Distinction Between Proliferative Lupus Nephritis and Membranous Lupus Nephritis Based on Inflammation, NETosis, and Glomerular Exostosin
Abstract
1. Introduction
2. Method for Literature Search
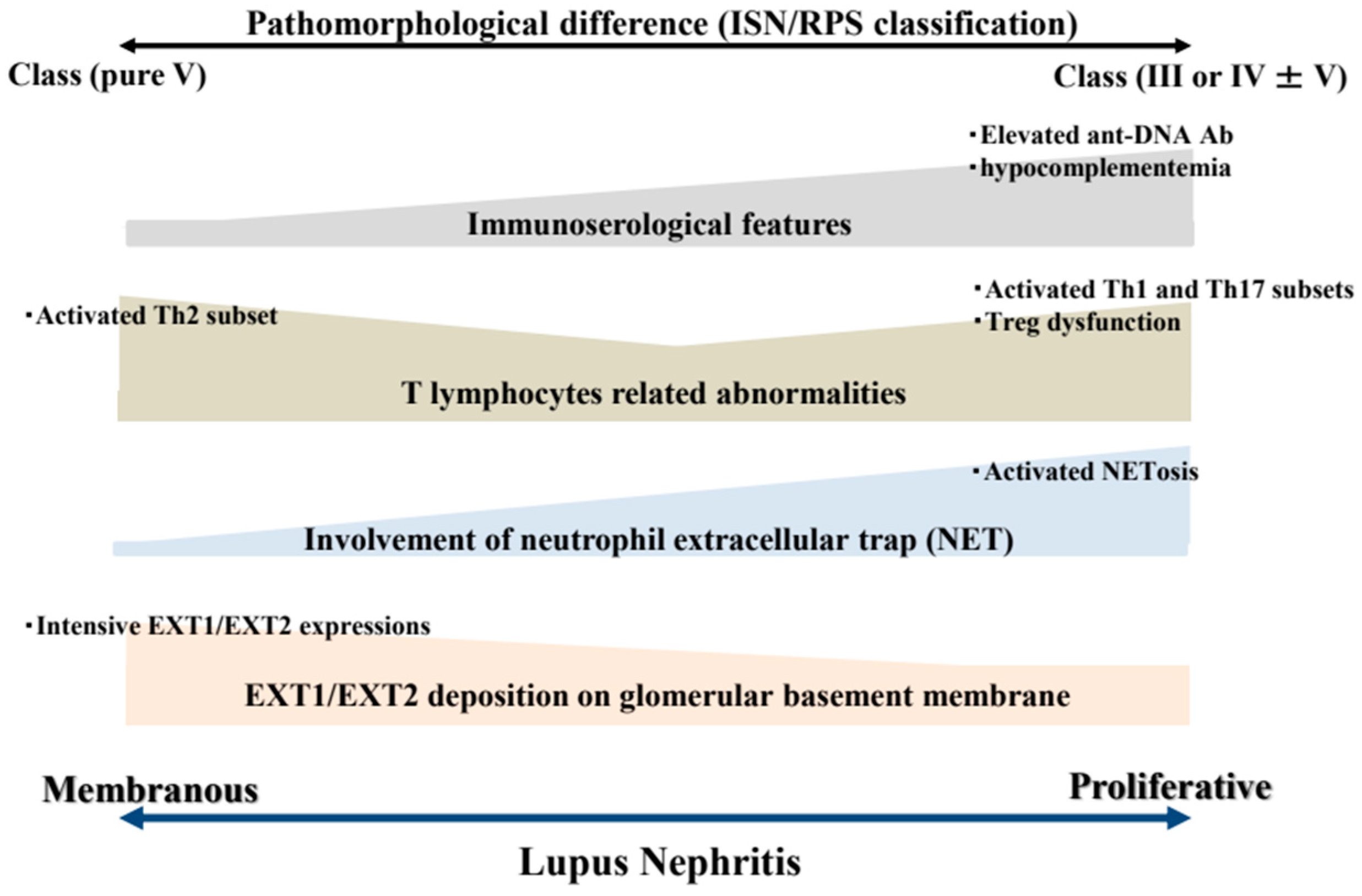
3. Pathomorphological Differences Between PLN and MLN
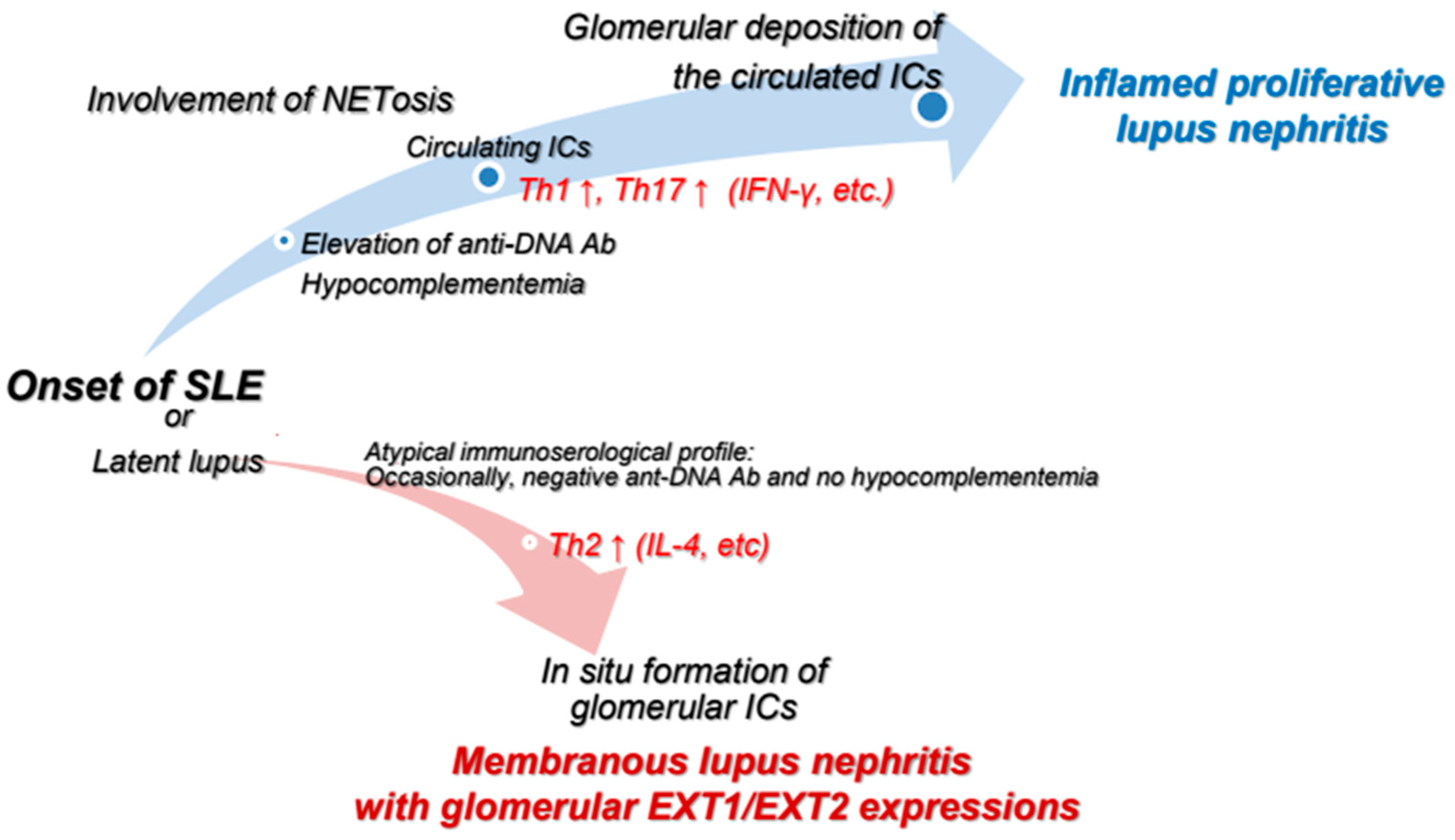
4. Dissimilarities Between PLN and MLN Based on T Lymphocyte-Related Abnormalities
5. Dissimilarities Between PLN and MLN Based on Involvement of NETosis
6. Dissimilarities Between PLN and MLN Based on Glomerular Expressions of Exostosin 1 and 2
7. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tsokos, G.C. Systemic lupus erythematosus. N. Engl. J. Med. 2011, 365, 2110–2121. [Google Scholar] [CrossRef] [PubMed]
- Yap, D.Y.H.; Chan, T.M. B Cell Abnormalities in Systemic Lupus Erythematosus and Lupus Nephritis-Role in Pathogenesis and Effect of Immunosuppressive Treatments. Int. J. Mol. Sci. 2019, 20, 6231. [Google Scholar] [CrossRef]
- Moysidou, E.; Christodoulou, M.; Lioulios, G.; Stai, S.; Karamitsos, T.; Dimitroulas, T.; Fylaktou, A.; Stangou, M. Lymphocytes Change Their Phenotype and Function in Systemic Lupus Erythematosus and Lupus Nephritis. Int. J. Mol. Sci. 2024, 25, 10905. [Google Scholar] [CrossRef]
- Roveta, A.; Parodi, E.L.; Brezzi, B.; Tunesi, F.; Zanetti, V.; Merlotti, G.; Francese, A.; Maconi, A.G.; Quaglia, M. Lupus Nephritis from Pathogenesis to New Therapies: An Update. Int. J. Mol. Sci. 2024, 25, 8981. [Google Scholar] [CrossRef]
- Rees, F.; Doherty, M.; Grainge, M.J.; Lanyon, P.; Zhang, W. The worldwide incidence and prevalence of systemic lupus erythematosus: A systematic review of epidemiological studies. Rheumatology 2017, 56, 1945–1961. [Google Scholar] [CrossRef]
- Lai, B.; Luo, S.F.; Lai, J.H. Therapeutically targeting proinflammatory type I interferons in systemic lupus erythematosus: Efficacy and insufficiency with a specific focus on lupus nephritis. Front. Immunol. 2024, 15, 1489205. [Google Scholar] [CrossRef]
- Fanouriakis, A.; Tziolos, N.; Bertsias, G.; Boumpas, D.T. Update οn the diagnosis and management of systemic lupus erythematosus. Ann. Rheum. Dis. 2021, 80, 14–25. [Google Scholar] [CrossRef]
- Parikh, S.V.; Almaani, S.; Brodsky, S.; Rovin, B.H. Update on Lupus Nephritis: Core Curriculum 2020. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2020, 76, 265–281. [Google Scholar] [CrossRef]
- Chen, P.M.; Tsokos, G.C. Mitochondria in the Pathogenesis of Systemic Lupus Erythematosus. Curr. Rheumatol. Rep. 2022, 24, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, H.; Yang, H.; Zhou, J.; Zhao, L.; Zhang, F. Glucose metabolism and glycosylation link the gut microbiota to autoimmune diseases. Front. Immunol. 2022, 13, 952398. [Google Scholar] [CrossRef]
- Caielli, S.; Wan, Z.; Pascual, V. Systemic Lupus Erythematosus Pathogenesis: Interferon and Beyond. Annu. Rev. Immunol. 2023, 41, 533–560. [Google Scholar] [CrossRef]
- Lech, M.; Anders, H.J. The pathogenesis of lupus nephritis. J. Am. Soc. Nephrol. JASN 2013, 24, 1357–1366. [Google Scholar] [CrossRef]
- Yu, F.; Haas, M.; Glassock, R.; Zhao, M.H. Redefining lupus nephritis: Clinical implications of pathophysiologic subtypes. Nat. reviews. Nephrol. 2017, 13, 483–495. [Google Scholar] [CrossRef]
- Ward, F.; Bargman, J.M. Membranous Lupus Nephritis: The Same, But Different. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 2016, 68, 954–966. [Google Scholar] [CrossRef]
- Bajema, I.M.; Wilhelmus, S.; Alpers, C.E.; Bruijn, J.A.; Colvin, R.B.; Cook, H.T.; D’Agati, V.D.; Ferrario, F.; Haas, M.; Jennette, J.C.; et al. Revision of the International Society of Nephrology/Renal Pathology Society classification for lupus nephritis: Clarification of definitions, and modified National Institutes of Health activity and chronicity indices. Kidney Int. 2018, 93, 789–796. [Google Scholar] [CrossRef]
- Miyake, K.; Akahoshi, M.; Nakashima, H. Th subset balance in lupus nephritis. J. Biomed. Biotechnol. 2011, 2011, 980286. [Google Scholar] [CrossRef]
- Miyake, K.; Adachi, K.; Watanabe, M.; Sasatomi, Y.; Ogahara, S.; Abe, Y.; Ito, K.; Dan Justin, Y.K.; Saito, T.; Nakashima, H.; et al. Parasites alter the pathological phenotype of lupus nephritis. Autoimmunity 2014, 47, 538–547. [Google Scholar] [CrossRef]
- Selvaraja, M.; Abdullah, M.; Arip, M.; Chin, V.K.; Shah, A.; Amin Nordin, S. Elevated interleukin-25 and its association to Th2 cytokines in systemic lupus erythematosus with lupus nephritis. PLoS ONE 2019, 14, e0224707. [Google Scholar] [CrossRef]
- Calvani, N.; Tucci, M.; Richards, H.B.; Tartaglia, P.; Silvestris, F. Th1 cytokines in the pathogenesis of lupus nephritis: The role of IL-18. Autoimmun. Rev. 2005, 4, 542–548. [Google Scholar] [CrossRef]
- Sigdel, K.R.; Duan, L.; Wang, Y.; Hu, W.; Wang, N.; Sun, Q.; Liu, Q.; Liu, X.; Hou, X.; Cheng, A.; et al. Serum Cytokines Th1, Th2, and Th17 Expression Profiling in Active Lupus Nephritis-IV: From a Southern Chinese Han Population. Mediat. Inflamm. 2016, 2016, 4927530. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Iyoda, M.; Suzuki, T.; Tachibana, S.; Kanazawa, N.; Matsumoto, K.; Honda, H. Immunopathological analysis of the expression of glomerular exostosin 1 and exostosin 2 in Japanese patients with lupus nephritis. Virchows Arch. Int. J. Pathol. 2021, 479, 997–1005. [Google Scholar] [CrossRef]
- Sethi, S.; Madden, B.J.; Debiec, H.; Charlesworth, M.C.; Gross, L.; Ravindran, A.; Hummel, A.M.; Specks, U.; Fervenza, F.C.; Ronco, P. Exostosin 1/Exostosin 2-Associated Membranous Nephropathy. J. Am. Soc. Nephrol. JASN 2019, 30, 1123–1136. [Google Scholar] [CrossRef]
- Kudo, T.; Nakazawa, D.; Watanabe-Kusunoki, K.; Kanda, M.; Shiratori-Aso, S.; Abe, N.; Nishio, S.; Koga, J.I.; Iwasaki, S.; Tsuji, T.; et al. Regulation of NETosis and Inflammation by Cyclophilin D in Myeloperoxidase-Positive Antineutrophil Cytoplasmic Antibody-Associated Vasculitis. Arthritis Rheumatol. 2023, 75, 71–83. [Google Scholar] [CrossRef] [PubMed]
- Nishi, H.; Mayadas, T.N. Neutrophils in lupus nephritis. Curr. Opin. Rheumatol. 2019, 31, 193–200. [Google Scholar] [CrossRef]
- Mistry, P.; Kaplan, M.J. Cell death in the pathogenesis of systemic lupus erythematosus and lupus nephritis. Clin. Immunol. 2017, 185, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Shiratori-Aso, S.; Nakazawa, D. The involvement of NETs in ANCA-associated vasculitis. Front. Immunol. 2023, 14, 1261151. [Google Scholar] [CrossRef]
- Ravindran, A.; Casal Moura, M.; Fervenza, F.C.; Nasr, S.H.; Alexander, M.P.; Fidler, M.E.; Herrera Hernandez, L.P.; Zhang, P.; Grande, J.P.; Cornell, L.D.; et al. In Patients with Membranous Lupus Nephritis, Exostosin-Positivity and Exostosin-Negativity Represent Two Different Phenotypes. J. Am. Soc. Nephrol. JASN 2021, 32, 695–706. [Google Scholar] [CrossRef] [PubMed]
- Sethi, S. New ‘Antigens’ in Membranous Nephropathy. J. Am. Soc. Nephrol. JASN 2021, 32, 268–278. [Google Scholar] [CrossRef]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. Kidney Int. 2004, 65, 521–530. [Google Scholar] [CrossRef]
- Weening, J.J.; D’Agati, V.D.; Schwartz, M.M.; Seshan, S.V.; Alpers, C.E.; Appel, G.B.; Balow, J.E.; Bruijn, J.A.; Cook, T.; Ferrario, F.; et al. The classification of glomerulonephritis in systemic lupus erythematosus revisited. J. Am. Soc. Nephrol. JASN 2004, 15, 241–250. [Google Scholar] [CrossRef]
- Ubara, Y.; Sawa, N.; Wada, T.; Kono, K.; Ohashi, K. Lupus nephritis and related renal disease: Review from case series. Clin. Exp. Nephrol. 2024, 29, 507–520. [Google Scholar] [CrossRef]
- Tektonidou, M.G.; Sotsiou, F.; Nakopoulou, L.; Vlachoyiannopoulos, P.G.; Moutsopoulos, H.M. Antiphospholipid syndrome nephropathy in patients with systemic lupus erythematosus and antiphospholipid antibodies: Prevalence, clinical associations, and long-term outcome. Arthritis Rheum. 2004, 50, 2569–2579. [Google Scholar] [CrossRef]
- Tektonidou, M.G. Renal involvement in the antiphospholipid syndrome (APS)-APS nephropathy. Clin. Rev. Allergy Immunol. 2009, 36, 131–140. [Google Scholar] [CrossRef]
- Cervera, R.; Serrano, R.; Pons-Estel, G.J.; Ceberio-Hualde, L.; Shoenfeld, Y.; de Ramón, E.; Buonaiuto, V.; Jacobsen, S.; Zeher, M.M.; Tarr, T.; et al. Morbidity and mortality in the antiphospholipid syndrome during a 10-year period: A multicentre prospective study of 1000 patients. Ann. Rheum. Dis. 2015, 74, 1011–1018. [Google Scholar] [CrossRef] [PubMed]
- Barbhaiya, M.; Zuily, S.; Naden, R.; Hendry, A.; Manneville, F.; Amigo, M.C.; Amoura, Z.; Andrade, D.; Andreoli, L.; Artim-Esen, B.; et al. 2023 ACR/EULAR antiphospholipid syndrome classification criteria. Ann. Rheum. Dis. 2023, 82, 1258–1270. [Google Scholar] [CrossRef] [PubMed]
- Tektonidou, M.G. Antiphospholipid Syndrome Nephropathy: From Pathogenesis to Treatment. Front. Immunol. 2018, 9, 1181. [Google Scholar] [CrossRef]
- Hihara, K.; Iyoda, M.; Tachibana, S.; Iseri, K.; Saito, T.; Yamamoto, Y.; Suzuki, T.; Wada, Y.; Matsumoto, K.; Shibata, T. Anti-Phospholipase A2 Receptor (PLA2R) Antibody and Glomerular PLA2R Expression in Japanese Patients with Membranous Nephropathy. PLoS ONE 2016, 11, e0158154. [Google Scholar] [CrossRef] [PubMed]
- Frangou, E.; Vassilopoulos, D.; Boletis, J.; Boumpas, D.T. An emerging role of neutrophils and NETosis in chronic inflammation and fibrosis in systemic lupus erythematosus (SLE) and ANCA-associated vasculitides (AAV): Implications for the pathogenesis and treatment. Autoimmun. Rev. 2019, 18, 751–760. [Google Scholar] [CrossRef]
- Kaul, A.; Gordon, C.; Crow, M.K.; Touma, Z.; Urowitz, M.B.; van Vollenhoven, R.; Ruiz-Irastorza, G.; Hughes, G. Systemic lupus erythematosus. Nat. reviews. Dis. Primers 2016, 2, 16039. [Google Scholar] [CrossRef]
- Weidenbusch, M.; Kulkarni, O.P.; Anders, H.J. The innate immune system in human systemic lupus erythematosus. Clin. Sci. 2017, 131, 625–634. [Google Scholar] [CrossRef]
- Canny, S.P.; Jackson, S.W. B Cells in Systemic Lupus Erythematosus: From Disease Mechanisms to Targeted Therapies. Rheum. Dis. Clin. North Am. 2021, 47, 395–413. [Google Scholar] [CrossRef]
- Tenbrock, K.; Rauen, T. T cell dysregulation in SLE. Clin. Immunol. 2022, 239, 109031. [Google Scholar] [CrossRef]
- Wang, Y.; Ito, S.; Chino, Y.; Goto, D.; Matsumoto, I.; Murata, H.; Tsutsumi, A.; Hayashi, T.; Uchida, K.; Usui, J.; et al. Laser microdissection-based analysis of cytokine balance in the kidneys of patients with lupus nephritis. Clin. Exp. Immunol. 2010, 159, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Koga, T.; Ichinose, K.; Kawakami, A.; Tsokos, G.C. Current Insights and Future Prospects for Targeting IL-17 to Treat Patients With Systemic Lupus Erythematosus. Front. Immunol. 2020, 11, 624971. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhu, M.; Jiao, C.; Xu, X.; Xu, F.; Liang, D.; Liu, Z.; Chen, Y.; Zhang, H. Association of regulatory T cells with renal outcomes in patients with proliferative lupus nephritis. Lupus 2023, 32, 1237–1244. [Google Scholar] [CrossRef]
- Kamal, M.; Gabr, H.; Anwar, S.; Bastawy, S.; Salah, L. The relation of CD4+CD25+Foxp3+ regulatory T cells concentration with disease activity and damage index in systemic lupus erythematosus. Lupus 2022, 31, 463–471. [Google Scholar] [CrossRef]
- Chen, P.M.; Tsokos, G.C. T Cell Abnormalities in the Pathogenesis of Systemic Lupus Erythematosus: An Update. Curr. Rheumatol. Rep. 2021, 23, 12. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Fang, J.Q.; Zhang, Z.S.; Chi, Z.; Fang, J.; Xu, D.Y.; Lu, K.Z.; Qian, M.Q.; Zhang, D.Y.; Guo, J.P.; et al. TcpC inhibits neutrophil extracellular trap formation by enhancing ubiquitination mediated degradation of peptidylarginine deiminase 4. Nat. Commun. 2021, 12, 3481. [Google Scholar] [CrossRef]
- Justiz-Vaillant, A.A.; Williams-Persad, A.F.; Arozarena-Fundora, R.; Gopaul, D.; Soodeen, S.; Asin-Milan, O.; Thompson, R.; Unakal, C.; Akpaka, P.E. Chronic Granulomatous Disease (CGD): Commonly Associated Pathogens, Diagnosis and Treatment. Microorganisms 2023, 11, 2233. [Google Scholar] [CrossRef]
- de Bont, C.M.; Koopman, W.J.H.; Boelens, W.C.; Pruijn, G.J.M. Stimulus-dependent chromatin dynamics, citrullination, calcium signalling and ROS production during NET formation. Biochim. Et Biophys. Acta-Mol. Cell Res. 2018, 1865, 1621–1629. [Google Scholar] [CrossRef]
- Belambri, S.A.; Rolas, L.; Raad, H.; Hurtado-Nedelec, M.; Dang, P.M.; El-Benna, J. NADPH oxidase activation in neutrophils: Role of the phosphorylation of its subunits. Eur. J. Clin. Investig. 2018, 48 (Suppl. S2), e12951. [Google Scholar] [CrossRef]
- Al Kuwaiti, A.A.; Al Dhaheri, A.D.; Al Hassani, M.; Ruszczak, Z.; Alrustamani, A.; Abuhammour, W.; El Ghazali, G.; Al-Hammadi, S.; Shendi, H.M. Chronic granulomatous disease in the United Arab Emirates: Clinical and molecular characteristics in a single center. Front. Immunol. 2023, 14, 1228161. [Google Scholar] [CrossRef]
- Hakkim, A.; Fürnrohr, B.G.; Amann, K.; Laube, B.; Abed, U.A.; Brinkmann, V.; Herrmann, M.; Voll, R.E.; Zychlinsky, A. Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis. Proc. Natl. Acad. Sci. USA 2010, 107, 9813–9818. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, E.; Yalavarthi, S.; Berthier, C.C.; Hodgin, J.B.; Khandpur, R.; Lin, A.M.; Rubin, C.J.; Zhao, W.; Olsen, S.H.; Klinker, M.; et al. Netting neutrophils induce endothelial damage, infiltrate tissues, and expose immunostimulatory molecules in systemic lupus erythematosus. J. Immunol. 2011, 187, 538–552. [Google Scholar] [CrossRef]
- Leffler, J.; Martin, M.; Gullstrand, B.; Tydén, H.; Lood, C.; Truedsson, L.; Bengtsson, A.A.; Blom, A.M. Neutrophil extracellular traps that are not degraded in systemic lupus erythematosus activate complement exacerbating the disease. J. Immunol. 2012, 188, 3522–3531. [Google Scholar] [CrossRef] [PubMed]
- Guillotin, F.; Fortier, M.; Portes, M.; Demattei, C.; Mousty, E.; Nouvellon, E.; Mercier, E.; Chea, M.; Letouzey, V.; Gris, J.C.; et al. Vital NETosis vs. suicidal NETosis during normal pregnancy and preeclampsia. Front. Cell Dev. Biol. 2022, 10, 1099038. [Google Scholar] [CrossRef]
- Li, J.; Chen, J.; Sun, J.; Li, K. The Formation of NETs and Their Mechanism of Promoting Tumor Metastasis. J. Oncol. 2023, 2023, 7022337. [Google Scholar] [CrossRef]
- Yang, D.; Liu, J. Neutrophil Extracellular Traps: A New Player in Cancer Metastasis and Therapeutic Target. J. Exp. Clin. Cancer Res. CR 2021, 40, 233. [Google Scholar] [CrossRef]
- Juha, M.; Molnár, A.; Jakus, Z.; Ledó, N. NETosis: An emerging therapeutic target in renal diseases. Front. Immunol. 2023, 14, 1253667. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, M.; Khan, M.A.; Palaniyar, N. Neutrophil Extracellular Trap Formation: Physiology, Pathology, and Pharmacology. Biomolecules 2019, 9, 365. [Google Scholar] [CrossRef]
- Yousefi, S.; Mihalache, C.; Kozlowski, E.; Schmid, I.; Simon, H.U. Viable neutrophils release mitochondrial DNA to form neutrophil extracellular traps. Cell Death Differ. 2009, 16, 1438–1444. [Google Scholar] [CrossRef]
- Lood, C.; Blanco, L.P.; Purmalek, M.M.; Carmona-Rivera, C.; De Ravin, S.S.; Smith, C.K.; Malech, H.L.; Ledbetter, J.A.; Elkon, K.B.; Kaplan, M.J. Neutrophil extracellular traps enriched in oxidized mitochondrial DNA are interferogenic and contribute to lupus-like disease. Nat. Med. 2016, 22, 146–153. [Google Scholar] [CrossRef]
- Mayadas, T.N.; Rosetti, F.; Ernandez, T.; Sethi, S. Neutrophils: Game changers in glomerulonephritis? Trends Mol. Med. 2010, 16, 368–378. [Google Scholar] [CrossRef]
- Orme, J.; Mohan, C. Macrophages and neutrophils in SLE-An online molecular catalog. Autoimmun. Rev. 2012, 11, 365–372. [Google Scholar] [CrossRef]
- Banchereau, R.; Hong, S.; Cantarel, B.; Baldwin, N.; Baisch, J.; Edens, M.; Cepika, A.M.; Acs, P.; Turner, J.; Anguiano, E.; et al. Personalized Immunomonitoring Uncovers Molecular Networks that Stratify Lupus Patients. Cell 2016, 165, 551–565. [Google Scholar] [CrossRef]
- Kimura, H.; Mii, A.; Shoji, J.; Arakawa, Y.; Shimizu, A. Immunohistochemical detection of citrullinated histone H3-positive neutrophils is useful for identifying active glomerular and interstitial lesions in antineutrophil cytoplasmic antibody-associated vasculitis. Histopathology 2021, 78, 520–531. [Google Scholar] [CrossRef]
- Rao, A.N.; Kazzaz, N.M.; Knight, J.S. Do neutrophil extracellular traps contribute to the heightened risk of thrombosis in inflammatory diseases? World J. Cardiol. 2015, 7, 829–842. [Google Scholar] [CrossRef]
- Yalavarthi, S.; Gould, T.J.; Rao, A.N.; Mazza, L.F.; Morris, A.E.; Núñez-Álvarez, C.; Hernández-Ramírez, D.; Bockenstedt, P.L.; Liaw, P.C.; Cabral, A.R.; et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: A newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol. 2015, 67, 2990–3003. [Google Scholar] [CrossRef]
- Ritis, K.; Doumas, M.; Mastellos, D.; Micheli, A.; Giaglis, S.; Magotti, P.; Rafail, S.; Kartalis, G.; Sideras, P.; Lambris, J.D. A novel C5a receptor-tissue factor cross-talk in neutrophils links innate immunity to coagulation pathways. J. Immunol. 2006, 177, 4794–4802. [Google Scholar] [CrossRef]
- Girardi, G.; Berman, J.; Redecha, P.; Spruce, L.; Thurman, J.M.; Kraus, D.; Hollmann, T.J.; Casali, P.; Caroll, M.C.; Wetsel, R.A.; et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J. Clin. Investig. 2003, 112, 1644–1654. [Google Scholar] [CrossRef]
- Wang, C.; Liu, Y.; Zhang, M.; Yang, F.; Xu, F.; Shi, S.; Zeng, C.; Chen, X.; Miao, Y.; Liu, Z.; et al. Glomerular Exostosin as a Subtype and Activity Marker of Class 5 Lupus Nephritis. Clin. J. Am. Soc. Nephrol. CJASN 2022, 17, 986–993. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, T.; Ema, C.; Isobe, S.; Fujikura, T.; Ohashi, N.; Kato, A.; Yasuda, H. Prevalence of neural epidermal growth factor-like 1- and exostosin 1/exostosin 2-associated membranous nephropathy: A single-center retrospective study in Japan. Sci. Rep. 2022, 12, 2967. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, T.; Takahashi, H.; Takeuchi, K.; Sakamoto, E.; Tominaga, K.; Sakurabayashi, S.; Abe, T.; Sano, T.; Wada, Y.; Kuwahara, N.; et al. Rare case of exostosin 1/exostosin 2-related membranous lupus nephritis concomitant with dual ANCA- and anti-GBM antibody-associated crescentic glomerulonephritis effectively diagnosed by mass spectrometry: A case report. BMC Nephrol. 2023, 24, 218. [Google Scholar] [CrossRef]
- Miyasaka, R.; Wada, Y.; Takeuchi, K.; Abe, T.; Uchitsubo, R.; Kawamura, S.; Sakurabayashi, S.; Naito, S.; Aoyama, T.; Shimizu, A.; et al. Lupus-like membranous nephropathy during the postpartum period expressing glomerular antigens exostosin 1/exostosin 2 and phospholipase A2 receptor: A case report. CEN Case Rep. 2024, 13, 318–325. [Google Scholar] [CrossRef] [PubMed]
- Hanrotel-Saliou, C.; Segalen, I.; Le Meur, Y.; Youinou, P.; Renaudineau, Y. Glomerular antibodies in lupus nephritis. Clin. Rev. Allergy Immunol. 2011, 40, 151–158. [Google Scholar] [CrossRef]
- Almaani, S.; Meara, A.; Rovin, B.H. Update on Lupus Nephritis. Clin. J. Am. Soc. Nephrol. CJASN 2017, 12, 825–835. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wada, Y.; Okawa, H.; Abe, T.; Takeuchi, K.; Kamata, M.; Takeuchi, E.; Suenaga, T.; Iyoda, M.; Takeuchi, Y. Distinction Between Proliferative Lupus Nephritis and Membranous Lupus Nephritis Based on Inflammation, NETosis, and Glomerular Exostosin. Int. J. Mol. Sci. 2025, 26, 8769. https://doi.org/10.3390/ijms26188769
Wada Y, Okawa H, Abe T, Takeuchi K, Kamata M, Takeuchi E, Suenaga T, Iyoda M, Takeuchi Y. Distinction Between Proliferative Lupus Nephritis and Membranous Lupus Nephritis Based on Inflammation, NETosis, and Glomerular Exostosin. International Journal of Molecular Sciences. 2025; 26(18):8769. https://doi.org/10.3390/ijms26188769
Chicago/Turabian StyleWada, Yukihiro, Hiroyuki Okawa, Tetsuya Abe, Kazuhiro Takeuchi, Mariko Kamata, Emiko Takeuchi, Tadahiro Suenaga, Masayuki Iyoda, and Yasuo Takeuchi. 2025. "Distinction Between Proliferative Lupus Nephritis and Membranous Lupus Nephritis Based on Inflammation, NETosis, and Glomerular Exostosin" International Journal of Molecular Sciences 26, no. 18: 8769. https://doi.org/10.3390/ijms26188769
APA StyleWada, Y., Okawa, H., Abe, T., Takeuchi, K., Kamata, M., Takeuchi, E., Suenaga, T., Iyoda, M., & Takeuchi, Y. (2025). Distinction Between Proliferative Lupus Nephritis and Membranous Lupus Nephritis Based on Inflammation, NETosis, and Glomerular Exostosin. International Journal of Molecular Sciences, 26(18), 8769. https://doi.org/10.3390/ijms26188769





