From Pharmacological Treatment to Neuromodulation: A Comprehensive Approach to Managing Gilles de la Tourette Syndrome
Abstract
1. Introduction
2. A Glimpse into the Complexity of GTS
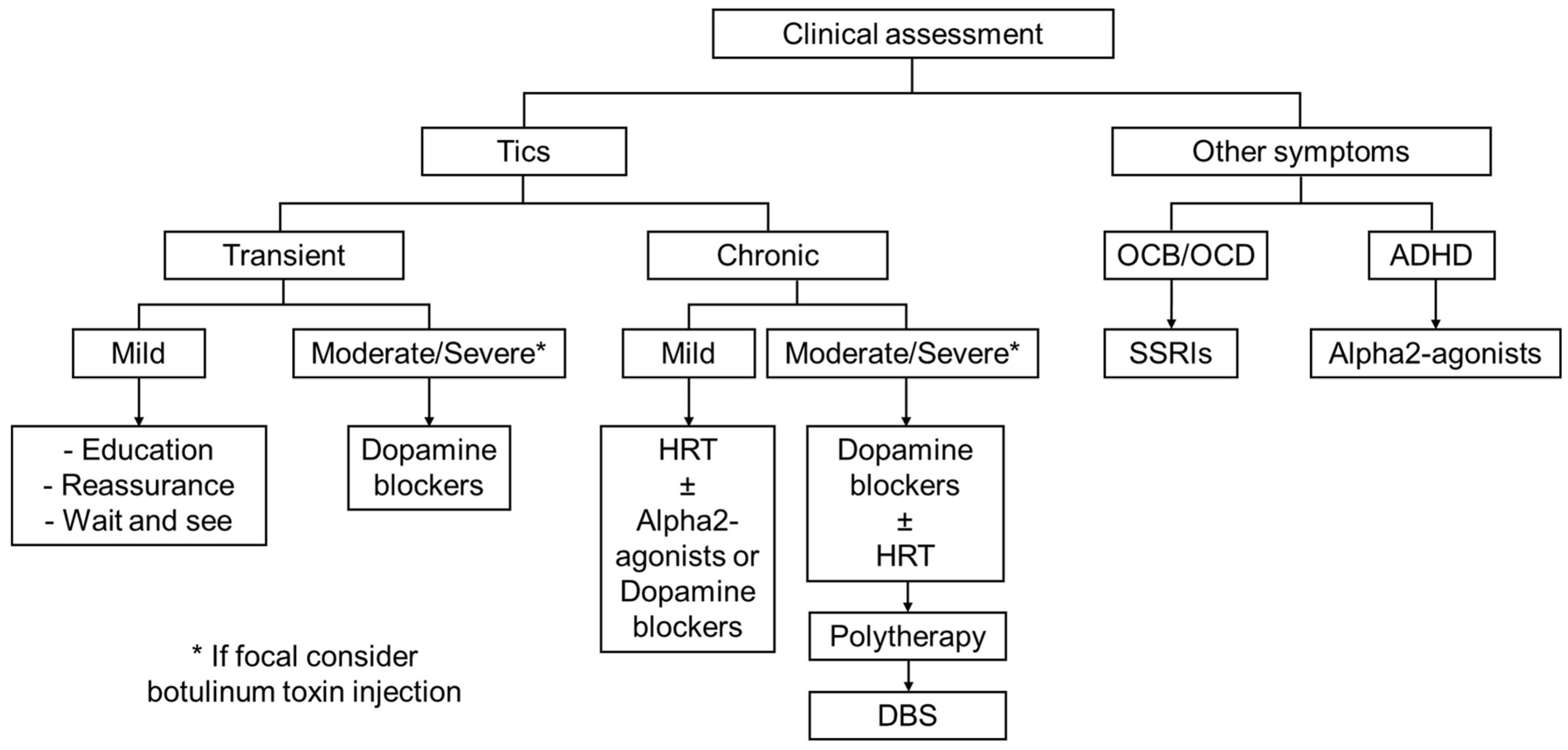
3. General Approach to Tics in GTS
4. Habit Reversal Therapy (HRT)
5. Pharmacology of Tics
5.1. Dopamine Receptor Antagonists
5.2. Monoamine Depletors
5.3. Alpha-2 Receptor Agonists
5.4. Botulinum Toxin
5.5. Antiseizure Drugs
5.6. Cannabinoids
5.7. GABAergic Drugs
5.8. Nicotine
6. Pharmacology of Non-Motor Symptoms
6.1. Obsessive–Compulsive Spectrum
6.2. Attention Deficit and Hyperactivity Spectrum
7. The Case for Neuromodulation in GTS
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Nilles, C.; Hartmann, A.; Roze, E.; Martino, D.; Pringsheim, T. Tourette Syndrome and Other Tic Disorders of Childhood. Handb. Clin. Neurol. 2023, 196, 457–474. [Google Scholar] [CrossRef]
- Ha, A.D.; Jankovic, J. An Introduction to Dyskinesia—The Clinical Spectrum. Int. Rev. Neurobiol. 2011, 98, 1–29. [Google Scholar] [CrossRef]
- Robertson, M.M. A Personal 35 Year Perspective on Gilles de La Tourette Syndrome: Assessment, Investigations, and Management. Lancet Psychiatry 2015, 2, 88–104, Correction in Lancet Psychiatry, 2, 291. [Google Scholar] [CrossRef]
- Lamanna, J.; Ferro, M.; Spadini, S.; Racchetti, G.; Malgaroli, A. The Dysfunctional Mechanisms Throwing Tics: Structural and Functional Changes in Tourette Syndrome. Behav. Sci. 2023, 13, 688. [Google Scholar] [CrossRef] [PubMed]
- Maia, T.V.; Conceição, V.A. Dopaminergic Disturbances in Tourette Syndrome: An Integrative Account. Biol. Psychiatry 2018, 84, 332–344. [Google Scholar] [CrossRef] [PubMed]
- Mahone, E.M.; Puts, N.A.; Edden, R.A.E.; Ryan, M.; Singer, H.S. GABA and Glutamate in Children with Tourette Syndrome: A 1H MR Spectroscopy Study at 7 T. Psychiatry Res. Neuroimaging 2018, 273, 46–53. [Google Scholar] [CrossRef]
- Steeves, T.D.L.; Fox, S.H. Neurobiological Basis of Serotonin-Dopamine Antagonists in the Treatment of Gilles de La Tourette Syndrome. Prog. Brain Res. 2008, 172, 495–513. [Google Scholar] [CrossRef] [PubMed]
- Castellan Baldan, L.; Williams, K.A.; Gallezot, J.D.; Pogorelov, V.; Rapanelli, M.; Crowley, M.; Anderson, G.M.; Loring, E.; Gorczyca, R.; Billingslea, E.; et al. Histidine Decarboxylase Deficiency Causes Tourette Syndrome: Parallel Findings in Humans and Mice. Neuron 2014, 81, 77–90, Erratum in Neuron, 82, 1186–1187. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, C.; Zhong, M.; Che, F.; Guan, C.; Zheng, X.; Liu, S. Role of Histidine Decarboxylase Gene in the Pathogenesis of Tourette Syndrome. Brain Behav. 2022, 12, e2511. [Google Scholar] [CrossRef]
- Lin, W.-D.; Tsai, F.-J.; Chou, I.-C. Current Understanding of the Genetics of Tourette Syndrome. Biomed. J. 2022, 45, 271–279. [Google Scholar] [CrossRef]
- Felling, R.J.; Singer, H.S. Neurobiology of Tourette Syndrome: Current Status and Need for Further Investigation. J. Neurosci. 2011, 31, 12387–12395. [Google Scholar] [CrossRef] [PubMed]
- Müller, N. Tourette’s Syndrome: Clinical Features, Pathophysiology, and Therapeutic Approaches. Dialogues Clin. Neurosci. 2007, 9, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Roessner, V.; Eichele, H.; Stern, J.S.; Skov, L.; Rizzo, R.; Debes, N.M.; Nagy, P.; Cavanna, A.E.; Termine, C.; Ganos, C.; et al. European Clinical Guidelines for Tourette Syndrome and Other Tic Disorders—Version 2.0. Part III: Pharmacological Treatment. Eur. Child. Adolesc. Psychiatry 2022, 31, 425–441. [Google Scholar] [CrossRef] [PubMed]
- Dodel, I.; Reese, J.P.; Müller, N.; Münchau, A.; Balzer-Geldsetzer, M.; Wasem, J.; Oertel, W.H.; Dodel, R.; Müller-Vahl, K. Cost of Illness in Patients with Gilles de La Tourette’s Syndrome. J. Neurol. 2010, 257, 1055–1061. [Google Scholar] [CrossRef]
- Wellen, B.C.M.; Bootes, K.R.; Braley, E.I.; Conelea, C.A.; Woods, D.W.; Himle, M.B. Caregiver Perspectives on the Health Care System for Tic Disorders: Utilization and Barriers. J. Dev. Behav. Pediatr. 2023, 44, E581–E589. [Google Scholar] [CrossRef]
- Iverson, A.M.; Black, K.J. Why Tic Severity Changes from Then to Now and from Here to There. J. Clin. Med. 2022, 11, 5930. [Google Scholar] [CrossRef]
- Groth, C. Tourette Syndrome in a Longitudinal Perspective. Clinical Course of Tics and Comorbidities, Coexisting Psychopathologies, Phenotypes and Predictors. Dan. Med. J. 2018, 65, B5465. [Google Scholar]
- McGuire, J.F.; Piacentini, J.; Brennan, E.A.; Lewin, A.B.; Murphy, T.K.; Small, B.J.; Storch, E.A. A Meta-Analysis of Behavior Therapy for Tourette Syndrome. J. Psychiatr. Res. 2014, 50, 106–112. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Rickards, H. The Psychopathological Spectrum of Gilles de La Tourette Syndrome. Neurosci. Biobehav. Rev. 2013, 37, 1008–1015. [Google Scholar] [CrossRef]
- Dell’Osso, B.; Marazziti, D.; Albert, U.; Pallanti, S.; Gambini, O.; Tundo, A.; Zanaboni, C.; Servello, D.; Rizzo, R.; Scalone, L.; et al. Parsing the Phenotype of Obsessive-Compulsive Tic Disorder (OCTD): A Multidisciplinary Consensus. Int. J. Psychiatry Clin. Pract. 2017, 21, 156–159. [Google Scholar] [CrossRef]
- Pringsheim, T.; Okun, M.S.; Müller-Vahl, K.; Martino, D.; Jankovic, J.; Cavanna, A.E.; Woods, D.W.; Robinson, M.; Jarvie, E.; Roessner, V.; et al. Practice Guideline Recommendations Summary: Treatment of Tics in People with Tourette Syndrome and Chronic Tic Disorders. Neurology 2019, 92, 896–906. [Google Scholar] [CrossRef] [PubMed]
- Szejko, N.; Lombroso, A.; Bloch, M.H.; Landeros-Weisenberger, A.; Leckman, J.F. Refractory Gilles de La Tourette Syndrome—Many Pieces That Define the Puzzle. Front. Neurol. 2020, 11, 589511. [Google Scholar] [CrossRef] [PubMed]
- Willford, S.; Deeb, W. Scoping Review of Multidisciplinary Care in Tourette Syndrome. Mov. Disord. Clin. Pract. 2023, 10, 868–877. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Treatment of Tics Associated with Tourette Syndrome. J. Neural Transm. 2020, 127, 843–850. [Google Scholar] [CrossRef]
- Dy-Hollins, M.E.; Chibnik, L.B.; Tracy, N.A.; Osiecki, L.; Budman, C.L.; Cath, D.C.; Grados, M.A.; King, R.A.; Lyon, G.J.; Rouleau, G.A.; et al. Sex Differences in Natural History and Health Outcomes among Individuals with Tic Disorders. Neurology 2025, 104, e210249. [Google Scholar] [CrossRef]
- Baizabal-Carvallo, J.F.; Jankovic, J. Sex Differences in Patients with Tourette Syndrome. CNS Spectr. 2023, 28, 205–211. [Google Scholar] [CrossRef]
- Azrin, N.H.; Nunn, R.G. Habit-Reversal: A Method of Eliminating Nervous Habits and Tics. Behav. Res. Ther. 1973, 11, 619–628. [Google Scholar] [CrossRef]
- Dutta, N.; Cavanna, A.E. The Effectiveness of Habit Reversal Therapy in the Treatment of Tourette Syndrome and Other Chronic Tic Disorders: A Systematic Review. Funct. Neurol. 2013, 28, 7–12. [Google Scholar]
- Himle, M.B.; Woods, D.W.; Piacentini, J.C.; Walkup, J.T. Brief Review of Habit Reversal Training for Tourette Syndrome. J. Child Neurol. 2006, 21, 719–725. [Google Scholar] [CrossRef]
- Deckersbach, T.; Chou, T.; Britton, J.C.; Carlson, L.E.; Reese, H.E.; Siev, J.; Scahill, L.; Piacentini, J.C.; Woods, D.W.; Walkup, J.T.; et al. Neural Correlates of Behavior Therapy for Tourette’s Disorder. Psychiatry Res. Neuroimaging 2014, 224, 269–274. [Google Scholar] [CrossRef]
- Kapur, S.; Agid, O.; Mizrahi, R.; Li, M. How Antipsychotics Work—From Receptors to Reality. NeuroRx 2006, 3, 10–21. [Google Scholar] [CrossRef]
- Hartmann, A.; Worbe, Y. Pharmacological Treatment of Gilles de La Tourette Syndrome. Neurosci. Biobehav. Rev. 2013, 37, 1157–1161. [Google Scholar] [CrossRef]
- Hassaballa, A.; Balk, R.A. Torsade de Pointes Associated with the Administration of Intravenous Haloperidol: A Review of the Literature and Practical Guidelines for Use. Expert Opin. Drug Saf. 2003, 2, 543–547. [Google Scholar] [CrossRef]
- Huhn, M.; Nikolakopoulou, A.; Schneider-Thoma, J.; Krause, M.; Samara, M.; Peter, N.; Arndt, T.; Bäckers, L.; Rothe, P.; Cipriani, A.; et al. Comparative Efficacy and Tolerability of 32 Oral Antipsychotics for the Acute Treatment of Adults with Multi-Episode Schizophrenia: A Systematic Review and Network Meta-Analysis. Focus 2020, 18, 443–455. [Google Scholar] [CrossRef] [PubMed]
- Huys, D.; Hardenacke, K.; Poppe, P.; Bartsch, C.; Baskin, B.; Kuhn, J. Update on the Role of Antipsychotics in the Treatment of Tourette Syndrome. Neuropsychiatry Dis. Treat. 2012, 8, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Meltzer, H.Y.; Massey, B.W. The Role of Serotonin Receptors in the Action of Atypical Antipsychotic Drugs. Curr. Opin. Pharmacol. 2011, 11, 59–67. [Google Scholar] [CrossRef] [PubMed]
- De Hert, M.; Detraux, J.; Van Winkel, R.; Yu, W.; Correll, C.U. Metabolic and Cardiovascular Adverse Effects Associated with Antipsychotic Drugs. Nat. Rev. Endocrinol. 2012, 8, 114–126. [Google Scholar] [CrossRef]
- Dayabandara, M.; Hanwella, R.; Ratnatunga, S.; Seneviratne, S.; Suraweera, C.; de Silva, V.A. Antipsychotic-Associated Weight Gain: Management Strategies and Impact on Treatment Adherence. Neuropsychiatry Dis. Treat. 2017, 13, 2231. [Google Scholar] [CrossRef]
- Grajales, D.; Ferreira, V.; Valverde, Á.M. Second-Generation Antipsychotics and Dysregulation of Glucose Metabolism: Beyond Weight Gain. Cells 2019, 8, 1336. [Google Scholar] [CrossRef]
- Gaffney, G.R.; Perry, P.J.; Lund, B.C.; Bever-Stille, K.A.; Arndt, S.; Kuperman, S. Risperidone Versus Clonidine in the Treatment of Children and Adolescents with Tourette’s Syndrome. J. Am. Acad. Child. Adolesc. Psychiatry 2002, 41, 330–336. [Google Scholar] [CrossRef]
- Sallee, F.; Kohegyi, E.; Zhao, J.; McQuade, R.; Cox, K.; Sanchez, R.; Van Beek, A.; Nyilas, M.; Carson, W.; Kurlan, R. Randomized, Double-Blind, Placebo-Controlled Trial Demonstrates the Efficacy and Safety of Oral Aripiprazole for the Treatment of Tourette’s Disorder in Children and Adolescents. J. Child. Adolesc. Psychopharmacol. 2017, 27, 771–781. [Google Scholar] [CrossRef] [PubMed]
- Pringsheim, T.; Ho, J.; Sarna, J.R.; Hammer, T.; Patten, S. Feasibility and Relevance of Antipsychotic Safety Monitoring in Children with Tourette Syndrome: A Prospective Longitudinal Study. J. Clin. Psychopharmacol. 2017, 37, 498–504. [Google Scholar] [CrossRef] [PubMed]
- Bačar Bole, C.; Nagode, K.; Pišlar, M.; Mrhar, A.; Grabnar, I.; Vovk, T. Potential Drug-Drug Interactions among Patients with Schizophrenia Spectrum Disorders: Prevalence, Association with Risk Factors, and Replicate Analysis in 2021. Medicina 2023, 59, 284. [Google Scholar] [CrossRef] [PubMed]
- Milosavljević, F.; Bukvić, N.; Pavlović, Z.; Miljević, Č.; Pešić, V.; Molden, E.; Ingelman-Sundberg, M.; Leucht, S.; Jukić, M.M. Association of CYP2C19 and CYP2D6 Poor and Intermediate Metabolizer Status with Antidepressant and Antipsychotic Exposure: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2021, 78, 270–280. [Google Scholar] [CrossRef]
- Gilbert, D.L.; Dubow, J.S.; Cunniff, T.M.; Wanaski, S.P.; Atkinson, S.D.; Mahableshwarkar, A.R. Ecopipam for Tourette Syndrome: A Randomized Trial. Pediatrics 2023, 151, e2022059574. [Google Scholar] [CrossRef]
- Gilbert, D.L.; Murphy, T.K.; Jankovic, J.; Budman, C.L.; Black, K.J.; Kurlan, R.M.; Coffman, K.A.; McCracken, J.T.; Juncos, J.; Grant, J.E.; et al. Ecopipam, a D1 Receptor Antagonist, for Treatment of Tourette Syndrome in Children: A Randomized, Placebo-Controlled Crossover Study. Mov. Disord. 2018, 33, 1272–1280. [Google Scholar] [CrossRef]
- Makhoul, K.; Jankovic, J. Real-World Experience with VMAT2 Inhibitors in Tourette Syndrome. J. Neurol. 2023, 270, 4518–4522. [Google Scholar] [CrossRef]
- Porta, M.; Sassi, M.; Cavallazzi, M.; Fornari, M.; Brambilla, A.; Servello, D. Tourette’s Syndrome and Role of Tetrabenazine: Review and Personal Experience. Clin. Drug Investig. 2008, 28, 443–459. [Google Scholar] [CrossRef]
- Jankovic, J.; Glaze, D.G.; Frost, J.D. Effect of Tetrabenazine on Tics and Sleep of Gilles de La Tourette’s Syndrome. Neurology 1984, 34, 688–692. [Google Scholar] [CrossRef]
- Schneider, F.; Bradbury, M.; Baillie, T.A.; Stamler, D.; Hellriegel, E.; Cox, D.S.; Loupe, P.S.; Savola, J.M.; Rabinovich-Guilatt, L. Pharmacokinetic and Metabolic Profile of Deutetrabenazine (TEV-50717) Compared with Tetrabenazine in Healthy Volunteers. Clin. Transl. Sci. 2020, 13, 707–717. [Google Scholar] [CrossRef]
- Jankovic, J.; Jimenez-Shahed, J.; Budman, C.; Coffey, B.; Murphy, T.; Shprecher, D.; Stamler, D. Deutetrabenazine in Tics Associated with Tourette Syndrome. Tremor Other Hyperkinet. Mov. 2016, 6, 422. [Google Scholar] [CrossRef]
- Farber, R.H.; Angelov, A.; Kim, K.; Carmack, T.; Thai-Cuarto, D.; Roberts, E. Clinical Development of Valbenazine for Tics Associated with Tourette Syndrome. Expert Rev. Neurother. 2021, 21, 393–404. [Google Scholar] [CrossRef]
- Kaur, N.; Kumar, P.; Jamwal, S.; Deshmukh, R.; Gauttam, V. Tetrabenazine: Spotlight on Drug Review. Ann. Neurosci. 2016, 23, 176–185. [Google Scholar] [CrossRef]
- Støve, S.I.; Skjevik, Å.A.; Teigen, K.; Martinez, A. Inhibition of VMAT2 by β2-Adrenergic Agonists, Antagonists, and the Atypical Antipsychotic Ziprasidone. Commun. Biol. 2022, 5, 1283. [Google Scholar] [CrossRef] [PubMed]
- Giovannitti, J.A.; Thoms, S.M.; Crawford, J.J. Alpha-2 Adrenergic Receptor Agonists: A Review of Current Clinical Applications. Anesth. Prog. 2015, 62, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Seideman, M.F.; Seideman, T.A. A Review of the Current Treatment of Tourette Syndrome. J. Pediatr. Pharmacol. Ther. 2020, 25, 401–412. [Google Scholar] [CrossRef] [PubMed]
- Neuchat, E.E.; Bocklud, B.E.; Kingsley, K.; Barham, W.T.; Luther, P.M.; Ahmadzadeh, S.; Shekoohi, S.; Cornett, E.M.; Kaye, A.D. The Role of Alpha-2 Agonists for Attention Deficit Hyperactivity Disorder in Children: A Review. Neurol. Int. 2023, 15, 697–707. [Google Scholar] [CrossRef]
- Scahill, L.; Chappell, P.B.; Kim, Y.S.; Schultz, R.T.; Katsovich, L.; Shepherd, E.; Arnsten, A.F.T.; Cohen, D.J.; Leckman, J.F. A Placebo-Controlled Study of Guanfacine in the Treatment of Children with Tic Disorders and Attention Deficit Hyperactivity Disorder. Am. J. Psychiatry 2001, 158, 1067–1074. [Google Scholar] [CrossRef]
- Prince, J.B.; Wilens, T.E.; Biederman, J.; Spencer, T.J.; Wozniak, J.R. Clonidine for Sleep Disturbances Associated with Attention-Deficit Hyperactivity Disorder: A Systematic Chart Review of 62 Cases. J. Am. Acad. Child Adolesc. Psychiatry 1996, 35, 599–605. [Google Scholar] [CrossRef]
- Bloch, M.H.; Panza, K.E.; Landeros-Weisenberger, A.; Leckman, J.F. Meta-Analysis: Treatment of Attention-Deficit/Hyperactivity Disorder in Children with Comorbid Tic Disorders. J. Am. Acad. Child Adolesc. Psychiatry 2009, 48, 884–893. [Google Scholar] [CrossRef]
- Simpson, L.L. The Origin, Structure, and Pharmacological Activity of Botulinum Toxin. Pharmacol. Rev. 1981, 33, 155–188. [Google Scholar] [CrossRef]
- Porta, M.; Maggioni, G.; Ottaviani, F.; Schindler, A. Treatment of Phonic Tics in Patients with Tourette’s Syndrome Using Botulinum Toxin Type A. Neurol. Sci. 2004, 24, 420–423. [Google Scholar] [CrossRef] [PubMed]
- Marras, C.; Andrews, D.; Sime, E.; Lang, A.E. Botulinum Toxin for Simple Motor Tics: A Randomized, Double-Blind, Controlled Clinical Trial. Neurology 2001, 56, 605–610. [Google Scholar] [CrossRef] [PubMed]
- Hull, M.; Parnes, M.; Jankovic, J. Botulinum Neurotoxin Injections in Children with Self-Injurious Behaviors. Toxins 2023, 15, 236. [Google Scholar] [CrossRef] [PubMed]
- Kwak, C.H.; Hanna, P.A.; Jankovic, J. Botulinum Toxin in the Treatment of Tics. Arch. Neurol. 2000, 57, 1190–1193. [Google Scholar] [CrossRef]
- Sills, G.J.; Rogawski, M.A. Mechanisms of Action of Currently Used Antiseizure Drugs. Neuropharmacology 2020, 168, 107966. [Google Scholar] [CrossRef]
- Cavanna, A.E.; Nani, A. Antiepileptic Drugs and Tourette Syndrome. Int. Rev. Neurobiol. 2013, 112, 373–389. [Google Scholar] [CrossRef]
- Jankovic, J.; Jimenez-Shahed, J.; Brown, L.W. A Randomised, Double-Blind, Placebo-Controlled Study of Topiramate in the Treatment of Tourette Syndrome. J. Neurol. Neurosurg. Psychiatry 2010, 81, 70–73. [Google Scholar] [CrossRef]
- Smith-Hicks, C.L.; Bridges, D.D.; Paynter, N.P.; Singer, H.S. A Double Blind Randomized Placebo Control Trial of Levetiracetam in Tourette Syndrome. Mov. Disord. 2007, 22, 1764–1770. [Google Scholar] [CrossRef]
- Hedderick, E.F.; Morris, C.M.; Singer, H.S. Double-Blind, Crossover Study of Clonidine and Levetiracetam in Tourette Syndrome. Pediatr. Neurol. 2009, 40, 420–425. [Google Scholar] [CrossRef]
- Prisco, L.; Ganau, M.; Bigotto, F.; Zornada, F. Trigeminal Neuralgia: Successful Antiepileptic Drug Combination Therapy in Three Refractory Cases. Drug Healthc. Patient Saf. 2011, 3, 43–45. [Google Scholar] [CrossRef] [PubMed]
- Legare, C.A.; Raup-Konsavage, W.M.; Vrana, K.E. Therapeutic Potential of Cannabis, Cannabidiol, and Cannabinoid-Based Pharmaceuticals. Pharmacology 2022, 107, 131–149. [Google Scholar] [CrossRef] [PubMed]
- Müller-Vahl, K.R.; Koblenz, A.; Jöbges, M.; Kolbe, H.; Emrich, H.M.; Schneider, U. Influence of Treatment of Tourette Syndrome with Δ9-Tetrahydrocannabinol (Δ9-THC) on Neuropsychological Performance. Pharmacopsychiatry 2001, 34, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Szejko, N.; Saramak, K.; Lombroso, A.; Müller-Vahl, K.R. Cannabis-Based Medicine in Treatment of Patients with Gilles de La Tourette Syndrome. Neurol. Neurochir. Pol. 2022, 56, 28–38. [Google Scholar] [CrossRef]
- Murray, R.M.; Englund, A.; Abi-Dargham, A.; Lewis, D.A.; Di Forti, M.; Davies, C.; Sherif, M.; McGuire, P.; D’Souza, D.C. Cannabis-Associated Psychosis: Neural Substrate and Clinical Impact. Neuropharmacology 2017, 124, 89–104. [Google Scholar] [CrossRef]
- Lima, L.; Salazar, M.; Trejo, E. Modulation of 5HT1A Receptors in the Hippocampus and the Raphe Area of Rats Treated with Clonazepam. Prog. Neuropsychopharmacol. Biol. Psychiatry 1993, 17, 663–677. [Google Scholar] [CrossRef]
- Goetz, C.G. Clonidine and Clonazepam in Tourette Syndrome. Adv. Neurol. 1992, 58, 245–251. [Google Scholar]
- Truong, D.D.; Bressman, S.; Shale, H.; Fahn, S. Clonazepam, Haloperidol, and Clonidine in Tic Disorders. South. Med. J. 1988, 81, 1103–1105. [Google Scholar] [CrossRef]
- Awaad, Y. Tics in Tourette Syndrome: New Treatment Options. J. Child Neurol. 1999, 14, 316–319. [Google Scholar] [CrossRef]
- Singer, H.S.; Wendlandt, J.; Krieger, M.; Giuliano, J. Baclofen Treatment in Tourette Syndrome: A Double-Blind, Placebo-Controlled, Crossover Trial. Neurology 2001, 56, 599–604. [Google Scholar] [CrossRef]
- Dani, J.A.; Bertrand, D. Nicotinic Acetylcholine Receptors and Nicotinic Cholinergic Mechanisms of the Central Nervous System. Annu. Rev. Pharmacol. Toxicol. 2007, 47, 699–729. [Google Scholar] [CrossRef] [PubMed]
- Devor, E.J.; Isenberg, K.E. Nicotine and Tourette’s Syndrome. Lancet 1989, 334, 1046. [Google Scholar] [CrossRef] [PubMed]
- Silver, A.A.; Shytle, R.D.; Philipp, M.K.; Wilkinson, B.J.; McConville, B.; Sanberg, P.R. Transdermal Nicotine and Haloperidol in Tourette’s Disorder: A Double-Blind Placebo-Controlled Study. J. Clin. Psychiatry 2001, 62, 707–714. [Google Scholar] [CrossRef] [PubMed]
- Sanberg, P.R.; Silver, A.A.; Shytle, R.D.; Philipp, M.K.; Cahill, D.W.; Fogelson, H.M.; McConville, B.J. Nicotine for the Treatment of Tourette’s Syndrome. Pharmacol. Ther. 1997, 74, 21–25. [Google Scholar] [CrossRef]
- Howson, A.L.; Batth, S.; Ilivitsky, V.; Boisjoli, A.; Jaworski, M.; Mahoney, C.; Knott, V.J. Clinical and Attentional Effects of Acute Nicotine Treatment in Tourette’s Syndrome. Eur. Psychiatry 2004, 19, 102–112. [Google Scholar] [CrossRef]
- Can, A.; Vermilion, J.; Mink, J.W.; Morrison, P. Pharmacological Treatment of Tourette Disorder in Children. J. Child Adolesc. Psychopharmacol. 2024, 34, 346–352. [Google Scholar] [CrossRef]
- Rua, A.; Damásio, J. Tics Induced by Sertraline: Case Report and Literature Review. Mov. Disord. Clin. Pract. 2014, 1, 243–244. [Google Scholar] [CrossRef]
- Bonnier, C.; Nassogne, M.C.; Evrard, P. Ketanserin Treatment of Tourette’s Syndrome in Children. Am. J. Psychiatry 1999, 156, 1122–1123. [Google Scholar] [CrossRef]
- Toren, P.; Weizman, A.; Ratner, S.; Cohen, D.; Laor, N. Ondansetron Treatment in Tourette’s Disorder: A 3-Week, Randomized, Double-Blind, Placebo-Controlled Study. J. Clin. Psychiatry 2005, 66, 499–503. [Google Scholar] [CrossRef]
- Billnitzer, A.; Jankovic, J. Pilot Study to Evaluate Pimavanserin for the Treatment of Motor and Behavioral Symptoms of Tourette Syndrome. Mov. Disord. Clin. Pract. 2021, 8, 694–700. [Google Scholar] [CrossRef]
- Nafisa, D.; Kakunje, A. Aripiprazole-Induced Obsessive-Compulsive Symptoms. Ind. Psychiatry J. 2022, 31, 158–161. [Google Scholar] [CrossRef] [PubMed]
- Rapinesi, C.; Kotzalidis, G.D.; Ferracuti, S.; Sani, G.; Girardi, P.; Del Casale, A. Brain Stimulation in Obsessive-Compulsive Disorder (OCD): A Systematic Review. Curr. Neuropharmacol. 2019, 17, 787–807. [Google Scholar] [CrossRef] [PubMed]
- Allen, A.J.; Kurlan, R.M.; Gilbert, D.L.; Coffey, B.J.; Linder, S.L.; Lewis, D.W.; Winner, P.K.; Dunn, D.W.; Dure, L.S.; Sallee, F.R.; et al. Atomoxetine Treatment in Children and Adolescents with ADHD and Comorbid Tic Disorders. Neurology 2005, 65, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Mink, J.W.; Walkup, J.; Frey, K.A.; Como, P.; Cath, D.; DeLong, M.R.; Erenberg, G.; Jankovic, J.; Juncos, J.; Leckman, J.F.; et al. Patient Selection and Assessment Recommendations for Deep Brain Stimulation in Tourette Syndrome. Mov. Disord. 2006, 21, 1831–1838. [Google Scholar] [CrossRef]
- McIntyre, C.C.; Savasta, M.; Kerkerian-Le Goff, L.; Vitek, J.L. Uncovering the Mechanism(s) of Action of Deep Brain Stimulation: Activation, Inhibition, or Both. Clin. Neurophysiol. 2004, 115, 1239–1248. [Google Scholar] [CrossRef]
- Casagrande, S.C.B.; Cury, R.G.; Alho, E.J.L.; Fonoff, E.T. Deep Brain Stimulation in Tourette’s Syndrome: Evidence to Date. Neuropsychiatry Dis. Treat. 2019, 15, 1061–1075. [Google Scholar] [CrossRef]
- Vandehey, N.T.; Garell, P.C.; Hampel, J.A.; Murali, D.; Smith, E.M.; Davidson, R.; Converse, A.K.; Nickles, R.J.; Christian, B.T. PET Measurement of Changes in D2/D3 Dopamine Receptor Binding in a Nonhuman Primate during Chronic Deep Brain Stimulation of the Bed Nucleus of the Stria Terminalis. J. Neurosci. Methods 2009, 176, 129–135. [Google Scholar] [CrossRef][Green Version]
- Vandewalle, V.; Van Der Linden, C.; Groenewegen, H.J.; Caemaert, J. Stereotactic Treatment of Gilles de La Tourette Syndrome by High Frequency Stimulation of Thalamus. Lancet 1999, 353, 724. [Google Scholar] [CrossRef]
- Schrock, L.E.; Mink, J.W.; Woods, D.W.; Porta, M.; Servello, D.; Visser-Vandewalle, V.; Silburn, P.A.; Foltynie, T.; Walker, H.C.; Shahed-Jimenez, J.; et al. Tourette Syndrome Deep Brain Stimulation: A Review and Updated Recommendations. Mov. Disord. 2015, 30, 448–471. [Google Scholar] [CrossRef]
- Najera, R.A.; Provenza, N.; Dang, H.; Katlowitz, K.A.; Hertz, A.; Reddy, S.; Shofty, B.; Bellows, S.T.; Storch, E.A.; Goodman, W.K.; et al. Dual-Target Deep Brain Stimulation for Obsessive-Compulsive Disorder and Tourette Syndrome. Biol. Psychiatry 2023, 93, e53–e55. [Google Scholar] [CrossRef]
- Saleh, C.; Gonzalez, V.; Cif, L.; Coubes, P. Deep Brain Stimulation of the Globus Pallidus Internus and Gilles de La Tourette Syndrome: Toward Multiple Networks Modulation. Surg. Neurol. Int. 2012, 3 (Suppl. 2), S127. [Google Scholar] [CrossRef]
- Servello, D.; Zekaj, E.; Saleh, C.; Dina, C.Z.; Porta, M. Sixteen Years of Deep Brain Stimulation in Tourette’s Syndrome: A Critical Review. J. Neurosurg. Sci. 2016, 60, 218–229. [Google Scholar]
- Servello, D.; Zekaj, E.; Saleh, C.; Lange, N.; Porta, M. Deep Brain Stimulation in Gilles de la Tourette Syndrome: What Does the Future Hold? A Cohort of 48 Patients. Neurosurgery 2016, 78, 91–100. [Google Scholar] [CrossRef]
- Szejko, N.; Worbe, Y.; Hartmann, A.; Visser-Vandewalle, V.; Ackermans, L.; Ganos, C.; Porta, M.; Leentjens, A.F.G.; Mehrkens, J.H.; Huys, D.; et al. European Clinical Guidelines for Tourette Syndrome and Other Tic Disorders—Version 2.0. Part IV: Deep Brain Stimulation. Eur. Child Adolesc. Psychiatry 2022, 31, 443–461. [Google Scholar] [CrossRef] [PubMed]
- Dowd, R.S.; Pourfar, M.; Mogilner, A.Y. Deep Brain Stimulation for Tourette Syndrome: A Single-Center Series. J. Neurosurg. 2018, 128, 596–604. [Google Scholar] [CrossRef] [PubMed]
- Saleh, C.; Hasler, G. Deep Brain Stimulation for Psychiatric Disorders: Is There an Impact on Social Functioning? Surg. Neurol. Int. 2017, 8, 134. [Google Scholar] [CrossRef] [PubMed]
- Diamond, A.; Kenney, C.; Jankovic, J. Effect of Vagal Nerve Stimulation in a Case of Tourette’s Syndrome and Complex Partial Epilepsy. Mov. Disord. 2006, 21, 1273–1275. [Google Scholar] [CrossRef]
- Grados, M.; Huselid, R.; Duque-Serrano, L. Transcranial Magnetic Stimulation in Tourette Syndrome: A Historical Perspective, Its Current Use and the Influence of Comorbidities in Treatment Response. Brain Sci. 2018, 8, 129. [Google Scholar] [CrossRef]
- Eapen, V.; Baker, R.; Walter, A.; Raghupathy, V.; Wehrman, J.J.; Sowman, P.F. The Role of Transcranial Direct Current Stimulation (TDCS) in Tourette Syndrome: A Review and Preliminary Findings. Brain Sci. 2017, 7, 161. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monfrini, E.; Saleh, C.; Servello, D.; Jaszczuk, P.; Porta, M. From Pharmacological Treatment to Neuromodulation: A Comprehensive Approach to Managing Gilles de la Tourette Syndrome. Int. J. Mol. Sci. 2025, 26, 8831. https://doi.org/10.3390/ijms26188831
Monfrini E, Saleh C, Servello D, Jaszczuk P, Porta M. From Pharmacological Treatment to Neuromodulation: A Comprehensive Approach to Managing Gilles de la Tourette Syndrome. International Journal of Molecular Sciences. 2025; 26(18):8831. https://doi.org/10.3390/ijms26188831
Chicago/Turabian StyleMonfrini, Edoardo, Christian Saleh, Domenico Servello, Phillip Jaszczuk, and Mauro Porta. 2025. "From Pharmacological Treatment to Neuromodulation: A Comprehensive Approach to Managing Gilles de la Tourette Syndrome" International Journal of Molecular Sciences 26, no. 18: 8831. https://doi.org/10.3390/ijms26188831
APA StyleMonfrini, E., Saleh, C., Servello, D., Jaszczuk, P., & Porta, M. (2025). From Pharmacological Treatment to Neuromodulation: A Comprehensive Approach to Managing Gilles de la Tourette Syndrome. International Journal of Molecular Sciences, 26(18), 8831. https://doi.org/10.3390/ijms26188831







