In Vitro Evaluation of Antimicrobial Amyloidogenic Peptides for the Treatment of Early and Mature Bacterial Biofilms
Abstract
1. Introduction
2. Results
2.1. Determination of Antibacterial Properties of Peptides in Liquid Medium
2.2. Results of Antimicrobial Activity Testing of Peptides on Agar
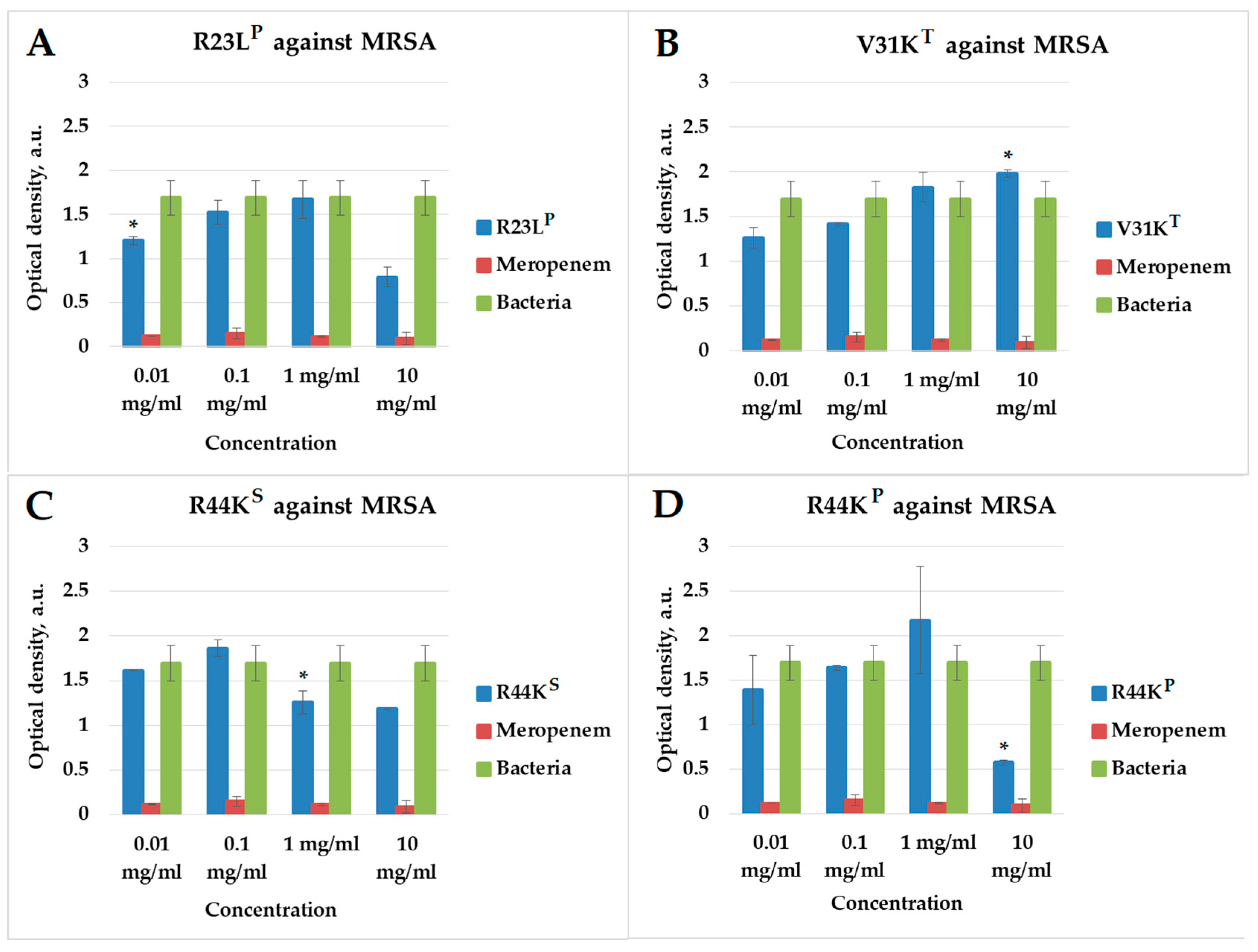
2.3. Effect of Peptides on Early Biofilm Formation
2.4. Effect of Peptides on Mature Biofilm Formation
3. Discussion
4. Materials and Methods
4.1. Peptide Synthesis and Preparation
4.2. Bacterial Strains
4.3. Assessment of Antibacterial Activity of Peptides During Early Stages of Biofilm Formation
4.4. Assessment of Antibacterial Activity of Peptides in Mature Biofilms
4.5. Determination of Antibacterial Activity of Peptides on 0.7% Agar
4.6. Determination of Antibacterial Activity of Peptides in Liquid Medium
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AMP | antimicrobial peptide |
| CPP | cell-penetrating peptide |
| MRSA | methicillin-resistant S. aureus |
References
- Salam, M.A.; Al-Amin, M.Y.; Salam, M.T.; Pawar, J.S.; Akhter, N.; Rabaan, A.A.; Alqumber, M.A.A. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare 2023, 11, 1946. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Chauhan, A.; Ranjan, A.; Mathkor, D.M.; Haque, S.; Ramniwas, S.; Tuli, H.S.; Jindal, T.; Yadav, V. Emerging challenges in antimicrobial resistance: Implications for pathogenic microorganisms, novel antibiotics, and their impact on sustainability. Front. Microbiol. 2024, 15, 1403168. [Google Scholar] [CrossRef]
- MacNair, C.R.; Rutherford, S.T.; Tan, M.-W. Alternative therapeutic strategies to treat antibiotic-resistant pathogens. Nat. Rev. Microbiol. 2024, 22, 262–275. [Google Scholar] [CrossRef]
- Silva, V.; Almeida, L.; Gaio, V.; Cerca, N.; Manageiro, V.; Caniça, M.; Capelo, J.L.; Igrejas, G.; Poeta, P. Biofilm Formation of Multidrug-Resistant MRSA Strains Isolated from Different Types of Human Infections. Pathogens 2021, 10, 970. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Therapeutic Potential of Antimicrobial Peptides in Polymicrobial Biofilm-Associated Infections. Int. J. Mol. Sci. 2021, 22, 482. [Google Scholar] [CrossRef]
- Shahrour, H.; Ferreira, D.A.; Sheridan, L.; Fitzgerald-Hughes, D.; O’Gara, J.P.; Devocelle, M.; Kelly, H.; O’Neill, E. Potent antimicrobial activity of hydrogel loaded with the antimicrobial peptide, D-Bac8c2,5 Leu, against monospecies and polymicrobial biofilms of Staphylococcus aureus and Pseudomonas aeruginosa. Front. Microbiol. 2025, 16, 1571649. [Google Scholar] [CrossRef]
- Talapko, J.; Meštrović, T.; Juzbašić, M.; Tomas, M.; Erić, S.; Horvat Aleksijević, L.; Bekić, S.; Schwarz, D.; Matić, S.; Neuberg, M.; et al. Antimicrobial Peptides—Mechanisms of Action, Antimicrobial Effects and Clinical Applications. Antibiotics 2022, 11, 1417. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Song, Y. Mechanism of Antimicrobial Peptides: Antimicrobial, Anti-Inflammatory and Antibiofilm Activities. Int. J. Mol. Sci. 2021, 22, 11401. [Google Scholar] [CrossRef] [PubMed]
- Böhning, J.; Tarafder, A.K.; Bharat, T.A.M. The role of filamentous matrix molecules in shaping the architecture and emergent properties of bacterial biofilms. Biochem. J. 2024, 481, 245–263. [Google Scholar] [CrossRef]
- Shree, P.; Singh, C.K.; Sodhi, K.K.; Surya, J.N.; Singh, D.K. Biofilms: Understanding the structure and contribution towards bacterial resistance in antibiotics. Med. Microecol. 2023, 16, 100084. [Google Scholar] [CrossRef]
- Singh, S.; Datta, S.; Narayanan, K.B.; Rajnish, K.N. Bacterial exo-polysaccharides in biofilms: Role in antimicrobial resistance and treatments. J. Genet. Eng. Biotechnol. 2021, 19, 140. [Google Scholar] [CrossRef]
- Cavallo, I.; Sivori, F.; Mastrofrancesco, A.; Abril, E.; Pontone, M.; Di Domenico, E.G.; Pimpinelli, F. Bacterial Biofilm in Chronic Wounds and Possible Therapeutic Approaches. Biology 2024, 13, 109. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Yin, R.; Cheng, J.; Lin, J. Bacterial Biofilm Formation on Biomaterials and Approaches to Its Treatment and Prevention. Int. J. Mol. Sci. 2023, 24, 11680. [Google Scholar] [CrossRef] [PubMed]
- Vetrivel, A.; Ramasamy, M.; Vetrivel, P.; Natchimuthu, S.; Arunachalam, S.; Kim, G.-S.; Murugesan, R. Pseudomonas aeruginosa Biofilm Formation and Its Control. Biologics 2021, 1, 312–336. [Google Scholar] [CrossRef]
- Ballén, V.; Cepas, V.; Ratia, C.; Gabasa, Y.; Soto, S.M. Clinical Escherichia coli: From Biofilm Formation to New Antibiofilm Strategies. Microorganisms 2022, 10, 1103. [Google Scholar] [CrossRef]
- Xu, Q.; Hu, X.; Wang, Y. Alternatives to Conventional Antibiotic Therapy: Potential Therapeutic Strategies of Combating Antimicrobial-Resistance and Biofilm-Related Infections. Mol. Biotechnol. 2021, 63, 1103–1124. [Google Scholar] [CrossRef] [PubMed]
- Jalalifar, S.; Razavi, S.; Mirzaei, R.; Irajian, G.; Pooshang Bagheri, K. A hope for ineffective antibiotics to return to treatment: Investigating the anti-biofilm potential of melittin alone and in combination with penicillin and oxacillin against multidrug resistant-MRSA and -VRSA. Front. Microbiol. 2024, 14, 1269392. [Google Scholar] [CrossRef]
- Dar, D.; Dar, N.; Cai, L.; Newman, D.K. Spatial transcriptomics of planktonic and sessile bacterial populations at single-cell resolution. Science 2021, 373, eabi4882. [Google Scholar] [CrossRef]
- Naaz, T.; Lahiri, D.; Pandit, S.; Nag, M.; Gupta, P.K.; Al-Dayan, N.; Rai, N.; Chaubey, K.K.; Gupta, A.K. Antimicrobial Peptides Against Microbial Biofilms: Efficacy, Challenges, and Future Prospect. Int. J. Pept. Res. Ther. 2023, 29, 48. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Antibiofilm peptides against oral biofilms. J. Oral Microbiol. 2017, 9, 1327308. [Google Scholar] [CrossRef]
- Di Martino, P. Extracellular polymeric substances, a key element in understanding biofilm phenotype. AIMS Microbiol. 2018, 4, 274–288. [Google Scholar] [CrossRef] [PubMed]
- Ploux, L.; Ponche, A.; Anselme, K. Bacteria/Material Interfaces: Role of the Material and Cell Wall Properties. J. Adhes. Sci. Technol. 2010, 24, 2165–2201. [Google Scholar] [CrossRef]
- Akbey, Ü.; Andreasen, M. Functional amyloids from bacterial biofilms—Structural properties and interaction partners. Chem. Sci. 2022, 13, 6457–6477. [Google Scholar] [CrossRef] [PubMed]
- Byeon, C.-H.; Wang, P.C.; Byeon, I.-J.L.; Akbey, Ü. Solution-state NMR assignment and secondary structure propensity of the full length and minimalistic-truncated prefibrillar monomeric form of biofilm forming functional amyloid FapC from Pseudomonas aeruginosa. Biomol. NMR Assign. 2023, 17, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Schwartz, K.; Syed, A.K.; Stephenson, R.E.; Rickard, A.H.; Boles, B.R. Functional Amyloids Composed of Phenol Soluble Modulins Stabilize Staphylococcus aureus Biofilms. PLoS Pathog. 2012, 8, e1002744. [Google Scholar] [CrossRef]
- Dueholm, M.S.; Søndergaard, M.T.; Nilsson, M.; Christiansen, G.; Stensballe, A.; Overgaard, M.T.; Givskov, M.; Tolker-Nielsen, T.; Otzen, D.E.; Nielsen, P.H. Expression of Fap amyloids in Pseudomonas aeruginosa, P. fluorescens, and P. putida results in aggregation and increased biofilm formation. Microbiologyopen 2013, 2, 365–382. [Google Scholar] [CrossRef]
- Chapman, M.R.; Robinson, L.S.; Pinkner, J.S.; Roth, R.; Heuser, J.; Hammar, M.; Normark, S.; Hultgren, S.J. Role of Escherichia coli Curli Operons in Directing Amyloid Fiber Formation. Science 2002, 295, 851–855. [Google Scholar] [CrossRef]
- Matilla-Cuenca, L.; Toledo-Arana, A.; Valle, J. Anti-Biofilm Molecules Targeting Functional Amyloids. Antibiotics 2021, 10, 795. [Google Scholar] [CrossRef]
- Chen, D.; Liu, X.; Chen, Y.; Lin, H. Amyloid peptides with antimicrobial and/or microbial agglutination activity. Appl. Microbiol. Biotechnol. 2022, 106, 7711–7720. [Google Scholar] [CrossRef]
- Petersen, E.N.; Pavel, M.A.; Wang, H.; Hansen, S.B. Disruption of palmitate-mediated localization; a shared pathway of force and anesthetic activation of TREK-1 channels. Biochim. Biophys. Acta—Biomembr. 2020, 1862, 183091. [Google Scholar] [CrossRef]
- Walter, J.-C.; Kissmann, A.-K.; Gruber, D.; Alpízar-Pedraza, D.; Martell-Huguet, E.M.; Preising, N.; Rodriguez-Alfonso, A.; Ständker, L.; Kleber, C.; Knoll, W.; et al. Antimicrobial Activity of the Peptide C14R Against Ab Initio Growing and Preformed Biofilms of Candida albicans, Candida parapsilosis and Candidozyma auris. Biomolecules 2025, 15, 322. [Google Scholar] [CrossRef]
- Sauer, K.; Stoodley, P.; Goeres, D.M.; Hall-Stoodley, L.; Burmølle, M.; Stewart, P.S.; Bjarnsholt, T. The biofilm life cycle: Expanding the conceptual model of biofilm formation. Nat. Rev. Microbiol. 2022, 20, 608–620. [Google Scholar] [CrossRef]
- Su, Y.; Yrastorza, J.T.; Matis, M.; Cusick, J.; Zhao, S.; Wang, G.; Xie, J. Biofilms: Formation, Research Models, Potential Targets, and Methods for Prevention and Treatment. Adv. Sci. 2022, 9, 2203291. [Google Scholar] [CrossRef] [PubMed]
- Grishin, S.Y.; Dzhus, U.F.; Glukhov, A.S.; Selivanova, O.M.; Surin, A.K.; Galzitskaya, O.V. Identification of Amyloidogenic Regions in Pseudomonas aeruginosa Ribosomal S1 Protein. Int. J. Mol. Sci. 2021, 22, 7291. [Google Scholar] [CrossRef] [PubMed]
- Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Panfilov, A.V.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Glyakina, A.V.; et al. Multiple Antimicrobial Effects of Hybrid Peptides Synthesized Based on the Sequence of Ribosomal S1 Protein from Staphylococcus aureus. Int. J. Mol. Sci. 2022, 23, 524. [Google Scholar] [CrossRef] [PubMed]
- Vivès, E.; Brodin, P.; Lebleu, B. A Truncated HIV-1 Tat Protein Basic Domain Rapidly Translocates through the Plasma Membrane and Accumulates in the Cell Nucleus. J. Biol. Chem. 1997, 272, 16010–16017. [Google Scholar] [CrossRef]
- Derossi, D.; Joliot, A.H.; Chassaing, G.; Prochiantz, A. The third helix of the Antennapedia homeodomain translocates through biological membranes. J. Biol. Chem. 1994, 269, 10444–10450. [Google Scholar] [CrossRef]
- Surin, A.K.; Malykhina, A.I.; Slizen, M.V.; Kochetov, A.P.; Suvorina, M.Y.; Biryulyov, V.E.; Grishin, S.Y.; Galzitskaya, O.V. Proteomic Analysis of Thermus thermophilus Cells after Treatment with Antimicrobial Peptide. Bacteria 2024, 3, 299–313. [Google Scholar] [CrossRef]
- Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Vershinin, N.A.; Gurina, E.V.; Zakharova, A.A.; Azev, V.N.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; et al. Enhancing the Antimicrobial Properties of Peptides through Cell-Penetrating Peptide Conjugation: A Comprehensive Assessment. Int. J. Mol. Sci. 2023, 24, 16723. [Google Scholar] [CrossRef]
- Kravchenko, S.V.; Domnin, P.A.; Grishin, S.Y.; Zakhareva, A.P.; Zakharova, A.A.; Mustaeva, L.G.; Gorbunova, E.Y.; Kobyakova, M.I.; Surin, A.K.; Poshvina, D.V.; et al. Optimizing Antimicrobial Peptide Design: Integration of Cell-Penetrating Peptides, Amyloidogenic Fragments, and Amino Acid Residue Modifications. Int. J. Mol. Sci. 2024, 25, 6030. [Google Scholar] [CrossRef]
- Pokharel, K.; Dawadi, B.R.; Shrestha, L.B. Role of Biofilm in Bacterial Infection and Antimicrobial Resistance. J. Nepal Med. Assoc. 2022, 60, 836–840. [Google Scholar] [CrossRef]
- Gondil, V.S.; Subhadra, B. Biofilms and their role on diseases. BMC Microbiol. 2023, 23, 203. [Google Scholar] [CrossRef]
- Xu, Z.; Liang, Y.; Lin, S.; Chen, D.; Li, B.; Li, L.; Deng, Y. Crystal Violet and XTT Assays on Staphylococcus aureus Biofilm Quantification. Curr. Microbiol. 2016, 73, 474–482. [Google Scholar] [CrossRef]
- Grela, E.; Kozłowska, J.; Grabowiecka, A. Current methodology of MTT assay in bacteria—A review. Acta Histochem. 2018, 120, 303–311. [Google Scholar] [CrossRef]
- Batoni, G.; Maisetta, G.; Esin, S. Antimicrobial peptides and their interaction with biofilms of medically relevant bacteria. Biochim. Biophys. Acta—Biomembr. 2016, 1858, 1044–1060. [Google Scholar] [CrossRef] [PubMed]
- Laverty, G.; Gorman, S.; Gilmore, B. Biomolecular Mechanisms of Pseudomonas aeruginosa and Escherichia coli Biofilm Formation. Pathogens 2014, 3, 596–632. [Google Scholar] [CrossRef]
- Mirani, Z.A.; Fatima, A.; Urooj, S.; Aziz, M.; Khan, M.N.; Abbas, T. Relationship of cell surface hydrophobicity with biofilm formation and growth rate: A study on Pseudomonas aeruginosa, Staphylococcus aureus, and Escherichia coli. Iran. J. Basic Med. Sci. 2018, 21, 760–769. [Google Scholar] [CrossRef]
- Hernández-Cuellar, E.; Tsuchiya, K.; Valle-Ríos, R.; Medina-Contreras, O. Differences in Biofilm Formation by Methicillin-Resistant and Methicillin-Susceptible Staphylococcus aureus Strains. Diseases 2023, 11, 160. [Google Scholar] [CrossRef]
- Berditsch, M.; Afonin, S.; Vladimirova, T.; Wadhwani, P.; Ulrich, A.S. Antimicrobial Peptides can Enhance the Risk of Persistent Infections. Front. Immunol. 2012, 3, 222. [Google Scholar] [CrossRef] [PubMed]
- Vasilchenko, A.S.; Rogozhin, E.A. Sub-inhibitory Effects of Antimicrobial Peptides. Front. Microbiol. 2019, 10, 1160. [Google Scholar] [CrossRef] [PubMed]
- Galzitskaya, O.V.; Kravchenko, S.V.; Grishin, S.Y.; Zakhareva, A.P.; Mustaeva, L.G.; Gorbunova, E.Y.; Surin, A.K.; Azev, V.N. Combinatorial Effects of CPP-Modified Antimicrobial Peptides: Synergistic and Additive Interactions Against Pathogenic Bacteria. Int. J. Mol. Sci. 2025, 26, 5968. [Google Scholar] [CrossRef] [PubMed]
| Peptide | MRSA (SA 180-F Strain) | S. aureus (129B Strain) | E. coli (MG1655 Strain) | P. aeruginosa (2943 Strain) |
|---|---|---|---|---|
| R23IT | ↓ (1 mg/mL) | ↑ | ↑ | ↓ (10 mg/mL) |
| R23LP | ↓ (0.1 mg/mL) | ↓ (1 mg/mL) | ↓ (0.1 mg/mL) | ↓ (10 mg/mL) |
| V31KT | ↓ (1 mg/mL) | − | − | − |
| R44KS | ↓ (0.1 mg/mL) | ↓ (10 mg/mL) | ↓ (0.1 mg/mL) | ↓ (10 mg/mL) |
| R44KP | ↓ (0.1 mg/mL) | ↑ | ↓ (0.1 mg/mL) | ↓ (10 mg/mL) |
| V31KS | ↓ (0.1 mg/mL) | ↑ | ↓ (0.1 mg/mL) | ↓ (10 mg/mL) |
| I31KP | ↓ (1 mg/mL) | ↓ (0.1 mg/mL) | − | ↑ |
 ↓—Decrease in the parameter (optical density) compared to the negative control.
↓—Decrease in the parameter (optical density) compared to the negative control.  −—No decrease in the parameter.
−—No decrease in the parameter.  ↑—Reverse effect (increase in the optical density compared to the control).
↑—Reverse effect (increase in the optical density compared to the control).| Peptide | Concentration | MRSA (SA 180-F Strain) | S. aureus (129B Strain) | E. coli (MG1655 Strain) | P. aeruginosa (2943 Strain) |
|---|---|---|---|---|---|
| R23IT | 1 mg/mL | + | + | − | + |
| 0.1 mg/mL | − | − | − | − | |
| 0.01 mg/mL | − | − | − | − | |
| 0.001 mg/mL | − | − | − | − | |
| R23LP | 1 mg/mL | + | + | + | + |
| 0.1 mg/mL | − | − | − | − | |
| 0.01 mg/mL | − | − | − | − | |
| 0.001 mg/mL | − | − | − | − | |
| V31KT | 1 mg/mL | − | − | − | − |
| 0.1 mg/mL | − | − | − | − | |
| 0.01 mg/mL | − | − | − | − | |
| 0.001 mg/mL | − | − | − | − | |
| R44KS | 1 mg/mL | + | + | − | + |
| 0.1 mg/mL | − | − | − | − | |
| 0.01 mg/mL | − | − | − | − | |
| 0.001 mg/mL | − | − | − | − | |
| R44KP | 1 mg/mL | − | + | − | − |
| 0.1 mg/mL | − | − | − | − | |
| 0.01 mg/mL | − | − | − | − | |
| 0.001 mg/mL | − | − | − | − | |
| V31KS | 1 mg/mL | − | − | + | − |
| 0.1 mg/mL | − | − | − | − | |
| 0.01 mg/mL | − | − | − | − | |
| 0.001 mg/mL | − | − | − | − |
| Peptide | MRSA (SA 180-F Strain) | S. aureus (129B Strain) | E. coli (MG1655 Strain) | P. aeruginosa (2943 Strain) |
|---|---|---|---|---|
| After crystal violet staining | ||||
| R23IT | − | ↑ | ↑ | − |
| R23LP | ↓ (10 mg/mL) | ↑ | ↑ | ↓ (10 mg/mL) |
| V31KT | ↑ | ↑ | ↑ | − |
| R44KS | ↓ (1 mg/mL) | ↑ | ↑ | ↓ (10 mg/mL) |
| R44KP | ↓ (10 mg/mL) | ↑ | ↑ | ↓ (10 mg/mL) |
| V31KS | ↓ (10 mg/mL) | ↑ | ↑ | ↓ (10 mg/mL) |
| I31KP | − | ↑ | ↑ | − |
| After MTT assay | ||||
| R23IT | ↑ | ↑ | ↑ | − |
| R23LP | ↑ | ↑ | ↑ | ↑ |
| V31KT | ↑ | ↑ | − | − |
| R44KS | ↑ | ↑ | − | − |
| R44KP | ↑ | ↑ | ↑ | ↓ (10 mg/mL) |
| V31KS | ↑ | ↑ | − | ↓ (10 mg/mL) |
| I31KP | ↑ | ↑ | ↑ | − |
 ↓—Decrease in the parameter (optical density) compared to the negative control.
↓—Decrease in the parameter (optical density) compared to the negative control.  −—No decrease in the optical density.
−—No decrease in the optical density.  ↑—Reverse effect (increase in the optical density compared to the control).
↑—Reverse effect (increase in the optical density compared to the control).| Peptide | MRSA (SA 180-F Strain) | S. aureus (129B Strain) | E. coli (MG1655 Strain) | P. aeruginosa (2943 Strain) |
|---|---|---|---|---|
| After crystal violet staining | ||||
| R23IT | − | − | − | − |
| R23LP | − | ↑ | ↑ | ↑ |
| V31KT | − | ↑ | ↑ | ↑ |
| R44KS | ↑ | ↑ | ↑ | ↑ |
| R44KP | ↑ | ↑ | − | − |
| V31KS | ↑ | ↑ | − | ↑ |
| I31KP | ↑ | ↑ | − | ↑ |
| After MTT assay | ||||
| R23IT | − | − | ↑ | − |
| R23LP | ↓ (0.01 mg/mL) | − | − | ↑ |
| V31KT | ↓ (0.01 mg/mL) | − | − | ↑ |
| R44KS | ↓ (0.01 mg/mL) | − | ↑ | ↑ |
| R44KP | ↓ (0.01 mg/mL) | − | ↑ | − |
| V31KS | ↓ (0.01 mg/mL) | − | ↑ | − |
| I31KP | ↓ (0.01 mg/mL) | ↓ (0.01 mg/mL) | − | ↑ |
 ↓—Decrease in the parameter (optical density) compared to the negative control.
↓—Decrease in the parameter (optical density) compared to the negative control.  −—No decrease in the optical density.
−—No decrease in the optical density.  ↑—Reverse effect (increase in the optical density compared to the control).
↑—Reverse effect (increase in the optical density compared to the control).Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Domnin, P.A.; Grishin, S.Y.; Surin, A.K.; Ermolaeva, S.A.; Galzitskaya, O.V. In Vitro Evaluation of Antimicrobial Amyloidogenic Peptides for the Treatment of Early and Mature Bacterial Biofilms. Int. J. Mol. Sci. 2025, 26, 8767. https://doi.org/10.3390/ijms26188767
Domnin PA, Grishin SY, Surin AK, Ermolaeva SA, Galzitskaya OV. In Vitro Evaluation of Antimicrobial Amyloidogenic Peptides for the Treatment of Early and Mature Bacterial Biofilms. International Journal of Molecular Sciences. 2025; 26(18):8767. https://doi.org/10.3390/ijms26188767
Chicago/Turabian StyleDomnin, Pavel A., Sergei Y. Grishin, Alexey K. Surin, Svetlana A. Ermolaeva, and Oxana V. Galzitskaya. 2025. "In Vitro Evaluation of Antimicrobial Amyloidogenic Peptides for the Treatment of Early and Mature Bacterial Biofilms" International Journal of Molecular Sciences 26, no. 18: 8767. https://doi.org/10.3390/ijms26188767
APA StyleDomnin, P. A., Grishin, S. Y., Surin, A. K., Ermolaeva, S. A., & Galzitskaya, O. V. (2025). In Vitro Evaluation of Antimicrobial Amyloidogenic Peptides for the Treatment of Early and Mature Bacterial Biofilms. International Journal of Molecular Sciences, 26(18), 8767. https://doi.org/10.3390/ijms26188767






