and Cytotoxicities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 136, 761–770. [Google Scholar] [CrossRef] [PubMed]
- Remko, M.; Fitz, D.; Broer, R.; Rode, B.M. Effect of Metal Ions (Ni2+, Cu2+ and Zn2+) and Water Coordination on the Structure of L-Phenylalanine, L-Tyrosine, L-Tryptophan and Their Zwitterionic Forms. J. Mol. Model. 2011, 17, 3117–3128. [Google Scholar] [CrossRef] [PubMed]
- Boča, R.; Štofko, J.; Imrich, R. Ab Initio Study of Molecular Properties of L-Tyrosine. J. Mol. Model. 2023, 29, 245. [Google Scholar] [CrossRef]
- İnci, D.; Aydın, R.; Vatan, Ö.; Yılmaz, D.; Gençkal, H.M.; Zorlu, Y.; Cavaş, T. Binary and Ternary New Water Soluble Copper(II) Complexes of L -Tyrosine and Substituted 1,10-Phenanthrolines: Effect of Substitution on DNA Interactions and Cytotoxicities. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 313–324. [Google Scholar] [CrossRef]
- İnci, D.; Aydın, R.; Vatan, Ö.; Huriyet, H.; Zorlu, Y.; Çoşut, B.; Çinkılıç, N. Cu (II) Tyrosinate Complexes Con-taining Methyl Substituted Phenanthrolines: Synthesis, X-ray Crystal Structures, Biomolecular Interactions, Antioxidant Activity, ROS Generation and Cytotoxicity. Appl. Organomet. Chem. 2019, 33, e4652. [Google Scholar] [CrossRef]
- Zong, Z.; Huang, G.-M.; Zhang, X.; Fan, Y.-H.; Bi, C.-F.; Zhang, D.-M.; Yan, X.-C.; Zhang, N. Synthesis, Crystal Structure, Theoretical Calculation, and DNA Binding of a Copper (II) Complex with 3,5-Dibromo-L-Tyrosine. Inorg. Nano-Met. Chem. 2020, 51, 1241–1248. [Google Scholar] [CrossRef]
- Jain, S.; John, A.; George, C.E.; Johnson, R.P. Tyrosine-Derived Polymers as Potential Biomaterials: Synthesis Strategies, Properties, and Applications. Biomacromolecules 2023, 24, 531–565. [Google Scholar] [CrossRef] [PubMed]
- Jastrzab, R.; Lomozik, L. Effectiveness of Phosphate Groups in Noncovalent Interactions in Binary Adenosine Nucleotides/Phosphoserine Aqueous Systems. J. Solut. Chem. 2009, 38, 35–46. [Google Scholar] [CrossRef]
- Ding, T.; Song, G.; Liu, X.; Xu, M.; Li, Y. Nucleotides as Optimal Candidates for Essential Nutrients in Living Organisms: A Review. J. Funct. Foods 2021, 82, 104498. [Google Scholar] [CrossRef]
- Delgado-García, E.; Piedras, P.; Gómez-Baena, G.; García-Magdaleno, I.M.; Pineda, M.; Gálvez-Valdivieso, G. Nucleoside Metabolism Is Induced in Common Bean During Early Seedling Development. Front. Plant Sci. 2021, 12, 651015. [Google Scholar] [CrossRef]
- Jastrzab, R.; Runka, T.; Skowronek, P.; Lomozik, L. The Effect of Spermine Concentration on the Solution Structure of Complexes Formed in Copper(II)/Adenosine 5′-Triphosphate/Phosphoserine System. J. Inorg. Biochem. 2010, 104, 868–887. [Google Scholar] [CrossRef] [PubMed]
- Yegutkin, G.G. Adenosine Metabolism in the Vascular System. Biochem. Pharmacol. 2021, 187, 114373. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xia, Y. Beneficial and Detrimental Role of Adenosine Signaling in Diseases and Therapy. J. Appl. Physiol. 2015, 119, 1173–1182. [Google Scholar] [CrossRef]
- Chang, C.-P.; Wu, K.-C.; Lin, C.-Y.; Chern, Y. Emerging Roles of Dysregulated Adenosine Homeostasis in Brain Disorders with a Specific Focus on Neurodegenerative Diseases. J. Biomed. Sci. 2021, 28, 70. [Google Scholar] [CrossRef] [PubMed]
- Kutryb-Zając, B.; Kawecka, A.; Nasadiuk, K.; Braczko, A.; Stawarska, K.; Caiazzo, E.; Koszałka, P.; Cicala, C. Drugs Targeting Adenosine Signaling Pathways: A Current View. Biomed. Pharmacother. 2023, 165, 115184. [Google Scholar] [CrossRef]
- Mondal, S.; Hsiao, K.; Goueli, S.A. Utility of Adenosine Monophosphate Detection System for Monitoring the Activities of Diverse Enzyme Reactions. Assay Drug Dev. Technol. 2017, 15, 330–341. [Google Scholar] [CrossRef]
- Cho, J.; Kim, S. Selective Sensing of Adenosine Monophosphate (AMP) by a Calix[6]Triazolium-Based Col-orimetric Sensing Ensemble. RSC Adv. 2022, 12, 32784–32789. [Google Scholar] [CrossRef]
- Borges, P.A.; Waclawiak, I.; Georgii, J.L.; Fraga-Junior, V.d.S.; Barros, J.F.; Lemos, F.S.; Russo-Abrahão, T.; Saraiva, E.M.; Takiya, C.M.; Coutinho-Silva, R.; et al. Adenosine Diphosphate Improves Wound Healing in Diabetic Mice Through P2Y12 Receptor Activation. Front. Immunol. 2021, 12, 651740. [Google Scholar] [CrossRef] [PubMed]
- Woulfe, D.; Yang, J.; Brass, L. ADP and Platelets: The End of the Beginning. J. Clin. Investig. 2001, 107, 1503–1505. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, S.K.; Goslar, J.; Bregier-Jarzebowska, R.; Gasowska, A.; Zalewska, A.; Lomozik, L. Copper(II) Ions Interactions in the Systems with Triamines and ATP. Potentiometric and Spectroscopic Studies. J. Inorg. Biochem. 2017, 177, 89–100. [Google Scholar] [CrossRef]
- Yegutkin, G.G. Nucleotide- and Nucleoside-Converting Ectoenzymes: Important Modulators of Purinergic Signalling Cascade. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2008, 1783, 673–694. [Google Scholar] [CrossRef]
- Bregier-Jarzebowska, R.; Hoffmann, S.K.; Łomozik, L.; Gasowska, A.; Stegient-Nowicka, J. Studies of Ternary Complexes Formed in the Biocoordination Systems Including Copper(II) Ions, Polyamines and l-Lysine. Polyhedron 2019, 173, 114137. [Google Scholar] [CrossRef]
- Gabryel-Skrodzka, M.; Nowak, M.; Teubert, A.; Jastrzab, R. Coordination Chemistry of Phosphate Groups in Systems Including Copper(II) Ions, Phosphoethanolamine and Pyrimidine Nucleotides. Int. J. Mol. Sci. 2022, 23, 13718. [Google Scholar] [CrossRef]
- Zabiszak, M.; Frymark, J.; Nowak, M.; Grajewski, J.; Stachowiak, K.; Kaczmarek, M.T.; Jastrząb, R. Influence of d-Electron Divalent Metal Ions in Complex Formation with L-Tartaric and L-Malic Acids. Molecules 2021, 26, 5290. [Google Scholar] [CrossRef]
- Wang, P.; Yuan, Y.; Xu, K.; Zhong, H.; Yang, Y.; Jin, S.; Yang, K.; Qi, X. Biological Applications of Copper-Containing Materials. Bioact. Mater. 2021, 6, 916–927. [Google Scholar] [CrossRef]
- Zehra, S.; Tabassum, S.; Arjmand, F. Biochemical Pathways of Copper Complexes: Progress over the Past 5 Years. Drug Discov. Today 2021, 26, 1086–1096. [Google Scholar] [CrossRef] [PubMed]
- Iakovidis, I.; Delimaris, I.; Piperakis, S.M. Copper and Its Complexes in Medicine: A Biochemical Approach. Mol. Biol. Int. 2011, 2011, 594529. [Google Scholar] [CrossRef]
- Copeland, K.L.; Pellock, S.J.; Cox, J.R.; Cafiero, M.L.; Tschumper, G.S. Examination of Tyrosine/Adenine Stacking Interactions in Protein Complexes. J. Phys. Chem. B 2013, 117, 14001–14008. [Google Scholar] [CrossRef]
- Sipos, P.; Kiss, T. Spectroscopic and Potentiometric Study of Protonation and Copper(II) Complex Formation of 3-Amino-L-Tyrosine. J. Chem. Soc. Dalton Trans. 1990, 9, 2909. [Google Scholar] [CrossRef]
- Sadowska, P.; Jankowski, W.; Bregier-Jarzębowska, R.; Pietrzyk, P.; Jastrząb, R. Deciphering the Impact of Nucleosides and Nucleotides on Copper Ion and Dopamine Coordination Dynamics. Int. J. Mol. Sci. 2024, 25, 9137. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, Y.; Yang, G. Copper (II) Complex of 1,10-Phenanthroline and L-Tyrosine with DNA Oxidative Cleavage Activity in the Gallic Acid. Biometals 2011, 24, 737–745. [Google Scholar] [CrossRef]
- de Moraes Silva, A.; Mercê, A.L.R.; Mangrich, A.S.; Téllez Souto, C.A.; Felcman, J. Potentiometric and Spectroscopic Study of Mixed Copper(II) Complexes with Amino Acids and Either Adenosine 5′ Triphosphate or Phosphocreatine. Polyhedron 2006, 25, 1319–1326. [Google Scholar] [CrossRef]
- Barr, M.L.; Baumgartner, E.; Kustin, K. Kinetics Of Formation And Stability Constants Of Mono And Bis L -Tyrosine Complexes of Cu(II), Co(II), and Ni(II). J. Coord. Chem. 1973, 2, 263–270. [Google Scholar] [CrossRef]
- İnci, D.; Aydın, R. Potentiometric Studies on Complexation of Cu(II) Ion with Methyl/Nitro-Substituted 1,10-Phenanthrolines and Selected Amino Acids. J. Solut. Chem. 2017, 46, 124–138. [Google Scholar] [CrossRef]
- İnci, D.; Aydın, R. Stabilities of the Ternary Complexes of Copper(II) with Substituted 1,10-Phenanthrolines and Some Amino Acids in Aqueous Solution. J. Solut. Chem. 2014, 43, 711–726. [Google Scholar] [CrossRef]
- İnci, D.; Aydın, R. Structures, Hydrolysis, Stabilities of Palladium(II) Complexes Containing Biologically Active Ligands and Species Distribution in Aqueous Solution. J. Mol. Struct. 2019, 1187, 23–37. [Google Scholar] [CrossRef]
- Madden, W.; Del Carpio, E.; Hernández, L.; Echevarria, L.; Lubes, V. Mixed-Ligand Complexes of Copper(II) with 1, 10’-Phenanthroline and Amino Acids. Phys. Chem. Liq. 2022, 60, 786–807. [Google Scholar] [CrossRef]
- Martell, A.E.; Smith, R.M. Critical Stability Constants: Volume 1: Aminoacids; Plenum Press: New York, NY, USA, 1974. [Google Scholar]
- Kiss, T.; Gergely, A. Complex-Forming Properties of Tyrosine Isomers with Transition Metal Ions. J. Chem. Soc. Dalton Trans. 1984, 9, 1951–1957. [Google Scholar] [CrossRef]
- Letter, J.E.; Bauman, J.E. Thermodynamic Study of the Complexation and Coordinated Ligand Deprotonation Reactions for a Series of Tryosine Isomers with Copper(II). J. Am. Chem. Soc. 1970, 92, 443–447. [Google Scholar] [CrossRef]
- Łomozik, L.; Ga̧sowska, A.; Bregier-Jarzȩbowska, R. Reactions of Complexation of Co(II), Ni(II), Cu(II), Cd(II) and Hg(II) Ions with Adenosine 5′-Diphosphate. Pol. J. Chem. 2004, 78, 2023–2035. [Google Scholar]
- Lomozik, L.; Gasowska, A.; Bregier-Jarzebowska, R.; Jastrzab, R. Coordination Chemistry of Polyamines and Their Interactions in Ternary Systems Including Metal Ions, Nucleosides and Nucleotides. Coord. Chem. Rev. 2005, 249, 2335–2350. [Google Scholar] [CrossRef]
- Kim, S.-H.; Martin, R.B. Binding Sites and Stabilities of Transition Metal Ions with Nucleosides and Related Ligands. Inorg. Chim. Acta 1984, 91, 19–24. [Google Scholar] [CrossRef]
- Kinjo, Y.; Tribolet, R.; Corfu, N.A.; Sigel, H. Stability and Structure of Xanthosine-Metal Ion Complexes in Aqueous Solution, Together with Intramolecular Adenosine-Metal Ion Equilibria. Inorg. Chem. 1989, 28, 1480–1489. [Google Scholar] [CrossRef]
- Gasowska, A.; Lomozik, L. Mode of Coordination and Stability of Cu(II) and Zn(II) Complexes with Adenosine, Deoxyadenosine, Cytidine and Deoxycytidine. Monatshefte Chem. Mon. 1995, 126, 13–22. [Google Scholar] [CrossRef]
- Gąsowska, A.; Łomozik, L. Cobalt(II), nickel(II) and copper(II) complexes with adenosine 5′-monophosphate and cytidine 5′-monophosphate in aqueous solutions and in solid. Pol. J. Chem. 1999, 73, 465–474. [Google Scholar]
- Gąsowska, A.; Bregier-Jarzebowska, R.; Lomozik, L. Interaction Centers of Purine and Pyrimidine Nucleotides in Their Reactions with Cu(II), Ni(II), Co(II), Cd(II) and Hg(III) Ions. Pol. J. Chem. 2002, 76, 773–783. [Google Scholar]
- Yamauchi, O.; Odani, A. Structure-Stability Relationship in Ternary Copper(II) Complexes Involving Aromatic Amines and Tyrosine or Related Amino Acids. Intramolecular Aromatic Ring Stacking and its Regulation through Tyrosine Phosphorylation. J. Am. Chem. Soc. 1985, 107, 5938–5945. [Google Scholar] [CrossRef]
- Gabryel-Skrodzka, M.; Nowak, M.; Grajewski, J.; Jastrząb, R. Biocoordination Reactions in Copper(II) Ions and Phosphocholine Systems Including Pyrimidine Nucleosides and Nucleotides. Sci. Rep. 2023, 13, 10787. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Gągor, A.; Jezierska, J.; Duczmal, M. Structural, Spectroscopic and Magnetic Properties of a Novel Copper(II) L-Tyrosinato Complex. RSC Adv. 2014, 4, 63150–63161. [Google Scholar] [CrossRef]
- Wojciechowska, A.; Rojek, T.; Malik−Gajewska, M.; Jerzykiewicz, M.; Wysokiński, R.; Gągor, A.; Rytlewski, P.; Staszak, Z.; Duczmal, M. Crystal and Molecular Structure Stabilized by Weak Interaction in Unique 3,5-Diiodo-L-Tyrosinato Copper(II) Complex–Synthesis, Experimental and Theoretical Studies. Mater. Sci. Eng. B 2020, 262, 114723. [Google Scholar] [CrossRef]
- Wojciechowska, A.; de Graaf, C.; Rojek, T.; Jerzykiewicz, M.; Malik, M.; Gągor, A.; Duczmal, M. A Rare Diio-do-L-Tyrosine Copper(II) Complexes—Crystal and Molecular Structure of Materials Stabilized by Weak Interactions. Polyhedron 2022, 219, 115780. [Google Scholar] [CrossRef]
- Arena, G.; Calì, R.; Cucinotta, V.; Musumeci, S.; Rizzarelli, E.; Sammartano, S. Thermodynamics of Metal Complexes with Ligand—Ligand Interaction. Mixed Complexes of Copper(II) and Zinc(II) with Adenosine 5′-Triphosphate and L-Phenylalanine or L-Tyrosine. Thermochim. Acta 1984, 74, 77–86. [Google Scholar] [CrossRef]
- Lomozik, L.; Jastrzab, R.; Gasowska, A. Interactions in Binary and Ternary Systems Including Cu(II), Uridine, Uridine 5′-Monophosphate or Diamine. Polyhedron 2000, 19, 1145–1154. [Google Scholar] [CrossRef]
- Lomozik, L.; Bregier-Jarzebowska, R.; Gasowska, A.; Hoffmann, S.K.; Zalewska, A. Biocoordination Reactions in Copper(II) Ions and l-Glutamic Acid Systems Including Tetramines: 1,11-Diamino-4,8-Diazaundecane or 1,12-Diamino-4,9-Diazadodecane. Inorg. Chim. Acta 2018, 482, 905–913. [Google Scholar] [CrossRef]
- Gabryel-Skrodzka, M.; Nowak, M.; Stachowiak, K.; Zabiszak, M.; Ogawa, K.; Jastrzab, R. The Influence of pH on Complexation Process of Copper(II) Phosphoethanolamine to Pyrimidine Nucleosides. Materials 2021, 14, 4309. [Google Scholar] [CrossRef]
- Bregier-Jarzebowska, R.; Gasowska, A.; Lomozik, L. Complexes of Cu(II) Ions and Noncovalent Interactions in Systems with L-Aspartic Acid and Cytidine-5’-Monophosphate. Bioinorg. Chem. Appl. 2008, 2008, 253971. [Google Scholar] [CrossRef]
- Lomozik, L.; Bolewski, L.; Dworczak, R. Complex Formation in Copper(II) Ternary Systems Involving Polyamines and Diaminocarboxylates Studied by Potentiometric and Spectroscopic Techniques. J. Coord. Chem. 1997, 41, 261–274. [Google Scholar] [CrossRef]
- Barbucci, R.; Campbell, M.J.M. An Investigation of the Structures of Copper(II) Polyamine Complexes in Aqueous Solution by a Combined Evaluation of the EPR and Thermodynamic Parameters. Inorg. Chim. Acta 1976, 16, 113–120. [Google Scholar] [CrossRef]
- Aydın, R.; İnci, D. Potentiometric and Spectrophotometric Studies of the Complexation of Lanthanum(III) with Adrenaline, Noradrenaline, and Dopamine. J. Chem. Eng. Data 2012, 57, 967–973. [Google Scholar] [CrossRef]
- Spałek, T.; Pietrzyk, P.; Sojka, Z. Application of the Genetic Algorithm Joint with the Powell Method to Non-linear Least-Squares Fitting of Powder EPR Spectra. J. Chem. Inf. Model. 2005, 45, 18–29. [Google Scholar] [CrossRef]
- Rigaku, O.D. CrysAlis PRO; Rigaku Oxford Diffraction Ltd.: Yarnton, UK, 2024. [Google Scholar]
- Dolomanov, O.V.; Bourhis, L.J.; Gildea, R.J.; Howard, J.A.K.; Puschmann, H. OLEX2: A Complete Structure Solution, Refinement and Analysis Program. Appl. Crystallogr. 2009, 42, 339–341. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELXT—Integrated Space-Group and Crystal-Structure Determination. Acta Crystallogr. Sect. A Found. Adv. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Sheldrick, G.M. Crystal Structure Refinement with SHELXL. Acta Crystallogr. Sect. C Struct. Chem. 2015, 71, 3–8. [Google Scholar] [CrossRef]
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermo-chemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar] [CrossRef]
- Dunning, T.H.; Hay, P.J. Gaussian Basis Sets for Molecular Calculations. In Methods of Electronic Structure Theory; Springer: Boston, MA, USA, 1977; pp. 1–27. [Google Scholar]
- Bregier-Jarzębowska, R.; Malczewska-Jaskóła, K.; Jankowski, W.; Jasiewicz, B.; Hoffmann, M.; Gąsowska, A.; Jastrząb, R. Experimental and Quantum-Chemical Studies of Anabasine Complexes with Copper(II) and Zinc(II) Ions. Polyhedron 2015, 85, 841–848. [Google Scholar] [CrossRef]
- Malczewska-Jaskóła, K.; Jankowski, W.; Warżajtis, B.; Jasiewicz, B.; Hoffmann, M.; Rychlewska, U. Chalco-genated (S)-(−)-Nicotine Derivatives as Chiral Linkers for 1D Coordination Polymers. Polyhedron 2015, 100, 404–411. [Google Scholar] [CrossRef]
- Jankowski, W.; Kurek, J.; Barczyński, P.; Hoffmann, M. Quantum-Chemical, NMR, FT IR, and ESI MS Studies of Complexes of Colchicine with Zn(II). J. Mol. Model. 2017, 23, 127. [Google Scholar] [CrossRef]
- Musumeci, C.; Wałęsa-Chorab, M.; Gorczyński, A.; Markiewicz, G.; Bogucki, A.; Świetlik, R.; Hnatejko, Z.; Jankowski, W.; Hoffmann, M.; Orgiu, E.; et al. Generation of Low-Dimensional Architectures through the Self-Assembly of Pyromellitic Diimide Derivatives. ACS Omega 2017, 2, 1672–1678. [Google Scholar] [CrossRef] [PubMed]
- Jankowski, W.; Długosz, R.; Pruski, B.; Koroniak, H.; Hoffmann, M. On the Stabilization of Organic Peroxides by Plasticizers: Quantum Chemical Study on Interactions of 2,2-Dihydroperoxybutane with Dimethyl Phthalate. J. Mol. Struct. 2022, 1261, 132864. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Foresman, J.; Frisch, A. Exploring Chemistry with Electronic Structure Methods, 3rd ed.; Gaussian Inc.: Pittsburg, PA, USA, 2015. [Google Scholar]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H. Gaussian 16 Revision C.01; Gaussian, Inc.: Wallingford, CT, USA, 2016. [Google Scholar]
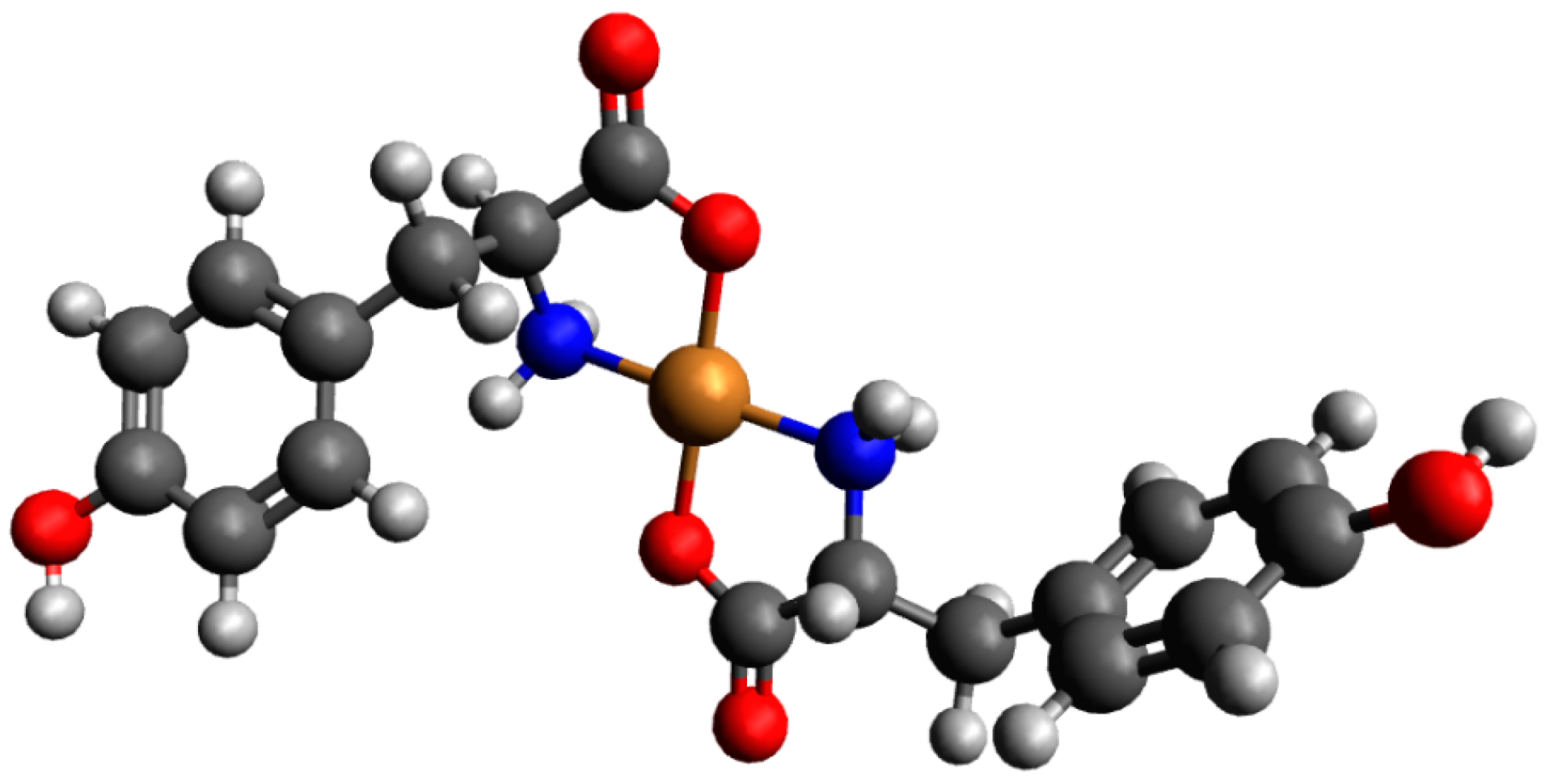
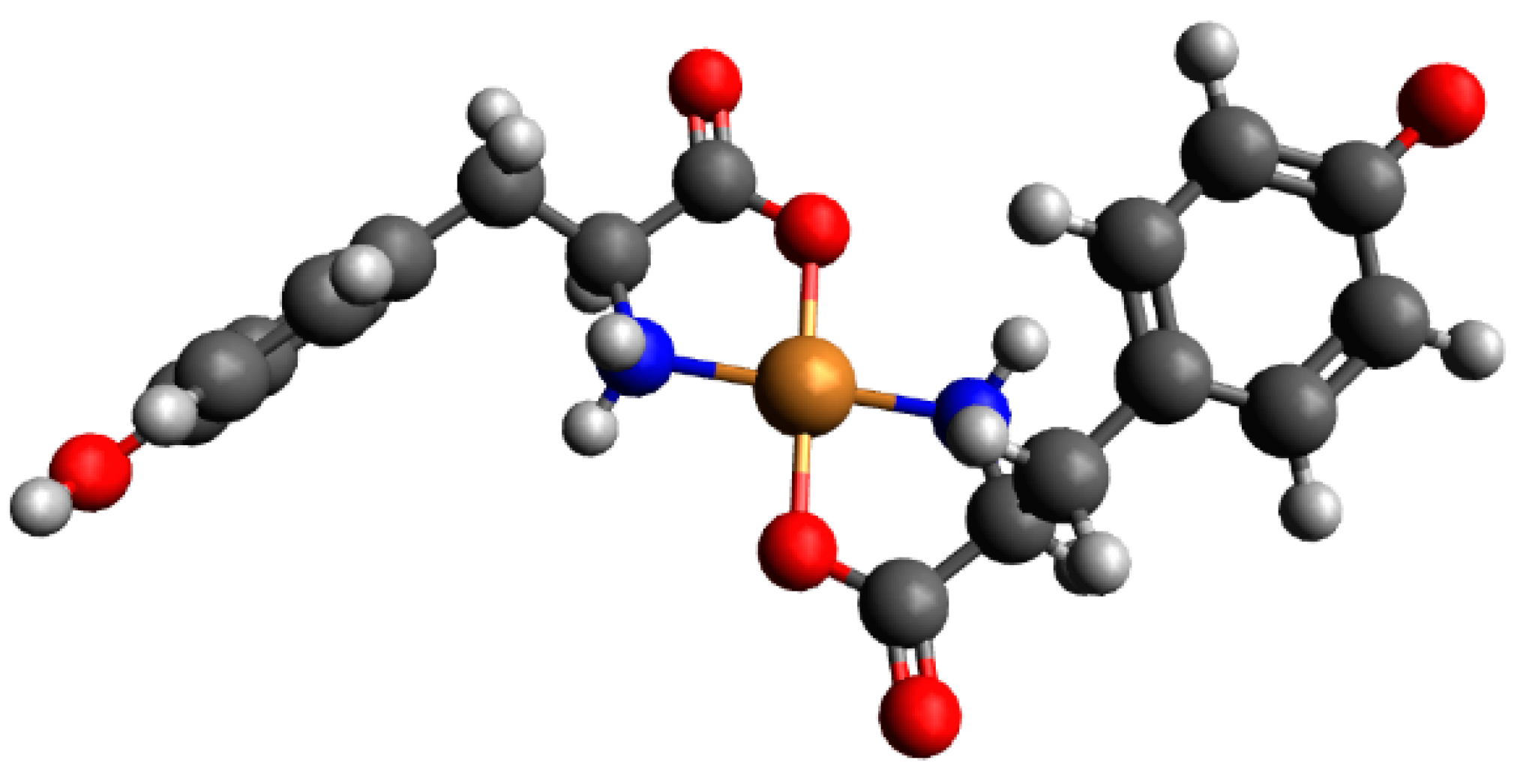
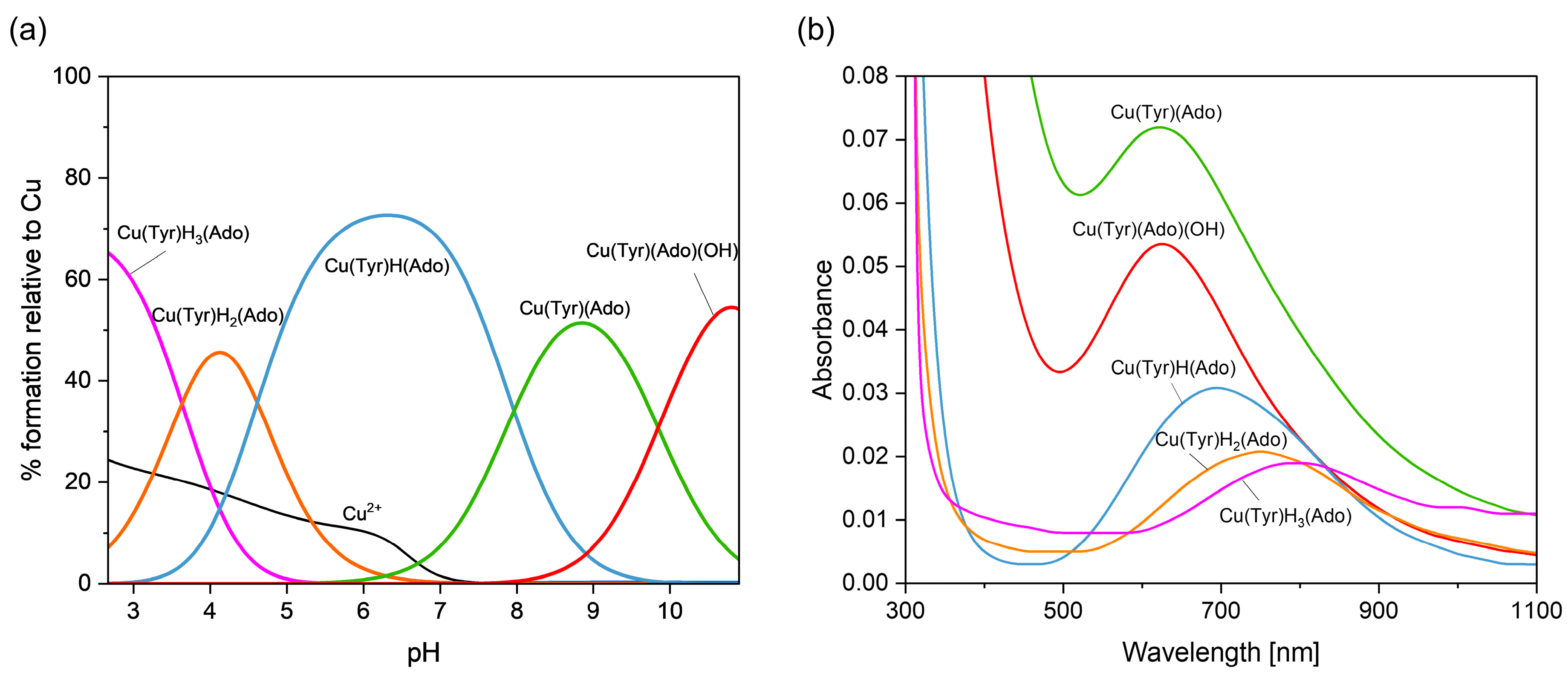
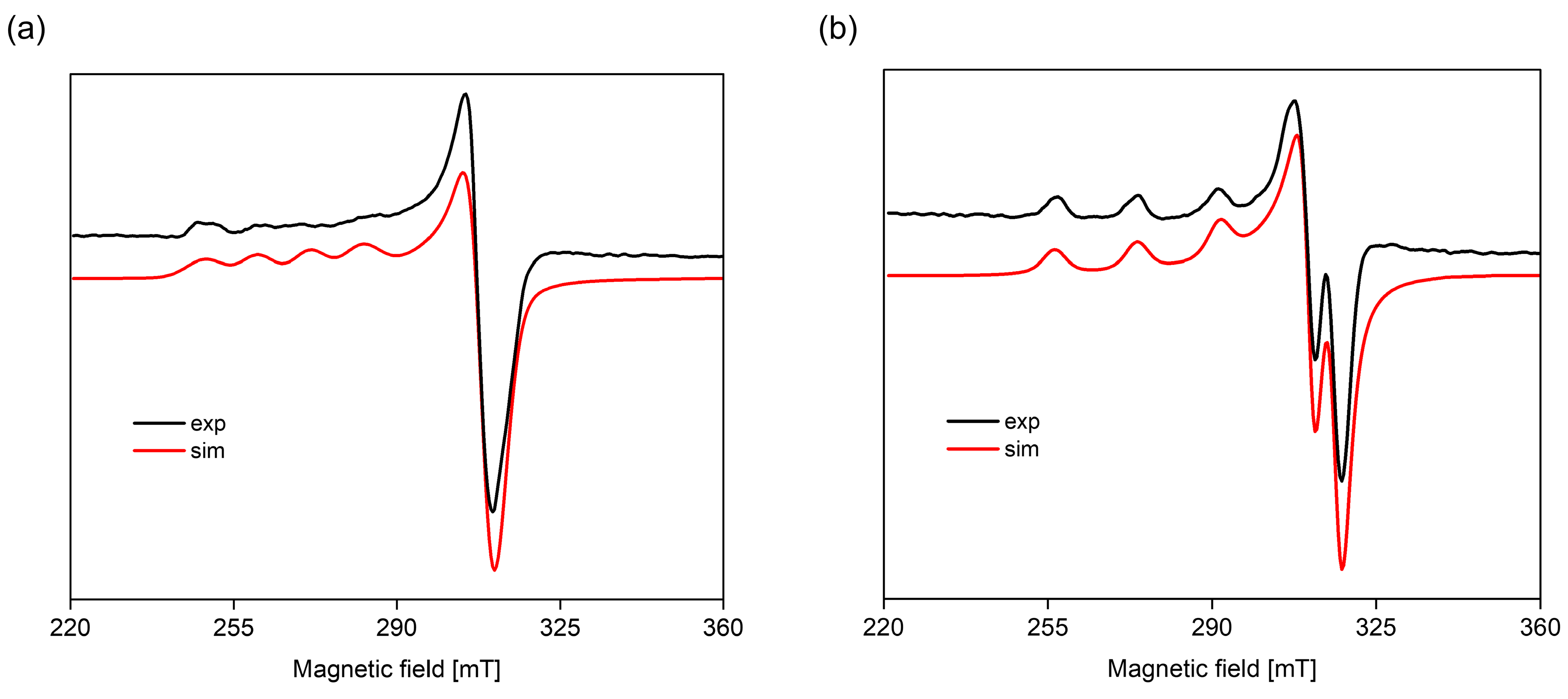

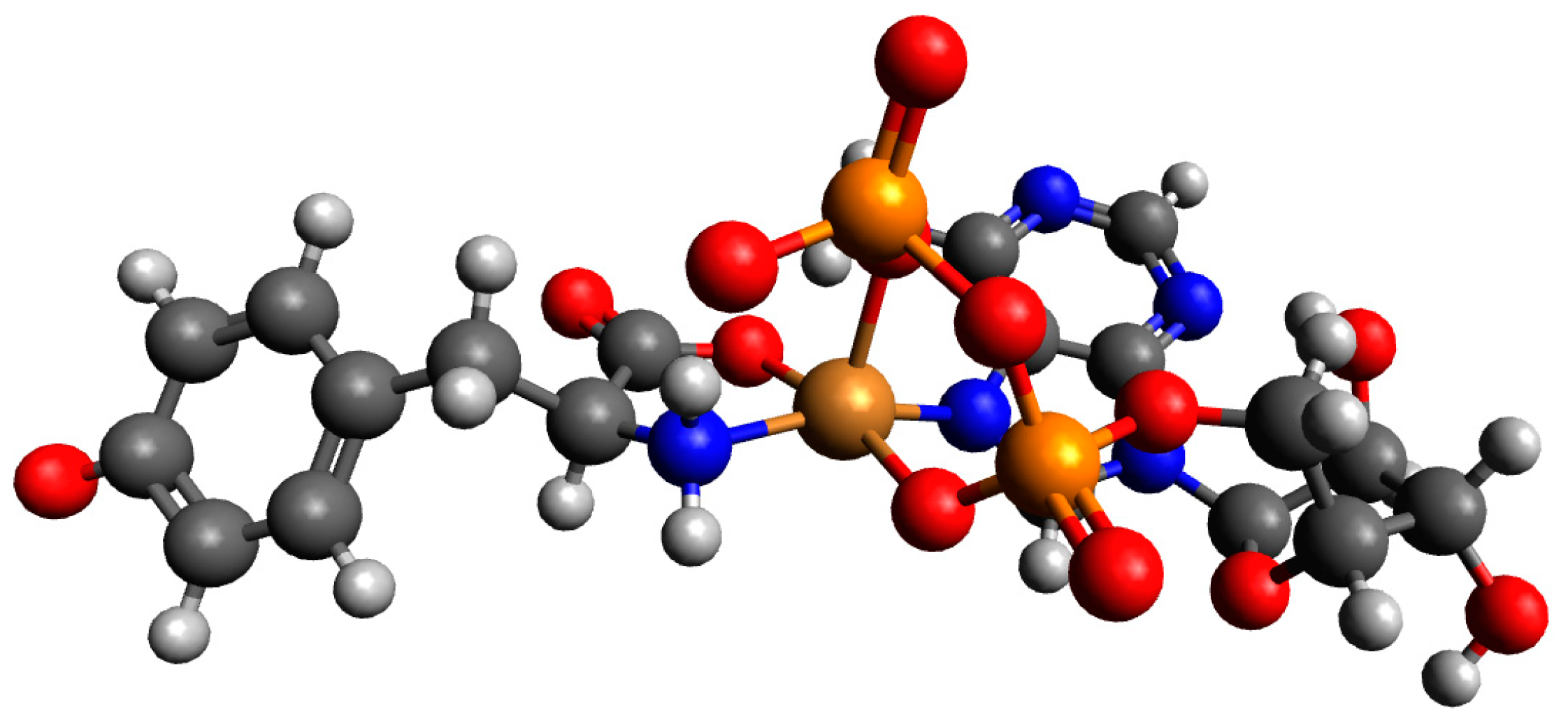
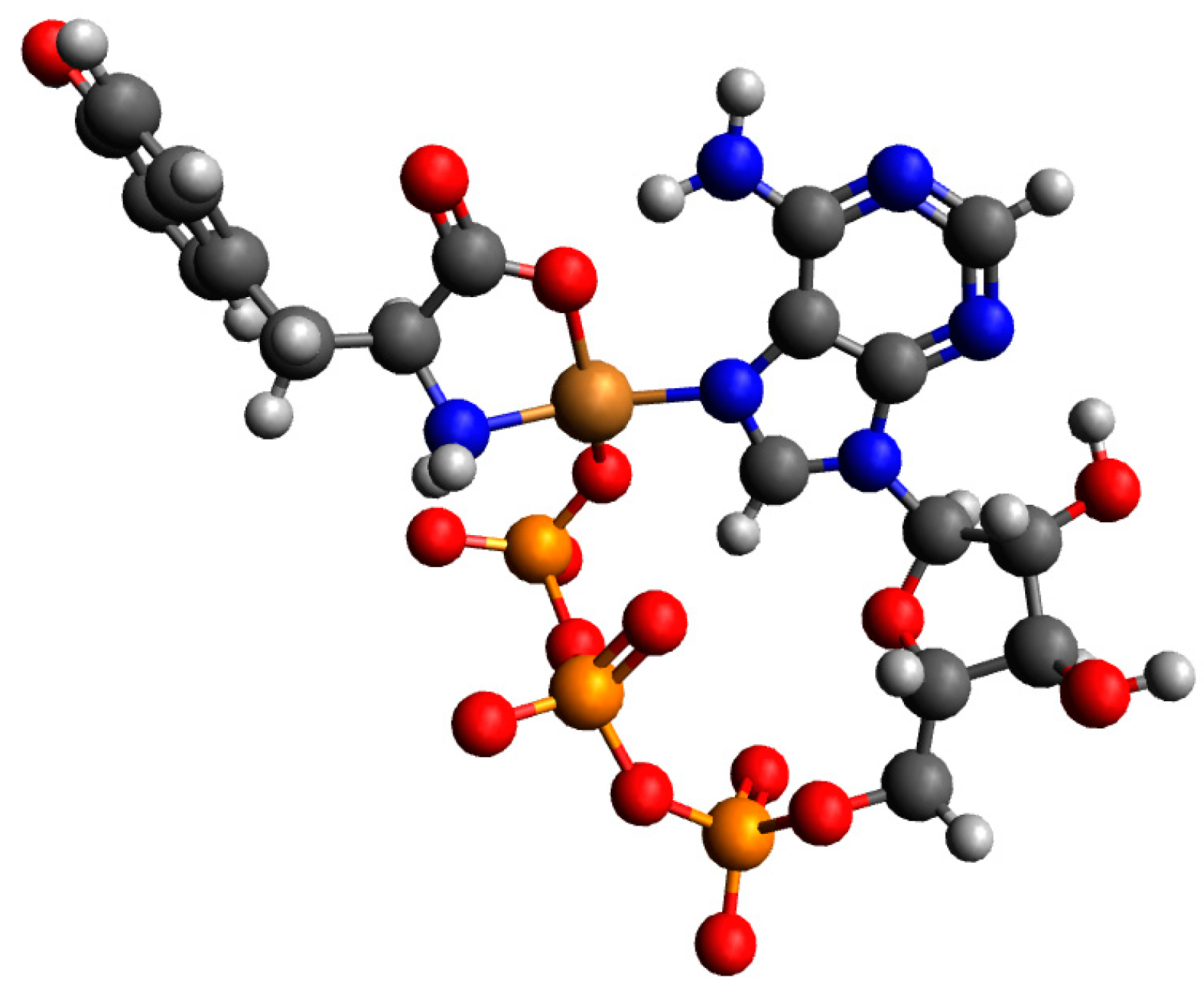

| System | Species | Counterions | logβ | logKe | Ref. |
|---|---|---|---|---|---|
| Tyr | H3(Tyr)+ | OH− | 21.81 ± 0.03 | 2.52 | 21.61 [39], 22.48 [40], 21.47 [38] |
| H2(Tyr) | - | 19.29 ± 0.02 | 9.01 | 19.18 [39], 19.47 [40], 19.27 [38], 19.66 [41] | |
| H(Tyr)− | H+ | 10.28 ± 0.02 | 10.28 | 10.16 [39], 10.12 [40], 10.22 [38], 10.47 [41] | |
| Cu(II)/Tyr | CuH2(Tyr)2+ | 2NO3− | 22.39 ± 0.05 | 3.10 | - |
| CuH(Tyr)+ | NO3− | 18.54 ± 0.03 | 8.26 | 18.28 [41], 17.79 [42], 19.34 [43] | |
| CuH2(Tyr)2 | - | 35.38 ± 0.03 | 6.56 | 35.68 [41], 34.64 [42], 36.41 [43] | |
| CuH(Tyr)22− | 2H+ | 25.99 ± 0.03 | 7.45 | 25.32 [42], 25.47 [51] |
| Species | pH | λmax [nm] | g|| | g┴ | A|| [10−4 cm−1] | A┴ [10−4 cm−1] | Chromophore |
|---|---|---|---|---|---|---|---|
| CuH2(Tyr) | 3.0 | 780 | 2.349 | 2.066 | 140 | 10 | {1O} |
| CuH(Tyr) | 4.8 | 670 | 2.307 | 2.059 | 174 | 13 | {1N,1O} |
| CuH2(Tyr)2 | 7.8 | 620 | 2.257 | 2.054 | 181 | 10 | {2N,2O} |
| CuH(Tyr)2 | 10.2 | 620 | 2.257 | 2.052 | 184 | 10 | {2N,2O} |
| Systems | Species | Counterions | logβ |
|---|---|---|---|
| Cu(II)/Tyr/Ado | Cu(Tyr)H3(Ado)3+ | 3NO3− | 31.40 ± 0.07 |
| Cu(Tyr)H2(Ado)2+ | 2NO3− | 27.77 ± 0.06 | |
| Cu(Tyr)H(Ado)+ | NO3− | 23.15 ± 0.06 | |
| Cu(Tyr)(Ado) | - | 15.19 ± 0.08 | |
| Cu(Tyr)(Ado)(OH)− | H+ | 5.35 ± 0.08 | |
| Cu(II)/Tyr/AMP | Cu(Tyr)H3(AMP)+ | NO3− | 32.11 ± 0.07 |
| Cu(Tyr)H2(AMP) | - | 27.59 ± 0.07 | |
| Cu(Tyr)H(AMP)− | H+ | 22.14 ± 0.03 | |
| Cu(Tyr)(AMP)2− | 2H+ | 14.87 ± 0.04 | |
| Cu(Tyr)(AMP)(OH)3− | 3H+ | 4.62 ± 0.05 | |
| Cu(II)/Tyr/ADP | Cu(Tyr)H3(ADP) | - | 33.65 ± 0.03 |
| Cu(Tyr)H2(ADP)− | H+ | 29.73 ± 0.03 | |
| Cu(Tyr)H(ADP)2− | 2H+ | 25.11 ± 0.02 | |
| Cu(Tyr)(ADP)3− | 3H+ | 19.01 ± 0.02 | |
| Cu(Tyr)(ADP)(OH)4− | 4H+ | 10.14 ± 0.02 | |
| Cu(Tyr)(ADP)(OH)25− | 5H+ | −0.27 ± 0.02 | |
| Cu(II)/Tyr/ATP | Cu(Tyr)H4(ATP) | - | 38.44 ± 0.04 |
| Cu(Tyr)H3(ATP)− | H+ | 34.76 ± 0.04 | |
| Cu(Tyr)H2(ATP)2− | 2H+ | 30.78 ± 0.04 | |
| Cu(Tyr)H(ATP)3− | 3H+ | 24.51 ± 0.02 | |
| Cu(Tyr)(ATP)4− | 4H+ | 14.56 ± 0.07 |
| Systems | Species | pH | λmax [nm] | g|| | g┴ | A|| [10−4 cm−1] | A┴ [10−4 cm−1] |
|---|---|---|---|---|---|---|---|
| Cu(II)/Tyr/Ado | Cu(Tyr)H3(Ado) | 3.0 | 790 | 2.402 | 2.069 | 130 | 5 |
| Cu(Tyr)H2(Ado) | 4.1 | 750 | 2.370 | 2.066 | 120 | 8 | |
| Cu(Tyr)H(Ado) | 6.2 | 700 | 2.301 | 2.069 | 174 | 8 | |
| Cu(Tyr)(Ado) | 8.8 | 635 | 2.251 | 2.055 | 185 | 5 | |
| Cu(Tyr)(Ado)(OH) | 10.5 | 625 | 2.250 | 2.051 | 190 | 4 | |
| Cu(II)/Tyr/AMP | Cu(Tyr)H(AMP) | 6.6 | 670 | 2.301 | 2.059 | 180 | 11 |
| Cu(Tyr)(AMP) | 8.6 | 650 | 2.300 | 2.063 | 174 | 5 | |
| Cu(Tyr)(AMP)(OH) | 10.7 | 635 | 2.258 | 2.057 | 190 | 7 | |
| Cu(II)/Tyr/ADP | Cu(Tyr)H(ADP) | 5.6 | 725 | 2.362 | 2.068 | 138 | 13 |
| Cu(Tyr)(ADP) | 7.5 | 675 | 2.325 | 2.067 | 146 | 11 | |
| Cu(Tyr)(ADP)(OH) | 9.6 | 650 | 2.322 | 2.038 | 124 | 22 | |
| Cu(Tyr)(ADP)(OH)2 | 10.5 | 640 | 2.256 | 2.053 | 186 | 9 | |
| Cu(II)/Tyr/ATP | Cu(Tyr)H4(ATP) | 2.5 | 800 | 2.397 | 2.072 | 129 | 4 |
| Cu(Tyr)H2(ATP) | 5.2 | 760 | 2.363 | 2.066 | 147 | 8 | |
| Cu(Tyr)H(ATP) | 8.0 | 705 | 2.316 | 2.047 | 155 | 6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, P.; Bregier-Jarzębowska, R.; Jankowski, W.; Gołdyn, M.R.; Jastrząb, R. Characterization of the Binding Modes of Cu2+ Ions with Tyrosine and Ado, AMP, ADP, and ATP: A Comprehensive Potentiometric, Spectroscopic, and Computational Approach. Int. J. Mol. Sci. 2025, 26, 8865. https://doi.org/10.3390/ijms26188865
Sadowska P, Bregier-Jarzębowska R, Jankowski W, Gołdyn MR, Jastrząb R. Characterization of the Binding Modes of Cu2+ Ions with Tyrosine and Ado, AMP, ADP, and ATP: A Comprehensive Potentiometric, Spectroscopic, and Computational Approach. International Journal of Molecular Sciences. 2025; 26(18):8865. https://doi.org/10.3390/ijms26188865
Chicago/Turabian StyleSadowska, Patrycja, Romualda Bregier-Jarzębowska, Wojciech Jankowski, Mateusz R. Gołdyn, and Renata Jastrząb. 2025. "Characterization of the Binding Modes of Cu2+ Ions with Tyrosine and Ado, AMP, ADP, and ATP: A Comprehensive Potentiometric, Spectroscopic, and Computational Approach" International Journal of Molecular Sciences 26, no. 18: 8865. https://doi.org/10.3390/ijms26188865
APA StyleSadowska, P., Bregier-Jarzębowska, R., Jankowski, W., Gołdyn, M. R., & Jastrząb, R. (2025). Characterization of the Binding Modes of Cu2+ Ions with Tyrosine and Ado, AMP, ADP, and ATP: A Comprehensive Potentiometric, Spectroscopic, and Computational Approach. International Journal of Molecular Sciences, 26(18), 8865. https://doi.org/10.3390/ijms26188865






