Effects of Maternal Clofibrate Supplementation During Gestation and Lactation on Intestinal Fatty Acid Oxidation of Suckling Piglets
Abstract
1. Introduction
2. Results
2.1. FA Oxidation
2.1.1. Effects of Maternal Supplementation of Clofibrate on FA Metabolism in the Intestinal Mucosa of Piglets During the Neonatal-Suckling Period
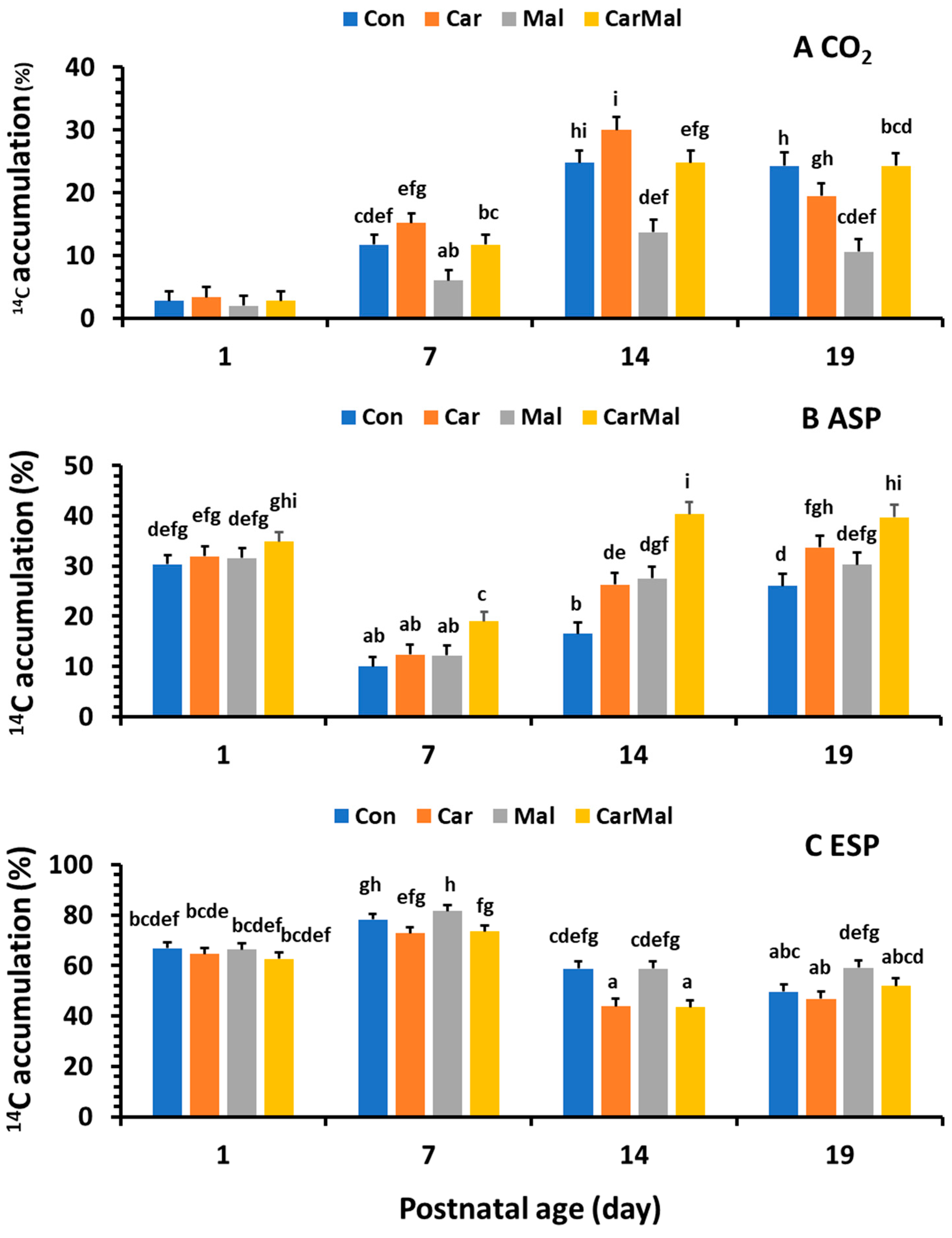
2.1.2. Effects of Maternal Supplementation of Clofibrate on Distribution (%) of CO2, ASP and ESP in Total FA Oxidation and Metabolism in the Intestinal Mucosa of Piglets During the Neonatal-Suckling Period
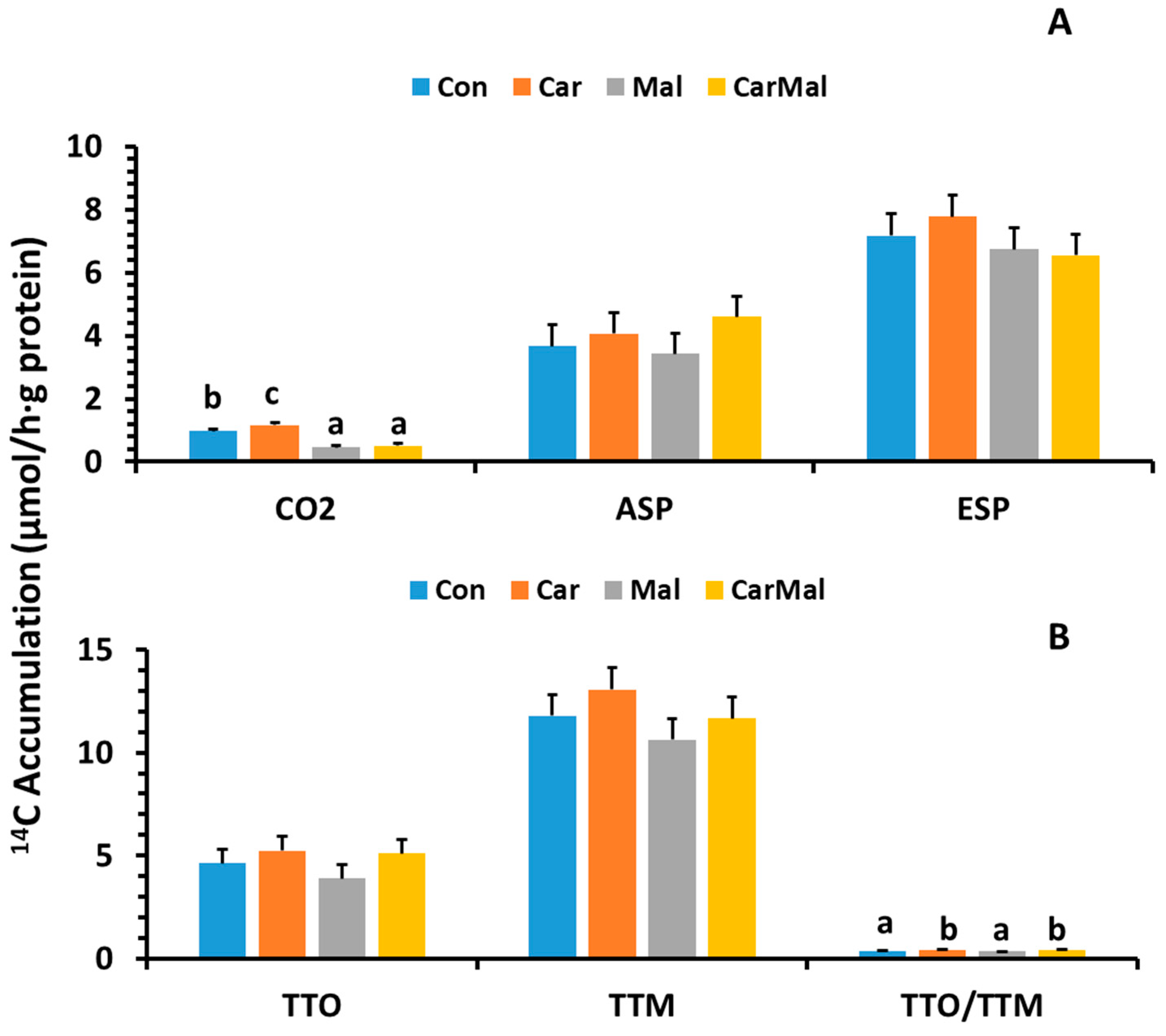
2.1.3. Effects of Carnitine and Malonate on FA Metabolism in the Intestinal Mucosa of Piglets During the Neonatal-Suckling Period
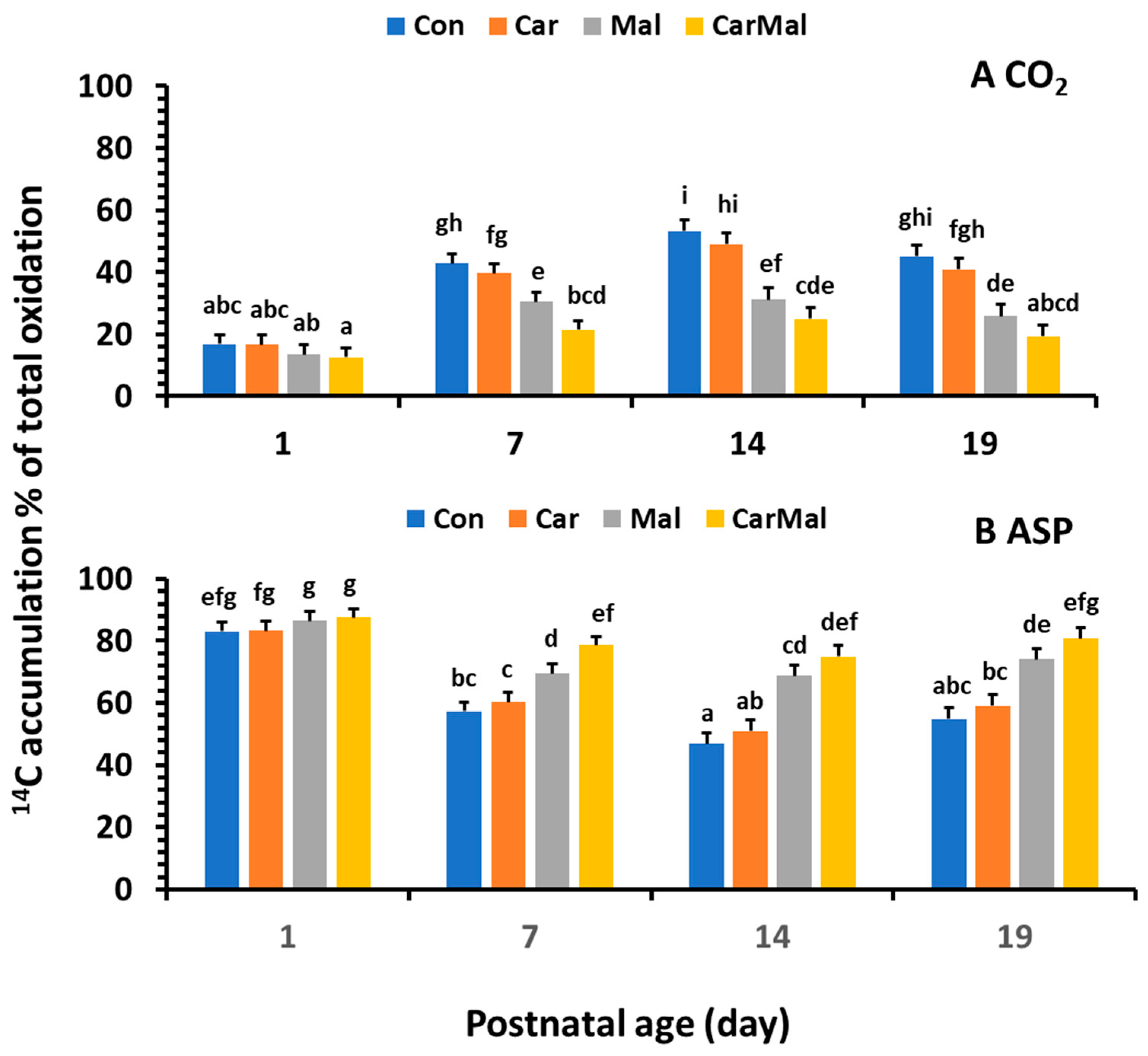
2.1.4. Effects of Carnitine and Malonate on Distribution (%) of CO2, ASP, and ESP in Total FA Oxidation and Metabolism in the Intestinal Mucosa of Piglets During the Neonatal-Suckling Period
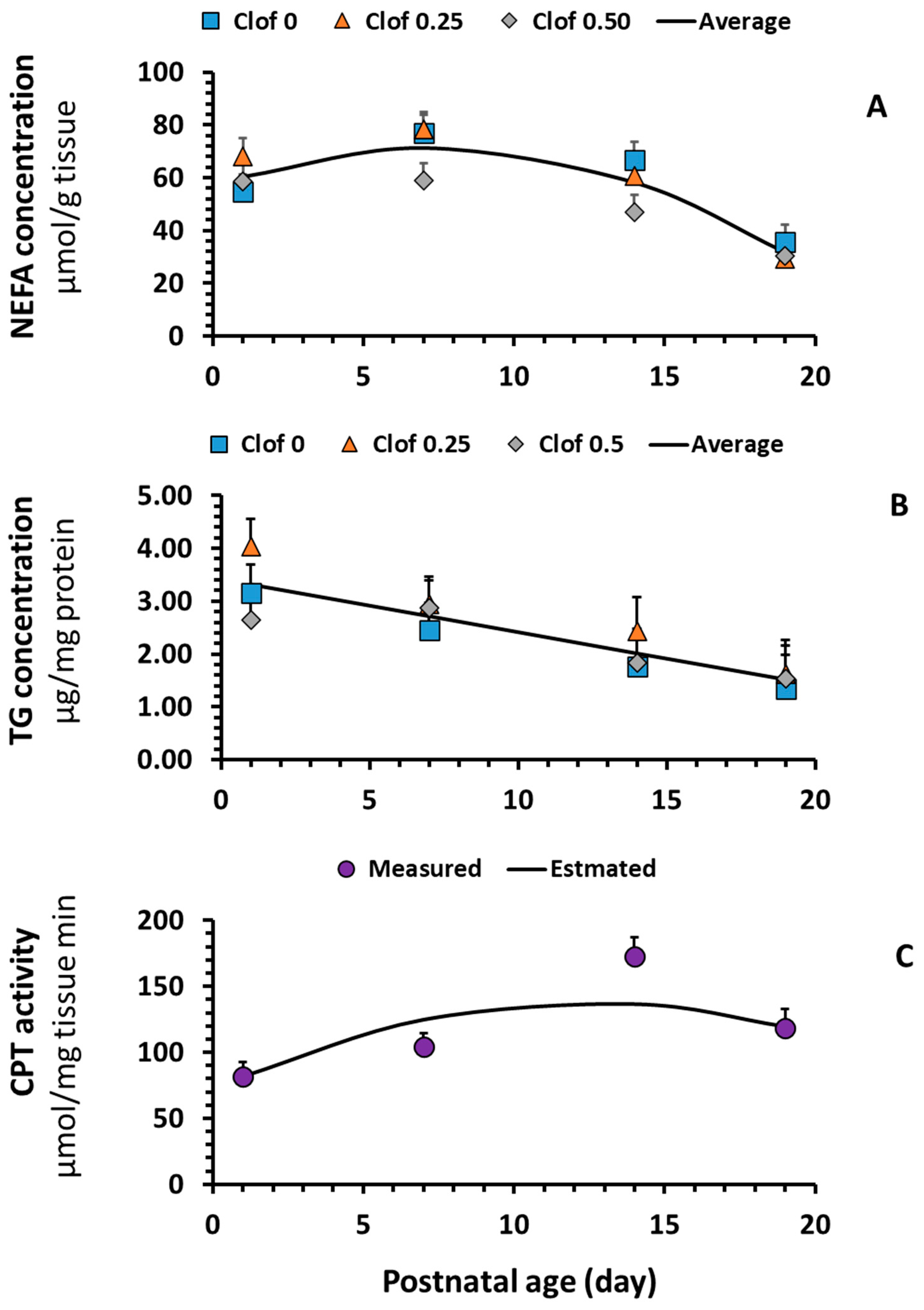
2.2. Non-Esterified Fatty Acid (NEFA) and Triglyceride (TG) Concentrations
2.3. Carnitine Palmitoyltransferase (CPT) Enzyme Activity
2.4. Gene Expression (qPCR)
3. Discussion
3.1. The Effect of Maternal Clofibrate on Intestinal FA Metabolism in Suckling Piglets
3.2. The Effect of Providing Carnitine and Inhibiting TCA Activity on Intestinal FA Metabolism in Suckling Pigs
4. Materials and Methods
4.1. Animals and Treatments
4.2. FA Metabolism Measurements
4.3. Non-Esterified Fatty Acid (NEFA) and Triglyceride (TG) Assays
4.4. Enzymatic Assay
4.5. RNA Isolation and RT-qPCR
4.6. Chemicals
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ACOX1 | Acyl-CoA oxidase 1 |
| ASP | Acid soluble products |
| CPT | Carnitine palmitoyltransferase |
| ESP | Esterified products |
| FA | Fatty acid |
| FABP2 | FA-binding protein 2 |
| HMGCS | 3-hydroxy-3-methylglutaryl-CoA synthase |
| NEFA | Non-Esterified Fatty Acids |
| PPARα | Peroxisome proliferator-activated receptor alpha |
| RXRα | Retinoid X receptor alpha |
| TCA | Tricarboxylic acid cycle |
| TG | Triglycerides |
References
- Herrera, E.; Amusquivar, E. Lipid metabolism in the fetus and the newborn. Diabetes Metab. Res. Rev. 2000, 16, 202–210. [Google Scholar] [CrossRef]
- Girard, J.; Ferré, P.; Pégorier, J.P.; Duée, P.H. Adaptations of glucose and fatty acid metabolism during the perinatal period and suckling-weaning transition. Physiol. Rev. 1992, 72, 507–562. [Google Scholar] [CrossRef]
- Hahn, P.; Koldovskv, O. Utilization of Nutrients During Postnatal Development; Pergamon Press: Oxford, UK, 1966; p. 17. [Google Scholar]
- Weström, B.; Arévalo Sureda, E.; Pierzynowska, K.; Pierzynowski, S.G.; Pérez-Cano, F.J. The Immature Gut Barrier and Its Importance in Establishing Immunity in Newborn Mammals. Front. Immunol. 2020, 11, 1153. [Google Scholar] [CrossRef]
- Kimura, R.E. Neonatal intestinal metabolism. Clin. Perinatol. 1996, 23, 245–263. [Google Scholar] [CrossRef] [PubMed]
- Girard, J.; Duée, P.H.; Ferré, P.; Pégorier, J.P.; Escriva, F.; Decaux, J.F. Fatty acid oxidation and ketogenesis during development. Reprod. Nutr. Develop. 1985, 25, 303–319. [Google Scholar] [CrossRef] [PubMed]
- Small, G.M.; Hocking, T.J.; Strudee, A.P.; Burdett, K.; Connock, M.J. Enhancement by dietary clofibrate of peroxisomal palmityl-CoA oxidase in kidney and small intestine of albino mice and liver of genetically lean and obese mice. Life Sci. 1981, 28, 1875–1882. [Google Scholar] [CrossRef] [PubMed]
- Kimura, R.; Takahashi, N.; Murota, K.; Yamada, Y.; Niiya, S.; Kanzaki, N.; Murakami, Y.; Moriyama, T.; Goto, T.; Kawada, T. Activation of peroxisome proliferator-activated receptor-alpha (PPARalpha) suppresses postprandial lipidemia through fatty acid oxidation in enterocytes. Biochem. Biophys. Res. Commun. 2011, 410, 1–6. [Google Scholar] [CrossRef]
- Kimura, R.; Takahashi, N.; Lin, S.; Goto, T.; Murota, K.; Nakata, R.; Inoue, H.; Kawada, T. DHA attenuates postprandial hyperlipidemia via activating PPARalpha in intestinal epithelial cells. J. Lipid Res. 2013, 54, 3258–3268. [Google Scholar] [CrossRef]
- Karimian Azari, E.; Leitner, C.; Jaggi, T.; Langhans, W.; Mansouri, A. Possible role of intestinal fatty acid oxidation in the eating-inhibitory effect of the PPAR-alpha agonist Wy-14643 in high-fat diet fed rats. PLoS ONE 2013, 8, e74869. [Google Scholar] [CrossRef]
- Mochizuki, K.; Suruga, K.; Yagi, E.; Takase, S.; Goda, T. The expression of PPAR-associated genes is modulated through postnatal development of PPAR subtypes in the small intestine. Biochim. Biophys. Acta 2001, 1531, 68–76. [Google Scholar] [CrossRef]
- Ringseis, R.; Eder, K. Influence of pharmacological PPARalpha activators on carnitine homeostasis in proliferating and non-proliferating species. Pharmacol. Res. 2009, 60, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Shim, K.; Jacobi, S.; Odle, J.; Lin, X. Pharmacologic activation of peroxisome proliferator-activating receptor-α accelerates hepatic fatty acid oxidation in neonatal pigs. Oncotarget 2018, 9, 23900–23914. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Pike, B.; Wang, F.; Yang, L.; Meisner, P.; Huang, Y.; Odle, J.; Lin, X. Effects of maternal feeding of clofibrate on hepatic fatty acid metabolism in suckling piglet. J. Anim. Sci. Biotechnol. 2024, 15, 163. [Google Scholar] [CrossRef]
- Pike, B.; Zhao, J.; Hicks, J.A.; Wang, F.; Hagen, R.; Liu, H.C.; Odle, J.; Lin, X. Intestinal Carnitine Status and Fatty Acid Oxidation in Response to Clofibrate and Medium-Chain Triglyceride Supplementation in Newborn Pigs. Int. J. Mol. Sci. 2023, 24, 6066. [Google Scholar] [CrossRef]
- Lin, X.; Jacobi, S.; Odle, J. Transplacental induction of fatty acid oxidation in term fetal pigs by the peroxisome proliferator-activated receptor alpha agonist clofibrate. J. Anim. Sci. Biotechnol. 2015, 6, 11. [Google Scholar] [CrossRef]
- Simpson, A.E.; Brammar, W.J.; Pratten, M.K.; Cockcroft, N.; Elcombe, C.R. Placental transfer of the hypolipidemic drug, clofibrate, induces CYP4A expression in 18.5-day fetal rats. Drug Metab. Dispos. 1996, 24, 547–554. [Google Scholar][Green Version]
- Simpson, A.E.; Brammar, W.J.; Pratten, M.K.; Elcombe, C.R. Translactational induction of CYP4A expression in 10.5-day neonatal rats by the hypolipidemic drug clofibrate. Biochem. Pharmacol. 1995, 50, 2021–2032. [Google Scholar] [CrossRef]
- Nicot, C.; Hegardt, F.G.; Woldegiorgis, G.; Haro, D.; Marrero, P.F. Pig liver carnitine palmitoyltransferase I, with low Km for carnitine and high sensitivity to malonyl-CoA inhibition, is a natural chimera of rat liver and muscle enzymes. Biochemistry 2001, 40, 2260–2266. [Google Scholar] [CrossRef] [PubMed]
- Relat, J.; Nicot, C.; Gacias, M.; Woldegiorgis, G.; Marrero, P.F.; Haro, D. Pig muscle carnitine palmitoyltransferase I (CPTI beta), with low Km for carnitine and low sensitivity to malonyl-CoA inhibition, has kinetic characteristics similar to those of the rat liver (CPTI alpha) enzyme. Biochemistry 2004, 43, 12686–12691. [Google Scholar] [CrossRef]
- Voltti, H.; Hassinen, I.E. Effect of clofibrate on the hepatic concentrations of citric acid cycle intermediates and malonyl-CoA in the rat. Life Sci. 1981, 28, 47–51. [Google Scholar] [CrossRef] [PubMed]
- Békési, A.; Williamson, D.H. An explanation for ketogenesis by the intestine of the suckling rat: The presence of an active hydroxymethylglutaryl-coenzyme A pathway. Biol. Neonate 1990, 58, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Wallenius, V.; Elias, E.; Elebring, E.; Haisma, B.; Casselbrant, A.; Larraufie, P.; Spak, E.; Reimann, F.; le Roux, C.W.; Docherty, N.G.; et al. Suppression of enteroendocrine cell glucagon-like peptide (GLP)-1 release by fat-induced small intestinal ketogenesis: A mechanism targeted by Roux-en-Y gastric bypass surgery but not by preoperative very-low-calorie diet. Gut 2020, 69, 1423–1431. [Google Scholar] [CrossRef] [PubMed]
- Adams, S.H.; Alho, C.S.; Asins, G.; Hegardt, F.G.; Marrero, P.F. Gene expression of mitochondrial 3-hydroxy-3-methylglutaryl-CoA synthase in a poorly ketogenic mammal: Effect of starvation during the neonatal period of the piglet. Biochem. J. 1997, 324 Pt 1, 65–73. [Google Scholar] [CrossRef]
- Darcy-Vrillon, B.; Cherbuy, C.; Morel, M.T.; Durand, M.; Duée, P.H. Short chain fatty acid and glucose metabolism in isolated pig colonocytes: Modulation by NH4+. Mol. Cell Biochem. 1996, 156, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Pégorier, J.P.; Duée, P.H.; Girard, J.; Peret, J. Metabolic fate of non-esterified fatty acids in isolated hepatocytes from newborn and young pigs. Evidence for a limited capacity for oxidation and increased capacity for esterification. Biochem. J. 1983, 212, 93–97. [Google Scholar] [CrossRef]
- Manners, M.J.; Mccrea, M.R. Changes in the chemical composition of sow-reared piglets during the 1st month of life. Br. J. Nutr. 1963, 17, 495–513. [Google Scholar] [CrossRef]
- Cherbuy, C.; Guesnet, P.; Morel, M.T.; Kohl, C.; Thomas, M.; Duée, P.H.; Prip-Buus, C. Oleate metabolism in pig enterocytes is characterized by an increased oxidation rate in the presence of a high esterification rate within two days after birth. J. Nutr. 2012, 142, 221–226. [Google Scholar] [CrossRef]
- Trotter, P.J.; Storch, J. Nutritional control of fatty acid esterification in differentiating Caco-2 intestinal cells is mediated by cellular diacylglycerol concentrations. J. Nutr. 1993, 123, 728–736. [Google Scholar] [CrossRef]
- Suarez-Trujillo, A.; Luecke, S.M.; Logan, L.; Bradshaw, C.; Stewart, K.R.; Minor, R.C.; Ramires Ferreira, C.; Casey, T.M. Changes in sow milk lipidome across lactation occur in fatty acyl residues of triacylglycerol and phosphatidylglycerol lipids, but not in plasma membrane phospholipids. Animal 2021, 15, 100280. [Google Scholar] [CrossRef]
- Huang, W.Y.; Kummerow, F.A. Cholesterol and fatty acid synthesis in swine. Lipids 1976, 11, 34–41. [Google Scholar] [CrossRef]
- Qureshi, A.A.; Burger, W.C.; Elson, C.E.; Benevenga, N.J. Effects of cereals and culture filtrate of Trichoderma viride on lipid metabolism of swine. Lipids 1982, 17, 924–934. [Google Scholar] [CrossRef]
- Christiansen, R.Z.; Osmundsen, H.; Borrebaek, B.; Bremer, J. The effects of clofibrate feeding on the metabolism of palmitate and erucate in isolated hepatocytes. Lipids 1978, 13, 487–491. [Google Scholar] [CrossRef]
- Blonç, M.; Lima, J.; Balasch, J.C.; Tort, L.; Gravato, C.; Teles, M. Elucidating the Effects of the Lipids Regulators Fibrates and Statins on the Health Status of Finfish Species: A Review. Animals 2023, 13, 792. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Adams, S.H.; Odle, J. Acetate represents a major product of heptanoate and octanoate beta-oxidation in hepatocytes isolated from neonatal piglets. Biochem. J. 1996, 318 Pt 1, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Xi, L.; Matsey, G.; Odle, J. The effect of 5-aminoimidazole-4-carboxamide ribonucleoside (AICAR) on fatty acid oxidation in hepatocytes isolated from neonatal piglets. J. Anim. Sci. Biotechnol. 2012, 3, 30. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
| Clofibrate | Con | Clof 0.25 | Clof 0.50 | p-Value | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age | D1 | D7 | D14 | D19 | D1 | D7 | D14 | D19 | D1 | D7 | D14 | D19 | SEM | Clof | Age | Clof *Age |
| Oxidation | µmol/g protein. h | |||||||||||||||
| CO2 | 0.31 a | 0.73 bcd | 0.80 bcd | 0.60 abc | 0.65 bc | 0.93 de | 0.82 bcde | 0.96 de | 0.82 cde | 0.54 ab | 1.15 e | 1.03 de | 0.11 | 0.0018 + | 0.0020 | 0.0045 * |
| ASP | 8.9 b | 1.07 a | 1.01 a | 1.35 a | 11.96 c | 1.14 a | 1.04 a | 1.86 a | 15.34 d | 0.75 a | 1.29 a | 1.57 a | 1.13 | 0.0990 | 0.0001 +^ | 0.0310 |
| CO2 + ASP | 9.22 b | 1.79 a | 1.81 a | 1.94 a | 12.61 c | 2.07 a | 1.86 a | 2.82 a | 16.16 c | 1.29 a | 2.43 a | 2.60 a | 1.15 | 0.0588 | 0.0001 +^ | 0.0246 |
| Metabolism | µmol/g protein. h | |||||||||||||||
| ESP | 7.56 c | 22.83 e | 3.56 ab | 3.39 ab | 7.14 c | 16.17 d | 2.78 a | 3.53 ab | 7.41 c | 6.12 bc | 1.79 a | 2.37 a | 1.22 | 0.0001+ | 0.0001 +^ | 0.0001 * |
| CO2 + ASP + ESP | 16.81 b | 24.57 d | 5.36 a | 5.44 a | 19.87 bc | 18.24 bc | 4.63 a | 6.34 a | 23.57 cd | 7.42 a | 4.25 a | 4.96 a | 1.81 | 0.0408 | 0.0001 + | 0.0001 |
| Con | Clof 0.25 | Clof 0.50 | p-Value | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| D1 | D7 | D14 | D19 | D1 | D7 | D14 | D19 | D1 | D7 | D14 | D19 | SEM | Clof | Age | Clof * | |
| Oxidation | ||||||||||||||||
| CO2% | 16.51 | 33.10 | 36.15 | 28.24 | 11.52 | 36.29 | 40.34 | 32.12 | 16.69 | 31.37 | 42.38 | 38.09 | 2.89 | 0.2410 | 0.0001 +^ | 0.1514 |
| ASP% | 83.49 | 66.90 | 63.85 | 71.76 | 88.48 | 63.71 | 59.66 | 67.88 | 83.31 | 68.63 | 57.62 | 61.91 | 2.89 | 0.2410 | 0.0001 +^ | 0.1514 |
| C/A | 0.27 | 0.99 | 1.04 | 0.58 | 0.14 | 0.91 | 0.96 | 0.54 | 0.27 | 0.70 | 0.98 | 0.73 | 0.13 | 0.6779 | 0.0001 ^ | 0.0769 |
| Metabolism | ||||||||||||||||
| CO2% | 1.32 a | 6.51 b | 14.01 cde | 12.26 bcd | 2.87 a | 11.86 cd | 20.36 ef | 14.58 d | 3.63 a | 8.55 bc | 22.49 f | 20.43 e | 1.54 | 0.0001 + | 0.0001 +^ | 0.0016 * |
| ASP% c | 28.48 d | 11.28 a | 18.58 c | 36.16 f | 34.56 ef | 12.34 ab | 28.47 d | 29.64 de | 33.53 def | 16.53 bc | 35.88 f | 31.51 def | 1.87 | 0.0001 + | 0.0001 +^ | 0.0001 * |
| ESP% c | 70.19 ef | 80.33 g | 65.08 de | 51.58 bc | 62.57 d | 75.14 f | 50.15 bc | 55.78 c | 62.85 d | 73.90 f | 38.28 a | 48.05 b | 2.29 | 0.0001 + | 0.0001 +^ | 0.0001 * |
| O/M | 0.30 b | 0.20 a | 0.35 b | 0.48 cd | 0.37 b | 0.25 ab | 0.50 d | 0.44 c | 0.37 b | 0.26 ab | 0.62 e | 0.52 d | 0.02 | 0.0001 + | 0.0001 +^ | 0.0001 * |
| Clofibrate (%) | Age (Day) | Clof *Age | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genes | Con | Clof 0.25 | Clof 0.50 | SEM | p-Value | D1 | D7 | D14 | D19 | SEM | p-Value | p-Value |
| Fold | Fold | |||||||||||
| INTESTINE | ||||||||||||
| ACOX1 | 2.75 | 2.60 | 2.34 | 0.24 | 0.423 | 2.68 | 2.46 | 2.63 | 2.50 | 0.28 | 0.915 | 0.037 * |
| CPT1A | 1.01 | 1.04 | 1.19 | 0.19 | 0.737 | 1.48 b | 1.09 b | 0.58 a | 1.16 b | 0.22 | 0.046 ^ | 0.543 |
| CPT1B | 1.14 | 0.98 | 1.18 | 0.21 | 0.772 | 1.39 | 1.35 | 0.65 | 1.02 | 0.25 | 0.161 | 0.177 |
| FABP2 | 0.82 | 1.17 | 1.04 | 0.13 | 0.171 # | 0.56 a | 0.79 a | 1.25 b | 1.43 b | 0.15 | 0.001 + | 0.400 |
| PPARα | 1.23 | 0.89 | 1.10 | 0.15 | 0.266 | 1.28 b | 1.41 b | 0.96 ab | 0.66 a | 0.18 | 0.019 + | 0.106 |
| RXRα | 1.15 | 0.66 | 0.95 | 0.17 | 0.123 # | 1.22 bc | 1.34 c | 0.79 ab | 0.33 a | 0.20 | 0.003 + | 0.244 |
| CPT1A/CPT1B | ||||||||||||
| Ratio | 1.04 | 1.01 | 0.93 | 0.09 | 0.662 | 1.00 ab | 0.89 a | 0.84 a | 1.25 b | 0.09 | 0.034 ^ | 0.087 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pike, B.; Zhao, J.; Hicks, J.A.; Wang, F.; Meisner, P.; Yang, L.; Liu, H.-C.; Odle, J.; Lin, X. Effects of Maternal Clofibrate Supplementation During Gestation and Lactation on Intestinal Fatty Acid Oxidation of Suckling Piglets. Int. J. Mol. Sci. 2025, 26, 8691. https://doi.org/10.3390/ijms26178691
Pike B, Zhao J, Hicks JA, Wang F, Meisner P, Yang L, Liu H-C, Odle J, Lin X. Effects of Maternal Clofibrate Supplementation During Gestation and Lactation on Intestinal Fatty Acid Oxidation of Suckling Piglets. International Journal of Molecular Sciences. 2025; 26(17):8691. https://doi.org/10.3390/ijms26178691
Chicago/Turabian StylePike, Brandon, Jinan Zhao, Julie A. Hicks, Feng Wang, Paige Meisner, Lin Yang, Hsiao-Ching Liu, Jack Odle, and Xi Lin. 2025. "Effects of Maternal Clofibrate Supplementation During Gestation and Lactation on Intestinal Fatty Acid Oxidation of Suckling Piglets" International Journal of Molecular Sciences 26, no. 17: 8691. https://doi.org/10.3390/ijms26178691
APA StylePike, B., Zhao, J., Hicks, J. A., Wang, F., Meisner, P., Yang, L., Liu, H.-C., Odle, J., & Lin, X. (2025). Effects of Maternal Clofibrate Supplementation During Gestation and Lactation on Intestinal Fatty Acid Oxidation of Suckling Piglets. International Journal of Molecular Sciences, 26(17), 8691. https://doi.org/10.3390/ijms26178691






