Cinnarizine, a Calcium Channel Blocker, Partially Prevents the Striatal Dopamine Decline and Loss of Nigral Dopamine Neurons in the Lactacystin-Induced Rat Model of Parkinson’s Disease
Abstract
1. Introduction
2. Results
2.1. Effects of the In Vivo Study
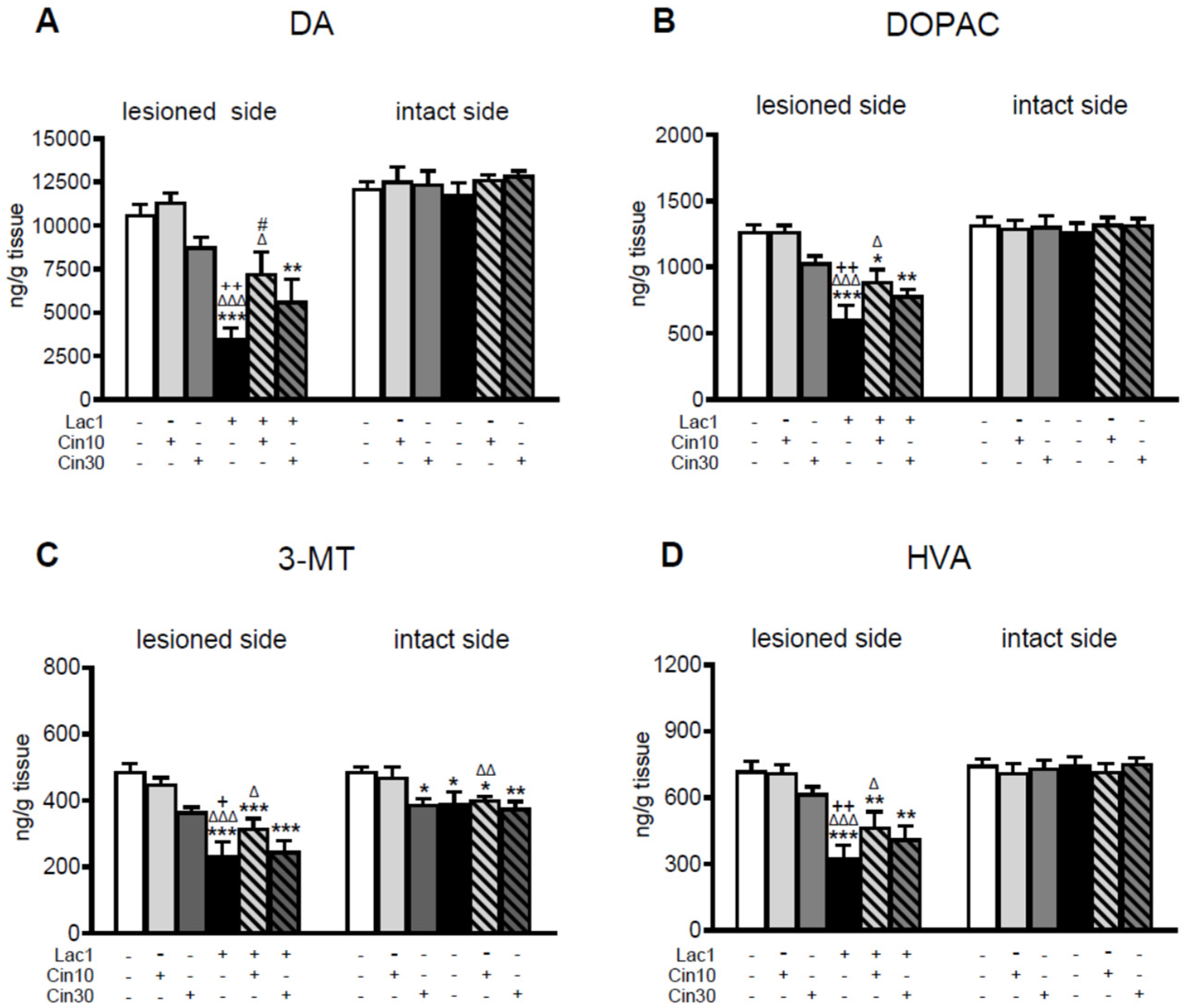
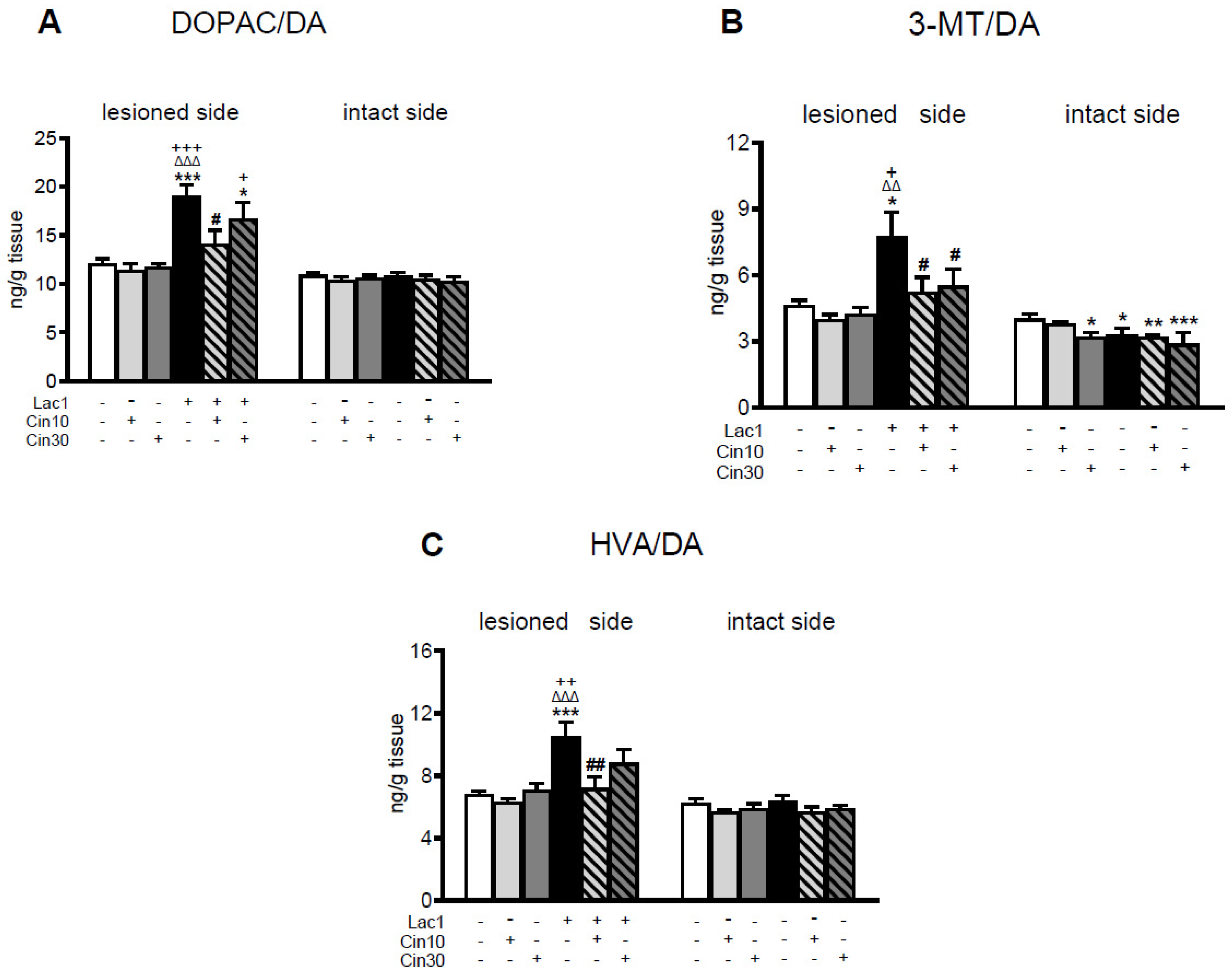
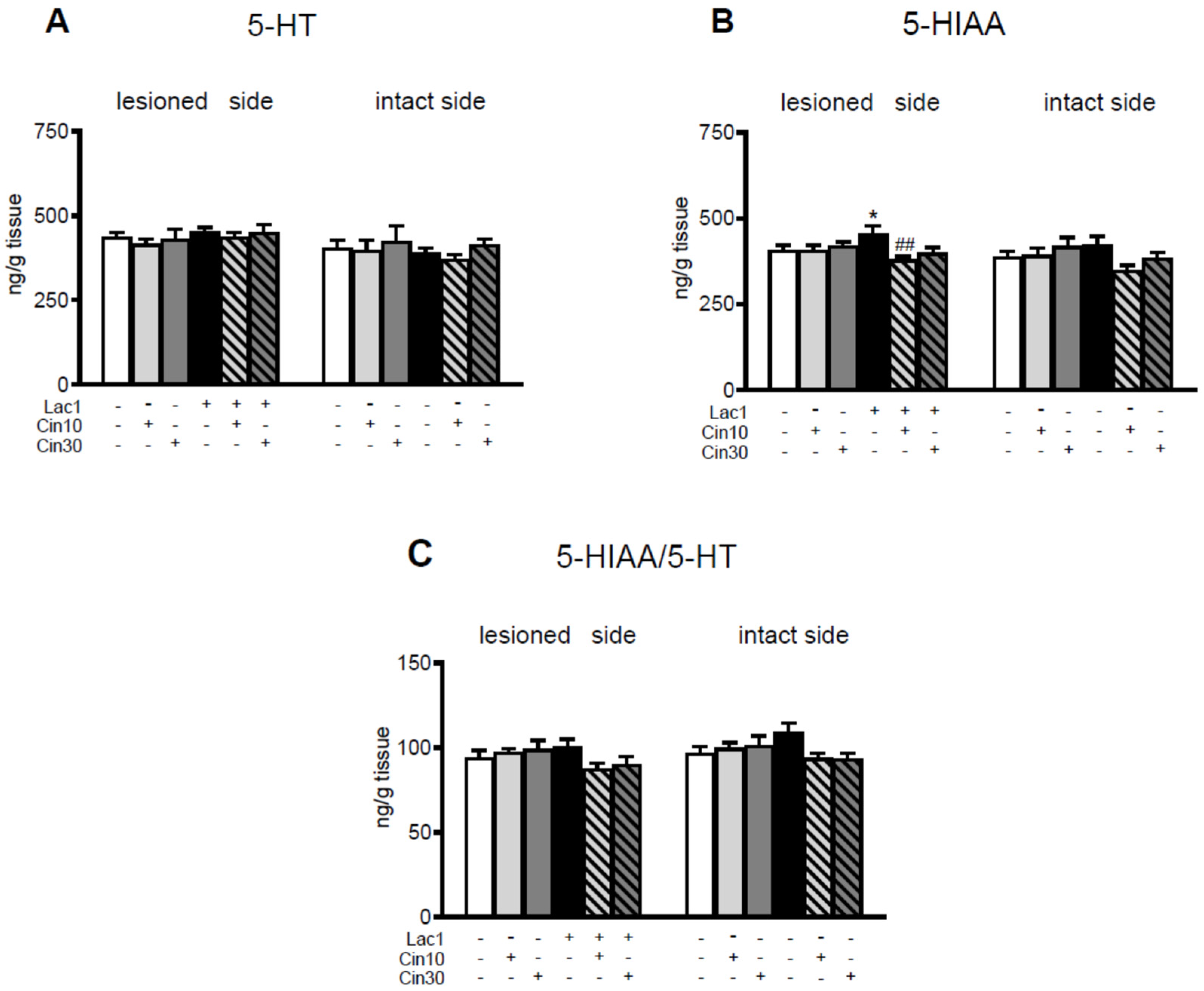
2.1.1. Impact of 10 and 30 mg/kg of Cinnarizine on DA and 5-HT Metabolism in the Striatum of a Lactacystin-Induced Rat Model of Parkinson’s Disease
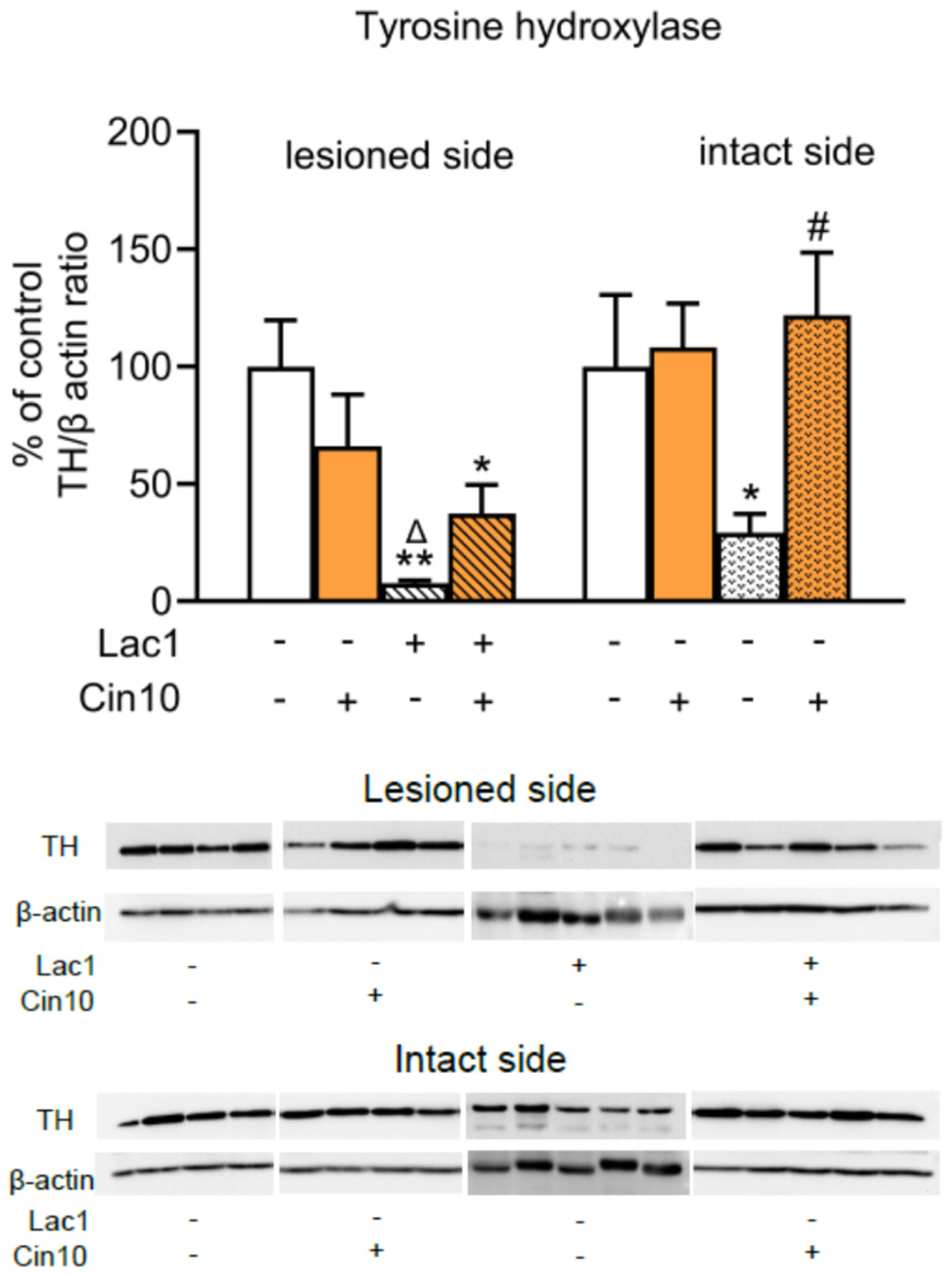
2.1.2. Impact of 10 mg/kg of Cinnarizine on the Tyrosine Hydroxylase (TH) Protein Level in the Substantia Nigra of Lactacystin-Pretreated Rats
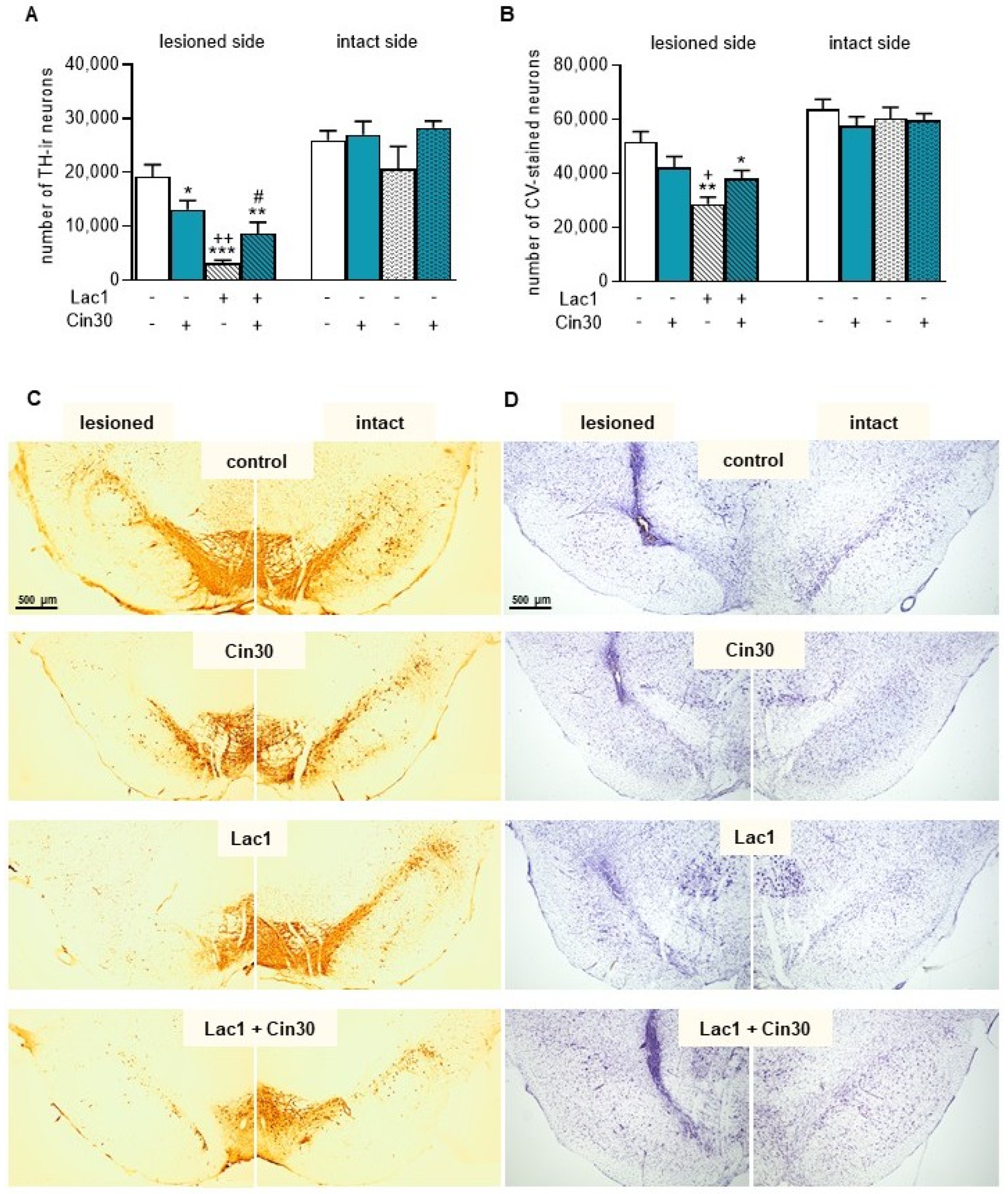
2.1.3. Impact of 30 mg/kg of Cinnarizine on the Number of TH-Ir and CV-Stained Neurons in the SN of Lactacystin-Treated Rats
2.2. Effects of the In Vitro Study
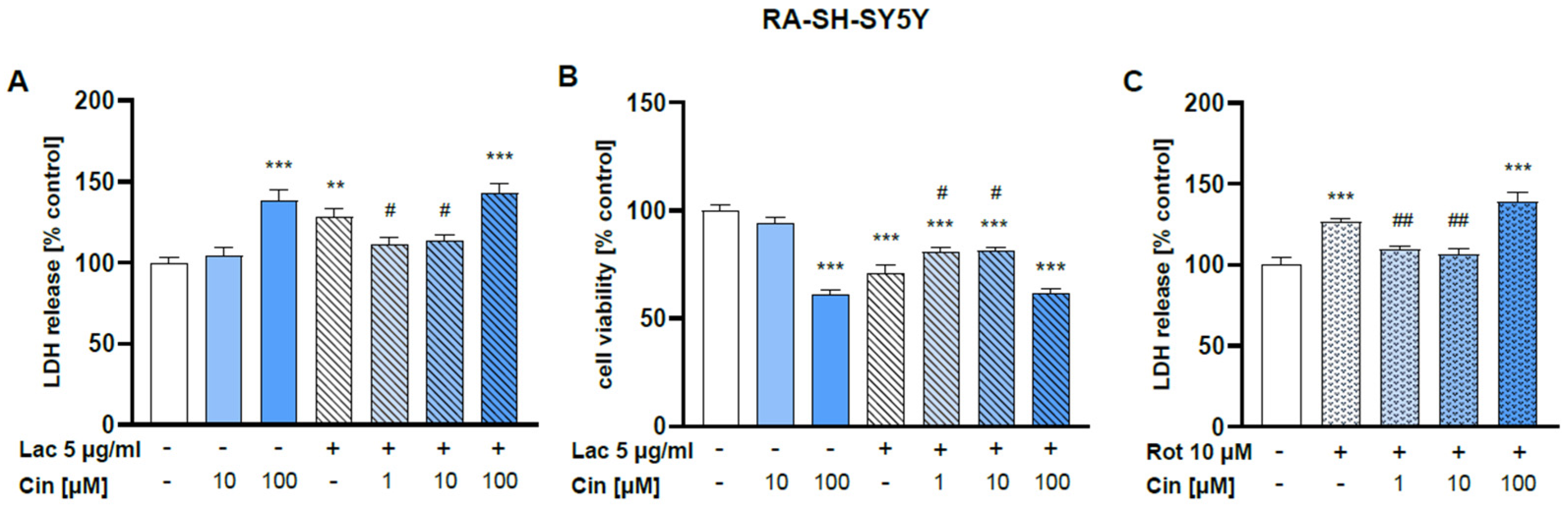
2.2.1. Effect of Cinnarizine on Lactacystin- and Rotenone-Induced Cell Damage in SH-SY5Y Cells
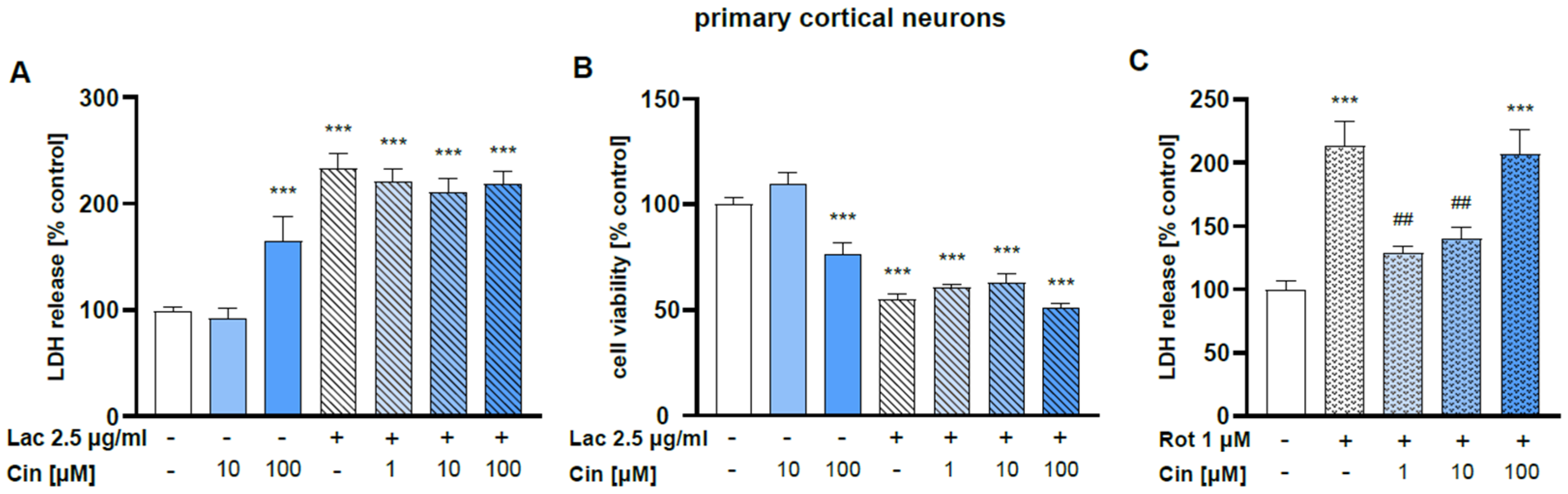
2.2.2. Effect of Cinnarizine on Lactacystin- or Rotenone-Induced Cell Damage in Primary Cortical Neurons
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. In Vivo Study
4.2.1. Surgery
4.2.2. Experimental Groups and Drug Administration
4.2.3. Dopamine and Serotonin Metabolism in the Striatal Homogenates
4.2.4. Western Blots of Tyrosine Hydroxylase (TH) Protein in the Substantia Nigra
4.2.5. Histological Analysis
Tissue Collection
TH Immunocytochemistry and Cresyl Violet (CV) Staining in the SN
Stereology Microscopic Analysis
4.3. In Vitro Experiments
4.3.1. Chemicals
4.3.2. SH-SY5Y Cell Culture
4.3.3. Primary Neuronal Cell Cultures
4.3.4. Cell Treatment
4.3.5. Measurement of Lactate Dehydrogenase (LDH) Release
4.3.6. Measurements of Cell Viability
4.4. Calculation and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-HIAA | 5-hydroxyindoleacetic acid |
| 5-HT | serotonin |
| 6-OHDA | 6-hydroxydopamine |
| Calb | calbindin |
| COMT | catechol-O-methyltransferase |
| CV | cresyl violet |
| DA | dopamine |
| DAT | dopamine transporter |
| DOPAC | 3,4-dihydroxyphenylacetic acid |
| HPLC | high-performance liquid chromatography |
| HVA | homovanillic acid |
| LDH | lactate dehydrogenase |
| L-VGCC | L-type voltage-gated Ca2+ channels |
| MAO | monoamine oxidase |
| MPTP | 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine |
| MT | 3-methoxytyramine |
| MTT | 3-[4,5-dimethylthylthiazol-2-yl]-2,5-diphenyltetrazolium bromide |
| PD | Parkinson’s disease |
| SN | substantia nigra |
| SNc | substantia nigra pars compacta |
| TH | tyrosine hydroxylase |
| TH-ir neurons | tyrosine hydroxylase immunoreactive neurons |
| T-VGCC | T-type voltage-gated Ca2+ channels |
| UPS | ubiquitin-proteasome system |
| VGCC | voltage-gated Ca2+ channels |
References
- Spillantini, M.G.; Schmidt, M.L.; Lee, V.M.; Trojanowski, J.Q.; Jakes, R.; Goedert, M. Alpha-synuclein in Lewy bodies. Nature 1997, 388, 839–840. [Google Scholar] [CrossRef]
- Moore, D.J.; West, A.B.; Dawson, V.L.; Dawson, T.M. Molecular pathophysiology of Parkinson’s disease. Annu. Rev. Neurosci. 2005, 28, 57–87. [Google Scholar] [CrossRef]
- Hol, E.M.; Fischer, D.F.; Ovaa, H.; Scheper, W. Ubiquitin proteasome system as a pharmacological target in neurodegeneration. Expert Rev. Neurother. 2006, 6, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Sas, K.; Robotka, H.; Toldi, J.; Vécsei, L. Mitochondria, metabolic disturbances, oxidative stress and the kynurenine system, with focus on neurodegenerative disorders. J. Neurol. Sci. 2007, 257, 221–239. [Google Scholar] [CrossRef] [PubMed]
- Surmeier, D.J.; Schumacker, P.T. Calcium, bioenergetics, and neuronal vulnerability in Parkinson’s disease. J. Biol. Chem. 2013, 288, 10736–10741. [Google Scholar] [CrossRef]
- Zaichick, S.V.; McGrath, K.M.; Caraveo, G. The role of Ca2+ signaling in Parkinson’s disease. Dis. Models Mech. 2017, 10, 519–535. [Google Scholar] [CrossRef]
- Goldberg, A.L. Protein degradation and protection against misfolded or damaged proteins. Nature 2003, 426, 895–899. [Google Scholar] [CrossRef]
- Yi, J.J.; Ehlers, M.D. Emerging roles for ubiquitin and protein degradation in neuronal function. Pharmacol. Rev. 2007, 59, 14–39. [Google Scholar] [CrossRef]
- Di Antonio, A.; Haghighi, A.P.; Portman, S.L.; Lee, J.D.; Amaranto, A.M.; Goodman, C.S. Ubiquitination-dependent mechanisms regulate synaptic growth and function. Nature 2001, 412, 449–452. [Google Scholar] [CrossRef]
- Leroy, E.; Boyer, R.; Auburger, G.; Leube, B.; Ulm, G.; Mezey, E.; Harta, G.; Brownstein, M.J.; Jonnalagada, S.; Chernova, T.; et al. The ubiquitin pathway in Parkinson’s disease. Nature 1998, 395, 451–452. [Google Scholar] [CrossRef] [PubMed]
- McNaught, K.S.; Belizaire, R.; Isacson, O.; Jenner, P.; Olanow, C.W. Altered proteasomal function in sporadic Parkinson’s disease. Exp. Neurol. 2003, 179, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Lindsten, K.; Dantuma, N.P. Monitoring the ubiquitin/proteasome system in conformational diseases. Ageing Res. Rev. 2003, 2, 433–449. [Google Scholar] [CrossRef]
- McNaught, K.S.; Olanow, C.W. Protein aggregation in the pathogenesis of familial and sporadic Parkinson’s disease. Neurobiol. Aging 2006, 27, 530–545. [Google Scholar] [CrossRef]
- Lim, K.L.; Tan, J.M. Role of the ubiquitin proteasome system in Parkinson’s disease. BMC Biochem. 2007, 8 (Suppl. S1), S13. [Google Scholar] [CrossRef]
- McNaught, K.S.; Jenner, P. Proteasomal function is impaired in substantia nigra in Parkinson’s disease. Neurosci. Lett. 2001, 297, 191–194. [Google Scholar] [CrossRef]
- Furukawa, Y.; Vigouroux, S.; Wong, H.; Guttman, M.; Rajput, A.H.; Ang, L.; Briand, M.; Kish, S.J.; Briand, Y. Brain proteasomal function in sporadic Parkinson’s disease and related disorders. Ann. Neurol. 2002, 51, 779–782. [Google Scholar] [CrossRef]
- McNaught, K.S.; Björklund, L.M.; Belizaire, R.; Isacson, O.; Jenner, P.; Olanow, C.W. Proteasome inhibition causes nigral degeneration with inclusion bodies in rats. Neuroreport 2002, 13, 1437–1441. [Google Scholar] [CrossRef]
- Lorenc-Koci, E.; Lenda, T.; Antkiewicz-Michaluk, L.; Wardas, J.; Domin, H.; Smiałowska, M.; Konieczny, J. Different effects of intranigral and intrastriatal administration of the proteasome inhibitor lactacystin on typical neurochemical and histological markers of Parkinson’s disease in rats. Neurochem. Int. 2011, 58, 839–849. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, J.; Czarnecka, A.; Lenda, T.; Kamińska, K.; Lorenc-Koci, E. Chronic L-DOPA treatment attenuates behavioral and biochemical deficits induced by unilateral lactacystin administration into the rat substantia nigra. Behav. Brain Res. 2014, 261, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Bentea, E.; Verbruggen, L.; Massie, A. The proteasome inhibition model of Parkinson’s Disease. J. Park. Dis. 2017, 7, 31–63. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, H.; Selvaraj, S.; Sukumaran, P.; Lei, S.; Birnbaumer, L.; Singh, B.B. Inhibition of L-type Ca2+ channels by TRPC1-STIM1 complex is essential for the protection of dopaminergic neurons. J. Neurosci. 2017, 37, 3364–3377. [Google Scholar] [CrossRef]
- Ortner, N.J.; Bock, G.; Dougalis, A.; Kharitonova, M.; Duda, J.; Hess, S.; Tuluc, P.; Pomberger, T.; Stefanova, N.; Pitterl, F.; et al. Lower affinity of isradipine for L-Type Ca2+ channels during substantia nigra dopamine neuron-like activity: Implications for neuroprotection in Parkinson’s Disease. J. Neurosci. 2017, 37, 6761–6777. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.M.; Xu, Y.Y.; Liu, S.; Ma, Z.G. Isradipine attenuates MPTP-induced dopamine neuron degeneration by inhibiting up-regulation of L-type calcium channels and iron accumulation in the substantia nigra of mice. Oncotarget 2017, 8, 47284–47295. [Google Scholar] [CrossRef]
- Liss, B.; Striessnig, J. The Potential of L-Type calcium channels as a drug target for neuroprotective therapy in Parkinson’s disease. Annu. Rev. Pharmacol. Toxicol. 2019, 59, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Westerink, R.H. Targeting exocytosis: Ins and outs of the modulation of quantal dopamine release. CNS Neurol. Disord. Drug Targets 2006, 5, 57–77. [Google Scholar] [CrossRef]
- Arundine, M.; Tymianski, M. Molecular mechanisms of calcium-dependent neurodegeneration in excitotoxicity. Cell Calcium 2003, 34, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Nanou, E.; Catterall, W.A. Calcium channels, synaptic plasticity, and neuropsychiatric disease. Neuron 2018, 98, 466–481. [Google Scholar] [CrossRef]
- Ashrafi, G.; de Juan-Sanz, J.; Farrell, R.J.; Ryan, T.A. Molecular tuning of the axonal mitochondrial Ca2+ uniporter ensures metabolic flexibility of neurotransmission. Neuron 2020, 105, 678–687.e5. [Google Scholar] [CrossRef]
- Mellström, B.; Savignac, M.; Gomez-Villafuertes, R.; Naranjo, J.R. Ca2+-operated transcriptional networks: Molecular mechanisms and in vivo models. Physiol. Rev. 2008, 88, 421–449. [Google Scholar] [CrossRef]
- Ma, H.; Cohen, S.; Li, B.; Tsien, R.W. Exploring the dominant role of Cav1 channels in signalling to the nucleus. Biosci. Rep. 2012, 33, 97–101. [Google Scholar] [CrossRef]
- Heck, J.; Palmeira Do Amaral, A.C.; Weißbach, S.; El Khallouqi, A.; Bikbaev, A.; Heine, M. More than a pore: How voltage-gated calcium channels act on different levels of neuronal communication regulation. Channels 2021, 5, 322–338. [Google Scholar] [CrossRef]
- Canzoniero, L.M.; Babcock, D.J.; Gottron, F.J.; Grabb, M.C.; Manzerra, P.; Snider, B.J.; Choi, D.W. Raising intracellular calcium attenuates neuronal apoptosis triggered by staurosporine or oxygen-glucose deprivation in the presence of glutamate receptor blockade. Neurobiol. Dis. 2004, 15, 520–528. [Google Scholar] [CrossRef]
- Berliocchi, L.; Bano, D.; Nicotera, P. Ca2+ signals and death programmes in neurons. Philos. Trans. R Soc. Lond. B Biol. Sci. 2005, 360, 2255–2258. [Google Scholar] [CrossRef]
- Wu, S.; Hyrc, K.L.; Moulder, K.L.; Lin, Y.; Warmke, T.; Snider, B.J. Cellular calcium deficiency plays a role in neuronal death caused by proteasome inhibitors. J. Neurochem. 2009, 109, 1225–1236. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Guzman, J.N.; Sanchez-Padilla, J. Calcium, cellular aging, and selective neuronal vulnerability in Parkinson’s disease. Cell Calcium 2010, 47, 175–182. [Google Scholar] [CrossRef]
- Brimblecombe, K.R.; Gracie, C.J.; Platt, N.J.; Cragg, S.J. Gating of dopamine transmission by calcium and axonal N, Q, T and L-type voltage-gated calcium channels differs between striatal domains. J. Physiol. 2015, 593, 929–946. [Google Scholar] [CrossRef]
- Grienberger, C.; Konnerth, A. Imaging calcium in neurons. Neuron 2012, 73, 862–885. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Cali, T.; Ottolini, D.; Carafoli, E. Neuronal calcium signaling: Function and dysfunction. Cell Mol. Life Sci. 2014, 71, 2787–2814. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Carafoli, E.; Cali, T. The plasma membrane calcium pumps: Focus on the role in (neuro)pathology. Biochem. Biophys. Res. Commun. 2017, 483, 1116–1124. [Google Scholar] [CrossRef] [PubMed]
- Britzolaki, A.; Saurine, J.; Flaherty, E.; Thelen, C.; Pitychoutis, P.M. The SERCA2: A gatekeeper of neuronal calcium homeostasis in the brain. Cell Mol. Neurobiol. 2018, 38, 981–994. [Google Scholar] [CrossRef]
- Damier, P.; Hirsch, E.C.; Agid, Y.; Graybiel, A.M. The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 1999, 122, 1437–1448. [Google Scholar] [CrossRef] [PubMed]
- Hurley, M.J.; Dexter, D.T. Voltage-gated calcium channels and Parkinson’s disease. Pharmacol. Ther. 2012, 133, 324–333. [Google Scholar] [CrossRef]
- Sukiasyan, N.; Hultborn, H.; Zhang, M. Distribution of calcium channel Ca(V)1.3 immunoreactivity in the rat spinal cord and brain stem. Neuroscience 2009, 159, 217–235. [Google Scholar] [CrossRef]
- Hurley, M.J.; Brandon, B.; Gentleman, S.M.; Dexter, D.T. Parkinson’s disease is associated with altered expression of CaV1 channels and calcium-binding proteins. Brain 2013, 136, 2077–2097. [Google Scholar] [CrossRef]
- Zamponi, G.W.; Striessnig, J.; Koschak, A.; Dolphin, A.C. The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 2015, 67, 821–870. [Google Scholar] [CrossRef]
- Hell, J.W.; Westenbroek, R.E.; Warner, C.; Ahlijanian, M.K.; Prystay, W.; Gilbert, M.M.; Snutch, T.P.; Catterall, W.A. Identification and differential subcellular localization of the neuronal class C and class D L-type calcium channel alpha 1 subunits. J. Cell Biol. 1993, 123, 949–962. [Google Scholar] [CrossRef]
- Sinnegger-Brauns, M.J.; Huber, I.G.; Koschak, A.; Wild, C.; Obermair, G.J.; Einzinger, U.; Hoda, J.C.; Sartori, S.B.; Striessnig, J. Expression and 1,4-dihydropyridine-binding properties of brain L-type calcium channel isoforms. Mol. Pharmacol. 2009, 75, 407–414. [Google Scholar] [CrossRef]
- Hurley, M.J.; Gentleman, S.M.; Dexter, D.T. Calcium CaV1 channel subtype mRNA expression in Parkinson’s disease examined by in situ hybridization. J. Mol. Neurosci. 2015, 55, 715–724. [Google Scholar] [CrossRef]
- Surmeier, D.J.; Guzman, J.N.; Sanchez-Padilla, J.; Goldberg, J.A. The origins of oxidant stress in Parkinson’s disease and therapeutic strategies. Antioxid. Redox Signal. 2011, 14, 1289–1301. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.S.; Guzman, J.N.; Ilijic, E.; Mercer, J.N.; Rick, C.; Tkatch, T.; Meredith, G.E.; Surmeier, D.J. ‘Rejuvenation’ protects neurons in mouse models of Parkinson’s disease. Nature 2007, 447, 1081–1086. [Google Scholar] [CrossRef] [PubMed]
- Guzman, J.N.; Sánchez-Padilla, J.; Chan, C.S.; Surmeier, D.J. Robust pacemaking in substantia nigra dopaminergic neurons. J. Neurosci. 2009, 29, 11011–11019. [Google Scholar] [CrossRef]
- Kupsch, A.; Gerlach, M.; Pupeter, S.C.; Sautter, J.; Dirr, A.; Arnold, G.; Opitz, W.; Przuntek, H.; Riederer, P.; Oertel, W.H. Pretreatment with nimodipine prevents MPTP induced neurotoxicity at the nigral, but not at the striatal level in mice. Neuroreport 1995, 6, 621–625. [Google Scholar] [CrossRef] [PubMed]
- Kupsch, A.; Sautter, J.; Schwarz, J.; Riederer, P.; Gerlach, M.; Oertel, W.H. 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurotoxicity in non-human primates is antagonized by pretreatment with nimodipine at the nigral, but not at the striatal level. Brain Res. 1996, 741, 185–196. [Google Scholar] [CrossRef]
- Ilijic, E.; Guzman, J.N.; Surmeier, D.J. The L-type channel antagonist isradipine is neuroprotective in a mouse model of Parkinson’s disease. Neurobiol. Dis. 2011, 43, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Becker, C.; Jick, S.S.; Meier, C.R. Use of antihypertensives and the risk of Parkinson disease. Neurology 2008, 70, 1438–1444. [Google Scholar] [CrossRef]
- Ritz, B.; Rhodes, S.L.; Qian, L.; Schernhammer, E.; Olsen, J.H.; Friis, S. L-type calcium channel blockers and Parkinson disease in Denmark. Ann. Neurol. 2010, 67, 600–606. [Google Scholar] [CrossRef]
- Pasternak, B.; Svanstrom, H.; Nielsen, N.M.; Fugger, L.; Melbye, M.; Hviid, A. Use of calcium channel blockers and Parkinson’s disease. Am. J. Epidemiol. 2012, 175, 627–635. [Google Scholar] [CrossRef]
- Gudala, K.; Kanukula, R.; Bansal, D. Reduced risk of Parkinson’s disease in users of calcium channel blockers: A meta-analysis. Int. J. Chronic Dis. 2015, 2015, 697404. [Google Scholar] [CrossRef]
- Lang, Y.; Gong, D.; Fan, Y. Calcium channel blocker use and risk of Parkinson’s disease: A meta-analysis. Pharmacoepidemiol. Drug Saf. 2015, 24, 559–566. [Google Scholar] [CrossRef]
- Mullapudi, A.; Gudala, K.; Boya, C.S.; Bansal, D. Risk of Parkinson’s disease in the users of antihypertensive agents: An evidence from the meta-analysis of observational studies. J. Neurodegener. Dis. 2016, 2016, 5780809. [Google Scholar] [CrossRef] [PubMed]
- Takada, M.; Kang, Y.; Imanishi, M. Immunohistochemical localization of voltage-gated calcium channels in substantia nigra dopamine neurons. Eur. J. Neurosci. 2001, 13, 757–762. [Google Scholar] [CrossRef] [PubMed]
- Wolfart, J.; Roeper, J. Selective coupling of T-type calcium channels to SK potassium channels prevents intrinsic bursting in dopaminergic midbrain neurons. J. Neurosci. 2002, 22, 3404–3413. [Google Scholar] [CrossRef] [PubMed]
- Yunker, A.M.; Sharp, A.H.; Sundarraj, S.; Ranganathan, V.; Copeland, T.D.; McEnery, M.W. Immunological characterization of T-type voltage-dependent calcium channel CaV3.1 (alpha1G) and CaV3.3 (alpha1I) isoforms reveal differences in their localization, expression, and neural development. Neuroscience 2003, 117, 321–335, Erratum in Neuroscience 2006, 142, 607. [Google Scholar] [CrossRef]
- Dufour, M.A.; Woodhouse, A.; Goaillard, J.M. Somatodendritic ion channel expression in substantia nigra pars compacta dopaminergic neurons across postnatal development. J. Neurosci. Res. 2014, 92, 981–999. [Google Scholar] [CrossRef] [PubMed]
- Gerfen, C.R.; Herkenham, M.; Thibault, J. The neostriatal mosaic: II. Patch and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J. Neurosci. 1987, 7, 3915–3934. [Google Scholar] [CrossRef]
- Yamada, T.; McGeer, P.L.; Baimbridge, K.G.; McGeer, E.G. Relative sparing in Parkinson’s disease of substantia nigra dopamine neurons containing calbindin-D28K. Brain Res. 1990, 526, 303–307. [Google Scholar] [CrossRef]
- German, D.C.; Manaye, K.F.; Sonsalla, P.K.; Brooks, B.A. Midbrain dopaminergic cell loss in Parkinson’s disease and MPTP-induced parkinsonism: Sparing of calbindin-D28k-containing cells. Ann. N. Y. Acad. Sci. 1992, 648, 42–62. [Google Scholar] [CrossRef]
- Yuan, H.H.; Chen, R.J.; Zhu, Y.H.; Peng, C.L.; Zhu, X.R. The neuroprotective effect of overexpression of calbindin-D(28k) in an animal model of Parkinson’s disease. Mol. Neurobiol. 2013, 47, 117–122. [Google Scholar] [CrossRef]
- Evans, R.C.; Zhu, M.; Khaliq, Z.M. Dopamine inhibition differentially controls excitability of substantia nigra dopamine neuron subpopulations through T-Type calcium channels. J. Neurosci. 2017, 37, 3704–3720. [Google Scholar] [CrossRef]
- Anderson, D.; Rehak, R.; Hameed, S.; Mehaffey, W.H.; Zamponi, G.W.; Turner, R.W. Regulation of the K(V)4.2 complex by Ca(V)3.1 calcium channels. Channels 2010, 4, 163–167. [Google Scholar] [CrossRef]
- Anderson, D.; Engbers, J.D.T.; Heath, N.C.; Bartoletti, T.M.; Mehaffey, W.H.; Gerald, W.; Zamponi, G.W.; Turner, R.W. The Cav3–Kv4 complex acts as a calcium sensor to maintain inhibitory charge transfer during extracellular calcium fluctuations. J. Neurosci. 2013, 33, 7811–7824. [Google Scholar] [CrossRef]
- Engbers, J.D.; Zamponi, G.W.; Turner, R.W. Modeling interactions between voltage-gated Ca2+ channels and KCa1.1 channels. Channels 2013, 7, 524–529. [Google Scholar] [CrossRef][Green Version]
- Rehak, R.; Bartoletti, T.M.; Engbers, J.D.T.; Berecki, G.; Turner, R.W.; Zamponi, G.W. Low voltage activation of KCa1.1 current by Cav3-KCa1.1 complexes. PLoS ONE 2013, 8, e61844. [Google Scholar] [CrossRef]
- Turner, R.W.; Zamponi, G.W. T-type channels buddy up. Pflugers Arch. 2014, 466, 661–675. [Google Scholar] [CrossRef][Green Version]
- Kopecky, B.J.; Liang, R.; Bao, J. T-type calcium channel blockers as neuroprotective agents. Pflugers Arch. 2014, 466, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Tai, C.H.; Yang, Y.C.; Pan, M.K.; Huang, C.S.; Kuo, C.C. Modulation of subthalamic T-type Ca2+ channels remedies locomotor deficits in a rat model of Parkinson disease. J. Clin. Investig. 2011, 121, 3289–3305. [Google Scholar] [CrossRef] [PubMed]
- Tabata, Y.; Imaizumi, Y.; Sugawara, M.; Andoh-Noda, T.; Banno, S.; Chai, M.; Sone, T.; Yamazaki, K.; Ito, M.; Tsukahara, K.; et al. T-type Calcium channels determine the vulnerability of dopaminergic neurons to mitochondrial stress in familial Parkinson disease. Stem Cell Rep. 2018, 11, 1171–1184. [Google Scholar] [CrossRef]
- Cohen, C.J.; Spires, S.; Van Skiver, D. Block of T-type Ca channels in Guinea pig atrial cells by antiarrhythmic agents and Ca channel antagonists. J. Gen. Physiol. 1992, 100, 703–728. [Google Scholar] [CrossRef] [PubMed]
- Gaggi, R.; Gianni, A.M. Effects of calcium antagonists on biogenic amines in discrete brain areas. Eur. J. Pharmacol. 1990, 181, 187–197. [Google Scholar] [CrossRef]
- Gaggi, R.; Gianni, A.M. Effects of brain biogenic amines of repeated treatments with calcium antagonists. Jpn. J. Pharmacol. 1991, 56, 33–41. [Google Scholar] [CrossRef]
- Gaggi, R.; Cont, R.; Gianni, A.M. Comparison among the effects of nifedipine, nimodipine and nisoldipine on the brain biogenic amines of normal or haloperidol treated rats. Gen Pharmacol. 1993, 24, 1091–1096. [Google Scholar] [CrossRef]
- Gaggi, R.; Dall’Olio, R.; Roncada, P.; Gianni, A.M. Peculiar effects of isradipine and darodipine on the rat brain dopaminergic system. Gen Pharmacol. 1995, 26, 303–308. [Google Scholar] [CrossRef]
- Brücke, T.; Wöber, C.; Podreka, I.; Wöber-Bingöl, C.; Asenbaum, S.; Aull, S.; Wenger, S.; Ilieva, D.; Harasko-van der Meer, C.; Wessely, P.; et al. D2 receptor blockade by flunarizine and cinnarizine explains extrapyramidal side effects. A SPECT study. J. Cereb. Blood Flow Metab. 1995, 15, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Dall’Igna, O.P.; Tort, A.B.; Souza, D.O.; Lara, D.R. Cinnarizine has an atypical antipsychotic profile in animal models of psychosis. J. Psychopharmacol. 2005, 19, 342–346. [Google Scholar] [CrossRef]
- Teive, H.A.; Troiano, A.R.; Germiniani, F.M.; Werneck, L.C. Flunarizine and cinnarizine-induced parkinsonism: A historical and clinical analysis. Park. Relat. Disord. 2004, 10, 243–245. [Google Scholar] [CrossRef]
- Seeman, P.; Corbett, R.; Van Tol, H.H. Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors. Neuropsychopharmacology 1997, 16, 93–110. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Seeman, P.; Tallerico, T. Antipsychotic drugs which elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol. Psychiatry 1998, 3, 123–134. [Google Scholar] [CrossRef] [PubMed]
- Kapur, S.; Seeman, P. Does fast dissociation from the dopamine D2 receptor explain the action of atypical antipsychotics? A new hypothesis. Am. J. Psychiatry 2001, 158, 360–369. [Google Scholar] [CrossRef]
- Okoro, E.O. Overlap in the pharmacology of L-type Ca2+-channel blockers and 5-HT2 receptor antagonists in rat aorta. J. Pharm. Pharmacol. 1999, 51, 953–957. [Google Scholar] [CrossRef]
- Bymaster, F.P.; Calligaro, D.O.; Falcone, J.F.; Marsh, R.D.; Moore, N.A.; Tye, N.C.; Seeman, P.; Wong, D.T. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 1996, 14, 87–96. [Google Scholar] [CrossRef]
- Neher, E.; Sakaba, T. Multiple roles of calcium ions in the regulation of neurotransmitter release. Neuron 2008, 59, 861–872. [Google Scholar] [CrossRef] [PubMed]
- Kehr, W. 3-Methoxytyramine as an indicator of impulse-induced dopamine release in rat brain in vivo. Naunyn-Schmiedeberg’s Arch. Pharmacol. 1976, 293, 209–215. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Otsu, Y.; Furune, Y.; Tamamoto, T. Different effects of L-, N- and T-type calcium channel blockers on striatal dopamine release measured by microdialysis in freely moving rats. Neurochem. Int. 1992, 21, 99–107. [Google Scholar] [CrossRef]
- Pileblad, E.; Carlsson, A. In vivo effects of the Ca2+-antagonist nimodipine on dopamine metabolism in mouse brain. J. Neural. Transm. 1986, 66, 171–187. [Google Scholar] [CrossRef]
- Pileblad, E.; Carlsson, A. The Ca++-antagonist nimodipine decreases and the Ca++-agonist bay K 8644 increases catecholamine synthesis in mouse brain. Neuropharmacology 1987, 26, 101–105. [Google Scholar] [CrossRef]
- Brimblecombe, K.R.; Connor-Robson, N.; Bataille, C.J.R.; Roberts, B.M.; Gracie, C.; O’Connor, B.; te Water Naude, R.; Karthik, G.; Russell, A.J.; Wade-Martins, R.; et al. Inhibition of striatal dopamine release by the L-type calcium channel inhibitor isradipine co-varies with risk factors for Parkinson’s. Eur. J. Neurosci. 2024, 59, 1242–1259. [Google Scholar] [CrossRef]
- McNaught, K.S.; Mytilineou, C.; Jnobaptiste, R.; Yabut, J.; Shashidharan, P.; Jennert, P.; Olanow, C.W. Impairment of the ubiquitin-proteasome system causes dopaminergic cell death and inclusion body formation in ventral mesencephalic cultures. J. Neurochem. 2002, 81, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Rideout, H.J.; Larsen, K.E.; Sulzer, D.; Stefanis, L. Proteasomal inhibition leads to formation of ubiquitin/alpha-synuclein-immunoreactive inclusions in PC12 cells. J. Neurochem. 2001, 78, 899–908. [Google Scholar] [CrossRef]
- Rideout, H.J.; Lang-Rollin, I.C.; Savalle, M.; Stefanis, L. Dopaminergic neurons in rat ventral midbrain cultures undergo selective apoptosis and form inclusions, but do not up-regulate iHSP70, following proteasomal inhibition. J. Neurochem. 2005, 93, 1304–1313. [Google Scholar] [CrossRef]
- Ding, Q.; Dimayuga, E.; Markesbery, W.R.; Keller, J.N. Proteasome inhibition induces reversible impairments in proteins synthesis. FASEB J. 2006, 20, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Ding, Q.; Cecarini, V.; Keller, J.N. Interplay between protein synthesis and degradation in the CNS: Physiological and pathological implications. Trends Neurosci. 2007, 30, 31–36. [Google Scholar] [CrossRef]
- Hakim, V.; Cohen, L.D.; Zuchman, R.; Ziv, T.; Ziv, N.E. The effects of proteasomal inhibition on synaptic proteostasis. EMBO J. 2016, 35, 2238–2262. [Google Scholar] [CrossRef] [PubMed]
- Landowski, T.H.; Megli, C.J.; Nullmeyer, K.D.; Lynch, R.M.; Dorr, R.T. Mitochondrial-mediated disregulation of Ca2+ is a critical determinant of Velcade (PS-341/bortezomib) cytotoxicity in myeloma cell lines. Cancer Res. 2005, 65, 3828–3836. [Google Scholar] [CrossRef]
- Lee, C.S.; Tee, L.Y.; Warmke, T.; Vinjamoori, A.; Cai, A.; Fagan, A.M.; Snider, B.J. A proteasomal stress response: Pretreatment with proteasome inhibitors increases proteasome activity and reduces neuronal vulnerability to oxidative injury. J. Neurochem. 2004, 91, 996–1006. [Google Scholar] [CrossRef]
- Nawrocki, S.T.; Carew, J.S.; Pino, M.S.; Highshaw, R.A.; Dunner, K., Jr.; Huang, P.; Abbruzzese, J.L.; McConkeyet, D.J. Bortezomib sensitizes pancreatic cancer cells to endoplasmic reticulum stress-mediated apoptosis. Cancer Res. 2005, 65, 11658–11666. [Google Scholar] [CrossRef]
- Verkhratsky, A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005, 85, 201–279. [Google Scholar] [CrossRef]
- Villegas, R.; Martinez, N.W.; Lillo, J.; Pihan, P.; Hernandez, D.; Twiss, J.L.; Court, F.A. Calcium release from intra-axonal endoplasmic reticulum leads to axon degeneration through mitochondrial dysfunction. J. Neurosci. 2014, 34, 7179–7189. [Google Scholar] [CrossRef]
- Őztürk, Z.; O’Kane, C.J.; Perez-Moreno, J.J. Axonal endoplasmic reticulum dynamics and its roles in neurodegeneration. Front Neurosci. 2020, 14, 48. [Google Scholar] [CrossRef] [PubMed]
- Brini, M.; Coletto, L.; Pierobon, N.; Kraev, N.; Guerini, D.; Carafoli, E. A comparative functional analysis of plasma membrane Ca2+ pump isoforms in intact cells. J. Biol. Chem. 2003, 278, 24500–24508. [Google Scholar] [CrossRef] [PubMed]
- Malli, R.; Frieden, M.; Osibow, K.; Zoratti, C.; Mayer, M.; Demaurex, N.; Graier, W.F. Sustained Ca2+ transfer across mitochondria is essential for mitochondrial Ca2+ buffering, sore-operated Ca2+ entry, and Ca2+ store refilling. J. Biol. Chem. 2003, 278, 44769–44779. [Google Scholar] [CrossRef]
- Tong, J.X.; Rich, K.M. Diphenylpiperazines enhance regeneration after facial nerve injury. J. Neurocytol. 1997, 26, 339–347. [Google Scholar] [CrossRef] [PubMed]
- Elimadi, A.; Bouillot, L.; Sapena, R.; Tillement, J.P.; Morin, D. Dose-related inversion of cinnarizine and flunarizine effects on mitochondrial permeability transition. Eur. J. Pharmacol. 1998, 348, 115–121. [Google Scholar] [CrossRef] [PubMed]
- Agholme, L.; Lindström, T.; Kägedal, K.; Marcusson, J.; Hallbeck, M. An in vitro model for neuroscience: Differentiation of SH-SY5Y cells into cells with morphological and biochemical characteristics of mature neurons. J. Alzheimers Dis. 2010, 20, 1069–1082. [Google Scholar] [CrossRef] [PubMed]
- Cheung, Y.T.; Lau, W.K.; Yu, M.; Lai, C.S.; Yeung, S.C.; So, K.F.; Chang, R.C. Effects of all-trans-retinoic acid on human SH-SY5Y neuroblastoma as in vitro model in neurotoxicity research. Neurotoxicology 2009, 30, 127–135. [Google Scholar] [CrossRef]
- Lopes, F.M.; Schröder, R.; da Frota, M.L.; Zanotto-Filho, A., Jr.; Műller, C.B.; Pires, A.S.; Meurer, R.T.; Colpo, G.D.; Gelain, D.P.; Kapczinski, F.; et al. Comparison between proliferative and neuron-like SH-SY5Ycells as an in vitro model for Parkinson disease studies. Brain Res. 2010, 1337, 85–94. [Google Scholar] [CrossRef]
- Yong-Kee, C.J.; Salomonczyk, D.; Nash, J.E. Development and validation of a screening assay for the evaluation of putative neuroprotective agents in the treatment of Parkinson’s disease. Neurotox. Res. 2011, 19, 519–526. [Google Scholar] [CrossRef]
- Yong-Kee, C.J.; Sidorova, E.; Hanif, A.; Perera, G.; Nash, J.E. Mitochondrial dysfunction precedes other sub-cellular abnormalities in an in vitro model linked with cell death in Parkinson’s disease. Neurotox. Res. 2012, 21, 185–194. [Google Scholar] [CrossRef]
- Mann, V.M.; Cooper, J.M.; Krige, D.; Daniel, S.E.; Schapira, A.H.; Marsden, C.D. Brain, skeletal muscle and platelet homogenate mitochondrial function in Parkinson’s disease. Brain 1992, 115, 333–342. [Google Scholar] [CrossRef]
- Schapira, A.H.; Gu, M.; Taanman, J.W.; Tabrizi, S.J.; Seaton, T.; Cleeter, M.; Cooper, J.M. Mitochondria in the etiology and pathogenesis of Parkinson’s disease. Ann. Neurol. 1998, 44 (Suppl. S1), S89–S98. [Google Scholar] [CrossRef]
- Terzioglu, M.; Galter, D. Parkinson’s disease: Genetic versus toxin-induced rodent models. FEBS J. 2008, 275, 1384–1391. [Google Scholar] [CrossRef]
- Navarro, A.; Boveris, A. Brain mitochondrial dysfunction and oxidative damage in Parkinson’s disease. J. Bioenerg. Biomembr. 2009, 41, 517–521. [Google Scholar] [CrossRef]
- Duty, S.; Jenner, P. Animal models of Parkinson’s disease: A source of novel treatments and clues to the cause of the disease. Br. J. Pharmacol. 2011, 164, 1357–1391. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates; Academic Press: San Diego, CA, USA, 1989. [Google Scholar]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Konieczny, J.; Jantas, D.; Lenda, T.; Domin, H.; Czarnecka, A.; Kuter, K.; Śmiałowska, M.; Lasoń, W.; Lorenc-Koci, E. Lack of neuroprotective effect of celastrol under conditions of proteasome inhibition by lactacystin in in vitro and in vivo studies: Implications for Parkinson’s disease. Neurotox Res. 2014, 26, 255–273. [Google Scholar] [CrossRef]
- Czarnecka, A.; Lenda, T.; Domin, H.; Konieczny, J.; Śmiałowska, M.; Lorenc-Koci, E. Alterations in the expression of nNOS in the substantia nigra and subthalamic nucleus of 6-OHDA-lesioned rats: The effects of chronic treatment with l-DOPA and the nitric oxide donor, molsidomine. Brain Res. 2013, 1541, 92–105. [Google Scholar] [CrossRef] [PubMed]
- Jantas, D.; Roman, A.; Kuśmierczyk, J.; Lorenc-Koci, E.; Konieczny, J.; Lenda, T.; Lasoń, W. The extent of neurodegeneration and neuroprotection in two chemical in vitro models related to Parkinson’s disease is critically dependent on cell culture conditions. Neurotox. Res. 2013, 24, 41–54. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lorenc-Koci, E.; Lenda, T.; Konieczny, J.; Jantas, D.; Domin, H. Cinnarizine, a Calcium Channel Blocker, Partially Prevents the Striatal Dopamine Decline and Loss of Nigral Dopamine Neurons in the Lactacystin-Induced Rat Model of Parkinson’s Disease. Int. J. Mol. Sci. 2025, 26, 8833. https://doi.org/10.3390/ijms26188833
Lorenc-Koci E, Lenda T, Konieczny J, Jantas D, Domin H. Cinnarizine, a Calcium Channel Blocker, Partially Prevents the Striatal Dopamine Decline and Loss of Nigral Dopamine Neurons in the Lactacystin-Induced Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences. 2025; 26(18):8833. https://doi.org/10.3390/ijms26188833
Chicago/Turabian StyleLorenc-Koci, Elżbieta, Tomasz Lenda, Jolanta Konieczny, Danuta Jantas, and Helena Domin. 2025. "Cinnarizine, a Calcium Channel Blocker, Partially Prevents the Striatal Dopamine Decline and Loss of Nigral Dopamine Neurons in the Lactacystin-Induced Rat Model of Parkinson’s Disease" International Journal of Molecular Sciences 26, no. 18: 8833. https://doi.org/10.3390/ijms26188833
APA StyleLorenc-Koci, E., Lenda, T., Konieczny, J., Jantas, D., & Domin, H. (2025). Cinnarizine, a Calcium Channel Blocker, Partially Prevents the Striatal Dopamine Decline and Loss of Nigral Dopamine Neurons in the Lactacystin-Induced Rat Model of Parkinson’s Disease. International Journal of Molecular Sciences, 26(18), 8833. https://doi.org/10.3390/ijms26188833






