Molecular Characterization of Three Novel Large Deletions Causing α0-Thalassemia
Abstract
1. Introduction
2. Results
3. Discussion
3.1. Clinical Implications and Genetic Counseling
3.2. Functional Relevance of HS-40 and Related Regulatory Mutations
Limitations and Future Perspectives
4. Materials and Methods
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| NGS | Next-Generation Sequencing |
| HPLC | High-Performance Liquid Chromatography |
| CE | Capillary Electrophoresis |
| MLPA | Multiplex Ligation-dependent Probe Amplification |
References
- Harteveld, C.L.; Higgs, D.R. Alpha-thalassaemia. Orphanet J. Rare Dis. 2010, 5, 13. [Google Scholar] [CrossRef] [PubMed]
- Higgs, D.R. The molecular basis of α-thalassemia. Cold Spring Harb. Perspect. Med. 2013, 3, a011718. [Google Scholar] [CrossRef] [PubMed]
- Farashi, S.; Harteveld, C.L. Molecular basis of α-thalassemia. Blood Cells Mol. Dis. 2018, 70, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Giardine, B.; Borg, J.; Viennas, E.; Pavlidis, C.; Moradkhani, K.; Joly, P.; Bartsakoulia, M.; Riemer, C.; Miller, W.; Tzimas, G.; et al. Updates of the HbVar database of human hemoglobin variants and thalassemia mutations. Nucleic Acids Res. 2014, 42, D1063–D1069. [Google Scholar] [CrossRef]
- Kountouris, P.; Lederer, C.W.; Fanis, P.; Feleki, X.; Old, J.; Kleanthous, M. IthaGenes: An interactive database for haemoglobin variations and epidemiology. PLoS ONE 2014, 9, e103020. [Google Scholar] [CrossRef] [PubMed]
- Traeger-Synodinos, J.; Harteveld, C.L. Advances in technologies for screening and diagnosis of hemoglobinopathies. Biomolecules 2021, 11, 1552. [Google Scholar] [CrossRef] [PubMed]
- Coelho, A.; Picanço, I.; Seuanes, F.; Seixas, M.T.; Faustino, P. Novel large deletions in the human alpha-globin gene cluster: Clarifying the HS-40 long-range regulatory role in the native chromosome environment. Blood Cells Mol. Dis. 2010, 45, 147–153. [Google Scholar] [CrossRef]
- Viprakasit, V.; Ekwattanakit, S. Clinical classification, screening and diagnosis for thalassemia. Hematol. Oncol. Clin. N. Am. 2018, 32, 193–211. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Liu, Y.; Chu, Y.; Zhu, Y.; Chen, B.; Yu, H. Identification of large deletions in the α-globin gene cluster using next-generation sequencing: Molecular characterization of two novel deletions causing α0-thalassemia. Mol. Genet. Genom. Med. 2022, 10, e1911. [Google Scholar]
- Lai, K.; Huang, G.; Su, L.; He, Y. The molecular characterization and hematological features of patients with HbH disease caused by novel large deletions of the α-globin gene cluster. Ann. Hematol. 2020, 99, 1803–1810. [Google Scholar]
- Capasso, S.; Cardiero, G.; Musollino, G.; Prezioso, R.; Testa, R.; Dembech, S.; Piluso, G.; Nigro, V.; Digilio, F.A.; Lacerra, G. Functional analysis of three new alpha-thalassemia deletions involving MCS-R2 reveals the presence of an additional enhancer element in the 5’ boundary region. PLoS Genet. 2023, 19, e1010727. [Google Scholar] [CrossRef] [PubMed]
- De, D.; Dutta, S.; Panja, S.; Ghosh, M.K. Molecular screening of alpha thalassemia in microcytic anemia: A prospective study from Eastern India. J. Clin. Diagn. Res. 2022, 16, BC04–BC08. [Google Scholar]
- Baird, D.C.; Batten, S.H.; Sparks, S.K. Alpha- and Beta-thalassemia: Rapid Evidence Review. Am. Fam. Physician 2022, 105, 272–280. [Google Scholar]
- Higgs, D.R.; Bowden, D.K. Clinical and laboratory features of the alpha-thalassemia syndromes. In Disorders of Hemoglobin: Genetics, Pathophysiology, and Clinical Management; Steinberg, M.H., Forget, P.G., Higgs, D.R., Nagel, R.L., Eds.; Cambridge University Press: Cambridge, UK, 2001; pp. 431–469. [Google Scholar]
- Gielen, M.; Harteveld, C.L.; van Vliet, M.; De Loos, J.M.; Heller, R.; Boonstra, A.H.; van Wijk, R.; Solari, A.; Giordano, P.C. Characterization of α-globin gene cluster deletions with gap-PCR, MLPA and next-generation sequencing. Int. J. Lab. Hematol. 2018, 40, 647–653. [Google Scholar]
- Higgs, D.R.; Engel, J.D.; Stamatoyannopoulos, G. Thalassaemia. Lancet 2012, 379, 373–383. [Google Scholar] [CrossRef]
- De Sanctis, V.; Kattamis, C.; Canatan, D.; Soliman, A.T.; Elsedfy, H.; Karimi, M.; Daar, S.; Wali, Y.; Yassin, M.; Soliman, N.; et al. β-Thalassemia Distribution in the Old World: An Ancient Disease Seen from a Historical Standpoint. Mediterr. J. Hematol. Infect. Dis. 2017, 9, e2017018. [Google Scholar] [CrossRef] [PubMed]
- Canatan, D.; Vives Corrons, J.L.; Piacentini, G.; Kara, F.; Keskinkılıç, B.; Tezel, B.; Külekçi Uğur, A.; Babayiğit, M.; Krishnevskaya, E.; Millimaggi, G.; et al. Immigration and screening programs for hemoglobinopathies in Italy, Spain and Turkey. Acta Biomed. 2021, 92, e2021410. [Google Scholar]
- Vichinsky, E.P. Alpha thalassemia major—New mutations, intrauterine management, and outcomes. Hematol. Am. Soc. Hematol. Educ. Program 2009, 2009, 35–41. [Google Scholar] [CrossRef]
- Iolascon, A.; De Falco, L.; Beaumont, C. Molecular basis of inherited microcytic anemia due to defects in iron acquisition or heme synthesis. Haematologica 2009, 94, 395–408. [Google Scholar] [CrossRef]
- Harteveld, C.L.; Voskamp, A.; Phylipsen, M.; Wijk, R.; Leijtens, L.C.; Reesink-Peters, N.A.; Boonstra, A.; Giordano, P.C.A. Nine unknown rearrangements in the alpha-globin gene cluster: Improved detection with MLPA. Br. J. Haematol. 2013, 163, 280–286. [Google Scholar]
- Old, J.M. Screening and genetic diagnosis of haemoglobin disorders. Blood Rev. 2003, 17, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Vernimmen, D.; Bickmore, W.A. The Hierarchy of Transcriptional Activation: From Enhancer to Promoter. Trends Genet. 2015, 31, 696–708. [Google Scholar] [CrossRef]
- Li, Q.; Peterson, K.R.; Fang, X.; Stamatoyannopoulos, G. Locus control regions. Blood 2002, 100, 3077–3086. [Google Scholar] [CrossRef]
- Tufarelli, C.; Frischauf, A.M. Genomic deletions of the human alpha-globin gene cluster caused by recombination between Alu repeats. Hum. Genet. 1998, 102, 359–361. [Google Scholar]
- Loeber, J.G.; Platis, D.; Zetterström, R.H.; Almashanu, S.; Boemer, F.; Bonham, J.R.; Borde, P.; Brincat, I.; Cheillan, D.; Dekkers, E.; et al. Neonatal Screening in Europe Revisited: An ISNS Perspective on the Current State and Developments Since 2010. Int. J. Neonatal. Screen. 2021, 7, 15. [Google Scholar] [CrossRef]
- Giordano, P.C. Strategies for basic laboratory diagnostics of the hemoglobinopathies in multi-ethnic societies: Interpretation of results and pitfalls. Int. J. Lab Hematol. 2013, 35, 465–479. [Google Scholar] [CrossRef]
- He, S.; Qin, Q.; Huang, P.; Zhang, S.; Yi, S.; Lin, L.; Zuo, Y.; Chen, Q.; Deng, J.; Zheng, C.; et al. Characterization of a Large Novel α-Globin Gene Cluster Deletion Causing α0-Thalassemia in a Chinese Family. Hemoglobin 2017, 41, 297–299. [Google Scholar] [CrossRef]
- Mantere, T.; Kersten, S.; Hoischen, A. Long-Read Sequencing Emerging in Medical Genetics. Front. Genet. 2019, 10, 426. [Google Scholar] [CrossRef] [PubMed]
- Tesio, N.; Bauer, D.E. Molecular Basis and Genetic Modifiers of Thalassemia. Hematol. Oncol. Clin. N. Am. 2023, 37, 273–299. [Google Scholar] [CrossRef] [PubMed]
- Levy, S.; Myers, R.M. Advancements in Clinical Genome Sequencing. Annu. Rev. Genom. Hum. Genet. 2016, 17, 95–115. [Google Scholar] [CrossRef]
- Fucharoen, S.; Winichagoon, P. Haemoglobinopathies in southeast Asia. Indian J. Med. Res. 2011, 134, 498–506. [Google Scholar]
- Villegas, A.; Ropero, P.; González, F.A.; Anguita, E.; Espinós, D. The thalassemia syndromes: Molecular characterization in the Spanish population. Hemoglobin 2001, 273–283. [Google Scholar] [CrossRef]
- Vichinsky, E.P. Alpha thalassemia major—New insights and challenges. Blood 2020, 136, 389–391. [Google Scholar]
- Chong, S.S.; Boehm, C.D.; Higgs, D.R.; Cutting, G.R. Single-tube multiplex-PCR screen for common deletional determinants of alpha-thalassemia. Blood 2000, 95, 360–362. [Google Scholar] [CrossRef]
- Bowden, D.K.; Vickers, M.A.; Higgs, D.R. A PCR-based strategy to detect the common severe determinants of alpha-thalassemia. Br. J. Haematol. 1992, 81, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Hassan, S.; Bahar, R.; Johan, M.F.; Mohamed Hashim, E.K.; Abdullah, W.Z.; Esa, E.; Abdul Hamid, F.S.; Zulkafli, Z. Next-Generation Sequencing (NGS) and Third-Generation Sequencing (TGS) for the Diagnosis of Thalassemia. Diagnostics 2023, 13, 373. [Google Scholar] [CrossRef] [PubMed]
- Colosimo, A.; Gatta, V.; Guida, V.; Leodori, E.; Foglietta, E.; Rinaldi, S.; Cappabianca, M.P.; Amato, A.; Stuppia, L.; Dallapiccola, B. Application of MLPA assay to characterize unsolved α-globin gene rearrangements. Blood Cells Mol. Dis. 2011, 46, 139–144. [Google Scholar] [CrossRef] [PubMed]
- Vernimmen, D.; De Gobbi, M.; Sloane-Stanley, J.A.; Wood, W.G.; Higgs, D.R. Long-range chromosomal interactions regulate the timing of the transition between poised and active gene expression. EMBO J. 2007, 26, 2041–2051. [Google Scholar] [CrossRef]
- Hay, D.; Hughes, J.R.; Babbs, C.; Davies, J.O.J.; Graham, B.J.; Hanssen, L.; Kassouf, M.T.; Marieke Oudelaar, A.M.; Sharpe, J.A.; Suciu, M.C.; et al. Genetic dissection of the α-globin super-enhancer in vivo. Nat. Genet. 2016, 48, 895–903. [Google Scholar] [CrossRef]
- Vernimmen, D.; Marques-Kranc, F.; Sharpe, J.A.; Sloane-Stanley, J.A.; Wood, W.G.; Wallace, H.A.; Smith, A.J.; Higgs, D.R. Chromosome looping at the human alpha-globin locus is mediated via the major upstream regulatory element (HS-40). Blood 2009, 114, 4253–4260. [Google Scholar] [CrossRef]
- Daniel, Y.; Elion, J.; Allaf, B.; Badens, C.; Bouva, M.J.; Brincat, I.; Cela, E.; Coppinger, C.; de Montalembert, M.; Gulbis, B.; et al. Newborn Screening for Sickle Cell Disease in Europe. Int. J. Neonatal Screen. 2019, 5, 15. [Google Scholar] [CrossRef]
- Huisman, T.H.J.; Carver, M.F.H.; Baysal, E. A Syllabus of Human Hemoglobin Variants, 2nd ed.; The Sickle Cell Anemia Foundation: Augusta, Georgia, 1996. [Google Scholar]
- Liau, W.S.; Ngoc, P.C.; Sanda, T. Roles of the RUNX1 Enhancer in Normal Hematopoiesis and Leukemogenesis. Adv. Exp. Med. Biol. 2017, 962, 139–147. [Google Scholar]
- Buenrostro, J.D.; Wu, B.; Chang, H.Y.; Greenleaf, W.J. ATAC-seq: A method for assaying chromatin accessibility genome-wide. Curr. Protoc. Mol. Biol. 2015, 109, 21.29.1–21.29.9. [Google Scholar] [CrossRef] [PubMed]
- Mardis, E.R. DNA sequencing technologies: 2006–2016. Nat. Protoc. 2017, 12, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Voss, T.C.; Hager, G.L. Dynamic regulation of transcriptional states by chromatin and transcription factors. Nat. Rev. Genet. 2014, 15, 69–81. [Google Scholar] [CrossRef] [PubMed]

| Patient | Sex/Year | RBC (106/µL) | Hb (g/dL) | MCV (fL) | MCH (pg) | RDW (%) | HbA2 (%) HPLC | HbF (%) HPLC | Hb A2 (%) C.E |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Male/51 | 7.0 | 15.5 | 69.0 | 18.0 | 15.3 | 2.5 | <0.5 | 2.1 |
| 2 | Female/10 | 6.3 | 12.4 | 63.0 | 19.6 | 15.6 | 2.9 | 0.6 | 2.3 |
| 3 | Male/3 | 6.1 | 10.8 | 57.7 | 17.7 | 20.2 | 2.7 | 0.7 | 2.4 |
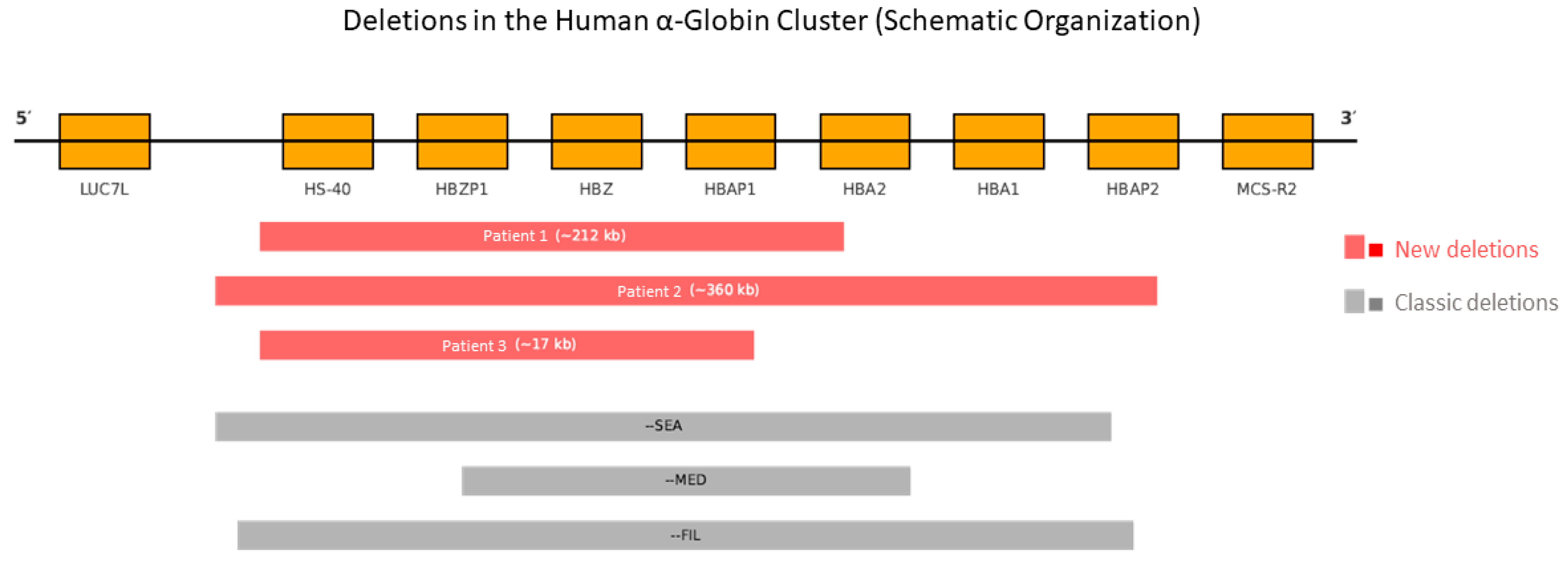
| Patient | Deletion (MLPA Probe P140 HBA) | Estimated Size | Affected Region (Genes and Elements) | Type of Deletion | NGS Confirmation |
|---|---|---|---|---|---|
| 1 | Probes 1–30 | ~212 kb | HBZ, HBA2, HBA1, HS-40 | Heterozygous | Yes |
| 2 | Probes 1–33 | ~360 kb | HBZ, HBA2, HBA1, HS-40 | Heterozygous | Yes |
| 3 | Probes 1–29 | ~17 kb | HBZ, HBA2, HBA1, HS-40 | Heterozygous | Yes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-Benito, S.; Ricard Andrés, M.P.; Murúzabal, M.J.; Nieto, J.M.; González, F.A.; Ortega-Montero, B.; Villegas, A.; Benavente, C.; Ropero, P. Molecular Characterization of Three Novel Large Deletions Causing α0-Thalassemia. Int. J. Mol. Sci. 2025, 26, 8783. https://doi.org/10.3390/ijms26188783
Ferrer-Benito S, Ricard Andrés MP, Murúzabal MJ, Nieto JM, González FA, Ortega-Montero B, Villegas A, Benavente C, Ropero P. Molecular Characterization of Three Novel Large Deletions Causing α0-Thalassemia. International Journal of Molecular Sciences. 2025; 26(18):8783. https://doi.org/10.3390/ijms26188783
Chicago/Turabian StyleFerrer-Benito, Sara, María Pilar Ricard Andrés, María José Murúzabal, Jorge M. Nieto, Fernando A. González, Belén Ortega-Montero, Ana Villegas, Celina Benavente, and Paloma Ropero. 2025. "Molecular Characterization of Three Novel Large Deletions Causing α0-Thalassemia" International Journal of Molecular Sciences 26, no. 18: 8783. https://doi.org/10.3390/ijms26188783
APA StyleFerrer-Benito, S., Ricard Andrés, M. P., Murúzabal, M. J., Nieto, J. M., González, F. A., Ortega-Montero, B., Villegas, A., Benavente, C., & Ropero, P. (2025). Molecular Characterization of Three Novel Large Deletions Causing α0-Thalassemia. International Journal of Molecular Sciences, 26(18), 8783. https://doi.org/10.3390/ijms26188783






