Metabolomic Effects and Their Relationship with Intracellular/Extracellular Concentrations of Casiopeinas® in Triple-Negative Mesenchymal Breast Cancer
Abstract
1. Introduction
2. Results
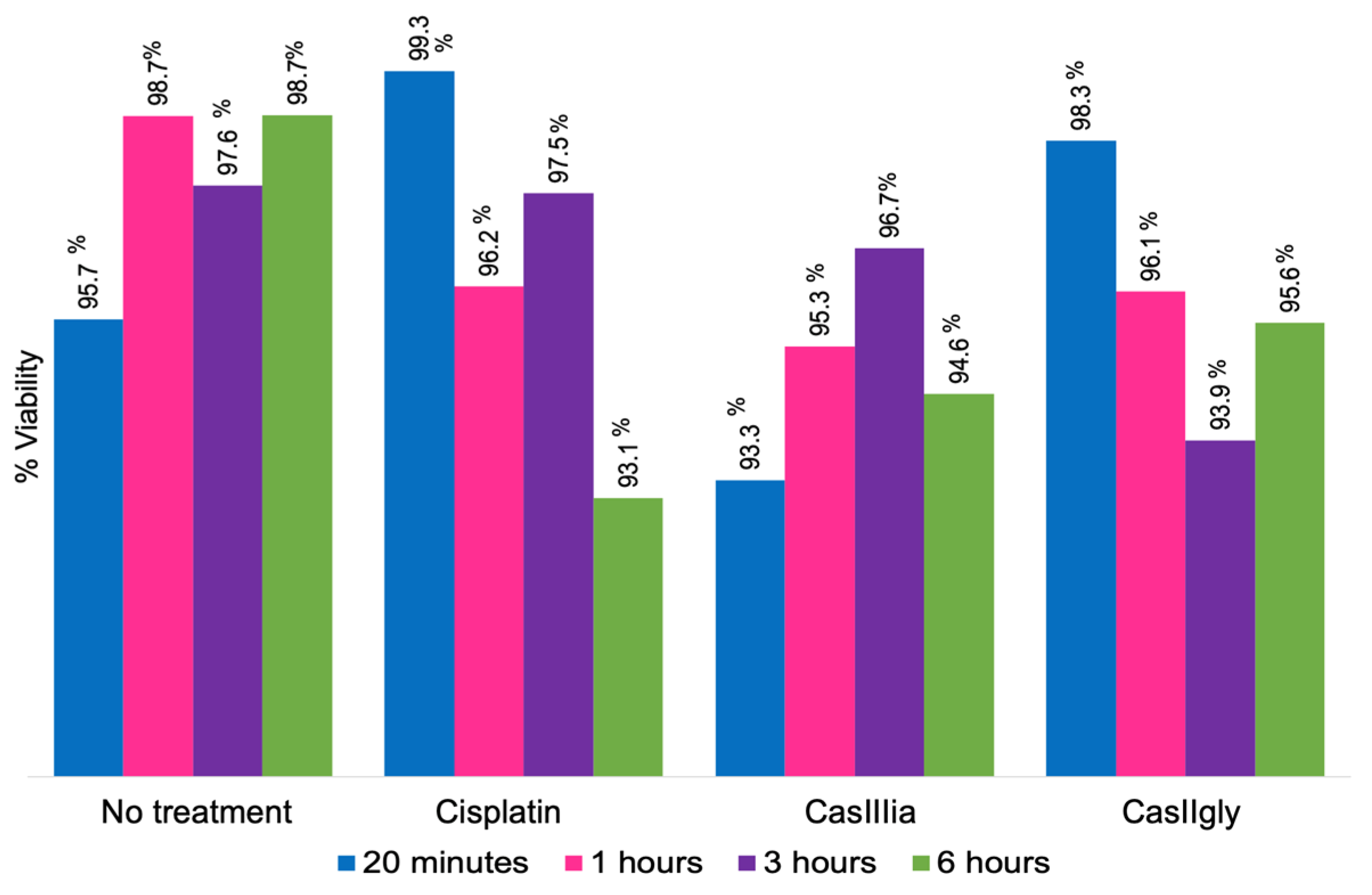
2.1. Treatment of MDA-MB-21 Cells
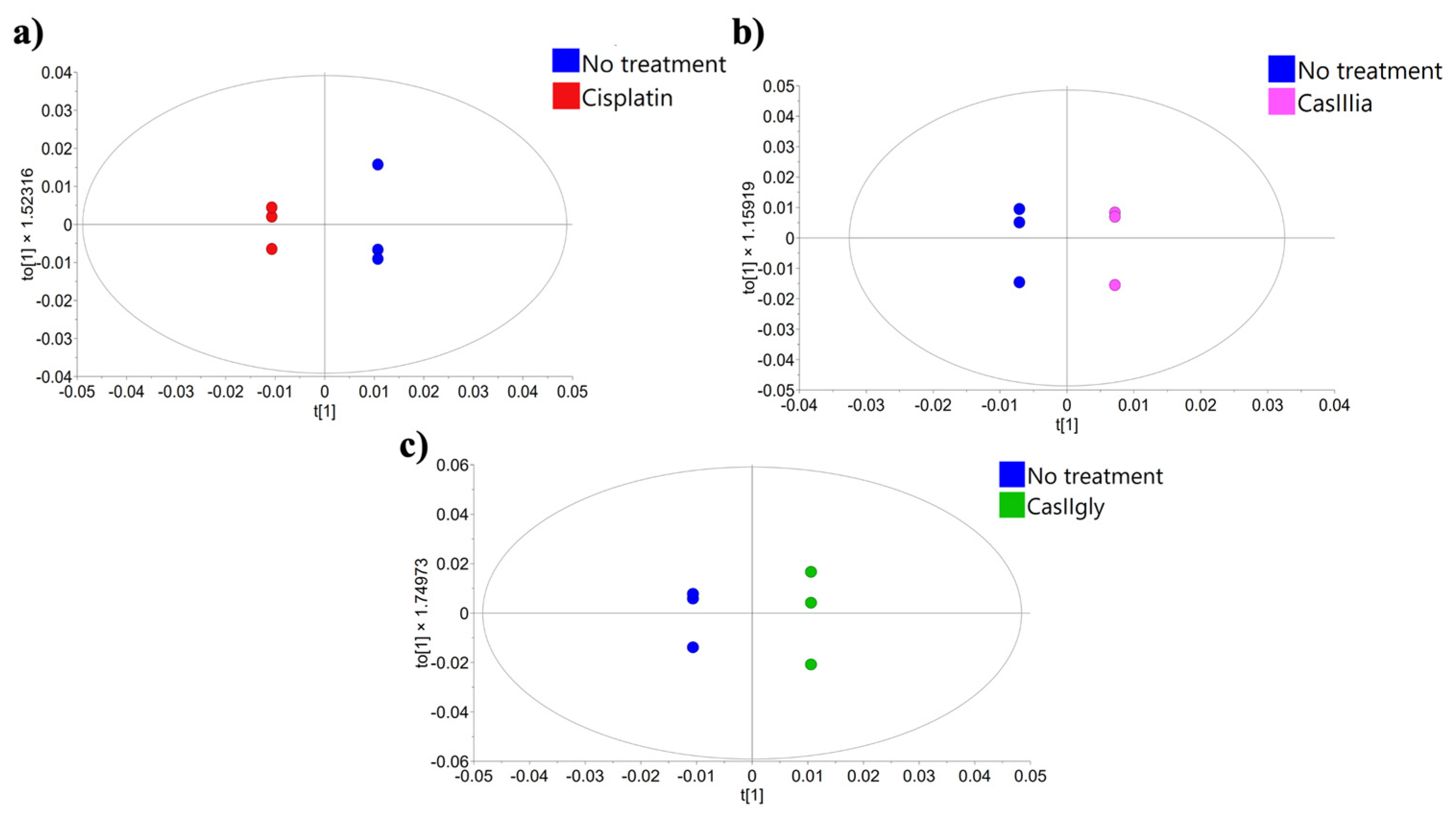
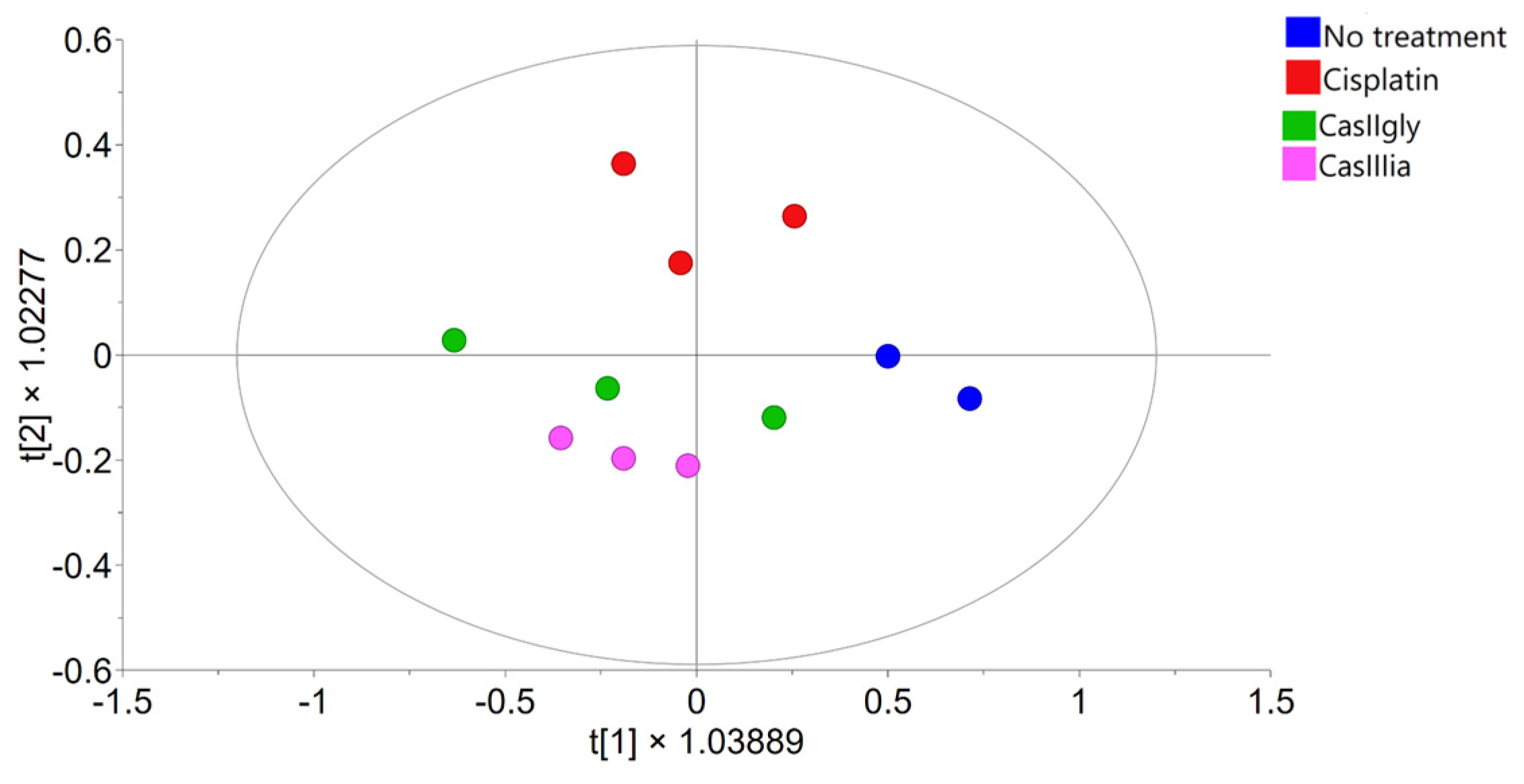
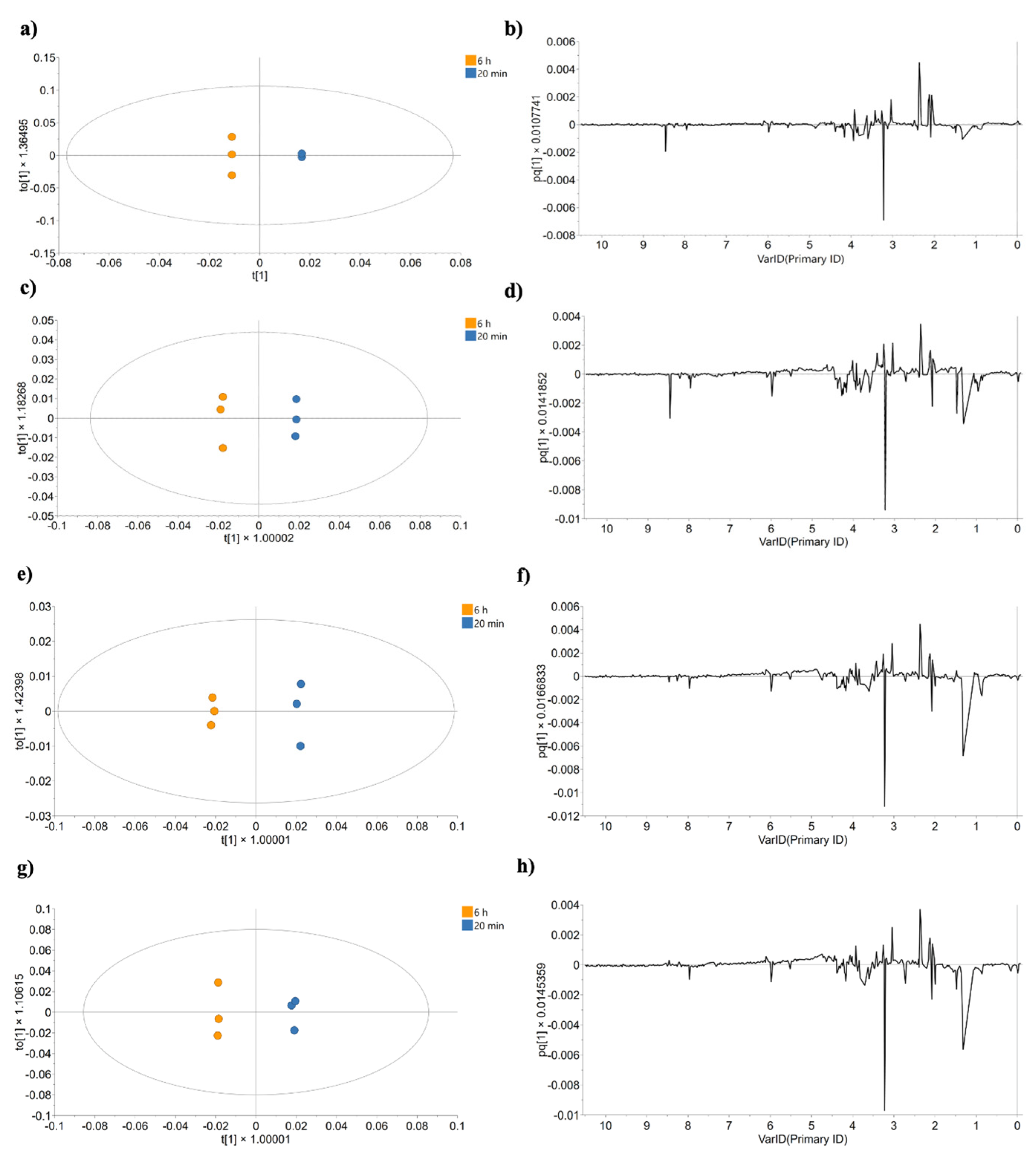
2.2. Statistical Data Processing
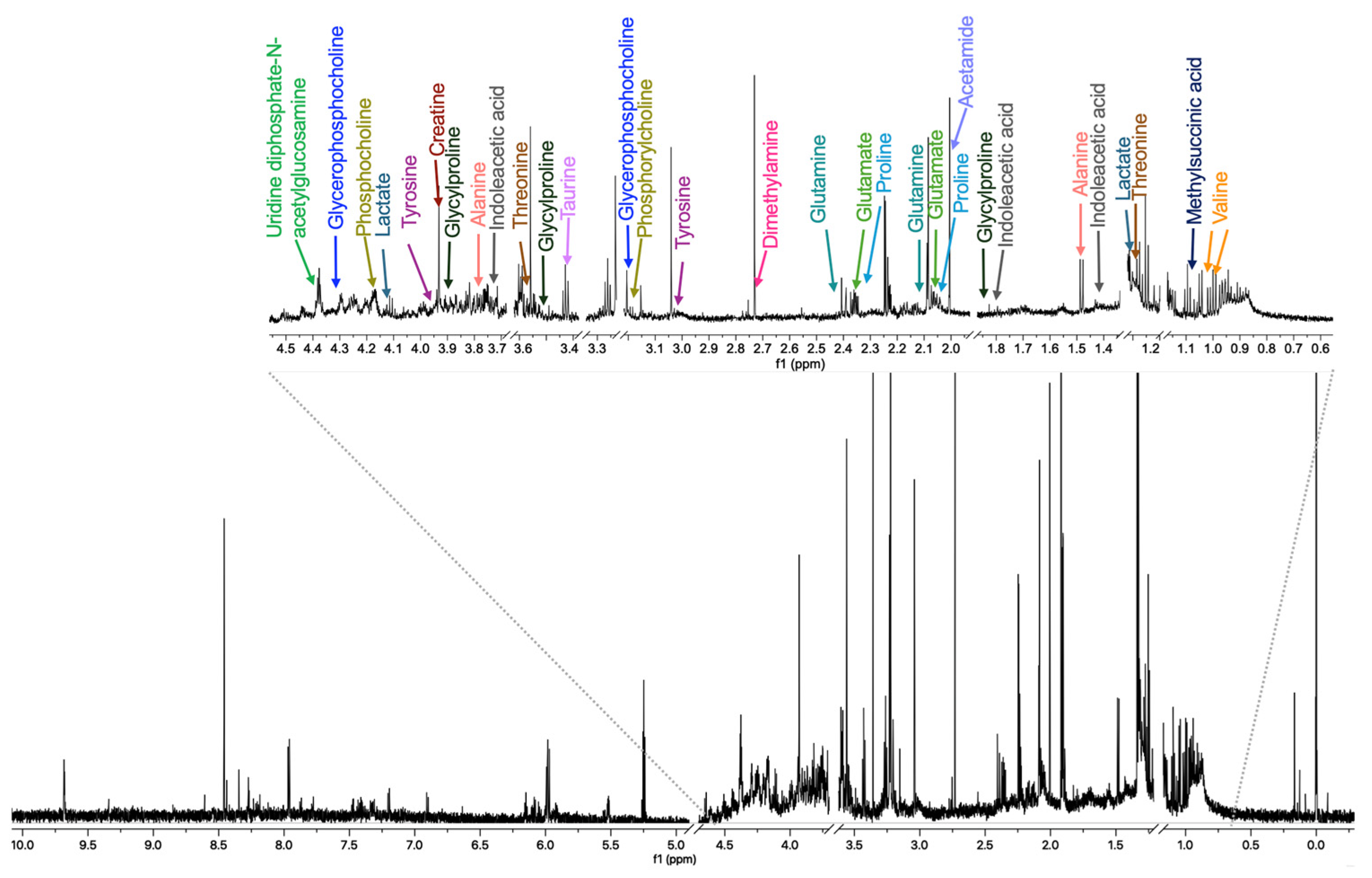
2.3. Metabolite Identification and Quantification
2.4. Analysis of Altered Pathways
2.4.1. Analysis of Effects up to 20 min Post-Treatment
2.4.2. Analysis of Effects up to 6 h Post-Treatment
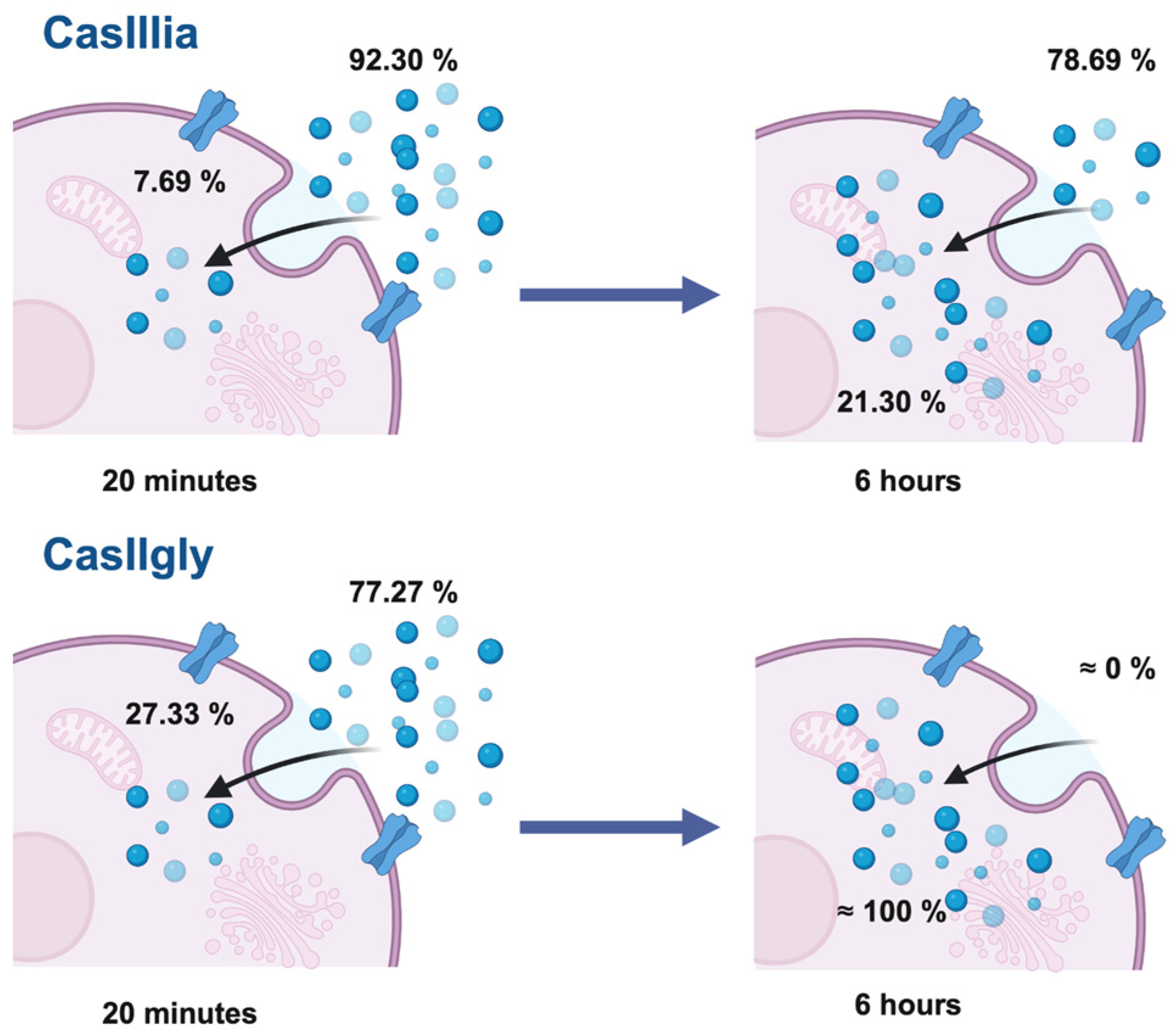
2.5. Quantification of Copper Associated with Casiopeinas®
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Determination of the Half-Maximal Inhibitory Concentration (IC50)
4.3. Viability Assays
4.4. Intracellular Metabolite Extraction
4.5. 1H-NMR Analysis
4.6. Statistical Analysis
4.7. Metabolite and Metabolic Pathway Identification
4.8. ICP-MS Analysis
Copper Quantification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TNBC | Triple-Negative Breast Cancer |
| EMT | Epithelial–mesenchymal transition |
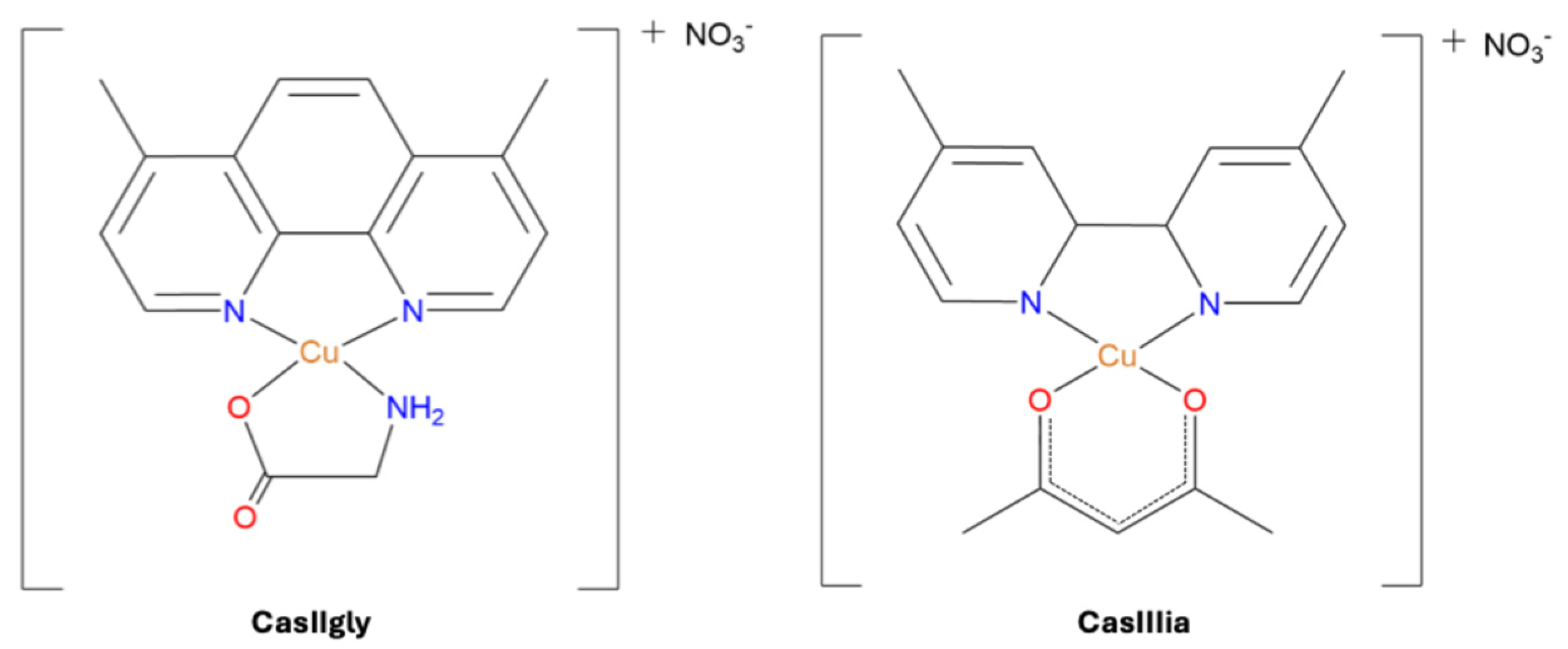
| CasIIIia | 4,4′-dimethyl-2,2′-bipridine acetylacetonate copper(II) monohydrate nitrate |
| CasIIgly | 4,7-dimethyl-1,10-phenanthroline glycinate copper(II) nitrate dihydrate |
| ATP | Adenosine triphosphate |
| NADPH | Nicotinamide adenine dinucleotide phosphate |
| HER2 | Human epidermal growth factor receptor 2 |
| BL1 | Basal-like 1 |
| BL2 | Basal-like 2 |
| M | Mesenchymal-like |
| LAR | Luminal androgen receptor |
| MDA-MB-231 | Breast adenocarcinoma |
| TCA cycle | Citric acid cycle |
| GSH | Glutathione |
| FAO | Fatty acid oxidation |
| DNA | Deoxyribonucleic acid |
| ROS | Reactive oxygen species |
| HeLa | Human cervical adenocarcinoma |
| PI3K | Phosphatidylinositol-3-kinase |
| AKT | Protein kinase B |
| mTOR | Mammalian target of rapamycin |
| PI3K/AKT/mTOR pathway | Phosphatidylinositol 3-kinase/protein kinase B/mammalian target of rapamycin pathway |
| 1H-NMR | Proton nuclear magnetic resonance |
| ICP-MS | Inductively coupled plasma mass spectrometry |
| IC50 | Half-maximal inhibitory concentration |
| SD | Standard deviation |
| PCA | Principal component analysis |
| PLS-DA | Partial least squares discriminant analysis |
| OPLS-DA | Orthogonal partial least squares discriminant analysis |
| VIP | Variable importance in projection |
| DMEM-F12 | Dulbecco’s Modified Eagle Medium |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide |
| TSP | Sodium 3-(trimethylsilyl)(2H4)propanoate |
| R2 | Goodness of fit parameter |
| Q2 | Variation predicted by a component |
| QEA | Quantitative enrichment analysis |
| PBS | Phosphate-Buffered Saline |
| HPS | High-Purity Standard |
References
- Mjos, K.D.; Orvig, C. Metallodrugs in Medicinal Inorganic Chemistry. Chem. Rev. 2014, 114, 4540–4563. [Google Scholar] [CrossRef] [PubMed]
- Kabir, E.; Noyon, M.R.O.K.; Hossain, M.A. Synthesis, Biological and Medicinal Impacts of Metallodrugs: A Study. Results Chem. 2023, 5, 100935. [Google Scholar] [CrossRef]
- Anthony, E.J.; Bolitho, E.M.; Bridgewater, H.E.; Carter, O.W.L.; Donnelly, J.M.; Imberti, C.; Lant, E.C.; Lermyte, F.; Needham, R.J.; Palau, M.; et al. Metallodrugs Are Unique: Opportunities and Challenges of Discovery and Development. Chem. Sci. 2020, 11, 12888–12917. [Google Scholar] [CrossRef]
- Adhikari, S.; Nath, P.; Das, A.; Datta, A.; Baildya, N.; Duttaroy, A.K.; Pathak, S. A Review on Metal Complexes and Its Anti-Cancer Activities: Recent Updates from in Vivo Studies. Biomed. Pharmacother. 2024, 171, 116211. [Google Scholar] [CrossRef] [PubMed]
- Bernal, G.; Aquea, G.; Ramírez-Rivera, S. Metal-Based Molecules in the Treatment of Cancer: From Bench to Bedside. Oncol. Res. 2025, 33, 759–779. [Google Scholar] [CrossRef]
- Lucaciu, R.L.; Hangan, A.C.; Sevastre, B.; Oprean, L.S. Metallo-Drugs in Cancer Therapy: Past, Present and Future. Molecules 2022, 27, 6485. [Google Scholar] [CrossRef]
- Garufi, A.; Scarpelli, F.; Ricciardi, L.; Aiello, I.; D’Orazi, G.; Crispini, A. New Copper-Based Metallodrugs with Anti-Invasive Capacity. Biomolecules 2023, 13, 1489. [Google Scholar] [CrossRef]
- Pereira, D.; Alves, N.; Sousa, Â.; Valente, J.F.A. Metal-Based Approaches to Fight Cervical Cancer. Drug Discov. Today 2024, 29, 104073. [Google Scholar] [CrossRef]
- González-Ballesteros, M.M.; Mejía, C.; Ruiz-Azuara, L. Metallodrugs: An Approach against Invasion and Metastasis in Cancer Treatment. FEBS Open Bio 2022, 12, 880–899. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Biological Hallmarks of Cancer. In Holland-Frei Cancer Medicine; Wiley: Hoboken, NJ, USA, 2017; pp. 1–10. [Google Scholar]
- Meirson, T.; Gil-Henn, H.; Samson, A.O. Invasion and Metastasis: The Elusive Hallmark of Cancer. Oncogene 2020, 39, 2024–2026. [Google Scholar] [CrossRef] [PubMed]
- Pavlova, N.N.; Zhu, J.; Thompson, C.B. The Hallmarks of Cancer Metabolism: Still Emerging. Cell Metab. 2022, 34, 355–377. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar] [CrossRef]
- Plathow, C.; Weber, W.A. Tumor Cell Metabolism Imaging. J. Nucl. Med. 2008, 49, 43S–63S. [Google Scholar] [CrossRef]
- Wang, Y.; Xia, Y.; Lu, Z. Metabolic Features of Cancer Cells 06 Biological Sciences 0601 Biochemistry and Cell Biology. Cancer Commun. 2018, 38, 65. [Google Scholar]
- Romero-Garcia, S.; Lopez-Gonzalez, J.S.; Báez-Viveros, J.L.; Aguilar-Cazares, D.; Prado-Garcia, H. Tumor Cell Metabolism: An Integral View. Cancer Biol. Ther. 2011, 12, 939–948. [Google Scholar] [CrossRef]
- DeBerardinis, R.J.; Lum, J.J.; Hatzivassiliou, G.; Thompson, C.B. The Biology of Cancer: Metabolic Reprogramming Fuels Cell Growth and Proliferation. Cell Metab. 2008, 7, 11–20. [Google Scholar] [CrossRef]
- Tufail, M.; Jiang, C.H.; Li, N. Altered Metabolism in Cancer: Insights into Energy Pathways and Therapeutic Targets. Mol. Cancer 2024, 23, 203. [Google Scholar] [CrossRef]
- Newton, E.E.; Mueller, L.E.; Treadwell, S.M.; Morris, C.A.; Machado, H.L. Molecular Targets of Triple-Negative Breast Cancer: Where Do We Stand? Cancers 2022, 14, 482. [Google Scholar] [CrossRef]
- Yin, L.; Duan, J.J.; Bian, X.W.; Yu, S.C. Triple-Negative Breast Cancer Molecular Subtyping and Treatment Progress. Breast Cancer Res. 2020, 22, 61. [Google Scholar] [CrossRef] [PubMed]
- Dogra, A.K.; Prakash, A.; Gupta, S.; Gupta, M. Prognostic Significance and Molecular Classification of Triple Nega tive Breast Cancer: A Systematic Review. Eur. J. Breast Health 2025, 21, 101–114. [Google Scholar]
- Zhao, S.; Zuo, W.-J.; Shao, Z.-M.; Jiang, Y.-Z. Molecular Subtypes and Precision Treatment of Triple-Negative Breast Cancer. Ann. Transl. Med. 2020, 8, 499. [Google Scholar] [CrossRef]
- Lehmann, B.D.; Pietenpol, J.A.; Tan, A.R. Triple-Negative Breast Cancer: Molecular Subtypes and New Targets for Therapy; American Society of Clinical Oncology: Alexandria, VA, USA, 2015. [Google Scholar]
- Mezi, S.; Botticelli, A.; Pomati, G.; Cerbelli, B.; Scagnoli, S.; Amirhassankhani, S.; D’amati, G.; Marchetti, P. Standard of Care and Promising New Agents for the Treatment of Mesenchymal Triple-Negative Breast Cancer. Cancers 2021, 13, 1080. [Google Scholar] [CrossRef]
- Liu, S.; Dong, Y.; Wang, Y.; Hu, P.; Wang, J.; Wang, R.Y. Pristimerin Exerts Antitumor Activity against MDA-MB-231 Triple-Negative Breast Cancer Cells by Reversing of Epithelial-Mesenchymal Transition via Downregulation of Integrin Β3. Biomed. J. 2021, 44, S84–S92. [Google Scholar] [CrossRef]
- Kalluri, R.; Weinberg, R.A. The Basics of Epithelial-Mesenchymal Transition. J. Clin. Investig. 2009, 119, 1420–1428. [Google Scholar] [CrossRef] [PubMed]
- Nie, F.; Sun, X.; Sun, J.; Zhang, J.; Wang, Y. Epithelial-Mesenchymal Transition in Colorectal Cancer Metastasis and Progression: Molecular Mechanisms and Therapeutic Strategies. Cell Death Discov. 2025, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Shum, H.C.E.; Wu, K.; Vadgama, J. From Interaction to Intervention: How Mesenchymal Stem Cells Affect and Target Triple-Negative Breast Cancer. Biomedicines 2023, 11, 1182. [Google Scholar] [CrossRef] [PubMed]
- Castaneda, M.; den Hollander, P.; Kuburich, N.A.; Rosen, J.M.; Mani, S.A. Mechanisms of Cancer Metastasis. Semin. Cancer Biol. 2022, 87, 17–31. [Google Scholar] [CrossRef]
- Roshanzamir, F.; Robinson, J.L.; Cook, D.; Hossein Karimi-Jafari, M.; Nielsen, J. Metastatic Triple Negative Breast Cancer Adapts Its Metabolism to Destination Tissues While Retaining Key Metabolic Signatures. Proc. Natl. Acad. Sci. USA 2022, 119, e2205456119. [Google Scholar] [CrossRef]
- Obidiro, O.; Battogtokh, G.; Akala, E.O. Triple Negative Breast Cancer Treatment Options and Limitations: Future Outlook. Pharmaceutics 2023, 15, 1796. [Google Scholar] [CrossRef]
- Munkácsy, G.; Santarpia, L.; Győrffy, B. Therapeutic Potential of Tumor Metabolic Reprogramming in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2023, 24, 6945. [Google Scholar] [CrossRef]
- Nong, S.; Han, X.; Xiang, Y.; Qian, Y.; Wei, Y.; Zhang, T.; Tian, K.; Shen, K.; Yang, J.; Ma, X. Metabolic Reprogramming in Cancer: Mechanisms and Therapeutics. MedComm 2023, 4, e218. [Google Scholar] [CrossRef]
- Garg, P.; Singhal, G.; Horne, D.; Salgia, R.; Singhal, S.S. Metabolic Reprogramming in Breast Cancer: Pathways Driving Progression, Drug Resistance, and Emerging Therapeutics. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2025, 1880, 189396. [Google Scholar] [CrossRef]
- Kooshan, Z.; Cárdenas-Piedra, L.; Clements, J.; Batra, J. Glycolysis, the Sweet Appetite of the Tumor Microenvironment. Cancer Lett. 2024, 600, 217156. [Google Scholar] [CrossRef] [PubMed]
- Sun, X.; Wang, M.; Wang, M.; Yu, X.; Guo, J.; Sun, T.; Li, X.; Yao, L.; Dong, H.; Xu, Y. Metabolic Reprogramming in Triple-Negative Breast Cancer. Front. Oncol. 2020, 10, 428. [Google Scholar] [CrossRef]
- Ogrodzinski, M.P.; Bernard, J.J.; Lunt, S.Y. Deciphering Metabolic Rewiring in Breast Cancer Subtypes. Transl. Res. 2017, 189, 105–122. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Zou, T.; Shen, X.; Nelson, P.J.; Li, J.; Wu, C.; Yang, J.; Zheng, Y.; Bruns, C.; Zhao, Y.; et al. Lipid Metabolism in Cancer Progression and Therapeutic Strategies. MedComm 2021, 2, 27–59. [Google Scholar] [CrossRef] [PubMed]
- Chu, X.; Korzekwa, K.; Elsby, R.; Fenner, K.; Galetin, A.; Lai, Y.; Matsson, P.; Moss, A.; Nagar, S.; Rosania, G.R.; et al. Intracellular Drug Concentrations and Transporters: Measurement, Modeling, and Implications for the Liver. Clin. Pharmacol. Ther. 2013, 94, 126–141. [Google Scholar] [CrossRef]
- Nedeljković, M.; Damjanović, A. Mechanisms of Chemotherapy Resistance in Triple-Negative Breast Cancer-How We Can Rise to the Challenge. Cells 2019, 8, 957. [Google Scholar] [CrossRef]
- Solans, B.X.; Ruiz-Ramirez, L.; Martinez, A.; Gasque, L.; Moreno-Esparza, R. Mixed Chelate Complexes. II. Structures of L-Alaninato(Aqua)(4,7-Diphenyl-l,10-Phenanthroline)Copper(II) Nitrite Monohydrate and Aqua(4,7-Dimethyl-l,10-Phenanthroline)(Glycinato)(Nitrato)Copper(II). Acta Cryst. 1993, 423, 890–893. [Google Scholar]
- Ruiz-Azuara, L. Process to Obtain New Mixed Copper Aminoacidate Complexes from Phenanthrolines and Their Alkyl Derivatives to be Used as Anticancerigenic Agents. EP Patent 0 434 445 A3, 20 December 1990. [Google Scholar]
- Carvallo-Chaigneau, F.; Trejo-Solís, C.; Gómez-Ruiz, C.; Rodríguez-Aguilera, E.; MacÍas-Rosales, L.; Cortés-Barberena, E.; Cedillo-Peláez, C.; Gracia-Mora, I.; Ruiz-Azuara, L.; Madrid-Marina, V.; et al. Casiopeina III-Ia Induces Apoptosis in HCT-15 Cells in Vitro through Caspase-Dependent Mechanisms and Has Antitumor Effect In Vivo. BioMetals 2008, 21, 17–28. [Google Scholar] [CrossRef]
- Trejo-Solís, C.; Jimenez-Farfan, D.; Rodriguez-Enriquez, S.; Fernandez-Valverde, F.; Cruz-Salgado, A.; Ruiz-Azuara, L.; Sotelo, J. Copper Compound Induces Autophagy and Apoptosis of Glioma Cells by Reactive Oxygen Species and Jnk Activation. BMC Cancer 2012, 12, 156. [Google Scholar] [CrossRef]
- Bravo-Gómez, M.E.; García-Ramos, J.C.; Gracia-Mora, I.; Ruiz-Azuara, L. Antiproliferative Activity and QSAR Study of Copper(II) Mixed Chelate [Cu(N-N)(Acetylacetonato)]NO3 and [Cu(N-N)(Glycinato)]NO3 Complexes, (Casiopeínas®). J. Inorg. Biochem. 2009, 103, 299–309. [Google Scholar] [CrossRef]
- Ruiz-Azuara, L.; Bravo-Gómez, M.E. Copper Compounds in Cancer Chemotherapy. Curr. Med. Chem. 2010, 17, 3606–3615. [Google Scholar] [CrossRef] [PubMed]
- Espinal-Enríquez, J.; Hernández-Lemus, E.; Mejía, C.; Ruiz-Azuara, L. Network Analysis Shows Novel Molecular Mechanisms of Action for Copper-Based Chemotherapy. Front. Physiol. 2016, 6, 406. [Google Scholar] [CrossRef]
- García-Ramos, J.C.; Gutiérrez, A.G.; Vázquez-Aguirre, A.; Toledano-Magaña, Y.; Alonso-Sáenz, A.L.; Gómez-Vidales, V.; Flores-Alamo, M.; Mejía, C.; Ruiz-Azuara, L. The Mitochondrial Apoptotic Pathway Is Induced by Cu(II) Antineoplastic Compounds (Casiopeínas®) in SK-N-SH Neuroblastoma Cells after Short Exposure Times. BioMetals 2017, 30, 43–58. [Google Scholar] [CrossRef] [PubMed]
- Becco, L.; García-Ramos, J.C.; Azuara, L.R.; Gambino, D.; Garat, B. Analysis of the DNA Interaction of Copper Compounds Belonging to the Casiopeínas® Antitumoral Series. Biol. Trace Elem. Res. 2014, 161, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Müller, A.; De Vizcaya-Ruiz, A.; Plant, N.; Ruiz, L.; Dobrota, M. Mixed Chelate Copper Complex, Casiopeina IIgly®, Binds and Degrades Nucleic Acids: A Mechanism of Cytotoxicity. Chem. Biol. Interact. 2007, 165, 189–199. [Google Scholar] [CrossRef]
- Bravo-Gómez, M.E.; Campero-Peredo, C.; García-Conde, D.; Mosqueira-Santillán, M.J.; Serment-Guerrero, J.; Ruiz-Azuara, L. DNA-Binding Mode of Antitumoral Copper Compounds (Casiopeinas®) and Analysis of Its Biological Meaning. Polyhedron 2015, 102, 530–538. [Google Scholar] [CrossRef]
- Figueroa-DePaz, Y.; Resendiz-Acevedo, K.; Dávila-Manzanilla, S.G.; García-Ramos, J.C.; Ortiz-Frade, L.; Serment-Guerrero, J.; Ruiz-Azuara, L. DNA, a Target of Mixed Chelate Copper(II) Compounds (Casiopeinas®) Studied by Electrophoresis, UV–Vis and Circular Dichroism Techniques. J. Inorg. Biochem. 2022, 231, 111772. [Google Scholar] [CrossRef]
- Kachadourian, R.; Brechbuhl, H.M.; Ruiz-Azuara, L.; Gracia-Mora, I.; Day, B.J. Casiopeína IIgly-Induced Oxidative Stress and Mitochondrial Dysfunction in Human Lung Cancer A549 and H157 Cells. Toxicology 2010, 268, 176–183. [Google Scholar] [CrossRef]
- Gutiérrez, A.G.; Vázquez-Aguirre, A.; García-Ramos, J.C.; Flores-Alamo, M.; Hernández-Lemus, E.; Ruiz-Azuara, L.; Mejía, C. Copper(II) Mixed Chelate Compounds Induce Apoptosis through Reactive Oxygen Species in Neuroblastoma Cell Line Chp-212. J. Inorg. Biochem. 2013, 126, 17–25. [Google Scholar] [CrossRef]
- Alemón-Medina, R.; Breña-Valle, M.; Muñoz-Sánchez, J.L.; Gracia-Mora, M.I.; Ruiz-Azuara, L. Induction of Oxidative Damage by Copper-Based Antineoplastic Drugs (Casiopeínas®). Cancer Chemother. Pharmacol. 2007, 60, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Trejo-Solís, C.; Palencia, G.; Zúñiga, S.; Rodríguez-Ropon, A.; Osorio-Rico, L.; Luvia, S.T.; Gracia-Mora, I.; Marquez-Rosado, L.; Sánchez, A.; Moreno-García, M.E.; et al. Cas IIgly Induces Apoptosis in Glioma C6 Cells in Vitro and in Vivo through Caspase-Dependent and Caspase-Independent Mechanisms. Neoplasia 2005, 7, 563–574. [Google Scholar] [CrossRef]
- Mejia, C.; Ruiz-Azuara, L. Casiopeinas Iigly and IIIia Induce Apoptosis in Medulloblastoma Cells. Pathol. Oncol. Res. 2008, 14, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Marín-Hernández, A.; Gallardo-Pérez, J.C.; López-Ramírez, S.Y.; García-García, J.D.; Rodríguez-Zavala, J.S.; Ruiz-Ramírez, L.; Gracia-Mora, I.; Zentella-Dehesa, A.; Sosa-Garrocho, M.; MacÍas-Silva, M.; et al. Casiopeina II-Gly and Bromo-Pyruvate Inhibition of Tumor Hexokinase, Glycolysis, and Oxidative Phosphorylation. Arch. Toxicol. 2012, 86, 753–766. [Google Scholar] [CrossRef]
- de Anda-Jáuregui, G.; Espinal-Enríquez, J.; Hur, J.; Alcalá-Corona, S.A.; Ruiz-Azuara, L.; Hernández-Lemus, E. Identification of Casiopeina II-Gly Secondary Targets through a Systems Pharmacology Approach. Comput. Biol. Chem. 2019, 78, 127–132. [Google Scholar] [CrossRef]
- Miricescu, D.; Totan, A.; Stanescu-Spinu, I.-I.; Constantin Badoiu, S.; Stefani, C.; Greabu, M. Molecular Sciences PI3K/AKT/MTOR Signaling Pathway in Breast Cancer: From Molecular Landscape to Clinical Aspects. Int. J. Mol. Sci. 2020, 22, 173. [Google Scholar] [CrossRef]
- González-Ballesteros, M.M.; Sánchez-Sánchez, L.; Espinoza-Guillén, A.; Espinal-Enríquez, J.; Mejía, C.; Hernández-Lemus, E.; Ruiz-Azuara, L. Antitumoral and Antimetastatic Activity by Mixed Chelate Copper(II) Compounds (Casiopeínas®) on Triple-Negative Breast Cancer, In Vitro and In Vivo Models. Int. J. Mol. Sci. 2024, 25, 8803. [Google Scholar] [CrossRef]
- Aguilar-Jiménez, Z.; González-Ballesteros, M.; Dávila-Manzanilla, S.G.; Espinoza-Guillén, A.; Ruiz-Azuara, L. Development and In Vitro and In Vivo Evaluation of an Antineoplastic Copper(II) Compound (Casiopeina III-ia) Loaded in Nonionic Vesicles Using Quality by Design. Int. J. Mol. Sci. 2022, 23, 12756. [Google Scholar] [CrossRef] [PubMed]
- Resendiz-Acevedo, K.; García-Aguilera, M.E.; Esturau-Escofet, N.; Ruiz-Azuara, L. 1H -NMR Metabolomics Study of the Effect of Cisplatin and Casiopeina IIgly on MDA-MB-231 Breast Tumor Cells. Front. Mol. Biosci. 2021, 8, 742859. [Google Scholar] [CrossRef]
- Elena Bravo-Gómez, M.; Dávila-Manzanilla, S.; Flood-Garibay, J.; Ángel Muciño-Hernández, M.; Mendoza, Á.; Carlos García-Ramos, J.; Moreno-Esparza, R.; Ruiz-Azuara, L. Secondary Ligand Effects on the Cytotoxicity of Several Casiopeína’s Group II Compounds Secondary Ligand Effects on the Cytotoxicity of Several Casiopeína’s Group II Compounds. J. Mex. Chem. Soc. 2012, 56, 85–92. [Google Scholar]
- Dasari, S.; Bernard Tchounwou, P. Cisplatin in Cancer Therapy: Molecular Mechanisms of Action. Eur. J. Pharmacol. 2014, 740, 364–378. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Chen, Y.; Dubrulle, J.; Stossi, F.; Putluri, V.; Sreekumar, A.; Putluri, N.; Baluya, D.; Lai, S.Y.; Sandulache, V.C. Cisplatin Generates Oxidative Stress Which Is Accompanied by Rapid Shifts in Central Carbon Metabolism. Sci. Rep. 2018, 8, 4306. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ding, C.; Zhu, W.; Li, X.; Chen, T.; Liu, Q.; Zhou, S.; Zhang, T.C.; Ma, W. Chemotherapeutic Drugs Induce Oxidative Stress Associated with DNA Repair and Metabolism Modulation. Life Sci. 2022, 289, 120242. [Google Scholar] [CrossRef]
- Zhou, D.; Duan, Z.; Li, Z.; Ge, F.; Wei, R.; Kong, L. The Significance of Glycolysis in Tumor Progression and Its Relationship with the Tumor Microenvironment. Front. Pharmacol. 2022, 13, 1091779. [Google Scholar] [CrossRef] [PubMed]
- Farahzadi, R.; Valipour, B.; Fathi, E.; Pirmoradi, S.; Molavi, O.; Montazersaheb, S.; Sanaat, Z. Oxidative Stress Regulation and Related Metabolic Pathways in Epithelial–Mesenchymal Transition of Breast Cancer Stem Cells. Stem Cell Res. Ther. 2023, 14, 342. [Google Scholar] [CrossRef]
- Schwager, S.C.; Mosier, J.A.; Padmanabhan, R.S.; White, A.; Xing, Q.; Hapach, L.A.; Taufalele, P.V.; Ortiz, I.; Reinhart-King, C.A. Link between Glucose Metabolism and Epithelial-to-Mesenchymal Transition Drives Triple-Negative Breast Cancer Migratory Heterogeneity. iScience 2022, 25, 105190. [Google Scholar] [CrossRef]
- Stachowiak, M.R. Mechanisms of Actomyosin Contractility in Cells. Ph.D. Thesis, Columbia University, New York, NY, USA, 2011. [Google Scholar]
- Oh, M.; Batty, S.; Banerjee, N.; Kim, T.H. High Extracellular Glucose Promotes Cell Motility by Modulating Cell Deformability and Contractility via the CAMP-RhoA-ROCK Axis in Human Breast Cancer Cells. Mol. Biol. Cell 2023, 34, ar79. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Q.; Huang, Z.; Li, B.; Nice, E.C.; Huang, C.; Wei, L.; Zou, B. Targeting Glucose Metabolism Enzymes in Cancer Treatment: Current and Emerging Strategies. Cancers 2022, 14, 4568. [Google Scholar] [CrossRef]
- Luo, X.; Cheng, C.; Tan, Z.; Li, N.; Tang, M.; Yang, L.; Cao, Y. Emerging roles of lipid metabolism in cancer metastasis. Mol. Cancer 2017, 16, 76. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Ali, A.; Yachioui, D.E.I.; Le Dévédec, S.E.; Hankemeier, T. Lipid Dysregulation in Triple Negative Breast Cancer: Insights from Mass Spectrometry-Based Approaches. Prog. Lipid Res. 2025, 98, 101330. [Google Scholar] [CrossRef]
- Lampa, M.; Arlt, H.; He, T.; Ospina, B.; Reeves, J.; Zhang, B.; Murtie, J.; Deng, G.; Barberis, C.; Hoffmann, D.; et al. Glutaminase Is Essential for the Growth of Triple-Negative Breast Cancer Cells with a Deregulated Glutamine Metabolism Pathway and Its Suppression Synergizes with MTOR Inhibition. PLoS ONE 2017, 12, e0185092. [Google Scholar] [CrossRef]
- Zhang, T.; Hu, L.; Tang, J.F.; Xu, H.; Tian, K.; Wu, M.N.; Huang, S.Y.; Du, Y.M.; Zhou, P.; Lu, R.J.; et al. Metformin Inhibits the Urea Cycle and Reduces Putrescine Generation in Colorectal Cancer Cell Lines. Molecules 2021, 26, 1990. [Google Scholar] [CrossRef]
- White, E. Role of Metabolic Stress Responses of Apoptosis and Autophagy in Tumor Suppression. Ernst Scher. Found. Symp. Proc. 2007, 4, 23–34. [Google Scholar]
- Aguilar-Jiménez, Z.; Espinoza-Guillén, A.; Resendiz-Acevedo, K.; Fuentes-Noriega, I.; Mejía, C.; Ruiz-Azuara, L. The Importance of Being Casiopeina as Polypharmacologycal Profile (Mixed Chelate–Copper (II) Complexes and Their In Vitro and In Vivo Activities). Inorganics 2023, 11, 394. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid Colorimetric Assay for Cellular Growth and Survival: Application to Proliferation and Cytotoxicity Assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Strober, W. Trypan Blue Exclusion Test of Cell Viability. Curr. Protoc. Immunol. 2015, 111, A3.B.1–A3.B.3. [Google Scholar] [CrossRef]
- Beckonert, O.; Keun, H.C.; Ebbels, T.M.D.; Bundy, J.; Holmes, E.; Lindon, J.C.; Nicholson, J.K. Metabolic Profiling, Metabolomic and Metabonomic Procedures for NMR Spectroscopy of Urine, Plasma, Serum and Tissue Extracts. Nat. Protoc. 2007, 2, 2692–2703. [Google Scholar] [CrossRef] [PubMed]

| Compound | IC50 ± SD (μM) |
|---|---|
| Cisplatin | 56.25 ± 1.29 |
| CasIIIia | 26.62 ± 0.61 |
| CasIIgly | 3.49 ± 0.33 |
| Metabolic Pathway Identified | Metabolites Identified in Samples | Cisplatin | CasIIIia | CasIIgly | |
|---|---|---|---|---|---|
| Carbohydrates metabolism | Warburg effect | Lactate, glutamate, glutamine, uridine diphosphate-N-acetylglucosamine, succinate |  |  |  |
| Gluconeogenesis |  |  |  | ||
| Pyruvate metabolism |  |  |  | ||
| Citric acid cycle |  |  |  |
| Metabolic Pathway Identified | Metabolites Identified in Samples | Cisplatin | CasIIIia | CasIIgly | |
|---|---|---|---|---|---|
| Carbohydrates metabolism | Warburg effect | Cytidine, cytosine, lactate, glucose, glutamate, glutamine, uridine diphosphate-N-acetylglucosamine, succinate |  |  |  |
| Gluconeogenesis |  |  |  | ||
| Pyruvate metabolism |  |  |  | ||
| Citric acid cycle |  |  |  | ||
| Lipids metabolism | Sphingolipid metabolism | Acetylcholine, glycerophosphocholine, phosphocholine |  |  |  |
| Phospholipid biosynthesis |  |  |  | ||
| Phosphatidylcholine biosynthesis |  |  |  | ||
| Ketone body metabolism |  |  |  | ||
| Amino acid metabolism | Arginine metabolism | Acetylcysteine, alanine, arginine, proline, glutamate, glutamine, Glycylproline, N-acetylglutamine, valine, taurine, threonine |  |  |  |
| Tyrosine metabolism |  |  |  | ||
| Cysteine metabolism |  |  |  | ||
| Lysine metabolism |  |  |  | ||
| Histidine metabolism |  |  |  | ||
| Proline metabolism |  |  |  | ||
| Glutathione metabolism |  |  |  | ||
| Phenylacetate metabolism |  |  |  | ||
| Energy metabolism | Mitochondrial electron transport chain | Creatine, creatinine, formate, NADH, phosphocreatine, succinate |  |  |  |
| 20 min | 6 h | |||
|---|---|---|---|---|
| Intracellular (µg Cu/mL) | Extracellular (µg Cu/mL) | Intracellular (µg Cu/mL) | Extracellular (µg Cu/mL) | |
| Control (no treatment) | 0.028 ± 0.015 | 0.001 ± 0.003 | 0.016 ± 0.002 | 0.008 ± 0.008 |
| CasIIIia | 0.015 ± 0.001 | 0.183 ± 0.008 | 0.032 ± 0.001 | 0.116 ± 0.094 |
| CasIIgly | 0.021 ± 0.004 | 0.007 ± 0.005 | 0.033 ± 0.022 | 1 ND |
| Compound | Evaluation Time (µg Cu/mL) | Initial Copper Concentration Administered (µg Cu/mL) | Intracellular Copper Concentration (µg Cu/mL) | Extracellular Copper Concentration (µg Cu/mL) |
|---|---|---|---|---|
| CasIIIia | 20 min | 1.69 | 0.13 | 1.56 |
| 6 h | 0.36 | 1.33 | ||
| CasIIgly | 20 min | 0.22 | 0.17 | 0.05 |
| 6 h | ≈0.22 | ≈0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Resendiz-Acevedo, K.; García-Aguilera, M.E.; Tovar-Tovar, A.; Esturau-Escofet, N.; Ruiz-Azuara, L. Metabolomic Effects and Their Relationship with Intracellular/Extracellular Concentrations of Casiopeinas® in Triple-Negative Mesenchymal Breast Cancer. Int. J. Mol. Sci. 2025, 26, 8735. https://doi.org/10.3390/ijms26178735
Resendiz-Acevedo K, García-Aguilera ME, Tovar-Tovar A, Esturau-Escofet N, Ruiz-Azuara L. Metabolomic Effects and Their Relationship with Intracellular/Extracellular Concentrations of Casiopeinas® in Triple-Negative Mesenchymal Breast Cancer. International Journal of Molecular Sciences. 2025; 26(17):8735. https://doi.org/10.3390/ijms26178735
Chicago/Turabian StyleResendiz-Acevedo, Karen, Martha E. García-Aguilera, Araceli Tovar-Tovar, Nuria Esturau-Escofet, and Lena Ruiz-Azuara. 2025. "Metabolomic Effects and Their Relationship with Intracellular/Extracellular Concentrations of Casiopeinas® in Triple-Negative Mesenchymal Breast Cancer" International Journal of Molecular Sciences 26, no. 17: 8735. https://doi.org/10.3390/ijms26178735
APA StyleResendiz-Acevedo, K., García-Aguilera, M. E., Tovar-Tovar, A., Esturau-Escofet, N., & Ruiz-Azuara, L. (2025). Metabolomic Effects and Their Relationship with Intracellular/Extracellular Concentrations of Casiopeinas® in Triple-Negative Mesenchymal Breast Cancer. International Journal of Molecular Sciences, 26(17), 8735. https://doi.org/10.3390/ijms26178735







