Glial Cytokine and Metabolic Networks in Progressive Multiple Sclerosis: From Pathophysiology to Biomarkers and Therapeutic Strategies
Abstract
1. Introduction
What Is New in This Review
2. Review Methodology
2.1. Aim of the Study
2.2. Literature Search Strategy
2.3. Eligibility Criteria and Selection Process
2.4. Rationale for Narrative Review Format
2.5. Quality Assurance and Reporting Standards
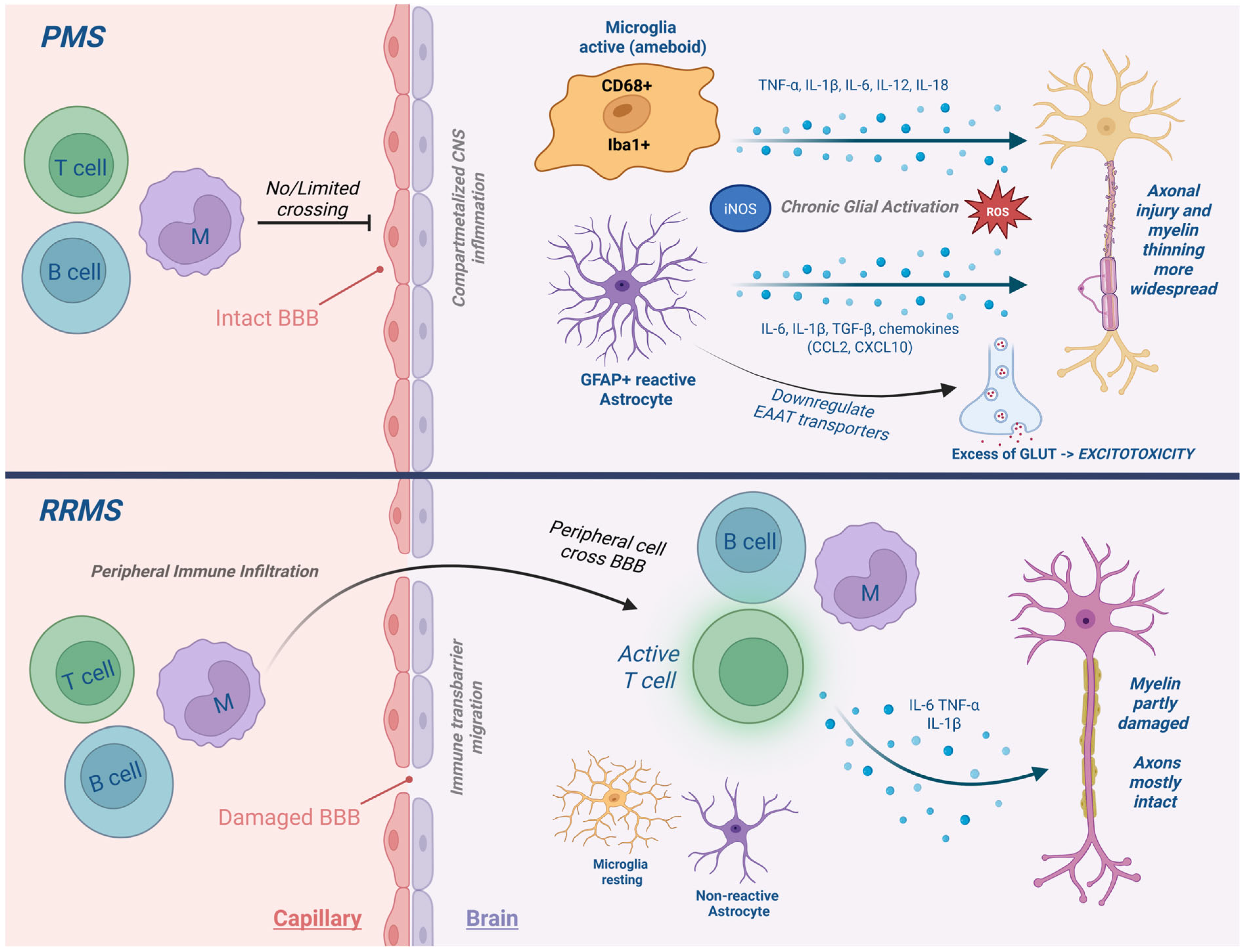
3. The Neuroimmune Landscape of PMS
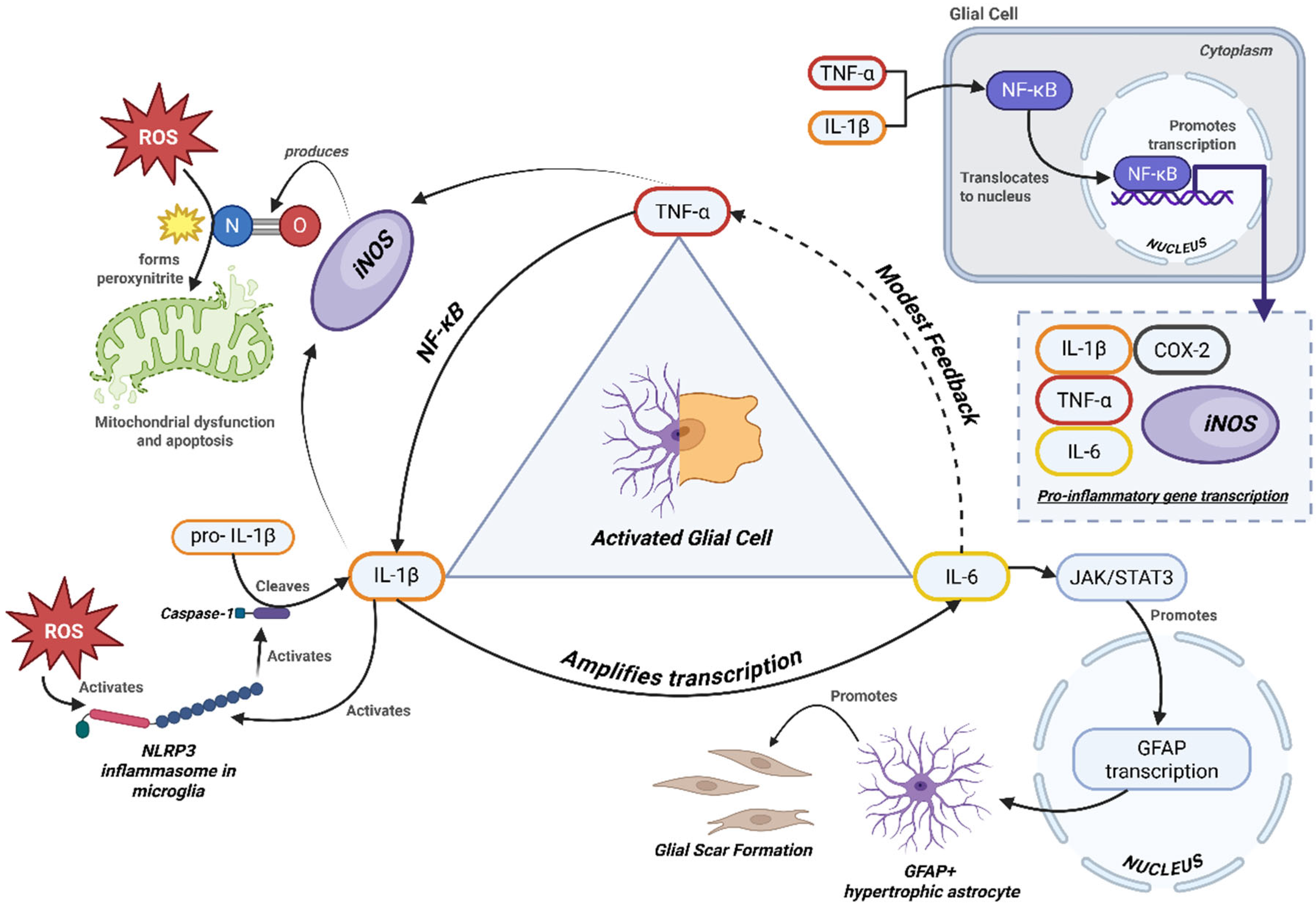
4. Key Cytokines and Inflammatory Mediators in PMS
Sex and Age-Related Modulation of Cytokine Activity in PMS
5. Cytokine Crosstalk and Disease Progression
Biomarkers and Imaging in Progressive Multiple Sclerosis
6. Therapeutic Implications and Future Directions
7. Comparative Insights from Other Neurodegenerative Diseases
8. Conceptual Framework
8.1. Implications for Patient Stratification
8.2. Implications for Endpoints and Design
8.3. Key Messages for Clinicians
- ▪
- Progressive MS is sustained by compartmentalized glial cytokine networks (TNF-α, IL-6, IL-1β).
- ▪
- Evidence is strongest for TNF-α and IL-6 in human tissue and CSF; IL-1β remains largely model-driven.
- ▪
- GFAP and NfL are validated progression biomarkers; combining them with TSPO-PET and iron-sensitive MRI improves specificity.
- ▪
- TSPO-PET requires binder-genotype handling and standardized analysis.
- ▪
- Iron rims on QSM MRI identify chronically active lesions driving progression.
- ▪
- BTK inhibitors (tolebrutinib) show first late-phase efficacy in non-relapsing SPMS.
- ▪
- Network-based interventions (AMPK activation, iron chelation, autophagy modulation) may outperform single-cytokine targeting.
- ▪
- Patient enrichment using GFAP, TSPO-PET, and PRL markers may accelerate PMS trial success.
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviation
| PMS | Progressive Multiple Sclerosis |
| RRMS | Relapsing–Remitting Multiple Sclerosis |
| MS | Multiple Sclerosis |
| CNS | Central Nervous System |
| TNF-α | Tumor Necrosis Factor-alpha |
| IL-6 | Interleukin-6 |
| IL-1β | Interleukin-1 beta |
| IFN-γ | Interferon gamma |
| iNOS | Inducible Nitric Oxide Synthase |
| COX-2 | Cyclooxygenase-2 |
| ROS | Reactive Oxygen Species |
| TSPO | Translocator Protein |
| PET | Positron Emission Tomography |
| MRI | Magnetic Resonance Imaging |
| NLRP3 | NOD-, LRR- and pyrin domain-containing protein 3 |
| GFAP | Glial Fibrillary Acidic Protein |
| NfL | Neurofilament Light Chain |
| BBB | Blood–Brain Barrier |
| DMT | Disease-Modifying Therapy |
| TLR | Toll-like Receptor |
| MAPK | Mitogen-Activated Protein Kinase |
| NF-κB | Nuclear Factor kappa-light-chain-enhancer of activated B cells |
| JAK/STAT | Janus Kinase/Signal Transducer and Activator of Transcription |
| mTOR | Mechanistic Target of Rapamycin |
| GSH | Glutathione |
| ATP | Adenosine Triphosphate |
| ISI | Institute for Scientific Information |
| SANRA | Scale for the Assessment of Narrative Review Articles |
References
- Haki, M.; Al-Biati, H.A.; Al-Tameemi, Z.S.; Ali, I.S.; Al-Hussaniy, H.A. Review of multiple sclerosis: Epidemiology, etiology, pathophysiology, and treatment. Medicine 2024, 103, e37297. [Google Scholar] [CrossRef]
- Walton, C.; King, R.; Rechtman, L.; Kaye, W.; Leray, E.; Marrie, R.A.; Robertson, N.; La Rocca, N.; Uitdehaag, B.; Van Der Mei, I.; et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult. Scler. J. 2020, 26, 1816–1821. [Google Scholar] [CrossRef]
- Lublin, F.D.; Reingold, S.C.; Cohen, J.A.; Cutter, G.R.; Sørensen, P.S.; Thompson, A.J.; Wolinsky, J.S.; Balcer, L.J.; Banwell, B. Defining the clinical course of multiple sclerosis: The 2013 revisions. Neurology 2014, 83, 278–286. [Google Scholar] [CrossRef]
- de Waal, H.; Stam, C.J.; Lansbergen, M.M.; Wieggers, R.L.; Kamphuis, P.J.G.H.; Scheltens, P.; Maestú, F.; van Straaten, E.C.W.; Valdes-Sosa, P.A. The Effect of Souvenaid on Functional Brain Network Organisation in Patients with Mild Alzheimer’s Disease: A Randomised Controlled Study. PLOS ONE 2014, 9, e86558. [Google Scholar] [CrossRef]
- Horn, T.; Schmid, S.C.; Seitz, A.K.; Grab, J.; Wolf, P.; Haller, B.; Retz, M.; Maurer, T.; Autenrieth, M.; Kübler, H.R.; et al. Clinical prognosticators of survival in patients with urothelial carcinoma of the bladder and lymph node metastases after cystectomy with curative intent. World J. Urol. 2014, 33, 813–819. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J.; Mahad, D. Progressive multiple sclerosis: Pathology and pathogenesis. Nat. Rev. Neurol. 2012, 8, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Siffrin, V. Pathways to Progressive Disability in Multiple Sclerosis: The Role of Glial Cells in Chronic CNS Inflammation. Glia 2025, 73, 1928–1950. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Du, S.; Zhao, L.; Jain, S.; Sahay, K.; Rizvanov, A.; Lezhnyova, V.; Khaibullin, T.; Martynova, E.; Khaiboullina, S.; et al. Autoreactive lymphocytes in multiple sclerosis: Pathogenesis and treatment target. Front. Immunol. 2022, 13, 996469. [Google Scholar] [CrossRef] [PubMed]
- Voon, C.C.; Wiltgen, T.; Wiestler, B.; Schlaeger, S.; Mühlau, M. Quantitative susceptibility mapping in multiple sclerosis: A systematic review and meta-analysis. NeuroImage: Clin. 2024, 42, 103598. [Google Scholar] [CrossRef]
- Elkjaer, M.L.; Waede, M.R.; Kingo, C.; Damsbo, K.; Illes, Z. Expression of Bruton’s tyrosine kinase in different type of brain lesions of multiple sclerosis patients and during experimental demyelination. Front. Immunol. 2023, 14, 1264128. [Google Scholar] [CrossRef]
- Ortiz, C.C.; Eroglu, C. Astrocyte signaling and interactions in Multiple Sclerosis. Curr. Opin. Cell Biol. 2023, 86, 102307. [Google Scholar] [CrossRef]
- Zhao, Y.; Huang, Y.; Cao, Y.; Yang, J. Astrocyte-Mediated Neuroinflammation in Neurological Conditions. Biomolecules 2024, 14, 1204. [Google Scholar] [CrossRef]
- Freedman, M.S.; Gnanapavan, S.; Booth, R.A.; Calabresi, P.A.; Khalil, M.; Kuhle, J.; Lycke, J.; Olsson, T. Guidance for use of neurofilament light chain as a cerebrospinal fluid and blood biomarker in multiple sclerosis management. EBioMedicine 2024, 101, 104970. [Google Scholar] [CrossRef]
- Marjo, N.; Jussi, L.; Markus, M.; Johan, R.; Saara, W.; Marcus, S.; Tanja, K.; Laura, A. Longitudinal accumulation of glial activation measured by TSPO-PET predicts later brain atrophy in multiple sclerosis. J. Neuroinflammation 2025, 22, 200. [Google Scholar] [CrossRef] [PubMed]
- Fox, R.J.; Bar-Or, A.; Traboulsee, A.; Oreja-Guevara, C.; Giovannoni, G.; Vermersch, P.; Syed, S.; Li, Y.; Vargas, W.S.; Turner, T.J.; et al. Tolebrutinib in Nonrelapsing Secondary Progressive Multiple Sclerosis. New Engl. J. Med. 2025, 392, 1883–1892. [Google Scholar] [CrossRef] [PubMed]
- Maccioni, L.; Brusaferri, L.; Barzon, L.; Schubert, J.J.; A Nettis, M.; Cousins, O.; Rosenzweig, I.; Mizuno, Y.; Vicente-Rodríguez, M.; Singh, N.; et al. A novel blood-free analytical framework for the quantification of neuroinflammatory load from TSPO PET imaging. J. Cereb. Blood Flow Metab. 2025. [Google Scholar] [CrossRef] [PubMed]
- Gillen, K.M.; Nguyen, T.D.; Dimov, A.; Kovanlikaya, I.; Luu, H.M.; Demmon, E.; Markowitz, D.M.; Bagnato, F.; Pitt, D.; A Gauthier, S.; et al. Quantitative susceptibility mapping is more sensitive and specific than phase imaging in detecting chronic active multiple sclerosis lesion rims: Pathological validation. Brain Commun. 2024, 7, fcaf011. [Google Scholar] [CrossRef]
- Sweeney, M.E.; Slusser, J.G.; Lynch, S.G.; Benedict, S.H.; Garcia, S.L.; Rues, L.; LeVine, S.M. Deferiprone modulates in vitro responses by peripheral blood T cells from control and relapsing–remitting multiple sclerosis subjects. Int. Immunopharmacol. 2011, 11, 1796–1801. [Google Scholar] [CrossRef]
- Galota, F.; Marcheselli, S.; De Biasi, S.; Gibellini, L.; Vitetta, F.; Fiore, A.; Smolik, K.; De Napoli, G.; Cardi, M.; Cossarizza, A.; et al. Impact of High-Efficacy Therapies for Multiple Sclerosis on B Cells. Cells 2025, 14, 606. [Google Scholar] [CrossRef]
- Garton, T.; Gadani, S.P.; Gill, A.J.; Calabresi, P.A. Neurodegeneration and demyelination in multiple sclerosis. Neuron 2024, 112, 3231–3251. [Google Scholar] [CrossRef]
- Guido, G.; Preziosa, P.; Filippi, M.; Rocca, M.A. TSPO PET in multiple sclerosis: Emerging insights into pathophysiology, prognosis, and treatment monitoring. Mult. Scler. Relat. Disord. 2025, 100, 106546. [Google Scholar] [CrossRef] [PubMed]
- Vermersch, P.; Airas, L.; Berger, T.; Deisenhammer, F.; Grigoriadis, N.; Hartung, H.-P.; Magyari, M.; Popescu, V.; Pozzilli, C.; Pugliatti, M.; et al. The role of microglia in multiple sclerosis: Implications for treatment with Bruton’s tyrosine kinase inhibitors. Front. Immunol. 2025, 16, 1495529. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, R.; Laroni, A.; Weiner, H.L. Role of the innate immune system in the pathogenesis of multiple sclerosis. J. Neuroimmunol. 2010, 221, 7–14. [Google Scholar] [CrossRef] [PubMed]
- Geladaris, A.; Häusler, D.; Weber, M.S. Microglia: The Missing Link to Decipher and Therapeutically Control MS Progression? Int. J. Mol. Sci. 2021, 22, 3461. [Google Scholar] [CrossRef]
- Monaco, S.; Nicholas, R.; Reynolds, R.; Magliozzi, R. Intrathecal Inflammation in Progressive Multiple Sclerosis. Int. J. Mol. Sci. 2020, 21, 8217. [Google Scholar] [CrossRef]
- Sen, M.K.; Mahns, D.A.; Coorssen, J.R.; Shortland, P.J. The roles of microglia and astrocytes in phagocytosis and myelination: Insights from the cuprizone model of multiple sclerosis. Glia 2022, 70, 1215–1250. [Google Scholar] [CrossRef]
- Microglia Phenotypes in Aging and Neurodegenerative Diseases. Available online: https://www.mdpi.com/2073-4409/11/13/2091 (accessed on 26 June 2025).
- Colombo, E.; Farina, C. Astrocytes: Key Regulators of Neuroinflammation. Trends Immunol. 2016, 37, 608–620. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Zhang, L.; Verkhratsky, A.; Shi, F.D. Chapter 8—Astrocytes and microglia in multiple sclerosis and neuromyelitis optica. In Handbook of Clinical Neurology; Verkhratsky, A., de Witte, L.D., Aronica, E., Hol, E.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2025; Volume 210, pp. 133–145. Available online: https://www.sciencedirect.com/science/article/pii/B9780443191022000016 (accessed on 26 June 2025).
- Dal-Bianco, A.; Grabner, G.; Kronnerwetter, C.; Weber, M.; Höftberger, R.; Berger, T.; Auff, E.; Leutmezer, F.; Trattnig, S.; Lassmann, H.; et al. Slow expansion of multiple sclerosis iron rim lesions: Pathology and 7 T magnetic resonance imaging. Acta Neuropathol. 2016, 133, 25–42. [Google Scholar] [CrossRef]
- Dal-Bianco, A.; Oh, J.; Sati, P.; Absinta, M. Chronic active lesions in multiple sclerosis: Classification, terminology, and clinical significance. Ther. Adv. Neurol. Disord. 2024, 17. [Google Scholar] [CrossRef]
- Loane, D.J.; Kumar, A. Microglia in the TBI brain: The good, the bad, and the dysregulated. Exp. Neurol. 2016, 275 Pt 3, 316–327. [Google Scholar] [CrossRef] [PubMed]
- Schuh, C.; Wimmer, I.; Hametner, S.; Haider, L.; Van Dam, A.-M.; Liblau, R.S.; Smith, K.J.; Probert, L.; Binder, C.J.; Bauer, J.; et al. Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta Neuropathol. 2014, 128, 247–266. [Google Scholar] [CrossRef] [PubMed]
- Olmos, G.; Lladó, J. Tumor Necrosis Factor Alpha: A Link between Neuroinflammation and Excitotoxicity. Mediat. Inflamm. 2014, 2014, 861231. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, R.; Ashbaugh, J.J.; Magliozzi, R.; Dellarole, A.; Karmally, S.; Szymkowski, D.E.; Bethea, J.R. Inhibition of soluble tumour necrosis factor is therapeutic in experimental autoimmune encephalomyelitis and promotes axon preservation and remyelination. Brain 2011, 134, 2736–2754. [Google Scholar] [CrossRef]
- Imitola, J.; Chitnis, T.; Khoury, S. Cytokines in multiple sclerosis: From bench to bedside. Pharmacol. Ther. 2005, 106, 163–177. [Google Scholar] [CrossRef]
- Cirac, A.; Tsaktanis, T.; Beyer, T.; Linnerbauer, M.; Andlauer, T.; Grummel, V.; Nirschl, L.; Loesslein, L.; Quintana, F.J.; Hemmer, B.; et al. The Aryl Hydrocarbon Receptor–Dependent TGF-α/VEGF-B Ratio Correlates With Disease Subtype and Prognosis in Multiple Sclerosis. Neurol. Neuroimmunol. Neuroinflammation 2021, 8. [Google Scholar] [CrossRef]
- Reali, C.; Magliozzi, R.; Roncaroli, F.; Nicholas, R.; Howell, O.W.; Reynolds, R. B cell rich meningeal inflammation associates with increased spinal cord pathology in multiple sclerosis. Brain Pathol. 2020, 30, 779–793. [Google Scholar] [CrossRef]
- Maimone, D.; Guazzi, G.C.; Annunziata, P. IL-6 detection in multiple sclerosis brain. J. Neurol. Sci. 1997, 146, 59–65. [Google Scholar] [CrossRef]
- Howell, O.W.; Reeves, C.A.; Nicholas, R.; Carassiti, D.; Radotra, B.; Gentleman, S.M.; Serafini, B.; Aloisi, F.; Roncaroli, F.; Magliozzi, R.; et al. Meningeal inflammation is widespread and linked to cortical pathology in multiple sclerosis. Brain 2011, 134, 2755–2771. [Google Scholar] [CrossRef]
- Navikas, V.; Matusevicius, D.; Söderström, M.; Fredrikson, S.; Kivisäkk, P.; Ljungdahl, Å.; Höjeberg, B.; Link, H. Increased interleukin-6 mRNA expression in blood and cerebrospinal fluid mononuclear cells in multiple sclerosis. J. Neuroimmunol. 1996, 64, 63–69. [Google Scholar] [CrossRef]
- Barclay, W.; Shinohara, M.L. Inflammasome activation in multiple sclerosis and experimental autoimmune encephalomyelitis (EAE). Brain Pathol. 2017, 27, 213–219. [Google Scholar] [CrossRef] [PubMed]
- García-Domínguez, M. Neuroinflammation: Mechanisms, Dual Roles, and Therapeutic Strategies in Neurological Disorders. Curr. Issues Mol. Biol. 2025, 47, 417. [Google Scholar] [CrossRef] [PubMed]
- Mendiola, A.S.; Cardona, A.E. The IL-1β phenomena in neuroinflammatory diseases. J. Neural Transm. 2017, 125, 781–795. [Google Scholar] [CrossRef] [PubMed]
- Sabirov, D.; Ogurcov, S.; Shulman, I.; Kabdesh, I.; Garanina, E.; Sufianov, A.; Rizvanov, A.; Mukhamedshina, Y. Comparative Analysis of Cytokine Profiles in Cerebrospinal Fluid and Blood Serum in Patients with Acute and Subacute Spinal Cord Injury. Biomedicines 2023, 11, 2641. [Google Scholar] [CrossRef]
- Martynova, E.; Goyal, M.; Johri, S.; Kumar, V.; Khaibullin, T.; Rizvanov, A.A.; Verma, S.; Khaiboullina, S.F.; Baranwal, M.; Xiao, C. Serum and Cerebrospinal Fluid Cytokine Biomarkers for Diagnosis of Multiple Sclerosis. Mediat. Inflamm. 2020, 2020, 2727042. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J. The molecular basis of neurodegeneration in multiple sclerosis. FEBS Lett. 2011, 585, 3715–3723. [Google Scholar] [CrossRef]
- Lassmann, H.; van Horssen, J. Oxidative stress and its impact on neurons and glia in multiple sclerosis lesions. Biochim. Biophys. Acta. 2016, 1862, 506–510. [Google Scholar] [CrossRef]
- Healy, L.M.; Stratton, J.A.; Kuhlmann, T.; Antel, J. The role of glial cells in multiple sclerosis disease progression. Nat. Rev. Neurol. 2022, 18, 237–248. [Google Scholar] [CrossRef]
- Al-Kuraishy, H.M.; Jabir, M.S.; Al-Gareeb, A.I.; Saad, H.M.; Batiha, G.E.-S.; Klionsky, D.J. The beneficial role of autophagy in multiple sclerosis: Yes or No? Autophagy 2023, 20, 259–274. [Google Scholar] [CrossRef]
- Guo, F.; Liu, X.; Cai, H.; Le, W. Autophagy in neurodegenerative diseases: Pathogenesis and therapy. Brain Pathol. 2017, 28, 3–13. [Google Scholar] [CrossRef]
- Misrielal, C.; Mauthe, M.; Reggiori, F.; Eggen, B.J.L. Autophagy in Multiple Sclerosis: Two Sides of the Same Coin. Front. Cell. Neurosci. 2020, 14. [Google Scholar] [CrossRef]
- Ren, W.; Sun, Y.; Zhao, L.; Shi, X. NLRP3 inflammasome and its role in autoimmune diseases: A promising therapeutic target. Biomed. Pharmacother. 2024, 175, 116679. [Google Scholar] [CrossRef] [PubMed]
- Rahman, M.d.A.; Rhim, H. Therapeutic implication of autophagy in neurodegenerative diseases. BMB Rep. 2017, 50, 345–354. [Google Scholar] [CrossRef] [PubMed]
- Harbo, H.F.; Gold, R.; Tintoré, M. Sex and gender issues in multiple sclerosis. Ther. Adv. Neurol. Disord. 2013, 6, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Gold, S.M. Sex-related Factors in Multiple Sclerosis: Genetic, Hormonal and Environmental Contributions. Nat. Rev. Neurol. 2012, 8, 255–263. [Google Scholar] [CrossRef]
- Scalfari, A.; Neuhaus, A.; Degenhardt, A.; Rice, G.P.; Muraro, P.A.; Daumer, M.; Ebers, G.C. The natural history of multiple sclerosis, a geographically based study 10: Relapses and long-term disability. Brain 2010, 133, 1914–1929. [Google Scholar] [CrossRef]
- Crabtree-Hartman, E. SEX DIFFERENCES IN MULTIPLE SCLEROSIS. Contin. Lifelong Learn. Neurol. 2010, 16, 193–210. [Google Scholar] [CrossRef]
- Magliozzi, R.; Howell, O.W.; Reeves, C.; Roncaroli, F.; Nicholas, R.; Serafini, B.; Aloisi, F.; Reynolds, R. A Gradient of neuronal loss and meningeal inflammation in multiple sclerosis. Ann. Neurol. 2010, 68, 477–493. [Google Scholar] [CrossRef]
- Bove, R.; Alwan, S.; Friedman, J.M.; Hellwig, K.; Houtchens, M.; Koren, G.; Lu, E.; McElrath , T.F.; Smyth, P.; Tremlett, H.; et al. Management of multiple sclerosis during pregnancy and the reproductive years: A systematic review. Obs. Gynecol. 2014, 124, 1157–1168. [Google Scholar] [CrossRef]
- Benítez-Fernández, R.; Melero-Jerez, C.; Gil, C.; de la Rosa, E.J.; Martínez, A.; de Castro, F. Dynamics of Central Remyelination and Treatment Evolution in a Model of Multiple Sclerosis with Optic Coherence Tomography. Int. J. Mol. Sci. 2021, 22, 2440. [Google Scholar] [CrossRef]
- Sofroniew, M.V. Astrocyte barriers to neurotoxic inflammation. Nat. Rev. Neurosci. 2015, 16, 249–263. [Google Scholar] [CrossRef]
- Hackett, A.R.; Lee, J.K. Understanding the NG2 Glial Scar after Spinal Cord Injury. Front. Neurol. 2016, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Ciubotaru, A.; Smihor, M.I.; Grosu, C.; Alexa, D.; Covali, R.; Anicăi, R.-C.; Păvăleanu, I.; Cucu, A.I.; Bobu, A.M.; Ghiciuc, C.M.; et al. Neurodegenerative Biomarkers in Multiple Sclerosis: At the Interface Between Research and Clinical Practice. Diagnostics 2025, 15, 1178. [Google Scholar] [CrossRef] [PubMed]
- Biernacki, T.; Kokas, Z.; Sandi, D.; Füvesi, J.; Fricska-Nagy, Z.; Faragó, P.; Kincses, T.Z.; Klivényi, P.; Bencsik, K.; Vécsei, L. Emerging Biomarkers of Multiple Sclerosis in the Blood and the CSF: A Focus on Neurofilaments and Therapeutic Considerations. Int. J. Mol. Sci. 2022, 23, 3383. [Google Scholar] [CrossRef] [PubMed]
- Paul, A.; Comabella, M.; Gandhi, R. Biomarkers in Multiple Sclerosis. Cold Spring Harb. Perspect. Med. 2019, 9, a029058. [Google Scholar] [CrossRef]
- Gill, A.J.; Schorr, E.M.; Gadani, S.P.; Calabresi, P.A. Emerging imaging and liquid biomarkers in multiple sclerosis. Eur. J. Immunol. 2023, 53, e2250228. [Google Scholar] [CrossRef]
- Öhrfelt, A.; Axelsson, M.; Malmeström, C.; Novakova, L.; Heslegrave, A.; Blennow, K.; Lycke, J.; Zetterberg, H. Soluble TREM-2 in cerebrospinal fluid from patients with multiple sclerosis treated with natalizumab or mitoxantrone. Mult. Scler. J. 2016, 22, 1587–1595. [Google Scholar] [CrossRef]
- Piccio, L.; Buonsanti, C.; Cella, M.; Tassi, I.; Schmidt, R.E.; Fenoglio, C.; Rinker, J.; Naismith, R.T.; Panina-Bordignon, P.; Passini, N.; et al. Identification of soluble TREM-2 in the cerebrospinal fluid and its association with multiple sclerosis and CNS inflammation. Brain 2008, 131, 3081–3091. [Google Scholar] [CrossRef]
- Dimisianos, N.; Rodi, M.; Kalavrizioti, D.; Georgiou, V.; Papathanasopoulos, P.; Mouzaki, A. Cytokines as Biomarkers of Treatment Response to IFNβin Relapsing-Remitting Multiple Sclerosis. Mult. Scler. Int. 2014, 2014, 436764. [Google Scholar] [CrossRef]
- Airas, L.; Rissanen, E.; Rinne, J. Imaging of microglial activation in MS using PET: Research use and potential future clinical application. Mult. Scler. J. 2016, 23, 496–504. [Google Scholar] [CrossRef]
- van der Weijden, C.W.J.; Meilof, J.F.; van der Hoorn, A.; de Vries, E.F.J.; Baron, W. The Future of PET Imaging in Multiple Sclerosis: Characterisation of Individual White Matter Lesions. J. Clin. Med. 2025, 14, 4439. [Google Scholar] [CrossRef]
- Oh, U.; Fujita, M.; Ikonomidou, V.N.; Evangelou, I.E.; Matsuura, E.; Harberts, E.; Ohayon, J.; Pike, V.W.; Zhang, Y.; Zoghbi, S.S.; et al. Translocator Protein PET Imaging for Glial Activation in Multiple Sclerosis. J. Neuroimmune Pharmacol. 2010, 6, 354–361. [Google Scholar] [CrossRef]
- Ching, A.S.C.; Kuhnast, B.; Damont, A.; Roeda, D.; Tavitian, B.; Dollé, F. Current paradigm of the 18-kDa translocator protein (TSPO) as a molecular target for PET imaging in neuroinflammation and neurodegenerative diseases. Insights into Imaging 2011, 3, 111–119. [Google Scholar] [CrossRef]
- Owen, D.R.; Yeo, A.J.; Gunn, R.N.; Song, K.; Wadsworth, G.; Lewis, A.; Rhodes, C.; Pulford, D.J.; Bennacef, I.; Parker, C.A.; et al. An 18-kDa Translocator Protein (TSPO) Polymorphism Explains Differences in Binding Affinity of the PET Radioligand PBR28. J. Cereb. Blood Flow Metab. 2012, 32, 1–5. [Google Scholar] [CrossRef]
- Mazziotti, V.; Crescenzo, F.; Turano, E.; Guandalini, M.; Bertolazzo, M.; Ziccardi, S.; Virla, F.; Camera, V.; Marastoni, D.; Tamanti, A.; et al. The contribution of tumor necrosis factor to multiple sclerosis: A possible role in progression independent of relapse? J. Neuroinflammation 2024, 21, 209. [Google Scholar] [CrossRef]
- Zahid, M.; Busmail, A.; Penumetcha, S.S.; Ahluwalia, S.; Irfan, R.; Khan, S.A.; Reddy, S.R.; Lopez, M.E.V.; Mohammed, L. Tumor Necrosis Factor Alpha Blockade and Multiple Sclerosis: Exploring New Avenues. Cureus 2021, 13, e18847. [Google Scholar] [CrossRef]
- van Oosten, B.W.; Barkhof, F.; Truyen, L.; Boringa, J.B.; Bertelsmann, F.W.; von Blomberg, B.; Woody, J.N.; Hartung, H.-P.; Polman, C.H. Increased MRI activity and immune activation in two multiple sclerosis patients treated with the monoclonal anti-tumor necrosis factor antibody cA2. Neurology 1996, 47, 1531–1534. [Google Scholar] [CrossRef]
- Eugenin, E.A.; Basilio, D.; Sáez, J.C.; Orellana, J.A.; Raine, C.S.; Bukauskas, F.; Bennett, M.V.L.; Berman, J.W. The Role of Gap Junction Channels During Physiologic and Pathologic Conditions of the Human Central Nervous System. J. Neuroimmune Pharmacol. 2012, 7, 499–518. [Google Scholar] [CrossRef]
- Wilbanks, B.; Maher, L.; Rodriguez, M. Glial cells as therapeutic targets in progressive multiple sclerosis. Expert Rev. Neurother. 2019, 19, 481–494. [Google Scholar] [CrossRef]
- Zahoor, I.; Mir, S.; Giri, S. Profiling Blood-Based Neural Biomarkers and Cytokines in Experimental Autoimmune Encephalomyelitis Model of Multiple Sclerosis Using Single-Molecule Array Technology. Int. J. Mol. Sci. 2025, 26, 3258. [Google Scholar] [CrossRef]
- De Keersmaecker, A.-V.; Van Doninck, E.; Popescu, V.; Willem, L.; Cambron, M.; Laureys, G.; Haeseleer, M.D.; Bjerke, M.; Roelant, E.; Lemmerling, M.; et al. A metformin add-on clinical study in multiple sclerosis to evaluate brain remyelination and neurodegeneration (MACSiMiSE-BRAIN): Study protocol for a multi-center randomized placebo controlled clinical trial. Front. Immunol. 2024, 15, 1362629. [Google Scholar] [CrossRef]
- Gelfand, J.M.; Cree, B.A.C.; Hauser, S.L. Ocrelizumab and Other CD20+ B-Cell-Depleting Therapies in Multiple Sclerosis. Neurotherapeutics 2017, 14, 835–841. [Google Scholar] [CrossRef]
- Weigel, K.J.; Lynch, S.G.; LeVine, S.M. Iron Chelation and Multiple Sclerosis. ASN Neuro 2014, 6, 43–63. [Google Scholar] [CrossRef]
- Lubetzki, C.; Zalc, B.; Williams, A.; Stadelmann, C.; Stankoff, B. Remyelination in multiple sclerosis: From basic science to clinical translation. Lancet Neurol. 2020, 19, 678–688. [Google Scholar] [CrossRef]
- Panitch, H.S. Investigational drug therapies for treatment of multiple sclerosis. Mult. Scler. J. 1996, 2, 66–77. [Google Scholar] [CrossRef]
- Vakrakou, A.G.; Alexaki, A.; Brinia, M.-E.; Anagnostouli, M.; Stefanis, L.; Stathopoulos, P. The mTOR Signaling Pathway in Multiple Sclerosis; from Animal Models to Human Data. Int. J. Mol. Sci. 2022, 23, 8077. [Google Scholar] [CrossRef]
- Fan, H.; Fu, Q.; Du, G.; Qin, L.; Shi, X.; Wang, D.; Yang, Y. Microglial Mayhem NLRP3 Inflammasome’s Role in Multiple Sclerosis Pathology. CNS Neurosci. Ther. 2024, 30, e70135. [Google Scholar] [CrossRef]
- Li, X.-L.; Zhang, B.; Liu, W.; Sun, M.-J.; Zhang, Y.-L.; Liu, H.; Wang, M.-X. Rapamycin Alleviates the Symptoms of Multiple Sclerosis in Experimental Autoimmune Encephalomyelitis (EAE) Through Mediating the TAM-TLRs-SOCS Pathway. Front. Neurol. 2020, 11, 590884. [Google Scholar] [CrossRef]
- Linker, R.A.; Gold, R. Dimethyl Fumarate for Treatment of Multiple Sclerosis: Mechanism of Action, Effectiveness, and Side Effects. Curr. Neurol. Neurosci. Rep. 2013, 13, 394. [Google Scholar] [CrossRef]
- Zhang, W.; Xiao, D.; Mao, Q.; Xia, H. Role of neuroinflammation in neurodegeneration development. Signal Transduct. Target. Ther. 2023, 8, 267. [Google Scholar] [CrossRef]
- Glass, C.K.; Saijo, K.; Winner, B.; Marchetto, M.C.; Gage, F.H. Mechanisms Underlying Inflammation in Neurodegeneration. Cell 2010, 140, 918–934. [Google Scholar] [CrossRef]
- Lassmann, H. Multiple Sclerosis Pathology. Cold Spring Harb. Perspect. Med. 2018, 8, a028936. [Google Scholar] [CrossRef]
- Dal Bianco, A.; Bradl, M.; Frischer, J.; Kutzelnigg, A.; Jellinger, K.; Lassmann, H. Multiple sclerosis and Alzheimer’s disease. Ann. Neurol. 2008, 63, 174–183. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; Khoury, J.E.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [PubMed]
- Frake, R.A.; Ricketts, T.; Menzies, F.M.; Rubinsztein, D.C. Autophagy and neurodegeneration. J. Clin. Invest. 2015, 125, 65–74. [Google Scholar] [CrossRef] [PubMed]
| Year/First Author | Primary Focus | Biomarkers/ Imaging Discussed | Therapeutics Discussed | Distinctive Contribution of Present Review |
|---|---|---|---|---|
| 2024—Garton et al. [20] | Immune–glia interactions in demyelination/degeneration with PMS focus | General biomarker overview; limited integration of iron, mitochondria, autophagy | Broad disease-modifying therapy overview | Mechanistic cell biology of glia; limited linkage between biomarkers and therapeutic targeting |
| 2024—Voon et al. [9] | Quantitative susceptibility mapping (QSM) methodology and sensitivity to iron/myelin | QSM and paramagnetic rim lesions (PRLs) | None | Imaging physics and lesion detection; no cytokine network integration |
| 2025—Guido et al. [21] | TSPO-PET in MS, validation, prognostic use | TSPO-PET longitudinal and standardization | None | Detailed TSPO-PET; lacks integration with cytokine/iron/autophagy axes |
| 2025—Vermersch et al. [22] | Microglia roles and targeting in MS | Selected biomarkers; narrative emphasis | Microglia-targeted agents | Microglial biology; minimal biomarker-to-trial framework |
| Present review | Glial cytokine network + metabolic/structural axes in PMS | GFAP, NfL, TSPO-PET, QSM PRLs | BTK inhibitors (incl. HERCULES), metabolic modulation, iron chelation, mTOR targeting | Unified network linking cytokines ↔ mitochondria ↔ iron ↔ autophagy; explicit evidence tiering; trial-stratification blueprint |
| Study Category | Typical Sample Size | Strengths | Common Bias Modes | Integration Guidance |
|---|---|---|---|---|
| Human in vivo (CSF/imaging, clinical trials) | 50–500 | Direct clinical translation | Selection bias, medication confounding, disease-stage heterogeneity | Prioritize replicated CSF/imaging results; caution if small n or mixed phenotypes |
| Human post-mortem | 10–80 | High-resolution cellular detail | Terminal-stage bias, lack of longitudinal dynamics | Useful for mechanism; avoid overgeneralizing prevalence |
| Animal models (EAE, cuprizone) | 8–30 per arm | Controlled mechanistic testing | Species differences, short disease course | Best for mechanistic plausibility; validate in human tissue |
| In vitro (glial culture) | 3–10 replicates | Molecular specificity | Artificial conditions, no systemic interaction | Interpret as hypothesis-generating only |
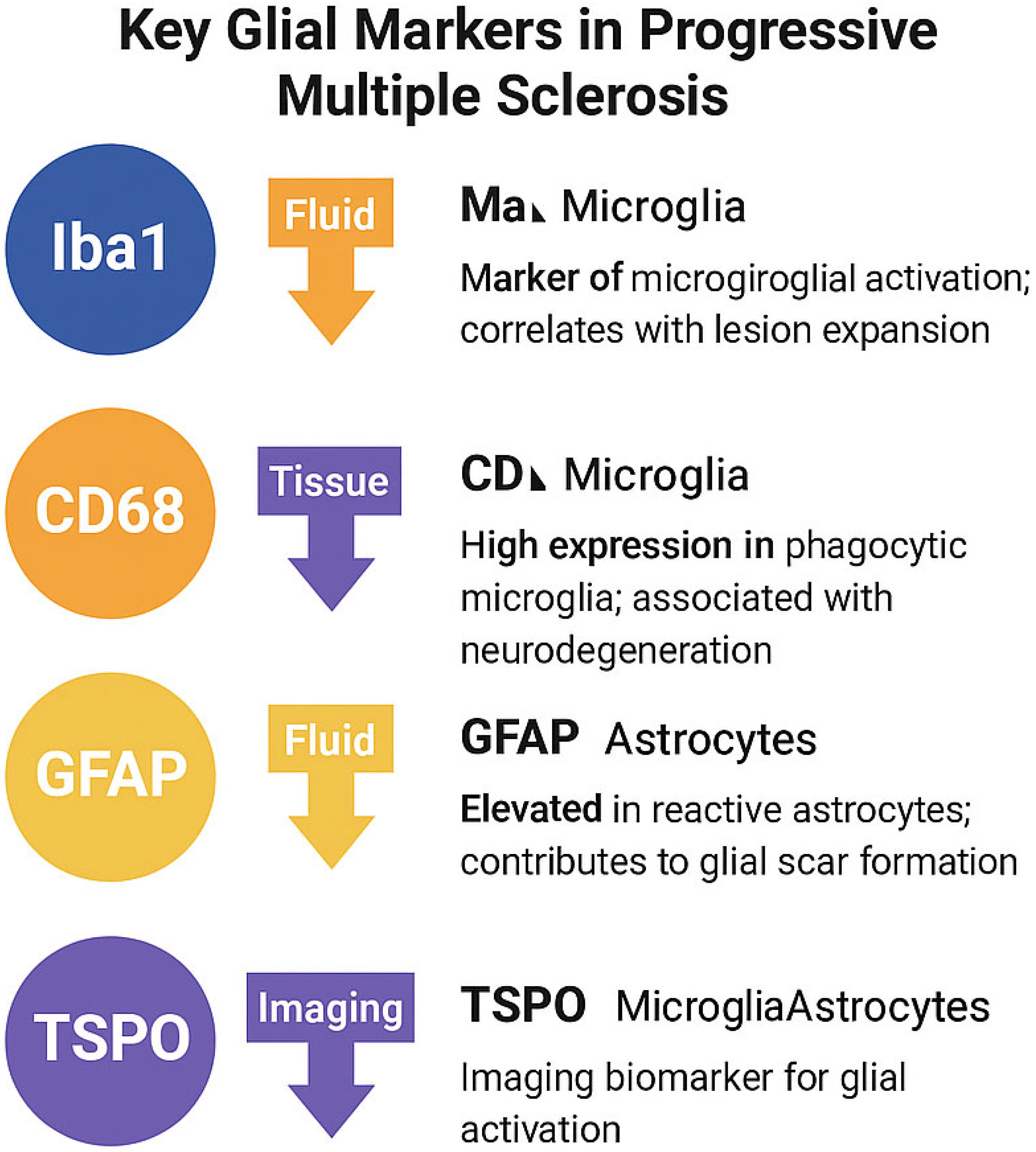
| Marker | Cell Type | Function | Relevance in PMS |
|---|---|---|---|
| Iba1 | Microglia | Motility, phagocytosis | Marker of microglial activation; correlates with lesion expansion |
| CD68 | Microglia | Lysosomal degradation | High expression in phagocytic microglia; associated with neurodegeneration |
| GFAP | Astrocytes | Cytoskeletal structure | Elevated in reactive astrocytes; contributes to glial scar formation |
| TSPO | Microglia/Astrocytes | Mitochondrial translocator | Imaging biomarker for glial activation (e.g., via PET radioligands) |
| Molecule/ Marker | What It Is | Primary Producing/ Expressing Cells in CNS | Role in Progressive MS (PMS) | Biomarker/ Assay Context | Notes | Study Design | Human/ Animal |
|---|---|---|---|---|---|---|---|
| TNF-α (TNFSF2) | Pro-inflammatory cytokine that signals via TNFR1 (death/inflammatory) and TNFR2 (repair/regulatory) receptors. | Microglia, astrocytes; infiltrating myeloid cells and T cells. | Chronically elevated in lesions/CSF; promotes glial activation, BBB permeability, and oligodendrocyte injury; TNFR1-associated neurotoxicity vs. TNFR2-associated repair. | Measured in CSF/serum (ELISA); lesion immunohistochemistry; single-cell transcriptomics in active rims. | Non-selective anti-TNF can exacerbate MS; selective TNFR1 blockade and TNFR2 agonism are proposed strategies. | Both (human tissue/CSF + preclinical) | Both |
| IL-6 (IL6) | Pleiotropic cytokine signaling via IL-6R/gp130, activating JAK/STAT3. | Astrocytes, microglia; meningeal B/plasma cells. | Overproduced intrathecally; sustains astrogliosis via STAT3, supports B cell survival; associated with cortical demyelination. | CSF/serum ELISA; intrathecal synthesis indices; transcriptomic detection in meningeal aggregates. | IL-6 receptor blockade (e.g., tocilizumab) modulates pathway in related disorders; role in PMS under investigation. | Both (human cohorts + preclinical) | Both |
| IL-1β (IL1B) | Pro-inflammatory cytokine matured by NLRP3 inflammasome (caspase-1-dependent processing). | Microglia (inflammasome-activated), infiltrating macrophages; astrocytes under stress. | Amplifies glial feedback loops and cytokine crosstalk; contributes to demyelination and chronic lesion activity. | CSF levels (often low but detectable); lesion immunostaining; peripheral monocyte-priming assays. | IL-1 receptor antagonists (e.g., anakinra) and NLRP3 inhibitors are mechanistically relevant; CNS penetration and efficacy remain under study. | Both (human tissue/CSF + preclinical) | Both |
| COX-2 (PTGS2) | Inducible cyclooxygenase isoform, catalyzes prostaglandin synthesis. | Activated microglia/macrophages, astrocytes, neurons. | Amplifies glial inflammatory cascades via prostaglandin signaling, contributes to excitotoxicity. | IHC/ISH for PTGS2; PGE2 levels in CSF/serum. | Often upregulated at rims of chronic active lesions; target of selective COX-2 inhibitors. | Reviews + pathology context | Both |
| iNOS (NOS2) | Inducible nitric oxide synthase producing high-output NO. | Activated microglia and macrophages; reactive astrocytes. | Excess NO/peroxynitrite damages mitochondria and oligodendrocytes, sustaining chronic neuroinflammation. | IHC for NOS2; nitrotyrosine adducts; NO metabolites. | Upregulated by IL-1β, TNF-α, IFN-γ; limited CNS-penetrant inhibitors available. | Reviews + human lesion pathology | Both |
| ROS (Reactive oxygen species) | Oxidants (superoxide, H2O2, OH·) from NOX2/mitochondria. | Activated microglia/macrophages, mitochondria. | Drives oxidative injury to lipids, DNA, proteins; central to chronic lesion expansion. | Oxidative adducts (4-HNE, MDA), 8-OHdG, nitrotyrosine in CSF/tissue. | Oxidative stress is a hallmark of PMS pathology. | Human pathology + reviews | Both |
| Iron/Ferritin (FTH1/FTL) | Iron storage proteins; ferritin reflects cellular iron load. | Microglia (iron-laden in rim lesions), oligodendrocytes. | Iron catalyzes ROS formation (Fenton chemistry); contributes to microglial priming and chronic lesion activity. | QSM MRI for iron; ferritin in CSF/serum; histopathology. | Paramagnetic rim lesions correlate with iron accumulation. | Human pathology + MRI validation | Human |
| LC3 (MAP1LC3A/B) | Autophagosome membrane protein; LC3-II reflects autophagy activity. | Neurons, oligodendrocytes, astrocytes, microglia. | Altered autophagy affects oligodendrocyte/myelin integrity and glial stress responses. | IHC/Western blot for LC3-I/II; EM puncta counts. | Interpreted with p62 (accumulates when autophagy impaired). | Reviews + preclinical EAE | Both |
| p62 (SQSTM1) | Autophagy adaptor binding ubiquitinated proteins to LC3. | Neurons, oligodendrocytes, astrocytes, microglia. | Build-up indicates defective autophagic flux; links to inflammasome activation. | IHC/Western blot for p62 aggregates; co-stain with LC3. | Intersects with Nrf2 signaling; imbalance implicated in PMS. | Reviews + preclinical EAE | Both |
| Iba1 (AIF1) | Actin-binding protein upregulated in activated microglia. | Microglia; infiltrating macrophages. | Marks activated microglia forming paramagnetic rims; mediates cytoskeletal remodeling and phagocytosis. | IHC for Iba1 to quantify microglial activation. | Used as imaging and pathological biomarker of active lesion rims. | Microglia reviews + PET studies | Both |
| GFAP | Astrocyte intermediate filament (reactive astrogliosis). | Astrocytes. | Astrogliosis walls off inflammation but promotes scar formation and axonal repair inhibition. | IHC for gliosis; serum/CSF GFAP (Simoa). | Serum GFAP elevated in PMS; biomarker of disease progression. | Prospective cohort + review | Human |
| CD68 | Lysosomal glycoprotein of phagocytes (macrophages/microglia). | Microglia and infiltrating macrophages. | Marks phagocytic, lipid-laden microglia at lesion rims; linked to chronic tissue breakdown. | IHC for CD68 in lesion rims; MRI-pathology correlations. | CD68+ rims co-localize with iron on QSM and predict chronic progression. | Human pathology + MRI-pathology validation | Human |
| Cytokine/Mediator | Central Claim | Evidence Tier | Sample Sizes (Approx.) | Anchor Citations |
|---|---|---|---|---|
| TNF-α | Chronically elevated in PMS lesions; drives glial activation and oligodendrocyte injury | Strong human (post-mortem + CSF studies) | n ≈ 120 (lesion cohorts); n >200 (CSF biomarker) | Brambilla 2011 [38]; Olmos 2014 [37]; Lassmann 2012 [6] |
| IL-6 | Intrathecal elevation linked to cortical atrophy and meningeal inflammation | Supportive human (CSF + autopsy); reinforced by preclinical | n ≈ 150 | Navikas 1996 [44]; Howell 2011 [43]; Reali 2020 [41] |
| IL-1β/NLRP3 | Upstream driver of TNF-α/IL-6; persistent inflammasome expression in PMS | Preclinical + post-mortem support only | n < 40 human autopsy; EAE models | Barclay 2020 [45]; Brambilla 2011 [38] |
| GFAP | Serum/CSF levels predict disability progression | Strong human evidence | n > 1000 pooled across cohorts | Freedman 2024 [13] |
| NfL | Non-specific neuroaxonal injury marker | Strong but non-specific human evidence | n > 3000 (pooled) | eBioMedicine 2024 guidance [13] |
| TSPO-PET | Detects microglial activation; predictive of later atrophy | Supportive human longitudinal | n ≈ 120 | Marjo 2025 [14]; Guido 2025 [21] |
| Biomarker | Modality | Cellular Source | Clinical Relevance |
|---|---|---|---|
| GFAP | Fluid (CSF/serum) | Astrocytes | Marker of astrogliosis; correlates with spinal cord atrophy and disease progression |
| Neurofilament light chain (NfL) | Fluid (CSF/serum) | Neurons/axons | General marker of neuroaxonal injury; widely used in clinical trials |
| sTREM2 | Fluid (CSF) | Microglia | Reflects microglial activation and phagocytic function |
| CD14 | Fluid (CSF) | Monocytes/microglia | Marker of innate immune activation; associated with inflammatory disease progression |
| IL-6, IL-8, CXCL13 | Fluid (CSF) | Activated glia, lymphocytes | Elevated in PMS; correlate with cortical atrophy and disease activity |
| TSPO-PET | Imaging | Microglia, astrocytes | Visualizes glial activation; increased uptake in PMS lesions and deep gray matter |
| QSM/SWI MRI | Imaging | Iron-retaining microglia | Detects paramagnetic rim lesions; identifies chronic active plaques |
| Therapeutic Agent | Primary Target | Mechanism of Action | Clinical Development Stage | CNS Penetration |
|---|---|---|---|---|
| Metformin | AMPK pathway, autophagy | Enhances mitochondrial function, restores autophagic flux, suppresses IL-6/STAT3 signaling in glial cells | Repurposed; Phase II trials in PMS | Yes (confirmed CNS activity) |
| Tolebrutinib | Bruton’s tyrosine kinase (BTK) | Modulates CNS-resident microglia and peripheral B cells; reduces glial inflammation | Phase III clinical trials | Yes (designed for CNS penetration) |
| Deferiprone | Microglial iron overload | Chelates excess iron, limits ROS production via inhibition of Fenton chemistry | Early-phase; off-label in neurodegeneration | Partial (limited BBB permeability) |
| Rapamycin | mTOR pathway | Enhances autophagic clearance, reduces astrocyte reactivity, supports proteostasis | Preclinical; limited human trials | Variable (CNS effects demonstrated in models) |
| Dimethyl Fumarate | Nrf2 activation | Activates antioxidant defenses, reduces ROS-mediated injury and glial priming | Approved for RRMS; under PMS evaluation | Yes (clinically proven CNS penetration) |
| Anakinra | IL-1 receptor antagonist | Inhibits IL-1β signaling and inflammasome activity | Approved for systemic diseases; early CNS studies | Low (limited BBB crossing) |
| Tocilizumab | IL-6 receptor blockade | Suppresses IL-6/STAT3 pathway, reduces reactive astrogliosis | Phase I-II in neuroinflammation | Low–Moderate (limited CNS access; variable results) |
| Molecule/Pathway | Other Diseases Involved | Mechanistic Similarity | Key Distinction in PMS | Therapeutic Implication |
|---|---|---|---|---|
| IL-6 | AD | STAT3 activation, astrocyte-driven inflammation | CNS-compartmentalized IL-6 from astrocytes | IL-6 receptor inhibitors may be applicable |
| COX-2 | AD, PD | Glial inflammation, synaptic dysfunction | Requires CNS-penetrant inhibitors due to chronic activation | COX-2 inhibitors may need reformulation for CNS access |
| TNF-α | ALS | Cytokine-mediated inflammation, peripheral in ALS | Produced mainly by CNS-resident microglia in PMS | Anti-TNF therapy limited; TNFR2 agonism is an alternative |
| Iron/ROS | PD, ALS | Oxidative stress via Fenton chemistry | Chronic iron retention and efflux dysfunction in microglia | Targeted CNS iron chelation strategies required |
| Autophagy (LC3, p62) | PD, AD | Impaired clearance of damaged proteins | Glial priming and mitochondrial dysfunction tightly linked | Metformin and rapamycin show promise across diseases |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohm, H.L.; Schuster, R.; Buciu, V.B.; Serban, D.-M.; Ciurescu, S.; Cornea, A.; Sharma, A.; Nistor, D.; Kundnani, N.R. Glial Cytokine and Metabolic Networks in Progressive Multiple Sclerosis: From Pathophysiology to Biomarkers and Therapeutic Strategies. Int. J. Mol. Sci. 2025, 26, 8817. https://doi.org/10.3390/ijms26188817
Hohm HL, Schuster R, Buciu VB, Serban D-M, Ciurescu S, Cornea A, Sharma A, Nistor D, Kundnani NR. Glial Cytokine and Metabolic Networks in Progressive Multiple Sclerosis: From Pathophysiology to Biomarkers and Therapeutic Strategies. International Journal of Molecular Sciences. 2025; 26(18):8817. https://doi.org/10.3390/ijms26188817
Chicago/Turabian StyleHohm, Henry Leonard, Rasmus Schuster, Victor Bogdan Buciu, Denis-Mihai Serban, Sebastian Ciurescu, Amalia Cornea, Abhinav Sharma, Daciana Nistor, and Nilima Rajpal Kundnani. 2025. "Glial Cytokine and Metabolic Networks in Progressive Multiple Sclerosis: From Pathophysiology to Biomarkers and Therapeutic Strategies" International Journal of Molecular Sciences 26, no. 18: 8817. https://doi.org/10.3390/ijms26188817
APA StyleHohm, H. L., Schuster, R., Buciu, V. B., Serban, D.-M., Ciurescu, S., Cornea, A., Sharma, A., Nistor, D., & Kundnani, N. R. (2025). Glial Cytokine and Metabolic Networks in Progressive Multiple Sclerosis: From Pathophysiology to Biomarkers and Therapeutic Strategies. International Journal of Molecular Sciences, 26(18), 8817. https://doi.org/10.3390/ijms26188817









