A Systematic Literature Review of Reproductive Toxicological Studies on Phthalates
Abstract
1. Introduction
2. Methodology
2.1. Eligibility Criteria
- Language: Studies not published in English.
- Studies with non-conserved reproductive mechanisms:
- o
- In vitro studies using animal cell lines.
- o
- In vivo studies employing animal models where experimental endpoints lack translational validity for human reproductive health (e.g., zebrafish (Danio rerio) and nematodes (C. elegans)).
- Co-exposure: Studies investigating phthalate exposure in combination with other chemicals.
- Irrelevant outcomes: Studies that did not directly report on fertility, reproductive health, or reproductive toxicity outcomes, such as research on gestational diabetes, fetal development, or pregnancy outcomes.
- Non-original research: Case reports, case series, conference papers, editorials, opinion pieces, letters to the editor, reviews, and meta-analyses.
2.2. Search Strategy
2.3. Study Selection Process
3. Results
3.1. Overview of Selected Studies
3.2. In Vivo Studies
| Animal Model | Phthalate(s) Used | Exposure (Dose, ROA, Duration) | Main Effects | Reference |
|---|---|---|---|---|
| Four-week-old female ICR mice | DEHP | 0, 500, 1000, 1500 mg/kg/day by gavage for 30 days |
| [54] |
| Four-week-old female Swiss Albino mice | DEP | 1500 mg/kg/day by gavage for 56 days |
| [55] |
| Twenty-eight-day-old female CD-1 mice | DBP | 10, 100, 1000 mg/kg/day orally for 10, 20, or 30 days |
| [56] |
| Thirty-three-day-old female CD-1 mice | DEHP, DiNP | 0.15, 1.5, 1500 ppm for each, orally for 1 month and 6 months |
| [51] |
| Six-week-old female CD-1 mice | DEHP, DiNP, (DEHP, DiNP, BBP, DBP, DiBP, DEP) mixture | 0.15, 1.5, and 1500 ppm, dietary exposure via rodent chow for 11 months |
| [52] |
| Newborn male ICR mice | DEHP | 30, 500 mg/kg/day orally from birth to postnatal day 21 |
| [53] |
| Eight-week-old male C57BL/6N mice | DBP | 0, 10, 100 mg/kg/day by gavage for 5 weeks |
| [54] |
| Eight- to nine-week-old male C57BL/6J mice | DBP, DEHP, (DBP + DEHP) mixture | 2.5 mg/kg/day for each group by subcutaneous osmotic pumps for 40 days |
| [55] |
| Male Sprague-Dawley rats | DEHP, DBP, BBP mixture | 16 mg/kg/day orally for 91 days |
| [47] |
| Twenty-eight-day-old male Fischer CDF344 rats | MEHP | 700 mg/kg single dose by gavage once | Increased MHC-II+ peritubular macrophages (CD68+) and PLZF+ spermatogonia, indicating immune activation and potential spermatogenesis disruption. | [56] |
3.3. In Vitro Studies
| Cell Line(s)/Sample(s) | Human Tissue Type | Phthalate(s) Used | Concentration and Time of Exposure | Main Effects | Reference |
|---|---|---|---|---|---|
| KGN | Ovarian Granulosa Tumor | MEHP | 0-800 μM for 24 h |
| [48] |
| KGN | Ovarian Granulosa Tumor | MEHP | 0–200 μM for 24 h |
| [39] |
| KGN | Ovarian Granulosa Tumor | DEHP | 0.01, 0.1, 1, 10 µM for 24 h |
| [62] |
| H295R | Adrenocortical Cancer | DBP, MBP | 0, 1, 10, 100, 500 µM each for 48 h |
| [59] |
| A2780, OVCAR5 | Ovarian Adenocarcinoma | DEHP | 0, 1, 10, 100 μg/mL (time not stated) |
| [63] |
| PNT1A | Prostate | DBP | 10−12 M to 10−6 M for 30 min, 2 h, 4 h |
| [58] |
| Granulosa Cells | Ovary | DEP, DEHP, DBP, DiNP, DiBP, BBzP mixture | 1, 10, 100, 500 μg/mL 24–48 h pre-hCG treatment, followed by 0, 6, 12, 24, or 36 h post-hCG |
| [64] |
| Human Ovarian Tissue; KGN, COV434, PA-1, Ovarian Primary Cells | Ovarian Granulosa Tumor: KGN, COV434 Ovarian Germ Cell Tumor: PA-1 Ovary: Human Ovarian Primary Cells | MEHP | 0.1X, 1X, 10X, 100X, 1000X (2.05 nM–20.51 mM) for 7 days |
| [57] |
| Human Sperm Cells | Semen | DBP, DnOP, and (DMP, DEP, BBP, DEHP, DnOP) mixture | DBP, DnOP: from 1 ng/mL to 1000 ng/mL for 2 h Mixture: 100 ng/mL for 2 h |
| [60] |
| Granulosa Cells | Ovary | DEHP | 50, 100, 200 μM for 24 h and 48 h |
| [65] |
| Human Sperm Cells | Semen | DEHP, MEHP, (DEHP, MEHP) mixtures | DEHP: 20 nM, 200 nM, 2 µM, 4 µM, 8 µM, MEHP: 1 nM, 10 nM, 100 nM, 1 µM, 20 µM for 1, 2, 4 h |
| [61] |
3.4. Epidemiological Studies
4. Discussion
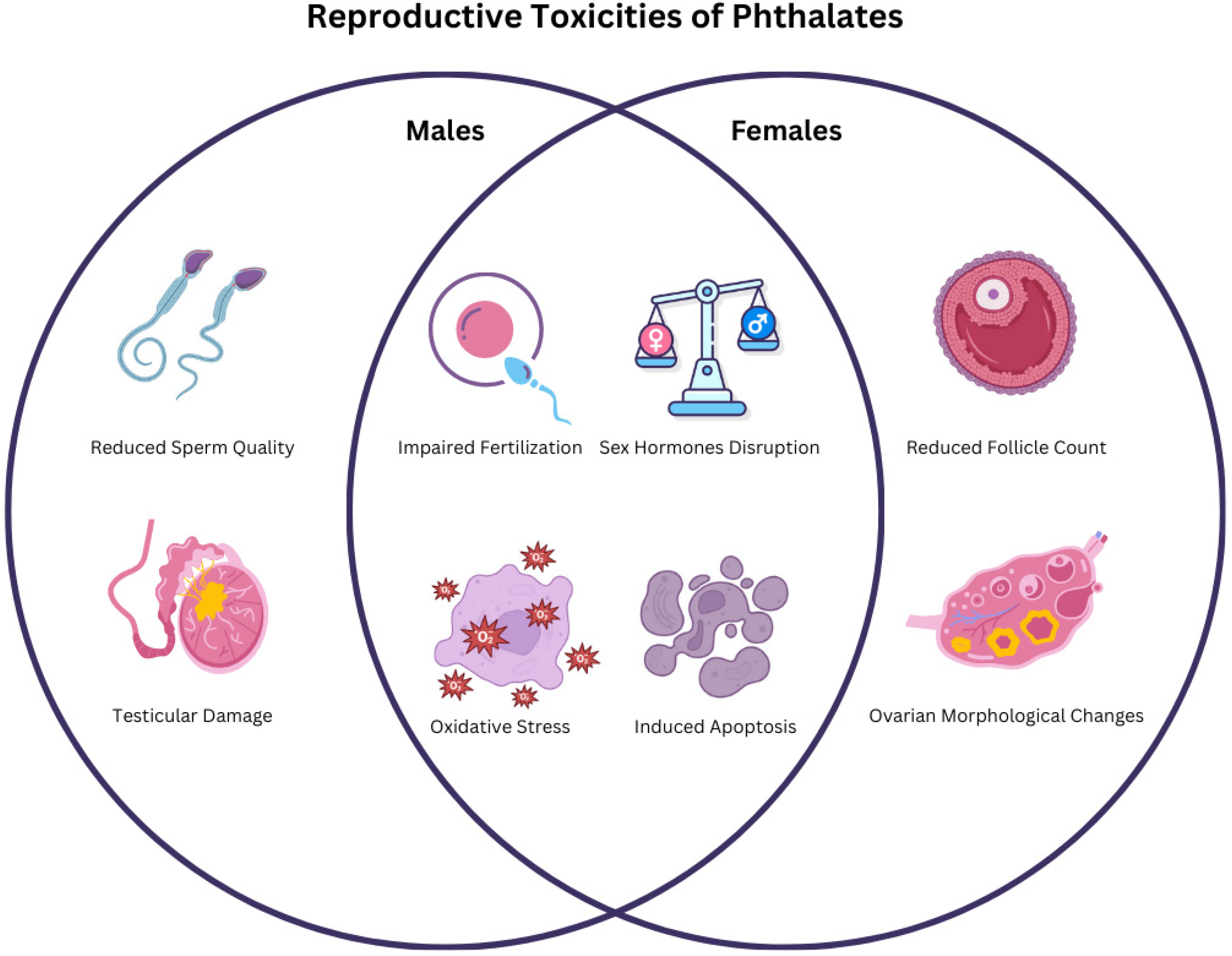
4.1. Synthesis of the Key Reproductive Toxicities of Phthalates
4.2. Strengths and Limitations of the Systematic Review
4.3. Research Gaps and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AAPs | Anti-Androgenic Phthalates |
| AMH | Anti-Müllerian Hormone |
| AR | Androgen Receptor |
| AFC | Antral Follicle Count |
| BTB | Blood–Testis Barrier |
| CAS | Chemical Abstracts Service |
| CI | Confidence Interval |
| COR | Cortisol |
| CORT | Corticosterone |
| CYP | Cytochrome P450 enzymes (e.g., CYP11A1, CYP17A1) |
| dbcAMP | Dibutyryl Cyclic AMP |
| DEGs | Differentially Expressed Genes |
| DUB | Deubiquitinating Enzyme |
| EDCs | Endocrine-Disrupting Chemicals |
| E2 | Estradiol |
| EPA | U.S. Environmental Protection Agency |
| ECHA | European Chemicals Agency |
| ERα | Estrogen Receptor alpha |
| EV-miRNAs | Extracellular Vesicle microRNAs |
| FF | Follicular Fluid |
| FSH | Follicle-Stimulating Hormone |
| GCs | Granulosa Cells |
| GSH | Glutathione |
| HMW | High Molecular Weight |
| IVF | In Vitro Fertilization |
| ICSI | Intracytoplasmic Sperm Injection |
| LMW | Low Molecular Weight |
| LH | Luteinizing Hormone |
| logP | Lipophilicity Index |
| MDA | Malondialdehyde |
| ML | Machine Learning |
| NF-κB | Nuclear Factor kappa B |
| NLRP3 | NLR Family Pyrin Domain Containing 3 |
| OR | Odds Ratio |
| PAEs | Phthalic Acid Esters |
| PCOS | Polycystic Ovary Syndrome |
| PCO | Polycystic Ovaries |
| PLA2 | Phospholipase A2 |
| PIWI | P-element Induced WImpy Testis Proteins (e.g., PIWIL1, PIWIL2) |
| POF | Premature Ovarian Failure |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PVC | Polyvinyl Chloride |
| ROA | Route of Administration |
| ROS | Reactive Oxygen Species |
| SAR | Structure–Activity Relationship |
| SEA | Sperm Epigenetic Aging |
| SOD | Superoxide Dismutase |
| StAR | Steroidogenic Acute Regulatory Protein |
| T | Testosterone |
| T-AOC | Total Antioxidant Capacity |
References
- Long, F.; Ren, Y.; Bi, F.; Wu, Z.; Zhang, H.; Li, J.; Gao, R.; Liu, Z.; Li, H. Contamination Characterization, Toxicological Properties, and Health Risk Assessment of Bisphenols in Multiple Media: Current Research Status and Future Perspectives. Toxics 2025, 13, 109. [Google Scholar] [CrossRef]
- Chianese, T.; Trinchese, G.; Leandri, R.; De Falco, M.; Mollica, M.P.; Scudiero, R.; Rosati, L. Glyphosate Exposure Induces Cytotoxicity, Mitochondrial Dysfunction and Activation of ERα and ERβ Estrogen Receptors in Human Prostate PNT1A Cells. Int. J. Mol. Sci. 2024, 25, 7039. [Google Scholar] [CrossRef]
- De Falco, M.; Laforgia, V. Combined Effects of Different Endocrine-Disrupting Chemicals (EDCs) on Prostate Gland. Int. J. Environ. Res. Public Health 2021, 18, 9772. [Google Scholar] [CrossRef] [PubMed]
- Combarnous, Y.; Nguyen, T.M.D. Comparative Overview of the Mechanisms of Action of Hormones and Endocrine Disruptor Compounds. Toxics 2019, 7, 5. [Google Scholar] [CrossRef]
- Kahn, L.G.; Philippat, C.; Nakayama, S.F.; Slama, R.; Trasande, L. Endocrine-disrupting chemicals: Implications for human health. Lancet Diabetes Endocrinol. 2020, 8, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Gong, Y.; Jiang, Q.; Yu, Y.; Zhang, J. Environmental endocrine disruptors and pregnane X receptor action: A review. Food Chem. Toxicol. 2023, 179, 113976. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Lee, Y.-S.; Moon, H.-B. Occurrence, distribution, and sources of phthalates and non-phthalate plasticizers in sediment from semi-enclosed bays of Korea. Mar. Pollut. Bull. 2020, 151, 110824. [Google Scholar] [CrossRef]
- U.S. Food and Drug Administration. Phthalates in Cosmetics; U.S. Food and Drug Administration: Silver Spring, MD, USA, 2022. [Google Scholar]
- Kashyap, D.; Agarwal, T. Concentration and factors affecting the distribution of phthalates in the air and dust: A global scenario. Sci. Total Environ. 2018, 635, 817–827. [Google Scholar] [CrossRef]
- Chang, W.-H.; Herianto, S.; Lee, C.-C.; Hung, H.; Chen, H.-L. The effects of phthalate ester exposure on human health: A review. Sci. Total Environ. 2021, 786, 147371. [Google Scholar] [CrossRef]
- Arrigo, F.; Impellitteri, F.; Piccione, G.; Faggio, C. Phthalates and their effects on human health: Focus on erythrocytes and the reproductive system. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 270, 109645. [Google Scholar] [CrossRef]
- Naveen, K.V.; Saravanakumar, K.; Zhang, X.; Sathiyaseelan, A.; Wang, M.-H. Impact of environmental phthalate on human health and their bioremediation strategies using fungal cell factory—A review. Environ. Res. 2022, 214, 113781. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Sahu, M. Phthalate pollution and remediation strategies: A review. J. Hazard. Mater. Adv. 2022, 6, 100065. [Google Scholar] [CrossRef]
- Wang, Y.; Qian, H. Phthalates and Their Impacts on Human Health. Healthcare 2021, 9, 603. [Google Scholar] [CrossRef]
- Alternatives to Certain Phthalates Partnership | US EPA. Available online: https://www.epa.gov/saferchoice/alternatives-certain-phthalates-partnership (accessed on 11 April 2025).
- Phthalates—ECHA. Available online: https://echa.europa.eu/hot-topics/phthalates (accessed on 11 April 2025).
- Turan, S. Endocrine disrupting chemicals and bone. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101495. [Google Scholar] [CrossRef]
- CHMP. Committee for Medicinal Products for Human Use (CHMP) Guideline on the Use of Phthalates as Excipients in Human Medicinal Products. 2014. Available online: www.ema.europa.eu/contact (accessed on 11 April 2025).
- Giuliani, A.; Zuccarini, M.; Cichelli, A.; Khan, H.; Reale, M. Critical Review on the Presence of Phthalates in Food and Evidence of Their Biological Impact. Int. J. Environ. Res. Public Health 2020, 17, 5655. [Google Scholar] [CrossRef] [PubMed]
- Miranda, C.V.; Gama, M.R.; Pizzolato, T.M. Analytical methodologies for the determination of phthalates in environmental matrices. Trends Environ. Anal. Chem. 2025, 45, e00248. [Google Scholar] [CrossRef]
- Corea-Téllez, K.S.; Bustamante-Montes, P.; García-Fábila, M.; Hernández-Valero, M.A.; Vázquez-Moreno, F. Estimated risks of water and saliva contamination by phthalate diffusion from plasticized polyvinyl chloride. J. Environ. Health 2008, 71, 34–39, 45. [Google Scholar]
- Yen, T.-H.; Lin-Tan, D.-T.; Lin, J.-L. Food safety involving ingestion of foods and beverages prepared with phthalate-plasticizer-containing clouding agents. J. Formos. Med. Assoc. 2011, 110, 671–684. [Google Scholar] [CrossRef]
- Koch, H.M.; Rüther, M.; Schütze, A.; Conrad, A.; Pälmke, C.; Apel, P.; Brüning, T.; Kolossa-Gehring, M. Phthalate metabolites in 24-h urine samples of the German Environmental Specimen Bank (ESB) from 1988 to 2015 and a comparison with US NHANES data from 1999 to 2012. Int. J. Hyg. Environ. Health 2017, 220, 130–141. [Google Scholar] [CrossRef]
- Fréry, N.; Santonen, T.; Porras, S.P.; Fucic, A.; Leso, V.; Bousoumah, R.; Duca, R.C.; El Yamani, M.; Kolossa-Gehring, M.; Ndaw, S.; et al. Biomonitoring of occupational exposure to phthalates: A systematic review. Int. J. Hyg. Environ. Health 2020, 229, 113548. [Google Scholar] [CrossRef]
- Ventrice, P.; Ventrice, D.; Russo, E.; De Sarro, G. Phthalates: European regulation, chemistry, pharmacokinetic and related toxicity. Environ. Toxicol. Pharmacol. 2013, 36, 88–96. [Google Scholar] [CrossRef]
- Isobe, T.; Ohkawara, S.; Mori, Y.; Jinno, H.; Tanaka-Kagawa, T.; Hanioka, N. Hydrolysis of dibutyl phthalate and di(2-ethylhexyl) phthalate in human liver, small intestine, kidney, and lung: An in vitro analysis using organ subcellular fractions and recombinant carboxylesterases. Chem.-Biol. Interact. 2023, 372, 110353. [Google Scholar] [CrossRef]
- Heudorf, U.; Mersch-Sundermann, V.; Angerer, J. Phthalates: Toxicology and exposure. Int. J. Hyg. Environ. Health 2007, 210, 623–634. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, H.; Kannan, K. A Review of Biomonitoring of Phthalate Exposures. Toxics 2019, 7, 21. [Google Scholar] [CrossRef]
- Kay, V.R.; Chambers, C.; Foster, W.G. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol. 2013, 43, 200–219. [Google Scholar] [CrossRef] [PubMed]
- Gore, A.C.; Chappell, V.A.; Fenton, S.E.; Flaws, J.A.; Nadal, A.; Prins, G.S.; Toppari, J.; Zoeller, R.T. Executive Summary to EDC-2: The Endocrine Society’s Second Scientific Statement on Endocrine-Disrupting Chemicals. Endocr. Rev. 2015, 36, 593–602. [Google Scholar] [CrossRef] [PubMed]
- Mariana, M.; Feiteiro, J.; Verde, I.; Cairrao, E. The effects of phthalates in the cardiovascular and reproductive systems: A review. Environ. Int. 2016, 94, 758–776. [Google Scholar] [CrossRef]
- Grindler, N.M.; Vanderlinden, L.; Karthikraj, R.; Kannan, K.; Teal, S.; Polotsky, A.J.; Powell, T.L.; Yang, I.V.; Jansson, T. Exposure to Phthalate, an Endocrine Disrupting Chemical, Alters the First Trimester Placental Methylome and Transcriptome in Women. Sci. Rep. 2018, 8, 6086. [Google Scholar] [CrossRef]
- Sedha, S.; Lee, H.; Singh, S.; Kumar, S.; Jain, S.; Ahmad, A.; Bin Jardan, Y.A.; Sonwal, S.; Shukla, S.; Simal-Gandara, J.; et al. Reproductive toxic potential of phthalate compounds—State of art review. Pharmacol. Res. 2021, 167, 105536. [Google Scholar] [CrossRef] [PubMed]
- Mesquita, I.; Lorigo, M.; Cairrao, E. Update about the disrupting-effects of phthalates on the human reproductive system. Mol. Reprod. Dev. 2021, 88, 650–672. [Google Scholar] [CrossRef]
- Axelsson, J.; Rylander, L.; Rignell-Hydbom, A.; Jönsson, B.A.G.; Lindh, C.H.; Giwercman, A. Phthalate exposure and reproductive parameters in young men from the general Swedish population. Environ. Int. 2015, 85, 54–60. [Google Scholar] [CrossRef]
- Woodward, M.J.; Obsekov, V.; Jacobson, M.H.; Kahn, L.G.; Trasande, L. Phthalates and Sex Steroid Hormones Among Men From NHANES, 2013–2016. J. Clin. Endocrinol. Metab. 2020, 105, e1225–e1234. [Google Scholar] [CrossRef]
- Hlisníková, H.; Petrovičová, I.; Kolena, B.; Šidlovská, M.; Sirotkin, A. Effects and Mechanisms of Phthalates’ Action on Reproductive Processes and Reproductive Health: A Literature Review. Int. J. Environ. Res. Public Health 2020, 17, 6811. [Google Scholar] [CrossRef]
- Panagiotou, E.M.; Ojasalo, V.; Damdimopoulou, P. Phthalates, ovarian function and fertility in adulthood. Best Pract. Res. Clin. Endocrinol. Metab. 2021, 35, 101552. [Google Scholar] [CrossRef]
- CPSC Prohibits Certain Phthalates in Children’s Toys and Child Care Products | CPSC.gov. Available online: https://www.cpsc.gov/Newsroom/News-Releases/2018/CPSC-Prohibits-Certain-Phthalates-in-Childrens-Toys-and-Child-Care-Products (accessed on 11 April 2025).
- Phthalates in Food Packaging and Food Contact Applications | FDA. Available online: https://www.fda.gov/food/food-additives-and-gras-ingredients-information-consumers/phthalates-food-packaging-and-food-contact-applications (accessed on 11 April 2025).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, H.; Cui, J.-G.; Wang, J.-X.; Chen, M.-S.; Wang, H.-R.; Li, X.-N.; Li, J.-L. Ferroptosis is critical for phthalates driving the blood-testis barrier dysfunction via targeting transferrin receptor. Redox Biol. 2023, 59, 102584. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Li, X.-N.; Zhang, H.; Cui, J.-G.; Wang, J.-X.; Chen, M.-S.; Li, J.-L. Phthalate-induced testosterone/androgen receptor pathway disorder on spermatogenesis and antagonism of lycopene. J. Hazard. Mater. 2022, 439, 129689. [Google Scholar] [CrossRef]
- Batool, S.; Aziz, R.; Shameem, S.; Shaheen, M.; Batool, S.; Aslam, I.; Iram, F. Curative Potentials of Garlic (Allium sativum) Extract against Di-(2-Ethylhexyl) Phthalate Induced Reproductive Toxicity in Female Mice. Proc. Pak. Acad. Sci. B. Life Environ. Sci. 2022, 59, 39–53. [Google Scholar] [CrossRef]
- Xu, B.; He, T.; Yang, H.; Dai, W.; Liu, L.; Ma, X.; Ma, J.; Yang, G.; Si, R.; Du, X.; et al. Activation of the p62-Keap1-Nrf2 pathway protects against oxidative stress and excessive autophagy in ovarian granulosa cells to attenuate DEHP-induced ovarian impairment in mice. Ecotoxicol. Environ. Saf. 2023, 265, 115534. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-C.; Xing, C.-H.; Xu, Y.; Pan, Z.-N.; Zhang, H.-L.; Zhang, Y.; Sun, S.-C. DEHP exposure to lactating mice affects ovarian hormone production and antral follicle development of offspring. J. Hazard. Mater. 2021, 416, 125862. [Google Scholar] [CrossRef]
- Sun, J.; Gan, L.; Lv, S.; Wang, T.; Dai, C.; Sun, J. Exposure to Di-(2-Ethylhexyl) phthalate drives ovarian dysfunction by inducing granulosa cell pyroptosis via the SLC39A5/NF-κB/NLRP3 axis. Ecotoxicol. Environ. Saf. 2023, 252, 114625. [Google Scholar] [CrossRef]
- Yang, L.; Jiang, L.; Sun, X.; Li, J.; Wang, N.; Liu, X.; Yao, X.; Zhang, C.; Deng, H.; Wang, S.; et al. DEHP induces ferroptosis in testes via p38α-lipid ROS circulation and destroys the BTB integrity. Food Chem. Toxicol. 2022, 164, 113046. [Google Scholar] [CrossRef]
- Liu, X.; Liu, Y.; Teng, Z.; Zhang, Y.; Wang, Z.; Zhu, P.; Xie, M.; Liu, F. Di-2-ethylhexyl phthalate (DEHP) exposure induces sperm quality and functional defects in mice. Chemosphere 2023, 312, 137216. [Google Scholar] [CrossRef]
- Zhao, Y.; Chen, M.-S.; Wang, J.-X.; Cui, J.-G.; Zhang, H.; Li, X.-N.; Li, J.-L. Connexin-43 is a promising target for lycopene preventing phthalate-induced spermatogenic disorders. J. Adv. Res. 2023, 49, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Zhang, L.; Zhu, Z.; Li, R.; Wang, S.; Wang, W.; Qin, Z.; Zhang, W.; Lopez-Malo, D. Overexpression of miR-506-3p Aggravates DBP-Induced Testicular Oxidative Stress in Rats by Downregulating ANXA5 via Nrf2/HO-1 Signaling Pathway. Oxidative Med. Cell. Longev. 2020, 2020, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.-Z.; Liu, L.-L.; Yue, J.-Z.; Lu, Z.-Y.; Zheng, J.; Jiang, M.-Z.; Lin, M.; Liu, J.; Gao, H.-T. Alleviative effect of quercetin against reproductive toxicity induced by chronic exposure to the mixture of phthalates in male rats. Ecotoxicol. Environ. Saf. 2024, 270, 115920. [Google Scholar] [CrossRef]
- Liu, L.-L.; Yue, J.-Z.; Lu, Z.-Y.; Deng, R.-Y.; Li, C.-C.; Yu, Y.-N.; Zhou, W.-J.; Lin, M.; Gao, H.-T.; Liu, J.; et al. Long-term exposure to the mixture of phthalates induced male reproductive toxicity in rats and the alleviative effects of quercetin. Toxicol. Appl. Pharmacol. 2024, 483, 116816. [Google Scholar] [CrossRef]
- Xu, B.; Zhang, Z.; Yang, H.; Ding, L.; Dai, W.; Liu, L.; Du, X.; Fu, X.; Pei, X. A novel perspective on di-hexyl phthalate (2-ethylhexyl)-induced reproductive toxicity in females: Lipopolysaccharide synergizes with mono-2-ethylhexyl ester to cause inflammatory apoptosis rather than autophagy in ovarian granulosa cells. Ecotoxicol. Environ. Saf. 2024, 276, 116319. [Google Scholar] [CrossRef]
- Ara, C.; Asmatullah; Ramzan, N.; Ali, S.; Shakir, H.A.; Liaqat, I.; Iqbal, A.; Yaseen, F.; Shahzad, N. Black coffee mitigates diethyl phthalate disrupted folliculogenesis, reduced gonadotropins, and ovarian lesions in female albino mice. Environ. Sci. Pollut. Res. 2022, 29, 47254–47266. [Google Scholar] [CrossRef]
- Colón-Díaz, M.; Ortiz-Santana, J.; Craig, Z.R. Data on the activity of DNA methyltransferase in the uteri of CD-1 mice exposed to dibutyl phthalate. Data Brief 2020, 28, 105061. [Google Scholar] [CrossRef]
- Panagiotou, E.M.; Damdimopoulos, A.; Li, T.; Moussaud-Lamodière, E.; Pedersen, M.; Lebre, F.; Pettersson, K.; Arnelo, C.; Papaikonomou, K.; Alfaro-Moreno, E.; et al. Exposure to the phthalate metabolite MEHP impacts survival and growth of human ovarian follicles in vitro. Toxicology 2024, 505, 153815. [Google Scholar] [CrossRef]
- Mileo, A.; Chianese, T.; Fasciolo, G.; Venditti, P.; Capaldo, A.; Rosati, L.; De Falco, M. Effects of Dibutylphthalate and Steroid Hormone Mixture on Human Prostate Cells. Int. J. Mol. Sci. 2023, 24, 14341. [Google Scholar] [CrossRef]
- Källsten, L.; Pierozan, P.; Martin, J.W.; Karlsson, O. Di-n-Butyl Phthalate and Its Monoester Metabolite Impairs Steroid Hormone Biosynthesis in Human Cells: Mechanistic In Vitro Studies. Cells 2022, 11, 3029. [Google Scholar] [CrossRef]
- Cosci, I.; Garolla, A.; Cabrelle, A.; Sut, S.; Dall’ACqua, S.; Ferlin, A.; Foresta, C.; De Toni, L. Lipophilic phthalic acid esters impair human sperm acrosomal reaction through the likely inhibition of phospholipase A2-signaling pathway. Biochem. Pharmacol. 2022, 205, 115249. [Google Scholar] [CrossRef]
- Sun, X.; Chen, W.; Weng, S.; Pan, T.; Hu, X.; Wang, F.; Xia, T.; Chen, H.; Luo, T. Effects of the environmental endocrine disruptors di-2-ethylhexyl phthalate and mono-2-ethylhexyl phthalate on human sperm function in vitro. Reprod. Fertil. Dev. 2020, 32, 629. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Leung, A.O.W.; Chu, L.H.; Wong, M.H. Phthalates contamination in China: Status, trends and human exposure-with an emphasis on oral intake. Environ. Pollut. 2018, 238, 771–782. [Google Scholar] [CrossRef]
- Wang, Y.; Du, Y.-Y.; Yao, W.; Deng, T.-R.; Guo, N.; Yin, L.; Yuan, X.-Q.; Guo, Q.-C.; Li, J.; Liao, H.-M.; et al. Associations between phthalate metabolites and cytokines in the follicular fluid of women undergoing in vitro fertilization. Ecotoxicol. Environ. Saf. 2023, 267, 115616. [Google Scholar] [CrossRef]
- Tian, M.; Wu, S.; Wang, Y.-X.; Liu, L.; Zhang, J.; Shen, H.; Lu, Y.; Bao, H.; Huang, Q. Associations of environmental phthalate exposure with male steroid hormone synthesis and metabolism: An integrated epidemiology and toxicology study. J. Hazard. Mater. 2022, 436, 129213. [Google Scholar] [CrossRef] [PubMed]
- Yuan, G.; Zeng, Y.; Hu, G.; Liu, Y.; Wei, L.; Liu, P.; Liu, G.; Cheng, J. Inverse association of certain seminal phthalate metabolites with semen quality may be mediated by androgen synthesis: A cross-sectional study from the South China. Environ. Int. 2021, 151, 106459. [Google Scholar] [CrossRef] [PubMed]
- Yang, P.; Deng, L.-J.; Xie, J.-Y.; Li, X.-J.; Wang, X.-N.; Sun, B.; Meng, T.-Q.; Xiong, C.-L.; Huang, Y.-C.; Wang, Y.-X.; et al. Phthalate exposure with sperm quality among healthy Chinese male adults: The role of sperm cellular function. Environ. Pollut. 2023, 331, 121755. [Google Scholar] [CrossRef]
- Yao, W.; Liu, C.; Qin, D.-Y.; Yuan, X.-Q.; Yao, Q.-Y.; Li, N.-J.; Huang, Y.; Rao, W.-T.; Deng, Y.-L.; Zeng, Q.; et al. Associations between Phthalate Metabolite Concentrations in Follicular Fluid and Reproductive Outcomes among Women Undergoing in Vitro Fertilization/Intracytoplasmic Sperm Injection Treatment. Environ. Health Perspect. 2023, 131, 127019. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, I. The relationship between urinary phthalate metabolites and polycystic ovary syndrome in women undergoing in vitro fertilization: Nested case-control study. Chemosphere 2022, 286, 131495. [Google Scholar] [CrossRef]
- Abdo, N.; Al-Khalaileh, H.; Alajlouni, M.; Hamadneh, J.; Alajlouni, A.M. Screening for phthalates biomarkers and its potential role in infertility outcomes in Jordan. J. Expo. Sci. Environ. Epidemiol. 2023, 33, 273–282. [Google Scholar] [CrossRef] [PubMed]
- Caporossi, L.; Viganò, P.; Paci, E.; Capanna, S.; Alteri, A.; Pigini, D.; Tranfo, G.; Papaleo, B. A Case–Control Study on the Effects of Plasticizers Exposure on Male Fertility. Int. J. Environ. Res. Public Health 2022, 20, 235. [Google Scholar] [CrossRef]
- Li, Y.; Xiao, N.; Liu, M.; Liu, Y.; He, A.; Wang, L.; Luo, H.; Yao, Y.; Sun, H. Dysregulation of steroid metabolome in follicular fluid links phthalate exposure to diminished ovarian reserve of childbearing-age women. Environ. Pollut. 2023, 330, 121730. [Google Scholar] [CrossRef]
- Li, Y.; Yao, Y.; Xiao, N.; Liu, Y.; Du, Y.; Liu, M.; Zhang, Q.; Zhao, H.; Zhang, T.; Zhang, H.; et al. The association of serum phthalate metabolites with biomarkers of ovarian reserve in women of childbearing age. Ecotoxicol. Environ. Saf. 2022, 242, 113909. [Google Scholar] [CrossRef]
- Burns, J.S.; Sergeyev, O.; Lee, M.M.; Williams, P.L.; Mínguez-Alarcón, L.; Plaku-Alakbarova, B.; Sokolov, S.; Kovalev, S.; Koch, H.M.; Lebedev, A.T.; et al. Associations of prepubertal urinary phthalate metabolite concentrations with pubertal onset among a longitudinal cohort of boys. Environ. Res. 2022, 212, 113218. [Google Scholar] [CrossRef]
- Liu, J.; Gao, D.; Li, Y.; Song, X.; Chen, M.; Ma, Q.; Wang, X.; Cui, M.; Guo, T.; Chen, L.; et al. Persistent high exposure to exogenous phthalates and endogenous sex hormones associated with early pubertal onset among children: A 3.5-year longitudinal cohort study in China. Ecotoxicol. Environ. Saf. 2023, 262, 115199. [Google Scholar] [CrossRef]
- Gokyer, D.; Laws, M.J.; Kleinhans, A.; Riley, J.K.; Flaws, J.A.; Babayev, E. Phthalates are detected in the follicular fluid of adolescents and oocyte donors with associated changes in the cumulus cell transcriptome. bioRxiv 2024. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Du, Y.; Guo, N.; Liu, F.; Deng, T.; Li, Y. Associations between urinary phthalate concentrations and antral follicle count among women undergoing In Vitro fertilization. Front. Endocrinol. 2024, 14, 1286391. [Google Scholar] [CrossRef]
- Mínguez-Alarcón, L.; Burns, J.; Williams, P.L.; Korrick, S.A.; Lee, M.M.; Bather, J.R.; Kovalev, S.V.; Sokolov, S.A.; Lebedev, A.T.; Smigulina, L.; et al. Urinary phthalate metabolite concentrations during four windows spanning puberty (prepuberty through sexual maturity) and association with semen quality among young Russian men. Int. J. Hyg. Environ. Health 2022, 243, 113977. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Romero, E.; Scheringer, M. A review of phthalate pharmacokinetics in human and rat: What factors drive phthalate distribution and partitioning? Drug Metab. Rev. 2019, 51, 314–329. [Google Scholar] [CrossRef] [PubMed]
- Youhanna, S.; Kemas, A.M.; Preiss, L.; Zhou, Y.; Shen, J.X.; Cakal, S.D.; Paqualini, F.S.; Goparaju, S.K.; Shafagh, R.Z.; Lind, J.U.; et al. Organotypic and Microphysiological Human Tissue Models for Drug Discovery and Development—Current State-of-the-Art and Future Perspectives. Pharmacol. Rev. 2022, 74, 141–206. [Google Scholar] [CrossRef]
- Wu, H.-T.; Liao, C.-C.; Peng, C.-F.; Lee, T.-Y.; Liao, P.-H. Exploring the application of machine learning to identify the correlations between phthalate esters and disease: Enhancing nursing assessments. Health Inf. Sci. Syst. 2024, 13, 10. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhong, Y.; Lauschke, V.M. Evaluating the synergistic use of advanced liver models and AI for the prediction of drug-induced liver injury. Expert Opin. Drug Metab. Toxicol. 2025, 21, 563–577. [Google Scholar] [CrossRef] [PubMed]
| Parent Phthalate | Primary Metabolite | Secondary Metabolite | |
|---|---|---|---|
| High Molecular Weight (HMW) Phthalates | DEHP | MEHP | MEHHP MEOHP MECPP |
| DiNP | MiNP | MHiNP MOiNP MCiNP | |
| DiDP | MiDP | MHiDP MOiDP MCiDP | |
| DnOP | MnOP | MCPP | |
| Low Molecular Weight (LMW) Phthalates | BBP | MBzP | - |
| DMP | MMP | - | |
| DEP | MEP | - | |
| DBP | MnBP | MHnBP | |
| DiBP | MiBP | MHiBP |
| Phthalate Name | Abbreviation | CAS Registry Number | Molecular Formula | Chemical Structure | Molecular Weight (g/mol) |
|---|---|---|---|---|---|
| Di-(2-ethylhexyl) phthalate | DEHP | 117-81-7 | C24H38O4 | 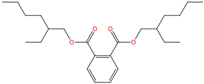 | 390.56 |
| Di-n-butyl phthalate | DBP | 84-74-2 | C16H22O4 | 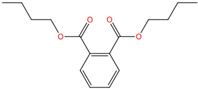 | 278.34 |
| Benzyl butyl phthalate | BBP | 85-68-7 | C19H20O4 | 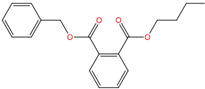 | 312.36 |
| Diethyl phthalate | DEP | 84-66-2 | C12H14O4 | 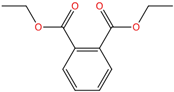 | 222.24 |
| Diisobutyl phthalate | DiBP | 84-69-5 | C16H22O4 | 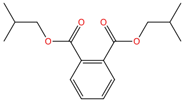 | 278.34 |
| Diisononyl phthalate | DiNP | 28553-12-0 | C26H42O4 |  | 418.61 |
| Diisodecyl phthalate | DiDP | 89-16-7 | C28H46O4 |  | 446.66 |
| Dimethyl phthalate | DMP | 131-11-3 | C10H10O4 | 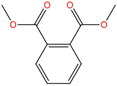 | 194.18 |
| Di-n-octyl phthalate | DnOP | 117-84-0 | C24H38O4 | 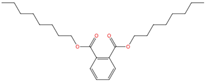 | 390.56 |
| Mono-(2-ethylhexyl) phthalate | MEHP | 4376-20-9 | C16H22O4 | 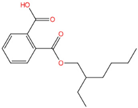 | 278.34 |
| Mono-2-ethyl-5-hydroxyhexyl phthalate | MEHHP | 40321-99-1 | C16H22O5 | 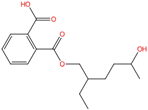 | 294.34 |
| Mono-n-butyl phthalate | MnBP | 131-70-4 | C12H14O4 | 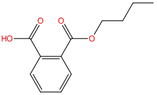 | 222.24 |
| Mono-benzyl phthalate | MBzP | 2528-16-7 | C15H12O4 | 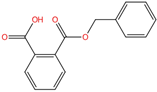 | 256.25 |
| Mono-n-octyl phthalate | MnOP | 5393-19-1 | C16H22O4 |  | 278.34 |
| Mono-ethyl phthalate | MEP | 2306-33-4 | C10H10O4 | 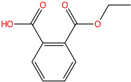 | 194.18 |
| Mono-isobutyl phthalate | MiBP | 30833-53-5 | C12H14O4 |  | 222.24 |
| Mono-methyl phthalate | MMP | 4376-18-5 | C9H8O4 |  | 180.16 |
| Mono-isopropyl phthalate | MiPrP | 35118-50-4 | C11H12O4 | 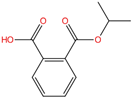 | 208.21 |
| Mono-isononyl phthalate | MiNP | 106610-61-1 | C17H24O4 | 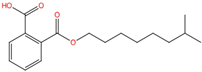 | 292.37 |
| Mono(2-ethyl-5-carboxypentyl) phthalate | MECPP | 40809-41-4 | C16H20O6 | 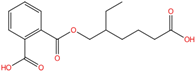 | 308.33 |
| Mono(2-ethyl-5-oxohexyl) phthalate | MEOHP | 40321-98-0 | C16H20O5 |  | 292.33 |
| Mono [2-(carboxymethyl)hexyl phthalate | MCMHP | 82975-93-7 | C16H20O6 |  | 308.33 |
| Mono-3-carboxypropyl phthalate | MCPP | 66851-46-5 | C12H12O6 | 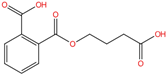 | 252.22 |
| Study Design | No. of Studies | Population | Phthalate/Phthalate Metabolite Examined | Association/Examined Outcome |
|---|---|---|---|---|
| C-S | 16 |
| MMP, MEP, MBP, MBzP, MEHP, MEHHP, MEOHP, MOP, MECPP, MCMHP, MCPP, MiBP, MnBP, DEHP, DEP, DBP, DnOP |
|
| C-C | 7 |
| DMP, MMP, DEP, MEP, DBP, MBP, BBzP, MBzP, DEHP, MEHP, MEHHP, MEOHP, MnBP, MiBP, MECPP |
|
| C | 7 |
| DEHP, DINP, DiNP, DiDP, MBzP, MnBP, MiBP, MCPP, MECPP, MEHP, MEHHP, MEOHP, MEP, MCOMHP, MCOMOP, MMP, MBP, MCMHP |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moghazy, M.; Papathanasiou, M.; Tzoupis, H.; Papavasileiou, K.D.; Xing, C.; Lauschke, V.M.; Afantitis, A.; Melagraki, G. A Systematic Literature Review of Reproductive Toxicological Studies on Phthalates. Int. J. Mol. Sci. 2025, 26, 8761. https://doi.org/10.3390/ijms26188761
Moghazy M, Papathanasiou M, Tzoupis H, Papavasileiou KD, Xing C, Lauschke VM, Afantitis A, Melagraki G. A Systematic Literature Review of Reproductive Toxicological Studies on Phthalates. International Journal of Molecular Sciences. 2025; 26(18):8761. https://doi.org/10.3390/ijms26188761
Chicago/Turabian StyleMoghazy, Muhammad, Marianthi Papathanasiou, Haralampos Tzoupis, Konstantinos D. Papavasileiou, Chen Xing, Volker M. Lauschke, Antreas Afantitis, and Georgia Melagraki. 2025. "A Systematic Literature Review of Reproductive Toxicological Studies on Phthalates" International Journal of Molecular Sciences 26, no. 18: 8761. https://doi.org/10.3390/ijms26188761
APA StyleMoghazy, M., Papathanasiou, M., Tzoupis, H., Papavasileiou, K. D., Xing, C., Lauschke, V. M., Afantitis, A., & Melagraki, G. (2025). A Systematic Literature Review of Reproductive Toxicological Studies on Phthalates. International Journal of Molecular Sciences, 26(18), 8761. https://doi.org/10.3390/ijms26188761








