Trace Elements in Different Blood Products Used in Neonatal Transfusion: Arsenic and Selenium
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. ICP-MS Analysis
2.3. Quality Control
2.4. Estimation of Dose per Transfusion
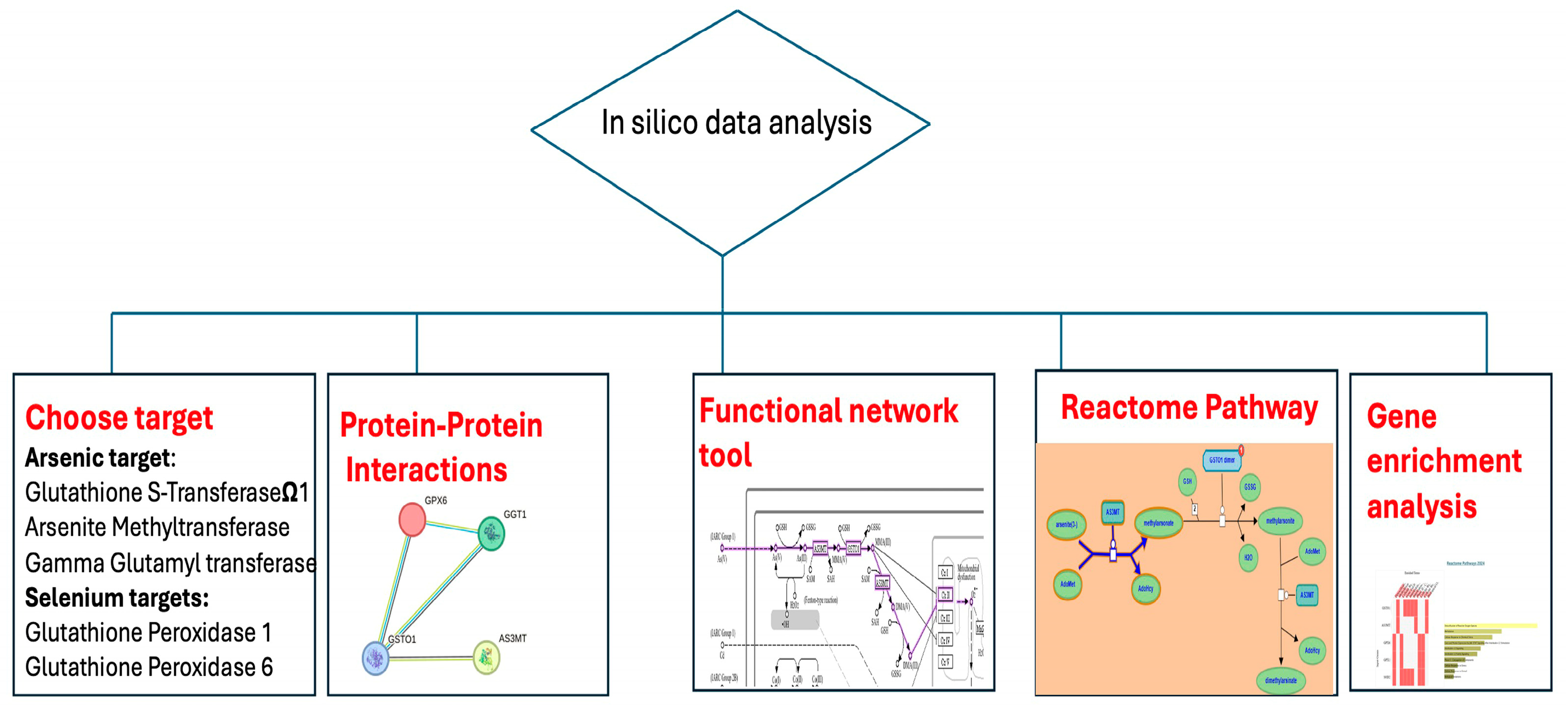
2.5. In Silico Analysis
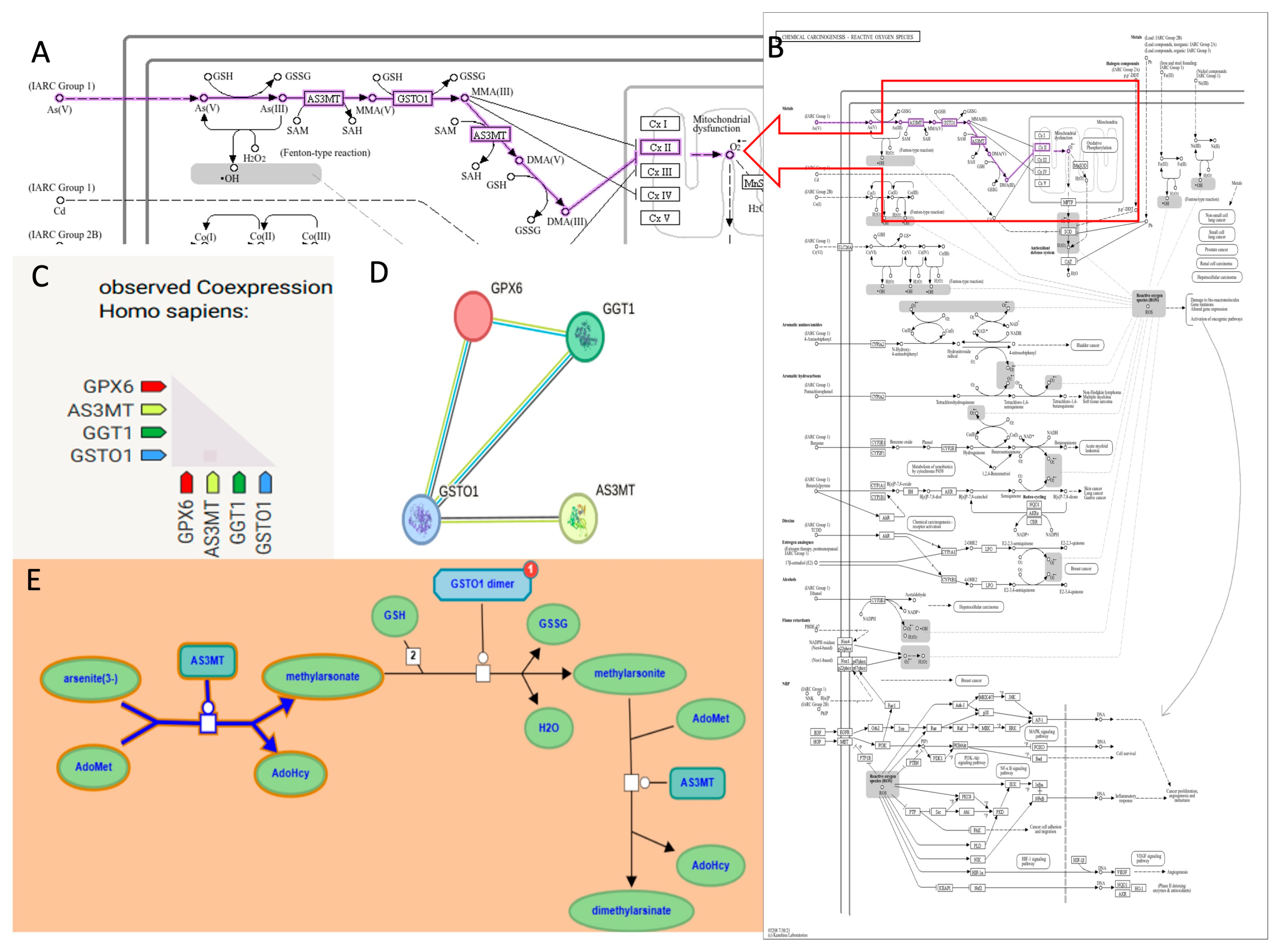
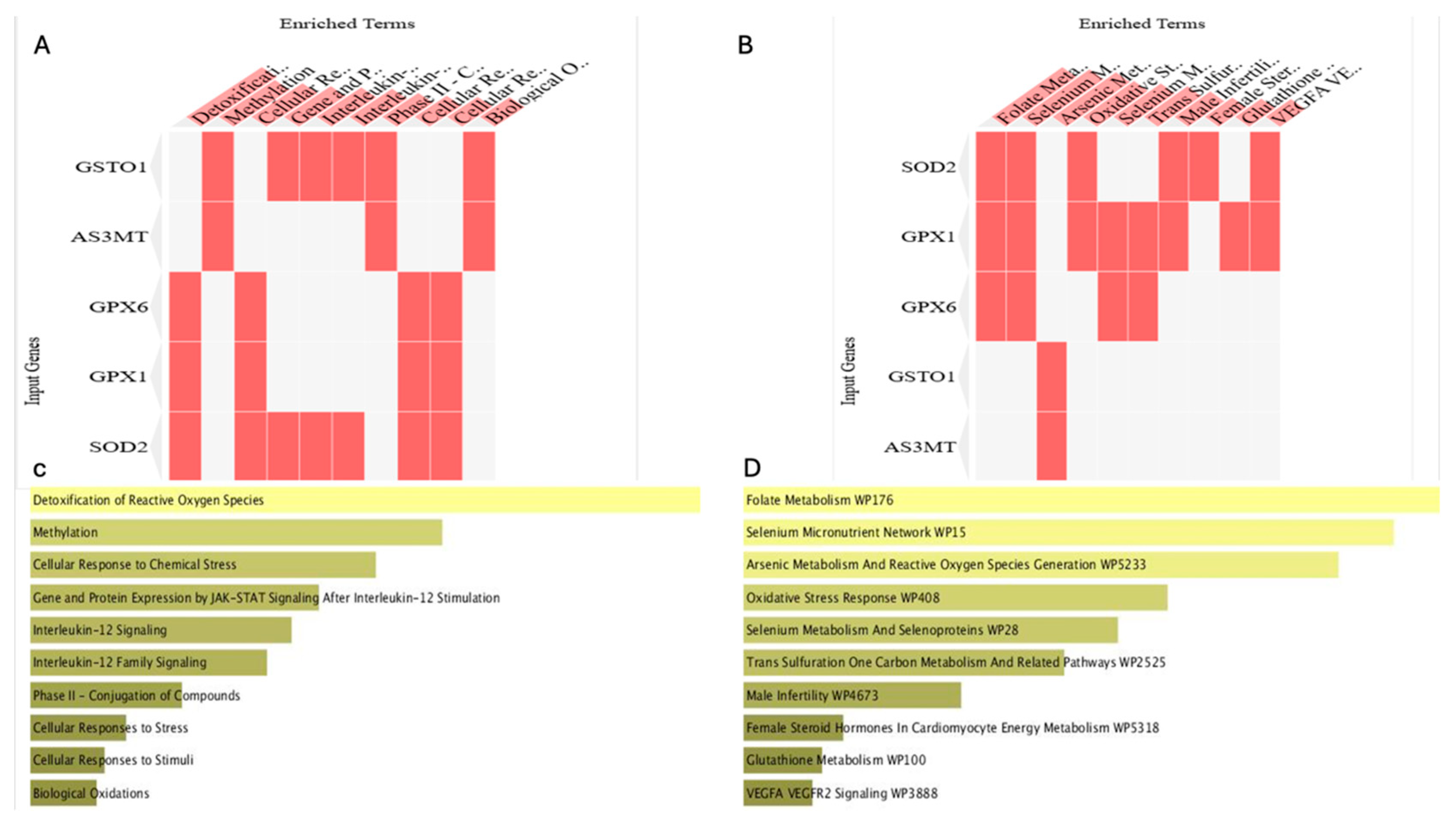
2.5.1. Protein–Protein Interaction and Functional Enrichment Analyses
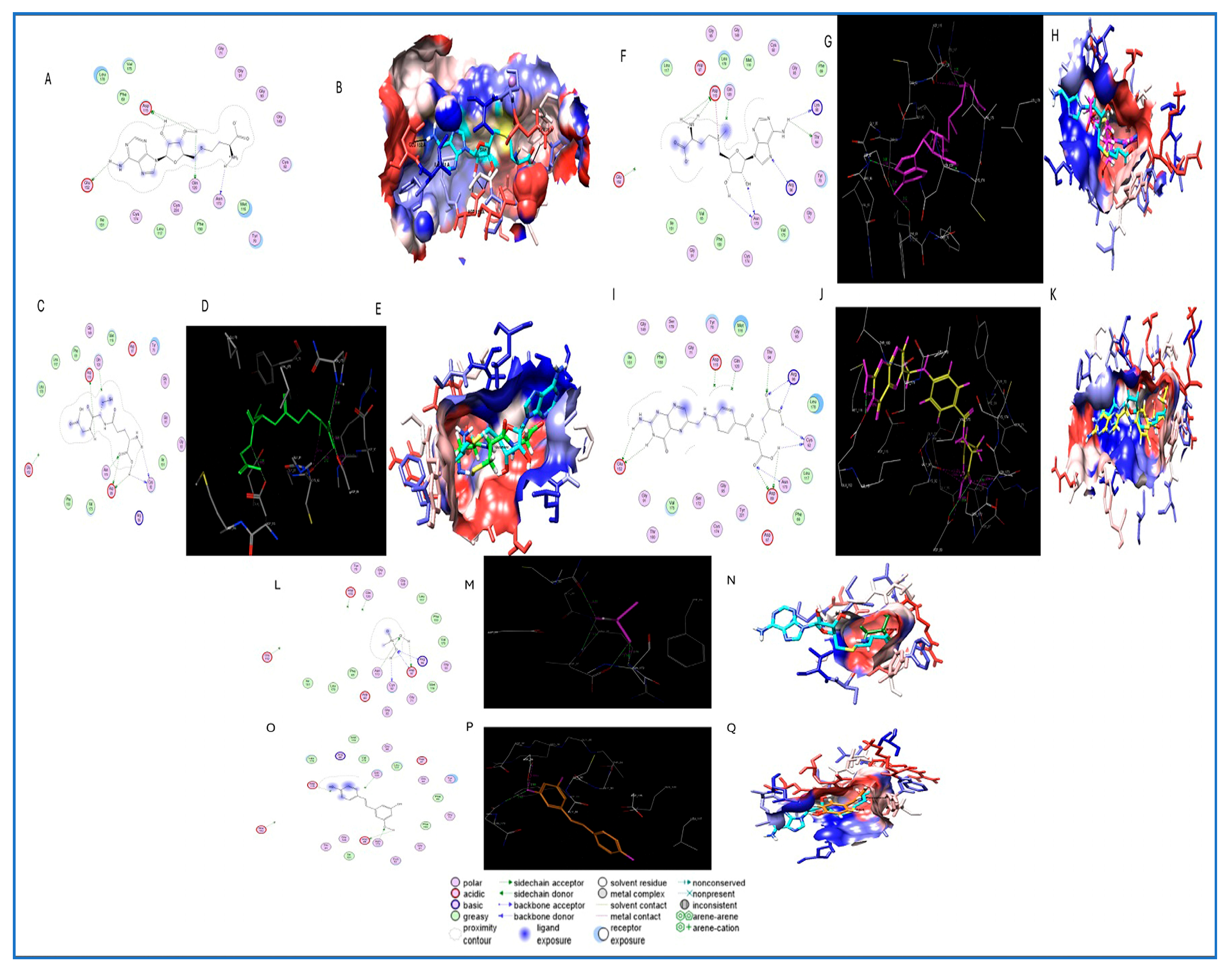
2.5.2. Ligand Preparation and Molecular Docking
2.6. Statistical Analysis
3. Results
3.1. Quality Control
3.2. Metal Concentrations in Donor Blood Units
3.3. Dose per Transfusion
3.4. Molecular Docking Analysis
4. Discussion
5. Conclusions
6. Further Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Girelli, G.; Antoncecchi, S.; Casadei, A.M.; Del Vecchio, A.; Isernia, P.; Motta, M.; Regoli, D.; Romagnoli, C.; Tripodi, G.; Velati, C. Recommendations for transfusion therapy in neonatology. Blood Transfus. 2015, 13, 484. [Google Scholar]
- Vassallo, R.; Bachowski, G.; Benjamin, R.; Borge, D.; Dodd, R.; Eder, A.; Eastvold, P.; Goldberg, C.; Hopkins, C.; Lima, J. A Compendium of Transfusion Practice Guidelines; American Red Cross: Washington, DC, USA, 2013. [Google Scholar]
- Falck, A.J.; Mooney, S.; Kapoor, S.S.; White, K.M.; Bearer, C.; El Metwally, D. Developmental exposure to environmental toxicants. Pediatr. Clin. 2015, 62, 1173–1197. [Google Scholar] [CrossRef]
- Aliasgharpour, M.; Rahnamaye Farzami, M. Trace elements in human nutrition: A review. J. Emerg. Health Care 2013, 2, 115–128. [Google Scholar]
- Balali-Mood, M.; Naseri, K.; Tahergorabi, Z.; Khazdair, M.R.; Sadeghi, M. Toxic mechanisms of five heavy metals: Mercury, lead, chromium, cadmium, and arsenic. Front. Pharmacol. 2021, 12, 643972. [Google Scholar] [CrossRef]
- Barone, G.; Dambrosio, A.; Storelli, A.; Garofalo, R.; Busco, V.P.; Storelli, M.M. Estimated dietary intake of trace metals from swordfish consumption: A human health problem. Toxics 2018, 6, 22. [Google Scholar] [CrossRef]
- Falck, A.J.; Medina, A.E.; Cummins-Oman, J.; El-Metwally, D.; Bearer, C.F. Mercury, lead, and cadmium exposure via red blood cell transfusions in preterm infants. Pediatr. Res. 2020, 87, 677–682. [Google Scholar] [CrossRef]
- Newman, N.; Carey, P.M. Donor blood lead levels and transfusion safety in a vulnerable population. Transfusion 2015, 55, 2544–2546. [Google Scholar] [CrossRef] [PubMed]
- Zubairi, H.; Visintainer, P.; Fleming, J.; Richardson, M.; Singh, R. Lead exposure in preterm infants receiving red blood cell transfusions. Pediatr. Res. 2015, 77, 814–818. [Google Scholar] [CrossRef][Green Version]
- Aly, S.M.; Elfiky, S.; Mohamed, Y.G.; Soliman, R.A.; Shalaby, N.; Beauval, N.; Gaulier, J.-M.; Allorge, D.; Omran, A. Lead, mercury, and cadmium concentrations in blood products transfused to neonates: Elimination not just mitigation. Toxics 2023, 11, 712. [Google Scholar] [CrossRef] [PubMed]
- Aly, S.M.; Omran, A.; Abdalla, M.O.; Gaulier, J.-M.; El-Metwally, D. Lead: A hidden “untested” risk in neonatal blood transfusion. Pediatr. Res. 2019, 85, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Zwolak, I. The role of selenium in arsenic and cadmium toxicity: An updated review of scientific literature. Biol. Trace Elem. Res. 2020, 193, 44–63. [Google Scholar] [CrossRef]
- Sundararajan, S.; Blatz, A.; Dearborn, D.; Varnes, A.; Bearer, C.; El Metwally, D. Toxic metal contamination of banked blood designated for neonatal transfusion. J. Clin. Toxicol. 2015, 5, 1–5. [Google Scholar] [CrossRef]
- Hasanato, R. Alterations in serum levels of copper, zinc, and selenium among children with sickle cell anemia. Turk. J. Med. Sci. 2019, 49, 1287–1291. [Google Scholar] [CrossRef]
- Yücel, U.M.; Başbuğan, Y.; Uyar, A.; Kömüroğlu, A.U.; Keleş, Ö.F. Use of an antiarrhythmic drug against acute selenium toxicity. J. Trace Elem. Med. Biol. 2020, 59, 126471. [Google Scholar] [CrossRef] [PubMed]
- Ronkart, S.N.; Laurent, V.; Carbonnelle, P.; Mabon, N.; Copin, A.; Barthélemy, J.-P. Speciation of five arsenic species (arsenite, arsenate, MMAAV, DMAAV and AsBet) in different kind of water by HPLC-ICP-MS. Chemosphere 2007, 66, 738–745. [Google Scholar] [CrossRef]
- Ochoa-Martínez, Á.C.; Ruiz-Vera, T.; Almendarez-Reyna, C.I.; Zarazúa, S.; Carrizales-Yáñez, L.; Pérez-Maldonado, I.N. Impact of arsenic exposure on clinical biomarkers indicative of cardiovascular disease risk in Mexican women. Ecotoxicol. Environ. Saf. 2019, 169, 678–686. [Google Scholar] [CrossRef] [PubMed]
- Lewchalermvong, K.; Rangkadilok, N.; Nookabkaew, S.; Suriyo, T.; Satayavivad, J. Arsenic speciation and accumulation in selected organs after oral administration of rice extracts in Wistar rats. J. Agric. Food Chem. 2018, 66, 3199–3209. [Google Scholar] [CrossRef] [PubMed]
- Thakur, M.; Rachamalla, M.; Niyogi, S.; Datusalia, A.K.; Flora, S.J.S. Molecular mechanism of arsenic-induced neurotoxicity including neuronal dysfunctions. Int. J. Mol. Sci. 2021, 22, 10077. [Google Scholar] [CrossRef]
- Liang, C.; Wu, X.; Huang, K.; Yan, S.; Li, Z.; Xia, X.; Pan, W.; Sheng, J.; Tao, R.; Tao, Y. Domain-and sex-specific effects of prenatal exposure to low levels of arsenic on children’s development at 6 months of age: Findings from the Ma’anshan birth cohort study in China. Environ. Int. 2020, 135, 105112. [Google Scholar] [CrossRef]
- Al-Ayadhi, L.Y. Heavy metals and trace elements in hair samples of autistic children in central Saudi Arabia. Neurosci. J. 2005, 10, 213–218. [Google Scholar]
- Chen, P.; Miah, M.R.; Aschner, M. Metals and neurodegeneration. F1000Research 2016, 5. [Google Scholar] [CrossRef]
- Baker, B.A.; Cassano, V.A.; Murray, C. Arsenic exposure, assessment, toxicity, diagnosis, and management: Guidance for occupational and environmental physicians. J. Occup. Environ. Med. 2018, 60, e634–e639. [Google Scholar] [CrossRef] [PubMed]
- American Cancer Society. Available online: https://www.cancer.org/cancer/risk-prevention/chemicals/arsenic.html (accessed on 31 August 2023).
- Tan, H.W.; Mo, H.-Y.; Lau, A.T.; Xu, Y.-M. Selenium species: Current status and potentials in cancer prevention and therapy. Int. J. Mol. Sci. 2018, 20, 75. [Google Scholar] [CrossRef] [PubMed]
- Tinggi, U. Essentiality and toxicity of selenium and its status in Australia: A review. Toxicol. Lett. 2003, 137, 103–110. [Google Scholar] [CrossRef]
- Zhang, J.; Ye, Z.; Huang, L.; Zhao, Q.; Dong, K.; Zhang, W. Significant biotransformation of arsenobetaine into inorganic arsenic in mice. Toxics 2023, 11, 91. [Google Scholar] [CrossRef]
- Bebars, G.M.; Kamel, B.A.; Allam, E. Comparison between preterm and full term neonatal cord selenium in correlation to maternal serum selenium levels. Egypt. Pediatr. Assoc. Gaz. 2018, 66, 96–99. [Google Scholar] [CrossRef]
- Makhoul, I.R.; Sammour, R.N.; Diamond, E.; Shohat, I.; Tamir, A.; Shamir, R. Selenium concentrations in maternal and umbilical cord blood at 24–42 weeks of gestation: Basis for optimization of selenium supplementation to premature infants. Clin. Nutr. 2004, 23, 373–381. [Google Scholar] [CrossRef] [PubMed]
- Tindell, R.; Tipple, T. Selenium: Implications for outcomes in extremely preterm infants. J. Perinatol. 2018, 38, 197–202. [Google Scholar] [CrossRef]
- Litov, R.E.; Combs, G.F., Jr. Selenium in pediatric nutrition. Pediatrics 1991, 87, 339–351. [Google Scholar] [CrossRef]
- Offor, S.J.; Orish, C.N.; Eze, E.C.; Frazzoli, C.; Orisakwe, O.E. Blood donation and heavy metal poisoning in developing nations: Any link? Transfus. Apher. Sci. 2021, 60, 103067. [Google Scholar] [CrossRef]
- Harmening, D.M. Modern Blood Banking & Transfusion Practices; FA Davis: Philadelphia, PA, USA, 2018. [Google Scholar]
- EMA; Committee for Medicinal Products for Human Use. Guideline on Bioanalytical Method Validation; EMEA/CHMP/EWP/192217/2009 Rev 1 Corr 2; EMA: Amsterdam, The Netherlands, 2012; Volume 44, pp. 1–23. Available online: https://www.ema.europa.eu/en/documents/scientific-guideline/guideline-bioanalytical-method-validation_en.pdf (accessed on 15 July 2025).
- ATSDR. Toxicological Profile for Arsenic; ATSDR: Atlanta, GA, USA, 2007.
- Averina, M.; Hervig, T.; Huber, S.; Kjær, M.; Kristoffersen, E.K.; Bolann, B. Environmental pollutants in blood donors: The multicentre Norwegian donor study. Transfus. Med. 2020, 30, 201–209. [Google Scholar] [CrossRef]
- Delage, G.; Gingras, S.; Rhainds, M. A population-based study on blood lead levels in blood donors. Transfusion 2015, 55, 2633–2640. [Google Scholar] [CrossRef]
- Lugon-Moulin, N.; Martin, F.; Krauss, M.R.; Ramey, P.B.; Rossi, L. Arsenic concentration in tobacco leaves: A study on three commercially important tobacco (Nicotiana tabacum L.) types. Water Air Soil Pollut. 2008, 192, 315–319. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ng, J.C.; Naidu, R. Chronic exposure of arsenic via drinking water and its adverse health impacts on humans. Environ. Geochem. Health 2009, 31 (Suppl. 1), 189–200. [Google Scholar] [CrossRef]
- Sun, Q.; Song, Y.; Liu, S.; Wang, F.; Zhang, L.; Xi, S.; Sun, G. Arsenic exposure levels in relation to different working departments in a copper mining and smelting plant. Atmos. Environ. 2015, 118, 1–6. [Google Scholar] [CrossRef]
- Wen, L.; Zhou, L.; Zhu, X.; Mei, Y. Epidemiological profile and determinants of whole blood heavy metal levels in occupationally exposed populations: A cross-sectional study in Hunan Province, China. Front. Public Health 2025, 13, 1635236. [Google Scholar] [CrossRef]
- Anastasiadi, A.T.; Stamoulis, K.; Kriebardis, A.G.; Tzounakas, V.L. Molecular modifications to mitigate oxidative stress and improve red blood cell storability. Front. Physiol. 2024, 15, 1499308. [Google Scholar] [CrossRef]
- D’Alessandro, A.; Liumbruno, G. Personalised transfusion medicine. Blood Transfus. 2019, 17, 255. [Google Scholar]
- Satyapal, G.K.; Kumar, N. Arsenic: Source, distribution, toxicity and bioremediation. In Arsenic Toxicity: Challenges and Solutions; Springer: Singapore, 2021; pp. 153–163. [Google Scholar]
- Verdon, C.P.; Caldwell, K.L.; Fresquez, M.R.; Jones, R.L. Determination of seven arsenic compounds in urine by HPLC-ICP-DRC-MS: A CDC population biomonitoring method. Anal. Bioanal. Chem. 2009, 393, 939–947. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Zavala, A.; Matoušek, T.; Drobná, Z.; Paul, D.S.; Walton, F.; Adair, B.M.; Dědina, J.; Thomas, D.J.; Stýblo, M. Speciation analysis of arsenic in biological matrices by automated hydride generation-cryotrapping-atomic absorption spectrometry with multiple microflame quartz tube atomizer (multiatomizer). J. Anal. At. Spectrom. 2008, 23, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Rajaković, L.V.; Todorović, Ž.; Rajaković-Ognjanović, V.N.; Onjia, A.E. Analytical methods for arsenic speciation analysis. J. Serbian Chem. Soc. 2013, 78, 1461–1479. [Google Scholar] [CrossRef]
- Scheer, J.; Findenig, S.; Goessler, W.; Francesconi, K.A.; Howard, B.; Umans, J.G.; Pollak, J.; Tellez-Plaza, M.; Silbergeld, E.K.; Guallar, E. Arsenic species and selected metals in human urine: Validation of HPLC/ICPMS and ICPMS procedures for a long-term population-based epidemiological study. Anal. Methods 2012, 4, 406–413. [Google Scholar] [CrossRef]
- Expert Committee on Food Additives; World Health Organization. Safety Evaluation of Certain Contaminants In Food: Prepared by the Seventy-Second Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); WHO Press: Geneva, Switzerland, 2011; Available online: http://apps.who.int/iris/bitstream/10665/44520/1/9789241660631_eng.pdf (accessed on 13 July 2025).
- World Health Organization. Preventing Disease Through Healthy Environments: Exposure to Arsenic: A Major Public Health Concern; WHO: Geneva, Switzerland, 2019; Available online: https://www.who.int/publications/i/item/WHO-CED-PHE-EPE-19.4.1 (accessed on 13 July 2025).
- Bermúdez, L.; García-Vicent, C.; López, J.; Torró, M.I.; Lurbe, E. Assessment of ten trace elements in umbilical cord blood and maternal blood: Association with birth weight. J. Transl. Med. 2015, 13, 291. [Google Scholar] [CrossRef] [PubMed]
- Knee, D.; Knoop, S.; Davis, A.T.; Rawson, B.; DiCarlo, A.; Olivero, R. Outcomes after implementing restrictive blood transfusion criteria in extremely premature infants. J. Perinatol. 2019, 39, 1089–1097. [Google Scholar] [CrossRef]
- Van Cauwenbergh, R.; Robberecht, H.; Van Vlaslaer, V.; De Smet, A.; Emonds, M.-P.; Hermans, N. Plasma selenium levels in healthy blood bank donors in the central-eastern part of Belgium. J. Trace Elem. Med. Biol. 2007, 21, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Zhang, M.; Tang, S.; Li, M.; Wu, R.; Wan, S.; Chen, L.; Wei, X.; Feng, S. Effects and impact of selenium on human health, a review. Molecules 2024, 30, 50. [Google Scholar] [CrossRef]
- Fairweather-Tait, S.J.; Bao, Y.; Broadley, M.R.; Collings, R.; Ford, D.; Hesketh, J.E.; Hurst, R. Selenium in human health and disease. Antioxid. Redox Signal. 2011, 14, 1337–1383. [Google Scholar] [CrossRef] [PubMed]
- McDonald, C.; Colebourne, K.; Faddy, H.M.; Flower, R.; Fraser, J.F. Plasma selenium status in a group of Australian blood donors and fresh blood components. J. Trace Elem. Med. Biol. 2013, 27, 352–354. [Google Scholar] [CrossRef]
- Khatami, S.-F.; Parvaresh, P.; Parvaresh, P.; Gharib, M. The investigation of effects of blood exchange transfusion on selenium in newborn infants by instrumental neutron activation analysis method. Iran. J. Pediatr. 2013, 23, 131. [Google Scholar] [PubMed]
- McDonald, C.; Fraser, J.; Shekar, K.; Dunster, K.; Thom, O.; Fung, Y.L. Transfusion of packed red blood cells reduces selenium levels and increases lipid peroxidation in an in vivo ovine model. Transfus. Med. 2014, 24, 50–54. [Google Scholar] [CrossRef]
- Patterson, B.H.; Combs, G.F., Jr.; Taylor, P.R.; Patterson, K.Y.; Moler, J.E.; Wastney, M.E. Selenium kinetics in humans change following 2 years of supplementation with selenomethionine. Front. Endocrinol. 2021, 12, 621687. [Google Scholar] [CrossRef] [PubMed]
- Hawkes, W.C.; Alkan, F.Z.; Oehler, L. Absorption, distribution and excretion of selenium from beef and rice in healthy North American men. J. Nutr. 2003, 133, 3434–3442. [Google Scholar] [CrossRef]
- Jiménez, M.P.H.; de la Calle, S.d.P.; Vives, C.C.; Sáez, D.E. Nutritional supplementation in pregnant, lactating women and young children following a plant-based diet: A narrative review of the evidence. Nutrition 2025, 136, 112778. [Google Scholar] [CrossRef]
- Gamble, M.V.; Liu, X.; Ahsan, H.; Pilsner, J.R.; Ilievski, V.; Slavkovich, V.; Parvez, F.; Chen, Y.; Levy, D.; Factor-Litvak, P. Folate and arsenic metabolism: A double-blind, placebo-controlled folic acid–supplementation trial in Bangladesh. Am. J. Clin. Nutr. 2006, 84, 1093–1101. [Google Scholar] [CrossRef]
- Martinez-Morata, I.; Parvez, F.; Wu, H.; Eunus, M.; Goldsmith, J.; Ilievski, V.; Slavkovich, V.; Balac, O.; Izuchukwu, C.; Glabonjat, R.A. Influence of folic acid and vitamin B12 supplementation on arsenic methylation: A double-blinded, placebo-controlled trial in Bangladeshi children. Environ. Int. 2024, 187, 108715. [Google Scholar] [CrossRef]
- Engström, K.S.; Broberg, K.; Concha, G.; Nermell, B.; Warholm, M.; Vahter, M. Genetic polymorphisms influencing arsenic metabolism: Evidence from Argentina. Environ. Health Perspect. 2007, 115, 599–605. [Google Scholar] [CrossRef] [PubMed]
- Naranmandura, H.; Suzuki, N.; Suzuki, K.T. Trivalent arsenicals are bound to proteins during reductive methylation. Chem. Res. Toxicol. 2006, 19, 1010–1018. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, Y.; Ge, M.; Jing, J.; Chen, Y.; Jiang, H.; Yu, H.; Li, N.; Zhang, Z. Protective effect of resveratrol on arsenic trioxide-induced nephrotoxicity in rats. Nutr. Res. Pract. 2014, 8, 220–226. [Google Scholar] [CrossRef]
- Zhang, W.; Xue, J.; Ge, M.; Yu, M.; Liu, L.; Zhang, Z. Resveratrol attenuates hepatotoxicity of rats exposed to arsenic trioxide. Food Chem. Toxicol. 2013, 51, 87–92. [Google Scholar] [CrossRef]
- Zhang, Y.; Gong, C.; Tao, L.; Zhai, J.; Huang, F.; Zhang, S. Involvement of SIRT1-mediated aging in liver diseases. Front. Cell Dev. Biol. 2025, 13, 1548015. [Google Scholar] [CrossRef] [PubMed]
| Element | Isotope | Dwell Time (s) | Sweeps per Replicate | Replicates | Internal Standard Used for Quantification | Measurement Mode | Sampling Volume (µL) | Sample Dilution | Calibration Specificity | LLOQ in Plasma, Platelets and WB (µg/L) | LLOQ in pRBCs (µg/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| As | 75 | 0.1 | 10 | 3 | 71Ga | KED * | 100 | 1/50 | None | 0.2 | 0.6 |
| Se | 78 | 0.3 | 10 | 3 | 89Y | KED | 100 | 1/50 | None | 5 | 15 |
| ClinChekwholeblood-Lot 2192 | ClinChekserum-Lot2062 | |||||||
|---|---|---|---|---|---|---|---|---|
| As | Se | As | Se | |||||
| Result * | Certified $ | Results | Certified | Results | Certified | Results | Certified | |
| Level 1 | 3.05–3.56 | 2.99 (2.39–3.59) | 71.4–87.3 | 84.8 (67.8–102) | 9.89–10.3 | 9.48 (7.58–11.4) | 51.9–55.6 | 57.7 (46.1–69.2) |
| Level 2 | 5.57–6.16 | 5.37 (4.29–6.44) | 139–163 | 163 (130–195) | 19.8–20.9 | 19.3 (15.4–23.2) | 102–104 | 105 (83.7–126) |
| Level 3 | 11.0–11.2 | 10.2 (8.14–12.2) | 168–179 | 205 (164–245) | ||||
| Plasma | Platelets | pRBCs | WB | p | ||
|---|---|---|---|---|---|---|
| As | ||||||
| Median | 0.4 | 0.4 | 0.9 | 0.5 | 0.00006 * | |
| IQR | 0.375 | 0.4 | 0.525 | 0.4 | ||
| Se | ||||||
| Median | 54.5 | 56.5 | 126.0 | 79.0 | <0.00001 * | |
| IQR | 17 | 17.75 | 25.5 | 15 | ||
| Dose per Transfusion | Blood Products (µg/kg/Day) | |||
|---|---|---|---|---|
| Plasma | Platelets | pRBCs | WB | |
| As | ||||
| Median | 0.01 | 0.01 | 0.02 | 0.05 |
| IQR | 0.0058 | 0.00625 | 0.0075 | 0.025 |
| Se | ||||
| Median | 0.9 | 0.9 | 2 | 7 |
| IQR | 0.283 | 0.296 | 0.425 | 1.25 |
| Molecular Target and PDB Code | Compound | Hydrogen Bond Analysis | Amino Acids Involved in the Lipophilic Analysis | ||
|---|---|---|---|---|---|
| N | H Bond | A | |||
| 6CX6 | SAH | 4 | ASP115 | 1.8A, 1.6A | PHE 150, ILE 151, VAL175, LEU 178, METH116, CYS92 |
| ASN173 | 2.3A | ||||
| GLU 152 | 2.5A | ||||
| Glutathione conf.3 | 4 | ASP115 | 2.9A | LEU52, VAL137, LEU140, LEU79, ILE113 | |
| ASN173 | 3.3A | ||||
| CYST92 | 2.7A | ||||
| ASP89 | 3A | ||||
| SAM conf.9 | 4 | ASN173 | 2.34A | PHE31, VAL60, LEU79, LEU52, CYS54, VAL137, LEU140 | |
| ASP115 | 2.25A | ||||
| ARG96 | 3A | ||||
| LYS68 | 3.1A | ||||
| THR94 | 2.14 | ||||
| Folic acid conf.3 | 6 | ASN173 | 2.7A | PHE31, LEU52, VAL60, ILE131, PHE112, LEU79, CYS186 | |
| ASP89 | 3.4A | ||||
| ARG96 | 2.7A | ||||
| THR94 | 2.8A | ||||
| CYST92 | 3A | ||||
| GLU152 | 2.38A | ||||
| MMAIII conf.4 | 4 | ASN173 | 2.26A | PHE31, VAL60, CYS54, CYS136, VAL137 | |
| CYS92 | 2.52A | ||||
| ASP97 | 2.35A | ||||
| ARG96 | 3.06A | ||||
| Resveratrol conf.6 | 3 | ASN173 | 2.02A | VAL 27, CYS54,PHE31, GLY55, LEU79, LEU140, CYS136 | |
| ASP89 | 2.8A | ||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aly, S.M.; Abdellatif, H.A.A.; Mohamed, Y.G.; Soliman, R.A.M.; Abdalla, M.O.; Ali, N.H.A.; Hashish, A.A.; Beauval, N.; Gaulier, J.-M.; Allorge, D.; et al. Trace Elements in Different Blood Products Used in Neonatal Transfusion: Arsenic and Selenium. Int. J. Mol. Sci. 2025, 26, 8853. https://doi.org/10.3390/ijms26188853
Aly SM, Abdellatif HAA, Mohamed YG, Soliman RAM, Abdalla MO, Ali NHA, Hashish AA, Beauval N, Gaulier J-M, Allorge D, et al. Trace Elements in Different Blood Products Used in Neonatal Transfusion: Arsenic and Selenium. International Journal of Molecular Sciences. 2025; 26(18):8853. https://doi.org/10.3390/ijms26188853
Chicago/Turabian StyleAly, Sanaa M., Hidi A. A. Abdellatif, Yasmine G. Mohamed, Radwa A. M. Soliman, Mohamed Osama Abdalla, Nada Hosny Ahmed Ali, Abdullah A. Hashish, Nicolas Beauval, Jean-Michel Gaulier, Delphine Allorge, and et al. 2025. "Trace Elements in Different Blood Products Used in Neonatal Transfusion: Arsenic and Selenium" International Journal of Molecular Sciences 26, no. 18: 8853. https://doi.org/10.3390/ijms26188853
APA StyleAly, S. M., Abdellatif, H. A. A., Mohamed, Y. G., Soliman, R. A. M., Abdalla, M. O., Ali, N. H. A., Hashish, A. A., Beauval, N., Gaulier, J.-M., Allorge, D., Shalaby, N., & Omran, A. (2025). Trace Elements in Different Blood Products Used in Neonatal Transfusion: Arsenic and Selenium. International Journal of Molecular Sciences, 26(18), 8853. https://doi.org/10.3390/ijms26188853







