Focal Segmental Glomerulosclerosis: Comprehensive Review and Exploration of the Dual Potential of Cyclodextrins in Therapeutic Optimization
Abstract
1. Introduction
2. Pathophysiology of FSGS
2.1. Primary FSGS
2.2. Secondary FSGS
2.2.1. Adaptive FSGS
2.2.2. Genetic FSGS
2.2.3. Virus-Mediated FSGS
2.2.4. FSGS Associated with the Use of Drugs or Psychoactive Substances
2.2.5. Apolipoprotein L1 (APOL1)-Associated FSGS
3. Clinical Manifestations and Diagnosis of FSGS
4. Conventional Treatment of FSGS
4.1. Symptomatic Therapy
4.2. Immunosuppressive Therapy
4.3. Renal Replacement Therapy
5. Exploring Therapeutic Strategies for FSGS Using Cyclodextrins
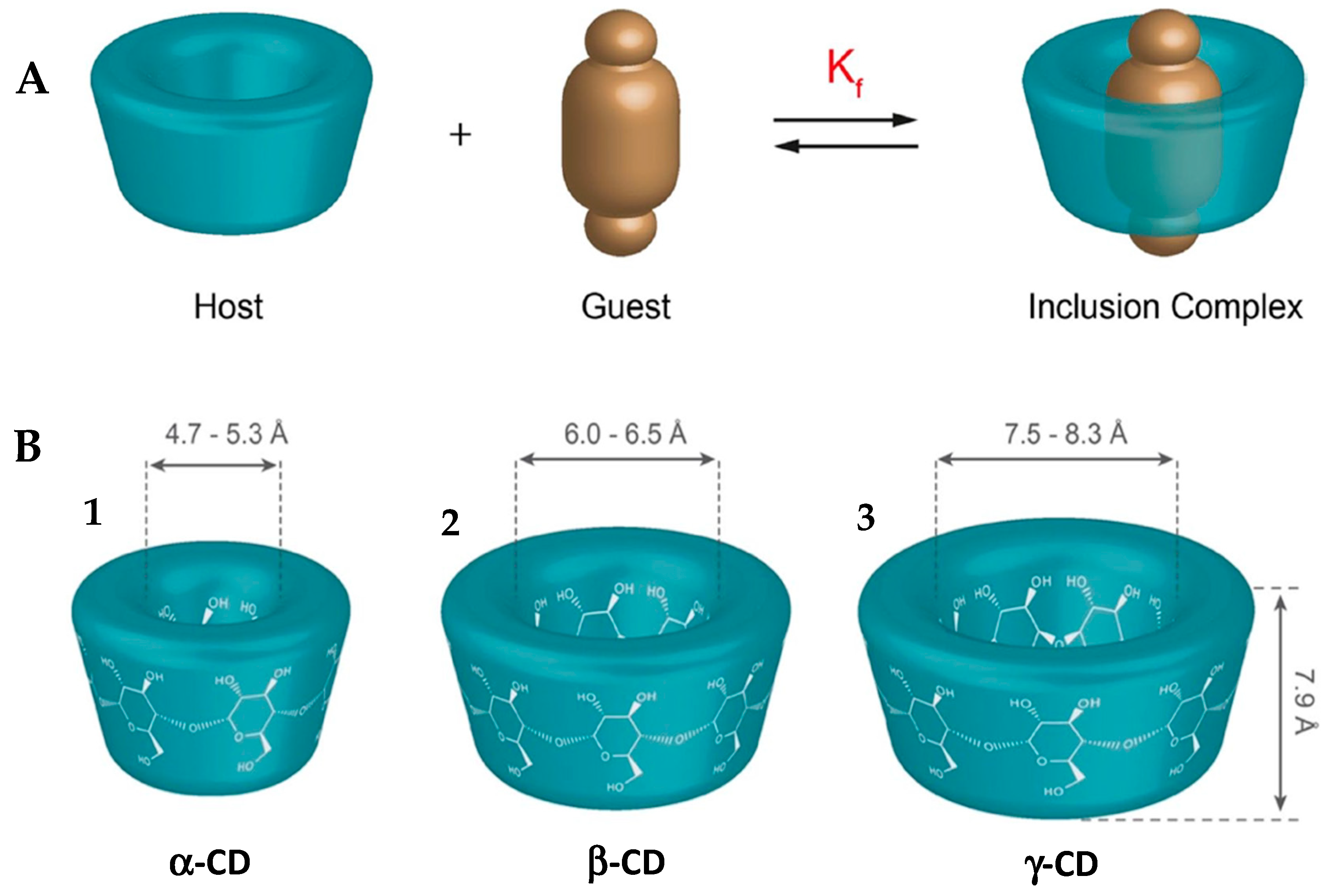
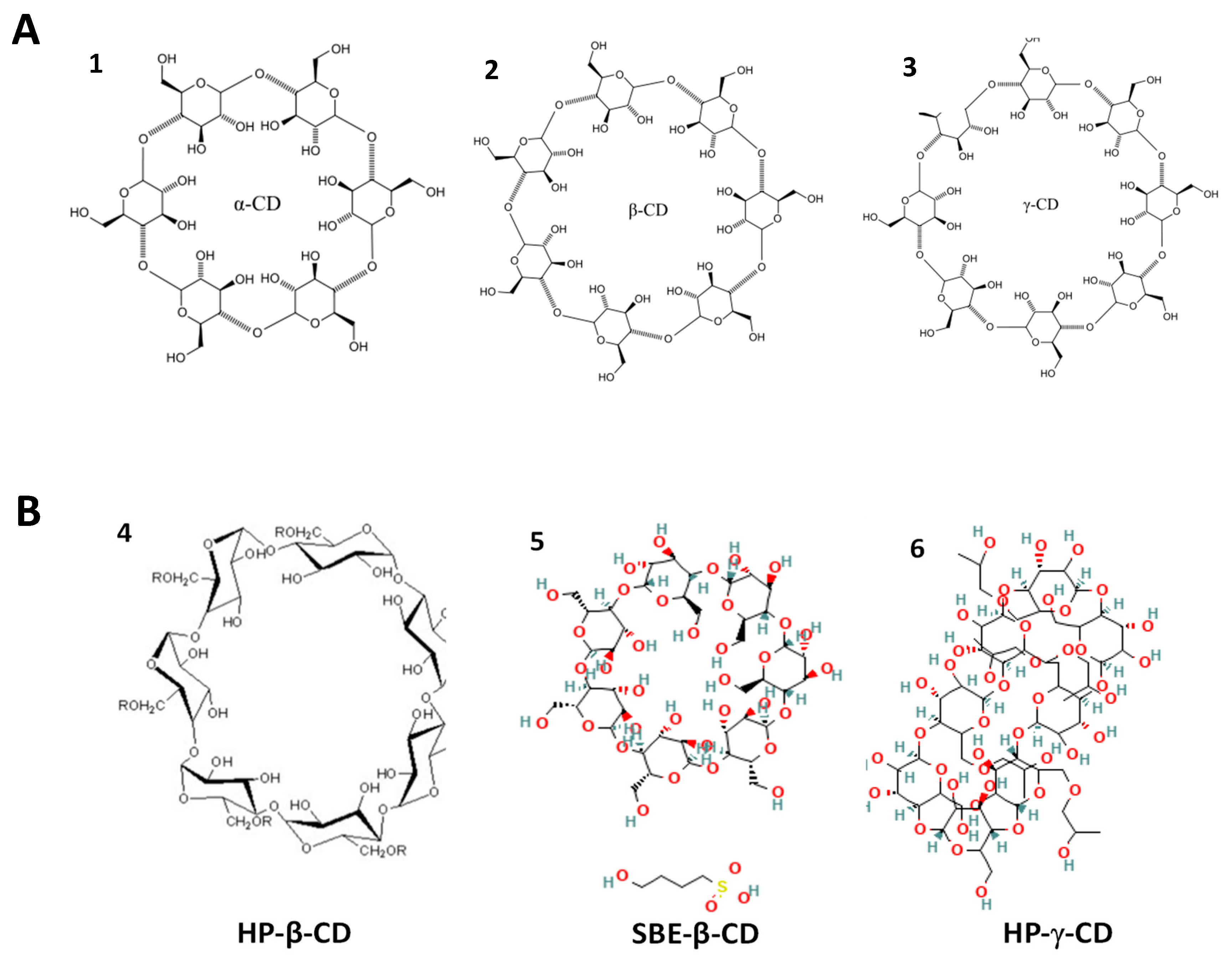
5.1. Cyclodextrins as Drug Transport Vehicles
5.1.1. Alpha Cyclodextrin (α-CD)
5.1.2. Beta Cyclodextrin (β-CD)
5.1.3. Gamma Cyclodextrin (γ-CD)
5.2. Cyclodextrins as Therapeutic Agents
5.2.1. Alpha Cyclodextrin (α-CD)
5.2.2. Beta Cyclodextrin (β-CD)
6. Final Considerations and Future Prospects
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gipson, D.S.; Troost, J.P.; Spino, C.; Attalla, S.; Tarnoff, J.; Massengill, S.; Lafayette, R.; Vega-Warner, V.; Adler, S.; Gipson, P.; et al. Comparing Kidney Health Outcomes in Children, Adolescents, and Adults with Focal Segmental Glomerulosclerosis. JAMA Netw. Open 2022, 5, 2228701. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.W.; Pippin, J.W.; Eng, D.G.; Lv, S.; Shankland, S.J.; Pun, S.H. Nanoparticles exhibit greater accumulation in kidney glomeruli during experimental glomerular kidney disease. Physiol. Rep. 2020, 8, e14545. [Google Scholar] [CrossRef]
- Pekkucuksen, N.T.; Liu, L.P.; Aly, R.; Shoemaker, L.R.; Alli, A.A. Extracellular vesicles from focal segmental glomerulo-sclerosis pediatric patients induce STAT3 activation and mesangial cell proliferation. PLoS ONE 2022, 17, e0274598. [Google Scholar] [CrossRef]
- Kim, D.W.; Jeon, H.; Kim, S.; Lee, W.; Kim, H.J.; Rhee, H.; Song, S.H.; Seong, E.Y.; Saranathan, M. Pembrolizumab-induced focal segmental glomerulosclerosis: A case report. Medicine 2021, 100, 27546. [Google Scholar] [CrossRef] [PubMed]
- Suresh, V.; Stillman, I.E.; Campbell, K.N.; Meliambro, K. Focal Segmental Glomerulosclerosis. Adv. Kidney Dis. Health 2024, 31, 275–289. [Google Scholar] [CrossRef]
- Newswire, G. Focal Segmental Glomerulosclerosis (FSGS) Epidemiology Forecast, 2024-2034—FSGS Prevalence Higher Among Males, Peaks in 18–64 Age Group Across the 7MM. Available online: https://www.globenewswire.com/news-release/2024/04/26/2870588/28124/en/Focal-Segmental-Glomerulosclerosis-FSGS-Epidemiology-Forecast-2024-2034-FSGS-Prevalence-Higher-Among-Males-Peaks-in-18-64-Age-Group-Across-the-7MM.html (accessed on 3 April 2025).
- Hogg, R.; Middleton, J.; Vehaskari, V.M. Focal segmental glomerulosclerosis-epidemiology aspects in children and adults. Pediatr. Nephrol. 2007, 22, 183–186. [Google Scholar] [CrossRef]
- Rosenberg, A.Z.; Kopp, J.B. Focal Segmental Glomerulosclerosis. Clin. J. Am. Soc. Nephrol. 2017, 12, 502–517. [Google Scholar] [CrossRef] [PubMed]
- de Cos, M.; Meliambro, K.; Campbell, K.N. Corrigendum to Novel Treatment Paradigms: Focal Segmental Glomerulosclerosis Kidney International Reports, Volume 8, Issue 1, January 2023, Pages 30–35. Kidney Int. Rep. 2023, 9, 949. [Google Scholar] [CrossRef]
- Jin Lim, B.; Won Yang, J.; Sung Do, W.; Fogo, A.B. Pathogenesis of Focal Segmental Glomerulosclerosis. J. Pathol. Transl. Med. 2016, 50, 405–410. [Google Scholar] [CrossRef]
- Shabaka, A.; Ribera, A.T.; Fernández-Juárez, G. Focal Segmental Glomerulosclerosis: State-of-the-Art and Clinical Perspective. Nephron 2020, 144, 413–427. [Google Scholar] [CrossRef]
- Navarro-Torres, M.; Wooden, B.; Santoriello, D.; Radhakrishnan, J.; Bomback, A.S. Podocytopathies. Adv. Kidney Dis. Health 2025, 32, 2–11. [Google Scholar] [CrossRef]
- Chávez Valencia, V.; Pérez-Vázquez, V.; Gómez García, A.; Vargas-Ortiz, K.; Villanueva Pérez, M.A.; Godínez Rubí, M.; Pazarín Villaseñor, L.; Gutiérrez Castellanos, S.; Cruz, C. Expresión de C4d en glomeruloesclerosis focal y segmentaria. Nefrología 2024, 44, 402–407. [Google Scholar] [CrossRef]
- Stryckers, M.; Oevelen, S.; Koshy, P.; Sprangers, B.; Craenenbroeck, A.H. Focal segmental glomerulosclerosis associated with the use of the IL-23 inhibitor guselkumab. Clin. Kidney J. 2023, 16, 1701–1702. [Google Scholar] [CrossRef] [PubMed]
- Welsh, G.I.; Saleem, M.A. The podocyte cytoskeleton—Key to a functioning glomerulus in health and disease. Nat. Rev. Nephrol. 2012, 8, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Jefferson, J.A.; Shankland, S.J. The Pathogenesis of Focal Segmental Glomerulosclerosis. Adv. Chronic Kidney Dis. 2014, 21, 408–416. [Google Scholar] [CrossRef] [PubMed]
- De Vriese, A.S.; Sethi, S.; Nath, K.A.; Glassock, R.J.; Fervenza, F.C. Differentiating Primary, Genetic, and Secondary FSGS in Adults: A Clinicopathologic Approach. J. Am. Soc. Nephrol. 2018, 29, 759–774. [Google Scholar] [CrossRef]
- Fogo, A.B. Causes and pathogenesis of focal segmental glomerulosclerosis. Nat. Rev. Nephrol. 2015, 11, 76–87. [Google Scholar] [CrossRef]
- Merscher, S.; Pedigo, C.E.; Mendez, A.J. Metabolism, Energetics, and Lipid Biology in the Podocyte—Cellular Cholesterol-Mediated Glomerular Injury. Front. Endocrinol. 2014, 5, 169. [Google Scholar] [CrossRef]
- Sim, J.J.; Batech, M.; Hever, A.; Harrison, T.N.; Avelar, T.; Kanter, M.H.; Jacobsen, S.J. Distribution of Biopsy-Proven Presumed Primary Glomerulonephropathies in 2000–2011 Among a Racially and Ethnically Diverse US Population. Am. J. Kidney Dis. 2016, 68, 533–544. [Google Scholar] [CrossRef]
- Kitiyakara, C.; Kopp, J.B.; Eggers, P. Trends in the epidemiology of focal segmental glomerulosclerosis. Semin. Nephrol. 2003, 23, 172–182. [Google Scholar] [CrossRef]
- Gauckler, P.; Zitt, E.; Regele, H.; Eller, K.; Säemann, M.D.; Lhotta, K.; Neumann, I.; Rudnicki, M.; Odler, B.; Kronbichler, A.; et al. Diagnosis and treatment of focal-segmental glomerulosclerosis—2023. Wien. Klin. Wochenschr. 2023, 135, 638–647. [Google Scholar] [CrossRef] [PubMed]
- Lempesis, I.G.; Georgakopoulou, V.E. Physiopathological mechanisms related to inflammation in obesity and type 2 diabetes mellitus. World J. Exp. Med. 2023, 13, 7–16. [Google Scholar] [CrossRef]
- Ritz, E.; Koleganova, N.; Piecha, G. Is there an obesity-metabolic syndrome related glomerulopathy? Curr. Opin. Nephrol. Hypertens. 2011, 20, 44–49, Erratum in Curr. Opin. Nephrol. Hypertens. 2011, 20, 209. [Google Scholar] [CrossRef] [PubMed]
- Podestà, M.A.; Ponticelli, C. Autoimmunity in Focal Segmental Glomerulosclerosis: A Long-Standing Yet Elusive Association. Front. Med. 2020, 7, 604961. [Google Scholar] [CrossRef]
- Trachtman, H. Emerging drugs for treatment of focal segmental glomerulosclerosis. Expert Opin. Emerg. Drugs 2020, 25, 367–375. [Google Scholar] [CrossRef]
- Jambhekar, S.S.; Breen, P. Cyclodextrins in pharmaceutical formulations I: Structure and physicochemical properties, for-mation of complexes, and types of complex. Drug Discov. Today 2016, 21, 356–362. [Google Scholar] [CrossRef]
- Lachowicz, M.; Stańczak, A.; Kołodziejczyk, M. Characteristic of Cyclodextrins: Their Role and Use in the Pharmaceutical Technology. Curr. Drug Targets 2020, 21, 1495–1510. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ye, L.; Xi, J.; Wang, J.; Feng, Z.G. Cyclodextrin polymers: Structure, synthesis, and use as drug carriers. Prog. Polym. Sci. 2021, 118, 101408. [Google Scholar] [CrossRef]
- Pullen, N.; Fornoni, A. Drug discovery in focal and segmental glomerulosclerosis. Kidney Int. 2016, 89, 1211–1220. [Google Scholar] [CrossRef]
- Korbet, S.M. Treatment of primary focal segmental glomerulosclerosis. Kidney Int. 2002, 62, 2301–2310. [Google Scholar] [CrossRef]
- Garcia, J.; Silva, J.P.d.; Marchi, L.F.d. Glomeruloesclerose focal e segmentar: Avanços no diagnóstico e consenso no tratamento. CuidArte Enferm 2020, 14, 265–269. [Google Scholar]
- Li, Y.; Fan, J.; Zhu, W.; Niu, Y.; Wu, M.; Zhang, A. Therapeutic Potential Targeting Podocyte Mitochondrial Dysfunction in Focal Segmental Glomerulosclerosis. Kidney Dis. 2023, 9, 254–264. [Google Scholar] [CrossRef]
- Ichikawa, I.; Fogo, A. Focal segmental glomerulosclerosis. Pediatr. Nephrol. 1996, 10, 374–391. [Google Scholar] [CrossRef]
- Saleh, Z. Focal Segmental Glomerulosclerosis. 2024. Available online: https://medizzy.com/feed/40232972 (accessed on 3 April 2025).
- Sadowski, C.E.; Lovric, S.; Ashraf, S.; Pabst, W.L.; Gee, H.Y.; Kohl, S.; Engelmann, S.; Vega-Warner, V.; Fang, H.; Halbritter, J.; et al. A single-gene cause in 29.5% of cases of steroid-resistant nephrotic syndrome. J. Am. Soc. Nephrol. 2015, 26, 1279–1289. [Google Scholar] [CrossRef] [PubMed]
- D’Agati, V.D.; Kaskel, F.J.; Falk, R.J. Focal segmental glomerulosclerosis. N. Engl. J. Med. 2011, 365, 2398–2411. [Google Scholar] [CrossRef] [PubMed]
- Korbet, S.M. Primary focal segmental glomerulosclerosis. J. Am. Soc. Nephrol. 1998, 9, 1333–1340. [Google Scholar] [CrossRef]
- Genovese, G.; Friedman, D.J.; Ross, M.D.; Lecordier, L.; Uzureau, P.; Freedman, B.I.; Bowden, D.W.; Langefeld, C.D.; Oleksyk, T.K.; Uscinski Knob, A.L.; et al. Association of trypanolytic ApoL1 variants with kidney disease in African Americans. Science 2010, 329, 841–845. [Google Scholar] [CrossRef]
- Friedman, D.J.; Pollak, M.R. APOL1 Nephropathy: From Genetics to Clinical Applications. Clin. J. Am. Soc. Nephrol. 2021, 16, 294–303. [Google Scholar] [CrossRef]
- Otalora, L.; Chavez, E.; Watford, D.; Tueros, L.; Correa, M.; Nair, V.; Ruiz, P.; Wahl, P.; Eddy, S.; Martini, S.; et al. Identification of glomerular and podocyte-specific genes and pathways activated by sera of patients with focal segmental glomerulosclerosis. PLoS ONE 2019, 14, e0222948. [Google Scholar] [CrossRef]
- Altintas, M.M.; Agarwal, S.; Sudhini, Y.; Zhu, K.; Wei, C.; Reiser, J. Pathogenesis of Focal Segmental Glomerulosclerosis and Related Disorders. Annu. Rev. Pathol. 2025, 20, 329–353. [Google Scholar] [CrossRef] [PubMed]
- Lan, X.; Rao, T.K.S.; Chander, P.N.; Skorecki, K.; Singhal, P.C. Apolipoprotein L1 (APOL1) Variants (Vs) a possible link between Heroin-associated Nephropathy (HAN) and HIV-associated Nephropathy (HIVAN. Front. Microbiol. 2015, 6, 571. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.Q.; Zhang, X.; Chen, X.L.; Cheng, L.; Wang, X. Application of nanotechnology in the treatment of glomerulonephritis: Current status and future perspectives. J. Nanobiotechnol. 2024, 22, 9. [Google Scholar] [CrossRef]
- Drexler, Y.; Molina, J.; Elfassy, T.; Ma, R.; Christoffersen, C.; Kurano, M.; Yatomi, Y.; Mariani, L.H.; Contreras, G.; Merscher, S.; et al. Identification of Glomerular and Plasma Apolipoprotein M as Novel Biomarkers in Glomerular Disease. Kidney Int. Reports 2023, 8, 884. [Google Scholar] [CrossRef] [PubMed]
- Nagata, M.; Kobayashi, N.; Hara, S. Focal segmental glomerulosclerosis; why does it occur segmentally? Pflugers Arch. Eur. J. Physiol. 2017, 469, 983–988. [Google Scholar] [CrossRef] [PubMed]
- Troost, J.P.; Trachtman, H.; Spino, C.; Kaskel, F.J.; Friedman, A.; Moxey-Mims, M.M.; Fine, R.N.; Gassman, J.J.; Kopp, J.B.; Walsh, L.; et al. Proteinuria Reduction and Kidney Survival in Focal Segmental Glomerulosclerosis. Am. J. Kidney Dis. 2021, 77, 216–225. [Google Scholar] [CrossRef]
- Yusuf, S.; Teo, K.K.; Pogue, J.; Dyal, L.; Copland, I.; Schumacher, H.; Dagenais, G.; Sleight, P.; Anderson, C. Telmisartan, ramipril, or both in patients at high risk for vascular events. N. Engl. J. Med. 2008, 358, 1547–1559. [Google Scholar]
- Bakris, G.L.; Weir, M.R. Angiotensin-converting enzyme inhibitor-associated elevations in serum creatinine: Is this a cause for concern? Arch. Intern. Med. 2000, 160, 685–693. [Google Scholar] [CrossRef]
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerular Diseases Work Group. KDIGO 2021 Clinical Practice Guideline for the Management of Glomerular Diseases. Kidney Int. 2021, 100, S1–S276. [Google Scholar] [CrossRef]
- National Institute for Health and Care Excellence (NICE). Chronic Kidney Disease: Assessment and Management; NICE: London, UK, 2021; Volume 203, ISBN 9781473142336. [Google Scholar]
- Angeletti, A.; Bin, S.; Magnasco, A.; Bruschi, M.; Cravedi, P.; Ghiggeri, G.M. Efficacy of combined rituximab and dara-tumumab treatment in posttransplant recurrent focal segmental glomerulosclerosis. Am. J. Transplant. 2024, 24, 688–692. [Google Scholar] [CrossRef]
- Campbell, K.N.; Pennese, N.; Zaffalon, A.; Magalhaes, B.; Faiella, M.; Caster, D.J.; Radhakrishnan, J.; Tesar, V.; Trachtman, H. Efficacy and Safety of ACE Inhibitor and Angiotensin Receptor Blocker Therapies in Primary Focal Segmental Glomerulo-sclerosis Treatment: A Systematic Review and Meta-Analysis. Kidney Med. 2022, 4, 100457. [Google Scholar] [CrossRef]
- Fogo, A.B. Sparsentan versus irbesartan in focal segmental glomerulosclerosis. Kidney Int. 2014, 105, 418–420. [Google Scholar]
- Costa, N.B.; Cândido, V.T.L.; Prudente, S.V.C.; Campos, D.C.; Roriz, V.H.B.; Silveira, M.A.M.; Pereira, H.P.O.C.; Nasci-mento, A.L.O. Glomerulonefrose segmentar e focal—Resposta à corticoterapia: Relato de caso. Rev. Contemp. 2024, 4, 3795. [Google Scholar] [CrossRef]
- Xing, C.; Zheng, X.; Deng, T.; Zeng, L.; Liu, X.; Chi, X. The Role of Cyclodextrin in the Construction of Nanoplatforms: From Structure, Function and Application Perspectives. Pharmaceutics 2023, 15, 1536. [Google Scholar] [CrossRef] [PubMed]
- Cid-Samamed, A.; Rakmai, J.; Mejuto, J.C.; Simal-Gandara, J.; Astray, G. Cyclodextrins inclusion complex: Preparation methods, analytical techniques and food industry applications. Food Chem. 2022, 384, 132467. [Google Scholar] [CrossRef]
- Nicolaescu, O.E.; Belu, I.; Mocanu, A.G.; Manda, V.C.; Rău, G.; Pîrvu, A.S.; Ionescu, C.; Ciulu-Costinescu, F.; Popescu, M.; Ciocîlteu, M. V Cyclodextrins: Enhancing Drug Delivery, Solubility and Bioavailability for Modern Therapeutics. Pharmaceutics 2025, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Ianiski, F.R.; Oliveira, A.L.D.E.; Mezzomo, N.J.; Franceschi, I.D.D.E.; Carmo, G.M.D.O.; Cremonese, C.R.; Baldissera, M.D.; Zanon, J.P.; Kolling, J.; Friederich, J.D.; et al. β-Cyclodextrins alter the energy metabolism-related enzyme activities in rats. An. Acad. Bras. Cienc. 2023, 95, e20201783. [Google Scholar] [CrossRef]
- Trotta, F.; Loftsson, T.; Gaud, R.S.; Trivedi, R.; Shende, P. Integration of cyclodextrins and associated toxicities: A roadmap for high quality biomedical applications. Carbohydr. Polym. 2022, 295, 119880. [Google Scholar] [CrossRef]
- Almeida, B.; Domingues, C.; Mascarenhas-Melo, F.; Silva, I.; Jarak, I.; Veiga, F.; Figueiras, A. The Role of Cyclodextrins in COVID-19 Therapy—A Literature Review. Int. J. Mol. Sci. 2023, 24, 2974. [Google Scholar] [CrossRef]
- Coisne, C.; Hallier-Vanuxeem, D.; Boucau, M.C.; Hachani, J.; Tilloy, S.; Bricout, H.; Monflier, E.; Wils, D.; Serpelloni, M.; Parissaux, X.; et al. β-cyclodextrins decrease cholesterol release and ABC-associated transporter expression in smooth muscle cells and aortic endothelial cells. Front. Physiol. 2016, 7, 185. [Google Scholar] [CrossRef]
- Kfoury, M.; Lichtfouse, E.; Fourmentin, S. The revival of cyclodextrins as active pharmaceutical ingredients. Environ. Chem. Lett. 2024, 23, 1–6. [Google Scholar] [CrossRef]
- Dura, A.; Rosell, C.M. Physico-chemical properties of corn starch modified with cyclodextrin glycosyltransferase. Int. J. Biol. Macromol. 2016, 87, 466–472. [Google Scholar] [CrossRef]
- Paiva-Santos, A.C.; Ferreira, L.; Peixoto, D.; Silva, F.; Soares, M.J.; Zeinali, M.; Zafar, H.; Mascarenhas-Melo, F.; Raza, F.; Mazzola, P.G.; et al. Cyclodextrins as an encapsulation molecular strategy for volatile organic compounds—Pharmaceutical applications. Colloids Surf. B Biointerfaces 2022, 218, 112758. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Baldi, A. Exploring versatile applications of cyclodextrins: An overview. Drug Deliv. 2016, 23, 739–757. [Google Scholar] [CrossRef] [PubMed]
- Peeters, J.; Neeskens, P.; Tollenaere, J.P.; Remoortere, P.; Brewster, M.E. Characterization of the interaction of 2-hydroxypropyl-β-cyclodextrin with itraconazole at pH 2, 4, and 7. J. Pharm. Sci. 2002, 91, 1414–1422. [Google Scholar] [CrossRef]
- National Library of Medicine, PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/beta-Cyclodextrin-sulfobutyl-ether#section=2D-Structure (accessed on 20 June 2025).
- National Library of Medicine, PubChem. Available online: https://pubchem.ncbi.nlm.nih.gov/compound/Hydroxypropyl-gamma-cyclodextrin (accessed on 20 June 2025).
- Sielc. alpha-CYCLODEXTRIN. 2025. Available online: https://sielc.com/alpha-cyclodextrin (accessed on 20 June 2025).
- Sielc. beta-CYCLODEXTRIN. 2025. Available online: https://sielc.com/b-cyclodextrin (accessed on 20 June 2025).
- Sielc. Gamma-Cyclodextrin. 2025. Available online: https://sielc.com/gamma-cyclodextrin (accessed on 20 June 2025).
- Jóhannsdóttir, S.; Saokham, P.; Magnúsdóttir, A.; Loftsson, T. Cyclodextrin complexes of a globular protein and a lipophilic oligopeptide: The effect of structure and physicochemical properties. Pharmazie 2017, 72, 575–580. [Google Scholar] [PubMed]
- Sciortino, M.T.; Dannenberger, D.; Santos Braga, S. Molecular Mind Games: The Medicinal Action of Cyclodextrins in Neurodegenerative Diseases. Biomolecules 2023, 13, 666. [Google Scholar] [CrossRef]
- Cirri, M.; Maestrelli, F.; Mura, P.; Ghelardini, C.; Mannelli, L.D.C. Combined Approach of Cyclodextrin Complexation and Nanostructured Lipid Carriers for the Development of a Pediatric Liquid Oral Dosage Form of Hydrochlorothiazide. Pharmaceutics 2018, 10, 287. [Google Scholar] [CrossRef]
- Lopalco, A.; Manni, A.; Keeley, A.; Haider, S.; Li, W.; Lopedota, A.; Altomare, C.D.; Denora, N.; Tuleu, C. In Vivo Inves-tigation of (2-Hydroxypropyl)-β-cyclodextrin-Based Formulation of Spironolactone in Aqueous Solution for Paediatric Use. Pharma-ceutics 2022, 14, 780. [Google Scholar] [CrossRef]
- Fereidounpour, P.; Steinmann, C.; Larsen, K.L. Prediction of the free energy of binding for cyclodextrin-steroid complexes: Phase solubility and molecular dynamics studies. J. Incl. Phenom. Macrocycl. Chem. 2024, 104, 535–546. [Google Scholar] [CrossRef]
- Hasson, K.J.; Ghareeb, M.M. Long Term Stability and In-vitro Release Study of Telmisartan Complex included by Hydroxypropyl-beta-cyclodextrin in Directly Compressed Tablet Using Ion-pair Reversed Phase High-performance Liquid Chromatography. Int. J. Drug Deliv. Technol. 2021, 11, 974–979. [Google Scholar]
- Iqbal, A.; Zaman, M.; Amjad, M.W.; Adnan, S.; Raja, M.A.G.; Rizvi, S.F.H.; Mustafa, M.W.; Farooq, U.; Abbas, G.; Shah, S. Solid Lipid Nanoparticles of Mycophenolate Mofetil: An Attempt to Control the Release of an Immunosuppressant. Int. J. Nanomed. 2020, 15, 5603–5612. [Google Scholar] [CrossRef]
- Altamimi, M.A.; Elzayat, E.M.; Alhowyan, A.A.; Alshehri, S.; Shakeel, F. Effect of β-cyclodextrin and different surfactants on solubility, stability, and permeability of hydrochlorothiazide. J. Mol. Liq. 2018, 250, 323–328. [Google Scholar] [CrossRef]
- Mohanan, J.; Palanichamy, S.; Kuttalingam, A.; Narayanasamy, D. Development and Characterization of tacrolimus tablet formulations for sublingual administration. Int. J. Appl. Pharm. 2021, 13, 89–97. [Google Scholar] [CrossRef]
- Bíró, T.; Horvát, G.; Budai-Szűcs, M.; Csányi, E.; Urbán, E.; Facskó, A.; Szabó-Révész, P.; Csóka, I.; Aigner, Z. Development of prednisolone-containing eye drop formulations by cyclodextrin complexation and antimicrobial, mucoadhesive biopolymer. Drug Des. Devel. Ther. 2018, 12, 2529–2537. [Google Scholar] [CrossRef] [PubMed]
- Muankaew, C.; Jansook, P.; Loftsson, T. Evaluation of γ-cyclodextrin effect on permeation of lipophilic drugs: Application of cellophane/fused octanol membrane. Pharm. Dev. Technol. 2016, 22, 562–570. [Google Scholar] [CrossRef]
- Masmoudi, S.; Faouzi, M.A.; Meddah, B.; Elbarge, M.; Bouayad, H.; Cherrah, Y.; Bouklouze, A. Inclusion complex of hydrochlorothiazide-γ-Cyclodextrin: The effect on aqueous solubility, dissolution rate, bioavailability and the effect on intestinal permeability using Ussing Chamber Technique. Int. J. Pharm. Pharm. Sci. 2013, 5, 718–724. [Google Scholar]
- Thi, T.D.; Nauwelaerts, K.; Froeyen, M.; Baudemprez, L.; Speybroeck, M.; Augustijns, P.; Annaert, P.; Martens, J.; Humbeeck, J.; Mooter, G. Comparison of the complexation between methylprednisolone and different cyclodextrins in solution by 1H-NMR and molecular modeling studies. J. Pharm. Sci. 2010, 99, 3863–3873. [Google Scholar] [CrossRef]
- Amar, M.J.A.; Kaler, M.; Courville, A.B.; Shamburek, R.; Sampson, M.; Remaley, A.T. Randomized double blind clinical trial on the effect of oral α-cyclodextrin on serum lipids. Lipids Health Dis. 2017, 15, 115, Erratum in Lipids Health Dis. 2017, 16, 45. [Google Scholar] [CrossRef] [PubMed]
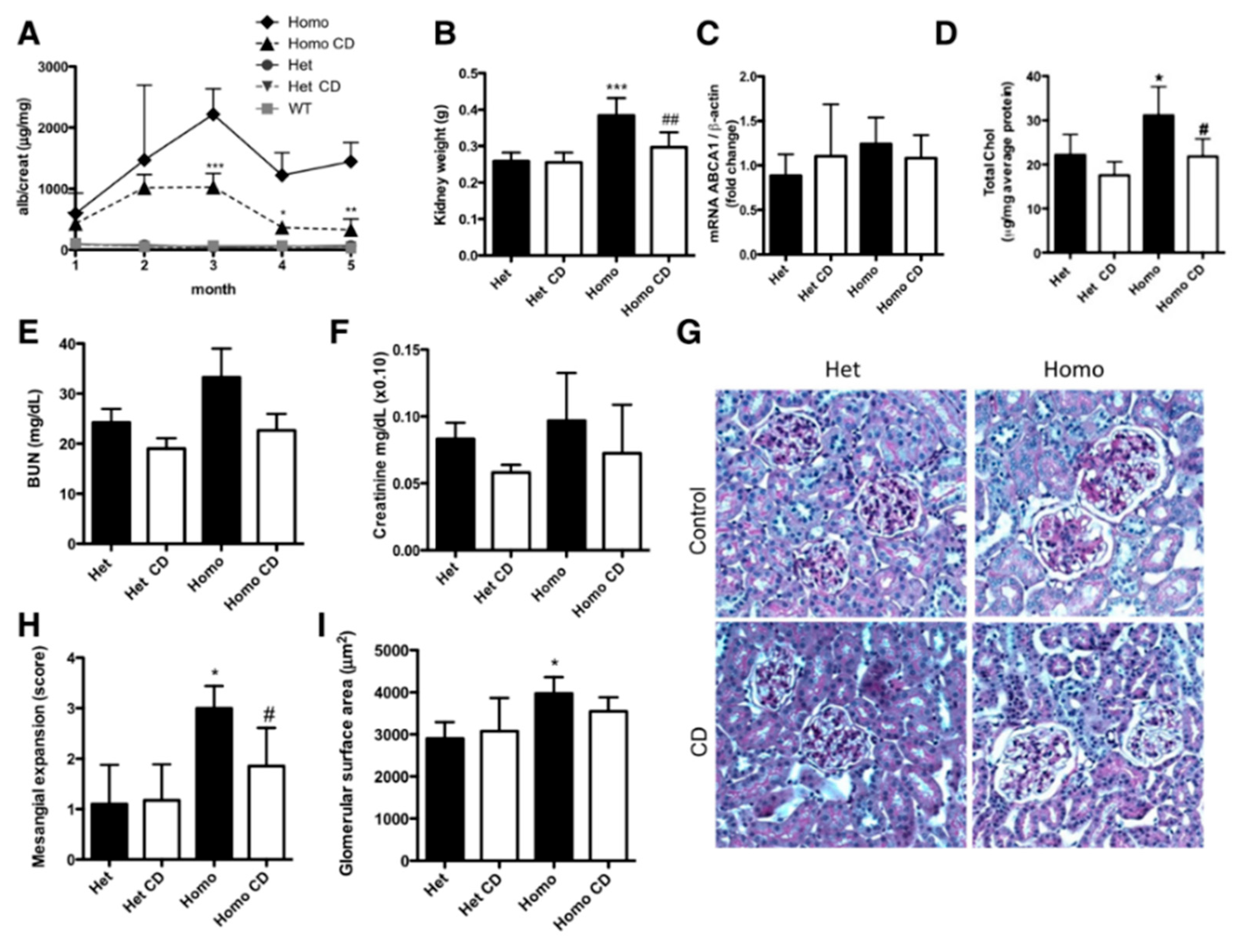
- Mitrofanova, A.; Molina, J.; Varona Santos, J.; Guzman, J.; Morales, X.A.; Ducasa, G.M.; Bryn, J.; Sloan, A.; Volosenco, I.; Kim, J.J.; et al. Hydroxypropyl-β-cyclodextrin protects from kidney disease in experimental Alport syndrome and focal segmental glomerulosclerosis. Kidney Int. 2018, 94, 1151–1159. [Google Scholar] [CrossRef]
- Merscher-Gomez, S.; Guzman, J.; Pedigo, C.E.; Lehto, M.; Aguillon-Prada, R.; Mendez, A.; Lassenius, M.I.; Forsblom, C.; Yoo, T.H.; Villarreal, R.; et al. Cyclodextrin protects podocytes in diabetic kidney disease. Diabetes 2013, 62, 3817–3827. [Google Scholar] [CrossRef]
- Müller-Deile, J.; Sarau, G.; Kotb, A.M.; Jaremenko, C.; Rolle-Kampczyk, U.E.; Daniel, C.; Kalkhof, S.; Christiansen, S.H.; Schiffer, M. Novel diagnostic and therapeutic techniques reveal changed metabolic profiles in recurrent focal segmental glomerulosclerosis. Sci. Rep. 2021, 11, 4577, Erratum in Sci. Rep. 2021, 11, 10693. [Google Scholar] [PubMed]
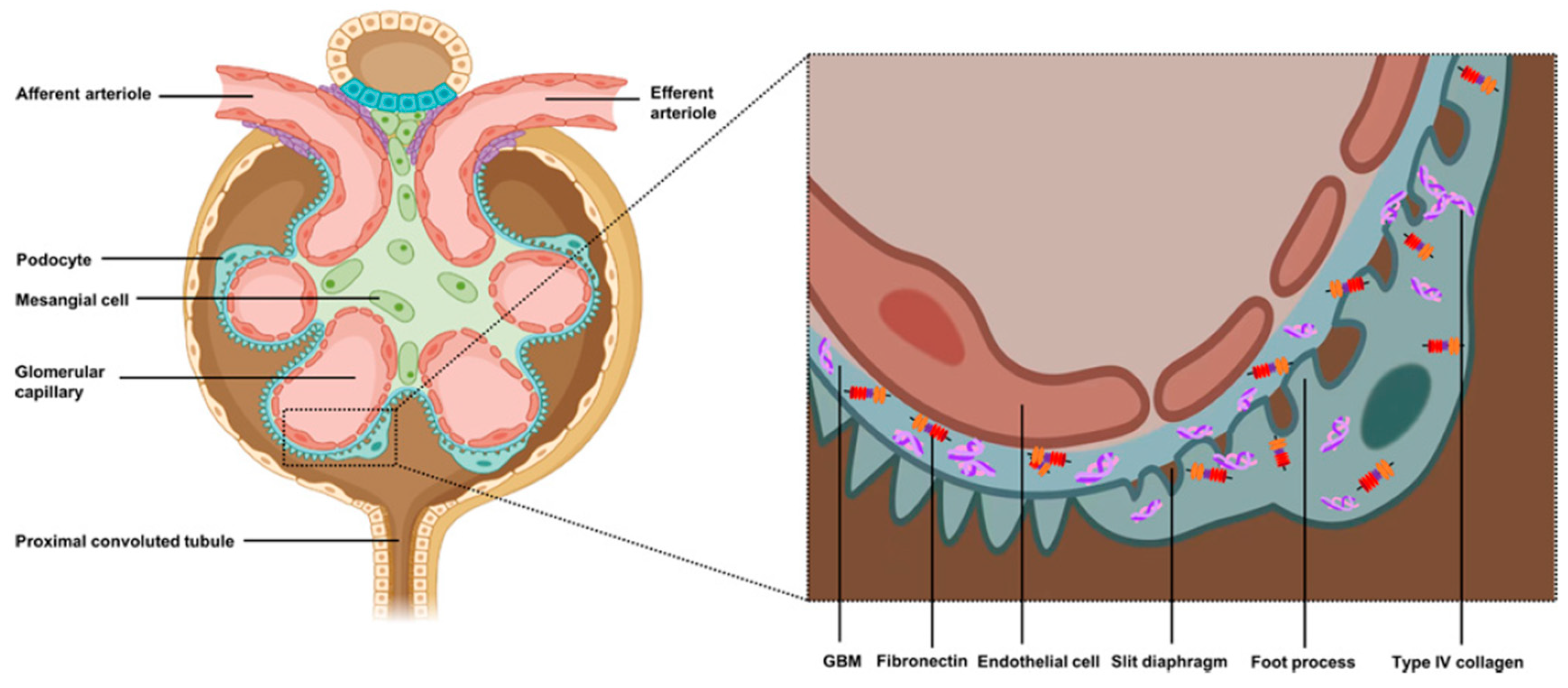


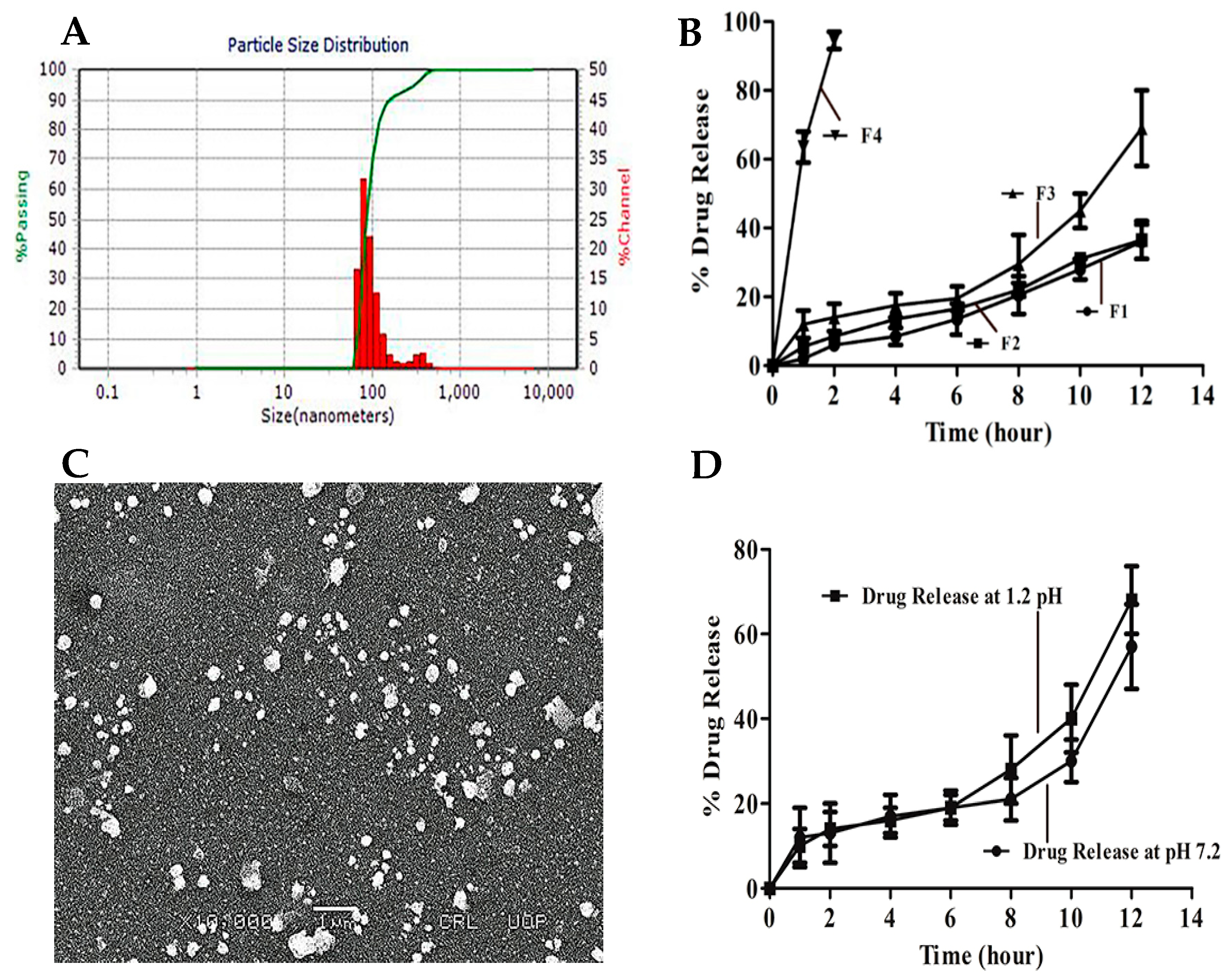
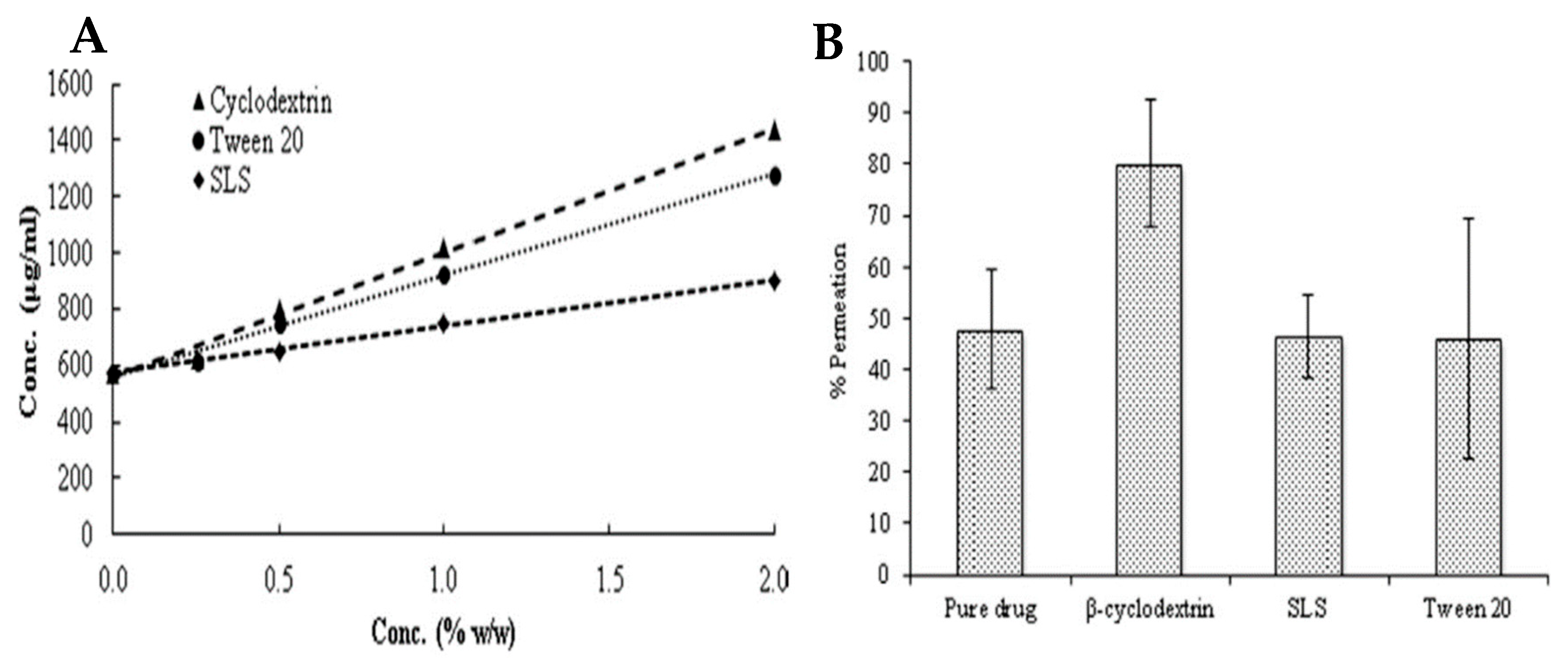
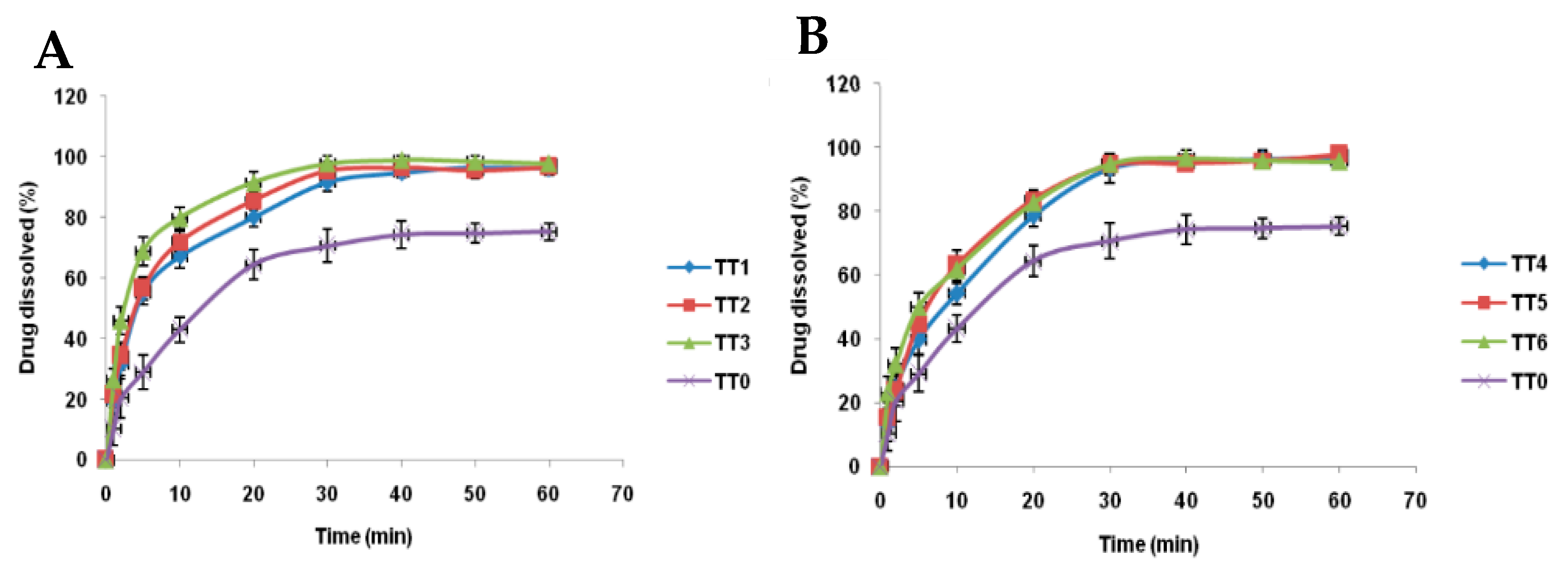
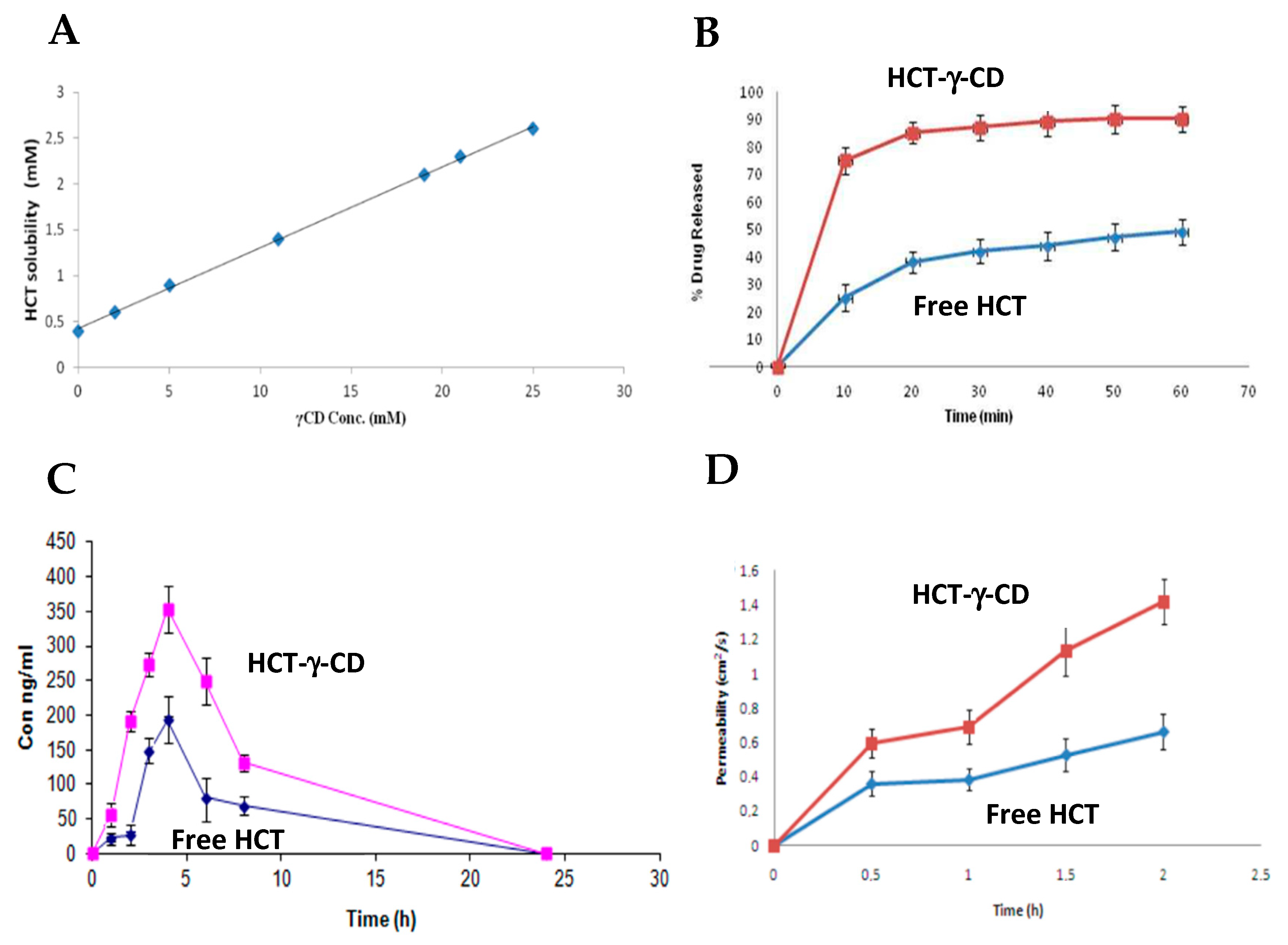
| Gene | Protein |
|---|---|
| NPHS1 | Nephrin |
| NPHS2 | Podocin |
| PLC 1ε | Phospholipase C 1ε |
| WT1 | Wilms tumor 1 |
| LAM 2β | Laminin 2β |
| PTP-RO | Protein tyrosine phosphatase receptor type O |
| ARHGDIA | Rho GDP dissociation inhibitor α |
| ADCK4 | AarF domain containing kinase 4 |
| EMP2 | Epithelial membrane protein 2 |
| ACTN4 | α-Actinin-4 |
| TRPC6 | Transient receptor potential cation channel 6 |
| CD2AP | CD2-associated protein |
| APOL1 | Apolipoprotein L1 |
| INF2 | Inverted formin-2 |
| MYO1E | Myosin 1E |
| PAX2 | Paired box gene 2 |
| ANLN | Anilin |
| CRB2 | Crumbs homolog 2 |
| Diagnostic Methods | Description | References | |
|---|---|---|---|
| Blood tests | Serum Creatinine and Glomerular Filtration Rate (GFR) | Assesses renal function; Creatinine may be normal early in the disease, but may increase as FSGS progresses to renal failure. | [32] |
| Serum albumin | It is usually reduced in patients with nephrotic syndrome associated with FSGS. | ||
| Circulating biomarkers | SuPAR (Soluble urokinase-type Plasminogen Activator Receptor) is a soluble receptor that, when at high levels, is associated with podocyte injury; It is the most relevant biomarker for FSGS and has strong diagnostic and prognostic potential. | [8] | |
| CD80 (Cluster of Differentiation 80) is a protein that helps differentiate FSGS from other podocyte pathologies, for example, Minimal Change Disease (MCD). | |||
| Urine tests | Urinary protein/ Creatinine ratio | Assesses proteinuria (>3.5 g/day) | [32] |
| Urinary biomarkers | Detects urinary podocin and nephrin, which indicate podocyte damage, and MCP-1 (Monocyte Chemoattractant Protein-1), which is a urinary inflammatory marker. | [10] | |
| Imaging exams | Helps rule out other causes of kidney disease. The kidneys may be normal in size in the early stages, but may lead to atrophy in later stages. | [8] | |
| Kidney biopsy | Optical microscopy | It detects glomerular size, microcystic tubular changes, tubular hypertrophy and morphological variants of FSGS. | [8] |
| Immunofluorescence microscopy | It rules out other primary glomerulopathies. | ||
| Electron microscopy | It reveals fusion of pedicels, cytoplasmic projections of podocytes, characteristics of FSGS, microvillous transformation of podocytes, tubuloreticular inclusions; It differentiates FSGS from MCD (it has diffused fusion without glomerular sclerosis). | ||
| Genetic testing | It allows for more appropriate therapy for patients, for example, avoiding glucocorticoids (except in genetic forms that may be responsive), a better prognosis (typical results of the native kidney and the likelihood of resorting to a kidney transplant) and identification of family history (identification of the disease in other family members and prenatal testing); Recommended when there is a family history to identify gene mutations associated with autosomal dominant mutations, such as Interferon (IFN) and α-Actinin-4 (ACTN4). | [8] | |
| Morphological Variant | Characterization | Type of FSGS |
|---|---|---|
| Not Otherwise Specified—NOS | The most common form, progressive podocyte injury | Primary |
| Collapsing | Aggressive form, with glomerular collapse and proliferation of epithelial cells. Associated with HIV, viral infections and genetic mutations | Primary; Virus-mediated; Associated with pharmaceuticals; Associated with APOL1 |
| Perihilar | Characterized by sclerosis predominantly around the hilum (point of entry and exit of blood vessels in the glomerulus) and is commonly associated with obesity and hypertension. Also associated with hyperfiltration | Adaptive |
| Cellular | It is the most difficult lesion to identify and is marked by the presence of an increase in cells within the glomeruli and is generally secondary to an inflammatory process | Primary; Adaptive |
| Tip-Lesion | Associated with intense proteinuria, with lesions close to the proximal tubule | Primary |
| Type of Remission | Proteinuria | GFR | Serum Albumin |
|---|---|---|---|
| Complete Remission (CR) | <0.3 g/day or <0.3 g/g creatinine | Stable | Disappearance of oedema, normalization of albumin (>3.5 g/dL) and lipids |
| Partial Remission (PR) | 0.3–3.5 g/day or 0.3–3.5 g/g creatinine, each with a >50% reduction in albuminuria from baseline | Stable | Disappearance of oedema, normalization of albumin and lipids |
| Response Types | Clinical and Laboratory Criteria |
|---|---|
| Resistance | Persistence of oedema, hypoalbuminemia, dyslipidemia and 24 h proteinuria >3.5 g |
| Recurrence | After the partial or total response, a new nephrotic outbreak appears (common: two relapses in six months or four relapses in 12 months) |
| Corticosteroid dependence | Two or more relapses during the corticosteroid dose reduction period, or two consecutive relapses, occurring within two weeks of the end of corticosteroid therapy |
| Type of Therapy | Pharmaco-Therapeutic Classification | Examples of Drugs | Drug Action in FSGS | References |
|---|---|---|---|---|
| Symptomatic | Angiotensin-Converting Enzyme Inhibitor (ACEi) | Ramipril | Treat blood pressure and reduce proteinuria | [8,53] |
| Angiotensin II Receptor Antagonist (ARA) | Telmisartan Ibersartan Sparsentan | [8,54] | ||
| Thiazide Diuretics | Hydrochloro-thiazide; Chlorthalidone | Control oedema and promote fluid excretion, boosting the effects of RAAS inhibitors | [8] | |
| Immunosuppressive | Glucocorticoids (GCs) | Prednisolone Methylprednisolone | They reduce inflammation and the immune response to prevent the formation of new scars | [22] |
| Calcineurin Inhibitors (ICNs) | Cyclosporine A Tacrolimus | Calcineurin is part of the T cell signaling pathway and participates in the activation of IL-2 production, promoting an immune response in various cell types | [55] | |
| Antiproliferation and antimetabolic medicines | Mycophenolate mofetil | It inhibits purine synthesis, which reduces the proliferation of T and B cells and, consequently, the immune response and proteinuria | [22,32] | |
| Biological medicines/Monoclonal antibodies | Baliximab Rituximab | Allow for the elimination of B cells and the interruption of interactions between B cells and T cells that cause proteinuria | [22,44] | |
| Alkylating agents | Cyclophosphamide | Interferes with DNA replication, causing cellular damage that can reduce the inflammatory and immune response, decreasing the progression of kidney disease and proteinuria | [22] |
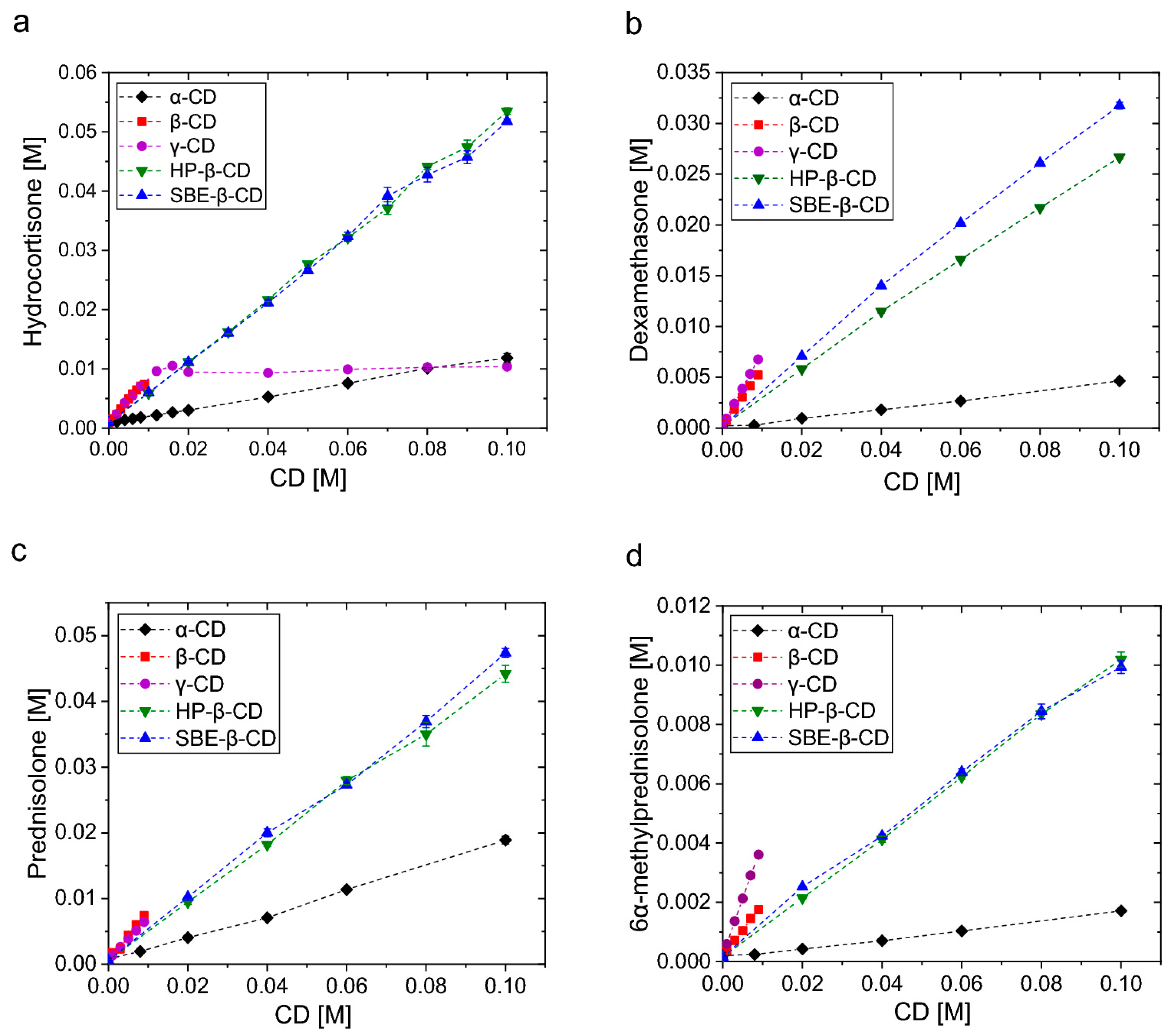
| Cyclodextrin | Alpha (α) | Beta (β) | Gamma (γ) |
|---|---|---|---|
| Height (nm) | 0.78 | 0.78 | 0.78 |
| Molecular Formula | C(36)H(60)O30 | C(42)H(70)O35 | C(48)H(80)O40 |
| Physicochemical characterization | Hydrophilic; Homogeneous and crystalline substances | Hydrophilic; Homogeneous and crystalline substances | Hydrophilic; Homogeneous and crystalline substances |
| Chemical composition | 6 units of cyclic oligosaccharides | 7 units of cyclic oligosaccharides | 8 units of cyclic oligosaccharides |
| Inner Diameter (nm) | 0.47–0.53 | 0.60–0.65 | 0.75–0.83 |
| Outer Diameter (nm) | 1.46 | 1.54 | 1.75 |
| Cyclodextrin | CE | Solubility (mg/mL) in the Presence of 5% (w/v) CDs | Solubility (mg/mL) in the Presence of 15% (w/v) CDs |
|---|---|---|---|
| α | 0.54 | 0.76 ± 0.02 | 4.22 ± 0.59 |
| β | 0.030 | Not Soluble | Not Soluble |
| γ | 0.0049 | 0.062 ± 0.001 | 0.11 ± 0.00 |
| Drug/Inclusion Complex | Drug Content (%w/w) | Aqueous Solubility (μg/mL) |
|---|---|---|
| Tacrolimus | N.A | 3.05 ± 0.1210 |
| Tacrolimus with β-CD | 96.84 ± 1.76 | 14.82 ± 0.8890 |
| Cyclodextrin | Drug/Active Ingredient | In Vitro Study | In Vivo Study | Results | References |
|---|---|---|---|---|---|
| α | Cyclosporine A | “Cyclodextrin complexes of a globular protein and a lipophilic oligopeptide: the effect of structure and physicochemical properties” | N.A | The solubility of the drug was increased; It was also found that the drug stabilized | [73] |
| Prednisolone | “Prediction of the free energy of binding for cyclodextrin-steroid complexes: phase solubility and molecular dynamics studies” | N.A | The solubility profile of the drug increased | [77] | |
| β | Hydrochlorothiazide | “Effect of β-cyclodextrin and different surfactants on solubility, stability, and permeability of hydrochlorothiazide” | N.A | β-Cyclodextrin increased the solubility, permeability and stability of the drug | [75] |
| “Combined Approach of Cyclodextrin Complexation and Nanostructured Lipid Carriers For the Development of Pediatric Liquid Oral Dosage Form of Hydrochlorothiazide” | N.A | Hydrochlorothiazide, when encapsulated in the (2-hydroxy)propyl-β-cyclodextrin is released in a more controlled and complete way, compared to when this drug is administered on its own without the use of any type of cyclodextrin complexation system | [80] | ||
| Mycophenolate Mofetil | “Solid Lipid Nanoparticles of Mycophenolate Mofetil: An Attempt to Control the Release of an Immunosuppressant” | N.A | β-Cyclodextrin increased the solubility of the drug and it is more easily released when combined with the β-cyclodextrin | [79] | |
| Spironolactone | N.A | “In Vivo Investigation of (2-Hydroxypropyl)-β-cyclodextrin Based Formulation of Spirinolactone in Aqueous Solution for Pediatric Use” | The (2-hydroxy)propyl-β-cyclodextrin increased the bioavailability, solubility and dissolution rate of spironolactone. There were no beneficial effects of this cyclodextrin in masking the flavor of oral formulations | [76] | |
| Cyclosporine A | “Cyclodextrin complexes of a globular protein and a lipophilic oligopeptide: the effect of structure and physicochemical properties” | N.A | The solubility of the drug was increased; It was also found that the drug stabilized | [73] | |
| Telmisartan | “Long Term Stability and In-vitro release study of telmisartan complex included by hydroxypropyl-beta-cyclodextrin in directly compressed tablet using ion-pair reversed phase high-performance liquid chromatography” | N.A | The dissolution rate of the drug increased; It was also found that due to CD the long-term stability of the drug improved, with no changes in its physical and chemical properties | [78] | |
| Tacrolimus | “Development and characterization of tacrolimus tablet formulations for sublingual administration” | N.A | There was an increase in the solubility of the drug as well as in the speed of disintegration; Dissolution profiles also showed improvements | [81] | |
| γ | Hydrochlorothiazide, Telmisartan and Ibersartan | “Evaluation of γ-cyclodextrin effect on permeation of lipophilic drugs: application of cellophane/fused octanol membrane” | N.A | γ-Cyclodextrin increased the permeability of all the drugs tested. However, this increase in permeability depended on the properties of each drug | [83] |
| Hydrochlorothiazide | N.A | “Inclusion complex of hydrochlorothiazide-γ-Cyclodextrin: The effect on aqueous solubility, dissolution rate, bioavailability and the effect on intestinal permeability using Ussing Chamber Technique” | γ-Cyclodextrin increased the aqueous solubility as well as the dissolution rate, bioavailability and permeability of the drug | [84] | |
| Cyclosporine A | “Cyclodextrin complexes of a globular protein and a lipophilic oligopeptide: the effect of structure and physicochemical properties” | N.A | The solubility of the drug was increased; It was also found that the drug stabilized | [73] | |
| Prednisolone | “Development of prednisolone-containing eye drop formulations by cyclodextrin complexation and antimicrobial, mucoadhesive biopolymer” | N.A | The solubility and the bioavailability of the drug were increased. In addition, there was an increase in the diffusion of this drug in the corneal cell membrane | [82] | |
| Methylprednisolone | “Comparison of the complexation between methylprednisolone and different cyclodextrins in solution by 1H-NMR and molecular modelling studies” | N.A | The aqueous solubility of the drug increased | [85] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mascarenhas-Melo, F.; Martins, B.; Monteiro, I.; Lohani, A.; Krambeck, K. Focal Segmental Glomerulosclerosis: Comprehensive Review and Exploration of the Dual Potential of Cyclodextrins in Therapeutic Optimization. Int. J. Mol. Sci. 2025, 26, 8760. https://doi.org/10.3390/ijms26188760
Mascarenhas-Melo F, Martins B, Monteiro I, Lohani A, Krambeck K. Focal Segmental Glomerulosclerosis: Comprehensive Review and Exploration of the Dual Potential of Cyclodextrins in Therapeutic Optimization. International Journal of Molecular Sciences. 2025; 26(18):8760. https://doi.org/10.3390/ijms26188760
Chicago/Turabian StyleMascarenhas-Melo, Filipa, Bruna Martins, Inês Monteiro, Alka Lohani, and Karolline Krambeck. 2025. "Focal Segmental Glomerulosclerosis: Comprehensive Review and Exploration of the Dual Potential of Cyclodextrins in Therapeutic Optimization" International Journal of Molecular Sciences 26, no. 18: 8760. https://doi.org/10.3390/ijms26188760
APA StyleMascarenhas-Melo, F., Martins, B., Monteiro, I., Lohani, A., & Krambeck, K. (2025). Focal Segmental Glomerulosclerosis: Comprehensive Review and Exploration of the Dual Potential of Cyclodextrins in Therapeutic Optimization. International Journal of Molecular Sciences, 26(18), 8760. https://doi.org/10.3390/ijms26188760








