Impaired Oxidative Stress Markers and Activities of Matrix Metalloproteinases in Plasma of Patients with Alzheimer’s Disease, Emphasizing Sex and APOE ε4 Allele Possession
Abstract
1. Introduction
2. Results
2.1. Study Population
2.2. Distribution of the APOE Gene Alleles in Study Participants
2.3. Alteration of Oxidative Stress Markers and Antioxidant Status in the Plasma of AD Patients
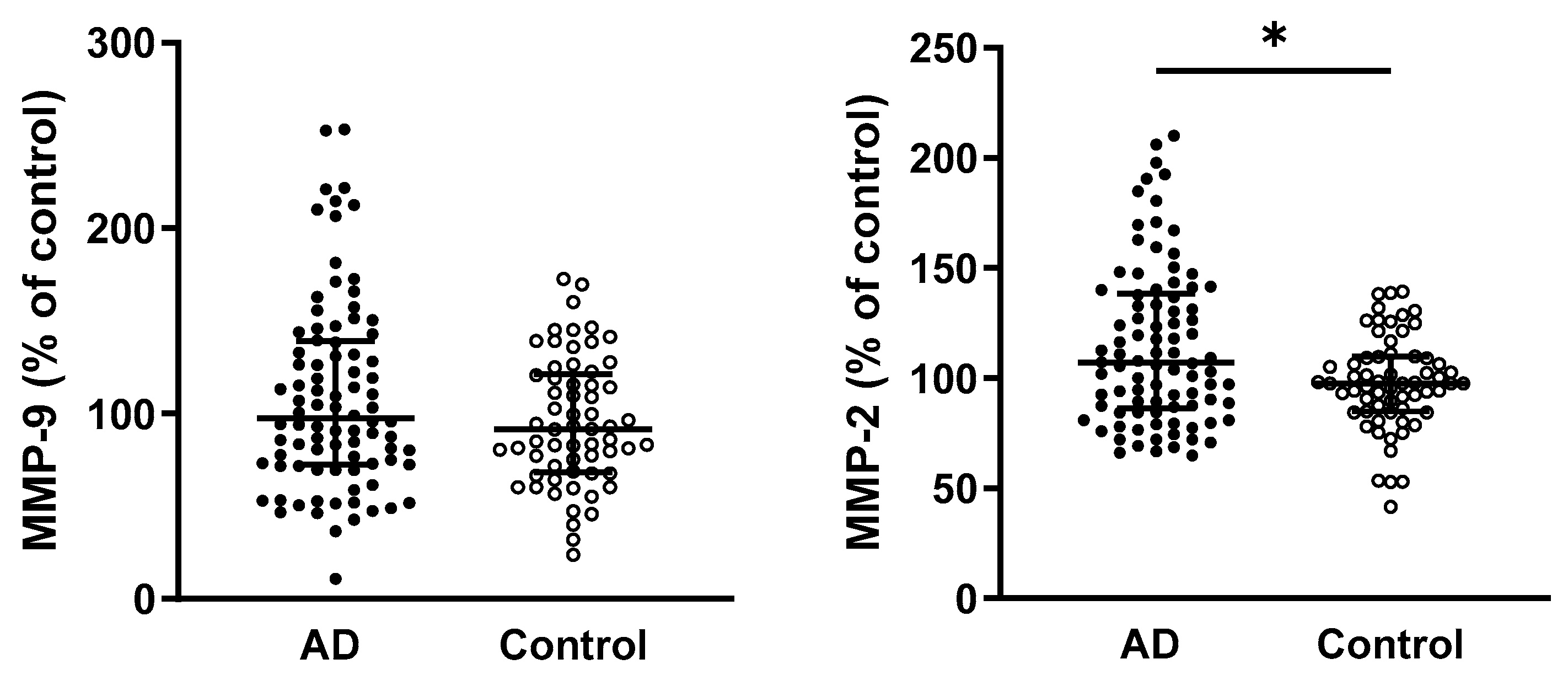
2.4. Elevated MMP-2 Activity, but Not MMP-9, in the Plasma of AD Patients
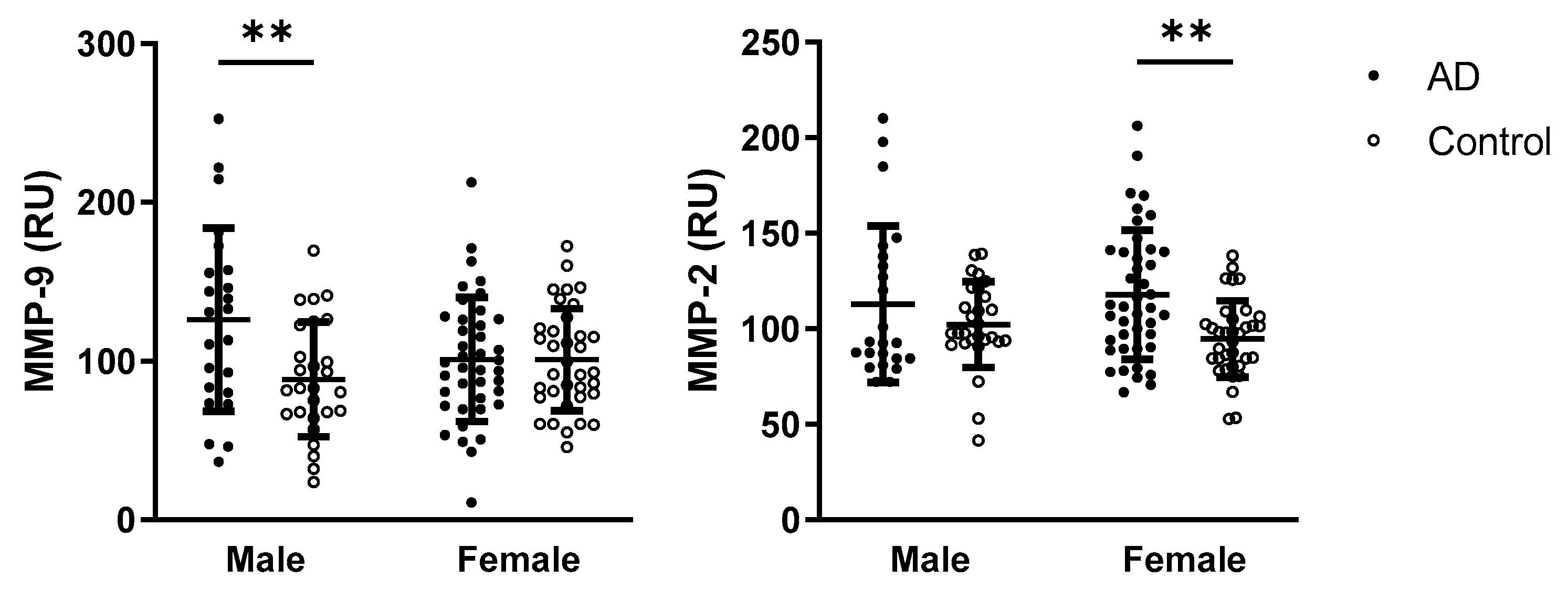
2.5. Sex Differences in Oxidative Stress Parameters and MMPs Activities
2.6. Emphasis on the Possession of APOE ε4 on Parameters of Oxidative Stress and Activities of Matrix Metalloproteinases
3. Discussion
4. Materials and Methods
4.1. Study Groups/Participants
4.2. Blood Collection
4.3. APOE Genotyping
4.4. Parameters of Oxidative Stress and Antioxidant Status
4.5. Activities of MMPs
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AD | Alzheimer’s disease |
| Aβ | Amyloid β |
| MMPs | Matrix metalloproteinases |
| APOE | Apolipoprotein E |
| MoCA | Montreal Cognitive Assessment |
| TBARS | Thiobarbituric acid reactive substances |
| AOPP | Advanced oxidation protein products |
| AGEs | Advanced glycation end products |
| GSH | Reduced glutathione |
| GSSG | Oxidized glutathione |
| TAC | Total antioxidant capacity |
| FRAP | Ferric reducing antioxidant power |
| SOD | Superoxide dismutase |
| CAT | Catalase |
| RU | Relative units |
References
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia Prevention, Intervention, and Care: 2020 Report of the Lancet Commission. Lancet 2023, 402, 1132, Erratum for: Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef] [PubMed]
- Long, S.; Benoist, C.; Weidner, W. World Alzheimer Report 2023: Reducing Dementia Risk: Never Too Early, Never Too Late, 9th ed.; Alzheimer’s Disease International: London, UK, 2023. [Google Scholar]
- Grobler, C.; van Tongeren, M.; Gettemans, J.; Kell, D.B.; Pretorius, E. Alzheimer’s Disease: A Systems View Provides a Unifying Explanation of Its Development. J. Alzheimers Dis. 2023, 91, 43–70. [Google Scholar] [CrossRef]
- Yamazaki, Y.; Zhao, N.; Caulfield, T.R.; Liu, C.-C.; Bu, G. Apolipoprotein E and Alzheimer Disease: Pathobiology and Targeting Strategies. Nat. Rev. Neurol. 2019, 15, 501–518. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-C.; Kanekiyo, T.; Xu, H.; Bu, G. Apolipoprotein E and Alzheimer Disease: Risk, Mechanisms and Therapy. Nat. Rev. Neurol. 2013, 9, 106–118, Erratum in Nat. Rev. Neurol. 2013, 9, 184. [Google Scholar] [CrossRef]
- Bu, G. Apolipoprotein E and Its Receptors in Alzheimer’s Disease: Pathways, Pathogenesis and Therapy. Nat. Rev. Neurosci. 2009, 10, 333–344. [Google Scholar] [CrossRef]
- Butterfield, D.A.; Mattson, M.P. Apolipoprotein E and Oxidative Stress in Brain with Relevance to Alzheimer’s Disease. Neurobiol. Dis. 2020, 138, 104795. [Google Scholar] [CrossRef]
- Dakterzada, F.; Jové, M.; Cantero, J.L.; Pamplona, R.; Piñoll-Ripoll, G. Plasma and Cerebrospinal Fluid Nonenzymatic Protein Damage Is Sustained in Alzheimer’s Disease. Redox Biol. 2023, 64, 102772. [Google Scholar] [CrossRef]
- Kharrazi, H.; Vaisi-Raygani, A.; Rahimi, Z.; Tavilani, H.; Aminian, M.; Pourmotabbed, T. Association between Enzymatic and Non-Enzymatic Antioxidant Defense Mechanism with Apolipoprotein E Genotypes in Alzheimer Disease. Clin. Biochem. 2008, 41, 932–936. [Google Scholar] [CrossRef]
- Tönnies, E.; Trushina, E. Oxidative Stress, Synaptic Dysfunction, and Alzheimer’s Disease. JAD 2017, 57, 1105–1121. [Google Scholar] [CrossRef]
- Chico, L.; Simoncini, C.; Lo Gerfo, A.; Rocchi, A.; Petrozzi, L.; Carlesi, C.; Volpi, L.; Tognoni, G.; Siciliano, G.; Bonuccelli, U. Oxidative Stress and APO E Polymorphisms in Alzheimer’s Disease and in Mild Cognitive Impairment. Free Radic. Res. 2013, 47, 569–576. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, P.; Polidori, M.C.; Metastasio, A.; Mariani, E.; Mattioli, P.; Cherubini, A.; Catani, M.; Cecchetti, R.; Senin, U.; Mecocci, P. Plasma Antioxidants Are Similarly Depleted in Mild Cognitive Impairment and in Alzheimer’s Disease. Neurobiol. Aging 2003, 24, 915–919. [Google Scholar] [CrossRef]
- Bourquard, T.; Lee, K.; Al-Ramahi, I.; Pham, M.; Shapiro, D.; Lagisetty, Y.; Soleimani, S.; Mota, S.; Wilhelm, K.; Samieinasab, M.; et al. Functional Variants Identify Sex-Specific Genes and Pathways in Alzheimer’s Disease. Nat. Commun. 2023, 14, 2765. [Google Scholar] [CrossRef]
- Calvo, N.; Einstein, G. Steroid Hormones: Risk and Resilience in Women’s Alzheimer Disease. Front. Aging Neurosci. 2023, 15, 1159435. [Google Scholar] [CrossRef] [PubMed]
- Fisher, D.W.; Bennett, D.A.; Dong, H. Sexual Dimorphism in Predisposition to Alzheimer’s Disease. Neurobiol. Aging 2018, 70, 308–324. [Google Scholar] [CrossRef]
- Chang, Y.-T.; Chang, W.-N.; Tsai, N.-W.; Huang, C.-C.; Kung, C.-T.; Su, Y.-J.; Lin, W.-C.; Cheng, B.-C.; Su, C.-M.; Chiang, Y.-F.; et al. The Roles of Biomarkers of Oxidative Stress and Antioxidant in Alzheimer’s Disease: A Systematic Review. BioMed Res. Int. 2014, 2014, 182303. [Google Scholar] [CrossRef] [PubMed]
- Schrag, M.; Mueller, C.; Zabel, M.; Crofton, A.; Kirsch, W.M.; Ghribi, O.; Squitti, R.; Perry, G. Oxidative Stress in Blood in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis. Neurobiol. Dis. 2013, 59, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Zhong, C. Oxidative Stress in Alzheimer’s Disease. Neurosci. Bull. 2014, 30, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Martín-Aragón, S.; Bermejo-Bescós, P.; Benedí, J.; Felici, E.; Gil, P.; Ribera, J.M.; Villar, Á.M. Metalloproteinase’s Activity and Oxidative Stress in Mild Cognitive Impairment and Alzheimer’s Disease. Neurochem. Res. 2009, 34, 373–378. [Google Scholar] [CrossRef]
- Radosinska, D.; Radosinska, J. The Link Between Matrix Metalloproteinases and Alzheimer’s Disease Pathophysiology. Mol. Neurobiol. 2024, 62, 885–899. [Google Scholar] [CrossRef]
- Amontree, M.; Deasy, S.; Turner, R.S.; Conant, K. Matrix Disequilibrium in Alzheimer’s Disease and Conditions That Increase Alzheimer’s Disease Risk. Front. Neurosci. 2023, 17, 1188065. [Google Scholar] [CrossRef]
- Wang, X.-X.; Tan, M.-S.; Yu, J.-T.; Tan, L. Matrix Metalloproteinases and Their Multiple Roles in Alzheimer’s Disease. BioMed Res. Int. 2014, 2014, 908636. [Google Scholar] [CrossRef]
- Rivera, S.; García-González, L.; Khrestchatisky, M.; Baranger, K. Metalloproteinases and Their Tissue Inhibitors in Alzheimer’s Disease and Other Neurodegenerative Disorders. Cell. Mol. Life Sci. 2019, 76, 3167–3191. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Alloza, M.; Prada, C.; Lattarulo, C.; Fine, S.; Borrelli, L.A.; Betensky, R.; Greenberg, S.M.; Frosch, M.P.; Bacskai, B.J. Matrix Metalloproteinase Inhibition Reduces Oxidative Stress Associated with Cerebral Amyloid Angiopathy in Vivo in Transgenic Mice. J. Neurochem. 2009, 109, 1636–1647. [Google Scholar] [CrossRef] [PubMed]
- Nasreddine, Z.S.; Phillips, N.A.; Bédirian, V.; Charbonneau, S.; Whitehead, V.; Collin, I.; Cummings, J.L.; Chertkow, H. The Montreal Cognitive Assessment, MoCA: A Brief Screening Tool For Mild Cognitive Impairment. J. Am. Geriatr. Soc. 2019, 67, 1991, Erratum for: J. Am. Geriatr. Soc. 2005, 53, 695–699. [Google Scholar] [CrossRef]
- Mittal, K.; Katare, D.P. Shared Links between Type 2 Diabetes Mellitus and Alzheimer’s Disease: A Review. Diabetes Metab. Syndr. Clin. Res. Rev. 2016, 10, S144–S149. [Google Scholar] [CrossRef]
- De La Monte, S.M. Contributions of Brain Insulin Resistance and Deficiency in Amyloid-Related Neurodegeneration in Alzheimer’s Disease. Drugs 2012, 72, 49–66. [Google Scholar] [CrossRef]
- De La Monte, S.M.; Tong, M. Brain Metabolic Dysfunction at the Core of Alzheimer’s Disease. Biochem. Pharmacol. 2014, 88, 548–559. [Google Scholar] [CrossRef]
- Dhapola, R.; Beura, S.K.; Sharma, P.; Singh, S.K.; HariKrishnaReddy, D. Oxidative Stress in Alzheimer’s Disease: Current Knowledge of Signaling Pathways and Therapeutics. Mol. Biol. Rep. 2024, 51, 48. [Google Scholar] [CrossRef]
- Cervellati, C.; Romani, A.; Seripa, D.; Cremonini, E.; Bosi, C.; Magon, S.; Passaro, A.; Bergamini, C.M.; Pilotto, A.; Zuliani, G. Oxidative Balance, Homocysteine, and Uric Acid Levels in Older Patients with Late Onset Alzheimer’s Disease or Vascular Dementia. J. Neurol. Sci. 2014, 337, 156–161. [Google Scholar] [CrossRef]
- Martínez De Toda, I.; Miguélez, L.; Vida, C.; Carro, E.; De La Fuente, M. Altered Redox State in Whole Blood Cells from Patients with Mild Cognitive Impairment and Alzheimer’s Disease. JAD 2019, 71, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ramassamy, C.; Averill, D.; Beffert, U.; Bastianetto, S.; Theroux, L.; Lussier-Cacan, S.; Cohn, J.S.; Christen, Y.; Davignon, J.; Quirion, R.; et al. Oxidative Damage and Protection by Antioxidants in the Frontal Cortex of Alzheimer’s Disease Is Related to the Apolipoprotein E Genotype. Free Radic. Biol. Med. 1999, 27, 544–553. [Google Scholar] [CrossRef]
- Gella, A.; Durany, N. Oxidative Stress in Alzheimer Disease. Cell Adhes. Migr. 2009, 3, 88–93. [Google Scholar] [CrossRef]
- Puertas, M.C.; Martínez-Martos, J.M.; Cobo, M.P.; Carrera, M.P.; Mayas, M.D.; Ramírez-Expósito, M.J. Plasma Oxidative Stress Parameters in Men and Women with Early Stage Alzheimer Type Dementia. Exp. Gerontol. 2012, 47, 625–630. [Google Scholar] [CrossRef]
- Cristalli, D.O.; Arnal, N.; Marra, F.A.; De Alaniz, M.J.T.; Marra, C.A. Peripheral Markers in Neurodegenerative Patients and Their First-Degree Relatives. J. Neurol. Sci. 2012, 314, 48–56. [Google Scholar] [CrossRef]
- Fawver, J.N.; Schall, H.E.; Petrofes Chapa, R.D.; Zhu, X.; Murray, I.V.J. Amyloid-β Metabolite Sensing: Biochemical Linking of Glycation Modification and Misfolding. JAD 2012, 30, 63–73. [Google Scholar] [CrossRef]
- Ayoub, S.; Arabi, M.; Al-Najjar, Y.; Laswi, I.; Outeiro, T.F.; Chaari, A. Glycation in Alzheimer’s Disease and Type 2 Diabetes: The Prospect of Dual Drug Approaches for Therapeutic Interventions. Mol. Neurobiol. 2025. [Google Scholar] [CrossRef]
- Sasaki, N.; Fukatsu, R.; Tsuzuki, K.; Hayashi, Y.; Yoshida, T.; Fujii, N.; Koike, T.; Wakayama, I.; Yanagihara, R.; Garruto, R.; et al. Advanced Glycation End Products in Alzheimer’s Disease and Other Neurodegenerative Diseases. Am. J. Pathol. 1998, 153, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Zhang, M.; Xu, L.; Liu, J.; Yang, P.; Li, M.; Qin, J. Fluorescent Advanced Glycation End Products in Type 2 Diabetes and Its Association with Diabetes Duration, Hemoglobin A1c, and Diabetic Complications. Front. Nutr. 2022, 9, 1083872. [Google Scholar] [CrossRef] [PubMed]
- Horie, K.; Miyata, T.; Yasuda, T.; Takeda, A.; Yasuda, Y.; Maeda, K.; Sobue, G.; Kurokawa, K. Immunohistochemical Localization of Advanced Glycation End Products, Pentosidine, and Carboxymethyllysine in Lipofuscin Pigments of Alzheimer’s Disease and Aged Neurons. Biochem. Biophys. Res. Commun. 1997, 236, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.; Nakamura, K.; Inoue, H.; Kikuchi, S.; Takeuchi, M. Serum or Cerebrospinal Fluid Levels of Glyceraldehyde-Derived Advanced Glycation End Products (AGEs) May Be a Promising Biomarker for Early Detection of Alzheimer’s Disease. Med. Hypotheses 2005, 64, 1205–1207. [Google Scholar] [CrossRef]
- Choei, H.; Sasaki, N.; Takeuchi, M.; Yoshida, T.; Ukai, W.; Yamagishi, S.; Kikuchi, S.; Saito, T. Glyceraldehyde-Derived Advanced Glycation End Products in Alzheimer’s Disease. Acta Neuropathol. 2004, 108, 189–193. [Google Scholar] [CrossRef] [PubMed]
- Fournet, M.; Bonté, F.; Desmoulière, A. Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis. 2018, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Zafrilla, P.; Mulero, J.; Xandri, J.; Santo, E.; Caravaca, G.; Morillas, J. Oxidative Stress in Alzheimer Patients in Different Stages of the Disease. CMC 2006, 13, 1075–1083. [Google Scholar] [CrossRef]
- Vural, H.; Demirin, H.; Kara, Y.; Eren, I.; Delibas, N. Alterations of Plasma Magnesium, Copper, Zinc, Iron and Selenium Concentrations and Some Related Erythrocyte Antioxidant Enzyme Activities in Patients with Alzheimer’s Disease. J. Trace Elem. Med. Biol. 2010, 24, 169–173. [Google Scholar] [CrossRef]
- Chen, J.J.; Thiyagarajah, M.; Song, J.; Chen, C.; Herrmann, N.; Gallagher, D.; Rapoport, M.J.; Black, S.E.; Ramirez, J.; Andreazza, A.C.; et al. Altered Central and Blood Glutathione in Alzheimer’s Disease and Mild Cognitive Impairment: A Meta-Analysis. Alzheimer’s Res. Ther. 2022, 14, 23. [Google Scholar] [CrossRef]
- Casado, Á.; Encarnación López-Fernández, M.; Concepción Casado, M.; De La Torre, R. Lipid Peroxidation and Antioxidant Enzyme Activities in Vascular and Alzheimer Dementias. Neurochem. Res. 2008, 33, 450–458. [Google Scholar] [CrossRef]
- Padurariu, M.; Ciobica, A.; Hritcu, L.; Stoica, B.; Bild, W.; Stefanescu, C. Changes of Some Oxidative Stress Markers in the Serum of Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Neurosci. Lett. 2010, 469, 6–10. [Google Scholar] [CrossRef] [PubMed]
- Tóthová, L.; Ostatníková, D.; Šebeková, K.; Celec, P.; Hodosy, J. Sex Differences of Oxidative Stress Markers in Young Healthy Subjects Are Marker-Specific in Plasma but Not in Saliva. Ann. Human Biol. 2013, 40, 175–180. [Google Scholar] [CrossRef]
- Chen, C.; Arjomandi, M.; Balmes, J.; Tager, I.; Holland, N. Effects of Chronic and Acute Ozone Exposure on Lipid Peroxidation and Antioxidant Capacity in Healthy Young Adults. Environ. Health Perspect. 2007, 115, 1732–1737. [Google Scholar] [CrossRef]
- Benzie, I.F.F.; Chung, W.-Y. Total Antioxidant Power of Plasma: Male–Female Differences and Effect of Anticoagulant Used. Ann. Clin. Biochem. 1999, 36, 104–106. [Google Scholar] [CrossRef]
- Vassalle, C.; Masini, S.; Carpeggiani, C.; L’Abbate, A.; Boni, C.; CarloZucchelli, G. In Vivo Total Antioxidant Capacity: Comparison of Two Different Analytical Methods. Clin. Chem. Lab. Med. 2004, 42, 84–89. [Google Scholar] [CrossRef]
- Pedrosa, W.; de Sander Diniz, M.F.H.; Barreto, S.M.; Vidigal, P.G. Establishing a Blood Fructosamine Reference Range for the Brazilian Population Based on Data from ELSA—Brasil. Pract. Lab. Med. 2019, 13, e00111. [Google Scholar] [CrossRef]
- Yin, K.-J.; Cirrito, J.R.; Yan, P.; Hu, X.; Xiao, Q.; Pan, X.; Bateman, R.; Song, H.; Hsu, F.-F.; Turk, J.; et al. Matrix Metalloproteinases Expressed by Astrocytes Mediate Extracellular Amyloid-β Peptide Catabolism. J. Neurosci. 2006, 26, 10939–10948. [Google Scholar] [CrossRef] [PubMed]
- Zipfel, P.; Rochais, C.; Baranger, K.; Rivera, S.; Dallemagne, P. Matrix Metalloproteinases as New Targets in Alzheimer’s Disease: Opportunities and Challenges. J. Med. Chem. 2020, 63, 10705–10725. [Google Scholar] [CrossRef]
- Py, N.A.; Bonnet, A.E.; Bernard, A.; Marchalant, Y.; Charrat, E.; Checler, F.; Khrestchatisky, M.; Baranger, K.; Rivera, S. Differential Spatio-Temporal Regulation of MMPs in the 5xFAD Mouse Model of Alzheimerâ€TMs Disease: Evidence for a pro-Amyloidogenic Role of MT1-MMP. Front. Aging Neurosci. 2014, 6, 247. [Google Scholar] [CrossRef] [PubMed]
- Terni, B.; Ferrer, I. Abnormal Expression and Distribution of MMP2 at Initial Stages of Alzheimer’s Disease-Related Pathology. JAD 2015, 46, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Abe, K.; Chiba, Y.; Hattori, S.; Yoshimi, A.; Asami, T.; Katsuse, O.; Suda, A.; Hishimoto, A. Influence of Plasma Matrix Metalloproteinase Levels on Longitudinal Changes in Alzheimer’s Disease (AD) Biomarkers and Cognitive Function in Patients with Mild Cognitive Impairment Due to AD Registered in the Alzheimer’s Disease Neuroimaging Initiative Database. J. Neurol. Sci. 2020, 416, 116989. [Google Scholar] [CrossRef]
- Tsiknia, A.A.; Sundermann, E.E.; Reas, E.T.; Edland, S.D.; Brewer, J.B.; Galasko, D.; Banks, S.J. For the Alzheimer’s Disease Neuroimaging Initiative Sex Differences in Alzheimer’s Disease: Plasma MMP-9 and Markers of Disease Severity. Alzheimer’s Res. Ther. 2022, 14, 160. [Google Scholar] [CrossRef]
- Aksnes, M.; Edwin, T.H.; Saltvedt, I.; Eldholm, R.S.; Chaudhry, F.A.; Halaas, N.B.; Myrstad, M.; Watne, L.O.; Knapskog, A.-B. Sex-Specific Associations of Matrix Metalloproteinases in Alzheimer’s Disease. Biol. Sex Differ. 2023, 14, 35. [Google Scholar] [CrossRef]
- Paranjpe, M.D.; Belonwu, S.; Wang, J.K.; Oskotsky, T.; Gupta, A.; Taubes, A.; Zalocusky, K.A.; Paranjpe, I.; Glicksberg, B.S.; Huang, Y.; et al. Sex-Specific Cross Tissue Meta-Analysis Identifies Immune Dysregulation in Women with Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 735611. [Google Scholar] [CrossRef]
- Chowen, J.A.; Garcia-Segura, L.M. Role of Glial Cells in the Generation of Sex Differences in Neurodegenerative Diseases and Brain Aging. Mech. Ageing Dev. 2021, 196, 111473. [Google Scholar] [CrossRef]
- Santiago, J.A.; Potashkin, J.A. Biological and Clinical Implications of Sex-Specific Differences in Alzheimer’s Disease. In Sex and Gender Effects in Pharmacology; Tsirka, S.E., Acosta-Martinez, M., Eds.; Handbook of Experimental Pharmacology; Springer International Publishing: Cham, Switzerland, 2023; Volume 282, pp. 181–197. ISBN 978-3-031-42647-6. [Google Scholar]
- Duong, S.; Patel, T.; Chang, F. Dementia: What Pharmacists Need to Know. Can. Pharm. J. 2017, 150, 118–129. [Google Scholar] [CrossRef]
- Bennett, S.; Grant, M.M.; Aldred, S. Oxidative Stress in Vascular Dementia and Alzheimer’s Disease: A Common Pathology. JAD 2008, 17, 245–257. [Google Scholar] [CrossRef] [PubMed]
- Luca, M.; Luca, A.; Calandra, C. The Role of Oxidative Damage in the Pathogenesis and Progression of Alzheimer’s Disease and Vascular Dementia. Oxid. Med. Cell. Longev. 2015, 2015, 504678. [Google Scholar] [CrossRef]
- Jamali, Q.; Akinfala, A.; Upendram, A. Identification of Biomarkers for Vascular Dementia: A Literature Review. BJPsych Adv. 2024, 30, 89–96. [Google Scholar] [CrossRef]
- Lorenzl, S.; Büerger, K.; Hampel, H.; Beal, M.F. Profiles of Matrix Metalloproteinases and Their Inhibitors in Plasma of Patients with Dementia. Int. Psychogeriatr. 2008, 20, 67–76. [Google Scholar] [CrossRef]
- Hosoki, S.; Tanaka, T.; Ihara, M. Diagnostic and Prognostic Blood Biomarkers in Vascular Dementia: From the Viewpoint of Ischemic Stroke. Neurochem. Int. 2021, 146, 105015. [Google Scholar] [CrossRef]
- McKhann, G.; Drachman, D.; Folstein, M.; Katzman, R.; Price, D.; Stadlan, E.M. Clinical Diagnosis of Alzheimer’s Disease: Report of the NINCDS-ADRDA Work Group * under the Auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984, 34, 939. [Google Scholar] [CrossRef]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Cummings, J.L.; DeKosky, S.T.; Barberger-Gateau, P.; Delacourte, A.; Frisoni, G.; Fox, N.C.; Galasko, D.; et al. Revising the Definition of Alzheimer’s Disease: A New Lexicon. Lancet Neurol. 2010, 9, 1118–1127. [Google Scholar] [CrossRef] [PubMed]
- Durmanova, V.; Parnicka, Z.; Javor, J.; Minarik, G.; Vrazda, L.; Vaseckova, B.; Gmitterova, K.; Kralova, M.; Pecenak, J.; Filipcik, P.; et al. A Novel Association of Polymorphism in the ITGA4 Gene Encoding the VLA-4 α 4 Subunit with Increased Risk of Alzheimer’s Disease. Mediat. Inflamm. 2018, 2018, 7623823. [Google Scholar] [CrossRef] [PubMed]
- Kollarova, M.; Puzserova, A.; Balis, P.; Radosinska, D.; Tothova, L.; Bartekova, M.; Barancik, M.; Radosinska, J. Age- and Phenotype-Dependent Changes in Circulating MMP-2 and MMP-9 Activities in Normotensive and Hypertensive Rats. Int. J. Mol. Sci. 2020, 21, 7286. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. [13] Catalase in Vitro. In Methods in Enzymology; Elsevier: Amsterdam, The Netherlands, 1984; Volume 105, pp. 121–126. ISBN 978-0-12-182005-3. [Google Scholar]



| AD Patients (n = 104) | Controls (n = 73) | p-Value | |
|---|---|---|---|
| Age (years), mean ± SD | 78.9 ± 5.9 | 69.6 ± 6.6 | <0.0001 |
| Age at onset, mean ± SD | 76.55 ± 6.39 | N/A | N/A |
| Sex (male/female), n | 32/72 | 32/41 | 0.0825 |
| MoCA score, mean ± SD | 16.59 ± 5.8 | 27.5 ± 1.6 | <0.0001 |
| Hypertension, % | 73.07 | 63.01 | 0.1873 |
| Type 2 diabetes mellitus, % | 26 | 12.3 | 0.0361 |
| Tobacco smoker, % | 16.3 | 24.6 | 0.1845 |
| Genotypes | AD Patients (n = 104), n (%) | Controls (n = 72), n (%) | p-Value | χ2 Test |
|---|---|---|---|---|
| ε2/ε2 | 0 | 1 (1) | ||
| ε2/ε3 | 8 (8) | 11 (15) | ||
| ε2/ε4 | 4 (4) | 2 (3) | ||
| ε3/ε3 | 41 (39) | 47 (65) | ||
| ε3/ε4 | 39 (38) | 11 (15) | ||
| ε4/ε4 | 12 (11) | 0 | ||
| ε4+/ε4− carriers | 55/49 | 13/59 | <0.0001 | 21.77, 1 |
| Alleles | <0.0001 | 26.72, 2 | ||
| ε2 | 12 | 15 | ||
| ε3 | 129 | 116 | ||
| ε4 | 67 | 13 |
| AD Patients (n = 98–104) | Controls (n = 69–73) | |
|---|---|---|
| TBARS (µmol/L) | 0.61 ± 0.25 | 0.65 ± 0.22 |
| AOPP (µmol/L) | 0.631 (0.423; 0.935) ** | 0.460 (0.311; 0.679) |
| Fructosamine (µmol/L) | 5.431 (4.871; 6.276) ** | 5.123 (4.123; 5.761) |
| AGEs (g/L) | 0.0123 (0.010; 0.015) * | 0.0114 (0.010; 0.014) |
| GSH/GSSG ratio | 0.406 (0.325; 0.516) ** | 0.360 (0.302; 0.411) |
| AD Patients (n = 74–104) | Controls (n = 55–73) | |
|---|---|---|
| FRAP (µmol/L) | 942.6 ± 158.3 | 979.2 ± 199.2 |
| TAC (µmol/L) | 878.6 (701.5; 1032) **** | 1290 (1139; 1447) |
| CAT activity (% of control) | 94.11 ± 40.66 | 100 ± 30.48 |
| SOD activity (% of control) | 88.72 (80.8; 99.09) ** | 100 (82.82; 107.7) |
| Coefficient | Std. Error | p-Value | 95% CI | |
|---|---|---|---|---|
| AOPP | ||||
| Age | −0.00291 | 0.00172 | 0.09277 | −0.00631, 0.00049 |
| Disease | −0.15861 | 0.17065 | 0.35403 | −0.49559, 0.17837 |
| AxD | 0.00157 | 0.0023 | 0.49541 | −0.00297, 0.00611 |
| Fructosamine | ||||
| Age | 2.46 × 10−5 | 2.14 × 10−5 | 0.25123 | −1.8 × 10−5, 6.69 × 10−5 |
| Disease | 0.00483 | 0.00217 | 0.02723 | 0.00055, 0.00911 |
| AxD | −6.2 × 10−5 | 2.92 × 10−5 | 0.03458 | −0.00012, −4.6 × 10−6 |
| TAC | ||||
| Age | 8.92711 | 7.72268 | 0.21871 | −5.3556, 23.2098 |
| Disease | 1084.15 | 700.389 | 0.12381 | −300.065, 2468.36 |
| AxD | −20.0049 | −2.10814 | 0.03673 | −38.7587, −1.25062 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Radošinská, D.; Kollárová, M.; Shawkatová, I.; Ďurmanová, V.; Párnická, Z.; Javor, J.; Brandoburová, P.; Harsányi, Š.; Radošinská, J. Impaired Oxidative Stress Markers and Activities of Matrix Metalloproteinases in Plasma of Patients with Alzheimer’s Disease, Emphasizing Sex and APOE ε4 Allele Possession. Int. J. Mol. Sci. 2025, 26, 8790. https://doi.org/10.3390/ijms26188790
Radošinská D, Kollárová M, Shawkatová I, Ďurmanová V, Párnická Z, Javor J, Brandoburová P, Harsányi Š, Radošinská J. Impaired Oxidative Stress Markers and Activities of Matrix Metalloproteinases in Plasma of Patients with Alzheimer’s Disease, Emphasizing Sex and APOE ε4 Allele Possession. International Journal of Molecular Sciences. 2025; 26(18):8790. https://doi.org/10.3390/ijms26188790
Chicago/Turabian StyleRadošinská, Dominika, Marta Kollárová, Ivana Shawkatová, Vladimíra Ďurmanová, Zuzana Párnická, Juraj Javor, Petra Brandoburová, Štefan Harsányi, and Jana Radošinská. 2025. "Impaired Oxidative Stress Markers and Activities of Matrix Metalloproteinases in Plasma of Patients with Alzheimer’s Disease, Emphasizing Sex and APOE ε4 Allele Possession" International Journal of Molecular Sciences 26, no. 18: 8790. https://doi.org/10.3390/ijms26188790
APA StyleRadošinská, D., Kollárová, M., Shawkatová, I., Ďurmanová, V., Párnická, Z., Javor, J., Brandoburová, P., Harsányi, Š., & Radošinská, J. (2025). Impaired Oxidative Stress Markers and Activities of Matrix Metalloproteinases in Plasma of Patients with Alzheimer’s Disease, Emphasizing Sex and APOE ε4 Allele Possession. International Journal of Molecular Sciences, 26(18), 8790. https://doi.org/10.3390/ijms26188790






