Cold, Hot, and Lethal—The Tumour Microenvironment and the Immunology of Head and Neck Squamous Cell Carcinoma
Abstract
1. Introduction
1.1. Tumour Stem Cells
1.2. Metastasis
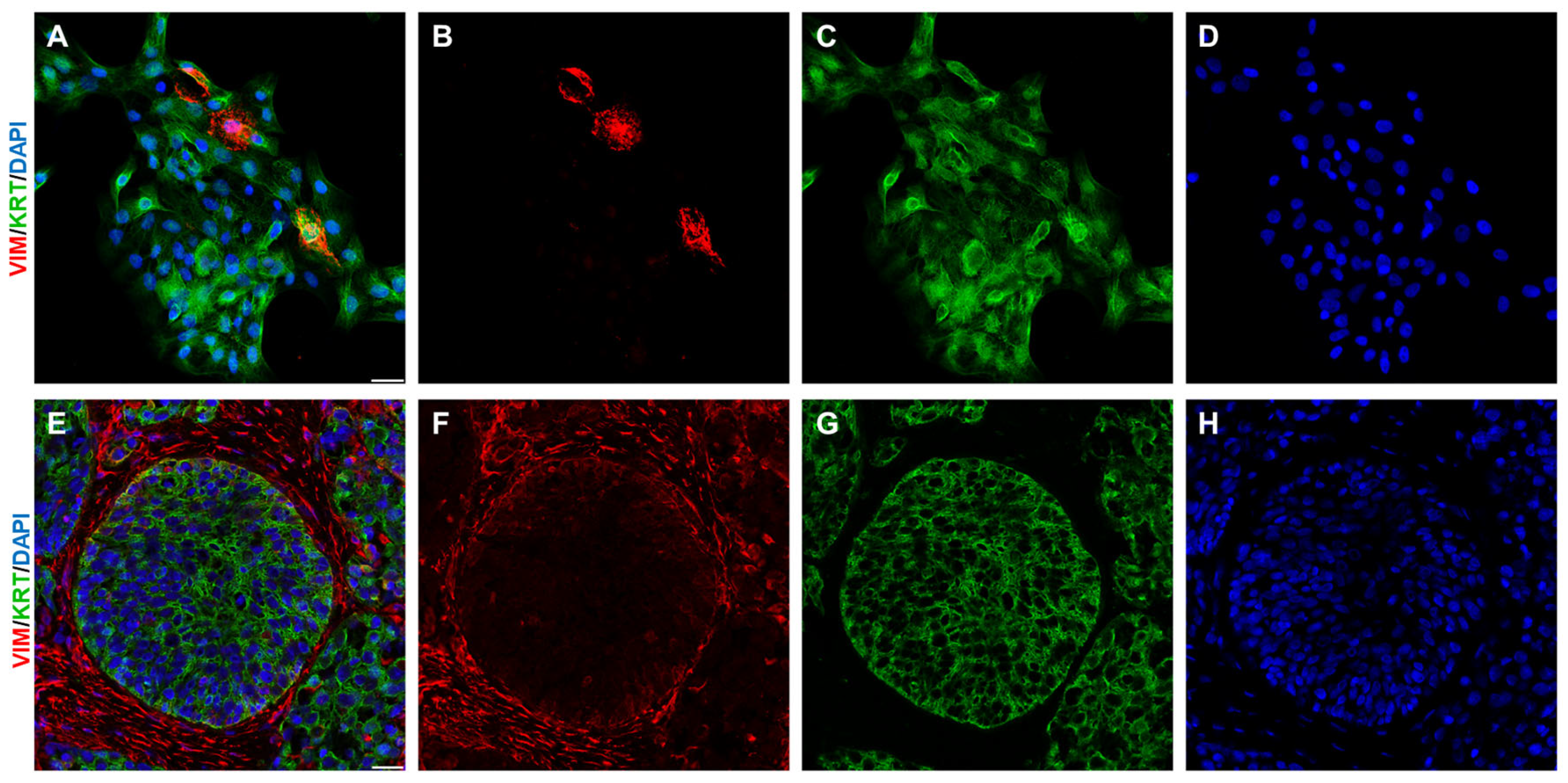
2. The Tumour Microenvironment and Its Components
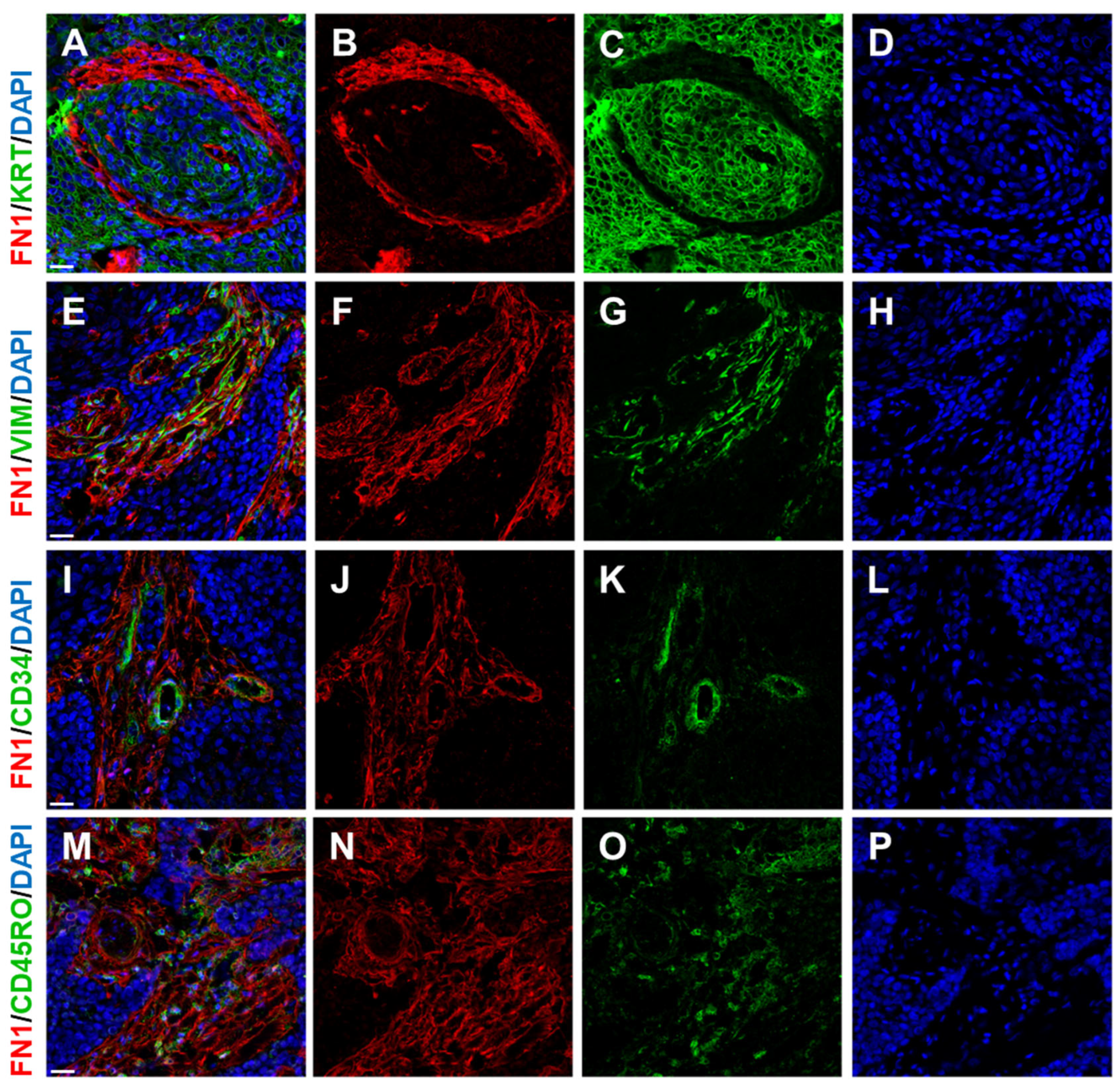
2.1. Immune Cells
2.2. Cancer Association Fibroblasts (CAFs)
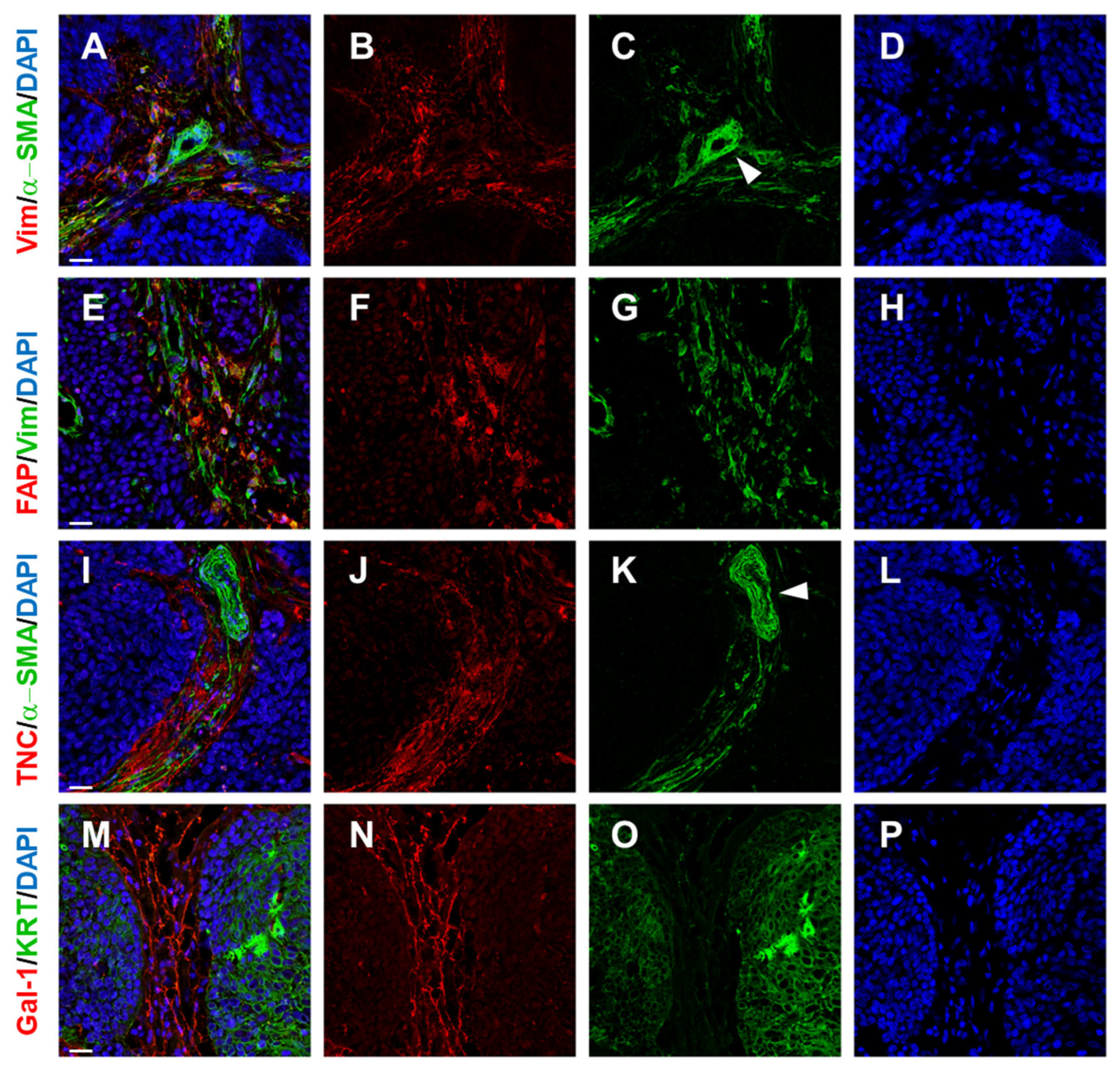
2.3. Blood Vessels
3. Intercellular Signalling in the Tumour Ecosystem
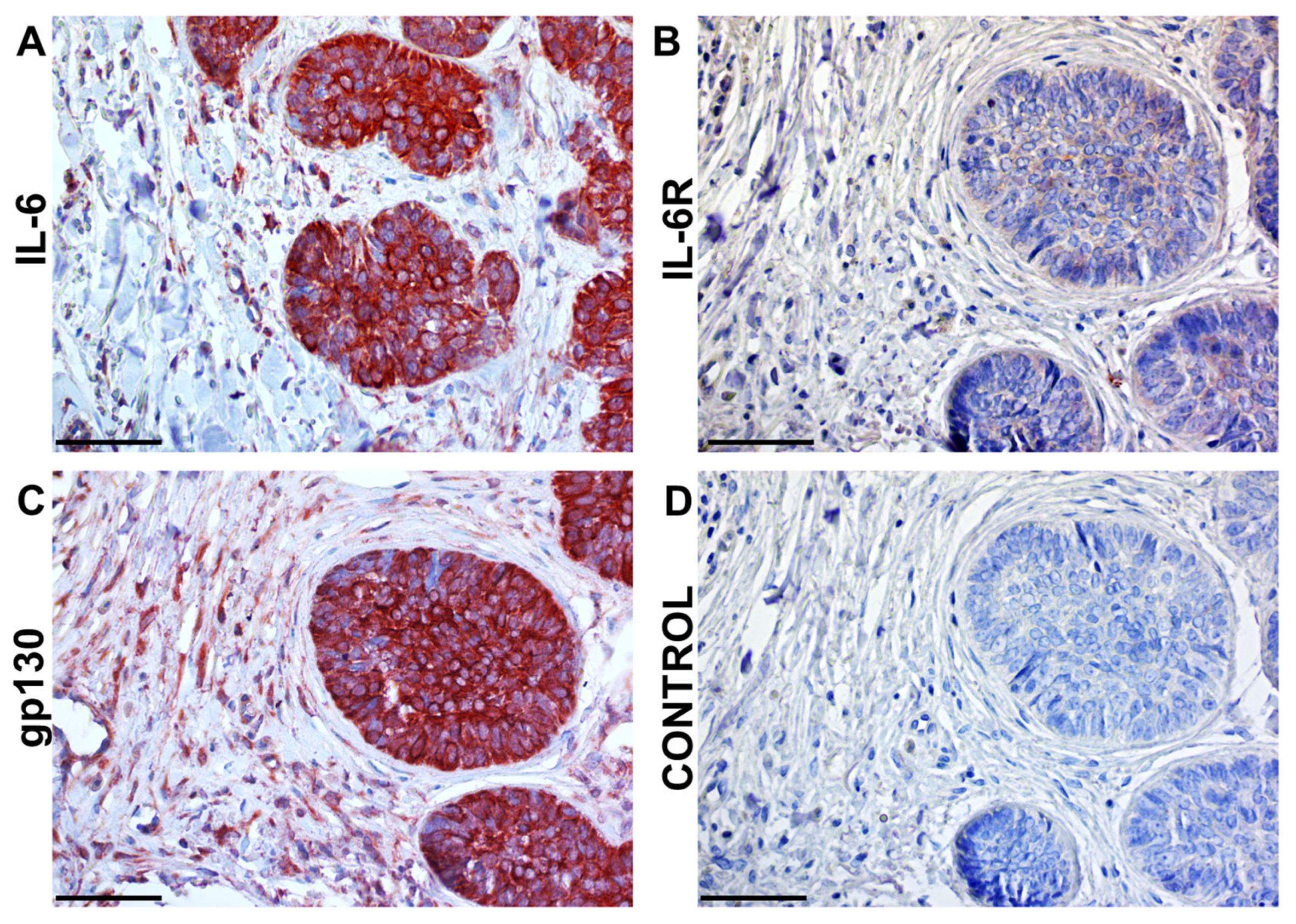
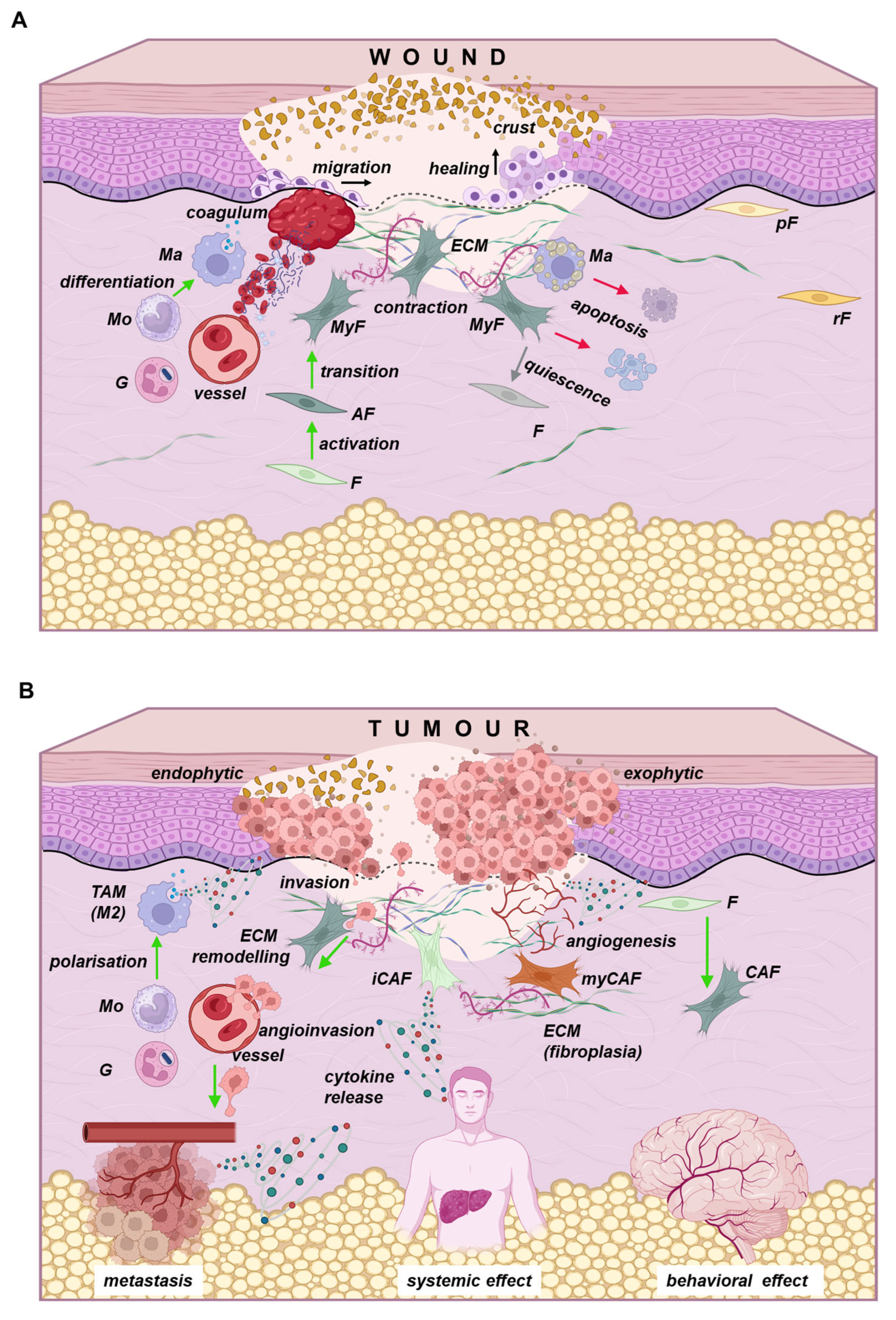
4. Similarity Between Cancer and Wound Healing
5. Systemic Effects of Tumours
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| a-SMA | a-Smooth Muscle Actin |
| ACT | Adoptive Cytotoxic T cell transfer |
| apCAFs | Antigen-Presenting Cancer-Associated Fibroblasts |
| BMP-4 | Bone Morphogenetic Protein 4 |
| CAFs | Cancer-Associated Fibroblasts |
| CCL | Chemokine (C-C motif) Ligand |
| CD | Cluster of Differentiation |
| CDK4-6 | Cyclin-Dependent Kinase 4-6 |
| CDKN2A | Cyclin-Dependent Kinase inhibitor 2A |
| CSCs | Cancer Stem Cells |
| CTLA-4 | Cytotoxic T lymphocyte-associated protein 4 |
| CXCL | C-X-C motive chemokine ligand |
| CXCR | C-X-C chemokine receptor |
| DPPIV | Dipeptidyl-peptidase IV |
| ECM | Extracellular Matrix |
| EGF | Epidermal Growth Factor |
| EMT | Epithelial–Mesenchymal Transition |
| FAP | Fibroblast Activation Protein |
| FGF | Fibroblast Growth Factor |
| FUS | Fused in Sarcoma protein |
| FUSIP1 | FUS-Interacting Protein 1 |
| Gal-1 | Galectin-1 |
| gp130 | Glycoprotein 130 |
| HGF | Hepatocyte Growth Factor |
| HIF | Hypoxia-Inducible Factor |
| HNSCC | Head and Neck Squamous Cell Carcinoma |
| HPV | Human Papillomavirus |
| iCAFs | Inflammation Cancer-Associated Fibroblasts |
| ICIs | Immune-Checkpoint Inhibitors |
| IGF-2 | Insulin-like Growth Factor-2 |
| IL- | Interleukin- |
| JAKs | Janus Activated Kinases |
| MAP3K2 | Mitogen-Activated Protein Kinase Kinase Kinase 2 |
| matCAFs | Matrix Cancer-Associated Fibroblasts |
| M-CSF | Macrophage Colony-Stimulating Factor |
| MDSCs | Myeloid-Derived Suppressor Cells |
| MET | Mesenchymal–Epithelial Transition |
| MIF | Macrophage Migration Inhibitory Factor |
| myCAFs | Myofibroblastic Cancer-Associated Fibroblasts |
| NF- κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| NG2 | Neuron-Glial antigen 2 |
| PD1-L | Programmed cell death protein 1 ligand |
| PD1-R | Programmed cell death protein 1 receptor |
| PDGFR | Platelet-Derived Growth Factor receptor |
| pRb | Retinoblastoma protein |
| PTPLAD1 | Protein Tyrosine Phosphatase-Like A Domain Containing 1 |
| RICs | Regeneration-initiating cells |
| ROCs | Regeneration-organising cells |
| SLC25A40 | Solute Carrier Family 25 Member 40 |
| SPIN1 | Spindlin 1 |
| STAT3 | Signal Transducer and Activator of Transcription 3 |
| TAMs | Tumour-Associated Macrophages |
| TGFβ | Transforming Growth Factor β |
| TME | Tumour Microenvironment |
| TNFa | Tumour Necrosis Factor α |
| TNFb | Tumour Necrosis Factor β |
| Treg | Regulatory T cells |
| TRIM23 | Tripartite Motive Containing 23 |
| VEGF | Vascular Endothelial Growth Factor |
References
- Liu, X.; Gao, X.L.; Liang, X.H.; Tang, Y.L. The Etiologic Spectrum of Head and Neck Squamous Cell Carcinoma in Young Patients. Oncotarget 2016, 7, 66226–66238. [Google Scholar] [CrossRef] [PubMed]
- Barsouk, A.; Aluru, J.S.; Rawla, P.; Saginala, K.; Barsouk, A. Epidemiology, Risk Factors, and Prevention of Head and Neck Squamous Cell Carcinoma. Med. Sci. 2023, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Novotný, J.; Bandúrová, V.; Strnad, H.; Chovanec, M.; Hradilová, M.; Šáchová, J.; Šteffl, M.; Grušanović, J.; Kodet, R.; Pačes, V.; et al. Analysis of HPV-Positive and HPV-Negative Head and Neck Squamous Cell Carcinomas and Paired Normal Mucosae Reveals Cyclin D1 Deregulation and Compensatory Effect of Cyclin D2. Cancers 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Birbrair, A. Tumor Microenvironment; Birbrair, A., Ed.; Springer International Publishing: Cham, Germany, 2020; Volume 1225, ISBN 978-3-030-35726-9. [Google Scholar]
- Landskron, G.; De La Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Gorelik, E.; Galili, U.; Raz, A. On the Role of Cell Surface Carbohydrates and Their Binding Proteins (Lectins) in Tumor Metastasis. Cancer Metastasis Rev. 2001, 20, 245–277. [Google Scholar] [CrossRef]
- Plzák, J.; Smetana, K.; Chovanec, M.; Betka, J. Glycobiology of Head and Neck Squamous Epithelia and Carcinomas. ORL 2005, 67, 61–69. [Google Scholar] [CrossRef]
- Solís, D.; Bovin, N.V.; Davis, A.P.; Jiménez-Barbero, J.; Romero, A.; Roy, R.; Smetana, K.; Gabius, H.J. A Guide into Glycosciences: How Chemistry, Biochemistry and Biology Cooperate to Crack the Sugar Code. Biochim. Biophys. Acta Gen. Subj. 2015, 1850, 186–235. [Google Scholar] [CrossRef]
- Mellman, I.; Chen, D.S.; Powles, T.; Turley, S.J. The Cancer-Immunity Cycle: Indication, Genotype, and Immunotype. Immunity 2023, 56, 2188–2205. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T Cell Exclusion, Immune Privilege, and the Tumor Microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, H.; Liu, C.; Wang, Z.; Wu, W.; Zhang, N.; Zhang, L.; Hu, J.; Luo, P.; Zhang, J.; et al. Immune Checkpoint Modulators in Cancer Immunotherapy: Recent Advances and Emerging Concepts. J. Hematol. Oncol. 2022, 15, 111. [Google Scholar] [CrossRef]
- Marin-Acevedo, J.A.; Dholaria, B.; Soyano, A.E.; Knutson, K.L.; Chumsri, S.; Lou, Y. Next Generation of Immune Checkpoint Therapy in Cancer: New Developments and Challenges. J. Hematol. Oncol. 2018, 11, 39. [Google Scholar] [CrossRef]
- Muzaffar, A.; Tajudin, A.A.; Syahir, A. A Cutting-Edge Solution to a Gordian Knot? Aptamers Targeting Cancer Stem Cell Markers for Strategic Cancer Therapy. Drug Discov. Today 2025, 30, 104365. [Google Scholar] [CrossRef]
- Lacina, L.; Plzak, J.; Kodet, O.; Szabo, P.; Chovanec, M.; Dvorankova, B.; Smetana, K. Cancer Microenvironment: What Can We Learn from the Stem Cell Niche. Int. J. Mol. Sci. 2015, 16, 24094–24110. [Google Scholar] [CrossRef] [PubMed]
- Motlík, J.; Klíma, J.; Dvořánková, B.; Smetana, K. Porcine Epidermal Stem Cells as a Biomedical Model for Wound Healing and Normal/Malignant Epithelial Cell Propagation. Theriogenology 2007, 67, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Čada, Z.; Bouček, J.; Dvořánková, B.; Chovanec, M.; Plzák, J.; Kodet, R.; Betka, J.; Pinot, G.L.; Gabius, H.J.; Smetana, K. Nucleostemin Expression in Squamous Cell Carcinoma of the Head and Neck. Anticancer Res. 2007, 27, 3279–3284. [Google Scholar]
- Fík, Z.; Dvorøánková, B.; Kodet, O.; Bouèek, J.; Betka, J.A.; Betka, J.; André, S.; Gabius, H.J.; Šnajdr, P.; Smetana, K.; et al. Towards Dissecting Molecular Routes of Intercellular Communication in the Tumour Microenvironment: Phenotypic Plasticity of Stem Cell-Associated Markers in Co-Culture (Carcinoma Cell/Fibroblast) Systems. Folia Biol. 2014, 60, 205–212. [Google Scholar] [CrossRef]
- Hamburger, A.W.; Salmon, S.E. Primary Bioassay of Human Tumor Stem Cells. Science 1977, 197, 461–463. [Google Scholar] [CrossRef]
- Smetana, K.; Lacina, L.; Szabo, P.; Dvoánková, B.; Broẑ, P.; Ŝedo, A. Ageing as an Important Risk Factor for Cancer. Anticancer Res. 2016, 36, 5009–5017. [Google Scholar] [CrossRef]
- Lacina, L.; Čoma, M.; Dvořánková, B.; Kodet, O.; Melegová, N.; Gál, P.; Smetana, K. Evolution of Cancer Progression in the Context of Darwinism. Anticancer Res. 2019, 39, 1–16. [Google Scholar] [CrossRef]
- Agudo, J. Immune Privilege of Skin Stem Cells: What Do We Know and What Can We Learn? Exp. Dermatol. 2021, 30, 522–528. [Google Scholar] [CrossRef]
- Agudo, J.; Park, E.S.; Rose, S.A.; Alibo, E.; Sweeney, R.; Dhainaut, M.; Kobayashi, K.S.; Sachidanandam, R.; Baccarini, A.; Merad, M.; et al. Quiescent Tissue Stem Cells Evade Immune Surveillance. Immunity 2018, 48, 271–285.e5. [Google Scholar] [CrossRef]
- Galassi, C.; Musella, M.; Manduca, N.; Maccafeo, E.; Sistigu, A. The Immune Privilege of Cancer Stem Cells: A Key to Understanding Tumor Immune Escape and Therapy Failure. Cells 2021, 10, 2361. [Google Scholar] [CrossRef]
- Rodig, S.J.; Gusenleitner, D.; Jackson, D.G.; Gjini, E.; Giobbie-Hurder, A.; Jin, C.; Chang, H.; Lovitch, S.B.; Horak, C.; Weber, J.S.; et al. MHC Proteins Confer Differential Sensitivity to CTLA-4 and PD-1 Blockade in Untreated Metastatic Melanoma. Sci. Transl. Med. 2018, 10, eaar3342. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, K.; Venida, A.; Yano, J.; Biancur, D.E.; Kakiuchi, M.; Gupta, S.; Sohn, A.S.W.; Mukhopadhyay, S.; Lin, E.Y.; Parker, S.J.; et al. Autophagy Promotes Immune Evasion of Pancreatic Cancer by Degrading MHC-I. Nature 2020, 581, 100–105. [Google Scholar] [CrossRef] [PubMed]
- Miao, Y.; Yang, H.; Levorse, J.; Yuan, S.; Polak, L.; Sribour, M.; Singh, B.; Rosenblum, M.D.; Fuchs, E. Adaptive Immune Resistance Emerges from Tumor-Initiating Stem Cells. Cell 2019, 177, 1172–1186.e14. [Google Scholar] [CrossRef]
- Mani, S.A.; Guo, W.; Liao, M.J.; Eaton, E.N.; Ayyanan, A.; Zhou, A.Y.; Brooks, M.; Reinhard, F.; Zhang, C.C.; Shipitsin, M.; et al. The Epithelial-Mesenchymal Transition Generates Cells with Properties of Stem Cells. Cell 2008, 133, 704–715. [Google Scholar] [CrossRef]
- Tolde, O.; Gandalovičová, A.; Křížová, A.; Veselý, P.; Chmelík, R.; Rosel, D.; Brábek, J. Quantitative Phase Imaging Unravels New Insight into Dynamics of Mesenchymal and Amoeboid Cancer Cell Invasion. Sci. Rep. 2018, 8, 12020. [Google Scholar] [CrossRef]
- Gandalovičová, A.; Vomastek, T.; Rosel, D.; Brábek, J. Cell Polarity Signaling in the Plasticity of Cancer Cell Invasiveness. Oncotarget 2016, 7, 25022–25049. [Google Scholar] [CrossRef]
- Paget, S. THE DISTRIBUTION OF SECONDARY GROWTHS IN CANCER OF THE BREAST. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Kaplan, R.N.; Riba, R.D.; Zacharoulis, S.; Bramley, A.H.; Vincent, L.; Costa, C.; MacDonald, D.D.; Jin, D.K.; Shido, K.; Kerns, S.A.; et al. VEGFR1-Positive Haematopoietic Bone Marrow Progenitors Initiate the Pre-Metastatic Niche. Nature 2005, 438, 820–827. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, J.; Wang, F.; Fang, Y.; Yang, Y.; Zhou, Q.; Yuan, W.; Gu, X.; Hu, J.; Yang, S. Pre-Metastatic Niche: Formation, Characteristics and Therapeutic Implication. Signal Transduct. Target. Ther. 2024, 9, 236. [Google Scholar] [CrossRef]
- Shaw, P.; Dey Bhowmik, A.; Gopinatha Pillai, M.S.; Robbins, N.; Dwivedi, S.K.D.; Rao, G. Anoikis Resistance in Cancer: Mechanisms, Therapeutic Strategies, Potential Targets, and Models for Enhanced Understanding. Cancer Lett. 2025, 624, 217750. [Google Scholar] [CrossRef] [PubMed]
- Dvoránková, B.; Smetana, K.; Chovanec, M.; Lacina, L.; Stork, J.; Plzáková, Z.; Galovicová, M.; Gabius, H.J. Transient Expression of Keratin 19 Is Induced in Originally Negative Interfollicular Epidermal Cells by Adhesion of Suspended Cells. Int. J. Mol. Med. 2005, 16, 525–531. [Google Scholar] [PubMed]
- Gandalovičová, A.; Rosel, D.; Fernandes, M.; Veselý, P.; Heneberg, P.; Čermák, V.; Petruželka, L.; Kumar, S.; Sanz-Moreno, V.; Brábek, J. Migrastatics—Anti-Metastatic and Anti-Invasion Drugs: Promises and Challenges. Trends Cancer 2017, 3, 391–406. [Google Scholar] [CrossRef]
- Dvořánková, B.; Smetana, K.; Říhová, B.; Kučera, J.; Mateu, R.; Szabo, P. Cancer-Associated Fibroblasts Are Not Formed from Cancer Cells by Epithelial-to-Mesenchymal Transition in Nu/Nu Mice. Histochem. Cell Biol. 2015, 143, 463–469. [Google Scholar] [CrossRef]
- Dvořánková, B.; Szabo, P.; Lacina, L.; Kodet, O.; Matouškové, E.; Smetana, K. Fibroblasts Prepared from Different Types of Malignant Tumors Stimulate Expression of Luminal Marker Keratin 8 in the EM-G3 Breast Cancer Cell Line. Histochem. Cell Biol. 2012, 137, 679–685. [Google Scholar] [CrossRef]
- Mastronikolis, N.S.; Delides, A.; Kyrodimos, E.; Piperigkou, Z.; Spyropoulou, D.; Giotakis, E.; Tsiambas, E.; Karamanos, N.K. Insights into Metastatic Roadmap of Head and Neck Cancer Squamous Cell Carcinoma Based on Clinical, Histopathological and Molecular Profiles. Mol. Biol. Rep. 2024, 51, 597. [Google Scholar] [CrossRef]
- Klein, C.A. Cancer Progression and the Invisible Phase of Metastatic Colonization. Nat. Rev. Cancer 2020, 20, 681–694. [Google Scholar] [CrossRef]
- Nobre, A.R.; Dalla, E.; Yang, J.; Huang, X.; Wullkopf, L.; Risson, E.; Razghandi, P.; Anton, M.L.; Zheng, W.; Seoane, J.A.; et al. ZFP281 Drives a Mesenchymal-like Dormancy Program in Early Disseminated Breast Cancer Cells That Prevents Metastatic Outgrowth in the Lung. Nat. Cancer 2022, 3, 1165–1180. [Google Scholar] [CrossRef]
- Laudadio, I.; Bastianelli, A.; Fulci, V.; Carissimi, C.; Colantoni, E.; Palone, F.; Vitali, R.; Lorefice, E.; Cucchiara, S.; Negroni, A.; et al. ZNF281 Promotes Colon Fibroblast Activation in TGFβ1-Induced Gut Fibrosis. Int. J. Mol. Sci. 2022, 23, 10261. [Google Scholar] [CrossRef]
- Baeza-Hernández, G.; Cañueto, J. Intralesional Treatments for Invasive Cutaneous Squamous Cell Carcinoma. Cancers 2024, 16, 158. [Google Scholar] [CrossRef]
- Lippey, J.; Bousounis, R.; Behrenbruch, C.; McKay, B.; Spillane, J.; Henderson, M.A.; Speakman, D.; Gyorki, D.E. Intralesional PV-10 for in-Transit Melanoma—A Single-Center Experience. J. Surg. Oncol. 2016, 114, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Read, T.A.; Smith, A.; Thomas, J.; David, M.; Foote, M.; Wagels, M.; Barbour, A.; Smithers, B.M. Intralesional PV-10 for the Treatment of in-Transit Melanoma Metastases—Results of a Prospective, Non-Randomized, Single Center Study. J. Surg. Oncol. 2018, 117, 579–587. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Du, J.; Song, Q.; Zhang, C.; Wu, X. A Novel In Situ Dendritic Cell Vaccine Triggered by Rose Bengal Enhances Adaptive Antitumour Immunity. J. Immunol. Res. 2022, 2022, 1178874. [Google Scholar] [CrossRef]
- Sztandera, K.; Gorzkiewicz, M.; Bątal, M.; Arkhipova, V.; Knauer, N.; Sánchez-Nieves, J.; de la Mata, F.J.; Gómez, R.; Apartsin, E.; Klajnert-Maculewicz, B. Triazine–Carbosilane Dendrimersomes Enhance Cellular Uptake and Phototoxic Activity of Rose Bengal in Basal Cell Skin Carcinoma Cells. Int. J. Nanomed. 2022, 17, 1139–1154. [Google Scholar] [CrossRef]
- Greig, S.L. Talimogene Laherparepvec: First Global Approval. Drugs 2016, 76, 147–154. [Google Scholar] [CrossRef]
- DePalo, D.K.; Zager, J.S. Advances in Intralesional Therapy for Locoregionally Advanced and Metastatic Melanoma: Five Years of Progress. Cancers 2023, 15, 1404. [Google Scholar] [CrossRef]
- Pannhausen, J.; Wirtz, J.; Mantwill, K.; Holm, P.-S.; Schwamborn, K.; Jonigk, D.D.; Gschwend, J.E.; Rose, M.; Gaisa, N.T.; Nawroth, R. Oncolytic Virotherapy Provides a Potent Therapy Option for Squamous Bladder Cancer. Sci. Rep. 2025, 15, 13443. [Google Scholar] [CrossRef]
- Ogawa, F.; Takaoka, H.; Iwai, S.; Aota, K.; Yura, Y. Combined Oncolytic Virotherapy with Herpes Simplex Virus for Oral Squamous Cell Carcinoma. Anticancer Res. 2008, 28, 3637–3645. [Google Scholar]
- Wu, A.; Li, Z.; Wang, Y.; Chen, Y.; Peng, J.; Zhu, M.; Li, Y.; Song, H.; Zhou, D.; Zhang, C.; et al. Recombinant Measles Virus Vaccine RMV-Hu191 Exerts an Oncolytic Effect on Esophageal Squamous Cell Carcinoma via Caspase-3/GSDME-Mediated Pyroptosis. Cell Death Discov. 2023, 9, 171. [Google Scholar] [CrossRef]
- Coley, W.B. CONTRIBUTION TO THE KNOWLEDGE OF SARCOMA. Ann. Surg. 1891, 14, 199–220. [Google Scholar] [CrossRef]
- Kremenovic, M.; Chan, A.A.; Feng, B.; Bäriswyl, L.; Robatel, S.; Gruber, T.; Tang, L.; Lee, D.J.; Schenk, M. BCG Hydrogel Promotes CTSS-Mediated Antigen Processing and Presentation, Thereby Suppressing Metastasis and Prolonging Survival in Melanoma. J. Immunother. Cancer 2022, 10, e004133. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, L.; Shi, H.; Shen, Z.; Huang, D.; Tang, S.; He, Y.; Wang, G.; Pan, H.; Wang, Z. Thermosensitive Hydrogel Delivery of BCG Lysates and Tumor Antigens: A Novel Strategy for Melanoma Immunoprevention and Therapeutics. Biochem. Biophys. Res. Commun. 2025, 745, 151215. [Google Scholar] [CrossRef] [PubMed]
- Lardone, R.D.; Chan, A.A.; Lee, A.F.; Foshag, L.J.; Faries, M.B.; Sieling, P.A.; Lee, D.J. Mycobacterium Bovis Bacillus Calmette–Guérin Alters Melanoma Microenvironment Favoring Antitumor T Cell Responses and Improving M2 Macrophage Function. Front. Immunol. 2017, 8, 965. [Google Scholar] [CrossRef] [PubMed]
- Craig, D.J.; Nanavaty, N.S.; Devanaboyina, M.; Stanbery, L.; Hamouda, D.; Edelman, G.; Dworkin, L.; Nemunaitis, J.J. The Abscopal Effect of Radiation Therapy. Future Oncol. 2021, 17, 1683–1694. [Google Scholar]
- Rodríguez-Ruiz, M.E.; Vanpouille-Box, C.; Melero, I.; Formenti, S.C.; Demaria, S. Immunological Mechanisms Responsible for Radiation-Induced Abscopal Effect. Trends Immunol. 2018, 39, 644–655. [Google Scholar] [CrossRef]
- Yang, H.; Hu, Y.; Kong, D.; Chen, P.; Yang, L. Intralesional Bacillus Calmette–Guérin Injections and Hypo-Fractionated Radiation Synergistically Induce Systemic Antitumor Immune Responses. Int. Immunopharmacol. 2023, 114, 109542. [Google Scholar] [CrossRef]
- Wang, W.; Xu, H.; Ye, Q.; Tao, F.; Wheeldon, I.; Yuan, A.; Hu, Y.; Wu, J. Systemic Immune Responses to Irradiated Tumours via the Transport of Antigens to the Tumour Periphery by Injected Flagellate Bacteria. Nat. Biomed. Eng. 2022, 6, 44–53. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Guo, Z.-Q.; Cai, X.-T.; Rong, Z.-X.; Fang, Y.; Chen, J.-Q.; Zhuang, K.-M.; Ruan, M.-J.; Ma, S.-C.; Lin, L.-Y.; et al. PAI-1-Driven SFRP2high Cancer-Associated Fibroblasts Hijack the Abscopal Effect of Radioimmunotherapy. Cancer Cell 2025, 43, 856–874.e9. [Google Scholar] [CrossRef]
- Vidovic, D.; Helyer, L.K.; Pasternak, S.; Giacomantonio, C.A. Abscopal Responses in Patients with Metastatic Melanoma Involving Skin and Subcutaneous Tissues Treated with Intralesional IL2 plus BCG. Front. Oncol. 2023, 13, 1160269. [Google Scholar] [CrossRef]
- Plzák, J.; Lacina, L.; Chovanec, M.; Dvořánková, B.; Szabo, P.; Čada, Z.; Smetana, K. Epithelial-Stromal Interaction in Squamous Cell Epithelium-Derived Tumors: An Important New Player in the Control of Tumor Biological Properties. Anticancer Res. 2010, 30, 455–462. [Google Scholar]
- Valach, J.; Fík, Z.; Strnad, H.; Chovanec, M.; Plzák, J.; Čada, Z.; Szabo, P.; Šáchová, J.; Hroudová, M.; Urbanová, M.; et al. Smooth Muscle Actin-Expressing Stromal Fibroblasts in Head and Neck Squamous Cell Carcinoma: Increased Expression of Galectin-1 and Induction of Poor Prognosis Factors. Int. J. Cancer 2012, 131, 2499–2508. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Li, B. Interactions between Cancer Cells and Tumor-Associated Macrophages in Tumor Microenvironment. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2025, 1880, 189344. [Google Scholar] [CrossRef]
- Kurt, F.G.O.; Lasser, S.; Arkhypov, I.; Utikal, J.; Umansky, V. Enhancing Immunotherapy Response in Melanoma: Myeloid-Derived Suppressor Cells as a Therapeutic Target. J. Clin. Investig. 2023, 133, e170762. [Google Scholar] [CrossRef]
- von der Grün, J.; Rödel, F.; Brandts, C.; Fokas, E.; Guckenberger, M.; Rödel, C.; Balermpas, P. Targeted Therapies and Immune-Checkpoint Inhibition in Head and Neck Squamous Cell Carcinoma: Where Do We Stand Today and Where to Go? Cancers 2019, 11, 472. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.; Li, Y.; Zhao, W.; Wu, J.B.; Zhang, Z. PD-1/PD-L1 Checkpoint Inhibitors in Tumor Immunotherapy. Front. Pharmacol. 2021, 12, 731798. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Song, Y.; Min, Q.; Cheng, W.; Wang, J.; Fu, Y.; Yin, J. The Administration Sequences of Immune Checkpoint Inhibitors and Chemotherapy Cause Discrete Efficacy When Treating Non-Small Cell Lung Cancer: A Retrospective Study. Front. Immunol. 2025, 16, 1579420. [Google Scholar] [CrossRef] [PubMed]
- Carter, S.; Wigmore, T. Immunotherapy on ICU: A Narrative Review. Anaesthesia 2025, 80, 299–310. [Google Scholar] [CrossRef] [PubMed]
- Curry, J.M.; Sprandio, J.; Cognetti, D.; Luginbuhl, A.; Bar-Ad, V.; Pribitkin, E.; Tuluc, M. Tumor Microenvironment in Head and Neck Squamous Cell Carcinoma. Semin. Oncol. 2014, 41, 217–234. [Google Scholar] [CrossRef]
- Španko, M.; Strnadová, K.; Pavlíček, A.J.; Szabo, P.; Kodet, O.; Valach, J.; Dvořánková, B.; Smetana, K.; Lacina, L. Il-6 in the Ecosystem of Head and Neck Cancer: Possible Therapeutic Perspectives. Int. J. Mol. Sci. 2021, 22, 11027. [Google Scholar] [CrossRef]
- Wang, L.; Geng, H.; Liu, Y.; Liu, L.; Chen, Y.; Wu, F.; Liu, Z.; Ling, S.; Wang, Y.; Zhou, L. Hot and Cold Tumors: Immunological Features and the Therapeutic Strategies. MedComm 2023, 4, e343. [Google Scholar] [CrossRef]
- Tanaka, M.; Lum, L.; Hu, K.H.; Chaudhary, P.; Hughes, S.; Ledezma-Soto, C.; Samad, B.; Superville, D.; Ng, K.; Chumber, A.; et al. Tumor Cell Heterogeneity Drives Spatial Organization of the Intratumoral Immune Response. J. Exp. Med. 2025, 222, e20242282. [Google Scholar] [CrossRef]
- Talhouni, S.; Fadhil, W.; Mongan, N.P.; Field, L.; Hunter, K.; Makhsous, S.; Maciel-Guerra, A.; Kaur, N.; Nestarenkaite, A.; Laurinavicius, A.; et al. Activated Tissue Resident Memory T-Cells (CD8+CD103+CD39+) Uniquely Predict Survival in Left Sided “Immune-Hot” Colorectal Cancers. Front. Immunol. 2023, 14, 1057292. [Google Scholar] [CrossRef]
- Ren, S.; Lan, T.; Wu, F.; Chen, S.; Jiang, X.; Huo, C.; Li, Z.; Xie, S.; Wu, D.; Wang, R.; et al. Intratumoral CD103+ CD8+ T Cells Predict Response to Neoadjuvant Chemoimmunotherapy in Advanced Head and Neck Squamous Cell Carcinoma. Cancer Commun. 2023, 43, 1143–1163. [Google Scholar] [CrossRef]
- Patel, R.; Saab, K.; Luo, L.; Ma, Y.; Osman, R.A.; Williams, N.T.; Everitt, J.; Zelazowski, M.J.; Castro, P.; Decker, W.K.; et al. Nrf2 Hyperactivation as a Driver of Radiotherapy Resistance and Suppressed Antitumor Immunity in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2025; ahead of print. [Google Scholar] [CrossRef]
- Guan, L.; Nambiar, D.K.; Cao, H.; Viswanathan, V.; Kwok, S.; Hui, A.B.; Hou, Y.; Hildebrand, R.; von Eyben, R.; Holmes, B.J.; et al. NFE2L2 Mutations Enhance Radioresistance in Head and Neck Cancer by Modulating Intratumoral Myeloid Cells. Cancer Res. 2023, 83, 861–874. [Google Scholar] [CrossRef]
- Cederkvist, H.; Kolan, S.S.; Wik, J.A.; Sener, Z.; Skålhegg, B.S. Identification and Characterization of a Novel Glutaminase Inhibitor. FEBS Open Bio 2022, 12, 163–174. [Google Scholar] [CrossRef]
- Grünwald, B.T.; Devisme, A.; Andrieux, G.; Vyas, F.; Aliar, K.; McCloskey, C.W.; Macklin, A.; Jang, G.H.; Denroche, R.; Romero, J.M.; et al. Spatially Confined Sub-Tumor Microenvironments in Pancreatic Cancer. Cell 2021, 184, 5577–5592.e18. [Google Scholar] [CrossRef]
- Ma, C.; Yang, C.; Peng, A.; Sun, T.; Ji, X.; Mi, J.; Wei, L.; Shen, S.; Feng, Q. Pan-Cancer Spatially Resolved Single-Cell Analysis Reveals the Crosstalk between Cancer-Associated Fibroblasts and Tumor Microenvironment. Mol. Cancer 2023, 22, 170. [Google Scholar] [CrossRef]
- Pfeiferová, L.; Španko, M.; Šáchová, J.; Hradilová, M.; Pienta, K.J.; Valach, J.; Machoň, V.; Výmolová, B.; Šedo, A.; Bušek, P.; et al. The HOX Code of Human Adult Fibroblasts Reflects Their Ectomesenchymal or Mesodermal Origin. Histochem Cell Biol 2025, 163, 38. [Google Scholar] [CrossRef]
- Ciszewski, W.M.; Wawro, M.E.; Sacewicz-Hofman, I.; Sobierajska, K. Cytoskeleton Reorganization in EndMT—The Role in Cancer and Fibrotic Diseases. Int. J. Mol. Sci. 2021, 22, 11607. [Google Scholar] [CrossRef]
- Jia, H.; Chen, X.; Zhang, L.; Chen, M. Cancer Associated Fibroblasts in Cancer Development and Therapy. J. Hematol. Oncol. 2025, 18, 36. [Google Scholar] [CrossRef]
- Farrington-Rock, C.; Crofts, N.J.; Doherty, M.J.; Ashton, B.A.; Griffin-Jones, C.; Canfield, A.E. Chondrogenic and Adipogenic Potential of Microvascular Pericytes. Circulation 2004, 110, 2226–2232. [Google Scholar] [CrossRef]
- Lin, S.-L.; Kisseleva, T.; Brenner, D.A.; Duffield, J.S. Pericytes and Perivascular Fibroblasts Are the Primary Source of Collagen-Producing Cells in Obstructive Fibrosis of the Kidney. Am. J. Pathol. 2008, 173, 1617–1627. [Google Scholar] [CrossRef]
- Ning, X.; Zhang, H.; Wang, C.; Song, X. Exosomes Released by Gastric Cancer Cells Induce Transition of Pericytes Into Cancer-Associated Fibroblasts. Med. Sci. Monit. 2018, 24, 2350–2359. [Google Scholar] [CrossRef]
- Xu, Y.; Kovacic, J.C. Endothelial to Mesenchymal Transition in Health and Disease. Annu. Rev. Physiol. 2023, 85, 245–267. [Google Scholar] [CrossRef]
- Di Benedetto, P.; Ruscitti, P.; Berardicurti, O.; Vomero, M.; Navarini, L.; Dolo, V.; Cipriani, P.; Giacomelli, R. Endothelial-to-Mesenchymal Transition in Systemic Sclerosis. Clin. Exp. Immunol. 2021, 205, 12–27. [Google Scholar] [CrossRef]
- Schwartz, M.A.; Vestweber, D.; Simons, M. A Unifying Concept in Vascular Health and Disease. Science 2018, 360, 270–271. [Google Scholar] [CrossRef]
- Zhu, X.; Wang, Y.; Soaita, I.; Lee, H.-W.; Bae, H.; Boutagy, N.; Bostwick, A.; Zhang, R.-M.; Bowman, C.; Xu, Y.; et al. Acetate Controls Endothelial-to-Mesenchymal Transition. Cell Metab. 2023, 35, 1163–1178.e10. [Google Scholar] [CrossRef]
- O’Fee, K.; Burley, A.; Stewart, S.; Wilkins, A. Targeting Cancer-Associated Fibroblasts (CAFs) to Optimize Radiation Responses. Cancer J. 2025, 31, e0776. [Google Scholar] [CrossRef]
- Wei, W.-F.; Zhou, H.-L.; Chen, P.-Y.; Huang, X.-L.; Huang, L.; Liang, L.-J.; Guo, C.-H.; Zhou, C.-F.; Yu, L.; Fan, L.-S.; et al. Cancer-Associated Fibroblast-Derived PAI-1 Promotes Lymphatic Metastasis via the Induction of EndoMT in Lymphatic Endothelial Cells. J. Exp. Clin. Cancer Res. 2023, 42, 160. [Google Scholar] [CrossRef]
- Szabó, P.; Kolář, M.; Dvořánková, B.; Lacina, L.; Štork, J.; Vlček, Č.; Strnad, H.; Tvrdek, M.; Smetana, K. Mouse 3T3 Fibroblasts under the Influence of Fibroblasts Isolated from Stroma of Human Basal Cell Carcinoma Acquire Properties of Multipotent Stem Cells. Biol. Cell 2011, 103, 233–248. [Google Scholar] [CrossRef]
- Petersen, O.W.; Nielsen, H.L.; Gudjonsson, T.; Villadsen, R.; Rank, F.; Niebuhr, E.; Bissell, M.J.; Rønnov-Jessen, L. Epithelial to Mesenchymal Transition in Human Breast Cancer Can Provide a Nonmalignant Stroma. Am. J. Pathol. 2003, 162, 391–402. [Google Scholar] [CrossRef]
- Smetana, K.; Dvoránková, B.; Lacina, L.; Cada, Z.; Vonka, V. Human Hair Follicle and Interfollicular Keratinocyte Reactivity to Mouse HPV16-Transformed Cells: An in Vitro Study. Oncol. Rep. 2008, 20, 75–80. [Google Scholar] [CrossRef]
- Dvorankova, B.; Szabo, P.; Lacina, L.; Gal, P.; Uhrova, J.; Zima, T.; Kaltner, H.; André, S.; Gabius, H.J.; Sykova, E.; et al. Human Galectins Induce Conversion of Dermal Fibroblasts into Myofibroblasts and Production of Extracellular Matrix: Potential Application in Tissue Engineering and Wound Repair. Cells Tissues Organs 2011, 194, 469–480. [Google Scholar] [CrossRef]
- Novotný, J.; Strnadová, K.; Dvořánková, B.; Kocourková, Š.; Jakša, R.; Dundr, P.; Pačes, V.; Smetana, K.; Kolář, M.; Lacina, L. Single-Cell RNA Sequencing Unravels Heterogeneity of the Stromal Niche in Cutaneous Melanoma Heterogeneous Spheroids. Cancers 2020, 12, 3324. [Google Scholar] [CrossRef]
- Chen, B.; Chan, W.N.; Xie, F.; Mui, C.W.; Liu, X.; Cheung, A.H.K.; Lung, R.W.M.; Chow, C.; Zhang, Z.; Fang, C.; et al. The Molecular Classification of Cancer-associated Fibroblasts on a Pan-cancer Single-cell Transcriptional Atlas. Clin. Transl. Med. 2023, 13, e1516. [Google Scholar] [CrossRef]
- Wan, Y.; Hu, Q.; Sun, K.; Shi, J.; Liu, L.; Zhang, X.; Huang, J.; Gong, C.; Liu, J.; Wang, H.; et al. Heterogenous Cancer-Associated Fibroblasts Related Tumor Microenvironment Marked by CD10/KLF4/TIAM1 Were Identified in Pancreatic Adenocarcinoma by Integrated Transcriptomics. Front. Immunol. 2025, 16, 1557698. [Google Scholar] [CrossRef]
- Szabo, P.; Valach, J.; Smetana, K.; Dvoránková, B. Comparative Analysis of IL-8 and CXCL-1 Production by Normal and Cancer Stromal Fibroblasts. Folia Biol. 2013, 59, 134–137. [Google Scholar] [CrossRef]
- Jiang, L.; Gonda, T.A.; Gamble, M.V.; Salas, M.; Seshan, V.; Tu, S.; Twaddell, W.S.; Hegyi, P.; Lazar, G.; Steele, I.; et al. Global Hypomethylation of Genomic DNA in Cancer-Associated Myofibroblasts. Cancer Res. 2008, 68, 9900–9908. [Google Scholar] [CrossRef]
- Trylcova, J.; Busek, P.; Smetana, K.; Balaziova, E.; Dvorankova, B.; Mifkova, A.; Sedo, A. Effect of Cancer-Associated Fibroblasts on the Migration of Glioma Cells in Vitro. Tumor Biol. 2015, 36, 5873–5879. [Google Scholar] [CrossRef]
- Berndt, A.; Richter, P.; Kosmehl, H.; Franz, M. Tenascin-C and Carcinoma Cell Invasion in Oral and Urinary Bladder Cancer. Cell Adh. Migr. 2015, 9, 105–111. [Google Scholar] [CrossRef]
- Zivicova, V.; Gal, P.; Mifkova, A.; Novak, S.; Kaltner, H.; Kolar, M.; Strnad, H.; Sachova, J.; Hradilova, M.; Chovanec, M.; et al. Detection of Distinct Changes in Gene-Expression Profiles in Specimens of Tumors and Transition Zones of Tenascin-Positive/-Negative Head and Neck Squamous Cell Carcinoma. Anticancer Res. 2018, 38, 1279–1290. [Google Scholar] [CrossRef]
- Novák, Š.; Kolář, M.; Szabó, A.; Vernerová, Z.; Lacina, L.; Strnad, H.; Šáchová, J.; Hradilová, M.; Havránek, J.; Španko, M.; et al. Desmoplastic Crosstalk in Pancreatic Ductal Adenocarcinoma Is Reflected by Different Responses of Panc-1, MIAPaCa-2, PaTu-8902, and CAPAN-2 Cell Lines to Cancer-Associated/Normal Fibroblasts. Cancer Genom. Proteom. 2021, 18, 221–243. [Google Scholar] [CrossRef]
- Anastasia, A.; Formenti, L.; Ostano, P.; Minoli, L.; Resovi, A.; Morosi, L.; Fioravanti, C.; Micotti, E.; Matteo, C.; Scanziani, E.; et al. Stroma Gene Signature Predicts Responsiveness to Chemotherapy in Pancreatic Ductal Adenocarcinoma Patient-derived Xenograft Models. Mol. Oncol. 2025, 19, 1075–1091. [Google Scholar] [CrossRef]
- Bates, M.E.; Libring, S.; Reinhart-King, C.A. Forces Exerted and Transduced by Cancer-Associated Fibroblasts during Cancer Progression. Biol. Cell 2023, 115, e2200104. [Google Scholar] [CrossRef]
- Saint, A.; Van Obberghen-Schilling, E. The Role of the Tumor Matrix Environment in Progression of Head and Neck Cancer. Curr. Opin. Oncol. 2021, 33, 168–174. [Google Scholar] [CrossRef]
- Maller, O.; Drain, A.P.; Barrett, A.S.; Borgquist, S.; Ruffell, B.; Zakharevich, I.; Pham, T.T.; Gruosso, T.; Kuasne, H.; Lakins, J.N.; et al. Tumour-Associated Macrophages Drive Stromal Cell-Dependent Collagen Crosslinking and Stiffening to Promote Breast Cancer Aggression. Nat. Mater. 2021, 20, 548–559. [Google Scholar] [CrossRef]
- Miéville, A.; Fonta, C.M.; Leo, C.; Christe, L.; Goldhahn, J.; Singer, G.; Vogel, V. Fibronectin Fibers Progressively Lose Their Tension in Invasive Human Breast Carcinoma While Being Tensed in DCIS and Healthy Breast Tissue. Adv. Sci. 2025, 12, e04351. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen Reorganization at the Tumor-Stromal Interface Facilitates Local Invasion. BMC Med. 2006, 4, 38. [Google Scholar] [CrossRef]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human Breast Cancer Invasion and Aggression Correlates with ECM Stiffening and Immune Cell Infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar] [CrossRef] [PubMed]
- Gordon-Weeks, A.; Yuzhalin, A.E. Cancer Extracellular Matrix Proteins Regulate Tumour Immunity. Cancers 2020, 12, 3331. [Google Scholar] [CrossRef] [PubMed]
- Seclì, L.; Fusella, F.; Avalle, L.; Brancaccio, M. The Dark-Side of the Outside: How Extracellular Heat Shock Proteins Promote Cancer. Cell. Mol. Life Sci. 2021, 78, 4069–4083. [Google Scholar] [CrossRef]
- Jürgensen, H.J.; van Putten, S.; Nørregaard, K.S.; Bugge, T.H.; Engelholm, L.H.; Behrendt, N.; Madsen, D.H. Cellular Uptake of Collagens and Implications for Immune Cell Regulation in Disease. Cell. Mol. Life Sci. 2020, 77, 3161–3176. [Google Scholar] [CrossRef]
- Lieverse, R.I.Y.; Marcus, D.; van der Wiel, A.M.A.; Van Limbergen, E.J.; Theys, J.; Yaromina, A.; Lambin, P.; Dubois, L.J. Human Fibronectin Extra Domain B as a Biomarker for Targeted Therapy in Cancer. Mol. Oncol. 2020, 14, 1555–1568. [Google Scholar] [CrossRef]
- Lin, T.-C.; Yang, C.-H.; Cheng, L.-H.; Chang, W.-T.; Lin, Y.-R.; Cheng, H.-C. Fibronectin in Cancer: Friend or Foe. Cells 2019, 9, 27. [Google Scholar] [CrossRef]
- Wahbi, W.; Naakka, E.; Tuomainen, K.; Suleymanova, I.; Arpalahti, A.; Miinalainen, I.; Vaananen, J.; Grenman, R.; Monni, O.; Al-Samadi, A.; et al. The Critical Effects of Matrices on Cultured Carcinoma Cells: Human Tumor-Derived Matrix Promotes Cell Invasive Properties. Exp. Cell Res. 2020, 389, 111885. [Google Scholar] [CrossRef]
- Barker, T.H.; Engler, A.J. The Provisional Matrix: Setting the Stage for Tissue Repair Outcomes. Matrix Biol. 2017, 60–61, 1–4. [Google Scholar] [CrossRef]
- Gopal, S.; Veracini, L.; Grall, D.; Butori, C.; Schaub, S.; Audebert, S.; Camoin, L.; Baudelet, E.; Adwanska, A.; Beghelli-De La Forest Divonne, S.; et al. Fibronectin-Guided Migration of Carcinoma Collectives. Nat. Commun. 2017, 8, 14105. [Google Scholar] [CrossRef]
- Rick, J.W.; Chandra, A.; Dalle Ore, C.; Nguyen, A.T.; Yagnik, G.; Aghi, M.K. Fibronectin in Malignancy: Cancer-Specific Alterations, Protumoral Effects, and Therapeutic Implications. Semin. Oncol. 2019, 46, 284–290. [Google Scholar] [CrossRef]
- da Costa Santos, M.A.R.; dos Reis, J.S.; do Nascimento Santos, C.A.; da Costa, K.M.; Barcelos, P.M.; de Oliveira Francisco, K.Q.; Barbosa, P.A.G.N.; da Silva, E.D.S.; Freire-de-Lima, C.G.; Morrot, A.; et al. Expression of O-Glycosylated Oncofetal Fibronectin in Alternatively Activated Human Macrophages. Immunol. Res. 2023, 71, 92–104, Correction in Immunol. Res. 2023, 71, 105–106. [Google Scholar] [CrossRef]
- Dos Reis, J.S.; da Costa Santos, M.A.R.; da Costa, K.M.; Freire-de-Lima, C.G.; Morrot, A.; Previato, J.O.; Previato, L.M.; da Fonseca, L.M.; Freire-de-Lima, L. Increased Expression of the Pathological O-Glycosylated Form of Oncofetal Fibronectin in the Multidrug Resistance Phenotype of Cancer Cells. Matrix Biol. 2023, 118, 47–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, G.; Liu, H. Tenascin-C: A Key Regulator in Angiogenesis during Wound Healing. Biomolecules 2022, 12, 1689. [Google Scholar] [CrossRef] [PubMed]
- Cai, X.; Han, M.; Lou, F.; Sun, Y.; Yin, Q.; Sun, L.; Wang, Z.; Li, X.; Zhou, H.; Xu, Z.; et al. Tenascin C+ Papillary Fibroblasts Facilitate Neuro-Immune Interaction in a Mouse Model of Psoriasis. Nat. Commun. 2023, 14, 2004. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Midwood, K.S.; Varga, J. Tenascin-C in Fibrosis in Multiple Organs: Translational Implications. Semin. Cell Dev. Biol. 2022, 128, 130–136. [Google Scholar] [CrossRef] [PubMed]
- De Gregorio, C.; Ramos-Gonzalez, G.; Morales-Catalán, B.; Ezquer, F.; Ezquer, M. Paw Skin as a Translational Model for Investigating Fibrotic and Inflammatory Wound Healing Defects in Recessive Dystrophic Epidermolysis Bullosa. Int. J. Mol. Sci. 2025, 26, 4281. [Google Scholar] [CrossRef]
- Bonamonte, D.; Filoni, A.; De Marco, A.; Lospalluti, L.; Nacchiero, E.; Ronghi, V.; Colagrande, A.; Giudice, G.; Cazzato, G. Squamous Cell Carcinoma in Patients with Inherited Epidermolysis Bullosa: Review of Current Literature. Cells 2022, 11, 1365. [Google Scholar] [CrossRef]
- Spenle, C.; Loustau, T.; Murdamoothoo, D.; Erne, W.; Beghelli-De la Forest Divonne, S.; Veber, R.; Petti, L.; Bourdely, P.; Morgelin, M.; Brauchle, E.M.; et al. Tenascin-C Orchestrates an Immune-Suppressive Tumor Microenvironment in Oral Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 1122–1138. [Google Scholar] [CrossRef]
- Sanjurjo, L.; Schulkens, I.A.; Touarin, P.; Heusschen, R.; Aanhane, E.; Castricum, K.C.M.; De Gruijl, T.D.; Nilsson, U.J.; Leffler, H.; Griffioen, A.W.; et al. Chemokines Modulate Glycan Binding and the Immunoregulatory Activity of Galectins. Commun. Biol. 2021, 4, 1415. [Google Scholar] [CrossRef]
- Dong, S.; Zhang, S.; Zhao, P.; Lin, G.; Ma, X.; Xu, J.; Zhang, H.; Hu, J.; Zou, C. A Combined Analysis of Bulk and Single-Cell Sequencing Data Reveals That Depleted Extracellular Matrix and Enhanced Immune Processes Co-Contribute to Fluorouracil Beneficial Responses in Gastric Cancer. Front. Immunol. 2022, 13, 999551. [Google Scholar] [CrossRef]
- Raudenska, M.; Balvan, J.; Hanelova, K.; Bugajova, M.; Masarik, M. Cancer-Associated Fibroblasts: Mediators of Head and Neck Tumor Microenvironment Remodeling. Biochim. Biophys. Acta Rev. Cancer 2023, 1878, 188940. [Google Scholar] [CrossRef] [PubMed]
- Cumming, J.; Maneshi, P.; Dongre, M.; Alsaed, T.; Dehghan-Nayeri, M.J.; Ling, A.; Pietras, K.; Patthey, C.; Öhlund, D. Dissecting FAP+ Cell Diversity in Pancreatic Cancer Uncovers an Interferon-Response Subtype of Cancer-Associated Fibroblasts with Tumor-Restraining Properties. Cancer Res. 2025, 85, 2388–2411. [Google Scholar] [CrossRef] [PubMed]
- Kieffer, Y.; Hocine, H.R.; Gentric, G.; Pelon, F.; Bernard, C.; Bourachot, B.; Lameiras, S.; Albergante, L.; Bonneau, C.; Guyard, A.; et al. Single-Cell Analysis Reveals Fibroblast Clusters Linked to Immunotherapy Resistance in Cancer. Cancer Discov. 2020, 10, 1330–1351. [Google Scholar] [CrossRef] [PubMed]
- Puram, S.V.; Tirosh, I.; Parikh, A.S.; Patel, A.P.; Yizhak, K.; Gillespie, S.; Rodman, C.; Luo, C.L.; Mroz, E.A.; Emerick, K.S.; et al. Single-Cell Transcriptomic Analysis of Primary and Metastatic Tumor Ecosystems in Head and Neck Cancer. Cell 2017, 171, 1611–1624.e24. [Google Scholar] [CrossRef]
- Pradhan, R.N.; Krishnamurty, A.T.; Fletcher, A.L.; Turley, S.J.; Müller, S. A Bird’s Eye View of Fibroblast Heterogeneity: A Pan-Disease, Pan-Cancer Perspective. Immunol. Rev. 2021, 302, 299–320. [Google Scholar] [CrossRef]
- Jenkins, L.; Jungwirth, U.; Avgustinova, A.; Iravani, M.; Mills, A.; Haider, S.; Harper, J.; Isacke, C.M. Cancer-Associated Fibroblasts Suppress CD8+ T-Cell Infiltration and Confer Resistance to Immune-Checkpoint Blockade. Cancer Res. 2022, 82, 2904–2917. [Google Scholar] [CrossRef]
- Hou, W. Role of TGFβ-Activated Cancer-Associated Fibroblasts in the Resistance to Checkpoint Blockade Immunotherapy. Front. Oncol. 2025, 15, 1602452. [Google Scholar] [CrossRef]
- Yun, Z.; Giaccia, A.J. Tumor Deprivation of Oxygen and Tumor Suppressor Gene Function. In Tumor Suppressor Genes; Humana Press: Totowa, NJ, USA, 2003; pp. 485–504. [Google Scholar]
- Le, Q.T.; Shi, G.; Cao, H.; Nelson, D.W.; Wang, Y.; Chen, E.Y.; Zhao, S.; Kong, C.; Richardson, D.; O’Byrne, K.J.; et al. Galectin-1: A Link between Tumor Hypoxia and Tumor Immune Privilege. J. Clin. Oncol. 2005, 23, 8932–8941. [Google Scholar] [CrossRef]
- Brizel, D.M.; Dodge, R.K.; Clough, R.W.; Dewhirst, M.W. Oxygenation of Head and Neck Cancer: Changes during Radiotherapy and Impact on Treatment Outcome. Radiother. Oncol. 1999, 53, 113–117. [Google Scholar] [CrossRef]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The Role of Tumor Stroma in Cancer Progression and Prognosis: Emphasis on Carcinoma-Associated Fibroblasts and Non-Small Cell Lung Cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef]
- Comito, G.; Giannoni, E.; Segura, C.P.; Barcellos-De-Souza, P.; Raspollini, M.R.; Baroni, G.; Lanciotti, M.; Serni, S.; Chiarugi, P. Cancer-Associated Fibroblasts and M2-Polarized Macrophages Synergize during Prostate Carcinoma Progression. Oncogene 2014, 33, 2423–2431. [Google Scholar] [CrossRef]
- Krishnamurthy, S.; Warner, K.A.; Dong, Z.; Imai, A.; Nör, C.; Ward, B.B.; Helman, J.I.; Taichman, R.S.; Bellile, E.L.; McCauley, L.K.; et al. Endothelial Interleukin-6 Defines the Tumorigenic Potential of Primary Human Cancer Stem Cells. Stem Cells 2014, 32, 2845–2857. [Google Scholar] [CrossRef]
- Sattiraju, A.; Kang, S.; Giotti, B.; Chen, Z.; Marallano, V.J.; Brusco, C.; Ramakrishnan, A.; Shen, L.; Tsankov, A.M.; Hambardzumyan, D.; et al. Hypoxic Niches Attract and Sequester Tumor-Associated Macrophages and Cytotoxic T Cells and Reprogram Them for Immunosuppression. Immunity 2023, 56, 1825–1843.e6. [Google Scholar] [CrossRef] [PubMed]
- Seymour, L.W.; Ulbrich, K.; Steyger, P.S.; Brereton, M.; Subr, V.; Strohalm, J.; Duncan, R. Tumour Tropism and Anti-Cancer Efficacy of Polymer-Based Doxorubicin Prodrugs in the Treatment of Subcutaneous Murine B16F10 Melanoma. Br. J. Cancer 1994, 70, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Karras, P.; Bordeu, I.; Pozniak, J.; Nowosad, A.; Pazzi, C.; Van Raemdonck, N.; Landeloos, E.; Van Herck, Y.; Pedri, D.; Bervoets, G.; et al. A Cellular Hierarchy in Melanoma Uncouples Growth and Metastasis. Nature 2022, 610, 190–198, Correction in Nature 2022, 611, E4. [Google Scholar] [CrossRef] [PubMed]
- Anstee, J.E.; Feehan, K.T.; Opzoomer, J.W.; Dean, I.; Muller, H.P.; Bahri, M.; Cheung, T.S.; Liakath-Ali, K.; Liu, Z.; Choy, D.; et al. LYVE-1+ Macrophages Form a Collaborative CCR5-Dependent Perivascular Niche That Influences Chemotherapy Responses in Murine Breast Cancer. Dev. Cell 2023, 58, 1548–1561.e10. [Google Scholar] [CrossRef]
- Verginadis, I.I.; Avgousti, H.; Monslow, J.; Skoufos, G.; Chinga, F.; Kim, K.; Leli, N.M.; Karagounis, I.V.; Bell, B.I.; Velalopoulou, A.; et al. A Stromal Integrated Stress Response Activates Perivascular Cancer-Associated Fibroblasts to Drive Angiogenesis and Tumour Progression. Nat. Cell Biol. 2022, 24, 940–953. [Google Scholar] [CrossRef]
- Xiao, Z.; Todd, L.; Huang, L.; Noguera-Ortega, E.; Lu, Z.; Huang, L.; Kopp, M.; Li, Y.; Pattada, N.; Zhong, W.; et al. Desmoplastic Stroma Restricts T Cell Extravasation and Mediates Immune Exclusion and Immunosuppression in Solid Tumors. Nat. Commun. 2023, 14, 5110. [Google Scholar] [CrossRef]
- Puzianowska-Kuźnicka, M.; Owczarz, M.; Wieczorowska-Tobis, K.; Nadrowski, P.; Chudek, J.; Slusarczyk, P.; Skalska, A.; Jonas, M.; Franek, E.; Mossakowska, M. Interleukin-6 and C-Reactive Protein, Successful Aging, and Mortality: The PolSenior Study. Immun. Ageing 2016, 13, 21. [Google Scholar] [CrossRef]
- Wolf, J.; Rose-John, S.; Garbers, C. Interleukin-6 and Its Receptors: A Highly Regulated and Dynamic System. Cytokine 2014, 70, 11–20. [Google Scholar] [CrossRef]
- Neurath, L.; Sticherling, M.; Schett, G.; Fagni, F. Targeting Cytokines in Psoriatic Arthritis. Cytokine Growth Factor Rev. 2024, 78, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Rose-John, S.; Jenkins, B.J.; Garbers, C.; Moll, J.M.; Scheller, J. Targeting IL-6 Trans-Signalling: Past, Present and Future Prospects. Nat. Rev. Immunol. 2023, 23, 666–681. [Google Scholar] [CrossRef] [PubMed]
- Thuya, W.L.; Cao, Y.; Ho, P.C.-L.; Wong, A.L.-A.; Wang, L.; Zhou, J.; Nicot, C.; Goh, B.C. Insights into IL-6/JAK/STAT3 Signaling in the Tumor Microenvironment: Implications for Cancer Therapy. Cytokine Growth Factor Rev. 2025, ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Schaefer, E.J.; Dulipsingh, L.; Comite, F.; Jimison, J.; Grajower, M.M.; Lebowitz, N.E.; Lang, M.; Geller, A.S.; Diffenderfer, M.R.; He, L.; et al. Corona Virus Disease-19 Serology, Inflammatory Markers, Hospitalizations, Case Finding, and Aging. PLoS ONE 2021, 16, e0252818. [Google Scholar] [CrossRef]
- Gál, P.; Brábek, J.; Holub, M.; Jakubek, M.; Šedo, A.; Lacina, L.; Strnadová, K.; Dubový, P.; Hornychová, H.; Ryška, A.; et al. Autoimmunity, Cancer and COVID-19 Abnormally Activate Wound Healing Pathways: Critical Role of Inflammation. Histochem. Cell Biol. 2022, 158, 415–434. [Google Scholar] [CrossRef]
- Virtanen, A.; Spinelli, F.R.; Telliez, J.B.; O’Shea, J.J.; Silvennoinen, O.; Gadina, M. JAK Inhibitor Selectivity: New Opportunities, Better Drugs? Nat. Rev. Rheumatol. 2024, 20, 649–665. [Google Scholar] [CrossRef]
- Flynn, C.M.; Kespohl, B.; Daunke, T.; Garbers, Y.; Düsterhöft, S.; Rose-John, S.; Haybaeck, J.; Lokau, J.; Aparicio-Siegmund, S.; Garbers, C. Interleukin-6 Controls Recycling and Degradation, but Not Internalization of Its Receptors. J. Biol. Chem. 2021, 296, 100434. [Google Scholar] [CrossRef]
- Locke, F.L.; Neelapu, S.S.; Bartlett, N.L.; Lekakis, L.J.; Jacobson, C.A.; Braunschweig, I.; Oluwole, O.O.; Siddiqi, T.; Lin, Y.; Timmerman, J.M.; et al. Tocilizumab Prophylaxis Following Axicabtagene Ciloleucel in Relapsed or Refractory Large B-Cell Lymphoma. Transpl. Cell Ther. 2024, 30, 1065–1079. [Google Scholar] [CrossRef]
- Groza, Y.; Lacina, L.; Kuchař, M.; Rašková Kafková, L.; Zachová, K.; Janoušková, O.; Osička, R.; Černý, J.; Petroková, H.; Mierzwicka, J.M.; et al. Small Protein Blockers of Human IL-6 Receptor Alpha Inhibit Proliferation and Migration of Cancer Cells. Cell Commun. Signal. 2024, 22, 261. [Google Scholar] [CrossRef]
- Vandercappellen, J.; Van Damme, J.; Struyf, S. The Role of CXC Chemokines and Their Receptors in Cancer. Cancer Lett. 2008, 267, 226–244. [Google Scholar] [CrossRef]
- Ogura, M.; Takeuchi, H.; Kawakubo, H.; Nishi, T.; Fukuda, K.; Nakamura, R.; Takahashi, T.; Wada, N.; Saikawa, Y.; Omori, T.; et al. Clinical Significance of CXCL-8/CXCR-2 Network in Esophageal Squamous Cell Carcinoma. Surgery 2013, 154, 512–520. [Google Scholar] [CrossRef]
- Jobe, N.P.; Rösel, D.; Dvořánková, B.; Kodet, O.; Lacina, L.; Mateu, R.; Smetana, K.; Brábek, J. Simultaneous Blocking of IL-6 and IL-8 Is Sufficient to Fully Inhibit CAF-Induced Human Melanoma Cell Invasiveness. Histochem. Cell Biol. 2016, 146, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Jayatilaka, H.; Tyle, P.; Chen, J.J.; Kwak, M.; Ju, J.; Kim, H.J.; Lee, J.S.H.; Wu, P.H.; Gilkes, D.M.; Fan, R.; et al. Synergistic IL-6 and IL-8 Paracrine Signalling Pathway Infers a Strategy to Inhibit Tumour Cell Migration. Nat. Commun. 2017, 8, 15584. [Google Scholar] [CrossRef] [PubMed]
- Ray, C.M.P.; Yang, H.; Spangler, J.B.; Mac Gabhann, F. Mechanistic Computational Modeling of Monospecific and Bispecific Antibodies Targeting Interleukin-6/8 Receptors. PLoS Comput. Biol. 2024, 20, e1012157. [Google Scholar] [CrossRef] [PubMed]
- Kolář, M.; Szabo, P.; Dvořánková, B.; Lacina, L.; Gabius, H.J.; Strnad, H.; Šáchová, J.; Vlček, Č.; Plzák, J.; Chovanec, M.; et al. Upregulation of IL-6, IL-8 and CXCL-1 Production in Dermal Fibroblasts by Normal/Malignant Epithelial Cells in Vitro: Immunohistochemical and Transcriptomic Analyses. Biol. Cell 2012, 104, 738–751. [Google Scholar] [CrossRef]
- Nishihira, J. Macrophage Migration Inhibitory Factor (MIF): Its Essential Role in the Immune System and Cell Growth. J. Interferon Cytokine Res. 2000, 20, 751–762. [Google Scholar] [CrossRef]
- Gilliver, S.C.; Emmerson, E.; Bernhagen, J.; Hardman, M.J. MIF: A Key Player in Cutaneous Biology and Wound Healing. Exp. Dermatol. 2011, 20, 1–6. [Google Scholar] [CrossRef]
- Ashcroft, G.S.; Mills, S.J.; Lei, K.J.; Gibbons, L.; Jeong, M.J.; Taniguchi, M.; Burow, M.; Horan, M.A.; Wahl, S.M.; Nakayama, T. Estrogen Modulates Cutaneous Wound Healing by Downregulating Macrophage Migration Inhibitory Factor. J. Clin. Investig. 2003, 111, 1309–1318. [Google Scholar] [CrossRef]
- Calvin, M.; Dyson, M.; Rymer, J.; Young, S.R. The Effects of Ovarian Hormone Deficiency on Wound Contraction in a Rat Model. BJOG 1998, 105, 223–227. [Google Scholar] [CrossRef]
- Kubota, Y.; Takubo, K.; Shimizu, T.; Ohno, H.; Kishi, K.; Shibuya, M.; Saya, H.; Suda, T. M-CSF Inhibition Selectively Targets Pathological Angiogenesis and Lymphangiogenesis. J. Exp. Med. 2009, 206, 1089–1102. [Google Scholar] [CrossRef]
- Izzo, J.G.; Correa, A.M.; Wu, T.T.; Malhotra, U.; Chao, C.K.S.; Luthra, R.; Ensor, J.; Dekovich, A.; Liao, Z.; Hittelman, W.N.; et al. Pretherapy Nuclear Factor-ΚB Status, Chemoradiation Resistance, and Metastatic Progression in Esophageal Carcinoma. Mol. Cancer Ther. 2006, 5, 2844–2850. [Google Scholar] [CrossRef] [PubMed]
- Civenni, G.; Longoni, N.; Costales, P.; Dallavalle, C.; Inclán, C.G.; Albino, D.; Nuñez, L.E.; Morís, F.; Carbone, G.M.; Catapano, C.V. EC-70124, a Novel Glycosylated Indolocarbazole Multikinase Inhibitor, Reverts Tumorigenic and Stem Cell Properties in Prostate Cancer by Inhibiting STAT3 and NF-ΚB. Mol. Cancer Ther. 2016, 15, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Greten, F.R.; Arkan, M.C.; Bollrath, J.; Hsu, L.C.; Goode, J.; Miething, C.; Göktuna, S.I.; Neuenhahn, M.; Fierer, J.; Paxian, S.; et al. NF-ΚB Is a Negative Regulator of IL-1β Secretion as Revealed by Genetic and Pharmacological Inhibition of IKKβ. Cell 2007, 130, 918–931. [Google Scholar] [CrossRef] [PubMed]
- Graves, D.T.; Nooh, N.; Gillen, T.; Davey, M.; Patel, S.; Cottrell, D.; Amar, S. IL-1 Plays a Critical Role in Oral, But Not Dermal, Wound Healing. J. Immunol. 2001, 167, 5316–5320. [Google Scholar] [CrossRef]
- Spaeth, E.L.; Dembinski, J.L.; Sasser, A.K.; Watson, K.; Klopp, A.; Hall, B.; Andreeff, M.; Marini, F. Mesenchymal Stem Cell Transition to Tumor-Associated Fibroblasts Contributes to Fibrovascular Network Expansion and Tumor Progression. PLoS ONE 2009, 4, e4992. [Google Scholar] [CrossRef]
- Gál, P.; Varinská, L.; Fáber, L.; Novák, Š.; Szabo, P.; Mitrengová, P.; Mirossay, A.; Mučaji, P.; Smetana, K. How Signaling Molecules Regulate Tumor Microenvironment: Parallels to Wound Repair. Molecules 2017, 22, 1818. [Google Scholar] [CrossRef]
- Barondes, S.H.; Castronovo, V.; Cooper, D.N.W.; Cummings, R.D.; Drickamer, K.; Felzi, T.; Gitt, M.A.; Hirabayashi, J.; Hughes, C.; Kasai, K.; et al. Galectins: A Family of Animal β-Galactoside-Binding Lectins. Cell 1994, 76, 597–598. [Google Scholar] [CrossRef]
- Yang, R.Y.; Rabinovich, G.A.; Liu, F.T. Galectins: Structure, Function and Therapeutic Potential. Expert Rev. Mol. Med. 2008, 10, e17. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin Expression in Cancer Diagnosis and Prognosis: A Systematic Review. Biochim. Biophys. Acta Rev. Cancer 2015, 1855, 235–247. [Google Scholar] [CrossRef]
- Ito, K.; Stannard, K.; Gabutero, E.; Clark, A.M.; Neo, S.Y.; Onturk, S.; Blanchard, H.; Ralph, S.J. Galectin-1 as a Potent Target for Cancer Therapy: Role in the Tumor Microenvironment. Cancer Metastasis Rev. 2012, 31, 763–778. [Google Scholar] [CrossRef]
- Astorgues-Xerri, L.; Riveiro, M.E.; Tijeras-Raballand, A.; Serova, M.; Neuzillet, C.; Albert, S.; Raymond, E.; Faivre, S. Unraveling Galectin-1 as a Novel Therapeutic Target for Cancer. Cancer Treat. Rev. 2014, 40, 307–319. [Google Scholar] [CrossRef]
- Perillo, N.L.; Pace, K.E.; Seilhamer, J.J.; Baum, L.G. Apoptosis of T Cells Mediated by Galectin−1. Nature 1995, 378, 736–739. [Google Scholar] [CrossRef]
- Sanchez-Ruderisch, H.; Detjen, K.M.; Welzel, M.; André, S.; Fischer, C.; Gabius, H.-J.; Rosewicz, S. Galectin-1 Sensitizes Carcinoma Cells to Anoikis via the Fibronectin Receptor A5β1-Integrin. Cell Death Differ. 2011, 18, 806–816. [Google Scholar] [CrossRef] [PubMed]
- Ruvolo, P.P. Galectin 3 as a Guardian of the Tumor Microenvironment. Biochim. Biophys. Acta Mol. Cell Res. 2016, 1863, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Dvorak, H.F. Tumors: Wounds That Do Not Heal. Similarities between Tumor Stroma Generation and Wound Healing. N. Engl. J. Med. 1986, 315, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Busek, P.; Duke-Cohan, J.S.; Sedo, A. Does DPP-IV Inhibition Offer New Avenues for Therapeutic Intervention in Malignant Disease? Cancers 2022, 14, 2072. [Google Scholar] [CrossRef]
- Sindelka, R.; Naraine, R.; Abaffy, P.; Zucha, D.; Kraus, D.; Netusil, J.; Smetana, K.; Lacina, L.; Endaya, B.B.; Neuzil, J.; et al. Characterization of Regeneration Initiating Cells during Xenopus Laevis Tail Regeneration. Genome Biol. 2024, 25, 251. [Google Scholar] [CrossRef]
- Li, H.; Fan, X.; Houghton, J.M. Tumor Microenvironment: The Role of the Tumor Stroma in Cancer. J. Cell Biochem. 2007, 101, 805–815. [Google Scholar] [CrossRef]
- Hinz, B. Formation and Function of the Myofibroblast during Tissue Repair. J. Investig. Dermatol. 2007, 127, 526–537. [Google Scholar] [CrossRef]
- Lacina, L.; Kolář, M.; Pfeiferová, L.; Gál, P.; Smetana, K. Wound Healing: Insights into Autoimmunity, Ageing, and Cancer Ecosystems through Inflammation and IL-6 Modulation. Front. Immunol. 2024, 15, 1403570. [Google Scholar] [CrossRef]
- Jakovija, A.; Chtanova, T. Skin Immunity in Wound Healing and Cancer. Front. Immunol. 2023, 14, 1060258. [Google Scholar] [CrossRef] [PubMed]
- Robles, D.T.; Berg, D. Abnormal Wound Healing: Keloids. Clin. Dermatol. 2007, 25, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Živicová, V.; Lacina, L.; Mateu, R.; Smetana, K.; Kavková, R.; Krejcí, E.D.; Grim, M.; Kvasilová, A.; Borský, J.; Strnad, H.; et al. Analysis of Dermal Fibroblasts Isolated from Neonatal and Child Cleft Lip and Adult Skin: Developmental Implications on Reconstructive Surgery. Int. J. Mol. Med. 2017, 40, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Uberoi, A.; McCready-Vangi, A.; Grice, E.A. The Wound Microbiota: Microbial Mechanisms of Impaired Wound Healing and Infection. Nat. Rev. Microbiol. 2024, 22, 507–521. [Google Scholar] [CrossRef]
- Goswami, A.G.; Basu, S.; Banerjee, T.; Shukla, V.K. Biofilm and Wound Healing: From Bench to Bedside. Eur. J. Med. Res. 2023, 28, 157. [Google Scholar] [CrossRef]
- Yuan, Q.; Wu, H.; Tan, H.; Wang, X.; Cao, Y.; Chen, G. Oral Microbial Dysbiosis Driven by Periodontitis Facilitates Oral Squamous Cell Carcinoma Progression. Cancers 2025, 17, 2181. [Google Scholar] [CrossRef]
- Roberts, S.L.; Bhamra, R.; Ilankovan, V. Malignant Transformation Rate of Erosive Oral Lichen Planus: A Retrospective Study. Br. J. Oral Maxillofac. Surg. 2024, 62, 788–793. [Google Scholar] [CrossRef]
- Gallimidi, A.B.; Fischman, S.; Revach, B.; Bulvik, R.; Maliutina, A.; Rubinstein, A.M.; Nussbaum, G.; Elkin, M. Periodontal Pathogens Porphyromonas Gingivalis and Fusobacterium Nucleatum Promote Tumor Progression in an Oral-Specific Chemical Carcinogenesis Model. Oncotarget 2015, 6, 22613–22623. [Google Scholar] [CrossRef]
- Saikia, P.J.; Pathak, L.; Mitra, S.; Das, B. The Emerging Role of Oral Microbiota in Oral Cancer Initiation, Progression and Stemness. Front. Immunol. 2023, 14, 1198269. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Liu, J.; Geng, F.; Shi, X.; Li, Q.; Lu, Z.; Pan, Y. Fusobacterium Nucleatum Promotes Epithelial-Mesenchymal Transiton through Regulation of the LncRNA MIR4435-2HG/MiR-296-5p/Akt2/SNAI1 Signaling Pathway. FEBS J. 2020, 287, 4032–4047. [Google Scholar] [CrossRef]
- Panebianco, C.; Andriulli, A.; Pazienza, V. Pharmacomicrobiomics: Exploiting the Drug-Microbiota Interactions in Anticancer Therapies. Microbiome 2018, 6, 92. [Google Scholar] [CrossRef]
- Liu, X.; Sun, M.; Pu, F.; Ren, J.; Qu, X. Transforming Intratumor Bacteria into Immunopotentiators to Reverse Cold Tumors for Enhanced Immuno-Chemodynamic Therapy of Triple-Negative Breast Cancer. J. Am. Chem. Soc. 2023, 145, 26296–26307. [Google Scholar] [CrossRef]
- Yu, T.C.; Guo, F.; Yu, Y.; Sun, T.; Ma, D.; Han, J.; Qian, Y.; Kryczek, I.; Sun, D.; Nagarsheth, N.; et al. Fusobacterium Nucleatum Promotes Chemoresistance to Colorectal Cancer by Modulating Autophagy. Cell 2017, 170, 548–563.e16. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Huang, J.; Jiang, Z.; Tong, H.; Ma, X.; Liu, Y. Tumor Microbiome: Roles in Tumor Initiation, Progression, and Therapy. Mol. Biomed. 2025, 6, 9. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, Y. Intratumor Microbiome in Cancer Progression: Current Developments, Challenges and Future Trends. Biomark. Res. 2022, 10, 37. [Google Scholar] [CrossRef] [PubMed]
- Jiang, M.; Yang, Z.; Dai, J.; Wu, T.; Jiao, Z.; Yu, Y.; Ning, K.; Chen, W.; Yang, A. Intratumor Microbiome: Selective Colonization in the Tumor Microenvironment and a Vital Regulator of Tumor Biology. MedComm 2023, 4, e376. [Google Scholar] [CrossRef]
- Wong-Rolle, A.; Wei, H.K.; Zhao, C.; Jin, C. Unexpected Guests in the Tumor Microenvironment: Microbiome in Cancer. Protein Cell 2021, 12, 426–435. [Google Scholar] [CrossRef]
- Vétizou, M.; Pitt, J.M.; Daillère, R.; Lepage, P.; Waldschmitt, N.; Flament, C.; Rusakiewicz, S.; Routy, B.; Roberti, M.P.; Duong, C.P.M.; et al. Anticancer Immunotherapy by CTLA-4 Blockade Relies on the Gut Microbiota. Science 2015, 350, 1079–1084. [Google Scholar] [CrossRef]
- Routy, B.; Le Chatelier, E.; Derosa, L.; Duong, C.P.M.; Alou, M.T.; Daillère, R.; Fluckiger, A.; Messaoudene, M.; Rauber, C.; Roberti, M.P.; et al. Gut Microbiome Influences Efficacy of PD-1-Based Immunotherapy against Epithelial Tumors. Science 2018, 359, 91–97. [Google Scholar] [CrossRef]
- Borgers, J.S.W.; Burgers, F.H.; Terveer, E.M.; van Leerdam, M.E.; Korse, C.M.; Kessels, R.; Flohil, C.C.; Blank, C.U.; Schumacher, T.N.; van Dijk, M.; et al. Conversion of Unresponsiveness to Immune Checkpoint Inhibition by Fecal Microbiota Transplantation in Patients with Metastatic Melanoma: Study Protocol for a Randomized Phase Ib/IIa Trial. BMC Cancer 2022, 22, 1366. [Google Scholar] [CrossRef]
- Routy, B.; Lenehan, J.G.; Miller, W.H.; Jamal, R.; Messaoudene, M.; Daisley, B.A.; Hes, C.; Al, K.F.; Martinez-Gili, L.; Punčochář, M.; et al. Fecal Microbiota Transplantation plus Anti-PD-1 Immunotherapy in Advanced Melanoma: A Phase I Trial. Nat. Med. 2023, 29, 2121–2132, Correction in Nat. Med. 2024, 30, 604. [Google Scholar] [CrossRef] [PubMed]
- Zimmers, T.A.; Fishel, M.L.; Bonetto, A. STAT3 in the Systemic Inflammation of Cancer Cachexia. Semin. Cell Dev. Biol. 2016, 54, 28–41. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.A.; Starosta, S.; Ferrer, M.; Hou, J.; Chevy, Q.; Lucantonio, F.; Muñoz-Castañeda, R.; Zhang, F.; Zang, K.; Zhao, X.; et al. A Neuroimmune Circuit Mediates Cancer Cachexia-Associated Apathy. Science 2025, 388, eadm8857. [Google Scholar] [CrossRef] [PubMed]
- Yun, J.-Y.; Jung, J.Y.; Keam, B.; Lee, N.-R.; Kang, J.H.; Kim, Y.J.; Shim, H.-J.; Jung, K.H.; Koh, S.-J.; Ryu, H.; et al. Depression, Performance Status, and Discontinued Treatment Mediate an Association of Curability Belief with Prognosis in Advanced Cancer Patients. Sci. Rep. 2024, 14, 29098. [Google Scholar] [CrossRef]
- Emmons, H.A.; Wallace, C.W.; Fordahl, S.C. Interleukin-6 and Tumor Necrosis Factor-α Attenuate Dopamine Release in Mice Fed a High-Fat Diet, but Not Medium or Low-Fat Diets. Nutr. Neurosci. 2023, 26, 864–874. [Google Scholar] [CrossRef]
- Felger, J.C. The Role of Dopamine in Inflammation-Associated Depression: Mechanisms and Therapeutic Implications. In Inflammation-Associated Depression: Evidence, Mechanisms and Implications; Springer: Cham, Switzerland, 2016; pp. 199–219. [Google Scholar]
- Bauer, M.E.; Teixeira, A.L. Neuroinflammation in Mood Disorders: Role of Regulatory Immune Cells. Neuroimmunomodulation 2021, 28, 99–107. [Google Scholar] [CrossRef]
- Alpert, O.; Begun, L.; Issac, T.; Solhkhah, R. The Brain–Gut Axis in Gastrointestinal Cancers. J. Gastrointest. Oncol. 2021, 12, S301–S310. [Google Scholar] [CrossRef]
- Dai, H.; Yang, H.; Wang, R.; Wang, X.; Zhang, X. Modulating Gut Microbiota with Dietary Components: A Novel Strategy for Cancer–Depression Comorbidity Management. Nutrients 2025, 17, 1505. [Google Scholar] [CrossRef]
- Cash, E.; Albert, C.; Palmer, I.; Polzin, B.; Kabithe, A.; Crawford, D.; Bumpous, J.M.; Sephton, S.E. Depressive Symptoms, Systemic Inflammation, and Survival Among Patients With Head and Neck Cancer. JAMA Otolaryngol.–Head Neck Surg. 2024, 150, 405. [Google Scholar] [CrossRef]
- Gonçalves, D.C.; Gomes, S.P.; Seelaender, M. Metabolic, Inflammatory, and Molecular Impact of Cancer Cachexia on the Liver. Int. J. Mol. Sci. 2024, 25, 11945. [Google Scholar] [CrossRef]
- Strnadová, K.; Pfeiferová, L.; Přikryl, P.; Dvořánková, B.; Vlčák, E.; Frýdlová, J.; Vokurka, M.; Novotný, J.; Šáchová, J.; Hradilová, M.; et al. Exosomes Produced by Melanoma Cells Significantly Influence the Biological Properties of Normal and Cancer-Associated Fibroblasts. Histochem. Cell Biol. 2022, 157, 153–172. [Google Scholar] [CrossRef] [PubMed]
- Vokurka, M.; Lacina, L.; Brábek, J.; Kolář, M.; Ng, Y.Z.; Smetana, K. Cancer-Associated Fibroblasts Influence the Biological Properties of Malignant Tumours via Paracrine Secretion and Exosome Production. Int. J. Mol. Sci. 2022, 23, 964. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Li, J.; Bojmar, L.; Chen, H.; Li, Z.; Tobias, G.C.; Hu, M.; Homan, E.A.; Lucotti, S.; Zhao, F.; et al. Tumour Extracellular Vesicles and Particles Induce Liver Metabolic Dysfunction. Nature 2023, 618, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Tilg, H.; Adolph, T.E.; Dudek, M.; Knolle, P. Non-Alcoholic Fatty Liver Disease: The Interplay between Metabolism, Microbes and Immunity. Nat. Metab. 2021, 3, 1596–1607. [Google Scholar] [CrossRef]
- Stienstra, R.; Saudale, F.; Duval, C.; Keshtkar, S.; Groener, J.E.M.; van Rooijen, N.; Staels, B.; Kersten, S.; Müller, M. Kupffer Cells Promote Hepatic Steatosis Via Interleukin-1β–Dependent Suppression of Peroxisome Proliferator-Activated Receptor α Activity. Hepatology 2010, 51, 511–522. [Google Scholar] [CrossRef]
- Rubinstein, M.M.; Brown, K.A.; Iyengar, N.M. Targeting Obesity-Related Dysfunction in Hormonally Driven Cancers. Br. J. Cancer 2021, 125, 495–509. [Google Scholar] [CrossRef]
- Brown, K.A. Metabolic Pathways in Obesity-Related Breast Cancer. Nat. Rev. Endocrinol. 2021, 17, 350–363. [Google Scholar] [CrossRef]
- Caruso, A.; Gelsomino, L.; Panza, S.; Accattatis, F.M.; Naimo, G.D.; Barone, I.; Giordano, C.; Catalano, S.; Andò, S. Leptin: A Heavyweight Player in Obesity-Related Cancers. Biomolecules 2023, 13, 1084. [Google Scholar] [CrossRef]
- Pham, D.-V.; Park, P.-H. Tumor Metabolic Reprogramming by Adipokines as a Critical Driver of Obesity-Associated Cancer Progression. Int. J. Mol. Sci. 2021, 22, 1444. [Google Scholar] [CrossRef]
- Shi, H.; Hao, X.; Sun, Y.; Zhao, Y.; Wang, Y.; Cao, X.; Gong, Z.; Ji, S.; Lu, J.; Yan, Y.; et al. Exercise-inducible Circulating Extracellular Vesicle Irisin Promotes Browning and the Thermogenic Program in White Adipose Tissue. Acta Physiol. 2024, 240, e14103. [Google Scholar] [CrossRef]
- Huang, Q.; Wu, M.; Wu, X.; Zhang, Y.; Xia, Y. Muscle-to-Tumor Crosstalk: The Effect of Exercise-Induced Myokine on Cancer Progression. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2022, 1877, 188761. [Google Scholar] [CrossRef]
- Becher, T.; Palanisamy, S.; Kramer, D.J.; Eljalby, M.; Marx, S.J.; Wibmer, A.G.; Butler, S.D.; Jiang, C.S.; Vaughan, R.; Schöder, H.; et al. Brown Adipose Tissue Is Associated with Cardiometabolic Health. Nat. Med. 2021, 27, 58–65. [Google Scholar] [CrossRef] [PubMed]
- Seki, T.; Yang, Y.; Sun, X.; Lim, S.; Xie, S.; Guo, Z.; Xiong, W.; Kuroda, M.; Sakaue, H.; Hosaka, K.; et al. Brown-Fat-Mediated Tumour Suppression by Cold-Altered Global Metabolism. Nature 2022, 608, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, G.L.; Coelho, A.R.; Marques, R.; Oliveira, P.J. Cancer Cell Metabolism: Rewiring the Mitochondrial Hub. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2021, 1867, 166016. [Google Scholar] [CrossRef] [PubMed]
- Nishikawa, H.; Goto, M.; Fukunishi, S.; Asai, A.; Nishiguchi, S.; Higuchi, K. Cancer Cachexia: Its Mechanism and Clinical Significance. Int. J. Mol. Sci. 2021, 22, 8491. [Google Scholar] [CrossRef]
- Petruzzelli, M.; Schweiger, M.; Schreiber, R.; Campos-Olivas, R.; Tsoli, M.; Allen, J.; Swarbrick, M.; Rose-John, S.; Rincon, M.; Robertson, G.; et al. A Switch from White to Brown Fat Increases Energy Expenditure in Cancer-Associated Cachexia. Cell Metab. 2014, 20, 433–447. [Google Scholar] [CrossRef]
- Thompson, J.J.; McGovern, J.; Roxburgh, C.S.D.; Edwards, J.; Dolan, R.D.; McMillan, D.C. The Relationship between LDH and GLIM Criteria for Cancer Cachexia: Systematic Review and Meta-Analysis. Crit. Rev. Oncol. Hematol. 2024, 199, 104378. [Google Scholar] [CrossRef]
- Li, L.; Xing, M.; Wang, R.; Ding, X.; Wan, X.; Yu, X. The Predictive Values of Sarcopenia Screening Tools in Preoperative Elderly Patients with Colorectal Cancer: Applying the Diagnostic Criteria of EWGSOP2 and AWGS2019. BMC Geriatr. 2025, 25, 206. [Google Scholar] [CrossRef]
- Meza-Valderrama, D.; Marco, E.; Dávalos-Yerovi, V.; Muns, M.D.; Tejero-Sánchez, M.; Duarte, E.; Sánchez-Rodríguez, D. Sarcopenia, Malnutrition, and Cachexia: Adapting Definitions and Terminology of Nutritional Disorders in Older People with Cancer. Nutrients 2021, 13, 761. [Google Scholar] [CrossRef]
- Berriel Diaz, M.; Rohm, M.; Herzig, S. Cancer Cachexia: Multilevel Metabolic Dysfunction. Nat. Metab. 2024, 6, 2222–2245. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vyhnánková, S.; Lacina, L.; Chovanec, M.; Plzák, J.; Smetana, K., Jr.; Netušil, J.; Kolář, M.; Šindelka, R. Cold, Hot, and Lethal—The Tumour Microenvironment and the Immunology of Head and Neck Squamous Cell Carcinoma. Int. J. Mol. Sci. 2025, 26, 8844. https://doi.org/10.3390/ijms26188844
Vyhnánková S, Lacina L, Chovanec M, Plzák J, Smetana K Jr., Netušil J, Kolář M, Šindelka R. Cold, Hot, and Lethal—The Tumour Microenvironment and the Immunology of Head and Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences. 2025; 26(18):8844. https://doi.org/10.3390/ijms26188844
Chicago/Turabian StyleVyhnánková, Svatava, Lukáš Lacina, Martin Chovanec, Jan Plzák, Karel Smetana, Jr., Jiří Netušil, Michal Kolář, and Radek Šindelka. 2025. "Cold, Hot, and Lethal—The Tumour Microenvironment and the Immunology of Head and Neck Squamous Cell Carcinoma" International Journal of Molecular Sciences 26, no. 18: 8844. https://doi.org/10.3390/ijms26188844
APA StyleVyhnánková, S., Lacina, L., Chovanec, M., Plzák, J., Smetana, K., Jr., Netušil, J., Kolář, M., & Šindelka, R. (2025). Cold, Hot, and Lethal—The Tumour Microenvironment and the Immunology of Head and Neck Squamous Cell Carcinoma. International Journal of Molecular Sciences, 26(18), 8844. https://doi.org/10.3390/ijms26188844








