Beyond Killing: The Overlooked Contribution of Neutrophils to Tissue Repair
Abstract
1. Introduction
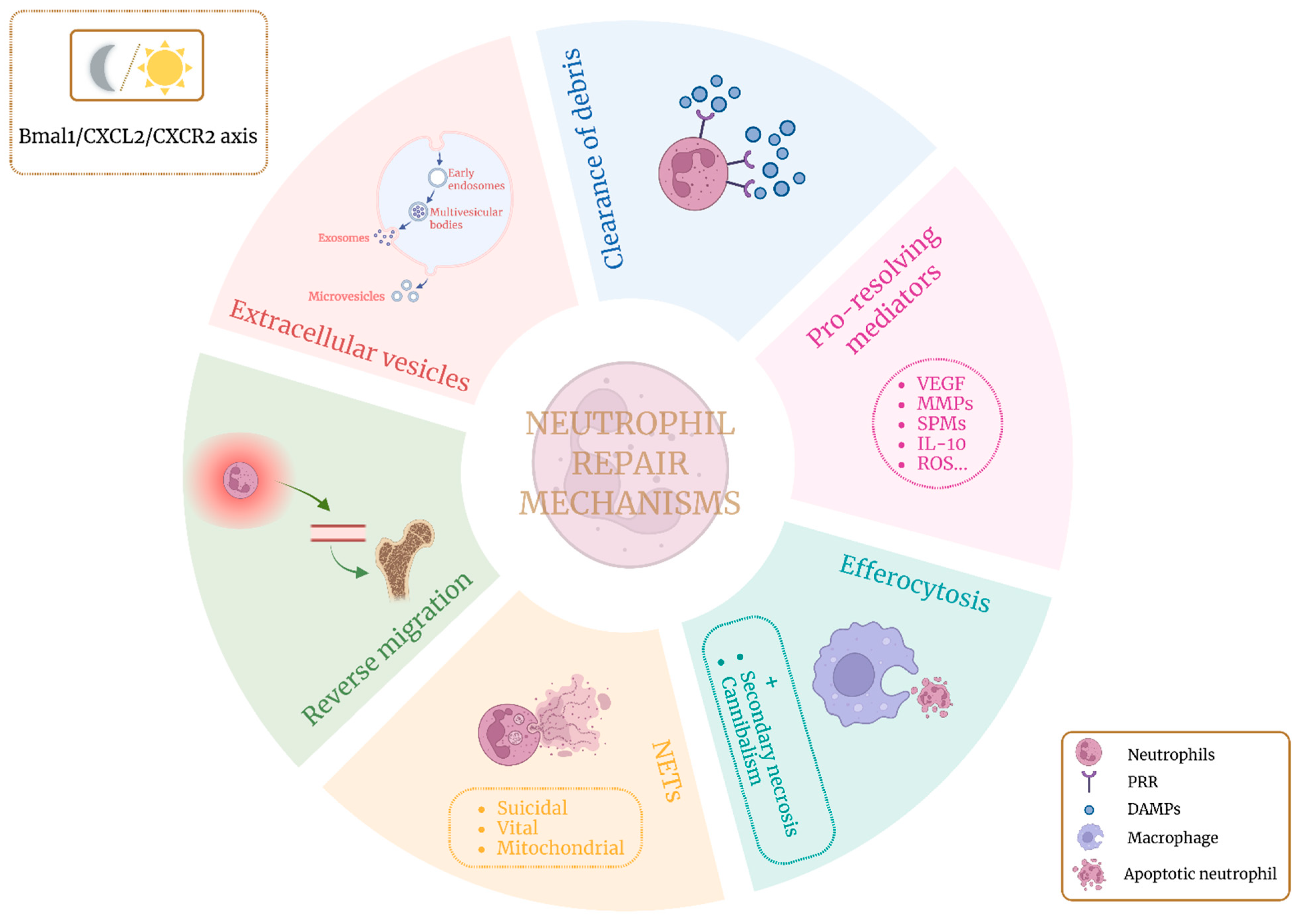
2. Neutrophils and Tissue Repair
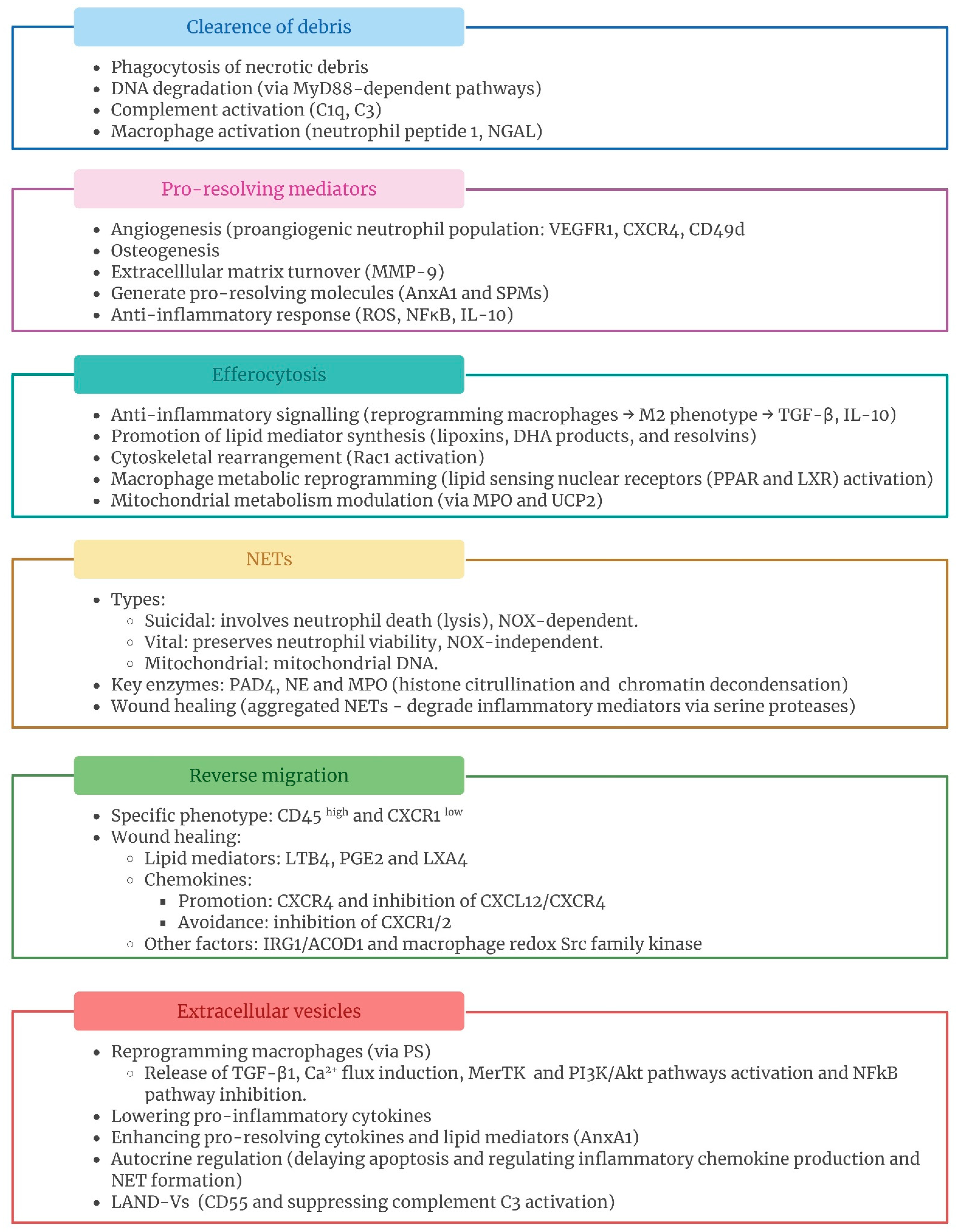
2.1. Clearance of Debris
2.2. Pro-Resolving Mediators
2.3. Efferocytosis
2.4. Neutrophil Extracellular Traps (NETs)
2.5. Neutrophil Reverse Migration
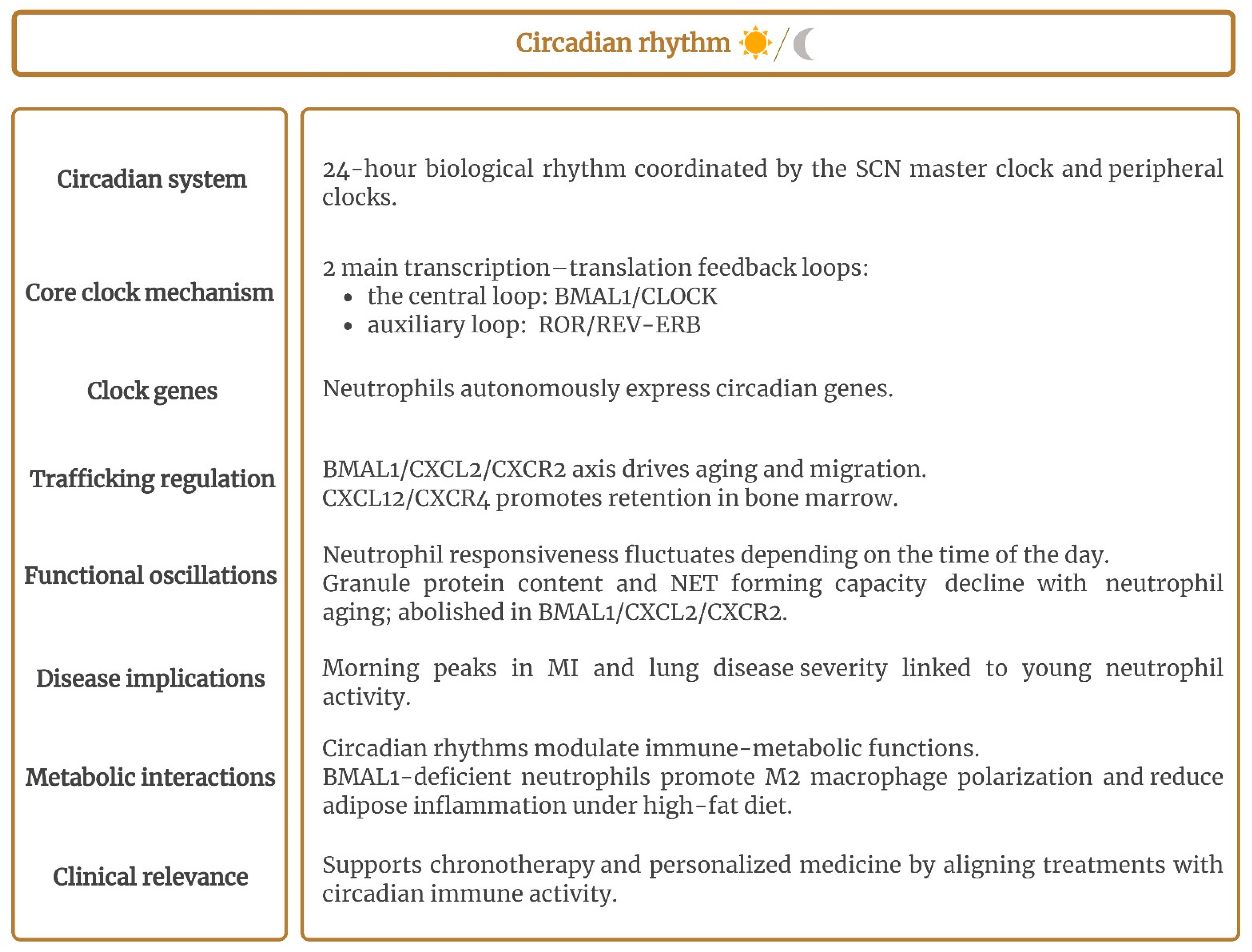
2.6. Circadian Rhythm
2.7. Neutrophil Extracellular Vesicles
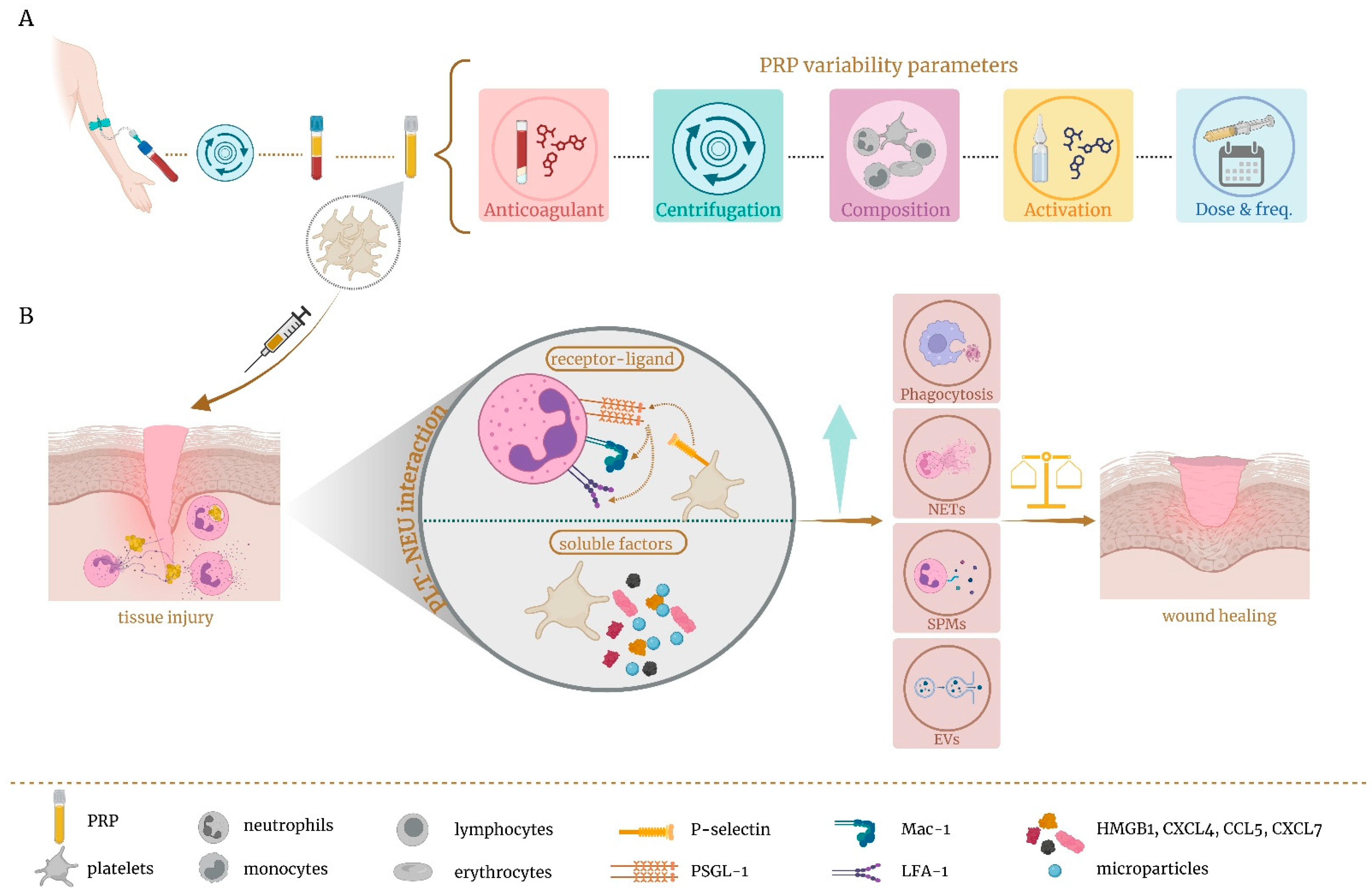
3. Role of Platelet-Rich Plasma as Immunomodulator of Local Neutrophils
4. Concluding Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 4-PBA | sodium 4-phenylbutyrate |
| AA | arachidonic acid |
| ACAMPs | apoptotic cell-associated molecular patterns |
| AggNETs | aggregated neutrophil extracellular traps |
| AMPK | AMP-activated protein kinase |
| AnxA1 | annexin 1 |
| APAP | acetaminophen |
| ATP | adenosine triphosphate |
| BLT1 | leukotriene B4 receptor 1 |
| BMAL1 | brain and muscle ARNT-like 1 |
| BMSCs | bone marrow mesenchymal stem cells |
| CCN1 | communication network factor 1 |
| CD49d | integrin alpha 4 |
| CD62L | L-selectin |
| CLOCK | circadian locomotor output cycles kaput |
| CNS | central nervous system |
| CX3CL1 | fractalkine |
| CXCR4 | C-X-C chemokine receptor type 4 |
| DAMPs | damage-associated molecular patterns |
| DHA | docosahexaenic acid |
| ECM | extracellular matrix |
| EPA | eicosapentaenoic acid |
| EVs | extracellular vesicles |
| FGF-2 | fibroblast growth factor-2 |
| GAS6 | growth arrest specific gene 6 |
| G-CSF | granulocyte colony-stimulating factor |
| GJA1 | gap junction alpha-1 protein |
| GPCRs | G protein-coupled receptors |
| HD | healthy donor |
| HIF-1 | hypoxia-inducible factor-1 |
| HMGB1 | high-mobility group box 1 |
| IL-10 | interleukin-10 |
| IRG1/ACOD1 | immune-responsive gene 1/aconitate decarboxylase 1 |
| JAM | junctional adhesion molecule |
| LAND-Vs | large aging-neutrophil-derived vesicles |
| LAP | LC3-asscociated phagocytosis |
| LTB4 | leukotriene B4 |
| LXA4 | stimulated lipoxin A4 |
| LXs | lipoxins |
| MFG-8 | milk fat globule protein |
| MI | myocardial infarction |
| miR | microRNA |
| MMPs | matrix metalloproteinases |
| MPO | myeloperoxidase |
| MR | mannose receptor (MR) |
| MSU | monosodium urate |
| n-3 DPA | n-3 docosapentaenoic acid |
| NE | neutrophil elastase |
| NE-BKO | neutrophil Bmal1 knockout |
| NETs | neutrophil extracellular traps |
| nEVs | neutrophil EVs |
| NGAL | neutrophil gelatinase-associated lipocalin |
| NOX | NADPH oxidase-2 |
| PAD4 | peptidylarginine deiminase 4 |
| PAI-1 | plasminogen activator inhibitor-1 |
| PAMPs | pathogen-associated molecular patterns |
| PGE2 | prostaglandin E2 |
| PMN | polymorphonuclear leukocyte |
| PPARγ | peroxisome proliferator-activated receptor gamma |
| PRP | platelet-rich plasma |
| PRRs | pattern recognition receptors |
| PS | phosphatidylserine |
| PSGL-1 | P-selectin glycoprotein ligand 1 |
| RA | rheumatoid arthritis |
| REV-ERB | reverse-Erb |
| ROR | retinoic acid-related orphan receptor |
| ROS | reactive oxygen species |
| rTEM | reverse transendothelial cell migration |
| RvD | resolvin D |
| SCN | suprachiasmatic nuclei |
| SOD2 | superoxide dismutase 2 |
| SPMs | specialized pro-resolving mediators |
| T2D | type 2 diabetes mellitus |
| TAM | Tyro/Axl/Mer |
| TGF-β | transforming growth factor-β1 |
| TIM | T-cell immunoglobulin domain and mucin domain |
| TIMP-1 | tissue inhibitor of metalloproteinases-1 |
| TLR2 | toll-like receptor 2 |
| UCP2 | uncoupling protein 2 |
| UTP | uridine 5′ triphosphate |
| VEGF | vascular endothelial growth factor |
| WD | Wallerian degeneration |
References
- Mayadas, T.N.; Cullere, X.; Lowell, C.A. The multifaceted functions of neutrophils. Annu. Rev. Pathol. 2014, 9, 181–218. [Google Scholar] [CrossRef]
- Peiseler, M.; Kubes, P. More friend than foe: The emerging role of neutrophils in tissue repair. J. Clin. Investig. 2019, 129, 2629–2639. [Google Scholar] [CrossRef]
- Wang, J. Neutrophils in tissue injury and repair. Cell Tissue Res. 2018, 371, 531–539. [Google Scholar] [CrossRef]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef]
- Kovtun, A.; Messerer, D.A.C.; Scharffetter-Kochanek, K.; Huber-Lang, M.; Ignatius, A. Neutrophils in Tissue Trauma of the Skin, Bone, and Lung: Two Sides of the Same Coin. J. Immunol. Res. 2018, 2018, 8173983. [Google Scholar] [CrossRef]
- Perez-Figueroa, E.; Alvarez-Carrasco, P.; Ortega, E.; Maldonado-Bernal, C. Neutrophils: Many Ways to Die. Front. Immunol. 2021, 12, 631821. [Google Scholar] [CrossRef]
- Nolan, E.; Malanchi, I. Connecting the dots: Neutrophils at the interface of tissue regeneration and cancer. Semin. Immunol. 2021, 57, 101598. [Google Scholar] [CrossRef]
- Cossío, I.; Lucas, D.; Hidalgo, A. Neutrophils as regulators of the hematopoietic niche. Blood 2019, 133, 2140–2148. [Google Scholar] [CrossRef]
- Bouchery, T.; Harris, N. Neutrophil-macrophage cooperation and its impact on tissue repair. Immunol. Cell Biol. 2019, 97, 289–298. [Google Scholar] [CrossRef]
- De Filippo, K.; Rankin, S.M. The Secretive Life of Neutrophils Revealed by Intravital Microscopy. Front. Cell Dev. Biol. 2020, 8, 603230. [Google Scholar] [CrossRef]
- Rizo-Tellez, S.A.; Filep, J.G. Beyond host defense and tissue injury: The emerging role of neutrophils in tissue repair. Am. J. Physiol. Cell Physiol. 2024, 326, C661–C683. [Google Scholar] [CrossRef]
- Carestia, A.; Kaufman, T.; Schattner, M. Platelets: New Bricks in the Building of Neutrophil Extracellular Traps. Front. Immunol. 2016, 7, 271. [Google Scholar] [CrossRef]
- Mosso-Pani, M.A.; Barreda, D.; Salazar, M.I. Dynamic regulation of neutrophil immunometabolism by platelet-derived metabolites. Front. Immunol. 2025, 16, 1542438. [Google Scholar] [CrossRef]
- Rayes, J.; Bourne, J.H.; Brill, A.; Watson, S.P. The dual role of platelet-innate immune cell interactions in thrombo-inflammation. Res. Pract. Thromb. Haemost. 2020, 4, 23–35. [Google Scholar] [CrossRef]
- Vermeren, S.; Elks, P.M.; Ellett, F. Editorial: Neutrophil Functions in Host Immunity, Inflammation and Tissue Repair. Front. Immunol. 2021, 12, 810346. [Google Scholar] [CrossRef]
- Kirsebom, F.C.M.; Kausar, F.; Nuriev, R.; Makris, S.; Johansson, C. Neutrophil recruitment and activation are differentially dependent on MyD88/TRIF and MAVS signaling during RSV infection. Mucosal Immunol. 2019, 12, 1244–1255. [Google Scholar] [CrossRef]
- Oliveira-Costa, K.M.; Menezes, G.B.; Paula Neto, H.A. Neutrophil accumulation within tissues: A damage x healing dichotomy. Biomed. Pharmacother. 2022, 145, 112422. [Google Scholar] [CrossRef]
- Crawford, L.; Wyatt, M.; Bryers, J.; Ratner, B. Biocompatibility Evolves: Phenomenology to Toxicology to Regeneration. Adv. Healthc. Mater. 2021, 10, e2002153. [Google Scholar] [CrossRef]
- Vandendriessche, S.; Mattos, M.S.; Bialek, E.L.; Schuermans, S.; Proost, P.; Marques, P.E. Complement activation drives the phagocytosis of necrotic cell debris and resolution of liver injury. Front. Immunol. 2024, 15, 1512470. [Google Scholar] [CrossRef]
- Schuermans, S.; Kestens, C.; Marques, P.E. Systemic mechanisms of necrotic cell debris clearance. Cell Death Dis. 2024, 15, 557. [Google Scholar] [CrossRef]
- Westman, J.; Grinstein, S.; Marques, P.E. Phagocytosis of Necrotic Debris at Sites of Injury and Inflammation. Front. Immunol. 2019, 10, 3030. [Google Scholar] [CrossRef]
- Oved, J.H.; Paris, A.J.; Gollomp, K.; Dai, N.; Rubey, K.; Wang, P.; Spruce, L.A.; Seeholzer, S.H.; Poncz, M.; Worthen, G.S. Neutrophils promote clearance of nuclear debris following acid-induced lung injury. Blood 2021, 137, 392–397. [Google Scholar] [CrossRef]
- Wang, J.; Hossain, M.; Thanabalasuriar, A.; Gunzer, M.; Meininger, C.; Kubes, P. Visualizing the function and fate of neutrophils in sterile injury and repair. Science 2017, 358, 111–116. [Google Scholar] [CrossRef]
- Franklin, R.J.M.; Simons, M. CNS remyelination and inflammation: From basic mechanisms to therapeutic opportunities. Neuron 2022, 110, 3549–3565. [Google Scholar] [CrossRef]
- Hammel, G.; Zivkovic, S.; Ayazi, M.; Ren, Y. Consequences and mechanisms of myelin debris uptake and processing by cells in the central nervous system. Cell Immunol. 2022, 380, 104591. [Google Scholar] [CrossRef]
- Tian, R.; Zhou, Y.; Ren, Y.; Zhang, Y.; Tang, W. Wallerian degeneration: From mechanism to disease to imaging. Heliyon 2025, 11, e40729. [Google Scholar] [CrossRef]
- Lindborg, J.A.; Mack, M.; Zigmond, R.E. Neutrophils Are Critical for Myelin Removal in a Peripheral Nerve Injury Model of Wallerian Degeneration. J. Neurosci. 2017, 37, 10258–10277. [Google Scholar] [CrossRef]
- Kou, Y.; Yuan, Y.; Li, Q.; Xie, W.; Xu, H.; Han, N. Neutrophil peptide 1 accelerates the clearance of degenerative axons during Wallerian degeneration by activating macrophages after peripheral nerve crush injury. Neural Regen. Res. 2024, 19, 1822–1827. [Google Scholar] [CrossRef]
- Yuan, Y.S.; Niu, S.P.; Yu, F.; Zhang, Y.J.; Han, N.; Lu, H.; Yin, X.F.; Xu, H.L.; Kou, Y.H. Intraoperative single administration of neutrophil peptide 1 accelerates the early functional recovery of peripheral nerves after crush injury. Neural Regen. Res. 2020, 15, 2108–2115. [Google Scholar] [CrossRef]
- Teixeira, C.F.; Zamunér, S.R.; Zuliani, J.P.; Fernandes, C.M.; Cruz-Hofling, M.A.; Fernandes, I.; Chaves, F.; Gutiérrez, J.M. Neutrophils do not contribute to local tissue damage, but play a key role in skeletal muscle regeneration, in mice injected with Bothrops asper snake venom. Muscle Nerve 2003, 28, 449–459. [Google Scholar] [CrossRef]
- Horckmans, M.; Ring, L.; Duchene, J.; Santovito, D.; Schloss, M.J.; Drechsler, M.; Weber, C.; Soehnlein, O.; Steffens, S. Neutrophils orchestrate post-myocardial infarction healing by polarizing macrophages towards a reparative phenotype. Eur. Heart J. 2017, 38, 187–197. [Google Scholar] [CrossRef]
- Jones, H.R.; Robb, C.T.; Perretti, M.; Rossi, A.G. The role of neutrophils in inflammation resolution. Semin. Immunol. 2016, 28, 137–145. [Google Scholar] [CrossRef]
- Yang, A.; Wu, Y.; Yu, G.; Wang, H. Role of specialized pro-resolving lipid mediators in pulmonary inflammation diseases: Mechanisms and development. Respir. Res. 2021, 22, 204. [Google Scholar] [CrossRef]
- Turner, T.C.; Sok, M.C.P.; Hymel, L.A.; Pittman, F.S.; York, W.Y.; Mac, Q.D.; Vyshnya, S.; Lim, H.S.; Kwong, G.A.; Qiu, P.; et al. Harnessing lipid signaling pathways to target specialized pro-angiogenic neutrophil subsets for regenerative immunotherapy. Sci. Adv. 2020, 6, eaba7702. [Google Scholar] [CrossRef]
- Mueller, M.D.; Lebovic, D.I.; Garrett, E.; Taylor, R.N. Neutrophils infiltrating the endometrium express vascular endothelial growth factor: Potential role in endometrial angiogenesis. Fertil. Steril. 2000, 74, 107–112. [Google Scholar] [CrossRef]
- Phillipson, M.; Kubes, P. The Healing Power of Neutrophils. Trends Immunol. 2019, 40, 635–647. [Google Scholar] [CrossRef]
- Massena, S.; Christoffersson, G.; Vagesjo, E.; Seignez, C.; Gustafsson, K.; Binet, F.; Herrera Hidalgo, C.; Giraud, A.; Lomei, J.; Westrom, S.; et al. Identification and characterization of VEGF-A-responsive neutrophils expressing CD49d, VEGFR1, and CXCR4 in mice and humans. Blood 2015, 126, 2016–2026. [Google Scholar] [CrossRef]
- Ohki, Y.; Heissig, B.; Sato, Y.; Akiyama, H.; Zhu, Z.; Hicklin, D.J.; Shimada, K.; Ogawa, H.; Daida, H.; Hattori, K.; et al. Granulocyte colony-stimulating factor promotes neovascularization by releasing vascular endothelial growth factor from neutrophils. FASEB J. 2005, 19, 2005–2007. [Google Scholar] [CrossRef]
- Ardi, V.C.; Kupriyanova, T.A.; Deryugina, E.I.; Quigley, J.P. Human neutrophils uniquely release TIMP-free MMP-9 to provide a potent catalytic stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2007, 104, 20262–20267. [Google Scholar] [CrossRef]
- Heissig, B.; Nishida, C.; Tashiro, Y.; Sato, Y.; Ishihara, M.; Ohki, M.; Gritli, I.; Rosenkvist, J.; Hattori, K. Role of neutrophil-derived matrix metalloproteinase-9 in tissue regeneration. Histol. Histopathol. 2010, 25, 765–770. [Google Scholar]
- Lindsey, M.; Wedin, K.; Brown, M.D.; Keller, C.; Evans, A.J.; Smolen, J.; Burns, A.R.; Rossen, R.D.; Michael, L.; Entman, M. Matrix-Dependent Mechanism of Neutrophil-Mediated Release and Activation of Matrix Metalloproteinase 9 in Myocardial Ischemia/Reperfusion. Circulation 2001, 103, 2181–2187. [Google Scholar] [CrossRef] [PubMed]
- Herath, T.D.K.; Larbi, A.; Teoh, S.H.; Kirkpatrick, C.J.; Goh, B.T. Neutrophil-mediated enhancement of angiogenesis and osteogenesis in a novel triple cell co-culture model with endothelial cells and osteoblasts. J. Tissue Eng. Regen. Med. 2018, 12, e1221–e1236. [Google Scholar] [CrossRef]
- Christoffersson, G.; Vagesjo, E.; Vandooren, J.; Liden, M.; Massena, S.; Reinert, R.B.; Brissova, M.; Powers, A.C.; Opdenakker, G.; Phillipson, M. VEGF-A recruits a proangiogenic MMP-9-delivering neutrophil subset that induces angiogenesis in transplanted hypoxic tissue. Blood 2012, 120, 4653–4662. [Google Scholar] [CrossRef]
- Muhs, B.E.; Gagne, P.; Plitas, G.; Shaw, J.P.; Shamamian, P. Experimental hindlimb ischemia leads to neutrophil-mediated increases in gastrocnemius MMP-2 and -9 activity: A potential mechanism for ischemia induced MMP activation. J. Surg. Res. 2004, 117, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Sato, Y.; Ohki, Y.; Akiyama, H.; Rosenkvist, J.; Gritli, I.; Okumura, K.; Ogawa, H.; Daida, H.; Heissig, B.; Hattori, K.; et al. Targeted deletion of matrix metalloproteinase-9 reduces neutrophil accumulation during G-CSF-induced neoangiogenesis. Cytom. Res. 2009, 19, 53–62. [Google Scholar]
- Harty, M.W.; Muratore, C.S.; Papa, E.F.; Gart, M.S.; Ramm, G.A.; Gregory, S.H.; Tracy, T.F., Jr. Neutrophil depletion blocks early collagen degradation in repairing cholestatic rat livers. Am. J. Pathol. 2010, 176, 1271–1281. [Google Scholar] [CrossRef]
- Alvarenga, D.M.; Mattos, M.S.; Lopes, M.E.; Marchesi, S.C.; Araujo, A.M.; Nakagaki, B.N.; Santos, M.M.; David, B.A.; De Souza, V.A.; Carvalho, E.; et al. Paradoxical Role of Matrix Metalloproteinases in Liver Injury and Regeneration after Sterile Acute Hepatic Failure. Cells 2018, 7, 247. [Google Scholar] [CrossRef]
- Basil, M.C.; Levy, B.D. Specialized pro-resolving mediators: Endogenous regulators of infection and inflammation. Nat. Rev. Immunol. 2016, 16, 51–67. [Google Scholar] [CrossRef]
- Burn, G.L.; Foti, A.; Marsman, G.; Patel, D.F.; Zychlinsky, A. The Neutrophil. Immunity 2021, 54, 1377–1391. [Google Scholar] [CrossRef] [PubMed]
- Perucci, L.O.; Sugimoto, M.A.; Gomes, K.B.; Dusse, L.M.; Teixeira, M.M.; Sousa, L.P. Annexin A1 and specialized proresolving lipid mediators: Promoting resolution as a therapeutic strategy in human inflammatory diseases. Expert Opin. Ther. Targets 2017, 21, 879–896. [Google Scholar] [CrossRef]
- Fattori, V.; Zaninelli, T.H.; Rasquel-Oliveira, F.S.; Casagrande, R.; Verri, W.A., Jr. Specialized pro-resolving lipid mediators: A new class of non-immunosuppressive and non-opioid analgesic drugs. Pharmacol. Res. 2020, 151, 104549. [Google Scholar] [CrossRef]
- Valente, M.; Dentoni, M.; Bellizzi, F.; Kuris, F.; Gigli, G.L. Specialized Pro-Resolving Mediators in Neuroinflammation: Overview of Studies and Perspectives of Clinical Applications. Molecules 2022, 27, 4836. [Google Scholar] [CrossRef] [PubMed]
- Serhan, C.N. Systems approach to inflammation resolution: Identification of novel anti-inflammatory and pro-resolving mediators. J. Thromb. Haemost. 2009, 7 (Suppl. 1), 44–48. [Google Scholar] [CrossRef]
- Park, J.; Langmead, C.J.; Riddy, D.M. New Advances in Targeting the Resolution of Inflammation: Implications for Specialized Pro-Resolving Mediator GPCR Drug Discovery. ACS Pharmacol. Transl. Sci. 2020, 3, 88–106. [Google Scholar] [CrossRef]
- Lin, R.; Yi, Z.; Wang, J.; Geng, S.; Li, L. Generation of resolving memory neutrophils through pharmacological training with 4-PBA or genetic deletion of TRAM. Cell Death Dis. 2022, 13, 345. [Google Scholar] [CrossRef]
- Flak, M.B.; Koenis, D.S.; Sobrino, A.; Smith, J.; Pistorius, K.; Palmas, F.; Dalli, J. GPR101 mediates the pro-resolving actions of RvD5n-3 DPA in arthritis and infections. J. Clin. Investig. 2020, 130, 359–373. [Google Scholar] [CrossRef]
- Norris, P.C.; Libreros, S.; Serhan, C.N. Resolution metabolomes activated by hypoxic environment. Sci. Adv. 2019, 5, eaax4895. [Google Scholar] [CrossRef]
- Dalli, J.; Norling, L.V.; Renshaw, D.; Cooper, D.; Leung, K.Y.; Perretti, M. Annexin 1 mediates the rapid anti-inflammatory effects of neutrophil-derived microparticles. Blood 2008, 112, 2512–2519. [Google Scholar] [CrossRef] [PubMed]
- Mainka, M.; George, S.; Angioni, C.; Ebert, R.; Goebel, T.; Kampschulte, N.; Krommes, A.; Weigert, A.; Thomas, D.; Schebb, N.H.; et al. On the biosynthesis of specialized pro-resolving mediators in human neutrophils and the influence of cell integrity. Biochim. Et Biophys. Acta (BBA)-Mol. Cell Biol. Lipids 2022, 1867, 159093. [Google Scholar] [CrossRef]
- Yang, W.; Tao, Y.; Wu, Y.; Zhao, X.; Ye, W.; Zhao, D.; Fu, L.; Tian, C.; Yang, J.; He, F.; et al. Neutrophils promote the development of reparative macrophages mediated by ROS to orchestrate liver repair. Nat. Commun. 2019, 10, 1076. [Google Scholar] [CrossRef] [PubMed]
- Marwick, J.A.; Mills, R.; Kay, O.; Michail, K.; Stephen, J.; Rossi, A.G.; Dransfield, I.; Hirani, N. Neutrophils induce macrophage anti-inflammatory reprogramming by suppressing NF-kappaB activation. Cell Death Dis. 2018, 9, 665. [Google Scholar] [CrossRef]
- Tosello Boari, J.; Amezcua Vesely, M.C.; Bermejo, D.A.; Ramello, M.C.; Montes, C.L.; Cejas, H.; Gruppi, A.; Acosta Rodriguez, E.V. IL-17RA signaling reduces inflammation and mortality during Trypanosoma cruzi infection by recruiting suppressive IL-10-producing neutrophils. PLoS Pathog. 2012, 8, e1002658. [Google Scholar] [CrossRef] [PubMed]
- Lewkowicz, N.; Mycko, M.P.; Przygodzka, P.; Cwiklinska, H.; Cichalewska, M.; Matysiak, M.; Selmaj, K.; Lewkowicz, P. Induction of human IL-10-producing neutrophils by LPS-stimulated Treg cells and IL-10. Mucosal Immunol. 2016, 9, 364–378. [Google Scholar] [CrossRef]
- Zhang, X.; Majlessi, L.; Deriaud, E.; Leclerc, C.; Lo-Man, R. Coactivation of Syk kinase and MyD88 adaptor protein pathways by bacteria promotes regulatory properties of neutrophils. Immunity 2009, 31, 761–771. [Google Scholar] [CrossRef]
- Gonzalez, L.A.; Melo-Gonzalez, F.; Sebastian, V.P.; Vallejos, O.P.; Noguera, L.P.; Suazo, I.D.; Schultz, B.M.; Manosalva, A.H.; Penaloza, H.F.; Soto, J.A.; et al. Characterization of the Anti-Inflammatory Capacity of IL-10-Producing Neutrophils in Response to Streptococcus pneumoniae Infection. Front. Immunol. 2021, 12, 638917. [Google Scholar] [CrossRef]
- Raudszus, L.; Bahreini, F.; Allan, S.; Kalies, K.U.; Caldwell, C.C.; Kalies, K. Nanoparticles containing intracellular proteins modulate neutrophil functional and phenotypic heterogeneity. Front. Immunol. 2024, 15, 1494400. [Google Scholar] [CrossRef]
- Park, Y.J.; Liu, G.; Tsuruta, Y.; Lorne, E.; Abraham, E. Participation of the urokinase receptor in neutrophil efferocytosis. Blood 2009, 114, 860–870. [Google Scholar] [CrossRef]
- Jun, J.I.; Kim, K.H.; Lau, L.F. The matricellular protein CCN1 mediates neutrophil efferocytosis in cutaneous wound healing. Nat. Commun. 2015, 6, 7386. [Google Scholar] [CrossRef] [PubMed]
- Brostjan, C.; Oehler, R. The role of neutrophil death in chronic inflammation and cancer. Cell Death Discov. 2020, 6, 26. [Google Scholar] [CrossRef]
- Loh, W.; Vermeren, S. Anti-Inflammatory Neutrophil Functions in the Resolution of Inflammation and Tissue Repair. Cells 2022, 11, 4076. [Google Scholar] [CrossRef] [PubMed]
- Dalli, J.; Serhan, C.N. Specific lipid mediator signatures of human phagocytes: Microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012, 120, e60–e72. [Google Scholar] [CrossRef]
- Saas, P.; Vetter, M.; Maraux, M.; Bonnefoy, F.; Perruche, S. Resolution therapy: Harnessing efferocytic macrophages to trigger the resolution of inflammation. Front. Immunol. 2022, 13, 1021413. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ding, W.; Zhao, M.; Liu, J.; Xu, Y.; Wan, J.; Wang, M. Mechanisms of efferocytosis in determining inflammation resolution: Therapeutic potential and the association with cardiovascular disease. Br. J. Pharmacol. 2022, 179, 5151–5171. [Google Scholar] [CrossRef]
- Xie, Y.; Yang, J.; Zhu, H.; Yang, R.; Fan, Y. The efferocytosis dilemma: How neutrophil extracellular traps and PI3K/Rac1 complicate diabetic wound healing. Cell Commun. Signal. 2025, 23, 103. [Google Scholar] [CrossRef] [PubMed]
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef] [PubMed]
- Karaji, N.; Sattentau, Q.J. Efferocytosis of Pathogen-Infected Cells. Front. Immunol. 2017, 8, 1863. [Google Scholar] [CrossRef]
- Silva, M.T. Secondary necrosis: The natural outcome of the complete apoptotic program. FEBS Lett. 2010, 584, 4491–4499. [Google Scholar] [CrossRef]
- Moon, B.; Yang, S.; Moon, H.; Lee, J.; Park, D. After cell death: The molecular machinery of efferocytosis. Exp. Mol. Med. 2023, 55, 1644–1651. [Google Scholar] [CrossRef]
- Ramos, C.; Oehler, R. Clearance of apoptotic cells by neutrophils in inflammation and cancer. Cell Death Discov. 2024, 10, 26. [Google Scholar] [CrossRef]
- Wei, K.H.; Lin, I.T.; Chowdhury, K.; Lim, K.L.; Liu, K.T.; Ko, T.M.; Chang, Y.M.; Yang, K.C.; Lai, S.B. Comparative single-cell profiling reveals distinct cardiac resident macrophages essential for zebrafish heart regeneration. eLife 2023, 12, e84679. [Google Scholar] [CrossRef]
- Cunningham, K.T.; Maizels, R.M. We are what we eat: Macrophages and efferocytosis. Trends Parasitol. 2024, 40, 446–448. [Google Scholar] [CrossRef]
- Mihaila, A.C.; Ciortan, L.; Tucureanu, M.M.; Simionescu, M.; Butoi, E. Anti-Inflammatory Neutrophils Reprogram Macrophages toward a Pro-Healing Phenotype with Increased Efferocytosis Capacity. Cells 2024, 13, 208. [Google Scholar] [CrossRef] [PubMed]
- Han, C.Z.; Ravichandran, K.S. Metabolic connections during apoptotic cell engulfment. Cell 2011, 147, 1442–1445. [Google Scholar] [CrossRef]
- Better, J.; Wetstein, M.; Estiri, M.; Malainou, C.; Ferrero, M.; Langelage, M.; Kuznetsova, I.; Vazquez-Armendariz, I.; Kimmig, L.; Mansouri, S.; et al. Neutrophil efferocytosis reprograms mitochondrial metabolism to switch alveolar macrophages to a pro-resolution phenotype at the cost of bacterial control. bioRxiv 2023. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Kim, S.Y.; Kim, M.J.; Lim, J.H.; Cho, M.S.; Kang, J.L. PPARgamma activation following apoptotic cell instillation promotes resolution of lung inflammation and fibrosis via regulation of efferocytosis and proresolving cytokines. Mucosal Immunol. 2015, 8, 1031–1046. [Google Scholar] [CrossRef]
- Lim, K.; Kim, T.H.; Trzeciak, A.; Amitrano, A.M.; Reilly, E.C.; Prizant, H.; Fowell, D.J.; Topham, D.J.; Kim, M. In situ neutrophil efferocytosis shapes T cell immunity to influenza infection. Nat. Immunol. 2020, 21, 1046–1057. [Google Scholar] [CrossRef] [PubMed]
- Gregoire, M.; Uhel, F.; Lesouhaitier, M.; Gacouin, A.; Guirriec, M.; Mourcin, F.; Dumontet, E.; Chalin, A.; Samson, M.; Berthelot, L.L.; et al. Impaired efferocytosis and neutrophil extracellular trap clearance by macrophages in ARDS. Eur. Respir. J. 2018, 52, 1702590. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Wang, J.; Park, Y.J.; Tsuruta, Y.; Lorne, E.F.; Zhao, X.; Abraham, E. High mobility group protein-1 inhibits phagocytosis of apoptotic neutrophils through binding to phosphatidylserine. J. Immunol. 2008, 181, 4240–4246. [Google Scholar] [CrossRef]
- Banerjee, S.; de Freitas, A.; Friggeri, A.; Zmijewski, J.W.; Liu, G.; Abraham, E. Intracellular HMGB1 negatively regulates efferocytosis. J. Immunol. 2011, 187, 4686–4694. [Google Scholar] [CrossRef]
- Banerjee, S.; Friggeri, A.; Liu, G.; Abraham, E. The C-terminal acidic tail is responsible for the inhibitory effects of HMGB1 on efferocytosis. J. Leukoc. Biol. 2010, 88, 973–979. [Google Scholar] [CrossRef]
- Friggeri, A.; Yang, Y.; Banerjee, S.; Park, Y.J.; Liu, G.; Abraham, E. HMGB1 inhibits macrophage activity in efferocytosis through binding to the alphavbeta3-integrin. Am. J. Physiol. Cell Physiol. 2010, 299, C1267–C1276. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, W.; Xu, Y.; Wu, D.; Gao, Z.; Zhou, J.; Qian, H.; He, B.; Wang, G. Extracellular HMGB1 Impairs Macrophage-Mediated Efferocytosis by Suppressing the Rab43-Controlled Cell Surface Transport of CD91. Front. Immunol. 2022, 13, 767630. [Google Scholar] [CrossRef]
- Jiang, S.; Park, D.W.; Stigler, W.S.; Creighton, J.; Ravi, S.; Darley-Usmar, V.; Zmijewski, J.W. Mitochondria and AMP-activated protein kinase-dependent mechanism of efferocytosis. J. Biol. Chem. 2013, 288, 26013–26026. [Google Scholar] [CrossRef]
- Kim, K.H.; Won, J.H.; Cheng, N.; Lau, L.F. The matricellular protein CCN1 in tissue injury repair. J. Cell Commun. Signal. 2018, 12, 273–279. [Google Scholar] [CrossRef]
- Rydell-Tormanen, K.; Uller, L.; Erjefalt, J.S. Neutrophil cannibalism—A back up when the macrophage clearance system is insufficient. Respir. Res. 2006, 7, 143. [Google Scholar] [CrossRef]
- Sachet, M.; Liang, Y.Y.; Oehler, R. The immune response to secondary necrotic cells. Apoptosis 2017, 22, 1189–1204. [Google Scholar] [CrossRef]
- Steiger, S.; Harper, J.L. Neutrophil cannibalism triggers transforming growth factor beta1 production and self regulation of neutrophil inflammatory function in monosodium urate monohydrate crystal-induced inflammation in mice. Arthritis Rheum. 2013, 65, 815–823. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil extracellular traps kill bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef] [PubMed]
- Boeltz, S.; Amini, P.; Anders, H.J.; Andrade, F.; Bilyy, R.; Chatfield, S.; Cichon, I.; Clancy, D.M.; Desai, J.; Dumych, T.; et al. To NET or not to NET:current opinions and state of the science regarding the formation of neutrophil extracellular traps. Cell Death Differ. 2019, 26, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Schoen, J.; Euler, M.; Schauer, C.; Schett, G.; Herrmann, M.; Knopf, J.; Yaykasli, K.O. Neutrophils’ Extracellular Trap Mechanisms: From Physiology to Pathology. Int. J. Mol. Sci. 2022, 23, 12855. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, M.; Magnelli, V.; Renò, F. NETosis in Wound Healing: When Enough Is Enough. Cells 2021, 10, 494. [Google Scholar] [CrossRef]
- Knopf, J.; Leppkes, M.; Schett, G.; Herrmann, M.; Munoz, L.E. Aggregated NETs Sequester and Detoxify Extracellular Histones. Front. Immunol. 2019, 10, 2176. [Google Scholar] [CrossRef]
- Wang, H.; Kim, S.J.; Lei, Y.; Wang, S.; Wang, H.; Huang, H.; Zhang, H.; Tsung, A. Neutrophil extracellular traps in homeostasis and disease. Signal Transduct. Target. Ther. 2024, 9, 235. [Google Scholar] [CrossRef]
- Daniel, C.; Leppkes, M.; Munoz, L.E.; Schley, G.; Schett, G.; Herrmann, M. Extracellular DNA traps in inflammation, injury and healing. Nat. Rev. Nephrol. 2019, 15, 559–575. [Google Scholar] [CrossRef]
- Melbouci, D.; Haidar Ahmad, A.; Decker, P. Neutrophil extracellular traps (NET): Not only antimicrobial but also modulators of innate and adaptive immunities in inflammatory autoimmune diseases. RMD Open 2023, 9, e003104. [Google Scholar] [CrossRef] [PubMed]
- Galluzzi, L.; Vitale, I.; Aaronson, S.A.; Abrams, J.M.; Adam, D.; Agostinis, P.; Alnemri, E.S.; Altucci, L.; Amelio, I.; Andrews, D.W.; et al. Molecular mechanisms of cell death: Recommendations of the Nomenclature Committee on Cell Death 2018. Cell Death Differ. 2018, 25, 486–541. [Google Scholar] [CrossRef]
- Yousefi, S.; Stojkov, D.; Germic, N.; Simon, D.; Wang, X.; Benarafa, C.; Simon, H.U. Untangling “NETosis” from NETs. Eur. J. Immunol. 2019, 49, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Yu, Y.; Ren, Y.; Xu, L.; Wang, H.; Ling, X.; Jin, L.; Hu, Y.; Zhang, H.; Miao, C.; et al. The emerging roles of neutrophil extracellular traps in wound healing. Cell Death Dis. 2021, 12, 984. [Google Scholar] [CrossRef]
- Stoimenou, M.; Tzoros, G.; Skendros, P.; Chrysanthopoulou, A. Methods for the Assessment of NET Formation: From Neutrophil Biology to Translational Research. Int. J. Mol. Sci. 2022, 23, 15823. [Google Scholar] [CrossRef] [PubMed]
- Retter, A.; Singer, M.; Annane, D. “The NET effect”: Neutrophil extracellular traps-a potential key component of the dysregulated host immune response in sepsis. Crit. Care 2025, 29, 59. [Google Scholar] [CrossRef]
- Huang, J.; Hong, W.; Wan, M.; Zheng, L. Molecular mechanisms and therapeutic target of NETosis in diseases. MedComm (2020) 2022, 3, e162. [Google Scholar] [CrossRef]
- Manda-Handzlik, A.; Cieloch, A.; Kuzmicka, W.; Mroczek, A.; Stelmaszczyk-Emmel, A.; Demkow, U.; Wachowska, M. Secretomes of M1 and M2 macrophages decrease the release of neutrophil extracellular traps. Sci. Rep. 2023, 13, 15633. [Google Scholar] [CrossRef]
- Biermann, M.H.; Podolska, M.J.; Knopf, J.; Reinwald, C.; Weidner, D.; Maueroder, C.; Hahn, J.; Kienhofer, D.; Barras, A.; Boukherroub, R.; et al. Oxidative Burst-Dependent NETosis Is Implicated in the Resolution of Necrosis-Associated Sterile Inflammation. Front. Immunol. 2016, 7, 557, Erratum in Front. Immunol. 2025, 16, 1593749. [Google Scholar] [CrossRef]
- Schauer, C.; Janko, C.; Munoz, L.E.; Zhao, Y.; Kienhofer, D.; Frey, B.; Lell, M.; Manger, B.; Rech, J.; Naschberger, E.; et al. Aggregated neutrophil extracellular traps limit inflammation by degrading cytokines and chemokines. Nat. Med. 2014, 20, 511–517. [Google Scholar] [CrossRef]
- Mahajan, A.; Gruneboom, A.; Petru, L.; Podolska, M.J.; Kling, L.; Maueroder, C.; Dahms, F.; Christiansen, S.; Gunter, L.; Krenn, V.; et al. Frontline Science: Aggregated neutrophil extracellular traps prevent inflammation on the neutrophil-rich ocular surface. J. Leukoc. Biol. 2019, 105, 1087–1098. [Google Scholar] [CrossRef]
- Hahn, J.; Schauer, C.; Czegley, C.; Kling, L.; Petru, L.; Schmid, B.; Weidner, D.; Reinwald, C.; Biermann, M.H.C.; Blunder, S.; et al. Aggregated neutrophil extracellular traps resolve inflammation by proteolysis of cytokines and chemokines and protection from antiproteases. FASEB J. 2019, 33, 1401–1414. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Peng, M.; Tong, M.; He, Y.; Hao, M.; Gao, H.L.; Lao, Y.; Xue, J.; Liu, M.; Zhong, Q.; et al. The globular domain of extracellular histones mediates cytotoxicity via membrane disruption mechanism. J. Biol. Chem. 2025, 301, 108038. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Peng, J.; Zhang, Z.; Chen, Y.; Liu, Z.; Jiang, L.; Jin, L.; Han, M.; Su, B.; Li, Y. Emerging therapeutic strategies targeting extracellular histones for critical and inflammatory diseases: An updated narrative review. Front. Immunol. 2024, 15, 1438984. [Google Scholar] [CrossRef] [PubMed]
- Tonello, S.; Rizzi, M.; Migliario, M.; Rocchetti, V.; Reno, F. Low concentrations of neutrophil extracellular traps induce proliferation in human keratinocytes via NF-kB activation. J. Dermatol. Sci. 2017, 88, 110–116. [Google Scholar] [CrossRef]
- Leppkes, M.; Lindemann, A.; Gößwein, S.; Paulus, S.; Roth, D.; Hartung, A.; Liebing, E.; Zundler, S.; Gonzalez-Acera, M.; Patankar, J.V.; et al. Neutrophils prevent rectal bleeding in ulcerative colitis by peptidyl-arginine deiminase-4-dependent immunothrombosis. Gut 2022, 71, 2414–2429. [Google Scholar] [CrossRef]
- Arampatzioglou, A.; Papazoglou, D.; Konstantinidis, T.; Chrysanthopoulou, A.; Mitsios, A.; Angelidou, I.; Maroulakou, I.; Ritis, K.; Skendros, P. Clarithromycin Enhances the Antibacterial Activity and Wound Healing Capacity in Type 2 Diabetes Mellitus by Increasing LL-37 Load on Neutrophil Extracellular Traps. Front. Immunol. 2018, 9, 2064. [Google Scholar] [CrossRef]
- Ribon, M.; Seninet, S.; Mussard, J.; Sebbag, M.; Clavel, C.; Serre, G.; Boissier, M.C.; Semerano, L.; Decker, P. Neutrophil extracellular traps exert both pro- and anti-inflammatory actions in rheumatoid arthritis that are modulated by C1q and LL-37. J. Autoimmun. 2019, 98, 122–131. [Google Scholar] [CrossRef]
- Bilyy, R.; Fedorov, V.; Vovk, V.; Leppkes, M.; Dumych, T.; Chopyak, V.; Schett, G.; Herrmann, M. Neutrophil Extracellular Traps Form a Barrier between Necrotic and Viable Areas in Acute Abdominal Inflammation. Front. Immunol. 2016, 7, 424. [Google Scholar] [CrossRef]
- Demkow, U. Molecular Mechanisms of Neutrophil Extracellular Trap (NETs) Degradation. Int. J. Mol. Sci. 2023, 24, 4896. [Google Scholar] [CrossRef] [PubMed]
- Farrera, C.; Fadeel, B. Macrophage clearance of neutrophil extracellular traps is a silent process. J. Immunol. 2013, 191, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, S.; Rosowski, E.E.; Huttenlocher, A. Neutrophil migration in infection and wound repair: Going forward in reverse. Nat. Rev. Immunol. 2016, 16, 378–391. [Google Scholar] [CrossRef] [PubMed]
- Hind, L.E.; Huttenlocher, A. Neutrophil Reverse Migration and a Chemokinetic Resolution. Dev. Cell 2018, 47, 404–405. [Google Scholar] [CrossRef]
- Xu, Q.; Zhao, W.; Yan, M.; Mei, H. Neutrophil reverse migration. J. Inflamm. 2022, 19, 22. [Google Scholar] [CrossRef]
- Hughes, J.; Johnson, R.J.; Mooney, A.; Hugo, C.; Gordon, K.; Savill, J. Neutrophil fate in experimental glomerular capillary injury in the rat. Emigration exceeds in situ clearance by apoptosis. Am. J. Pathol. 1997, 150, 223–234. [Google Scholar]
- Mathias, J.R.; Perrin, B.J.; Liu, T.X.; Kanki, J.; Look, A.T.; Huttenlocher, A. Resolution of inflammation by retrograde chemotaxis of neutrophils in transgenic zebrafish. J. Leukoc. Biol. 2006, 80, 1281–1288. [Google Scholar] [CrossRef]
- Deng, Q.; Huttenlocher, A. Leukocyte migration from a fish eye’s view. J. Cell Sci. 2012, 125, 3949–3956. [Google Scholar] [CrossRef]
- Holmes, G.R.; Anderson, S.R.; Dixon, G.; Robertson, A.L.; Reyes-Aldasoro, C.C.; Billings, S.A.; Renshaw, S.A.; Kadirkamanathan, V. Repelled from the wound, or randomly dispersed? Reverse migration behaviour of neutrophils characterized by dynamic modelling. J. R. Soc. Interface 2012, 9, 3229–3239. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Rocha-Gregg, B.; Huttenlocher, A. Signal integration in forward and reverse neutrophil migration: Fundamentals and emerging mechanisms. Curr. Opin. Cell Biol. 2021, 72, 124–130. [Google Scholar] [CrossRef]
- Nourshargh, S.; Renshaw, S.A.; Imhof, B.A. Reverse Migration of Neutrophils: Where, When, How, and Why? Trends Immunol. 2016, 37, 273–286. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, K.A.; Ayuso, J.M.; Kerr, S.C.; Huttenlocher, A.; Beebe, D.J. Microfluidic Systems to Study Neutrophil Forward and Reverse Migration. Front. Immunol. 2021, 12, 781535. [Google Scholar] [CrossRef]
- Buckley, C.D.; Ross, E.A.; McGettrick, H.M.; Osborne, C.E.; Haworth, O.; Schmutz, C.; Stone, P.C.; Salmon, M.; Matharu, N.M.; Vohra, R.K.; et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J. Leukoc. Biol. 2006, 79, 303–311. [Google Scholar] [CrossRef] [PubMed]
- Colom, B.; Bodkin, J.V.; Beyrau, M.; Woodfin, A.; Ody, C.; Rourke, C.; Chavakis, T.; Brohi, K.; Imhof, B.A.; Nourshargh, S. Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity 2015, 42, 1075–1086. [Google Scholar] [CrossRef]
- Li, B.; Han, X.; Ye, X.; Ni, J.; Wu, J.; Dai, J.; Wu, Z.; Chen, C.; Wan, R.; Wang, X.; et al. Substance P-regulated leukotriene B4 production promotes acute pancreatitis-associated lung injury through neutrophil reverse migration. Int. Immunopharmacol. 2018, 57, 147–156. [Google Scholar] [CrossRef]
- Hirano, Y.; Ode, Y.; Ochani, M.; Wang, P.; Aziz, M. Targeting junctional adhesion molecule-C ameliorates sepsis-induced acute lung injury by decreasing CXCR4(+) aged neutrophils. J. Leukoc. Biol. 2018, 104, 1159–1171. [Google Scholar] [CrossRef]
- Loynes, C.A.; Lee, J.A.; Robertson, A.L.; Steel, M.J.; Ellett, F.; Feng, Y.; Levy, B.D.; Whyte, M.K.B.; Renshaw, S.A. PGE(2) production at sites of tissue injury promotes an anti-inflammatory neutrophil phenotype and determines the outcome of inflammation resolution in vivo. Sci. Adv. 2018, 4, eaar8320. [Google Scholar] [CrossRef]
- Hamza, B.; Wong, E.; Patel, S.; Cho, H.; Martel, J.; Irimia, D. Retrotaxis of human neutrophils during mechanical confinement inside microfluidic channels. Integr. Biol. 2014, 6, 175–183. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, H.; Li, M.; Huang, C. Microfluidic devices for neutrophil chemotaxis studies. J. Transl. Med. 2020, 18, 168. [Google Scholar] [CrossRef]
- Wang, X.; Jodoin, E.; Jorgensen, J.; Lee, J.; Markmann, J.J.; Cataltepe, S.; Irimia, D. Progressive mechanical confinement of chemotactic neutrophils induces arrest, oscillations, and retrotaxis. J. Leukoc. Biol. 2018, 104, 1253–1261. [Google Scholar] [CrossRef]
- Isles, H.M.; Herman, K.D.; Robertson, A.L.; Loynes, C.A.; Prince, L.R.; Elks, P.M.; Renshaw, S.A. The CXCL12/CXCR4 Signaling Axis Retains Neutrophils at Inflammatory Sites in Zebrafish. Front. Immunol. 2019, 10, 1784. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Xie, F.; Tian, L.; Manno, S.H.; Manno, F.A.M., 3rd; Cheng, S.H. Prolonged neutrophil retention in the wound impairs zebrafish heart regeneration after cryoinjury. Fish Shellfish Immunol. 2019, 94, 447–454. [Google Scholar] [CrossRef]
- Elks, P.M.; van Eeden, F.J.; Dixon, G.; Wang, X.; Reyes-Aldasoro, C.C.; Ingham, P.W.; Whyte, M.K.; Walmsley, S.R.; Renshaw, S.A. Activation of hypoxia-inducible factor-1alpha (Hif-1alpha) delays inflammation resolution by reducing neutrophil apoptosis and reverse migration in a zebrafish inflammation model. Blood 2011, 118, 712–722. [Google Scholar] [CrossRef]
- Ji, J.; Zhong, H.; Li, Y.; Billiar, T.R.; Wilson, M.A.; Scott, M.J.; Fan, J. Correction: Ji et al. IRG1/ACOD1 promotes neutrophil reverse migration and alleviates local inflammation. J. Leukoc. Biol. 2024, 116, 854–863, Erratum in J. Leukoc. Biol. 2024, 116, 1215. [Google Scholar]
- Tauzin, S.; Starnes, T.W.; Becker, F.B.; Lam, P.Y.; Huttenlocher, A. Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. J. Cell Biol. 2014, 207, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Babatunde, K.A.; Oluwadamilola, B.F.; Ahmed, A.; Salgado-Pabon, W.; Beebe, D.J.; Kerr, S.C. A microphysiological system of sterile injury demonstrates neutrophil reverse migration via macrophage-derived extracellular vesicle crosstalk. bioRxiv 2024. [Google Scholar] [CrossRef]
- Aziz, I.S.; McMahon, A.M.; Friedman, D.; Rabinovich-Nikitin, I.; Kirshenbaum, L.A.; Martino, T.A. Circadian influence on inflammatory response during cardiovascular disease. Curr. Opin. Pharmacol. 2021, 57, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Chen, A.Q.; Xue, M.; Qiu, C.Z.; Zhang, H.Y.; Zhou, R.; Zhang, L.; Yin, Z.J.; Ren, D.L. Circadian clock1a coordinates neutrophil recruitment via nfe212a/duox-reactive oxygen species pathway in zebrafish. Cell Rep. 2023, 42, 113179. [Google Scholar] [CrossRef]
- Jerigova, V.; Zeman, M.; Okuliarova, M. Circadian Disruption and Consequences on Innate Immunity and Inflammatory Response. Int. J. Mol. Sci. 2022, 23, 13722. [Google Scholar] [CrossRef]
- Palomino-Segura, M.; Hidalgo, A. Circadian immune circuits. J. Exp. Med. 2021, 218, e20200798. [Google Scholar] [CrossRef]
- Shi, C.; Zhao, D.; Lyubenov, L.; Motrapu, M.; Li, N.; Steiger, S.; Mammadova-Bach, E.; Yang, L.; Liu, D.; Anders, H.J. Neutrophil circadian rhythm is associated with different outcomes of acute kidney injury due to cholesterol crystal embolism. Front. Cardiovasc. Med. 2022, 9, 974759. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Chen, P.; Qi, C. Circadian rhythm regulation in the immune system. Immunology 2024, 171, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Ella, K.; Csepanyi-Komi, R.; Kaldi, K. Circadian regulation of human peripheral neutrophils. Brain Behav. Immun. 2016, 57, 209–221. [Google Scholar] [CrossRef]
- Su, Z.; Hu, Q.; Li, X.; Wang, Z.; Xie, Y. The Influence of Circadian Rhythms on DNA Damage Repair in Skin Photoaging. Int. J. Mol. Sci. 2024, 25, 10926. [Google Scholar] [CrossRef]
- Ovadia, S.; Ozcan, A.; Hidalgo, A. The circadian neutrophil, inside-out. J. Leukoc. Biol. 2023, 113, 555–566. [Google Scholar] [CrossRef]
- Scheiermann, C.; Gibbs, J.; Ince, L.; Loudon, A. Clocking in to immunity. Nat. Rev. Immunol. 2018, 18, 423–437. [Google Scholar] [CrossRef] [PubMed]
- Adrover, J.M.; Del Fresno, C.; Crainiciuc, G.; Cuartero, M.I.; Casanova-Acebes, M.; Weiss, L.A.; Huerga-Encabo, H.; Silvestre-Roig, C.; Rossaint, J.; Cossio, I.; et al. A Neutrophil Timer Coordinates Immune Defense and Vascular Protection. Immunity 2019, 50, 390–402.e10, Erratum in Immunity 2019, 51, 966–967. [Google Scholar] [CrossRef]
- Aroca-Crevillen, A.; Adrover, J.M.; Hidalgo, A. Circadian Features of Neutrophil Biology. Front. Immunol. 2020, 11, 576. [Google Scholar] [CrossRef]
- Brooks, J.F., 2nd; Hooper, L.V. Interactions among microbes, the immune system, and the circadian clock. Semin. Immunopathol. 2020, 42, 697–708. [Google Scholar] [CrossRef]
- Leinweber, B.; Pilorz, V.; Olejniczak, I.; Skrum, L.; Begemann, K.; Heyde, I.; Stenger, S.; Sadik, C.D.; Oster, H. Bmal1 deficiency in neutrophils alleviates symptoms induced by high-fat diet. iScience 2025, 28, 112038. [Google Scholar] [CrossRef]
- Hriscu, M.L. Circadian phagocytic activity of neutrophils and its modulation by light. J. Appl. Biomed. 2004, 2, 199–211. [Google Scholar] [CrossRef]
- Adrover, J.M.; Aroca-Crevillén, A.; Crainiciuc, G.; Ostos, F.; Rojas-Vega, Y.; Rubio-Ponce, A.; Cilloniz, C.; Bonzón-Kulichenko, E.; Calvo, E.; Rico, D.; et al. Programmed ‘disarming’ of the neutrophil proteome reduces the magnitude of inflammation. Nat. Immunol. 2020, 21, 135–144. [Google Scholar] [CrossRef] [PubMed]
- Vázquez-Reyes, S.; García-Culebras, A.; Di, G.; Benito, B.D.; De Castro-Millán, F.J.; Ruiz-Sanchez, A.; Merino-Casamayor, E.; Parra-Pérez, C.; Nieto-Vaquero, C.; Moraga, A.; et al. Circadian modulation of neutrophil function determines collateral perfusion and outcome after ischemic stroke. bioRxiv 2025. [Google Scholar] [CrossRef]
- Cox, S.L.; O’Siorain, J.R.; Fagan, L.E.; Curtis, A.M.; Carroll, R.G. Intertwining roles of circadian and metabolic regulation of the innate immune response. Semin. Immunopathol. 2022, 44, 225–237. [Google Scholar] [CrossRef] [PubMed]
- Schloss, M.J.; Horckmans, M.; Nitz, K.; Duchene, J.; Drechsler, M.; Bidzhekov, K.; Scheiermann, C.; Weber, C.; Soehnlein, O.; Steffens, S. The time-of-day of myocardial infarction onset affects healing through oscillations in cardiac neutrophil recruitment. EMBO Mol. Med. 2016, 8, 937–948. [Google Scholar] [CrossRef]
- Suarez-Barrientos, A.; Lopez-Romero, P.; Vivas, D.; Castro-Ferreira, F.; Nunez-Gil, I.; Franco, E.; Ruiz-Mateos, B.; Garcia-Rubira, J.C.; Fernandez-Ortiz, A.; Macaya, C.; et al. Circadian variations of infarct size in acute myocardial infarction. Heart 2011, 97, 970–976. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Falcon-Pérez, J.M.; López-Sarrio, S.; González, E.; Alkhraisat, M.H. Advances in Platelet Rich Plasma-Derived Extracellular Vesicles for Regenerative Medicine: A Systematic-Narrative Review. Int. J. Mol. Sci. 2023, 24, 13043. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, G.; Gao, Y.; Dai, T.; Yu, J.; Liu, Y.; Bao, H.; She, J.; Hou, Y.; Kong, L.; et al. Extracellular Vesicles Derived from Neutrophils Accelerate Bone Regeneration by Promoting Osteogenic Differentiation of BMSCs. ACS Biomater. Sci. Eng. 2024, 10, 3868–3882. [Google Scholar] [CrossRef]
- Hurtado Gutiérrez, M.J.; Allard, F.L.; Mosha, H.T.; Dubois, C.M.; McDonald, P.P. Human Neutrophils Generate Extracellular Vesicles That Modulate Their Functional Responses. Cells 2022, 12, 136. [Google Scholar] [CrossRef]
- Zhou, Y.; Brechard, S. Neutrophil Extracellular Vesicles: A Delicate Balance between Pro-Inflammatory Responses and Anti-Inflammatory Therapies. Cells 2022, 11, 3318. [Google Scholar] [CrossRef]
- Marki, A.; Ley, K. The expanding family of neutrophil-derived extracellular vesicles. Immunol. Rev. 2022, 312, 52–60. [Google Scholar] [CrossRef]
- Gasser, O.; Hess, C.; Miot, S.; Deon, C.; Sanchez, J.C.; Schifferli, J.A. Characterisation and properties of ectosomes released by human polymorphonuclear neutrophils. Exp. Cell Res. 2003, 285, 243–257. [Google Scholar] [CrossRef] [PubMed]
- Turbica, I.; Gallais, Y.; Gueguen, C.; Tharinger, H.; Al Sabbagh, C.; Gorges, R.; Gary-Gouy, H.; Kerdine-Romer, S.; Pallardy, M.; Mascarell, L.; et al. Ectosomes from neutrophil-like cells down-regulate nickel-induced dendritic cell maturation and promote Th2 polarization. J. Leukoc. Biol. 2015, 97, 737–749. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.L.; Montero-Melendez, T.; Oggero, S.; Kaneva, M.K.; Chambers, D.; Pinto, A.L.; Nerviani, A.; Lucchesi, D.; Austin-Williams, S.; Hussain, M.T.; et al. Molecular Determinants of Neutrophil Extracellular Vesicles That Drive Cartilage Regeneration in Inflammatory Arthritis. Arthritis Rheumatol. 2024, 76, 1705–1718. [Google Scholar] [CrossRef] [PubMed]
- Headland, S.E.; Jones, H.R.; Norling, L.V.; Kim, A.; Souza, P.R.; Corsiero, E.; Gil, C.D.; Nerviani, A.; Dell’Accio, F.; Pitzalis, C.; et al. Neutrophil-derived microvesicles enter cartilage and protect the joint in inflammatory arthritis. Sci. Transl. Med. 2015, 7, 315ra190. [Google Scholar] [CrossRef]
- Gasser, O.; Schifferli, J.A. Activated polymorphonuclear neutrophils disseminate anti-inflammatory microparticles by ectocytosis. Blood 2004, 104, 2543–2548. [Google Scholar] [CrossRef]
- Eken, C.; Sadallah, S.; Martin, P.J.; Treves, S.; Schifferli, J.A. Ectosomes of polymorphonuclear neutrophils activate multiple signaling pathways in macrophages. Immunobiology 2013, 218, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Rhys, H.I.; Dell’Accio, F.; Pitzalis, C.; Moore, A.; Norling, L.V.; Perretti, M. Neutrophil Microvesicles from Healthy Control and Rheumatoid Arthritis Patients Prevent the Inflammatory Activation of Macrophages. EBioMedicine 2018, 29, 60–69. [Google Scholar] [CrossRef] [PubMed]
- Eken, C.; Martin, P.J.; Sadallah, S.; Treves, S.; Schaller, M.; Schifferli, J.A. Ectosomes released by polymorphonuclear neutrophils induce a MerTK-dependent anti-inflammatory pathway in macrophages. J. Biol. Chem. 2010, 285, 39914–39921. [Google Scholar] [CrossRef]
- Hsu, A.Y.; Huang, Q.; Pi, X.; Fu, J.; Raghunathan, K.; Ghimire, L.; Balasubramanian, A.; Xie, X.; Yu, H.; Loison, F.; et al. Neutrophil-derived vesicles control complement activation to facilitate inflammation resolution. Cell 2025, 188, 1623–1641.e26. [Google Scholar] [CrossRef]
- Dong, X.; Gao, J.; Zhang, C.Y.; Hayworth, C.; Frank, M.; Wang, Z. Neutrophil Membrane-Derived Nanovesicles Alleviate Inflammation To Protect Mouse Brain Injury from Ischemic Stroke. ACS Nano 2019, 13, 1272–1283. [Google Scholar] [CrossRef]
- Gao, J.; Dong, X.; Su, Y.; Wang, Z. Human neutrophil membrane-derived nanovesicles as a drug delivery platform for improved therapy of infectious diseases. Acta Biomater. 2021, 123, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wang, S.; Dong, X.; Leanse, L.G.; Dai, T.; Wang, Z. Co-delivery of resolvin D1 and antibiotics with nanovesicles to lungs resolves inflammation and clears bacteria in mice. Commun. Biol. 2020, 3, 680. [Google Scholar] [CrossRef]
- Gao, J.; Wang, S.; Wang, Z. High yield, scalable and remotely drug-loaded neutrophil-derived extracellular vesicles (EVs) for anti-inflammation therapy. Biomaterials 2017, 135, 62–73. [Google Scholar] [CrossRef]
- Kaiser, R.; Escaig, R.; Erber, J.; Nicolai, L. Neutrophil-Platelet Interactions as Novel Treatment Targets in Cardiovascular Disease. Front. Cardiovasc. Med. 2022, 8, 824112. [Google Scholar] [CrossRef]
- Lisman, T. Platelet-neutrophil interactions as drivers of inflammatory and thrombotic disease. Cell Tissue Res. 2018, 371, 567–576. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.; Onishi, K.; Jayaram, P.; Lana, J.F.; Mautner, K. Platelet-Rich Plasma: New Performance Understandings and Therapeutic Considerations in 2020. Int. J. Mol. Sci. 2020, 21, 7794. [Google Scholar] [CrossRef]
- Padilla, S.; Nurden, A.T.; Prado, R.; Nurden, P.; Anitua, E. Healing through the lens of immunothrombosis: Biology-inspired, evolution-tailored, and human-engineered biomimetic therapies. Biomaterials 2021, 279, 121205. [Google Scholar] [CrossRef]
- Anitua, E.; Alkhraisat, M.H.; Orive, G. Perspectives and challenges in regenerative medicine using plasma rich in growth factors. J. Control. Release 2012, 157, 29–38. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Zalduendo, M.; Tejero, R.; Orive, G. Progress in the Use of Autologous Regenerative Platelet-based Therapies in Implant Dentistry. Curr. Pharm. Biotechnol. 2016, 17, 402–413. [Google Scholar] [CrossRef]
- Everts, P.A.; Lana, J.F.; Alexander, R.W.; Dallo, I.; Kon, E.; Ambach, M.A.; van Zundert, A.; Podesta, L. Profound Properties of Protein-Rich, Platelet-Rich Plasma Matrices as Novel, Multi-Purpose Biological Platforms in Tissue Repair, Regeneration, and Wound Healing. Int. J. Mol. Sci. 2024, 25, 7914. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Alkhraisat, M.H. Effectiveness of platelet derivatives in neuropathic pain management: A systematic review. Biomed. Pharmacother. 2024, 180, 117507. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Troya, M.; Zalduendo, M.; Orive, G. Personalized plasma-based medicine to treat age-related diseases. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 74, 459–464. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, P.; Xue, X.; Zhang, Z.; Wang, L.; Jiang, Y.; Zhang, C.; Zhou, H.; Lv, S.; Shen, W.; et al. The role of platelet-rich plasma in biomedicine: A comprehensive overview. iScience 2025, 28, 111705. [Google Scholar] [CrossRef]
- Anitua, E.; Troya, M.; Alkhraisat, M.H. Immunoregulatory role of platelet derivatives in the macrophage-mediated immune response. Front. Immunol. 2024, 15, 1399130. [Google Scholar] [CrossRef]
- Patel, H.; Pundkar, A.; Shrivastava, S.; Chandanwale, R.; Jaiswal, A.M. A Comprehensive Review on Platelet-Rich Plasma Activation: A Key Player in Accelerating Skin Wound Healing. Cureus 2023, 15, e48943. [Google Scholar] [CrossRef] [PubMed]
- DeLong, J.M.; Russell, R.P.; Mazzocca, A.D. Platelet-rich plasma: The PAW classification system. Arthroscopy 2012, 28, 998–1009. [Google Scholar] [CrossRef]
- Kon, E.; Di Matteo, B.; Delgado, D.; Cole, B.J.; Dorotei, A.; Dragoo, J.L.; Filardo, G.; Fortier, L.A.; Giuffrida, A.; Jo, C.H.; et al. Platelet-rich plasma for the treatment of knee osteoarthritis: An expert opinion and proposal for a novel classification and coding system. Expert Opin. Biol. Ther. 2020, 20, 1447–1460. [Google Scholar] [CrossRef]
- Magalon, J.; Brandin, T.; Francois, P.; Degioanni, C.; De Maria, L.; Grimaud, F.; Veran, J.; Dignat-George, F.; Sabatier, F. Technical and biological review of authorized medical devices for platelets-rich plasma preparation in the field of regenerative medicine. Platelets 2021, 32, 200–208. [Google Scholar] [CrossRef]
- Braun, H.J.; Kim, H.J.; Chu, C.R.; Dragoo, J.L. The effect of platelet-rich plasma formulations and blood products on human synoviocytes: Implications for intra-articular injury and therapy. Am. J. Sports Med. 2014, 42, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Lee, A.J.; Gangi, L.R.; Zandkarimi, F.; Stockwell, B.R.; Hung, C.T. Red blood cell exposure increases chondrocyte susceptibility to oxidative stress following hemarthrosis. Osteoarthr. Cartil. 2023, 31, 1365–1376. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Malanga, G.A.; Paul, R.V.; Rothenberg, J.B.; Stephens, N.; Mautner, K.R. Assessing clinical implications and perspectives of the pathophysiological effects of erythrocytes and plasma free hemoglobin in autologous biologics for use in musculoskeletal regenerative medicine therapies. A review. Regen. Ther. 2019, 11, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Marathe, A.; Patel, S.J.; Song, B.; Sliepka, J.M.; Shybut, T.S.; Lee, B.H.; Jayaram, P. Double-Spin Leukocyte-Rich Platelet-Rich Plasma Is Predominantly Lymphocyte Rich With Notable Concentrations of Other White Blood Cell Subtypes. Arthrosc. Sports Med. Rehabil. 2022, 4, e335–e341. [Google Scholar] [CrossRef]
- Anitua, E.; Muruzabal, F.; de la Fuente, M.; Riestra, A.; Merayo-Lloves, J.; Orive, G. PRGF exerts more potent proliferative and anti-inflammatory effects than autologous serum on a cell culture inflammatory model. Exp. Eye Res. 2016, 151, 115–121. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.; Troya, M.; Padilla, S.; Orive, G. Leukocyte inclusion within a platelet rich plasma-derived fibrin scaffold stimulates a more pro-inflammatory environment and alters fibrin properties. PLoS ONE 2015, 10, e0121713. [Google Scholar] [CrossRef]
- Xu, Z.; Yin, W.; Zhang, Y.; Qi, X.; Chen, Y.; Xie, X.; Zhang, C. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci. Rep. 2017, 7, 43301. [Google Scholar] [CrossRef]
- Sundman, E.A.; Cole, B.J.; Fortier, L.A. Growth factor and catabolic cytokine concentrations are influenced by the cellular composition of platelet-rich plasma. Am. J. Sports Med. 2011, 39, 2135–2140. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Saita, Y.; Nishio, H.; Ikeda, H.; Takazawa, Y.; Nagao, M.; Takaku, T.; Komatsu, N.; Kaneko, K. Leukocyte concentration and composition in platelet-rich plasma (PRP) influences the growth factor and protease concentrations. J. Orthop. Sci. Off. J. Jpn. Orthop. Assoc. 2016, 21, 683–689. [Google Scholar] [CrossRef]
- McCarrel, T.M.; Minas, T.; Fortier, L.A. Optimization of leukocyte concentration in platelet-rich plasma for the treatment of tendinopathy. J. Bone Jt. Surg. Am. Vol. 2012, 94, e143. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; Gong, C.; Peng, X.; Liu, X.; Su, X.; Tao, X.; Li, Y.; Wen, Y.; Li, W. Efficacy and safety of platelet-rich plasma injections for the treatment of osteoarthritis: A systematic review and meta-analysis of randomized controlled trials. Front. Med. 2023, 10, 1204144. [Google Scholar] [CrossRef]
- Yan, R.; Gu, Y.; Ran, J.; Hu, Y.; Zheng, Z.; Zeng, M.; Heng, B.C.; Chen, X.; Yin, Z.; Chen, W.; et al. Intratendon Delivery of Leukocyte-Poor Platelet-Rich Plasma Improves Healing Compared With Leukocyte-Rich Platelet-Rich Plasma in a Rabbit Achilles Tendinopathy Model. Am. J. Sports Med. 2017, 45, 1909–1920. [Google Scholar] [CrossRef]
- Ngoc, D.N.; Drzewiecka, B.; Junkuszew, A.; Patkowski, K.; Szponder, T.; Szymczak, B.; Nowakiewicz, A.; Podwysocka, S.; Wessely-Szponder, J. Blood-derived products can moderate the activity of neutrophils isolated after biomaterial implantation in a sheep model. Med. Weter. 2024, 80, 381–387. [Google Scholar] [CrossRef]
- Anitua, E.; Zalduendo, M.; Troya, M.; Tierno, R.; Alkhraisat, M.H. The inclusion of leukocytes into platelet rich plasma reduces scaffold stability and hinders extracellular matrix remodelling. Ann. Anat. 2022, 240, 151853. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Zalduendo, M.; Troya, M.; Tierno, R.; Alkhraisat, M.H. Cellular composition modifies the biological properties and stability of platelet rich plasma membranes for tissue engineering. J. Biomed. Mater. Res. A 2023, 111, 1710–1721. [Google Scholar] [CrossRef] [PubMed]
- Anitua, E.; Zalduendo, M.M.; Prado, R.; Alkhraisat, M.H.; Orive, G. Morphogen and proinflammatory cytokine release kinetics from PRGF-Endoret fibrin scaffolds: Evaluation of the effect of leukocyte inclusion. J. Biomed. Mater. Res. A 2015, 103, 1011–1020. [Google Scholar] [CrossRef]
- De Matthaeis, A.; Bianchi, M.; Putzulu, R.; Maccauro, G. High-Dose Neutrophil-Depleted Platelet-Rich Plasma Therapy for Knee Osteoarthritis: A Retrospective Study. J. Clin. Med. 2024, 13, 4816. [Google Scholar] [CrossRef]
- Yoshida, M.; Saito, M. Neutrophil-Reduced Platelet Rich Plasma with Optimal Platelets Concentrations for Epicondylitis of the Elbow. Muscle Ligaments Tendons J. 2021, 11, 457. [Google Scholar] [CrossRef]
- Zdziennicka, J.; Junkuszew, A.; Latalski, M.; Swieca, M.; Wessely-Szponder, J. Long-term Interactions of Circulating Neutrophils with Titanium Implants, the Role of Platelets in Regulation of Leukocyte Function. Int. J. Mol. Sci. 2021, 22, 10060. [Google Scholar] [CrossRef]
- Liu, Q.; Zhu, W.; Wen, X.; Da, Y. The Role of Platelet-Neutrophil Interactions in Driving Autoimmune Diseases. Immunology 2025, 175, 1–15. [Google Scholar] [CrossRef]
- Wienkamp, A.K.; Erpenbeck, L.; Rossaint, J. Platelets in the NETworks interweaving inflammation and thrombosis. Front. Immunol. 2022, 13, 953129. [Google Scholar] [CrossRef]
- Margraf, A.; Zarbock, A. Platelets in Inflammation and Resolution. J. Immunol. 2019, 203, 2357–2367. [Google Scholar] [CrossRef] [PubMed]
- Rossaint, J.; Margraf, A.; Zarbock, A. Role of Platelets in Leukocyte Recruitment and Resolution of Inflammation. Front. Immunol. 2018, 9, 2712. [Google Scholar] [CrossRef]
- Assinger, A.; Laky, M.; Schabbauer, G.; Hirschl, A.M.; Buchberger, E.; Binder, B.R.; Volf, I. Efficient phagocytosis of periodontopathogens by neutrophils requires plasma factors, platelets and TLR2. J. Thromb. Haemost. 2011, 9, 799–809. [Google Scholar] [CrossRef]
- Miyabe, K.; Sakamoto, N.; Wu, Y.H.; Mori, N.; Sakamoto, H. Effects of platelet release products on neutrophilic phagocytosis and complement receptors. Thromb. Res. 2004, 114, 29–36. [Google Scholar] [CrossRef]
- Clark, S.R.; Ma, A.C.; Tavener, S.A.; McDonald, B.; Goodarzi, Z.; Kelly, M.M.; Patel, K.D.; Chakrabarti, S.; McAvoy, E.; Sinclair, G.D.; et al. Platelet TLR4 activates neutrophil extracellular traps to ensnare bacteria in septic blood. Nat. Med. 2007, 13, 463–469. [Google Scholar] [CrossRef]
- Rui, S.; Dai, L.; Zhang, X.; He, M.; Xu, F.; Wu, W.; Armstrong, D.G.; You, Y.; Xiao, X.; Ma, Y.; et al. Exosomal miRNA-26b-5p from PRP suppresses NETs by targeting MMP-8 to promote diabetic wound healing. J. Control. Release 2024, 372, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Elaskalani, O.; Abdol Razak, N.B.; Metharom, P. Neutrophil extracellular traps induce aggregation of washed human platelets independently of extracellular DNA and histones. Cell Commun. Signal. 2018, 16, 24. [Google Scholar] [CrossRef] [PubMed]
- Slaba, I.; Wang, J.; Kolaczkowska, E.; McDonald, B.; Lee, W.Y.; Kubes, P. Imaging the dynamic platelet-neutrophil response in sterile liver injury and repair in mice. Hepatology 2015, 62, 1593–1605. [Google Scholar] [CrossRef] [PubMed]
- Abdulnour, R.E.; Dalli, J.; Colby, J.K.; Krishnamoorthy, N.; Timmons, J.Y.; Tan, S.H.; Colas, R.A.; Petasis, N.A.; Serhan, C.N.; Levy, B.D. Maresin 1 biosynthesis during platelet-neutrophil interactions is organ-protective. Proc. Natl. Acad. Sci. USA 2014, 111, 16526–16531. [Google Scholar] [CrossRef] [PubMed]
- Ebaid, H. Neutrophil depletion in the early inflammatory phase delayed cutaneous wound healing in older rats: Improvements due to the use of un-denatured camel whey protein. Diagn. Pathol. 2014, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Nishio, N.; Okawa, Y.; Sakurai, H.; Isobe, K. Neutrophil depletion delays wound repair in aged mice. Age 2008, 30, 11–19. [Google Scholar] [CrossRef]
- Campbell, E.L.; Bruyninckx, W.J.; Kelly, C.J.; Glover, L.E.; McNamee, E.N.; Bowers, B.E.; Bayless, A.J.; Scully, M.; Saeedi, B.J.; Golden-Mason, L.; et al. Transmigrating neutrophils shape the mucosal microenvironment through localized oxygen depletion to influence resolution of inflammation. Immunity 2014, 40, 66–77. [Google Scholar] [CrossRef]
- Wang, X.; Cai, J.; Lin, B.; Ma, M.; Tao, Y.; Zhou, Y.; Bai, L.; Jiang, W.; Zhou, R. GPR34-mediated sensing of lysophosphatidylserine released by apoptotic neutrophils activates type 3 innate lymphoid cells to mediate tissue repair. Immunity 2021, 54, 1123–1136.e8. [Google Scholar] [CrossRef]
| Organ/Tissue | Mechanism | Specific Strategy | Reference |
|---|---|---|---|
| Adipose | Circadian rhythm | Bmal1 | [163] |
| Bone | EVs | SOD2 and GJA1 | [171] |
| Brain | Circadian rhythm | NETs | [166] |
| Brain | EVs | Drug delivery (RvD2) | [184] |
| Eye | NETs | aggNET | [115] |
| Heart | Circadian rhythm | CXCR2high | [168] |
| Clearance of debris | Macrophage activation | [31] | |
| Pro-resolving mediators | MMP-9 | [41] | |
| Reverse migration | CXCR1/2 | [145] | |
| Ischemic tissue | Pro-resolving mediators | MMP-2, MMP-9 | [44] |
| VEGF, MMP-9 | [38,45] | ||
| Joints | Efferocytosis | Cannibalism | [97] |
| EVs | (miR)-455-3p | [177] | |
| EVs | AnxA1 | [178] | |
| EVs | PS and AnxA1 | [181] | |
| NETs | aggNET | [114] | |
| Pro-resolving mediators | Resolvins | [55] | |
| Kidney | Circadian rhythm | CXCR2+ neutrophils | [154] |
| Reverse migration | Reverse migration | [129] | |
| Liver | Clearance of debris | Complement activation | [19] |
| DNA degradation | [23] | ||
| Pro-resolving mediators | MMPs | [46,47] | |
| ROS | [60] | ||
| Reverse migration | CXCR4 | [23] | |
| Lung | Circadian rhythm | Neutrophil proteome | [165] |
| Clearance of debris | DNA degradation | [22] | |
| Efferocytosis | PPARγ | [85] | |
| Cannibalism | [95] | ||
| Active migration and EGF | [86] | ||
| Neutralization HMGB1 | [87] | ||
| HMGB1 (inhibitory effects) | [90] | ||
| HMGB1(inhibitory effects) | [92] | ||
| Mitochondrial AMPK axis | [93] | ||
| Mitochondrial metabolism modulation | [84] | ||
| EVs | Drug delivery (piceatannol) | [187] | |
| Drug delivery (RvD1 and ceftazidime) | [186] | ||
| LAND-Vs | [183] | ||
| Pro-resolving mediators | IL-10 | [65] | |
| Reverse migration | IRG1/ACOD1 | [147] | |
| JAM-C | [139] | ||
| Muscle | Clearance of debris | Clearance of debris | [30] |
| Nervous system | Clearance of debris | Clearance of myelin debris | [25,27] |
| Macrophage activation | [28,29] | ||
| Pancreas | NETs | aggNET | [123] |
| Pro-resolving mediators | VEGF, MMP-9 | [43] | |
| Reverse migration | BLT1 | [138] | |
| Periodontium | NETs | aggNET | [116] |
| Peritoneum | EVs | Drug delivery (RvD1 and ceftazidime) | [185] |
| Rectum | NETs | NET-associated immunothrombi | [120] |
| Skin | Efferocytosis | CCN1 | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anitua, E.; Troya, M.; Alkhraisat, M.H. Beyond Killing: The Overlooked Contribution of Neutrophils to Tissue Repair. Int. J. Mol. Sci. 2025, 26, 8669. https://doi.org/10.3390/ijms26178669
Anitua E, Troya M, Alkhraisat MH. Beyond Killing: The Overlooked Contribution of Neutrophils to Tissue Repair. International Journal of Molecular Sciences. 2025; 26(17):8669. https://doi.org/10.3390/ijms26178669
Chicago/Turabian StyleAnitua, Eduardo, María Troya, and Mohammad H. Alkhraisat. 2025. "Beyond Killing: The Overlooked Contribution of Neutrophils to Tissue Repair" International Journal of Molecular Sciences 26, no. 17: 8669. https://doi.org/10.3390/ijms26178669
APA StyleAnitua, E., Troya, M., & Alkhraisat, M. H. (2025). Beyond Killing: The Overlooked Contribution of Neutrophils to Tissue Repair. International Journal of Molecular Sciences, 26(17), 8669. https://doi.org/10.3390/ijms26178669







