A Cytochrome P450 Facilitates Polyethylene Metabolism in a Microbial Community
Abstract
1. Introduction
2. Results
2.1. Bacterial Consortium Uses PE as a Sole Carbon Source
2.2. Surface Modifications of PE Plastics After Degradation
2.3. Byproducts Resulting from Bacterial Degradation of PE Plastic
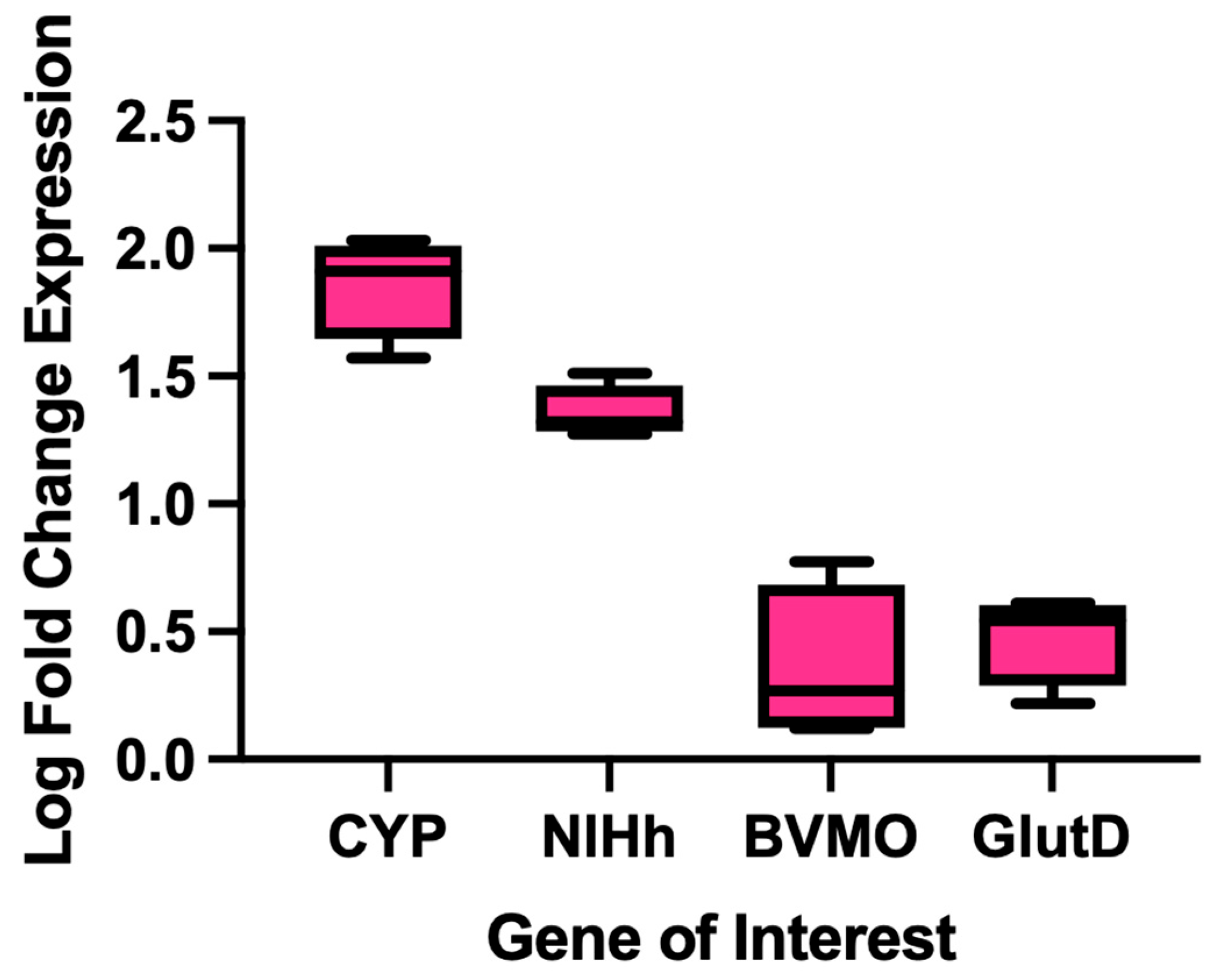
2.4. Identification of Enzymes Putatively Associated with PE Metabolism
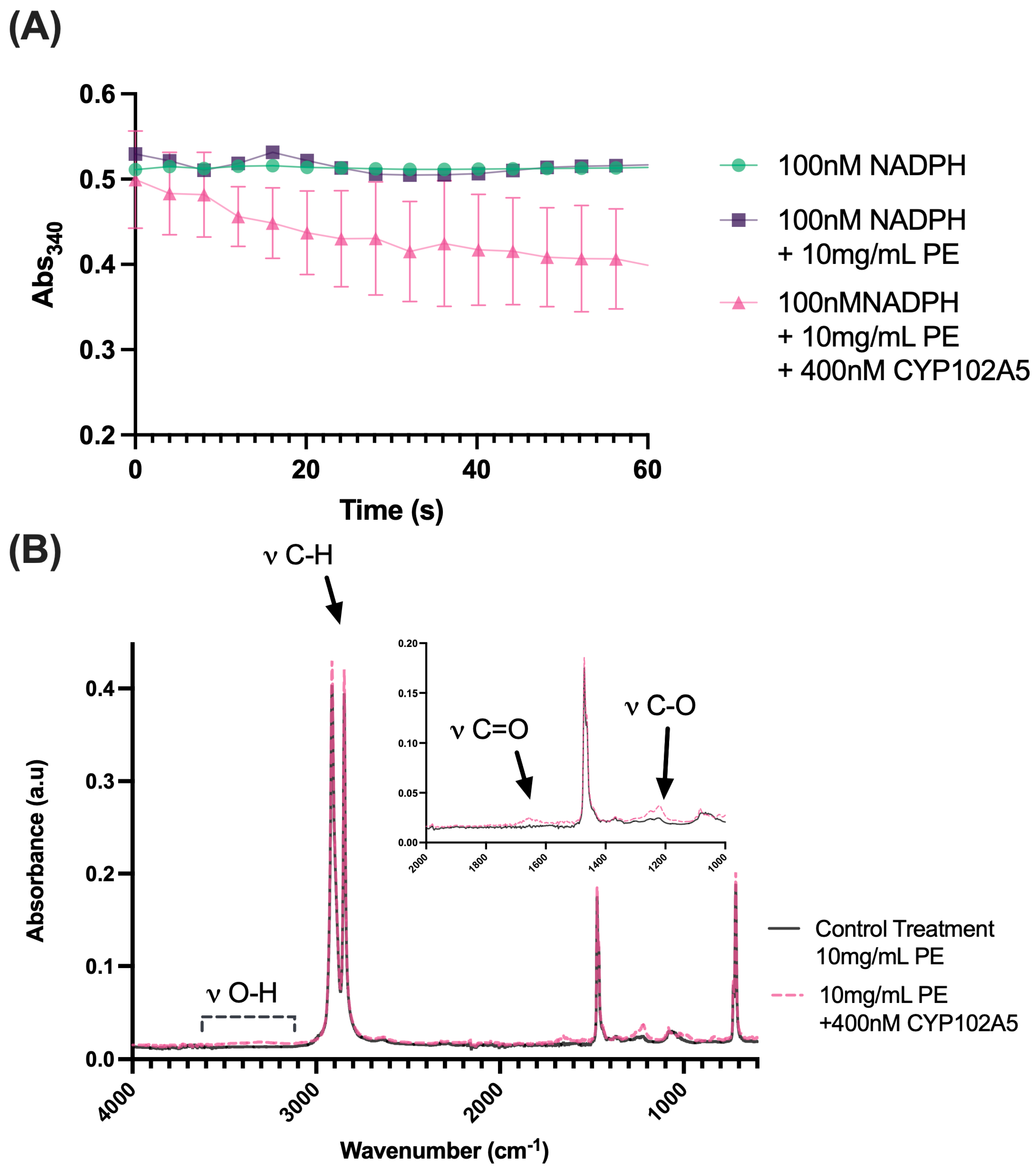
2.5. Purified CYP102 A5 Cytochrome P450 Reductase Enzymatic Activity in the Presence of PE Plastic
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains and Plasmids Utilized in This Study
4.2. Bacterial Growth
4.2.1. Pretreatment of PE Plastic
4.2.2. Growth of PE-Degrading Bacteria
4.2.3. Growth Conditions of E. coli Strains for Gibson Assembly, Plasmid Isolation, and Protein Overexpression
4.3. Measurement of Bacterial Growth for Evaluating PE Degradation
4.4. Weight Loss of PE Plastic and Water Absorbance Changes in Bacterial Culture
4.5. SEM Analysis of Bacteria-Degraded PE Plastic
4.6. FTIR Analysis of Bacteria-Degraded PE Plastic
4.7. Detection of PE Degradation Products
4.8. Gene of Interest Identification and Expression in PE Plastic Conditions
4.9. Expression and Purification of CYP102 A5
4.10. NADPH Consumption Assays of CYP102 A5
4.11. Long-Term NADPH-Regeneration Assay and FTIR Analysis of PE Plastic After Treatment with CYP102 A5
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PE | polyethylene |
| LDPE | low-density polyethylene |
| ATR-FTIR | attenuated total reflectance–Fourier transform infrared spectroscopy |
| GC/MS | gas chromatography–mass spectrometry |
| LCFBM | liquid carbon free basal medium |
| CFU | colony forming units |
References
- United States Envirometnal Protection Agency. Advancing Sustainable Materials Management: 2018 Tables and Figures 2020; United States Envirometnal Protection Agency: Washington, DC, USA, 2020. [Google Scholar]
- PlasticsEurope. Plastics-the Facts 2021: An Analysis of European Plastics Production, Demand, and Waste Data; PlasticsEurope: Brussels, Belgium, 2021. [Google Scholar]
- Di, J.; Reck, B.K.; Miatto, A.; Graedel, T.E. United States plastics: Large flows, short lifetimes, and negligible recycling. Resour. Conserv. Recycl. 2021, 167, 105440. [Google Scholar] [CrossRef]
- Hutley, T.J.; Ouederni, M. Polyolefins—The History and Economic Impact. In Polyolefin Compounds and Materials: Fundamentals and Industrial Applications; Springer Series on Polymer and Composite Materials; Al-Ali AlMa’adeed, M., Krupa, I., Eds.; Springer Series on Polymer and Composite Materials; Springer International Publishing: Cham, Switzerland, 2016; pp. 13–50. ISBN 978-3-319-25982-6. [Google Scholar]
- Lau, W.W.Y.; Shiran, Y.; Bailey, R.M.; Cook, E.; Stuchtey, M.R.; Koskella, J.; Velis, C.A.; Godfrey, L.; Boucher, J.; Murphy, M.B.; et al. Evaluating scenarios toward zero plastic pollution. Science 2020, 369, 1455–1461. [Google Scholar] [CrossRef]
- OECD. Global Plastics Outlook; OECD: Paris, France, 2022. [Google Scholar]
- Ory, N.C.; Sobral, P.; Ferreira, J.L.; Thiel, M. Amberstripe scad Decapterus muroadsi (Carangidae) fish ingest blue microplastics resembling their copepod prey along the coast of Rapa Nui (Easter Island) in the South Pacific subtropical gyre. Sci. Total. Environ. 2017, 586, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Balzani, P.; Galeotti, G.; Scheggi, S.; Masoni, A.; Santini, G.; Baracchi, D. Acute and chronic ingestion of polyethylene (PE) microplastics has mild effects on honey bee health and cognition. Environ. Pollut. 2022, 305, 119318. [Google Scholar] [CrossRef]
- Kim, S.W.; An, Y.-J. Edible Size of Polyethylene Microplastics and Their Effects on Springtail Behavior. Environ. Pollut. 2020, 266, 115255. [Google Scholar] [CrossRef]
- Park, E.-J.; Han, J.-S.; Seong, E.; Lee, G.-H.; Kim, D.-W.; Son, H.-Y.; Han, H.-Y.; Lee, B.-S. Repeated-oral dose toxicity of polyethylene microplastics and the possible implications on reproduction and development of the next generation. Toxicol. Lett. 2020, 324, 75–85. [Google Scholar] [CrossRef]
- Çobanoğlu, H.; Belivermiş, M.; Sıkdokur, E.; Kılıç, Ö.; Çayır, A. Genotoxic and cytotoxic effects of polyethylene microplastics on human peripheral blood lymphocytes. Chemosphere 2021, 272, 129805. [Google Scholar] [CrossRef]
- Gautam, R.; Jo, J.; Acharya, M.; Maharjan, A.; Lee, D.; Kc, P.B.; Kim, C.; Kim, K.; Kim, H.; Heo, Y. Evaluation of potential toxicity of polyethylene microplastics on human derived cell lines. Sci. Total Environ. 2022, 838, 156089. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.O.; Abrantes, N.; Gonçalves, F.; Nogueira, H.S.; Marques, J.; Gonçalves, A.M. Impacts of plastic products used in daily life on the environment and human health: What is known? Environ. Toxicol. Pharmacol. 2019, 72, 103239. [Google Scholar] [CrossRef]
- Bäckström, E.; Odelius, K.; Hakkarainen, M. Trash to Treasure: Microwave-Assisted Conversion of Polyethylene to Functional Chemicals. Ind. Eng. Chem. Res. 2017, 56, 14814–14821. [Google Scholar] [CrossRef]
- Oliveira, J.; Belchior, A.; da Silva, V.D.; Rotter, A.; Petrovski, Ž.; Almeida, P.L.; Lourenço, N.D.; Gaudêncio, S.P. Marine Environmental Plastic Pollution: Mitigation by Microorganism Degradation and Recycling Valorization. Front. Mar. Sci. 2020, 7, 567126. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef]
- Yao, Z.; Seong, H.J.; Jang, Y.-S. Environmental Toxicity and Decomposition of Polyethylene. Ecotoxicol. Environ. Saf. 2022, 242, 113933. [Google Scholar] [CrossRef]
- Verma, R.; Vinoda, K.S.; Papireddy, M.; Gowda, A.N.S. Toxic Pollutants from Plastic Waste—A Review. Procedia Environ. Sci. 2016, 35, 701–708. [Google Scholar] [CrossRef]
- Sen, S.K.; Raut, S. Microbial degradation of low density polyethylene (LDPE): A review. J. Environ. Chem. Eng. 2015, 3, 462–473. [Google Scholar] [CrossRef]
- Nowak, B.; Pająk, J.; Drozd-Bratkowicz, M.; Rymarz, G. Microorganisms participating in the biodegradation of modified polyethylene films in different soils under laboratory conditions. Int. Biodeterior. Biodegrad. 2011, 65, 757–767. [Google Scholar] [CrossRef]
- Santo, M.; Weitsman, R.; Sivan, A. The role of the copper-binding enzyme—Laccase—In the biodegradation of polyethylene by the actinomycete Rhodococcus ruber. Int. Biodeterior. Biodegrad. 2013, 84, 204–210. [Google Scholar] [CrossRef]
- Rajandas, H.; Parimannan, S.; Sathasivam, K.; Ravichandran, M.; Yin, L.S. A novel FTIR-ATR spectroscopy based technique for the estimation of low-density polyethylene biodegradation. Polym. Test. 2012, 31, 1094–1099. [Google Scholar] [CrossRef]
- Tribedi, P.; Sil, A.K. Low-Density Polyethylene Degradation by Pseudomonas Sp. AKS2 Biofilm. Environ. Sci. Pollut. Res. 2013, 20, 4146–4153. [Google Scholar] [CrossRef] [PubMed]
- Yun, S.-D.; Lee, C.O.; Kim, H.-W.; An, S.J.; Kim, S.; Seo, M.-J.; Park, C.; Yun, C.-H.; Chi, W.S.; Yeom, S.-J. Exploring a New Biocatalyst from Bacillus Thuringiensis JNU01 for Polyethylene Biodegradation. Environ. Sci. Technol. Lett. 2023, 10, 485–492. [Google Scholar] [CrossRef]
- Ghatge, S.; Yang, Y.; Ahn, J.-H.; Hur, H.-G. Biodegradation of polyethylene: A brief review. Appl. Biol. Chem. 2020, 63, 27. [Google Scholar] [CrossRef]
- Bardají, D.K.R.; Moretto, J.A.S.; Furlan, J.P.R.; Stehling, E.G. A mini-review: Current advances in polyethylene biodegradation. World J. Microbiol. Biotechnol. 2020, 36, 32. [Google Scholar] [CrossRef]
- Zeenat; Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics degradation by microbes: A sustainable approach. J. King Saud Univ. Sci. 2021, 33, 101538. [Google Scholar] [CrossRef]
- Yuan, Z.; Xu, X.-R. Chapter Six—Surface Characteristics and Biotoxicity of Airborne Microplastics. In Comprehensive Analytical Chemistry; Wang, J., Ed.; Airborne Microplastics: Analysis, Fate and Human Health Effects; Elsevier: Amsterdam, The Netherlands, 2023; Volume 100, pp. 117–164. [Google Scholar]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Peixoto, J.; Silva, L.P.; Krüger, R.H. Brazilian Cerrado soil reveals an untapped microbial potential for unpretreated polyethylene biodegradation. J. Hazard. Mater. 2017, 324, 634–644. [Google Scholar] [CrossRef] [PubMed]
- Gambarini, V.; Pantos, O.; Kingsbury, J.M.; Weaver, L.; Handley, K.M.; Lear, G. PlasticDB: A Database of Microorganisms and Proteins Linked to Plastic Biodegradation. Database 2022, 2022, baac008. Available online: https://academic.oup.com/database/article/doi/10.1093/database/baac008/6546196 (accessed on 13 February 2024). [PubMed]
- Restrepo-Flórez, J.-M.; Bassi, A.; Thompson, M.R. Microbial degradation and deterioration of polyethylene—A review. Int. Biodeterior. Biodegradation 2014, 88, 83–90. [Google Scholar] [CrossRef]
- Wei, R.; Zimmermann, W. Microbial enzymes for the recycling of recalcitrant petroleum-based plastics: How far are we? Microb. Biotechnol. 2017, 10, 1308–1322. [Google Scholar] [CrossRef]
- Rojo, F. Enzymes for Aerobic Degradation of Alkanes. In Handbook of Hydrocarbon and Lipid Microbiology; Timmis, K.N., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 781–797. ISBN 978-3-540-77584-3. [Google Scholar]
- Yeom, S.-J.; Le, T.-K.; Yun, C.-H. P450-driven plastic-degrading synthetic bacteria. Trends Biotechnol. 2022, 40, 166–179. [Google Scholar] [CrossRef]
- León-Zayas, R.; Roberts, C.; Vague, M.; Mellies, J.L.; Newton, I.L.G. Draft Genome Sequences of Five Environmental Bacterial Isolates That Degrade Polyethylene Terephthalate Plastic. Genome Announc. 2019, 8, e00237-19. [Google Scholar] [CrossRef]
- Edwards, S.; León-Zayas, R.; Ditter, R.; Laster, H.; Sheehan, G.; Anderson, O.; Beattie, T.; Mellies, J.L. Microbial Consortia and Mixed Plastic Waste: Pangenomic Analysis Reveals Potential for Degradation of Multiple Plastic Types via Previously Identified PET Degrading Bacteria. Int. J. Mol. Sci. 2022, 23, 5612. [Google Scholar] [CrossRef] [PubMed]
- Gilan, I.; Hadar, Y.; Sivan, A. Colonization, biofilm formation and biodegradation of polyethylene by a strain of Rhodococcus ruber. Appl. Microbiol. Biotechnol. 2004, 65, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Jeon, J.-M.; Park, S.-J.; Choi, T.-R.; Park, J.-H.; Yang, Y.-H.; Yoon, J.-J. Biodegradation of polyethylene and polypropylene by Lysinibacillus species JJY0216 isolated from soil grove. Polym. Degrad. Stab. 2021, 191, 109662. [Google Scholar] [CrossRef]
- Gerritse, J.; Leslie, H.A.; de Tender, C.A.; Devriese, L.I.; Vethaak, A.D. Fragmentation of plastic objects in a laboratory seawater microcosm. Sci. Rep. 2020, 10, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sutkar, P.R.; Gadewar, R.D.; Dhulap, V.P. Recent Trends in Degradation of Microplastics in the Environment: A State-of-the-Art Review. J. Hazard. Mater. Adv. 2023, 11, 100343. [Google Scholar] [CrossRef]
- Park, S.Y.; Kim, C.G. Biodegradation of micro-polyethylene particles by bacterial colonization of a mixed microbial consortium isolated from a landfill site. Chemosphere 2019, 222, 527–533. [Google Scholar] [CrossRef]
- Drnovská, H.; Lapčík, L.; Buršíková, V.; Zemek, J.; Barros-Timmons, A.M. Surface properties of polyethylene after low-temperature plasma treatment. Colloid Polym. Sci. 2003, 281, 1025–1033. [Google Scholar] [CrossRef]
- Elsamahy, T.; Sun, J.; Elsilk, S.E.; Ali, S.S. Biodegradation of low-density polyethylene plastic waste by a constructed tri-culture yeast consortium from wood-feeding termite: Degradation mechanism and pathway. J. Hazard. Mater. 2023, 448, 130944. [Google Scholar] [CrossRef]
- Montazer, Z.; Najafi, M.B.H.; Levin, D.B. Challenges with Verifying Microbial Degradation of Polyethylene. Polymers 2020, 12, 123. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Natarajan, K.; Hemambika, B.; Ramesh, N.; Sumathi, C.; Kottaimuthu, R.; Kannan, V.R. High-density polyethylene (HDPE)-degrading potential bacteria from marine ecosystem of Gulf of Mannar, India. Lett. Appl. Microbiol. 2010, 51, 205–211. [Google Scholar] [CrossRef]
- Nademo, Z.M.; Shibeshi, N.T.; Gemeda, M.T. Isolation and screening of low-density polyethylene (LDPE) bags degrading bacteria from Addis Ababa municipal solid waste disposal site “Koshe”. Ann. Microbiol. 2023, 73, 1–11. [Google Scholar] [CrossRef]
- Sandt, C.; Waeytens, J.; Deniset-Besseau, A.; Nielsen-Leroux, C.; Réjasse, A. Use and misuse of FTIR spectroscopy for studying the bio-oxidation of plastics. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2021, 258, 119841. [Google Scholar] [CrossRef]
- Khandare, S.D.; Agrawal, D.; Mehru, N.; Chaudhary, D.R. Marine bacterial based enzymatic degradation of low-density polyethylene (LDPE) plastic. J. Environ. Chem. Eng. 2022, 10, 107437. [Google Scholar] [CrossRef]
- Yao, Z.; Seong, H.J.; Jang, Y.-S. Degradation of low density polyethylene by Bacillus species. Appl. Biol. Chem. 2022, 65, 1–9. [Google Scholar] [CrossRef]
- Das, M.P.; Kumar, S. An Approach to Low-Density Polyethylene Biodegradation by Bacillus Amyloliquefaciens. 3 Biotech 2015, 5, 81–86. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. The Compostable Plastic Poly(lactic) Acid Causes a Temporal Shift in Fungal Communities in Maturing Compost. Compos. Sci. Util. 2017, 25, 211–219. [Google Scholar] [CrossRef]
- Sivan, A. New perspectives in plastic biodegradation. Curr. Opin. Biotechnol. 2011, 22, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Wilkes, R.A.; Aristilde, L. Degradation and Metabolism of Synthetic Plastics and Associated Products by Pseudomonas Sp.: Capabilities and Challenges. J. Appl. Microbiol. 2017, 123, 582–593. [Google Scholar] [CrossRef] [PubMed]
- Cai, Z.; Li, M.; Zhu, Z.; Wang, X.; Huang, Y.; Li, T.; Gong, H.; Yan, M. Biological Degradation of Plastics and Microplastics: A Recent Perspective on Associated Mechanisms and Influencing Factors. Microorganisms 2023, 11, 1661. [Google Scholar] [CrossRef]
- Khalil, M.A.; Alorabi, J.A.; Al-Otaibi, L.M.; Ali, S.S.; Elsilk, S.E. Antibiotic Resistance and Biofilm Formation in Enterococcus spp. Isolated from Urinary Tract Infections. Pathogens 2022, 12, 34. [Google Scholar] [CrossRef] [PubMed]
- Ndahebwa Muhonja, C.; Magoma, G.; Imbuga, M.; Makonde, H.M. Molecular Characterization of Low-Density Polyethene (LDPE) Degrading Bacteria and Fungi from Dandora Dumpsite, Nairobi, Kenya. Int. J. Microbiol. 2018, 2018, 4167845. [Google Scholar] [CrossRef]
- Woo, S.; Song, I.; Cha, H.J.; Johnson, K.N. Fast and Facile Biodegradation of Polystyrene by the Gut Microbial Flora of Plesiophthalmus davidis Larvae. Appl. Environ. Microbiol. 2020, 86, e01361-20. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, N.; Shang, Y.; Yao, M.; Ding, M.; Yuan, Y. Construction of Yarrowia lipolytica for degradation of low-density polyethylene. Process. Saf. Environ. Prot. 2025, 195, 106818. [Google Scholar] [CrossRef]
- Uddin, M.; Venkatesan, S.K.; Pal, S.K.; Vinu, R.; Sekar, K.; Kandasamy, R. Accelerating biodegradation efficiency of low-density polyethylene and its hazardous dissolved organic matter using unexplored polyolefin-respiring bacteria: New insights on degradation characterization, biomolecule influence and biotransformation pathways. J. Hazard. Mater. 2025, 492, 138144. [Google Scholar] [CrossRef]
- Aschenbrenner, J.C.; Ebrecht, A.C.; Tolmie, C.; Smit, M.S.; Opperman, D.J. Structure of the fungal hydroxylase, CYP505A30, and rational transfer of mutation data from CYP102A1 to alter regioselectivity. Catal. Sci. Technol. 2021, 11, 7359–7367. [Google Scholar] [CrossRef]
- Chen, M.M.; Snow, C.D.; Vizcarra, C.L.; Mayo, S.L.; Arnold, F.H. Comparison of random mutagenesis and semi-rational designed libraries for improved cytochrome P450 BM3-catalyzed hydroxylation of small alkanes. Protein Eng. Des. Sel. 2012, 25, 171–178. [Google Scholar] [CrossRef]
- Meinhold, P.; Peters, M.W.; Chen, M.M.Y.; Takahashi, K.; Arnold, F.H. Direct Conversion of Ethane to Ethanol by Engineered Cytochrome P450 BM3. ChemBioChem 2005, 6, 1765–1768. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.W.; Meinhold, P.; Glieder, A.; Arnold, F.H. Regio- and Enantioselective Alkane Hydroxylation with Engineered Cytochromes P450 BM-3. J. Am. Chem. Soc. 2003, 125, 13442–13450. [Google Scholar] [CrossRef]
- Chowdhary, P.K.; Alemseghed, M.; Haines, D.C. Cloning, expression and characterization of a fast self-sufficient P450: CYP102A5 from Bacillus cereus. Arch. Biochem. Biophys. 2007, 468, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Teufel, F.; Armenteros, J.J.A.; Johansen, A.R.; Gíslason, M.H.; Pihl, S.I.; Tsirigos, K.D.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 6.0 predicts all five types of signal peptides using protein language models. Nat. Biotechnol. 2022, 40, 1023–1025. [Google Scholar] [CrossRef]
- Fürst, M.J.L.J.; Gran-Scheuch, A.; Aalbers, F.S.; Fraaije, M.W. Baeyer–Villiger Monooxygenases: Tunable Oxidative Biocatalysts. ACS Catal. 2019, 9, 11207–11241. [Google Scholar] [CrossRef]
- Fiorentini, F.; Geier, M.; Binda, C.; Winkler, M.; Faber, K.; Hall, M.; Mattevi, A. Biocatalytic Characterization of Human FMO5: Unearthing Baeyer–Villiger Reactions in Humans. ACS Chem. Biol. 2016, 11, 1039–1048. [Google Scholar] [CrossRef]
- Zadjelovic, V.; Erni-Cassola, G.; Obrador-Viel, T.; Lester, D.; Eley, Y.; Gibson, M.I.; Dorador, C.; Golyshin, P.N.; Black, S.; Wellington, E.M.H.; et al. A Mechanistic Understanding of Polyethylene Biodegradation by the Marine Bacterium Alcanivorax. J. Hazard. Mater. 2022, 436, 129278. [Google Scholar] [CrossRef]
- Kim, H.R.; Lee, C.; Shin, H.; Kim, J.; Jeong, M.; Choi, D. Isolation of a polyethylene-degrading bacterium, Acinetobacter guillouiae, using a novel screening method based on a redox indicator. Heliyon 2023, 9, e15731. [Google Scholar] [CrossRef]
- Bhatia, S.K.; Bhatia, R.K.; Choi, Y.-K.; Kan, E.; Kim, Y.-G.; Yang, Y.-H. Biotechnological potential of microbial consortia and future perspectives. Crit. Rev. Biotechnol. 2018, 38, 1209–1229. [Google Scholar] [CrossRef]
- Salinas, J.; Carpena, V.; Martínez-Gallardo, M.R.; Segado, M.; Estrella-González, M.J.; Toribio, A.J.; Jurado, M.M.; López-González, J.A.; Suárez-Estrella, F.; López, M.J. Development of plastic-degrading microbial consortia by induced selection in microcosms. Front. Microbiol. 2023, 14, 1143769. [Google Scholar] [CrossRef] [PubMed]
- Roberts, C.; Edwards, S.; Vague, M.; León-Zayas, R.; Scheffer, H.; Chan, G.; Swartz, N.A.; Mellies, J.L.; McMahon, K. Environmental Consortium Containing Pseudomonas and Bacillus Species Synergistically Degrades Polyethylene Terephthalate Plastic. mSphere 2020, 5, e01151-20. [Google Scholar] [CrossRef]
- Awasthi, S.; Srivastava, P.; Singh, P.; Tiwary, D.; Mishra, P.K. Biodegradation of thermally treated high-density polyethylene (HDPE) by Klebsiella pneumoniae CH001. 3 Biotech 2017, 7, 332. [Google Scholar] [CrossRef] [PubMed]
- Khampratueng, P.; Rice, D.; Anal, A.K. Biodegradation of low-density polyethylene by the bacterial strains isolated from the dumping site community. Discov. Appl. Sci. 2024, 6, 348. [Google Scholar] [CrossRef]
- Kyaw, B.M.; Champakalakshmi, R.; Sakharkar, M.K.; Lim, C.S.; Sakharkar, K.R. Biodegradation of Low Density Polythene (LDPE) by Pseudomonas Species. Indian J. Microbiol. 2012, 52, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.K.; Devi, D. Characteristics investigation on biofilm formation and biodegradation activities of Pseudomonas aeruginosa strain ISJ14 colonizing low density polyethylene (LDPE) surface. Heliyon 2020, 6, e04398. [Google Scholar] [CrossRef]
- Almond, J.; Sugumaar, P.; Wenzel, M.N.; Hill, G.; Wallis, C. Determination of the carbonyl index of polyethylene and polypropylene using specified area under band methodology with ATR-FTIR spectroscopy. e-Polymers 2020, 20, 369–381. [Google Scholar] [CrossRef]
- Waterhouse, A.M.; Procter, J.B.; Martin, D.M.A.; Clamp, M.; Barton, G.J. Jalview Version 2-a Multiple Sequence Alignment Editor and Analysis Workbench. Bioinformatics 2009, 25, 1189–1191. [Google Scholar] [CrossRef] [PubMed]
- Kunzmann, P.; Mayer, B.E.; Hamacher, K. Substitution matrix based color schemes for sequence alignment visualization. BMC Bioinform. 2020, 21, 209. [Google Scholar] [CrossRef] [PubMed]
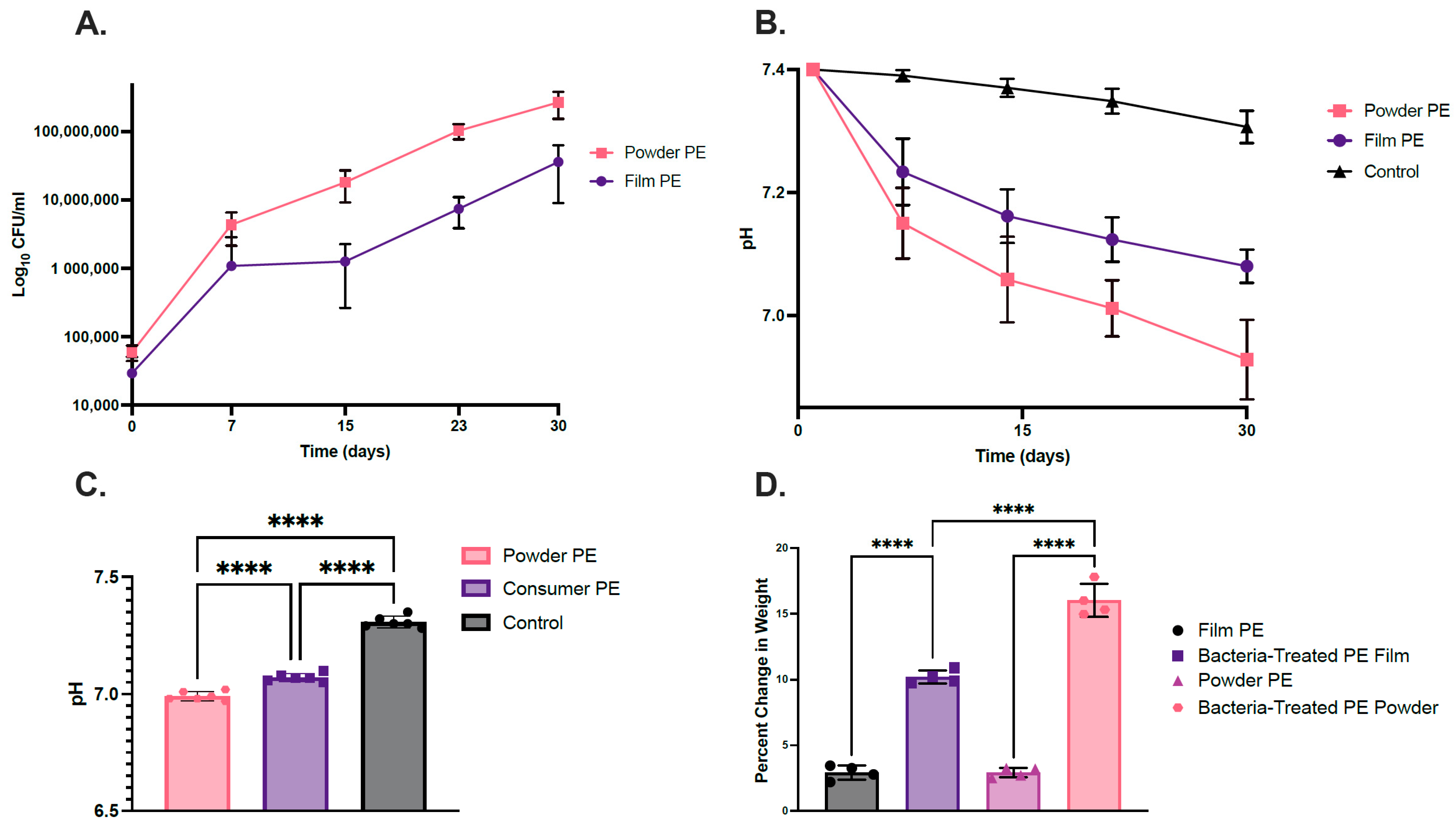
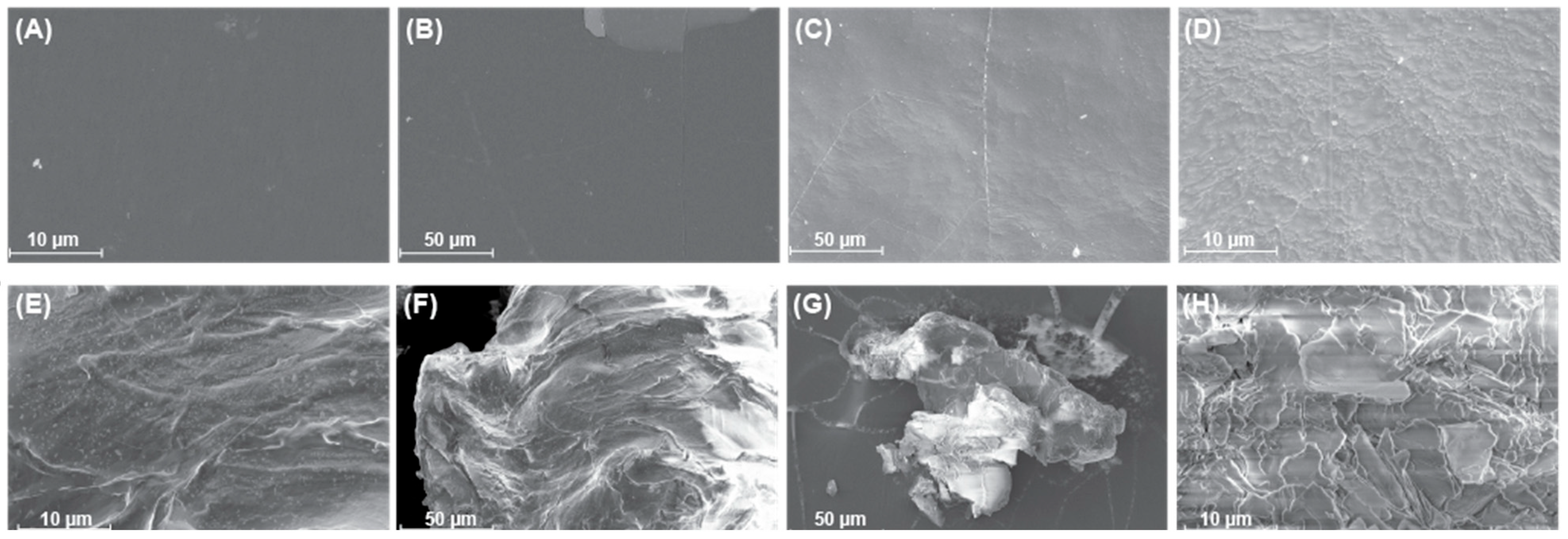
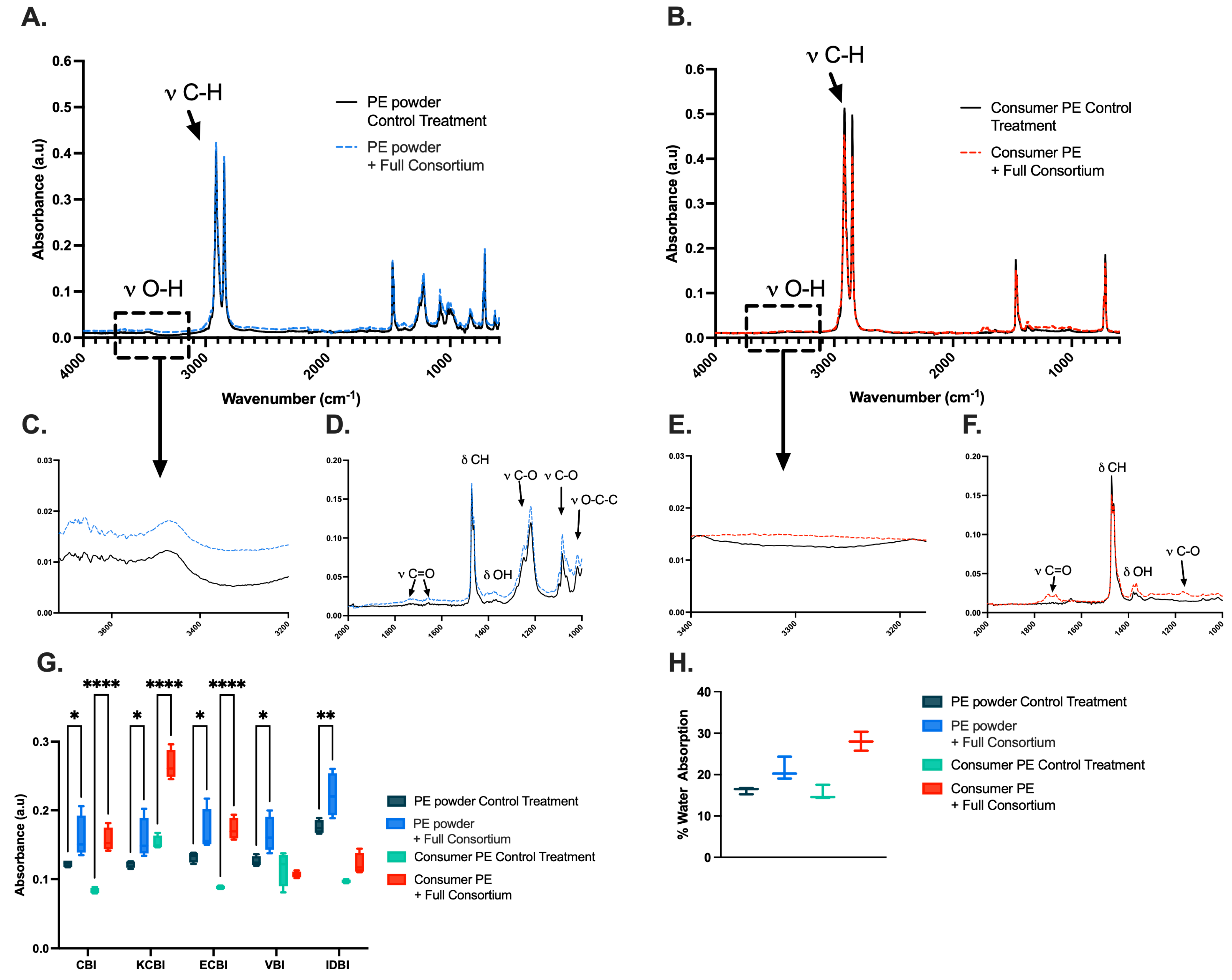
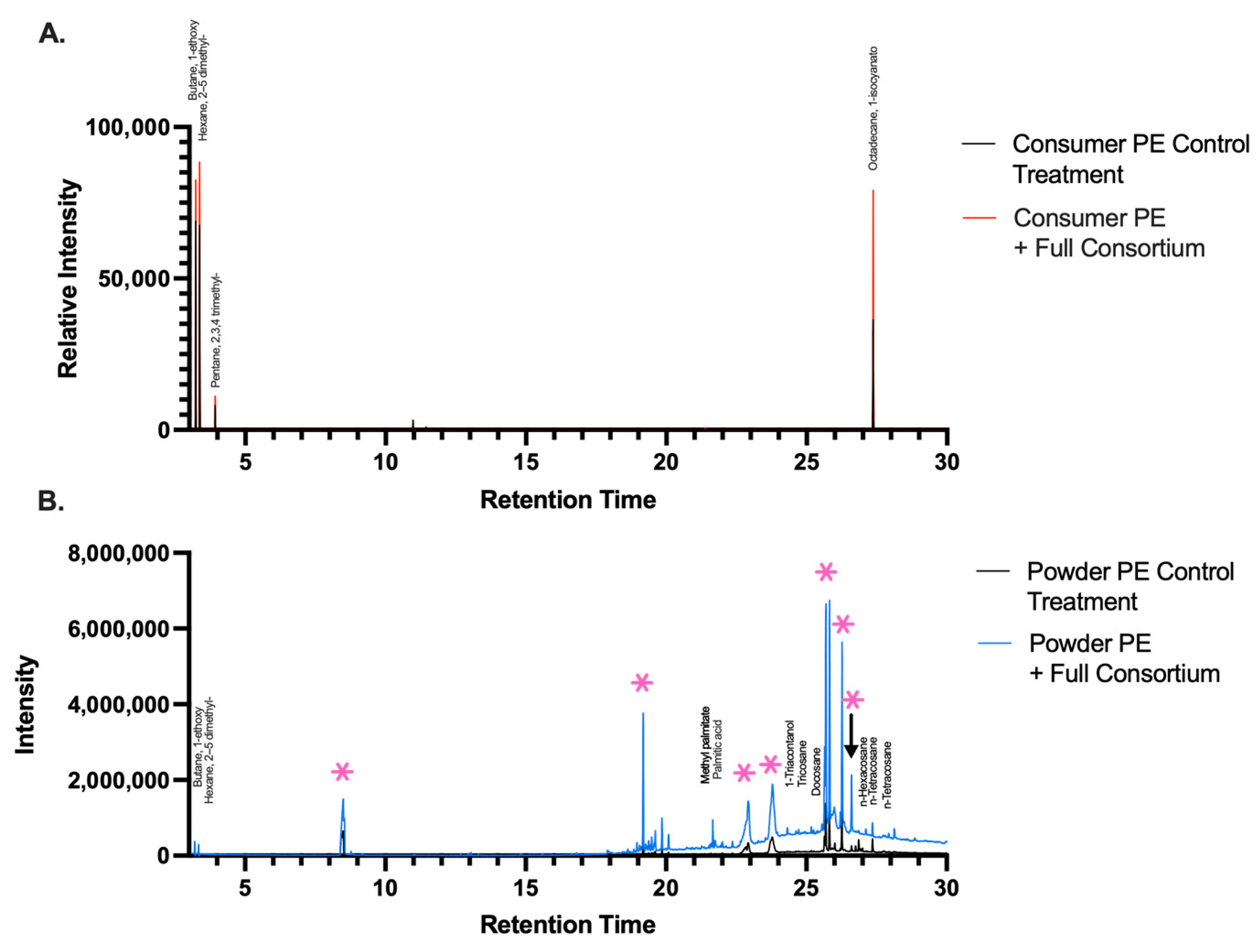
| Sample Type | Carbonyl Bond Index (I1714/I1463) | Keto Carbonyl Bond Index (I1715/I1465) | Internal Double Bond Index (I908/I1465) | Ester Carbonyl Index (I1740/I1465) | Vinyl Bond Index (I1650/I1465) |
|---|---|---|---|---|---|
| Untreated Powder | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.18 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 |
| Treated Powder | 0.16 ± 0.03 | 0.16 ± 0.03 | 0.22 ± 0.03 | 0.17 ± 0.03 | 0.16 ± 0.03 |
| Untreated Film | 0.08 ± 0.00 | 0.15 ± 0.01 | 0.10 ± 0.00 | 0.09 ± 0.00 | 0.12 ± 0.03 |
| Treated Film | 0.16 ± 0.02 | 0.27 ± 0.02 | 0.12 ± 0.02 | 0.17 ± 0.02 | 0.11 ± 0.01 |
| Sample Type | Carbonyl Bond Index (I1714/I1463) | Keto Carbonyl Bond Index (I1715/I1465) | Internal Double Bond Index (I908/I1465) | Ester Carbonyl Index (I1740/I1465) | Vinyl Bond Index (I1650/I1465) |
|---|---|---|---|---|---|
| Control powdered PE | 0.10 ± 0.02 | 0.12 ± 0.02 | 0.17 ± 0.02 | 0.15 ± 0.02 | 0.18 ± 0.01 |
| CYP102 A5, powdered PE | 0.14 ± 0.01 | 0.15 ± 0.01 | 0.18 ± 0.01 | 0.16 ± 0.01 | 0.19 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarara, M.; Ahuja, S.; Mellies, J.L. A Cytochrome P450 Facilitates Polyethylene Metabolism in a Microbial Community. Int. J. Mol. Sci. 2025, 26, 8775. https://doi.org/10.3390/ijms26188775
Tarara M, Ahuja S, Mellies JL. A Cytochrome P450 Facilitates Polyethylene Metabolism in a Microbial Community. International Journal of Molecular Sciences. 2025; 26(18):8775. https://doi.org/10.3390/ijms26188775
Chicago/Turabian StyleTarara, Madelyn, Shivani Ahuja, and Jay L. Mellies. 2025. "A Cytochrome P450 Facilitates Polyethylene Metabolism in a Microbial Community" International Journal of Molecular Sciences 26, no. 18: 8775. https://doi.org/10.3390/ijms26188775
APA StyleTarara, M., Ahuja, S., & Mellies, J. L. (2025). A Cytochrome P450 Facilitates Polyethylene Metabolism in a Microbial Community. International Journal of Molecular Sciences, 26(18), 8775. https://doi.org/10.3390/ijms26188775







