The Odorant-Binding Proteins AspiOBP1 and AspiOBP2 in Aleurocanthus spiniferus Are Involved in the Perception of Host Volatiles
Abstract
1. Introduction
2. Results
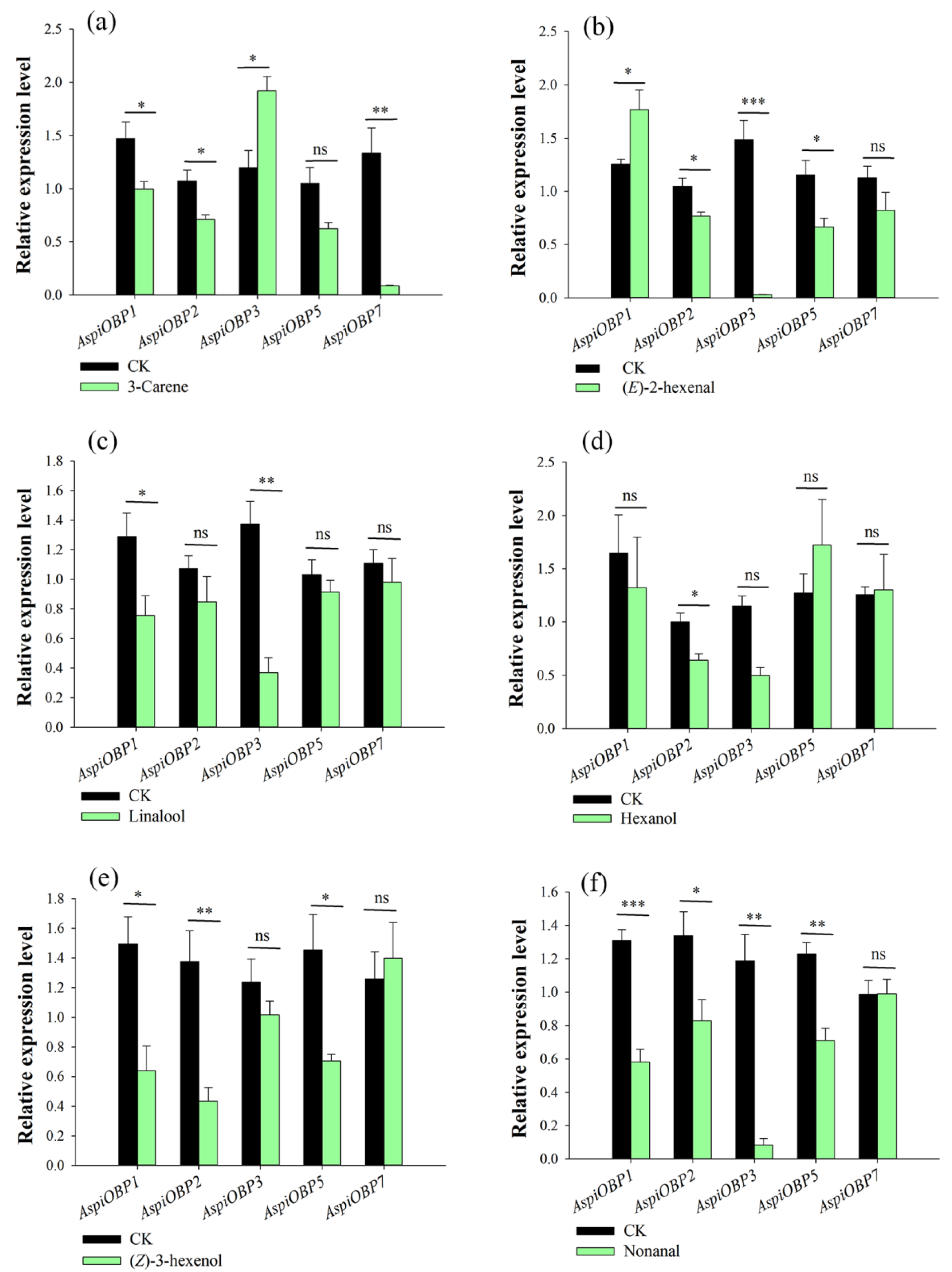
2.1. Host Volatiles-Induced Changes in AspiOBPs Expression
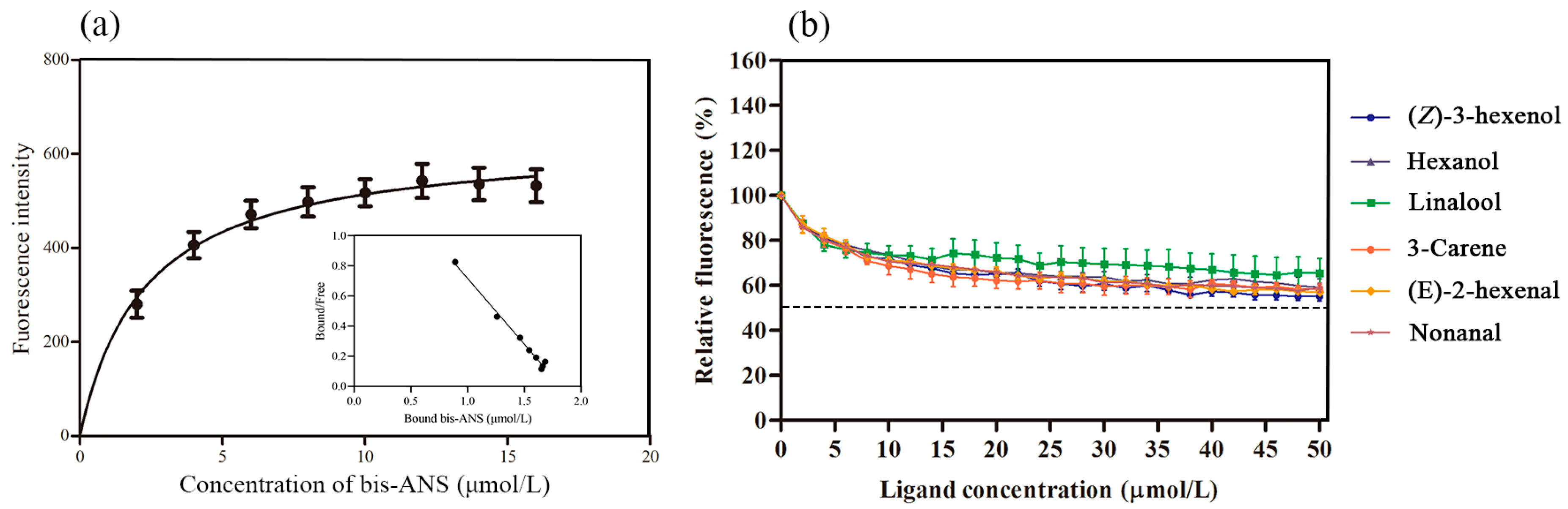
2.2. Fluorescence Binding Property Analysis
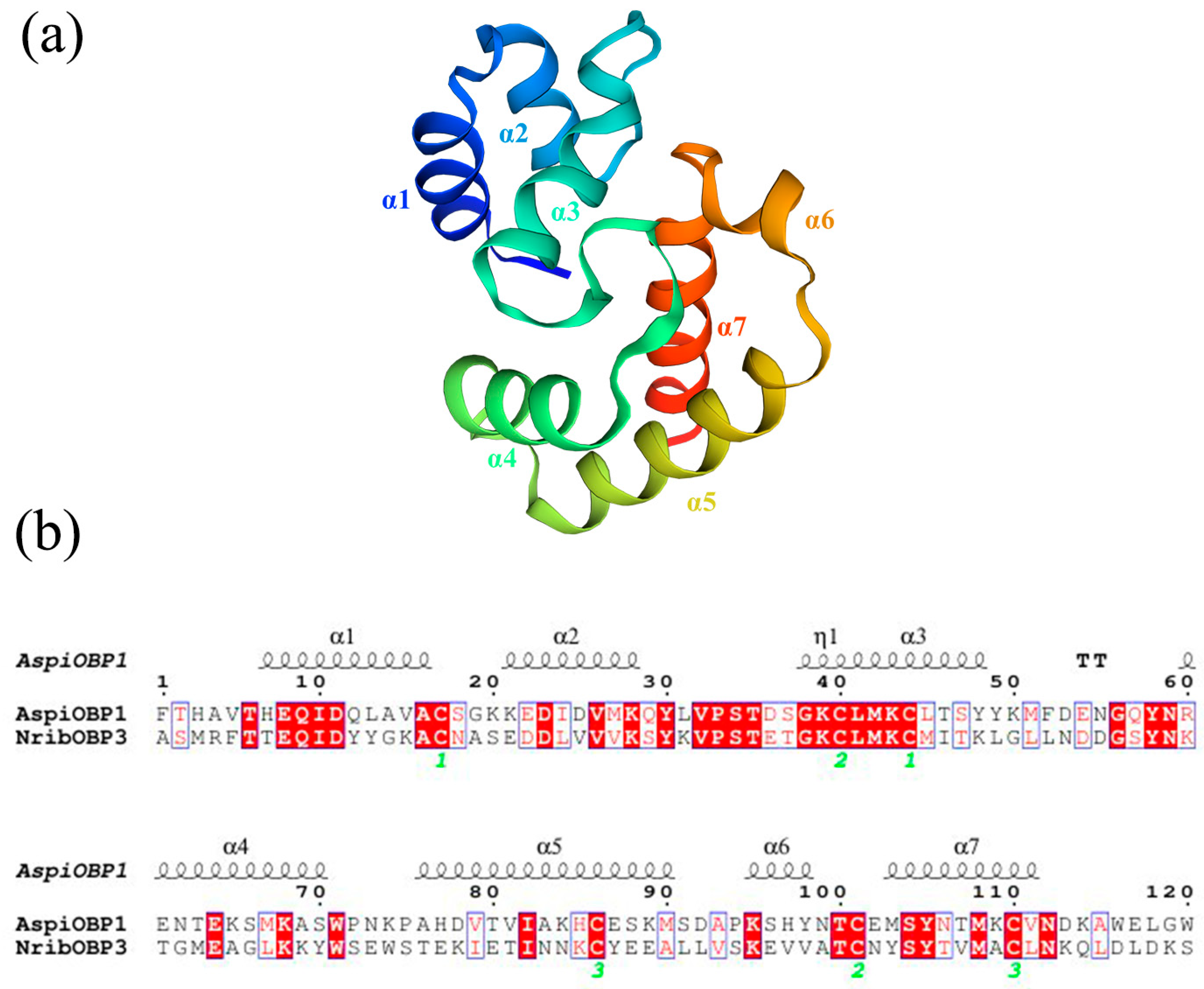
2.3. Three-Dimensional Modeling and Molecular Docking
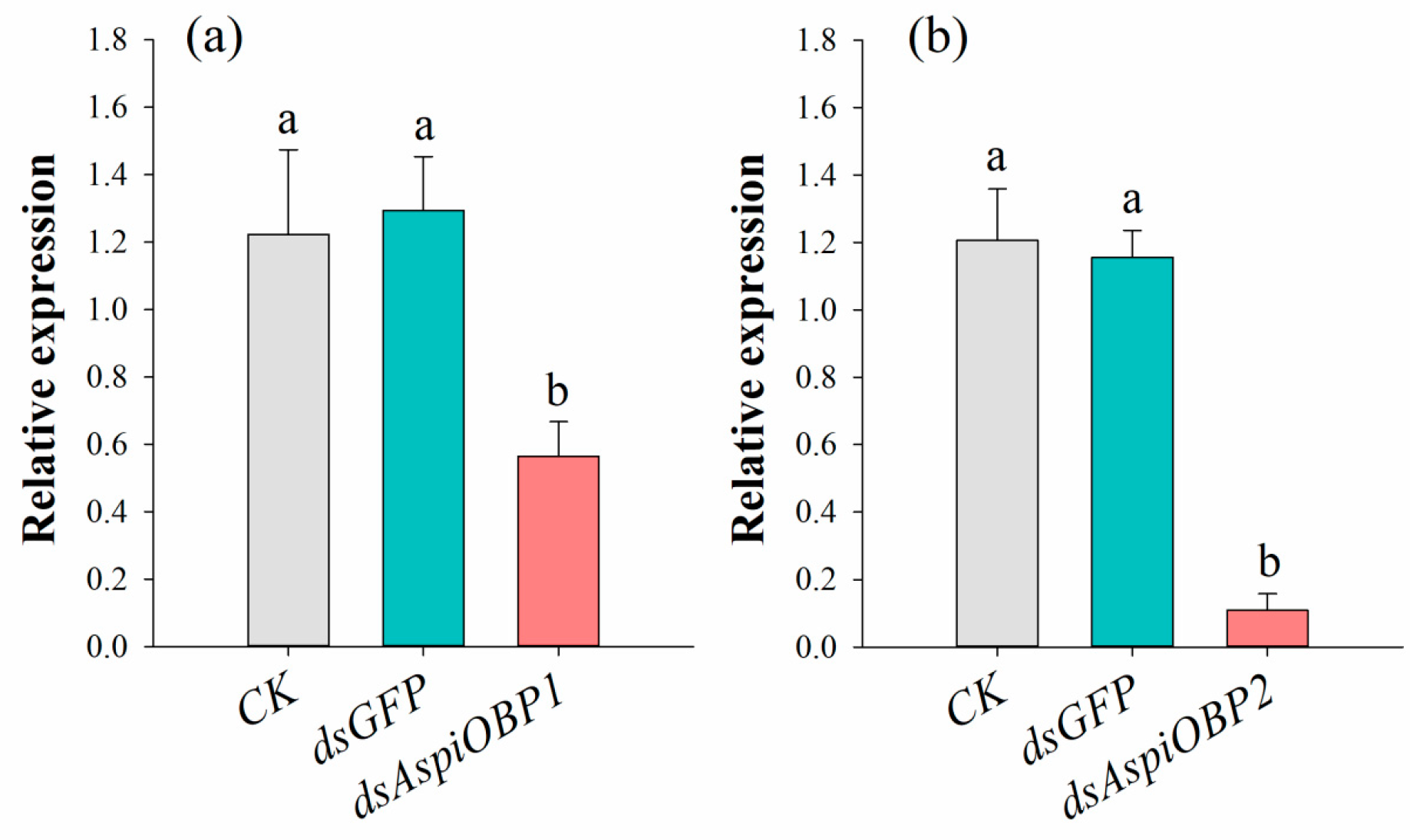
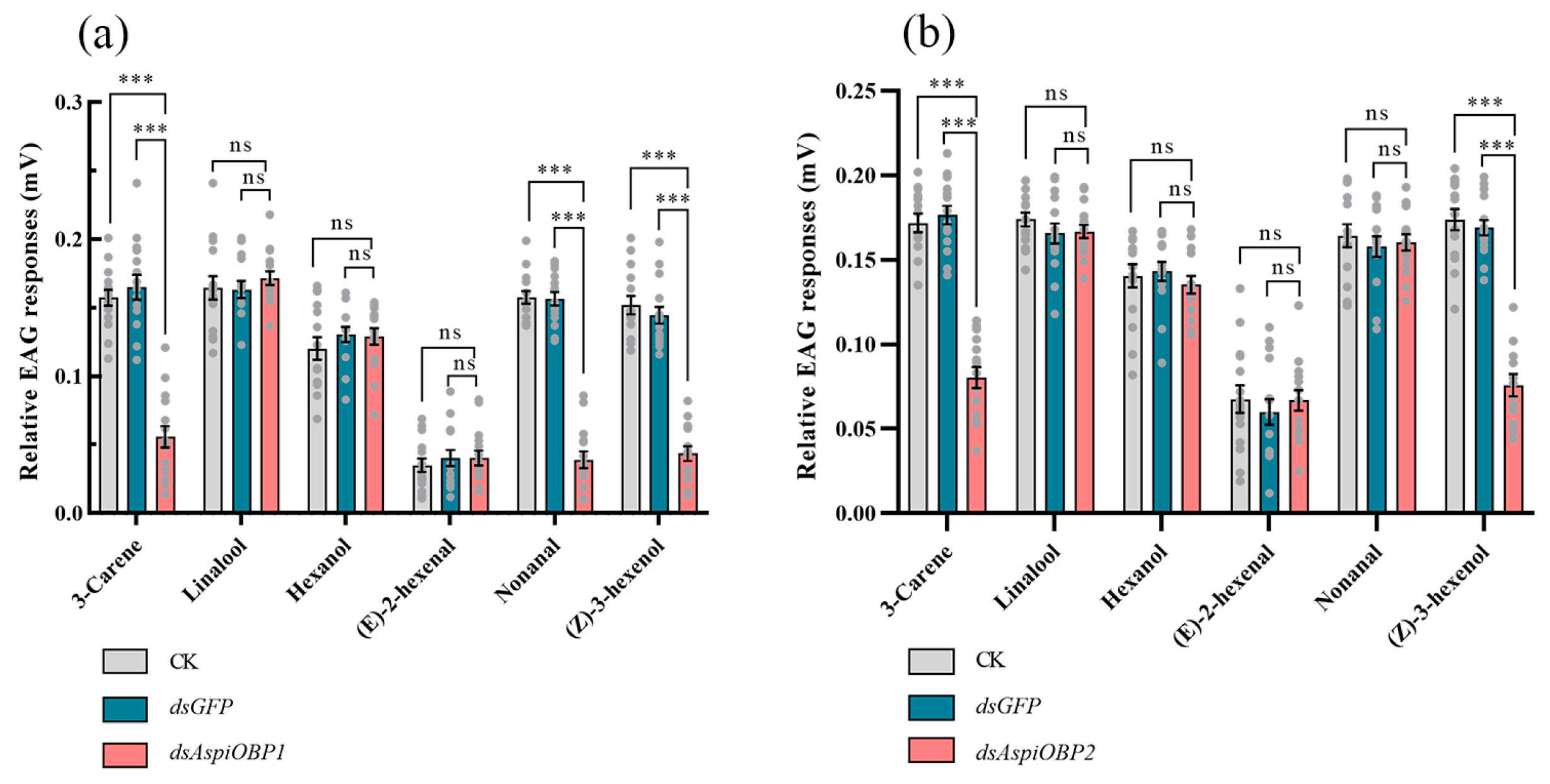
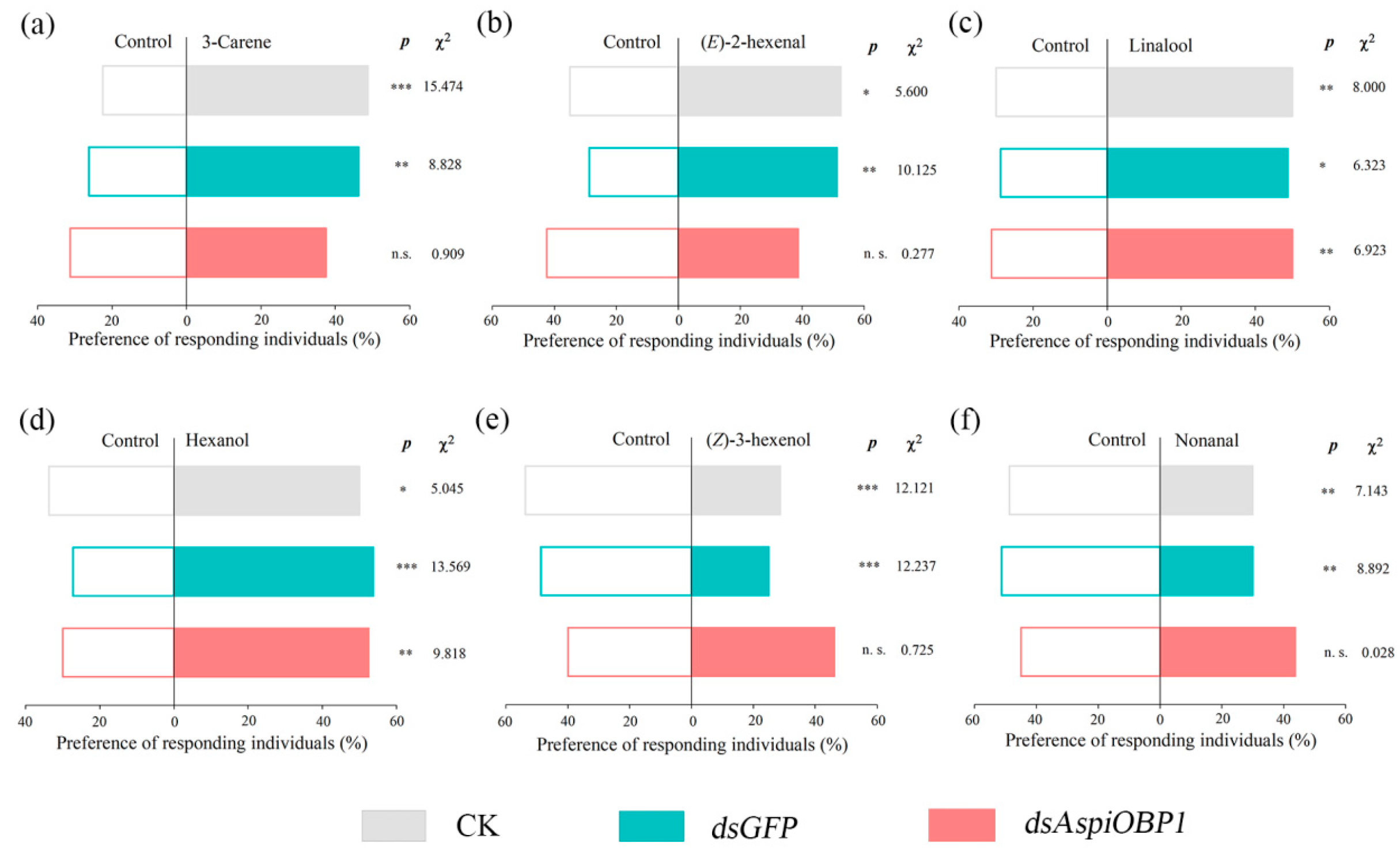
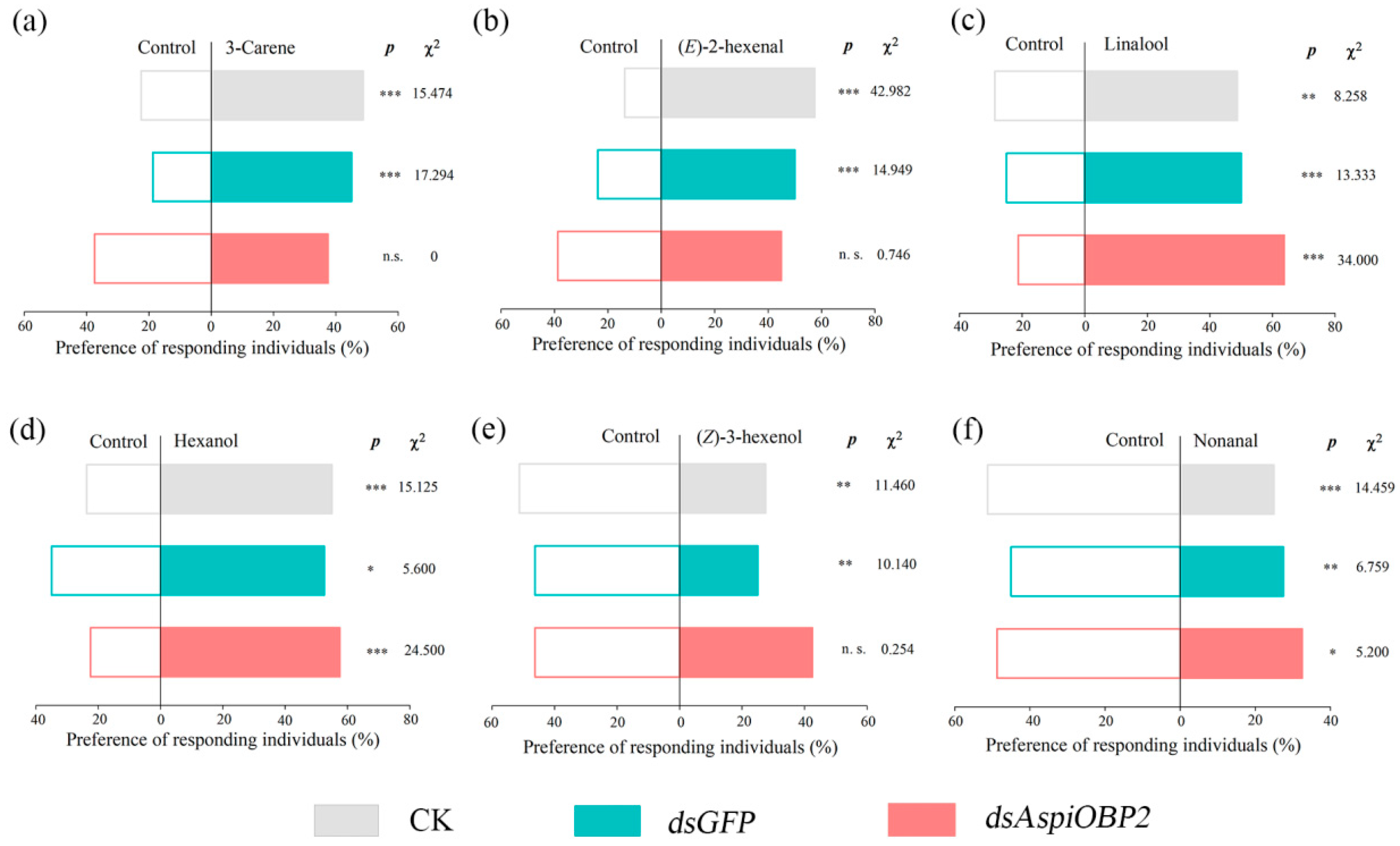
2.4. RNAi Experiments of AspiOBP1 and AspiOBP2
3. Discussion
4. Materials and Methods
4.1. Insect Collection and Chemicals
4.2. Formatting of Mathematical Components
4.3. Gene Cloning and Sequence Analysis
4.4. Expression and Purification of Recombinant Proteins
4.5. Fluorescence Competitive Binding Assay
4.6. Three-Dimensional Modeling and Molecular Docking
4.7. Synthesis of Double-Stranded RNA (dsRNA) and Treatment
4.8. EAG Tests
4.9. Two-Choice Olfactometry for dsRNA-Treated A. spiniferus
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AspiOBP | Aleurocanthus spiniferus odorant binding protein |
| CSPs | chemosensory proteins |
| OBPs | odorant binding proteins |
| EAG | electroantennogram |
| bis-ANS | 4,4′-Dianilino-1,1′-Binaphthyl-5,5′-Disulfonic Acid, Dipotassium Salt |
| SDS-PAGE | sodium dodecyl sulfate polyacrylamide gel electropheresis |
| IPTG | isopropyl-β-d-thiogalactoside |
| SRA | sequence read archive |
| ORF | open reading frame |
Appendix A
| VOCs | Gene | CK a | Treatment | t | df | p | Changes in OBP Gene Expression b | ||
|---|---|---|---|---|---|---|---|---|---|
| Mean | Standard Errors | Mean | Standard Errors | ||||||
| 3-Carene | AspiOBP1 | 1.4733 | 0.1551 | 0.9973 | 0.0676 | 2.815 | 6 | 0.048 | down |
| AspiOBP2 | 1.0433 | 0.1004 | 0.7099 | 0.0420 | 3.064 | 6 | 0.037 | down | |
| AspiOBP3 | 1.1977 | 0.1619 | 1.9209 | 0.1339 | 3.443 | 6 | 0.026 | up | |
| AspiOBP5 | 1.0493 | 0.1511 | 0.6223 | 0.0584 | 2.635 | 6 | 0.058 | ns | |
| AspiOBP7 | 1.3337 | 0.2355 | 0.0852 | 0.0071 | 5.298 | 6 | 0.006 | down | |
| (E)-2-hexenal | AspiOBP1 | 1.2560 | 0.0440 | 1.7670 | 0.1840 | 2.702 | 6 | 0.035 | up |
| AspiOBP2 | 1.0445 | 0.0781 | 0.7650 | 0.0380 | 3.218 | 6 | 0.018 | down | |
| AspiOBP3 | 1.4850 | 0.1825 | 0.0280 | 0.0011 | 7.985 | 6 | <0.001 | down | |
| AspiOBP5 | 1.1525 | 0.1362 | 0.6652 | 0.0826 | 3.059 | 6 | 0.022 | down | |
| AspiOBP7 | 1.1260 | 0.1094 | 0.8213 | 0.1701 | 1.507 | 6 | 0.183 | ns | |
| Linalool | AspiOBP1 | 1.2900 | 0.1580 | 0.7568 | 0.1344 | 2.571 | 6 | 0.042 | down |
| AspiOBP2 | 1.0725 | 0.0867 | 0.8475 | 0.1720 | 1.168 | 6 | 0.287 | ns | |
| AspiOBP3 | 1.3750 | 0.1529 | 0.3689 | 0.1017 | 5.479 | 6 | 0.002 | down | |
| AspiOBP5 | 1.0325 | 0.0988 | 0.9138 | 0.0803 | 0.932 | 6 | 0.387 | ns | |
| AspiOBP7 | 1.1088 | 0.0910 | 0.9816 | 0.1604 | 0.689 | 6 | 0.516 | ns | |
| Hexanol | AspiOBP1 | 1.6475 | 0.3590 | 1.3205 | 0.4746 | 0.549 | 6 | 0.603 | ns |
| AspiOBP2 | 1.0000 | 0.0832 | 0.6400 | 0.0619 | 3.473 | 6 | 0.013 | down | |
| AspiOBP3 | 1.1495 | 0.1310 | 0.4968 | 0.2750 | 2.143 | 6 | 0.076 | ns | |
| AspiOBP5 | 1.2708 | 0.1818 | 1.7229 | 0.4260 | 0.976 | 6 | 0.367 | ns | |
| AspiOBP7 | 1.2575 | 0.0723 | 1.3019 | 0.3317 | 0.131 | 6 | 0.900 | ns | |
| (Z)-3-hexenol | AspiOBP1 | 1.4925 | 0.1855 | 0.6390 | 0.1676 | 3.414 | 6 | 0.014 | down |
| AspiOBP2 | 1.3750 | 0.2081 | 0.4343 | 0.0899 | 4.151 | 6 | 0.006 | down | |
| AspiOBP3 | 1.2345 | 0.1575 | 1.0179 | 0.0915 | 1.189 | 6 | 0.279 | ns | |
| AspiOBP5 | 1.4538 | 0.2387 | 0.7066 | 0.0437 | 3.079 | 6 | 0.022 | down | |
| AspiOBP7 | 1.2565 | 0.1836 | 1.3991 | 0.2401 | 0.472 | 6 | 0.654 | ns | |
| Nonanal | AspiOBP1 | 1.3075 | 0.0660 | 0.5815 | 0.0765 | 7.188 | 6 | <0.001 | down |
| AspiOBP2 | 1.3368 | 0.1451 | 0.8300 | 0.0647 | 3.190 | 6 | 0.019 | down | |
| AspiOBP3 | 1.1860 | 0.1594 | 0.0846 | 0.0366 | 6.735 | 6 | 0.001 | down | |
| AspiOBP5 | 1.2275 | 0.0698 | 0.7113 | 0.0741 | 5.070 | 6 | 0.002 | down | |
| AspiOBP7 | 0.9885 | 0.0809 | 0.9920 | 0.0843 | 0.030 | 6 | 0.977 | ns | |
| Protein | α-Helix/% | Extended Strand/% | β-Turn/% | Random Coil/% |
|---|---|---|---|---|
| AspiOBP1 | 72.03 | 2.80 | 4.20 | 20.98 |
| Gene Name | Primer Sequence (5′–3′) | Remarks |
|---|---|---|
| AspiOBP1 | F: GTTTTTGTTTCGTTTGTTGCCCAGT | qPCR |
| R: TCGGAACCAGATACTGTTTCATTAC | ||
| AspiOBP2 | F: GGATGACGAGCCTTTGAA | |
| R: GTGGTCGGTGGTTTTCTA | ||
| AspiOBP3 | F: TGACGGGCACTATGTTGG | |
| R: CATTGTATCGGTGGCTGA | ||
| AspiOBP5 | F: CCCTGCTCGCTGCCTTTGT | |
| R: TGTCACTCCCGCCCAATCC | ||
| AspiOBP7 | F: ATCTGTGATAAGTGTGATGACGC | |
| R: ATGGCGTTCTGTATCCCT | ||
| RPS28 | F: AACCAGGACAAGTAGGCCAAG | |
| R: GCCTCGCCGATTTCCTTCTC | ||
| AspiOBP1 | F: GCCATGGCTGATATCGGATCCGTCACTCACGAACAAATTGATCAAC | Clone |
| R: TTGTCGACGGAGCTCGAATTCTTAAGATTTCGTGAACCATCCAAGC | ||
| AspiOBP2 | F: GCCATGGCTGATATCGGATCCGAGACATCAACGGTTGACTCTG | |
| R: TTGTCGACGGAGCTCGAATTCTCAAGGTTTTGGTGGAGGC | ||
| T7-dsAspiOBP1 | F: GGATCCTAATACGACTCACTATAGGNAAATGTTTGACGAGAATGGGC | RNAi |
| R: GGATCCTAATACGACTCACTATAGGNGTTATAGTGGGACTTCGGGGC | ||
| T7-dsAspiOBP2 | F: GGATCCTAATACGACTCACTATAGGNCCGTAGCAAATGGTGGCA | |
| R: GGATCCTAATACGACTCACTATAGGNTTCCGTTGGGCAGTTCAT | ||
| T7-dsGFP | F: GGATCCTAATACGACTCACTATAGGNTGGGCACAAATTTTCTGTC | |
| R: GGATCCTAATACGACTCACTATAGGNAAGGGTATCACCTTCAAAC |
| 3-Carene | Hexanol | (E)-2-Hexenal | (Z)-3-Hexenol | Nonanal | Linalool | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino Acid Residues | Distance | Amino Acid Residues | Distance | Amino Acid Residues | Distance | Amino Acid Residues | Distance | Amino Acid Residues | Distance | Amino Acid Residues | Distance |
| GLN-76 | 3.58 | GLN-76 | 3.50 | GLU-106 | 3.35 | TYR-77 | 3.46 | GLN-76 | 3.71 | GLU-80 | 3.46 |
| TYR-77 | 3.44 | TYR-77 | 3.95 | MET-109 | 3.42 | GLU-106 | 3.36 | GLN-76 | 3.54 | GLU-106 | 3.46 |
| TYR-77 | 3.24 | TYR-77 | 3.36 | MET-109 | 3.14 | TYR-117 | 3.12 | ||||
| ARG-79 | 3.79 | GLU-106 | 3.44 | TYR-117 | 3.30 | ||||||
| GLU-106 | 3.05 | MET-109 | 3.55 | ||||||||
| MET-109 | 3.40 | TYR-117 | 3.52 | ||||||||
| TYR-117 | 3.99 | ||||||||||
| Hexanol | (E)-2-Hexenal | (Z)-3-Hexenol | Nonanal | Linalool | |||||
|---|---|---|---|---|---|---|---|---|---|
| Amino Acid Residues | Distance | Amino Acid Residues | Distance | Amino Acid Residues | Distance | Amino Acid Residues | Distance | Amino Acid Residues | Distance |
| TYR-77 | 1.83 | ARG-79 | 1.85 | TYR-77 | 1.96 | GLN-76 | 2.11 | ASN-78 | 1.86 |
| ARG-79 | 2.03 | ARG-79 | 2.23 | ARG-79 | 2.53 | ||||
| GLU-80 | 1.73 | ||||||||
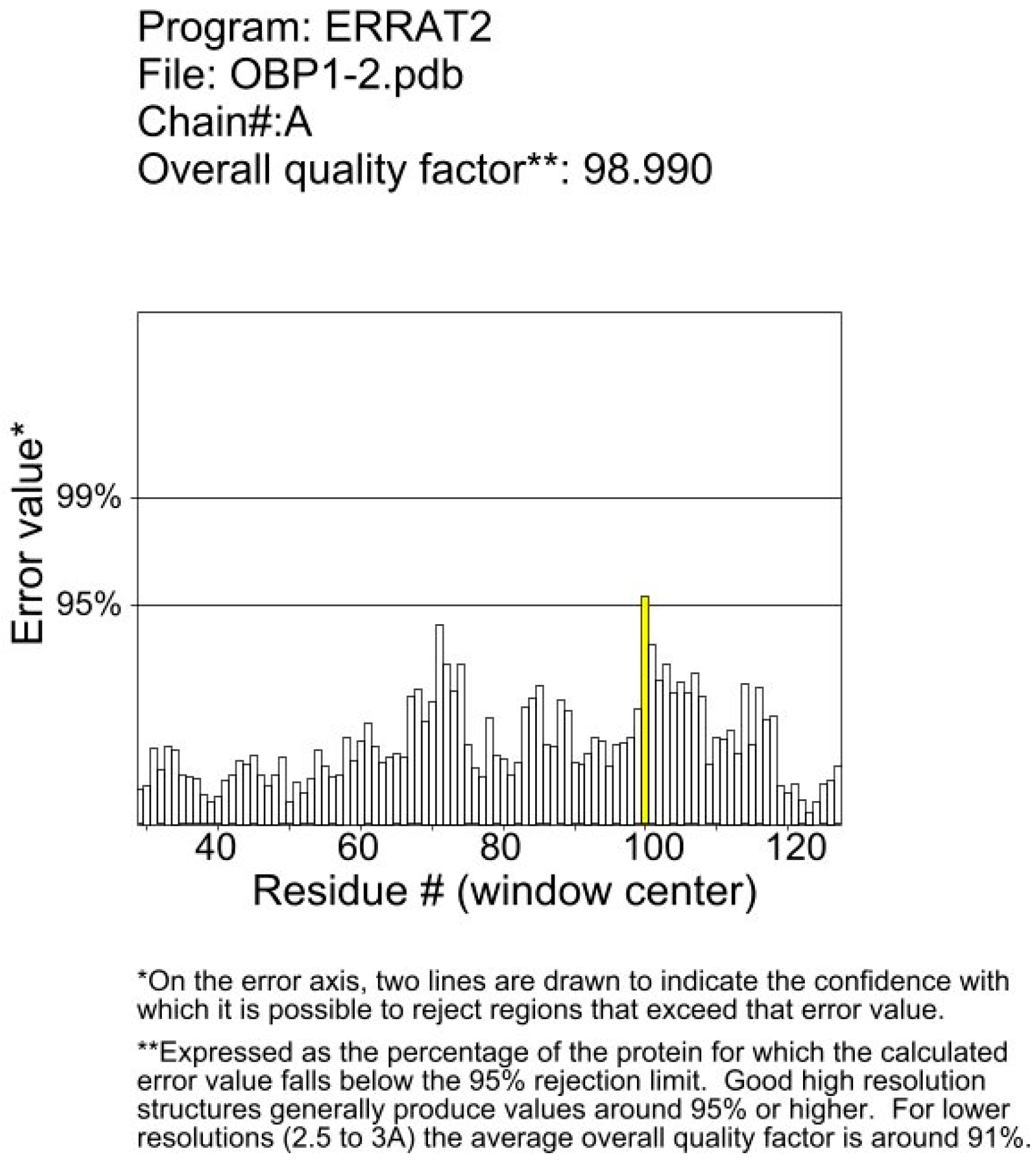
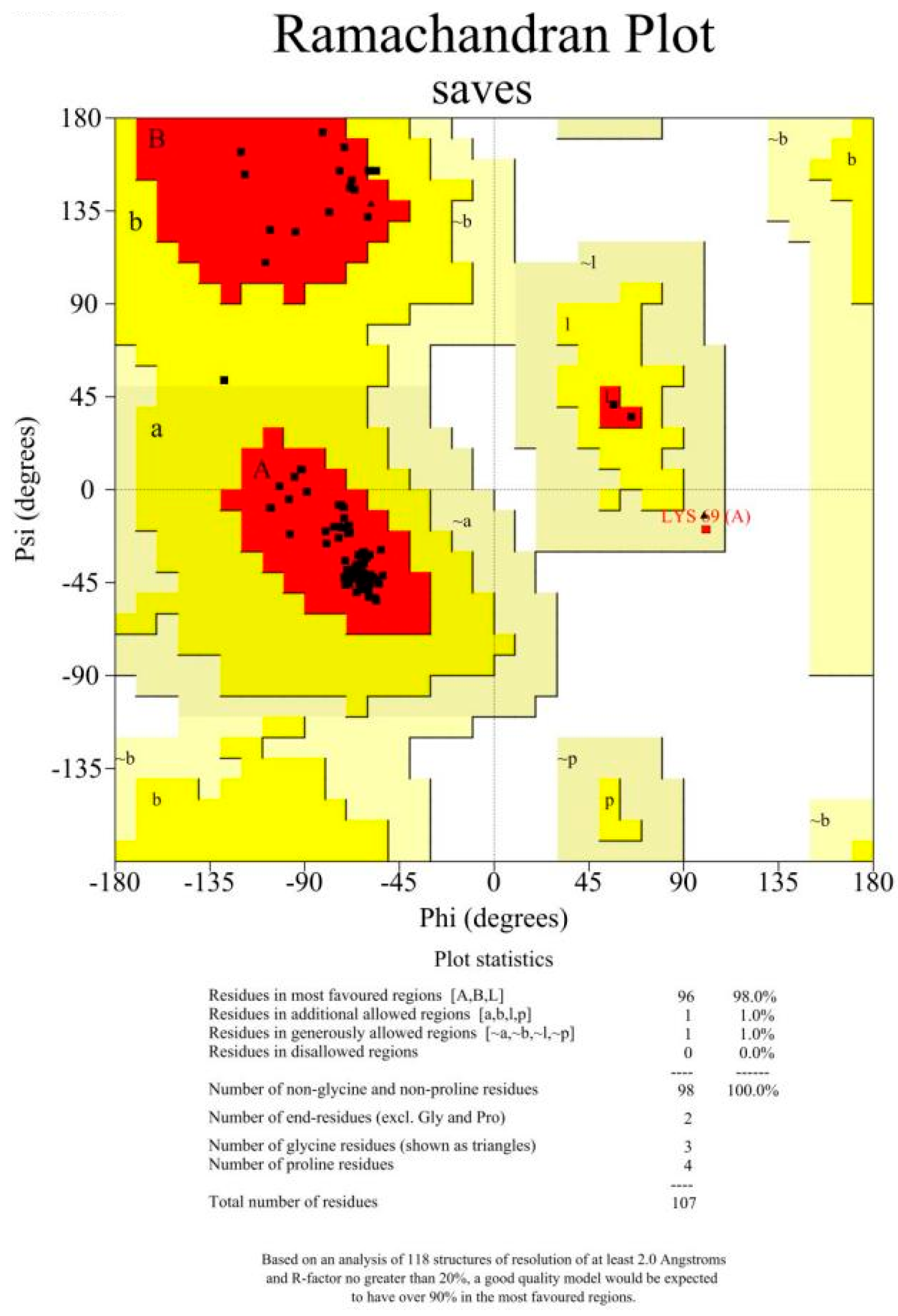
References
- Tang, X.T.; Tao, H.H.; Du, Y.Z. Microsatellite-based analysis of the genetic structure and diversity of Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) from tea plants in China. Gene 2015, 560, 107–113. [Google Scholar] [CrossRef]
- Van den Berg, M.A.; Greenland, J. Classical biological control of Aleurocanthus spiniferus (Hem.: Aleyrodidae), on citrus in Southern Africa. BioControl 1997, 42, 459–465. [Google Scholar] [CrossRef]
- Muniappan, R.; Purea, M.; Sengebau, F.; Reddy, G.V. Orange spiny whitefly, Aleurocanthus spiniferus (Quaintance) (Homoptera: Aleyrodidae), and its parasitoids in the Republic of Palau. Hawaii. Entomol. Soc. 2006, 38, 21–25. Available online: http://hdl.handle.net/10125/119 (accessed on 15 January 2025).
- Kanmiya, K.; Ueda, S.; Kasai, A.; Yamashita, K. Proposal of new specific status for tea-infesting populations of the nominal citrus spiny whitefly Aleurocanthus spiniferus (Homoptera: Aleyrodidae). Zootaxa 2011, 125, 25–44. [Google Scholar] [CrossRef]
- Cioffi, M.; Cornara, D.; Corrado, I.; Jansen, M.G.M.; Porcelli, F. The status of Aleurocanthus spiniferus from its unwanted introduction in Italy to date. Bull. Insectology 2013, 66, 273–281. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20133416562 (accessed on 15 January 2025).
- Porcelli, F. First record of Aleurocanthus spiniferus (Homoptera: Aleyrodidae) in Apulia, Southern Italy. OEPP/EPPO Bull. 2008, 38, 516–518. [Google Scholar] [CrossRef]
- Nugnes, F.; Laudonia, S.; Jesu, G.; Jansen, M.G.; Bernardo, U.; Porcelli, F. Aleurocanthus spiniferus (Hemiptera: Aleyrodidae) in some European countries: Diffusion, hosts, molecular characterization, and natural enemies. Insects 2020, 11, 42. [Google Scholar] [CrossRef]
- Streito, J.C.; Mendes, E.; Sanquer, E.; Strugarek, M.; Ouvrard, D.; Robin-Havret, V.; Poncet, L.; Lannou, C.; Rossi, J.P. Incursion preparedness, citizen science and early detection of invasive insects: The case of Aleurocanthus spiniferus (Hemiptera, Aleyrodidae) in France. Insects 2023, 14, 916. [Google Scholar] [CrossRef]
- Gillespie, P.S. A review of the whitefly genus Aleurocanthus Quaintance & Baker (Hemiptera: Aleyrodidae) in Australia. Zootaxa 2012, 3252, 1–42. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20133124601 (accessed on 20 January 2025). [CrossRef]
- Tian, Y.; Chen, Z.; Huang, X.; Zhang, L.; Zhang, Z. Evaluation of botanicals for management of piercing–sucking pests and the effect on beneficial arthropod populations in tea trees Camellia sinensis (L.) O. Kuntze (Theaceae). J. Insect Sci. 2020, 20, 27. [Google Scholar] [CrossRef]
- Zhou, J.J. Odorant-binding proteins in insects. Vitam. Horm. 2010, 83, 241–272. [Google Scholar] [CrossRef]
- Leal, W.S. Odorant reception in insects: Roles of receptors, binding proteins, and degrading enzymes. Annu. Rev. Entomol. 2013, 58, 373–391. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Chen, Z.Z.; Liu, M.Y.; Song, C.Y.; Jia, Z.F.; Liu, F.H.; Qu, C.; Dewer, Y.; Zhao, H.P.; Xu, Y.Y.; et al. Characterization of antennal chemosensilla and associated chemosensory genes in the orange spiny whitefly, Aleurocanthus spiniferus (Quaintanca). Front. Physiol. 2022, 13, 847895. [Google Scholar] [CrossRef]
- Venthur, H.; Mutis, A.; Zhou, J.J.; Quiroz, A. Ligand binding and homology modelling of insect odorant-binding proteins. Physiol. Entomol. 2014, 39, 183–198. [Google Scholar] [CrossRef]
- Wang, Z.; Shang, X.; Wu, Z.; Wei, J.; Tian, X.; Zhang, G. Identification of behaviorally active odorants for adult Chilo sacchariphagus based on the binding properties of odorant-binding proteins toward host volatiles. J. Agric. Food Chem. 2025, 73, 7669–7684. [Google Scholar] [CrossRef]
- Fan, J.; Francis, F.; Liu, Y.; Chen, J.L.; Cheng, D.F. An overview of odorant-binding protein functions in insect peripheral olfactory reception. Genet. Mol. Res. 2011, 10, 3056–3069. [Google Scholar] [CrossRef]
- Lizana, P.; Mutis, A.; Quiroz, A.; Venthur, H. Insights into chemosensory proteins from non-model insects: Advances and perspectives in the context of pest management. Front. Physiol. 2022, 13, 924750. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hou, M.; Liang, C.; Xu, Q.; Lu, Y.; Zhao, Z. Role of odorant binding protein C12 in the response of Tribolium castaneum to chemical agents. Pestic. Biochem. Physiol. 2024, 201, 105861. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tan, S.; Wang, Q.; Wang, F.; Zhang, Y. Key amino acids in odorant-binding protein OBP7 enable Bradysia odoriphaga to recognize host plant volatiles. Int. J. Biol. Macromol. 2025, 284, 138179. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.X.; Zwiebel, L.J.; Smith, D.P. Identification of a distinct family of genes encoding atypical odorant-binding proteins in the malaria vector mosquito, Anopheles gambiae. Insect Mol. Biol. 2003, 12, 549–560. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, G.; Huang, W.; Birkett, M.A.; Field, L.M.; Pickett, J.A.; Pelosi, P. Revisiting the odorant-binding protein LUSH of Drosophila melanogaster: Evidence for odour recognition and discrimination. Febs Lett. 2004, 558, 23–26. [Google Scholar] [CrossRef]
- Pelosi, P.; Mastrogiacomo, R.; Iovinella, I.; Tuccori, E.; Persaud, K.C. Structure and biotechnological applications of odorant-binding proteins. Appl. Microbiol. Biotechnol. 2014, 98, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Pelosi, P.; Zhou, J.J.; Ban, L.P.; Calvello, M. Soluble proteins in insect chemical communication. Cell. Mol. Life Sci. CMLS 2006, 63, 1658–1676. [Google Scholar] [CrossRef]
- Brito, N.F.; Moreira, M.F.; Melo, A.C. A look inside odorant binding proteins in insect chemoreception. J. Insect Physiol. 2016, 95, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Hekmat-Scafe, D.S.; Scafe, C.R.; McKinney, A.J.; Tanouye, M.A. Genome-wide analysis of the odorant-binding protein gene family in Drosophila melanogaster. Genome Res. 2002, 12, 1357–1369. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Z.; Yu, G.Q.; Yi, S.C.; Zhang, Y.; Kong, D.X.; Wang, M.Q. Structure-based analysis of the ligand-binding mechanism for DhelOBP21, a C-minus odorant binding protein, from Dastarcus helophoroides (Fairmaire; Coleoptera: Bothrideridae). Int. J. Biol. Sci. 2015, 11, 1281–1295. [Google Scholar] [CrossRef]
- Renou, M.; Anton, S. Insect olfactory communication in a complex and changing world. Curr. Opin. Insect Sci. 2020, 42, 1–7. [Google Scholar] [CrossRef]
- Li, L.; Wu, L.; Xu, Y.; Liu, F.; Zhao, H. Three odorant-binding proteins of small hive beetles, Aethina tumida, participate in the response of bee colony volatiles. Int. J. Biol. Macromol. 2024, 278, 134905. [Google Scholar] [CrossRef]
- Calín-Sánchez, Á.; Martínez, J.J.; Vázquez-Araújo, L.; Burló, F.; Melgarejo, P.; Carbonell-Barrachina, Á.A. Volatile composition and sensory quality of Spanish pomegranates (Punica granatum L.). J. Sci. Food Agric. 2011, 91, 586–592. [Google Scholar] [CrossRef]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E,E)-2,4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef]
- Goliáš, J.; Létal, J.; Kožíšková, J.; Dokoupil, L. Formation of volatiles in apricot (Prunus armeniaca L.) fruit during post-harvest ripening. Mitteilungen Klosterneubg. 2013, 63, 96–107. Available online: https://www.cabidigitallibrary.org/doi/full/10.5555/20143028343 (accessed on 20 January 2025).
- Velásquez, A.; Valenzuela, M.; Carvajal, M.; Fiaschi, G.; Avio, L.; Giovannetti, M.; D’Onofrio, C.; Seeger, M. The arbuscular mycorrhizal fungus Funneliformis mosseae induces changes and increases the concentration of volatile organic compounds in Vitis vinifera cv. Sangiovese leaf tissue. Plant Physiol. Biochem. 2020, 155, 437–443. [Google Scholar] [CrossRef]
- Sun, H.; Chen, W.; Jiang, Y.; He, Q.; Li, X.; Guo, Q.; Xiang, S.; Xi, W.; Liang, G. Characterization of volatiles in red-and white-fleshed loquat (Eriobotrya japonica) fruits by electronic nose and headspace solid-phase microextraction with gas chromatography-mass spectrometry. Food Sci. Technol. 2020, 40 (Suppl. S1), 21–32. [Google Scholar] [CrossRef]
- Wen, X.; Yang, K.; Piñero, J.C.; Wen, J. Contrasting behavioral and electrophysiological responses of Eucryptorrhynchus scrobiculatus and E. brandti (Coleoptera: Curculionidae) to volatiles emitted from the tree of heaven. Ailanthus altissima. Insects 2021, 12, 68. [Google Scholar] [CrossRef]
- Yamamoto, K.; Endo, S. Bombyx mori-derived aldo-keto reductase AKR2E8 detoxifies aldehydes present in mulberry leaves. Chem.-Biol. Interact. 2022, 351, 109717. [Google Scholar] [CrossRef]
- Jia, Z.; Li, Z.; Li, D.; Kang, Z.; Xu, Y.; Chen, Z. Two chemosensory proteins in Aleurocanthus spiniferus are involved in the recognition of host VOCs. Chem. Biol. Technol. Agric. 2024, 11, 183. [Google Scholar] [CrossRef]
- Jia, Z.; Ge, X.; Bian, Y.; Song, K.; Li, D.; Song, D.; Ding, S.; Xu, Y.; Chen, Z. Field application of tea volatiles mediating the selectivity of Aleurocanthus spiniferus on four tea cultivars. Plants 2025, 14, 2653. [Google Scholar] [CrossRef]
- von der Weid, B.; Rossier, D.; Lindup, M.; Tuberosa, J.; Widmer, A.; Col, J.D.; Kan, C.; Carleton, A.; Rodriguez, I. Large-scale transcriptional profiling of chemosensory neurons identifies receptor-ligand pairs in vivo. Nat. Neurosci. 2015, 18, 1455–1463. [Google Scholar] [CrossRef] [PubMed]
- Yin, J.; Wang, C.; Fang, C.; Zhang, S.; Cao, Y.; Li, K.; Leal, W.S. Functional characterization of odorant-binding proteins from the scarab beetle Holotrichia oblita based on semiochemical-induced expression alteration and gene silencing. Insect Biochem. Mol. Biol. 2019, 104, 11–19. [Google Scholar] [CrossRef]
- Liu, X.Q.; Jiang, H.B.; Fan, J.Y.; Liu, T.Y.; Meng, L.W.; Liu, Y.; Yu, H.Z.; Dou, W.; Wang, J.J. An odorant-binding protein of Asian citrus psyllid, Diaphorina citri, participates in the response of host plant volatiles. Pest Manag. Sci. 2021, 77, 3068–3079. [Google Scholar] [CrossRef]
- Hu, P.; Qiu, Z.; Chen, X.; Xu, Y.; Su, X.; Yang, Z. Olfactory proteins of Endoclita signifer larvae and their roles in host recognition. Chem. Biol. Technol. Agric. 2022, 9, 54. [Google Scholar] [CrossRef]
- He, P.; Chen, G.L.; Li, S.; Wang, J.; Ma, Y.F.; Pan, Y.F.; He, M. Evolution and functional analysis of odorant-binding proteins in three rice planthoppers: Nilaparvata lugens, Sogatella furcifera, and Laodelphax striatellus. Pest Manag. Sci. 2019, 75, 1606–1620. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Arena, S.; Spinelli, S.; Liu, D.; Zhang, G.; Wei, R.; Cambillau, C.; Scaloni, A.; Wang, G.; Pelosi, P. Reverse chemical ecology: Olfactory proteins from the giant panda and their interactions with putative pheromones and bamboo volatiles. Proc. Natl. Acad. Sci. USA 2017, 114, E9802–E9810. [Google Scholar] [CrossRef] [PubMed]
- Tang, H.; Xie, J.; Liu, J.; Khashaveh, A.; Liu, X.; Yi, C.; Zhao, D.; He, L.; Sun, Y.; Zhang, Y. Odorant-binding protein HvarOBP5 in ladybird Hippodamia variegata regulates the perception of semiochemicals from preys and habitat plants. J. Agric. Food Chem. 2023, 71, 1067–1076. [Google Scholar] [CrossRef]
- Liu, H.; Sun, X.; Shi, Z.; An, X.; Khashaveh, A.; Li, Y.; Gu, S.; Zhang, Y. Identification and functional analysis of odorant-binding proteins provide new control strategies for Apolygus lucorum. Int. J. Biol. Macromol. 2023, 224, 1129–1141. [Google Scholar] [CrossRef] [PubMed]
- Swarup, S.; Williams, T.I.; Anholt, R.R. Functional dissection of Odorant binding protein genes in Drosophila melanogaster. Genes Brain Behav. 2011, 10, 648–657. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, J.T.; Zhang, Y.J.; Chen, J.L.; Li, X.C.; Liang, P.; Gao, X.W.; Zhou, J.J.; Gu, S.H. Coordinative mediation of the response to alarm pheromones by three odorant binding proteins in the green peach aphid Myzus persicae. Insect Biochem. Mol. Biol. 2021, 130, 103528. [Google Scholar] [CrossRef]
- Sandler, B.H.; Nikonova, L.; Leal, W.S.; Clardy, J. Sexual attraction in the silkworm moth: Structure of the pheromone-binding-protein-bombykol complex. Chem. Biol. 2000, 7, 143–151. [Google Scholar] [CrossRef]
- Pelosi, P.; Iovinella, I.; Zhu, J.; Wang, G.; Dani, F.R. Beyond chemoreception: Diverse tasks of soluble olfactory proteins in insects. Biol. Rev. 2018, 93, 184–200. [Google Scholar] [CrossRef]
- Vincent, F.; Spinelli, S.; Ramoni, R.; Grolli, S.; Pelosi, P.; Cambillau, C.; Tegoni, M. Complexes of porcine odorant binding protein with odorant molecules belonging to different chemical classes. J. Mol. Biol. 2000, 300, 127–139. [Google Scholar] [CrossRef]
- Li, H.L.; Song, X.M.; Wu, F.; Qiu, Y.L.; Fu, X.B.; Zhang, L.Y.; Tan, J. Chemical structure of semiochemicals and key binding sites together determine the olfactory functional modes of odorant-binding protein 2 in Eastern honey bee, Apis cerana. Int. J. Biol. Macromol. 2020, 145, 876–884. [Google Scholar] [CrossRef]
- Del Mármol, J.; Yedlin, M.A.; Ruta, V. The structural basis of odorant recognition in insect olfactory receptors. Nature 2021, 597, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Li, D.; Yi, S.; Wei, Y.; Wang, M. Odorant-binding protein 19 in Monochamus alternatus involved in the recognition of a volatile strongly emitted from ovipositing host pines. Insect Sci. 2024, 31, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Rebijith, K.B.; Asokan, R.; Hande, H.R.; Kumar, N.K.; Krishna, V.; Vinutha, J.; Bakthavatsalam, N. RNA interference of odorant-binding protein 2 (OBP2) of the cotton aphid, Aphis gossypii (glover), resulted in altered electrophysiological responses. Appl. Biochem. Biotechnol. 2016, 178, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Y.; Zhu, X.Q.; Gu, S.H.; Zhou, Y.L.; Wang, S.Y.; Zhang, Y.J.; Guo, Y.Y. Silencing of odorant binding protein gene AlinOBP4 by RNAi induces declining electrophysiological responses of Adelphocoris lineolatus to six semiochemicals. Insect Sci. 2017, 24, 789–797. [Google Scholar] [CrossRef]
- Du, Y.; Xu, K.; Ma, W.; Su, W.; Tai, M.; Zhao, H.; Jiang, Y.; Li, X. Contact chemosensory genes identified in leg transcriptome of Apis cerana cerana (Hymenoptera: Apidae). J. Econ. Entomol. 2019, 112, 2015–2029. [Google Scholar] [CrossRef]
- Du, Y.; Chen, J. The odorant binding protein, SiOBP5, mediates alarm pheromone olfactory recognition in the red imported fire ant, Solenopsis invicta. Biomolecules 2021, 11, 1595. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Wei, H.S.; Duan, H.X.; Li, K.B.; Zhang, S.; Wei, Z.J.; Yin, J. The mechanism underlying OBP heterodimer formation and the recognition of odors in Holotrichia oblita faldermann. Int. J. Biol. Macromol. 2020, 152, 957–968. [Google Scholar] [CrossRef]
- Zhan, H.X.; Dewer, Y.; Zhang, J.P.; Tian, J.; Li, D.; Qu, C.; Yang, Z.; Li, F.; Luo, C. Odorant-binding protein 1 plays a crucial role in the olfactory response of Bemisia tabaci to R-curcumene. J. Agric. Food Chem. 2021, 69, 12785–12793. [Google Scholar] [CrossRef]
- Qu, Y.; Liu, X.; Zhao, X.; Qin, J.; Cao, Y.; Li, K.; Zhou, J.J.; Wang, S.; Yin, J. Evidence of the involvement of a plus-C odorant-binding protein HparOBP14 in host plant selection and oviposition of the scarab beetle Holotrichia parallela. Insects 2021, 12, 430. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Q.; Zhu, R.; Yao, W.C.; Yu, H.P.; Huang, J.R.; Wang, Z.; Sun, X.Y.; Yuan, D.H.; Sun, Y.Y.; Emam, S.S.; et al. Chemosensory protein 2 of male Athetis lepigone is involved in the perception of sex pheromones and maize volatiles. J. Agric. Food Chem. 2023, 71, 6277–6287. [Google Scholar] [CrossRef]
- Wang, W.W.; Han, K.R.; Jiang, X.F.; Liu, T.X.; Zhang, S.Z. Odorant-binding protein CrufOBP1 in Cotesia ruficrus females plays a pivotal role in the detection of Spodoptera frugiperda larvae. Int. J. Biol. Macromol. 2024, 274, 133491. [Google Scholar] [CrossRef]
- Gu, S.H.; Wang, S.Y.; Zhang, X.Y.; Ji, P.; Liu, J.T.; Wang, G.R.; Wu, K.M.; Guo, Y.Y.; Zhou, J.J.; Zhang, Y.J. Functional characterizations of chemosensory proteins of the Alfalfa plant bug Adelphocoris lineolatus indicate their involvement in host recognition. PLoS ONE 2012, 7, e42871. [Google Scholar] [CrossRef]
- Tang, R.; Su, M.W.; Zhang, Z.N. Electroantennogram responses of an invasive species fall webworm (Hyphantria cunea) to host volatile compounds. Chin. Sci. Bull. 2012, 57, 4560–4568. [Google Scholar] [CrossRef]
- Delorme, J.D.; Payne, T.L. Antennal olfactory responses of black turpentine beetle, Dendroctonus terebrans (Olivier), to bark beetle pheromones and host terpenes. J. Chem. Ecol. 1990, 16, 1321–1329. [Google Scholar] [CrossRef]
- Han, B.Y.; Chen, Z.M. Behavioral and electrophysiological responses ofnatural enemies to synomones from tea shoots and kairomones from tea aphids, Toxoptera aurantii. J. Chem. Ecol. 2002, 28, 2203–2219. [Google Scholar] [CrossRef]

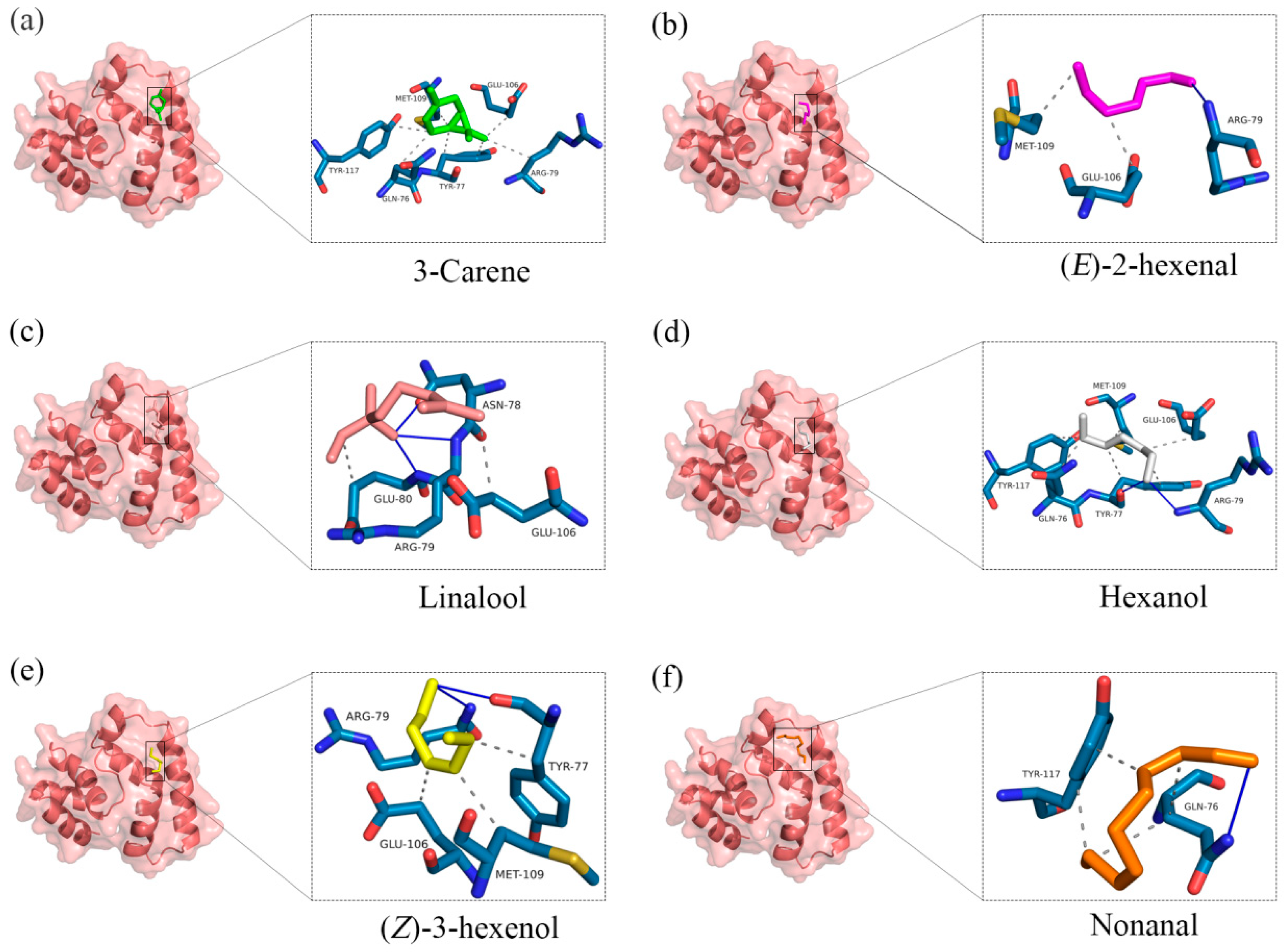
| Proteins | Ligands | Docking Energy (kcal/mol) |
|---|---|---|
| AspiOBP1 | 3-Carene | −3.07 |
| (E)-2-Hexenal | −2.47 | |
| (Z)-3-Hexenol | −3.10 | |
| Linalool | −1.78 | |
| Hexanol | −1.99 | |
| Nonanal | −1.89 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jia, Z.; Qin, Z.; Ge, X.; Xu, Y.; Chen, Z. The Odorant-Binding Proteins AspiOBP1 and AspiOBP2 in Aleurocanthus spiniferus Are Involved in the Perception of Host Volatiles. Int. J. Mol. Sci. 2025, 26, 8784. https://doi.org/10.3390/ijms26188784
Jia Z, Qin Z, Ge X, Xu Y, Chen Z. The Odorant-Binding Proteins AspiOBP1 and AspiOBP2 in Aleurocanthus spiniferus Are Involved in the Perception of Host Volatiles. International Journal of Molecular Sciences. 2025; 26(18):8784. https://doi.org/10.3390/ijms26188784
Chicago/Turabian StyleJia, Zhifei, Zeyu Qin, Xiaoyu Ge, Yongyu Xu, and Zhenzhen Chen. 2025. "The Odorant-Binding Proteins AspiOBP1 and AspiOBP2 in Aleurocanthus spiniferus Are Involved in the Perception of Host Volatiles" International Journal of Molecular Sciences 26, no. 18: 8784. https://doi.org/10.3390/ijms26188784
APA StyleJia, Z., Qin, Z., Ge, X., Xu, Y., & Chen, Z. (2025). The Odorant-Binding Proteins AspiOBP1 and AspiOBP2 in Aleurocanthus spiniferus Are Involved in the Perception of Host Volatiles. International Journal of Molecular Sciences, 26(18), 8784. https://doi.org/10.3390/ijms26188784






