Neuroprotective Effects of Low-Dose Graphenic Materials on SN4741 Embryonic Stem Cells Against ER Stress and MPTP-Induced Oxidative Stress
Abstract
1. Introduction
2. Results
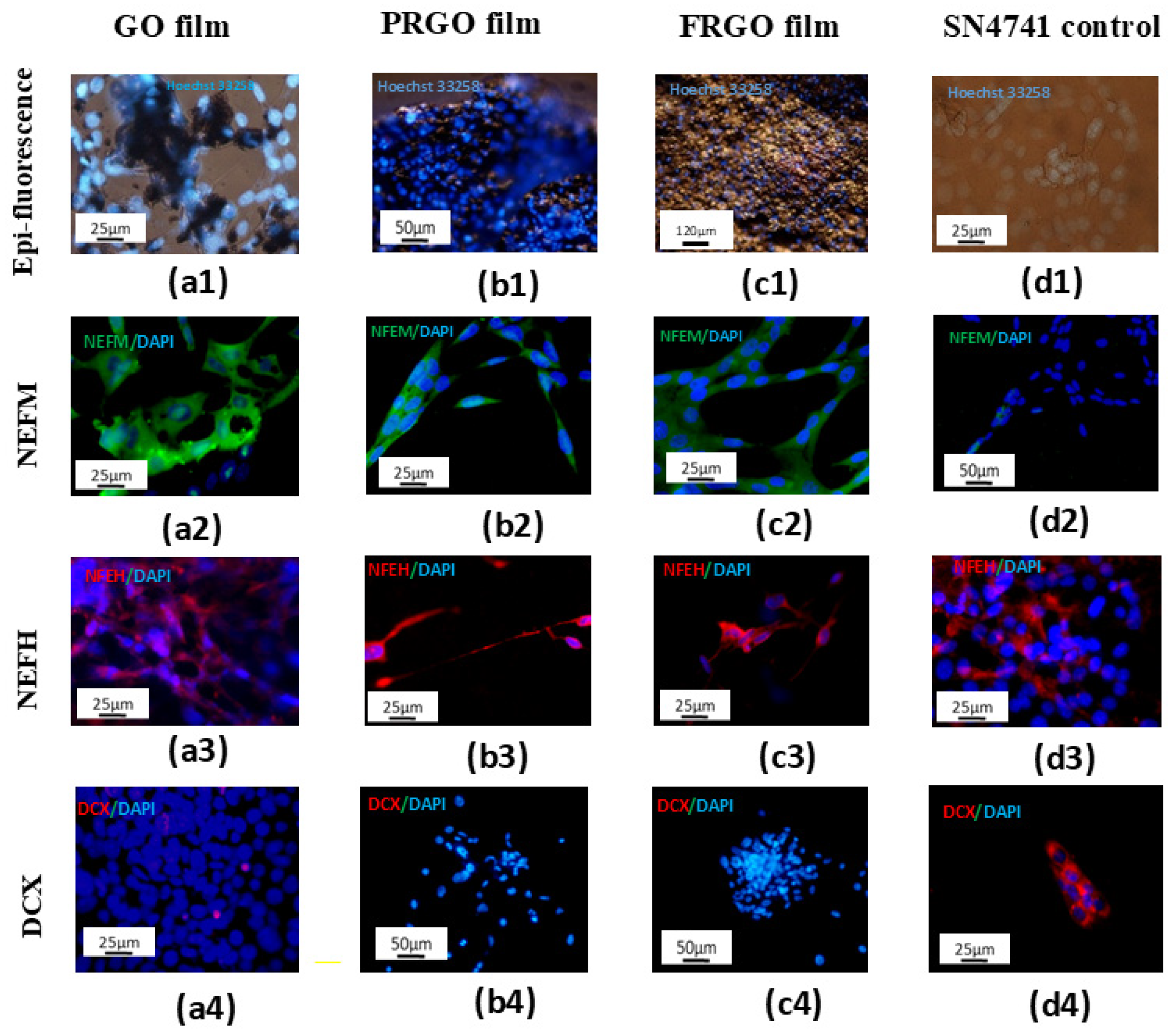
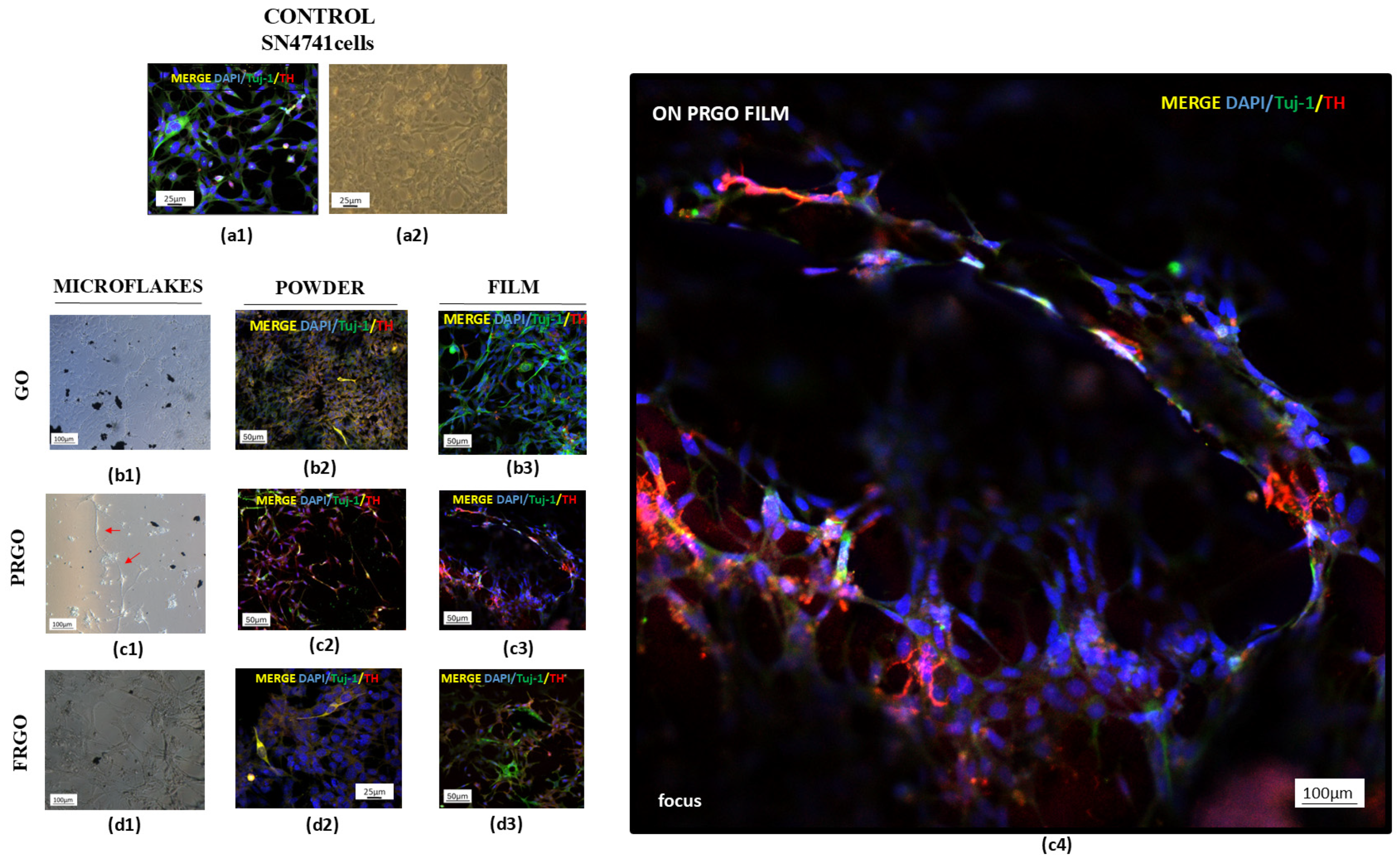
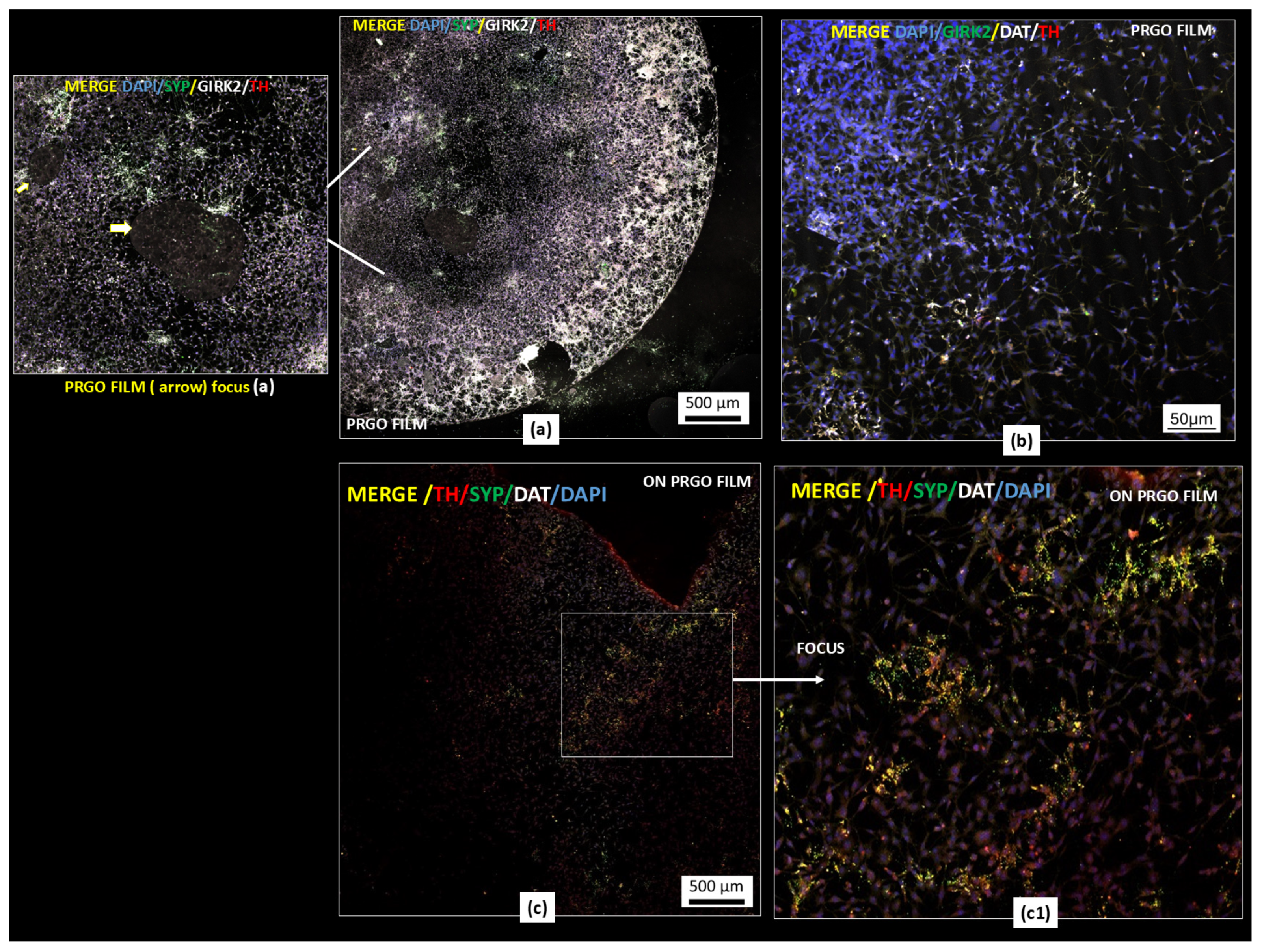
2.1. Maturation and Integrity of DA Phenotype in Culture with GMs
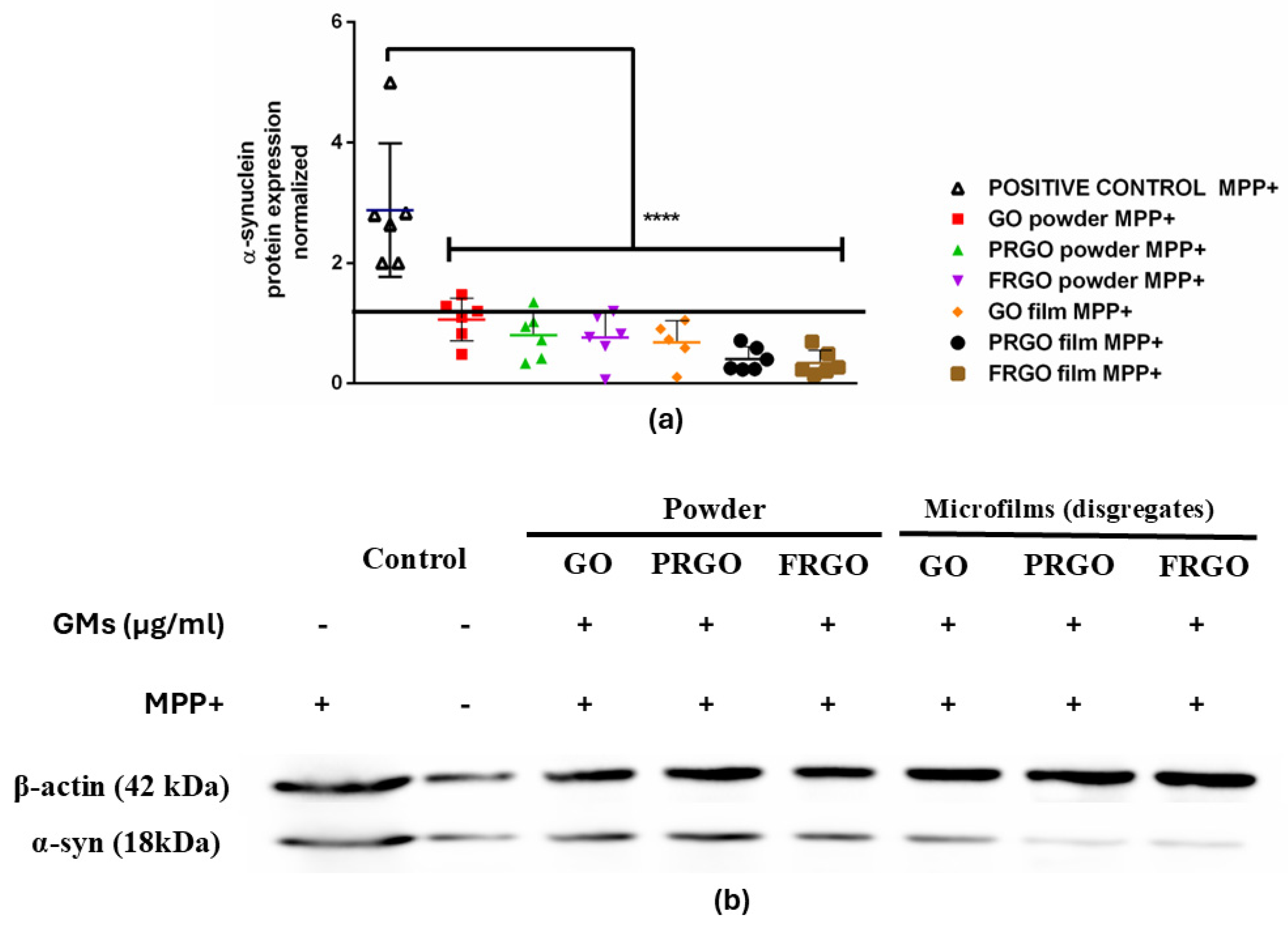
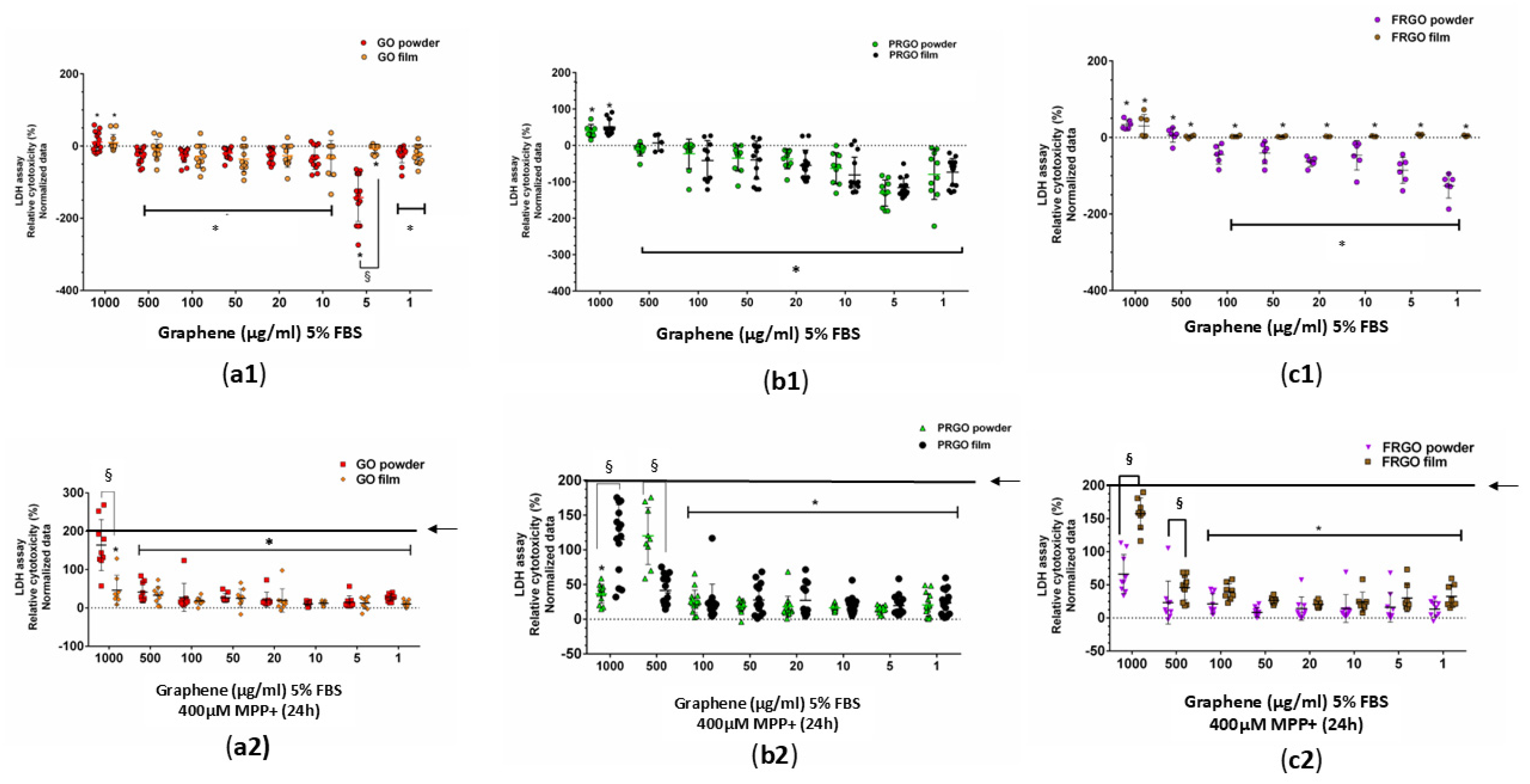
2.2. Neuroprotective Potential of Graphenic Materials Against MPP+-Induced Mitochondrial Dysfunction and α-Synuclein Accumulation
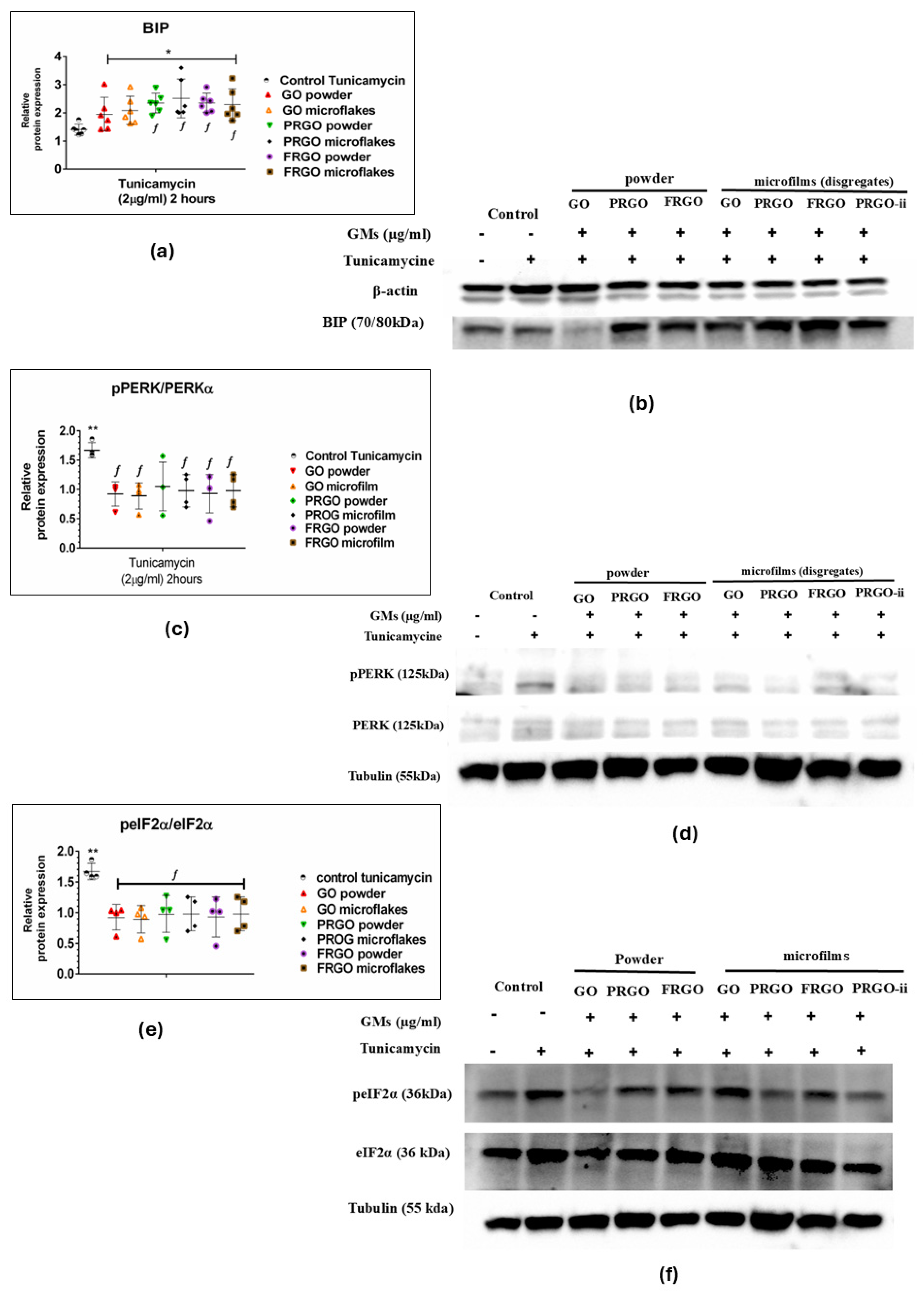
2.3. Neuroprotective Effects of GMs Against Tunicamycin-Induced Endoplasmic Reticulum Stress via Modulation of the Pre-Adaptive Stress Response
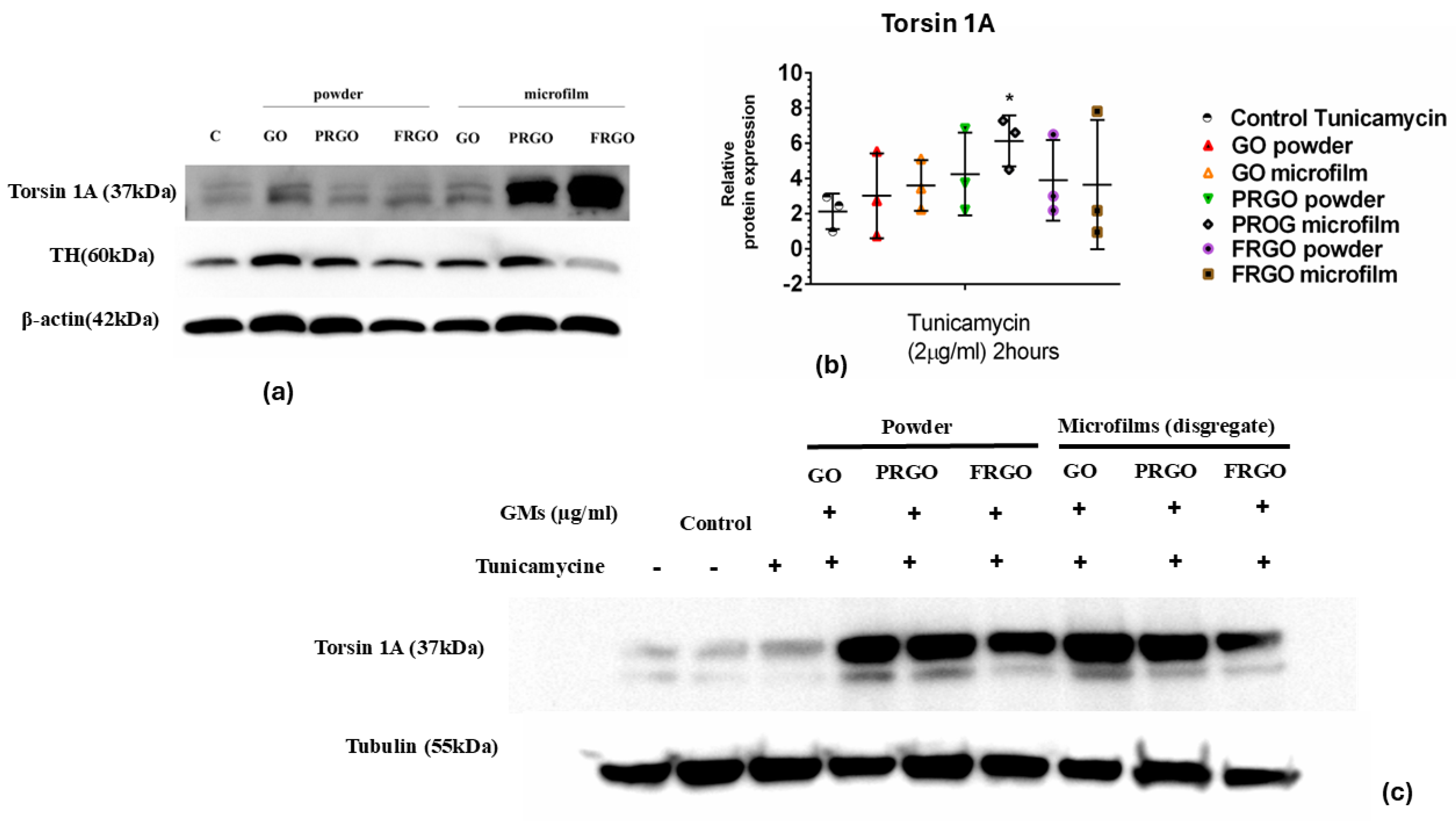
2.4. Exploring the Role of Torsin 1A in Tunicamycin-Induced ER Stress and the Modulatory Effects of GMs
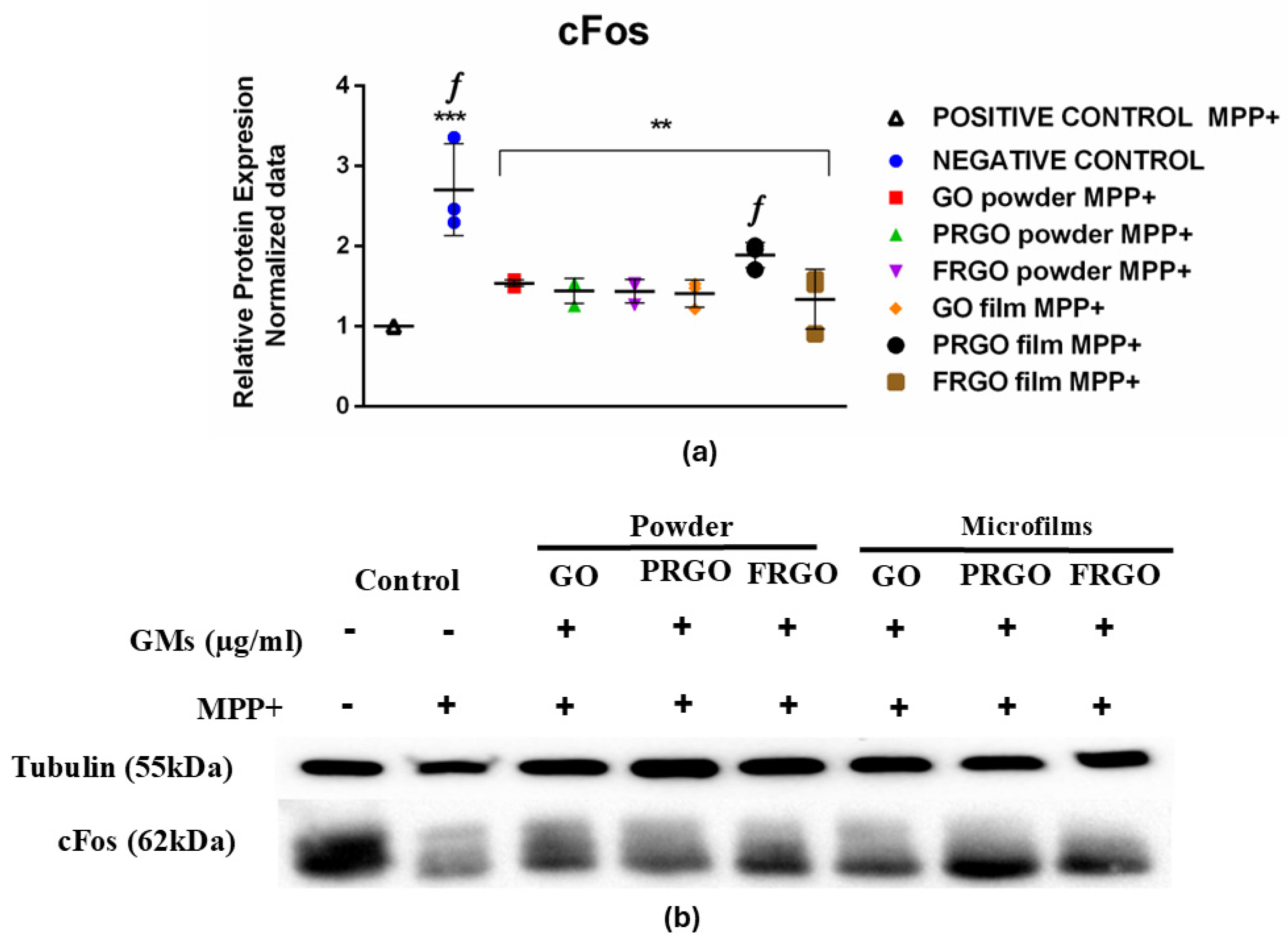
2.5. Analysis of cFos Response Concerning MPP+-Induced Oxidative Stress and the Protective Effects of GMs
3. Discussion
3.1. Dopaminergic Maturation
3.2. Neuroprotective Effect at Low-GM Concentrations
3.3. Oxidative Response of MPP+ in SN4741 with GMs
3.4. GM Maturation Related to Capacity to Respond to Stress Induced by Tunicamycin
3.5. Pre-Adaptative Response to Stress: A Potential Role of cFos
4. Materials and Methods
4.1. GM Production
4.2. Exposure of SN4741 Cells to GO, PRGO, and FRGO in Both Powder and Film Forms
4.3. LDH Assay: Study of Neuroprotective Capacity of GMs Against MPP+ Treatment
4.4. Western Blot Analysis of Proteins
4.5. Immunostaining Assay
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| GMs | graphenic materials |
| DCX | Doublecortin |
| ER | endoplasmic reticulum |
| FRGO | fully reduced graphene oxide |
| DAT | dopamine transporter |
| G | graphene |
| GIRK2 | G-protein-regulated inward-rectifier potassium channel 2 protein |
| GO | graphene oxide |
| IEG | immediate early gene |
| LDH | lactate dehydrogenase |
| MPP+ | 1-methyl-4-phenylpyridinium ion |
| MTPT | 1-methyl-4-phenylpyridinium |
| PD | Parkinson’s disease |
| PRGO | partially reduced graphene oxide |
| TH | tyrosine hydroxylase |
| Tuj-1 | beta-III tubulin |
| UPR | unfolded protein response |
| α-Syn | α-synuclein |
References
- Schapira, A.H.V.; Chaudhuri, K.R.; Jenner, P. Non-motor features of Parkinson disease. Nat. Rev. Neurosci. 2017, 18, 435–450, Erratum in Nat. Rev. Neurosci. 2017, 18, 509. https://doi.org/10.1038/nrn.2017.91. [Google Scholar] [CrossRef]
- Parish, C.L.; Arenas, E. Stem-cell-based strategies for the treatment of Parkinson’s disease. Neurodegener Dis. 2007, 4, 339–347. [Google Scholar] [CrossRef]
- Saffie-Awad, P.; Grant, S.M.; Makarious, M.B.; Elsayed, I.; Sanyaolu, A.O.; Crea, P.W.; Schumacher Schuh, A.F.; Levine, K.S.; Vitale, D.; Koretsky, M.J.; et al. Insights into ancestral diversity in Parkinson’s disease risk: A comparative assessment of polygenic risk scores. NPJ Park. Dis. 2025, 11, 201. [Google Scholar] [CrossRef]
- Yang, H.M. Mitochondrial Dysfunction in Neurodegenerative Diseases. Cells 2025, 14, 276. [Google Scholar] [CrossRef]
- Yuan, S.; She, D.; Jiang, S.; Deng, N.; Peng, J.; Ma, L. Endoplasmic reticulum stress and therapeutic strategies in metabolic, neurodegenerative diseases and cancer. Mol. Med. 2024, 30, 40. [Google Scholar] [CrossRef]
- Su, H.Y.; Waldron, R.T.; Gong, R.; Ramanujan, V.K.; Pandol, S.J.; Lugea, A. The Unfolded Protein Response Plays a Predominant Homeostatic Role in Response to Mitochondrial Stress in Pancreatic Stellate Cells. PLoS ONE 2016, 11, e0148999. [Google Scholar] [CrossRef] [PubMed]
- Yasser, M.B.; Hagag, R.S.; El-Sayed, N.M.; Hazem, R.M. Sinapic acid attenuates PERK-dependent ER stress signaling and resolves α-synuclein pathology in rotenone-induced Parkinson’s disease rat model. Neuropharmacology 2025, 279, 110621. [Google Scholar] [CrossRef] [PubMed]
- de Kloet, E.R.; Joëls, M.; Holsboer, F. Stress and the brain: From adaptation to disease. Nat. Rev. Neurosci. 2005, 6, 463–475. [Google Scholar] [CrossRef] [PubMed]
- Beauvais, G.; Rodriguez-Losada, N.; Ying, L.; Zakirova, Z.; Watson, J.L.; Readhead, B.; Gadue, P.; French, D.L.; Ehrlich, M.E.; Gonzalez-Alegre, P. Exploring the Interaction Between eIF2α Dysregulation, Acute Endoplasmic Reticulum Stress and DYT1 Dystonia in the Mammalian Brain. Neuroscience 2018, 371, 455–468. [Google Scholar] [CrossRef]
- Singh, R.; Kaur, N.; Choubey, V.; Dhingra, N.; Kaur, T. Endoplasmic reticulum stress and its role in various neurodegenerative diseases. Brain Res. 2024, 1826, 148742. [Google Scholar] [CrossRef]
- Li, X.; Zhuang, Y.; Zhang, Y.R.; Fan, K.K.; Chen, X.X.; Chen, X.X.; Liu, X.Y.; Sun, J.; Liu, L. Combined inhibition of dopamine D1/D2 receptors induces cognitive and emotional dysfunction through oxidative stress and dopaminergic neuron damage. Front. Behav. Neurosci. 2025, 19, 1621017. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Kong, X.; Qiao, J.; Wei, J. Decoding Parkinson’s Disease: The interplay of cell death pathways, oxidative stress, and therapeutic innovations. Redox Biol. 2025, 85, 103787. [Google Scholar] [CrossRef] [PubMed]
- Mudò, G.; Mäkelä, J.; Di Liberto, V.; Tselykh, T.V.; Olivieri, M.; Piepponen, P.; Eriksson, O.; Mälkiä, A.; Bonomo, A.; Kairisalo, M.; et al. Transgenic expression and activation of PGC-1α protect dopaminergic neurons in the MPTP mouse model of Parkinson’s disease. Cell. Mol. Life Sci. 2012, 69, 1153–1165. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Pan, R.Y.; Guan, F.; Yuan, Z. Lactate metabolism in neurodegenerative diseases. Neural Regen. Res. 2024, 19, 69–74. [Google Scholar] [CrossRef]
- Bandres-Ciga, S.; Ahmed, S.; Sabir, M.S.; Blauwendraat, C.; Adarmes-Gómez, A.D.; Bernal-Bernal, I.; Bonilla-Toribio, M.; Buiza-Rueda, D.; Carrillo, F.; Carrión-Claro, M.; et al. The Genetic Architecture of Parkinson Disease in Spain: Characterizing Population-Specific Risk, Differential Haplotype Structures, and Providing Etiologic Insight. Mov. Disord. 2019, 34, 1851–1863. [Google Scholar] [CrossRef]
- Li, J.; Chen, L.; Qin, Q.; Wang, D.; Zhao, J.; Gao, H.; Yuan, X.; Zhang, J.; Zou, Y.; Mao, Z.; et al. Upregulated hexokinase 2 expression induces the apoptosis of dopaminergic neurons by promoting lactate production in Parkinson’s disease. Neurobiol. Dis. 2022, 163, 105605. [Google Scholar] [CrossRef]
- Schirinzi, T.; Di Lazzaro, G.; Sancesario, G.M.; Summa, S.; Petrucci, S.; Colona, V.L.; Bernardini, S.; Pierantozzi, M.; Stefani, A.; Mercuri, N.B.; et al. Young-onset and late-onset Parkinson’s disease exhibit a different profile of fluid biomarkers and clinical features. Neurobiol. Aging. 2020, 90, 119–124. [Google Scholar] [CrossRef]
- Herdegen, T.; Leah, J.D. Inducible and Constitutive Transcription Factors in the Mammalian Nervous System: Control of Gene Expression by Jun, Fos and Krox, and CREB/ATF Proteins. Brain Res. Rev. 1998, 28, 370–490. [Google Scholar] [CrossRef]
- Kaufer, D.; Ogle, W.O.; Pincus, Z.S.; Clark, K.L.; Nicholas, A.C.; Dinkel, K.M.; Dumas, T.C.; Ferguson, D.; Lee, A.L.; Winters, M.A.; et al. Restructuring the neuronal stress response with anti-glucocorticoid gene delivery. Nat. Neurosci. 2004, 7, 947–953. [Google Scholar] [CrossRef]
- Rioja, J.; Santín, L.J.; Doña, A.; de Pablos, L.; Minano, F.J.; Gonzalez-Baron, S.; Aguirre, J.A. 5-HT1A receptor activation counteracts c-Fos immunoreactivity induced in serotonin neurons of the raphe nuclei after immobilization stress in the male rat. Neurosci. Lett. 2006, 397, 190–195. [Google Scholar] [CrossRef]
- Bahrami, S.; Drabløs, F. Gene regulation in the immediate-early response process. Adv. Biol. Regul. 2016, 62, 37–49. [Google Scholar] [CrossRef]
- Novoselov, K.S.; Geim, A.K.; Morozov, S.V.; Jiang, D.; Zhang, Y.; Dubonos, S.V.; Grigorieva, I.V.; Firsov, A.A. Electric field effect in atomically thin carbon films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, B.S. Culture of neural cells and stem cells on graphene. Tissue Eng. Regen. Med. 2013, 10, 39–46. [Google Scholar] [CrossRef]
- Rodríguez-Losada, N.; de la Rosa, J.; Larriva, M.; Wendelbo, R.; Aguirre, J.A.; Castresana, J.S.; Ballaz, S.J. Overexpression of alpha-synuclein promotes both cell proliferation and cell toxicity in human SH-SY5Y neuroblastoma cells. J. Adv. Res. 2020, 23, 37–45. [Google Scholar] [CrossRef]
- Rodriguez-Losada, N.; Wendelbob, R.; Ocaña, M.C.; Casares, A.D.; Guzman de Villoría, R.; Aguirre Gomez, J.A.; Arraez, M.A.; Gonzalez-Alegre, P.; Medina, M.A.; Arenas, E.; et al. Graphene Oxide and Reduced Derivatives, as Powder or Film Scaffolds, Differentially Promote Dopaminergic Neuron Differentiation and Survival. Front. Neurosci. 2020, 14, 570409. [Google Scholar] [CrossRef] [PubMed]
- Convertino, D.; Trincavelli, M.L.; Giacomelli, C.; Marchetti, L.; Coletti, C. Graphene-based nanomaterials for peripheral nerve regeneration. Front. Bioeng. Biotechnol. 2023, 11, 1306184. [Google Scholar] [CrossRef] [PubMed]
- Çağla, D.; Verdejo, R.; Majeed, S.; Boschetti-de-Fierro, A.; Méndez-Gómez, H.R.; Díaz-Guerra, E.; Fierro, D. In Vitro Evaluation of Biocompatibility of Uncoated Thermally Reduced Graphene and Carbon Nanotube-Loaded PVDF Membranes with Adult Neural Stem Cell-Derived Neurons and Glia. Front. Bioeng. Biotechnol. 2016, 4, 94–113. [Google Scholar] [CrossRef]
- Shimoke, K.; Utsumi, T.; Kishi, S.; Nishimura, M.; Sasaya, H.; Kudo, M.; Ikeuchi, T. Prevention of endoplasmic reticulum stress-induced cell death by brain-derived neurotrophic factor in cultured cerebral cortical neurons. Brain Res. 2004, 1028, 105–111. [Google Scholar] [CrossRef]
- Khaing, Z.Z.; Schmidt, C.E. Advances in natural biomaterials for nerve tissue repair. Neurosci. Lett. 2012, 519, 103–114. [Google Scholar] [CrossRef]
- Malhotra, R.; Halbig, C.E.; Sim, Y.F. Cytotoxicity survey of commercial graphene materials from worldwide. Npj 2D Mater. Appl. 2022, 6, 65. [Google Scholar] [CrossRef]
- Ikram, R.; Shamsuddin, S.A.A.; Mohamed Jan, B.; Abdul Qadir, M.; Kenanakis, G.; Stylianakis, M.M.; Anastasiadis, S.H. Impact of Graphene Derivatives as Artificial Extracellular Matrices on Mesenchymal Stem Cells. Molecules 2022, 27, 379. [Google Scholar] [CrossRef] [PubMed]
- Oz, T.; Kaushik, A.K.; Kujawska, M. Advances in graphene-based nanoplatforms and their application in Parkinson’s disease. Mater. Adv. 2023, 4, 6464–6477. [Google Scholar] [CrossRef]
- Farokhi, M.; Mottaghitalab, F.; Saeb, M.R.; Shojaei, S.; Zarrin, N.K.; Thomas, S.; Ramakrishna, S. Conductive Biomaterials as Substrates for Neural Stem Cells Differentiation towards Neuronal Lineage Cells. Macromol. Biosci. 2021, 21, e2000123. [Google Scholar] [CrossRef]
- Ásgrímsdóttir, E.S.; Arenas, E. Midbrain Dopaminergic Neuron Development at the Single Cell Level: In vivo and in Stem Cells. Front. Cell Dev. Biol. 2020, 8, 463. [Google Scholar] [CrossRef]
- Ye, T.; Yang, Y.; Bai, J.; Wu, F.Y.; Zhang, L.; Meng, L.Y.; Lan, Y. The mechanical, optical, and thermal properties of graphene influencing its pre-clinical use in treating neurological diseases. Front. Neurosci. 2023, 17, 1162493. [Google Scholar] [CrossRef]
- Kanakia, S.; Toussaint, J.D.; Mullick Chowdhury, S.; Tembulkar, T.; Lee, S.; Jiang, Y.P.; Lin, R.Z.; Shroyer, K.R.; Moore, W.; Sitharaman, B. Dose ranging, expanded acute toxicity and safety pharmacology studies for intravenously administered functionalized graphene nanoparticle formulations. Biomaterials 2014, 35, 7022–7031. [Google Scholar] [CrossRef]
- Romero-Zerbo, S.Y.; Valverde, N.; Claros, S.; Zamorano-Gonzalez, P.; Boraldi, F.; Lofaro, F.D.; Lara, E.; Pavia, J.; Garcia-Fernandez, M.; Gago, B.; et al. New molecular mechanisms to explain the neuroprotective effects of insulin-like growth factor II in a cellular model of Parkinson’s disease. J. Adv. Res. 2025, 67, 349–359. [Google Scholar] [CrossRef]
- Ni, M.; Lee, A.S. ER chaperones in mammalian development and human diseases. FEBS Lett. 2007, 581, 3641–3651. [Google Scholar] [CrossRef]
- Hetz, C.; Saxena, S. ER stress and the unfolded protein response in neurodegneration. Nat. Rev. Neurol. 2017, 13, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Eno, C.O.; Altman, B.J.; Zhu, Y.; Zhao, G.; Olberding, K.E.; Rathmell, J.C.; Li, C. ER stress modulates celular metabolism. Biochem. 2011, 435, 285–296. [Google Scholar] [CrossRef]
- Rutkowski, D.T.; Kaufman, R.J. A trip to the ER: Coping with stress. Trends Cell. Biol. 2004, 14, 20–28. [Google Scholar] [CrossRef]
- Luo, S.; Baumeister, P.; Yang, S.; Abcouwer, S.F.; Lee, A.S. Induction of Grp78/BiP by translational block: Activation of the Grp78 promoter by ATF4 through and upstream ATF/CRE site independent of the endoplasmic reticulum stress elements. J. Biol. Chem. 2003, 278, 37375–37385. [Google Scholar] [CrossRef]
- Liang, S.H.; Zhang, W.; McGrath, B.C.; Zhang, P.; Cavener, D.R. PERK (eIF2alpha kinase) is required to activate the stress-activated MAPKs and induce the expression of immediate-early genes upon disruption of ER calcium homoeostasis. Biochem. J. 2006, 393, 201–209. [Google Scholar] [CrossRef]
- Chen, X.; Shi, C.; He, M.; Xiong, S.; Xia, X. Endoplasmic reticulum stress: Molecular mechanism and therapeutic targets. Signal Transduct. Target. Ther. 2023, 8, 352. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Ye, R.; Barron, E.; Baumeister, P.; Mao, C.; Luo, S.; Fu, Y.; Luo, B.; Dubeau, L.; Hinton, D.R.; et al. Essential role of the unfolded protein response regulator GRP78/BiP in protection from neuronal apoptosis. Cell Death Differ. 2010, 17, 488–498. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Yang, Y.F.; Wu, A.T.; Yang, C.J.; Liu, Y.P.; Jan, Y.H.; Lee, C.H.; Hsiao, Y.W.; Yeh, C.T.; Shen, C.N.; et al. Endoplasmic reticulum ribosome-binding protein 1 (RRBP1) overexpression is frequently found in lung cancer patients and alleviates intracellular stress-induced apoptosis through the enhancement of GRP78. Oncogene 2013, 32, 4921–4931. [Google Scholar] [CrossRef]
- Plümpe, T.; Ehninger, D.; Steiner, B.; Klempin, F.; Jessberger, S.; Brandt, M.; Römer, B.; Rodriguez, G.R.; Kronenberg, G.; Kempermann, G. Variability of doublecortin-associated dendrite maturation in adult hippocampal neurogenesis is independent of the regulation of precursor cell proliferation. BMC Neurosci. 2006, 7, 77. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Si, Z.; Wang, L.; Zhang, L. DYT-TOR1A dystonia: An update on pathogenesis and treatment. Front. Neurosci. 2023, 17, 1216929. [Google Scholar] [CrossRef]
- Kovács, K.J. c-Fos as a transcription factor: A stressful (re)view from a functional map. Neurochem. Int. 1998, 33, 287–297. [Google Scholar] [CrossRef]
- Kovács, K.J. Measurement of immediate-early gene activation- c-fos and beyond. J. Neuroendocrinol. 2008, 20, 665–672. [Google Scholar] [CrossRef]
- Senba, E.; Matsunaga, K.; Tohyama, M.; Noguchi, K. Stress-induced c-fos expression in the rat brain: Activation mechanism of sympathetic pathway. Brain Res. Bull. 1993, 31, 329–344. [Google Scholar] [CrossRef]
- Khan, Z.U.; Carretero-Rey, M.; de León-López, C.A.M.; Navarro-Lobato, I. Memory-Associated Immediate Early Genes: Roles in Synaptic Function, Memory Processes, and Neurological Diseases. Mol. Neurobiol. 2025, in press. [Google Scholar] [CrossRef]
- Kania, E.; Pająk, B.; Orzechowski, A. Calcium homeostasis and ER stress in control of autophagy in cancer cells. Biomed. Res. Int. 2015, 2015, 352794. [Google Scholar] [CrossRef]
- Minatohara, K.; Akiyoshi, M.; Okuno, H. Role of Immediate-Early Genes in Synaptic Plasticity and Neuronal Ensembles Underlying the Memory Trace. Front. Mol. Neurosci. 2016, 8, 78. [Google Scholar] [CrossRef]
- Sheng, M.; Greenberg, M.E. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron 1990, 4, 477–485. [Google Scholar] [CrossRef]
- Bhurtel, S.; Katila, N.; Srivastav, S.; Neupane, S.; Choi, D.Y. Mechanistic comparison Between MPTP and rotenone neurotoxicity in mice. Neurotoxicology 2019, 71, 113–121. [Google Scholar] [CrossRef]
- Andersson, E.; Tryggvason, U.; Deng, Q.; Friling, S.; Alekseenko, Z.; Robert, B.; Perlmann, T.; Ericson, J. Identification of intrinsic determinants of midbrain dopamine neurons. Cell 2006, 124, 393–405. [Google Scholar] [CrossRef]
- Kambe, Y.; Miyata, A. Mitochondrial c-Fos May Increase the Vulnerability of Neuro2a Cells to Cellular Stressors. J. Mol. Neurosci. 2016, 59, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Losada, N.; Romero, P.; Estivill-Torrús, G.; Guzmán de Villoria, R.; Aguirre, J.A. Cell survival and differentiation with nanocrystalline glass-like carbon using substantia nigra dopaminergic cells derived from transgenic mouse embryos. PLoS ONE 2017, 12, e0173978. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S.; Offeman, R.E. Preparation of Graphitic Oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Cámara-Torres, M.; Sinha, R.; Eqtesadi, S.; Wendelbo, R.; Scatto, M.; Scopece, P.; Sanchez, A.; Villanueva, S.; Egizabal, A.; Álvarez, N.; et al. Effect of the reduced graphene oxide (rGO) compaction degree and concentration on rGO-polymer composite printability and cell interactions. Nanoscale 2021, 13, 14382–14398. [Google Scholar] [CrossRef] [PubMed]
- Son, J.H.; Chun, H.S.; Joh, T.H.; Cho, S.; Conti, B.; Lee, J.W. Neuroprotection and neuronal differentiation studies using substantia nigra dopaminergic cells derived from transgenic mouse embryos. J. Neurosci. 1999, 19, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Schaller, R.K.; Spiess, R.B.; Bier, F.F.; Bettag, U.; Cremer, C. Denaturation behaviour of DNA-protein-complexes detected in situ in metaphase chromosomes in suspension by Hoechst 33258 fluorescence. Biophys. Chem. 1990, 38, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Martin-Montañez, E.; Pavia, J.; Santin, L.J.; Boraldi, F.; Estivill-Torrus, G.; Aguirre, J.A.; Garcia-Fernandez, M. Involvement of IGF-II receptors in the antioxidant and neuroprotective effects of IGF-II on adult cortical neuronal cultures. Biochim. Biophys. Acta 2014, 1842, 1041–1051. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vallejo Perez, D.; Navarro, M.; Segura-Segura, B.; Wendelbo, R.; Bandrés-Ciga, S.; Arraez, M.A.; Arraez, C.; Rodriguez-Losada, N. Neuroprotective Effects of Low-Dose Graphenic Materials on SN4741 Embryonic Stem Cells Against ER Stress and MPTP-Induced Oxidative Stress. Int. J. Mol. Sci. 2025, 26, 8821. https://doi.org/10.3390/ijms26188821
Vallejo Perez D, Navarro M, Segura-Segura B, Wendelbo R, Bandrés-Ciga S, Arraez MA, Arraez C, Rodriguez-Losada N. Neuroprotective Effects of Low-Dose Graphenic Materials on SN4741 Embryonic Stem Cells Against ER Stress and MPTP-Induced Oxidative Stress. International Journal of Molecular Sciences. 2025; 26(18):8821. https://doi.org/10.3390/ijms26188821
Chicago/Turabian StyleVallejo Perez, David, Monica Navarro, Beatriz Segura-Segura, Rune Wendelbo, Sara Bandrés-Ciga, Miguel A. Arraez, Cinta Arraez, and Noela Rodriguez-Losada. 2025. "Neuroprotective Effects of Low-Dose Graphenic Materials on SN4741 Embryonic Stem Cells Against ER Stress and MPTP-Induced Oxidative Stress" International Journal of Molecular Sciences 26, no. 18: 8821. https://doi.org/10.3390/ijms26188821
APA StyleVallejo Perez, D., Navarro, M., Segura-Segura, B., Wendelbo, R., Bandrés-Ciga, S., Arraez, M. A., Arraez, C., & Rodriguez-Losada, N. (2025). Neuroprotective Effects of Low-Dose Graphenic Materials on SN4741 Embryonic Stem Cells Against ER Stress and MPTP-Induced Oxidative Stress. International Journal of Molecular Sciences, 26(18), 8821. https://doi.org/10.3390/ijms26188821




