Distinct Adipocyte Responses to Δ9-Tetrahydrocannabinol (THC) Exposure Govern Hepatic Lipid Accumulation in an Obesogenic Setting
Abstract
1. Introduction
2. Results
2.1. Proliferation and Differentiation Efficacy of THC Compared to ROSI
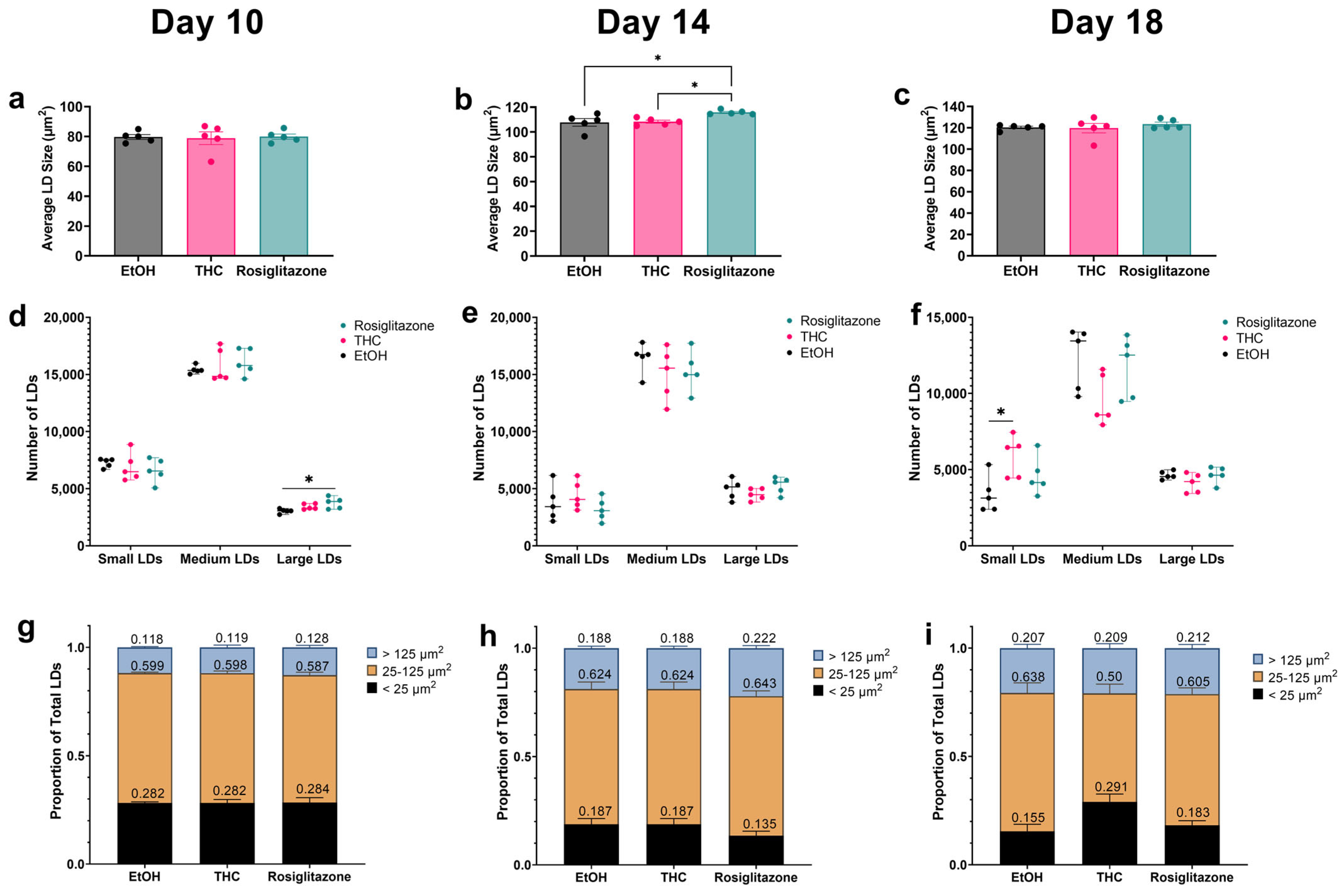
2.2. THC Promotes a Metabolically Favorable LD Distribution Pattern
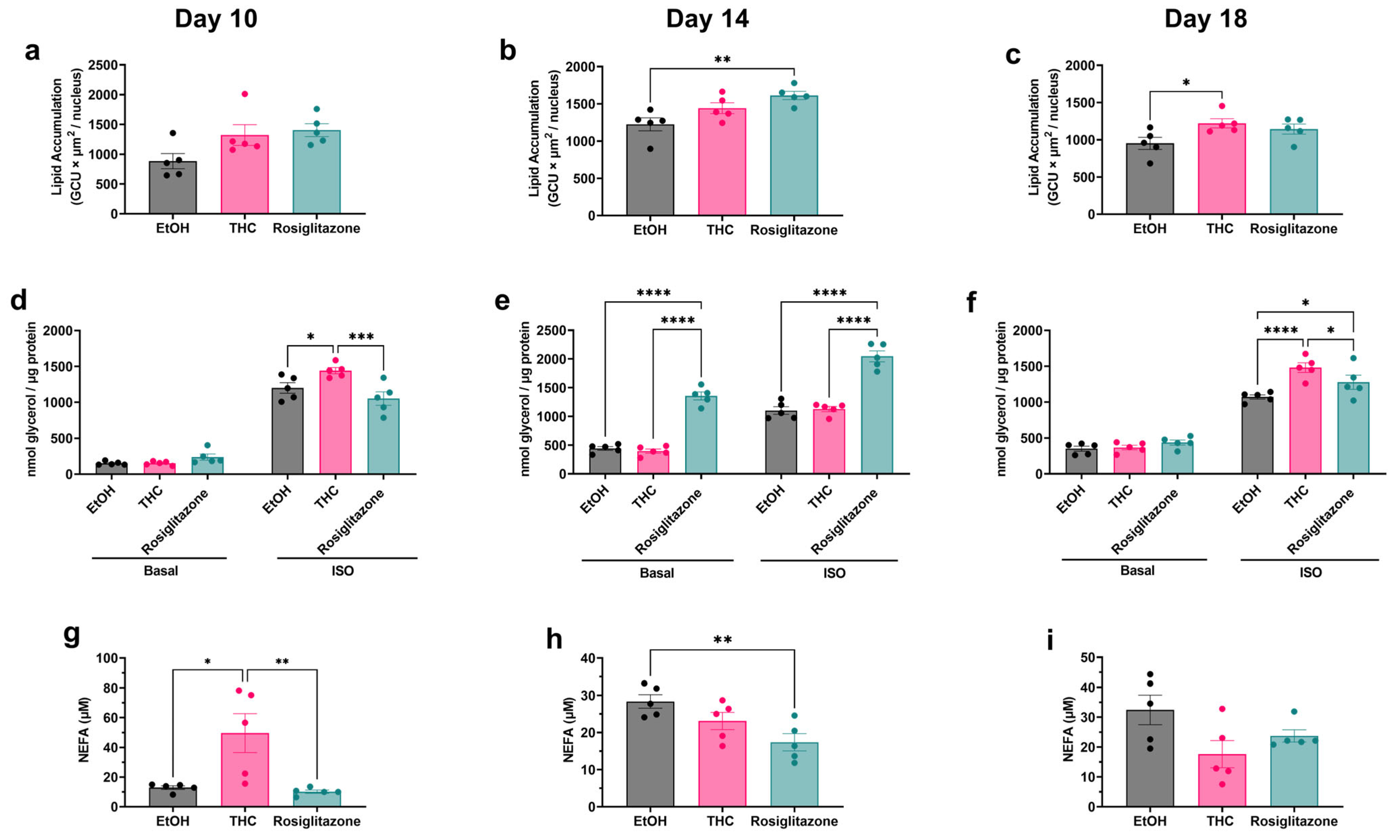
2.3. Assessment of THC’s Effect on Adipocyte Lipid Handling and FFA Buffering Capacity
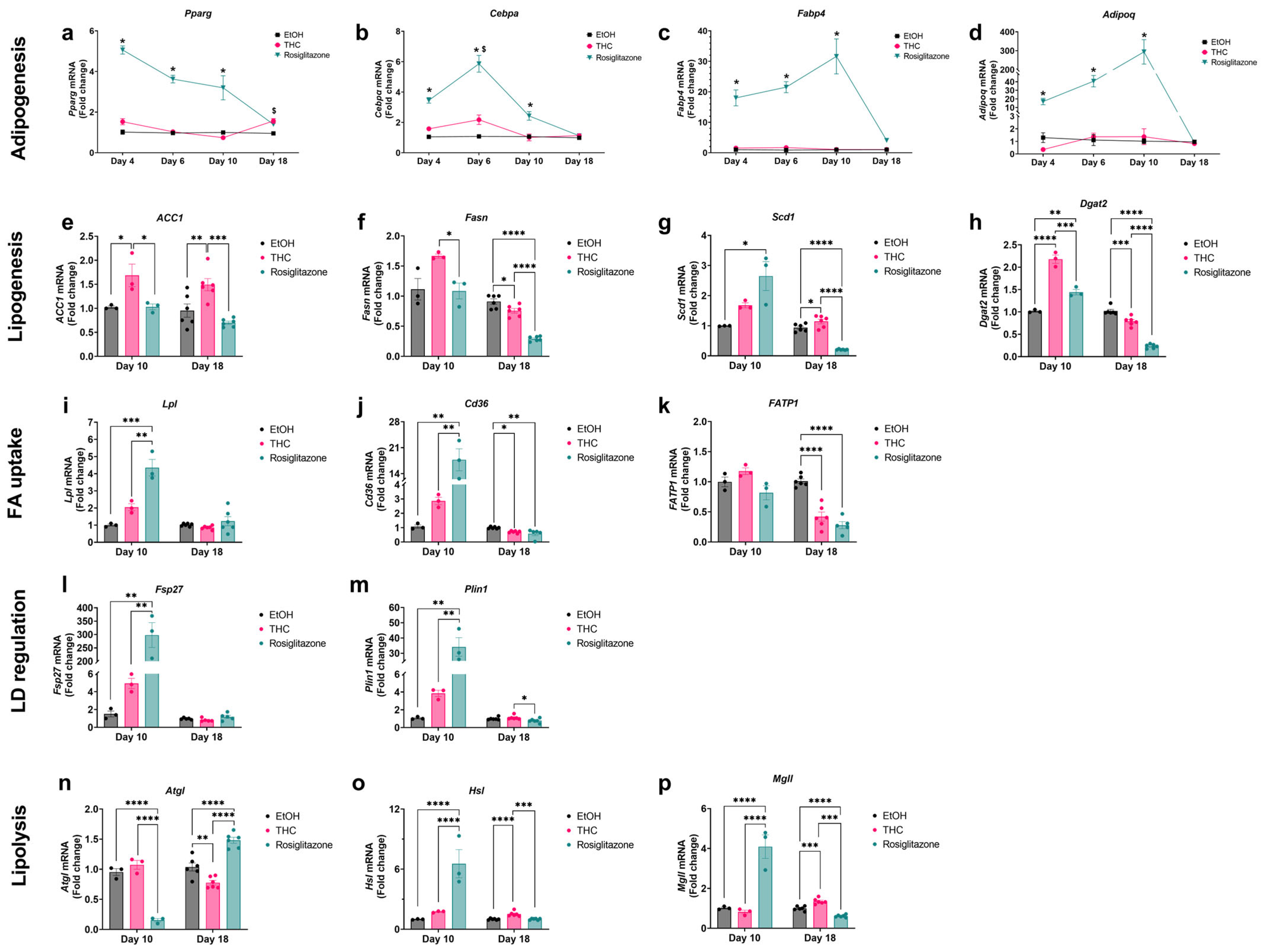
2.4. Gene Expression Analysis Reveals Differential Regulation of Adipogenic and Lipid Metabolism Pathways by THC and ROSI
2.5. Adipocyte-Hepatocyte Conditioned Medium Model Demonstrates the Functional Consequences of THC-Enhanced FFA Buffering
3. Discussion
4. Materials and Methods
4.1. 3T3-L1 Cell Culture and Differentiation
4.2. Cell Viability Assessment (MTT and Propidium Iodide Staining)
4.3. Cell Proliferation Assay
4.4. Oil Red O Staining and THC Dose–Response Assay
4.5. Live Cell Imaging and Staining
4.5.1. LD Dynamics and Lipogenic Capacity
4.5.2. Single Time Point Lipid Accumulation
4.5.3. Lipid Droplet Size Classification
4.6. Conditioned Medium Treatment of AML12 Cells
4.7. PPARγ Luciferase Reporter Assay
4.8. Lipolysis Assay
4.9. Non-Esterified Fatty Acids (NEFA) Quantification
4.10. RNA Isolation and Quantitative RT-PCR
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Lee, E.; Korf, H.; Vidal-Puig, A. An Adipocentric Perspective on the Development and Progression of Non-Alcoholic Fatty Liver Disease. J. Hepatol. 2023, 78, 1048–1062. [Google Scholar] [CrossRef]
- Rasouli, N.; Raue, U.; Miles, L.M.; Lu, T.; Di Gregorio, G.B.; Elbein, S.C.; Kern, P.A. Pioglitazone Improves Insulin Sensitivity through Reduction in Muscle Lipid and Redistribution of Lipid into Adipose Tissue. Am. J. Physiol. Endocrinol. Metab. 2005, 288, E930–E934. [Google Scholar] [CrossRef]
- Ghaben, A.L.; Scherer, P.E. Adipogenesis and Metabolic Health. Nat. Rev. Mol. Cell Biol. 2019, 20, 242–258. [Google Scholar] [CrossRef]
- Hansson, B.; Morén, B.; Fryklund, C.; Vliex, L.; Wasserstrom, S.; Albinsson, S.; Berger, K.; Stenkula, K.G. Adipose Cell Size Changes Are Associated with a Drastic Actin Remodeling. Sci. Rep. 2019, 9, 12941. [Google Scholar] [CrossRef]
- Wang, Q.A.; Tao, C.; Gupta, R.K.; Scherer, P.E. Tracking Adipogenesis during White Adipose Tissue Development, Expansion and Regeneration. Nat. Med. 2013, 19, 1338–1344. [Google Scholar] [CrossRef] [PubMed]
- Vishvanath, L.; Gupta, R.K. Contribution of Adipogenesis to Healthy Adipose Tissue Expansion in Obesity. J. Clin. Investig. 2019, 129, 4022–4031. [Google Scholar] [CrossRef]
- Tontonoz, P.; Hu, E.; Spiegelman, B.M. Stimulation of Adipogenesis in Fibroblasts by PPARγ2, a Lipid-Activated Transcription Factor. Cell 1994, 79, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Cariou, B.; Charbonnel, B.; Staels, B. Thiazolidinediones and PPARγ Agonists: Time for a Reassessment. Trends Endocrinol. Metab. 2012, 23, 205–215. [Google Scholar] [CrossRef] [PubMed]
- Gastaldelli, A.; Casolaro, A.; Ciociaro, D.; Frascerra, S.; Nannipieri, M.; Buzzigoli, E.; Ferrannini, E. Decreased Whole Body Lipolysis as a Mechanism of the Lipid-Lowering Effect of Pioglitazone in Type 2 Diabetic Patients. Am. J. Physiol. Endocrinol. Metab. 2009, 297, E225–E230. [Google Scholar] [CrossRef]
- Eitan, A.; Gover, O.; Sulimani, L.; Meiri, D.; Schwartz, B. The Effect of Orally Administered Δ9-Tetrahydrocannabinol (THC) and Cannabidiol (CBD) on Obesity Parameters in Mice. Int. J. Mol. Sci. 2023, 24, 13797. [Google Scholar] [CrossRef]
- Nguyen, M.T.A.; Satoh, H.; Favelyukis, S.; Babendure, J.L.; Imamura, T.; Sbodio, J.I.; Zalevsky, J.; Dahiyat, B.I.; Chi, N.-W.; Olefsky, J.M. JNK and Tumor Necrosis Factor-α Mediate Free Fatty Acid-Induced Insulin Resistance in 3T3-L1 Adipocytes. J. Biol. Chem. 2005, 280, 35361–35371. [Google Scholar] [CrossRef]
- Listenberger, L.L.; Han, X.; Lewis, S.E.; Cases, S.; Farese, R.V.; Ory, D.S.; Schaffer, J.E. Triglyceride Accumulation Protects against Fatty Acid-Induced Lipotoxicity. Proc. Natl. Acad. Sci. USA 2003, 100, 3077–3082. [Google Scholar] [CrossRef]
- Lin, L.; Jung, K.-M.; Lee, H.-L.; Le, J.; Colleluori, G.; Wood, C.; Palese, F.; Squire, E.; Ramirez, J.; Su, S. Adolescent Exposure to Low-Dose THC Disrupts Energy Balance and Adipose Organ Homeostasis in Adulthood. Cell Metab. 2023, 35, 1227–1241. [Google Scholar] [CrossRef]
- Cluny, N.L.; Keenan, C.M.; Reimer, R.A.; Foll, B.L.; Sharkey, K.A. Prevention of Diet-Induced Obesity Effects on Body Weight and Gut Microbiota in Mice Treated Chronically with Δ9-Tetrahydrocannabinol. PLoS ONE 2015, 10, e0144270. [Google Scholar] [CrossRef]
- Eitan, A.; Gover, O.; Sulimani, L.; Meiri, D.; Shterzer, N.; Mills, E.; Schwartz, B. The Effect of Oil-Based Cannabis Extracts on Metabolic Parameters and Microbiota Composition of Mice Fed a Standard and a High-Fat Diet. Int. J. Mol. Sci. 2024, 25, 1073. [Google Scholar] [CrossRef]
- Teixeira, D.; Pestana, D.; Faria, A.; Calhau, C.; Azevedo, I.; Monteiro, R. Modulation of Adipocyte Biology by Δ9-Tetrahydrocannabinol. Obesity 2010, 18, 2077–2085. [Google Scholar] [CrossRef] [PubMed]
- Gallant, M.; Odei-Addo, F.; Frost, C.L.; Levendal, R.-A. Biological Effects of THC and a Lipophilic Cannabis Extract on Normal and Insulin Resistant 3T3-L1 Adipocytes. Phytomedicine 2009, 16, 942–949. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E.; Tarling, E.J.; Bennett, A.J.; Kendall, D.A.; Randall, M.D. Novel Time-Dependent Vascular Actions of Δ9-Tetrahydrocannabinol Mediated by Peroxisome Proliferator-Activated Receptor Gamma. Biochem. Biophys. Res. Commun. 2005, 337, 824–831. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, S.E. An Update on PPAR Activation by Cannabinoids. Br. J. Pharmacol. 2016, 173, 1899–1910. [Google Scholar] [CrossRef]
- Hughes, T.S.; Chalmers, M.J.; Novick, S.; Kuruvilla, D.S.; Chang, M.R.; Kamenecka, T.M.; Rance, M.; Johnson, B.A.; Burris, T.P.; Griffin, P.R. Ligand and Receptor Dynamics Contribute to the Mechanism of Graded PPARγ Agonism. Structure 2012, 20, 139–150. [Google Scholar] [CrossRef]
- Di Marzo, V.; Piscitelli, F. The Endocannabinoid System and Its Modulation by Phytocannabinoids. Neurotherapeutics 2015, 12, 692–698. [Google Scholar] [CrossRef]
- Vara, D.; Morell, C.; Rodríguez-Henche, N.; Diaz-Laviada, I. Involvement of PPARγ in the Antitumoral Action of Cannabinoids on Hepatocellular Carcinoma. Cell Death Dis. 2013, 4, e618. [Google Scholar] [CrossRef]
- Carroll, C.B.; Zeissler, M.; Hanemann, C.O.; Zajicek, J.P. Δ9-tetrahydrocannabinol (Δ9-THC) Exerts a Direct Neuroprotective Effect in a Human Cell Culture Model of Parkinson’s Disease. Neuropathol. Appl. Neurobiol. 2012, 38, 535–547. [Google Scholar] [CrossRef]
- Gunasekaran, N. Reintoxication: The Release of Fat-Stored Δ9-Tetrahydrocannabinol (THC) into Blood Is Enhanced by Food Deprivation or ACTH Exposure. Br. J. Pharmacol. 2009, 158, 1330–1337. [Google Scholar] [CrossRef]
- Rosen, E.D.; Spiegelman, B.M. What We Talk about When We Talk about Fat. Cell 2014, 156, 20–44. [Google Scholar] [CrossRef]
- Beale, E.G.; Antoine, B.; Forest, C. Glyceroneogenesis in Adipocytes: Another Textbook Case. Trends Biochem. Sci. 2003, 28, 402–403. [Google Scholar] [CrossRef]
- Edens, N.K.; Leibel, R.L.; Hirsch, J. Mechanism of Free Fatty Acid Re-Esterification in Human Adipocytes in Vitro. J. Lipid Res. 1990, 31, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Lehrke, M.; Lazar, M.A. The Many Faces of PPARγ. Cell 2005, 123, 993–999. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.C.; Farese, R.V. Lipid Droplets and Cellular Lipid Metabolism. Annu. Rev. Biochem. 2012, 81, 687–714. [Google Scholar] [CrossRef] [PubMed]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-Tissue Plasticity in Health and Disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Guilherme, A.; Virbasius, J.V.; Puri, V.; Czech, M.P. Adipocyte Dysfunctions Linking Obesity to Insulin Resistance and Type 2 Diabetes. Nat. Rev. Mol. Cell Biol. 2008, 9, 367–377. [Google Scholar] [CrossRef]
- Kim, J.I.; Huh, J.Y.; Sohn, J.H.; Choe, S.S.; Lee, Y.S.; Lim, C.Y.; Jo, A.; Park, S.B.; Han, W.; Kim, J.B. Lipid-Overloaded Enlarged Adipocytes Provoke Insulin Resistance Independent of Inflammation. Mol. Cell. Biol. 2015, 35, 1686–1699. [Google Scholar] [CrossRef]
- Blüher, M. Adipose Tissue Dysfunction Contributes to Obesity Related Metabolic Diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2013, 27, 163–177. [Google Scholar] [CrossRef]
- Baldini, F.; Diab, F.; Serale, N.; Zeaiter, L.; Portincasa, P.; Diaspro, A.; Vergani, L. Adipocyte-Hepatocyte Crosstalk in Cellular Models of Obesity: Role of Soluble Factors. Life Sci. 2023, 317, 121464. [Google Scholar] [CrossRef] [PubMed]
- Niemeier, A.; Gafvels, M.; Heeren, J.; Meyer, N.; Angelin, B.; Beisiegel, U. VLDL Receptor Mediates the Uptake of Human Chylomicron Remnants in Vitro. J. Lipid Res. 1996, 37, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Römer, A.; Rawat, D.; Linn, T.; Petry, S.F. Preparation of Fatty Acid Solutions Exerts Significant Impact on Experimental Outcomes in Cell Culture Models of Lipotoxicity. Biol. Methods Protoc. 2022, 7, bpab023. [Google Scholar] [CrossRef]
- Zebisch, K.; Voigt, V.; Wabitsch, M.; Brandsch, M. Protocol for Effective Differentiation of 3T3-L1 Cells to Adipocytes. Anal. Biochem. 2012, 425, 88–90. [Google Scholar] [CrossRef]
- Ruhl, T.; Benic, S.; Plum, M.; Kim, B.-S.; Beier, J.P.; Schaefer, B. Δ9-Tetrahydrocannabinol Increases Growth Factor Release by Cultured Adipose Stem Cells and Adipose Tissue in Vivo. Tissue Eng. Regen. Med. 2025, 22, 225–235. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eitan, A.; Gover, O.; Schwartz, B. Distinct Adipocyte Responses to Δ9-Tetrahydrocannabinol (THC) Exposure Govern Hepatic Lipid Accumulation in an Obesogenic Setting. Int. J. Mol. Sci. 2025, 26, 8860. https://doi.org/10.3390/ijms26188860
Eitan A, Gover O, Schwartz B. Distinct Adipocyte Responses to Δ9-Tetrahydrocannabinol (THC) Exposure Govern Hepatic Lipid Accumulation in an Obesogenic Setting. International Journal of Molecular Sciences. 2025; 26(18):8860. https://doi.org/10.3390/ijms26188860
Chicago/Turabian StyleEitan, Adi, Ofer Gover, and Betty Schwartz. 2025. "Distinct Adipocyte Responses to Δ9-Tetrahydrocannabinol (THC) Exposure Govern Hepatic Lipid Accumulation in an Obesogenic Setting" International Journal of Molecular Sciences 26, no. 18: 8860. https://doi.org/10.3390/ijms26188860
APA StyleEitan, A., Gover, O., & Schwartz, B. (2025). Distinct Adipocyte Responses to Δ9-Tetrahydrocannabinol (THC) Exposure Govern Hepatic Lipid Accumulation in an Obesogenic Setting. International Journal of Molecular Sciences, 26(18), 8860. https://doi.org/10.3390/ijms26188860








