Monoclonal Antibodies: Historical Perspective and Current Trends in Biological Drug Development
Abstract
1. Introduction
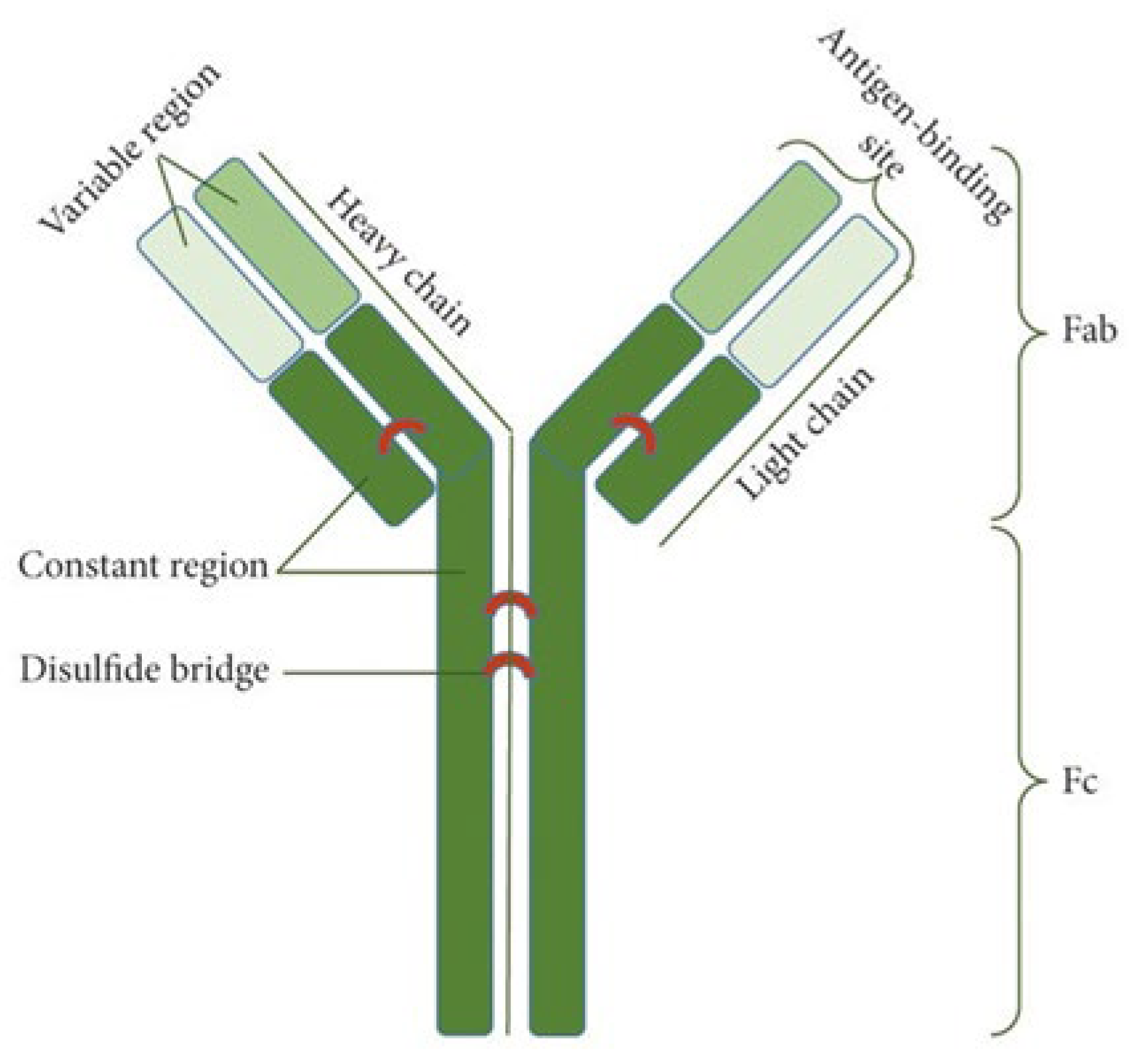
2. Basic Characteristics of Antibodies
2.1. Classification of Antibodies
- Mouse antibodies containing 100% mouse sequences;
- Chimeric antibodies having mouse-variable regions, which constitute about 25% of the entire sequence; the rest are human sequences;
- Humanized antibodies retain mostly human sequences, with mouse-derived CDRs and sometimes selected framework residues;
2.2. Mechanism of Action of Antibodies
3. The Methods of Producing Antibodies
3.1. Hybridoma Technology
3.2. Phage Display
3.3. Transgenic Animals
4. Development of Therapeutic Antibodies
5. New Perspectives for Miniature Antibodies—Nanobodies (Nbs)
6. Personal Opinion
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Abbreviations
| ADC | antibody–drug conjugate |
| ADCC | antibody-dependent cell-mediated cytotoxic reaction |
| BAC | bacterial artificial chromosome |
| bsAb | bispecific antibody |
| BTK | Bruton tyrosine kinase |
| CAR | chimeric antigen receptor |
| CDC | complement-dependent cytotoxicity |
| cDNA | complementary DNA |
| CDR | complementarity-determining region |
| c-erbB-2 | cellular homolog of the avian erythroblastosis virus oncogene B2 |
| cHL | classical Hodgkin lymphoma |
| CRC | colorectal cancer |
| CXCR7 | chemokine receptor type 7 |
| DAG | diacylglycerol |
| dMMR | deficient mismatch repair |
| EGFR | epidermal growth factor receptor |
| ELISA | enzyme-linked immunosorbent assay |
| EMA | European Medicines Agency |
| Fab | antigen-binding fragment |
| Fc | crystallizable fragment |
| FcR | Fc receptor |
| FDA | Food and Drug Administration |
| G3P | minor envelope protein |
| G8P | major envelope protein |
| GEF | guanine nucleotide exchange factor |
| HAMA | human anti-mouse antibody |
| HC | heavy chain |
| HER2 | human epidermal growth factor receptor 2 |
| HGF | hepatocyte growth factor |
| HGPRT | hypoxanthine–guanine phosphoribosyltransferase |
| HNSCC | head and neck squamous cell carcinoma |
| IgH | immunoglobulin heavy chain |
| IgL | immunoglobulin light chain |
| IL-2 | interleukin 2 |
| IP3 | cytosolic inositol-1,4,5 trisphosphate |
| ITAM | immunotyrosine-based activating motif |
| ITIM | immunotyrosine-based inhibitor motif |
| LC | light chain |
| LFIA | lateral flow immunoassay |
| mAb | monoclonal antibody |
| MAC | membrane-activating complex |
| MACS | magnetic cell sorting system |
| MAPK | mitogen-activated protein kinase |
| MCC | Merkel-cell carcinoma |
| MEK | serine–tyrosine–threonine kinase |
| MSI-H | microsatellite instability-high |
| mUC | metastatic urothelial carcinoma |
| Nbs | nanobody |
| NSCLC | non-small cell lung carcinoma |
| PCR | polymerase chain reaction |
| PD-1 | programmed death receptor-1 |
| PD-L1 | programmed death ligand 1 |
| PEG | polyethylene glycol |
| PH | pleckstrin homology |
| PI3K | phosphoinositide 3-kinase |
| PKC | protein kinase C |
| PLC-γ | phospho-lipase C-gamma |
| RAS | rat sarcoma virus |
| RCC | renal cell carcinoma |
| ROS | reactive oxygen species |
| scFv | single-chain Fv fragment |
| sdAb | single-domain antibody |
| SFK | SRC family kinase |
| SHIP | Src homology 2 domain-containing inositol 5′-phosphatase |
| SHP | Src homology 2 domain-containing protein tyrosine phosphatase |
| SYK | spleen tyrosine kinase |
| TNF-α | tumor necrosis factor-alpha |
| UCC | urothelial carcinoma |
| VEGFR2 | vascular endothelial growth factor receptor 2 |
| VSIV | vesicular stomatitis Indiana virus |
| YAC | yeast artificial chromosome |
References
- Milstein, C.; Kohler, G. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 1975, 256, 495–497. [Google Scholar] [CrossRef]
- Tsao, L.; Force, J.; Hartman, Z.C. Mechanisms of therapeutic antitumor monoclonal antibodies. Cancer Res. 2021, 81, 4641–4651. [Google Scholar] [CrossRef]
- Leavy, O. The birth of monoclonal antibodies. Nat. Immunol. 2016, 17 (Suppl. S1), S13. [Google Scholar] [CrossRef]
- Antibody Society. Available online: https://www.antibodysociety.org/resources/approved-antibodies/ (accessed on 16 May 2025).
- Chiu, M.L.; Goulet, D.R.; Teplyakov, A.; Gilliland, G.L. Antibody structure and function: The basis for engineering therapeutics. Antibodies 2019, 8, 55. [Google Scholar] [CrossRef] [PubMed]
- Chiu, M.L.; Gilliland, G.L. Engineering antibody therapeutics. Curr. Opin. Struct. Biol. 2016, 38, 163–173. [Google Scholar] [CrossRef]
- Stanfield, R.L.; Wilson, I.A. Antibody Structure. Microbiol. Spectr. 2014, 2, 49–62. [Google Scholar] [CrossRef] [PubMed]
- Golpour, M.; Hafezi, N.; Vatanpour, P.; Saeedi, M.; Hafezi, N.; Rafieiet, A. The perspective of therapeutic antibody marketing in Iran: Trend and estimation by 2025. Adv. Pharmacol. Pharm. Sci. 2021, 2021, 5569590. [Google Scholar] [CrossRef]
- Oostindie, S.C.; Lazar, G.A.; Schuurman, J.; Parren, P.W.H.I. Avidity in antibody effector functions and biotherapeutic drug design. Nat. Rev. Drug Discov. 2022, 21, 715–735. [Google Scholar] [CrossRef] [PubMed]
- Pelletier, J.P.R.; Mukhtar, F.; Maitta, R.W. Passive monoclonal and polyclonal antibody therapies. In Immunologic Concepts in Transfusion Medicine; Elsevier: Amsterdam, The Netherlands, 2020; Volume 16, pp. 251–348. [Google Scholar] [CrossRef]
- Keyt, B.A.; Baliga, R.; Sinclair, A.M.; Carroll, S.F.; Petersonet, M.S. Structure, Function, and Therapeutic Use of IgM Antibodies. Antibodies 2020, 9, 53. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.M.; Hwang, Y.C.; Liu, I.J.; Lee, C.C.; Tsai, H.-Z.; Li, H.-J.; Wuet, H.C. Development of therapeutic antibodies for the treatment of diseases. J. Biomed. Sci. 2020, 27, 1. [Google Scholar] [CrossRef]
- Sosnowska-Pasiarska, B.; Pasiarski, M.; Wożakowska-Kapłon, B. Monoclonal antibodies usage in cardiovascular diseases therapy. Chor. Serca I Naczyń 2014, 11, 342–347. [Google Scholar]
- Catapanoa, A.L.; Papadopoulosc, N. The safety of therapeutic monoclonal antibodies: Implications for cardiovascular disease and targeting the PCSK9 pathway. Atherosclerosis 2013, 228, 18–28. [Google Scholar] [CrossRef]
- Goulet, D.R.; Atkins, W.M. Considerations for the design of antibody-based therapeutics. J. Pharm. Sci. 2020, 109, 74–103. [Google Scholar] [CrossRef]
- Masuda, A.; Yoshida, M.; Shiomi, H.; Morita, Y.; Kutsumi, H.; Inokuchi, H.; Mizuno, S.; Nakamura, A.; Takai, T.; Blumberg, R.S.; et al. Role of Fc receptors as a therapeutic target. Inflamm. Allergy Drug Targets 2009, 8, 80–86. [Google Scholar] [CrossRef]
- Gogesch, P.; Dudek, S.; van Zandbergen, G.; Waibler, Z.; Anzaghe, M. The Role of Fc Receptors on the Effectiveness of Therapeutic Monoclonal Antibodies. Int. J. Mol. Sci. 2021, 22, 8947. [Google Scholar] [CrossRef]
- Bournazos, S.; Gupta, A.; Ravetch, J.V. The role of IgG Fc receptors in antibody-dependent enhancement. Nat. Rev. Immunol. 2020, 20, 633–643. [Google Scholar] [CrossRef]
- Mkaddem, B.S.; Benhamou, M.; Monteiro, R.C. Understanding Fc Receptor Involvement in Inflammatory Diseases: From Mechanisms to New Therapeutic Tools. Front. Immunol. 2019, 10, 811. [Google Scholar] [CrossRef] [PubMed]
- Tridandapani, S.; Siefker, K.; Teillaud, J.L.; Carter, J.E.; Wewers, M.D.; Andersonet, C.L. Regulated expression and inhibitory function of Fcgamma RIIb in human monocytic cells. J. Biol. Chem. 2002, 277, 5082–5089. [Google Scholar] [CrossRef] [PubMed]
- Getahun, A.; Cambier, J.C. Of ITIMs, ITAMs, and ITAMis: Revisiting immunoglobulin Fc receptor signaling. Immunol. Rev. 2015, 268, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Dunkelberger, J.; Song, W.C. Complement and its role in innate and adaptive immune responses. Cell Res. 2010, 20, 34–50. [Google Scholar] [CrossRef]
- Schartz, N.D.; Tenner, A.J. The good, the bad, and the opportunities of the complement system in neurodegenerative disease. J. Neuroinflamm. 2020, 17, 354–379. [Google Scholar] [CrossRef]
- Mitra, S.; Tomar, P.C. Hybridoma technology; advancements, clinical significance, and future aspects. J. Genet. Eng. Biotechnol. 2021, 19, 159–171. [Google Scholar] [CrossRef]
- Smith, S.A.; Crowe, J.E., Jr. Use of Human Hybridoma Technology To Isolate Human Monoclonal Antibodies. Microbiol. Spectr. 2015, 3, 141–156. [Google Scholar] [CrossRef]
- Paprocka, M. Monoclonal antibodies. Przeciwciała Monoklonalne Kosm. 1994, 43, 273–284. [Google Scholar]
- Samoilovich, S.R.; Dugan, C.B.; Macario, A.J. Hybridoma technology: New developments of practical interest. J. Immunol. Methods 1987, 101, 153–170. [Google Scholar] [CrossRef]
- Vienken, J.; Zimmermann, U. Electric field-induced fusion: Electro-hydraulic procedure for production of heterokaryon cells in high yield. FEBS Lett. 1982, 137, 11–13. [Google Scholar] [CrossRef]
- Greenfield, E.A. Electro cell fusion for hybridoma production. Cold Spring Harb. Protoc. 2019, 10, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Apiratmateekul, N.; Phunpae, P.; Kasinrerk, W. A modified hybridoma technique for production of monoclonal antibodies having desired isotypes. Cytotechnology 2009, 60, 45–51, Erratum in Cytotechnology 2009, 60, 53–54. https://doi.org/10.1007/s10616-009-9222-z. [Google Scholar] [CrossRef] [PubMed]
- Clementi, N.; Mancini, N.; Solforosi, L.; Castelli, M.; Clementi, M.; Burioniet, R. Phage display-based strategies for cloning and optimization of monoclonal antibodies directed against human pathogens. Int. J. Mol. Sci. 2012, 13, 8273–8292. [Google Scholar] [CrossRef] [PubMed]
- Ledsgaard, L.; Kilstrup, M.; Karatt-Vellatt, A.; McCafferty, J.; Laustsen, A.H. Basics of antibody phage display technology. Toxins 2018, 10, 236. [Google Scholar] [CrossRef]
- Brüggeman, M.; Osborn, M.J.; Ma, B.; Hayre, J.; Avis, S.; Lundstrom, B.; Buelowet, R. Human antibody production in transgenic animals. Arch. Immunol. Ther. Exp. 2015, 63, 101–108. [Google Scholar] [CrossRef]
- Chen, W.C.; Murawsky, C.M. Strategies for generating diverse antibody repertoires using transgenic animals expressing human antibodies. Front. Immunol. 2018, 9, 460–467. [Google Scholar] [CrossRef]
- Todd, P.A.; Brogden, R.N. Muromonab CD3. A review of its pharmacology and therapeutic potential. Drugs 1989, 37, 871–899. [Google Scholar] [CrossRef]
- Wilde, M.I.; Goa, K.L. Muromonab CD3: A reappraisal of its pharmacology and use as prophylaxis of solid organ transplant rejection. Drugs 1996, 51, 865–894. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, N.K.; Dwiwedi, P.; Jaykaran, C.; Rimplejeet, K.; Preeti, S.; Vinay, C. Monoclonal Antibodies: A Review. Curr. Clin. Pharmacol. 2018, 13, 85–99. [Google Scholar] [CrossRef] [PubMed]
- Kuus-Reichel, K.; Grauer, L.S.; Karavodin, L.M.; Knott, C.; Krusemeier, M.; Kay, N.E. Will immunogenicity limit the use, efficacy, and future development of therapeutic monoclonal antibodies? Clin. Diagn. Lab. Immunol. 1994, 1, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Krawczyk, P.; Wojas-Krawczyk, K. Przeciwciała monoklonalne przeciw immunologicznym punktom kontroli w leczeniu chorych na nowotwory. Onkol. Prak Klin. 2015, 2, 76–86. [Google Scholar]
- Grzywnowicz, M.; Giannopoulos, K. Znaczenie receptora programowanej śmierci 1 oraz jego ligandów w układzie immunologicznym oraz nowotworach. Acta Haematol. Pol. 2012, 43, 132–145. [Google Scholar] [CrossRef]
- Wysocki, P.J. Mechanizmy działania przeciwciał monoklonalnych w nowotworach litych. Onkol. Prak Klin. 2014, 10, 175–183. [Google Scholar]
- Malesa, A.; Nowak, J.; Skórka, K.; Karp, M.; Giannopoulos, K. Immunotherapy with monoclonal antibodies targeting the PD-1/PD-L1 pathway in cancer. Acta Haematol. Pol. 2018, 49, 207–227. [Google Scholar] [CrossRef]
- Varadan, V.; Gilmore, H.; Miskimen, K.L.S.; Tuck, D.; Parsai, S.; Awadallah, A.; Krop, I.E.; Winer, E.P.; Bossuyt, V.; Somlo, D.; et al. Immune signatures following single dose Trastuzumab Predict Pathologic response to Preoperative Trastuzumab and Chemotherapy in HER2-Positive early breast Cancer. Clin. Cancer Res. 2016, 22, 3249–3259. [Google Scholar] [CrossRef]
- Chaganty, B.K.R.; Qiu, S.; Gest, G.; Lu, Y.; Ivan, C.; Calin, G.A.; Weiner, L.M.; Fan, Z. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNγ secretion. Cancer Lett. 2018, 430, 47–56. [Google Scholar] [CrossRef]
- Stagg, J.; Loi, S.; Divisekera, U.; Smyth, M.J. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl. Acad. Sci. USA 2011, 108, 7142–7147. [Google Scholar] [CrossRef]
- Müller, P.; Kreuzaler, M.; Khan, K.; Thommen, D.S.; Martin, K.; Glatz, K.; Savic, S.; Harbeck, N.; Nitz, U.; Gluz, O.; et al. Trastuzumab emtansine (T-DM1) renders HER2 + breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 2015, 7, 315ra188. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Bai, Y.; Xu, J.; Lonardi, S.; Metges, J.P.; Yanez, P.; Wyrwicz, L.S.; Shen, L.; Ostapenko, Y.; et al. Pembrolizumab plus Trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: Interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet 2023, 402, 2197–2208. [Google Scholar] [CrossRef]
- Janjigian, Y.Y.; Kawazoe, A.; Yañez, P.; Li, N.; Lonardi, S.; Kolesnik, O.; Barajas, O.; Bai, Y.; Shen, L.; Tang, Y.; et al. KEYNOTE-811 trial of dual PD-1 and HER2 blockade in HER2-positive gastric cancer. Nature 2021, 600, 727–730. [Google Scholar] [CrossRef]
- Ali, D.S.; Gad, H.A.; Hathout, R.K. Enhancing Effector Jurkat Cell Activity and Increasing Cytotoxicity against A549 Cells Using Nivolumab as an Anti-PD-1 Agent Loaded on Gelatin Nanoparticles. Gels 2024, 10, 352. [Google Scholar] [CrossRef] [PubMed]
- Chis, A.A.; Dobrea, C.M.; Arseniu, A.M.; Frum, A.; Rus, L.-L.; Cormos, G.; Georgescu, C.; Morgovan, C.; Butuca, A.; Gligor, F.G.; et al. Antibody-Drug Conjugates-Evolution and Perspectives. Int. J. Mol. Sci. 2024, 25, 6969. [Google Scholar] [CrossRef] [PubMed]
- Maecker, H.; Jonnalagadda, V.; Bhakta, S.; Jammalamadaka, V.; Junutulaet, J.R. Exploration of the antibody-drug conjugate clinical landscape. MAbs 2023, 15, 2229101. [Google Scholar] [CrossRef]
- Suurs, F.; Lub-de Hooge, M.; de Vries, E.; Elisabeth, G.E.; de Groot, D.J.A. A review of bispecific antibodies and antibody constructs in oncology and clinical challenges. Pharmacol. Ther. 2019, 201, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Shim, H. Bispecific Antibodies and antibody–drug conjugates for cancer therapy: Technological considerations. Biomolecules 2020, 10, 360. [Google Scholar] [CrossRef] [PubMed]
- Jin, B.K.; Odongo, S.; Radwanska, M.; Magez, S. NANOBODIES®: A review of diagnostic and therapeutic applications. Int. J. Mol. Sci. 2023, 24, 5994. [Google Scholar] [CrossRef]
- Khilji, S.K.; Hoog, C.O.; Warschkau, D.D.; Lühle, J.; Goerdeler, F.; Freitag, A.; Seeberger, P.H.; Moscovitzet, O. Smaller size packs a stronger punch—Recent advances in small antibody fragments targeting tumour-associated carbohydrate antigens. Theranostics 2023, 13, 3041–3063. [Google Scholar] [CrossRef]
- Scully, M.; Cataland, S.R.; Peyvandi, F.; Coppo, P.; Knöbl, P.; Hovinga, J.A.K.; Metjian, A. Caplacizumab Treatment for Acquired Thrombotic Thrombocytopenic Purpura. N. Engl. J. Med. 2019, 380, 335–346. [Google Scholar] [CrossRef]
- Duggan, S. Caplacizumab: First Global Approval. Drugs 2018, 78, 1639–1642, Erratum in Drugs 2018, 78, 1955. [Google Scholar] [CrossRef] [PubMed]
- Hollifield, A.L.; Arnall, J.R.; Moore, D.C. Caplacizumab: An anti-von Willebrand factor antibody for the treatment of thrombotic thrombocytopenic purpura. Am. J. Health-Syst. Pharm. 2020, 77, 1201–1207. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.H.; Wang, B.Y.; Chen, L.J.; Fu, W.J.; Xu, J.; Liu, J.; Jin, S.W.; Chen, Y.X.; Cao, X.M.; Yang, Y.; et al. Four-year follow-up of LCAR-B38M in relapsed or refractory multiple myeloma: A phase 1, single-arm, open-label, multicenter study in China (LEGEND-2). J. Hematol. Oncol. 2022, 15, 86. [Google Scholar] [CrossRef]
- Berdeja, J.G.; Madduri, D.; Usmani, S.Z.; Jakubowiak, A.; Agha, M.; Cohen, A.D.; Stewart, A.K.; Hari, P.; Htut, M.; Lesokhin, A.; et al. Ciltacabtagene autoleucel, a B-cell maturation antigen-directed chimeric antigen receptor T-cell therapy in patients with relapsed or refractory multiple myeloma (CARTITUDE-1): A phase 1b/2 open-label study. Lancet 2021, 398, 314–324, Erratum in Lancet 2021, 398, 1216. [Google Scholar] [CrossRef]
- Mullard, A. FDA approves second BCMA-targeted CAR-T cell therapy. Nat. Rev. Drug Discov. 2022, 21, 249. [Google Scholar] [CrossRef]
- Keam, S.J. Ozoralizumab: First Approval. Drugs 2023, 83, 87–92. [Google Scholar] [CrossRef]
- Alexion Pharmaceuticals. Safety and Efficacy of ALXN1720 in Adults with Generalized Myasthenia Gravis. Identifier NCT05556096. Available online: https://clinicaltrials.gov/ct2/show/NCT05556096 (accessed on 9 February 2023).
- Keyaerts, M.; Xavier, C.; Heemskerk, J.; Devoogdt, N.; Everaert, H.; Ackaert, C.; Vanhoeij, M.; Duhoux, F.P.; Gevaert, T.; Simon, P.; et al. Phase I Study of 68Ga-HER2-Nanobody for PET/CT Assessment of HER2 Expression in Breast Carcinoma. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2016, 57, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Keyaerts, M.; Xavier, C.; Everaert, H.; Vaneycken, I.; Fontaine, C.; Decoster, L.; Vanhoeij, M.; Caveliers, V.; Lahoutte, T. Phase II trial of HER2-PET/CT using 68ga-anti-HER2 VHH1 for characterization of HER2 presence in brain metastases of breast cancer patients. Ann. Oncol. 2019, 30, iii25–iii26. [Google Scholar] [CrossRef]
- Hannon, C.W.; McCourt, C.; Lima, H.C.; Chen, S.; Bennett, C. Interventions for cutaneous disease in systemic lupus erythematosus. Cochrane Database Syst. Rev. 2021, 3, CD007478. [Google Scholar] [CrossRef]
- Ablynx, A. Phase IIb Study for ALX-0061 Monotherapy in Subjects with Rheumatoid Arthritis. Identifier NCT02518620. Available online: https://clinicaltrials.gov/ct2/show/NCT02518620 (accessed on 9 February 2023).
- Papp, K.A.; Weinberg, M.A.; Morris, A.; Reich, K. IL17A/F nanobody sonelokimab in patients with plaque psoriasis: A multicentre, randomised, placebo-controlled, phase 2b study. Lancet 2021, 397, 1564–1575, Erratum in Lancet 2021, 397, 2150. [Google Scholar] [CrossRef]
- VHsquared Ltd. A Six Week Efficacy, Safety and Tolerability Study of V565 in Crohn’s Disease (HarbOR). Identifier NCT02976129. Available online: https://clinicaltrials.gov/ct2/show/NCT02976129 (accessed on 9 February 2023).
- Sarker, S.A.; Jäkel, M.; Sultana, S.; Alam, N.H.; Bardhan, P.K.; Chisti, M.J.; Salam, M.A.; Theis, W.; Hammarström, L.; Frenken, L.G. Anti-rotavirus protein reduces stool output in infants with diarrhea: A randomized placebo-controlled trial. Gastroenterology 2013, 145, 740–748.e8. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, S.; Piedra, P.A.; Martinon-Torres, F.; Szymanski, H.; Brackeva, B.; Dombrecht, E.; Detalle, L.; Fleurinck, C. RESPIRE study group Nebulised ALX-0171 for respiratory syncytial virus lower respiratory tract infection in hospitalised children: A double-blind, randomised, placebo-controlled, phase 2b trial. Lancet Respir. Med. 2021, 9, 21–32. [Google Scholar] [CrossRef]
- Lumen Bioscience, Inc. LMN-101 in a Campylobacter Human Challenge Model. Identifier NCT04182490. Available online: https://clinicaltrials.gov/ct2/show/NCT04182490 (accessed on 9 February 2023).
- D’Huyvetter, M.; Vos, J.; Caveliers, V.; Vaneycken, I.; Heemskerk, J.; Duhoux, F.P.; Fontaine, C.; Vanhoeij, M.; Windhorst, A.D.; Aa, F.V.; et al. Phase I Trial of 131I-GMIB-Anti-HER2-VHH1, a New Promising Candidate for HER2-Targeted Radionuclide Therapy in Breast Cancer Patients. J. Nucl. Med. Off. Publ. Soc. Nucl. Med. 2021, 62, 1097–1105. [Google Scholar] [CrossRef]
- KGaA, M. Multiple Ascending Doses (MAD) of Anti-A Disintegrin and Metalloproteinase with Thrombospondin Motifs5 (Anti-ADAMTS-5) Nanobody in Participants with Knee Osteoarthritis (OA). Identifier NCT03583346. Available online: https://clinicaltrials.gov/ct2/show/NCT03583346 (accessed on 9 February 2023).
- Benmebarek, M.R.; Karches, C.H.; Cadilha, B.L.; Lesch, S.; Endres, S.; Kobold, S. Killing Mechanisms of Chimeric Antigen Receptor (CAR) T Cells. Int. J. Mol. Sci. 2019, 20, 1283. [Google Scholar] [CrossRef]
- Chakravarty, R.; Goel, S.; Cai, W. Nanobody: The “magic bullet” for molecular imaging? Theranostics 2014, 4, 386–398. [Google Scholar] [CrossRef]
- Kijanka, M.; Dorresteijn, B.; Oliveira, S.; van Bergen en Henegouwen, P.M. Nanobody-based cancer therapy of solid tumors. Nanomedicine 2015, 10, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Arezumand, R.; Alibakhshi, A.; Ranjbari, J.; Ramazani, A.; Muyldermans, S. Nanobodies as novel agents for targeting angiogenesis in solid cancers. Front. Immunol. 2017, 8, 1746. [Google Scholar] [CrossRef] [PubMed]
- Behdani, M.; Zeinali, S.; Khanahmad, H.; Karimipour, M.; Asadzadeh, N.; Azadmanesh, K.; Khabiri, A.; Schoonooghe, S.; Anbouhi, M.H.; Hassanzadeh-Ghassabeh, G.; et al. Generation and characterization of a functional nanobody against the vascular endothelial growth factor receptor-2; angiogenesis cell receptor. Mol. Immunol. 2012, 50, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Vosjan, M.J.; Vercammen, J.; Kolkman, J.A.; Stigter-van Walsum, M.; Revets, H.; van Dongen, G.A. Nanobodies targeting the hepatocyte growth factor: Potential new drugs for molecular cancer therapy. Mol. Cancer Ther. 2012, 11, 1017–1025. [Google Scholar] [CrossRef]
- Tijink, B.M.; Laeremans, T.; Budde, M.; Stigter-van Walsum, M.; Dreier, T.; de Haard, H.J.; Leemans, C.R.; van Dongen, G.A. Improved tumor targeting of antiepidermal growth factor receptor nanobodies through albumin binding: Taking advantage of modular nanobody technology. Mol. Cancer Ther. 2008, 7, 2288–2297. [Google Scholar] [CrossRef]
- Oliveira, S.; Heukers, R.; Sornkom, J.; Kok, R.J.; van Bergen, P.M. Targeting tumors with nanobodies for cancer imaging and therapy. J. Controll. Release 2013, 172, 607–617. [Google Scholar] [CrossRef]
- Oliveira, S.; Schiffelers, R.M.; van der Veeken, J.; van der Meel, R.; Vongpromek, R.; van Bergen En Henegouwen, P.M.; Storm, G.; Roovers, R.C. Downregulation of EGFR by a novel multivalent nanobody-liposome platform. J. Controll. Release 2010, 145, 165–175. [Google Scholar] [CrossRef]
- Talelli, M.; Rijcken, C.J.; Oliveira, S.; van der Meel, R.; van Bergen En Henegouwen, P.M.; Lammers, T.; van Nostrum, C.F.; Storm, G.; Hennink, W.E. Nanobody-shell functionalized thermosensitive core-crosslinked polymeric micelles for active drug targeting. J. Controll. Release 2011, 151, 183–192. [Google Scholar] [CrossRef]
- Altintas, I.; Heukers, R.; van der Meel, R.; Lacombe, M.; Amidi, M.; van Bergen En Henegouwen, P.M.; Hennink, W.E.; Schiffelers, R.M.; Kok, R.J. Nanobody-albumin nanoparticles (NANAPs) for the delivery of a multikinase inhibitor 17864 to EGFR overexpressing tumor cells. J. Controll. Release 2013, 165, 110–118. [Google Scholar] [CrossRef]
- van der Meel, R.; Oliveira, S.; Altintas, I.; Haselberg, R.; van der Veeken, J.; Roovers, R.C.; Henegouwen, P.M.v.B.E.; Storm, G.; Hennink, W.E.; Schiffelers, R.M.; et al. Tumor-targeted Nanobullets: Anti-EGFR nanobody-liposomes loaded with anti- IGF-1R kinase inhibitor for cancer treatment. J. Controll. Release 2012, 159, 281–289. [Google Scholar] [CrossRef]
- Talelli, M.; Iman, M.; Varkouhi, A.K.; Rijcken, C.J.; Schiffelers, R.M.; Etrych, T.; Ulbrich, K.; van Nostrum, C.F.; Lammers, T.; Storm, G.; et al. Core-crosslinked polymeric micelles with controlled release of covalently entrapped doxorubicin. Biomaterials 2010, 31, 7797–7804. [Google Scholar] [CrossRef] [PubMed]
- Jovčevska, I.; Serge Muyldermans, S. The Therapeutic Potential of Nanobodies. BioDrugs 2020, 34, 11–26. [Google Scholar] [CrossRef] [PubMed]


| Class | Form | Half-Life (Days) | Serum Concentration (mg/mL) | Percentage in Serum | Description |
|---|---|---|---|---|---|
| IgG | monomer | 23 | 8–16 | 80 | They play the most important role in the immune response in defending against microorganisms and viruses that have penetrated cells and cancer cells, initiating the death of these cells. |
| IgA | monomer, dimer, tetramer | 5.8 | 1.4–4 | 13 | They perform protective functions against pathogens in the mucous and serous membranes; they are found in tears, sweat, and secretions from the glands of the digestive, respiratory, and urinary tracts. |
| IgM | pentamer, hexamer | 5.1 | 0.5–2 | 6 | They are produced first in the initial phase of the immune response; their affinity for the antigen is low. |
| IgD | monomer | 2.8 | 0.04 | 0.001 | They are abundant on the surface of B lymphocytes (not in contact with the antigen) as their receptors; they are scarce in the serum; when bound to basophils and mast cells, they inhibit their degranulation and allergic reactions. |
| IgE | monomer | 2.5 | 0.00002–0.0005 | 0.000003 | They participate in defense against parasites and allergies; by binding to basophils and mast cells, they cause their degranulation, releasing, among others, histamine. |
| INN (Brand Name) | Target | Drug | Indication | Approval Year |
|---|---|---|---|---|
| Ibritumomab tiuxetan (Zevalin) 1 | CD20 | [90Y] | Follicular lymphoma | 2004 |
| Brentuximab vedotin (Adcetris) | CD30 | MMAE | Hodgkin’s lymphoma, systemic anaplastic large cell lymphoma, cutaneous T cell lymphoma | 2012 |
| Trastuzumab emtansine (Kadcyla) | HER-2 | DM1 | Breast cancer | 2013 |
| Gemtuzumab ozogamicin (Mylotarg) | CD33 | N-acetyl-gamma-calicheamicin | Acute myeloid leukemia | 2018 |
| Polatuzumab vedotin (Polivy) | CD79b | MMAE | Diffuse large B cell lymphoma | 2020 |
| Belantamab mafodotin (BLENREP) 1 | BCMA | mcMMAF | Multiple myeloma | 2020 |
| Trastuzumab deruxtecan (Enhertu) | HER-2 | deruxtecan | Breast cancer, non-small cell lung cancer, stomach cancer | 2021 |
| Sacituzumab govitecan (Trodelvy) | TROP-2 | SN-38 | Breast cancer | 2021 |
| Enfortumab vedotin (Padcev) | Nectin-4 | MMAE | Urothelial cancer | 2022 |
| Loncastuximab tesirine (Zynlonta) | CD19 | SG3249 (tesirine) | Diffuse large B cell lymphoma, high-grade B cell lymphoma | 2022 |
| INN (Brand Name) | Target | Indication | Approval Year |
|---|---|---|---|
| Catumaxomab (Removab) 1 | EpCAM, CD3 | Neoplastic ascites | 2009 |
| Blinatumomab (Blincyto) | CD19, CD3 | Acute lymphoblastic leukemia | 2015 |
| Amivantamab (Rybrevant) | EGFR, MET | Non-small cell lung cancer | 2021 |
| Faricimab (Vabysmo) | Ang-2, VEGR-A | Neovascular age-related macular degeneration, visual impairment due to diabetic macular edema | 2022 |
| Mosunetuzumab (Lunsumio) | CD20, CD3 | Follicular lymphoma | 2022 |
| Teclistamab (TECVAYLI) | CD3, BCMA | Multiple myeloma | 2022 |
| Glofitamab (Columvi) | CD20, CD3 | Diffuse large B cell lymphoma | 2023 |
| Epcoritamab (TEPKINLY) | CD20, CD3 | Diffuse large B cell lymphoma, follicular lymphoma | 2023 |
| Talquetamab (TALVEY) | Protein G (GPRC5D), CD3 | Multiple myeloma | 2023 |
| Elranatamab (Elrexfio) | CD3, BCMA | Multiple myeloma | 2023 |
| Odronekstamab (Ordspono) | CD20, CD3 | Diffuse large B cell lymphoma, follicular lymphoma | 2024 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Madej, B.; Tomaszewski, F.; Szmajda-Krygier, D.; Świechowski, R.; Jeleń, A.; Mirowski, M. Monoclonal Antibodies: Historical Perspective and Current Trends in Biological Drug Development. Int. J. Mol. Sci. 2025, 26, 8794. https://doi.org/10.3390/ijms26188794
Madej B, Tomaszewski F, Szmajda-Krygier D, Świechowski R, Jeleń A, Mirowski M. Monoclonal Antibodies: Historical Perspective and Current Trends in Biological Drug Development. International Journal of Molecular Sciences. 2025; 26(18):8794. https://doi.org/10.3390/ijms26188794
Chicago/Turabian StyleMadej, Barbara, Filip Tomaszewski, Dagmara Szmajda-Krygier, Rafał Świechowski, Agnieszka Jeleń, and Marek Mirowski. 2025. "Monoclonal Antibodies: Historical Perspective and Current Trends in Biological Drug Development" International Journal of Molecular Sciences 26, no. 18: 8794. https://doi.org/10.3390/ijms26188794
APA StyleMadej, B., Tomaszewski, F., Szmajda-Krygier, D., Świechowski, R., Jeleń, A., & Mirowski, M. (2025). Monoclonal Antibodies: Historical Perspective and Current Trends in Biological Drug Development. International Journal of Molecular Sciences, 26(18), 8794. https://doi.org/10.3390/ijms26188794







