From Immediate Impact to Enduring Change: A Transcriptomic Comparison of tDCS’s Temporal Effects and Its Long-Term Equivalence with TMS
Abstract
1. Introduction
2. Results
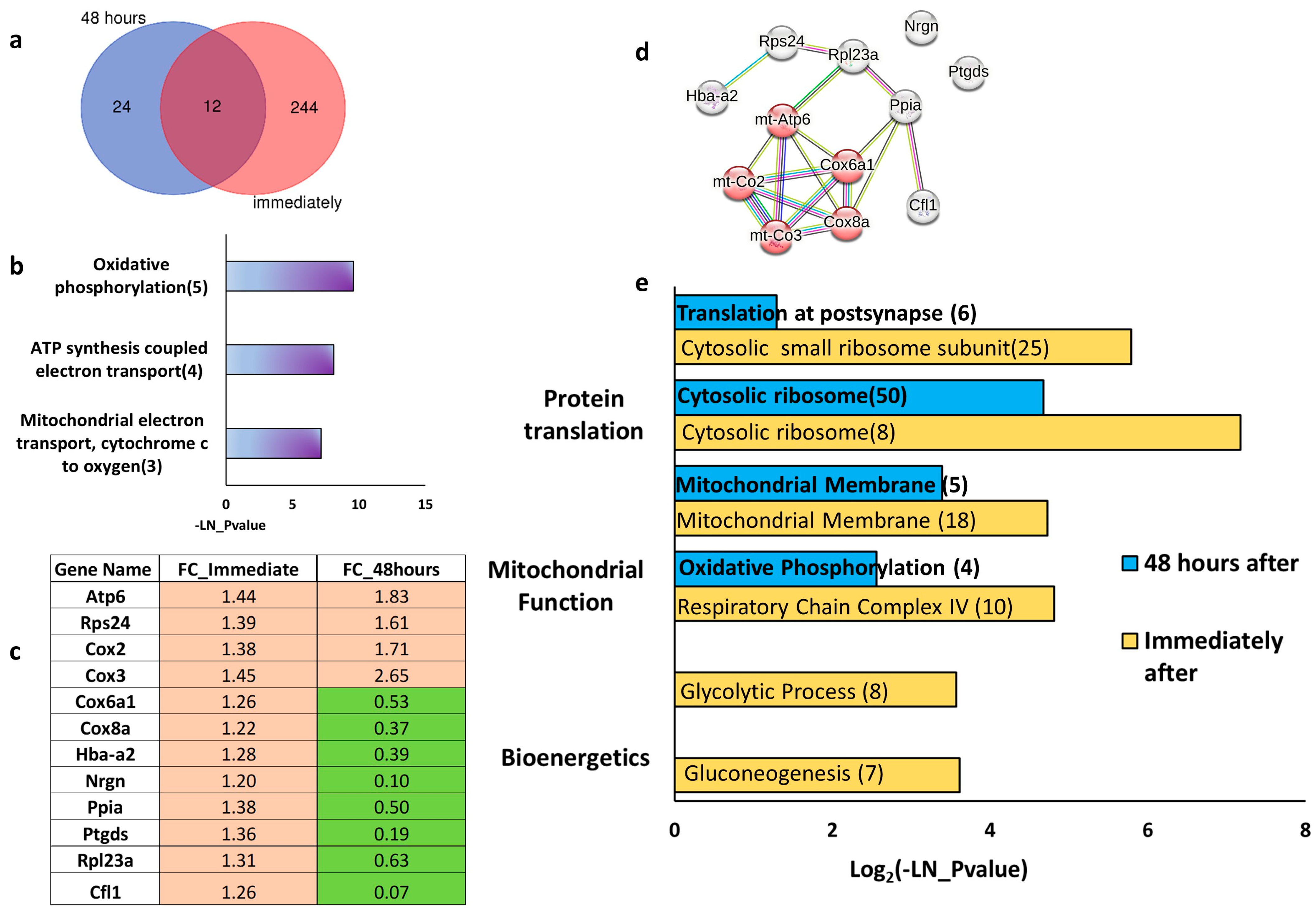
2.1. Sustained Effect of Transcranial Direct Current Stimulation (tDCS) on Gene Expression
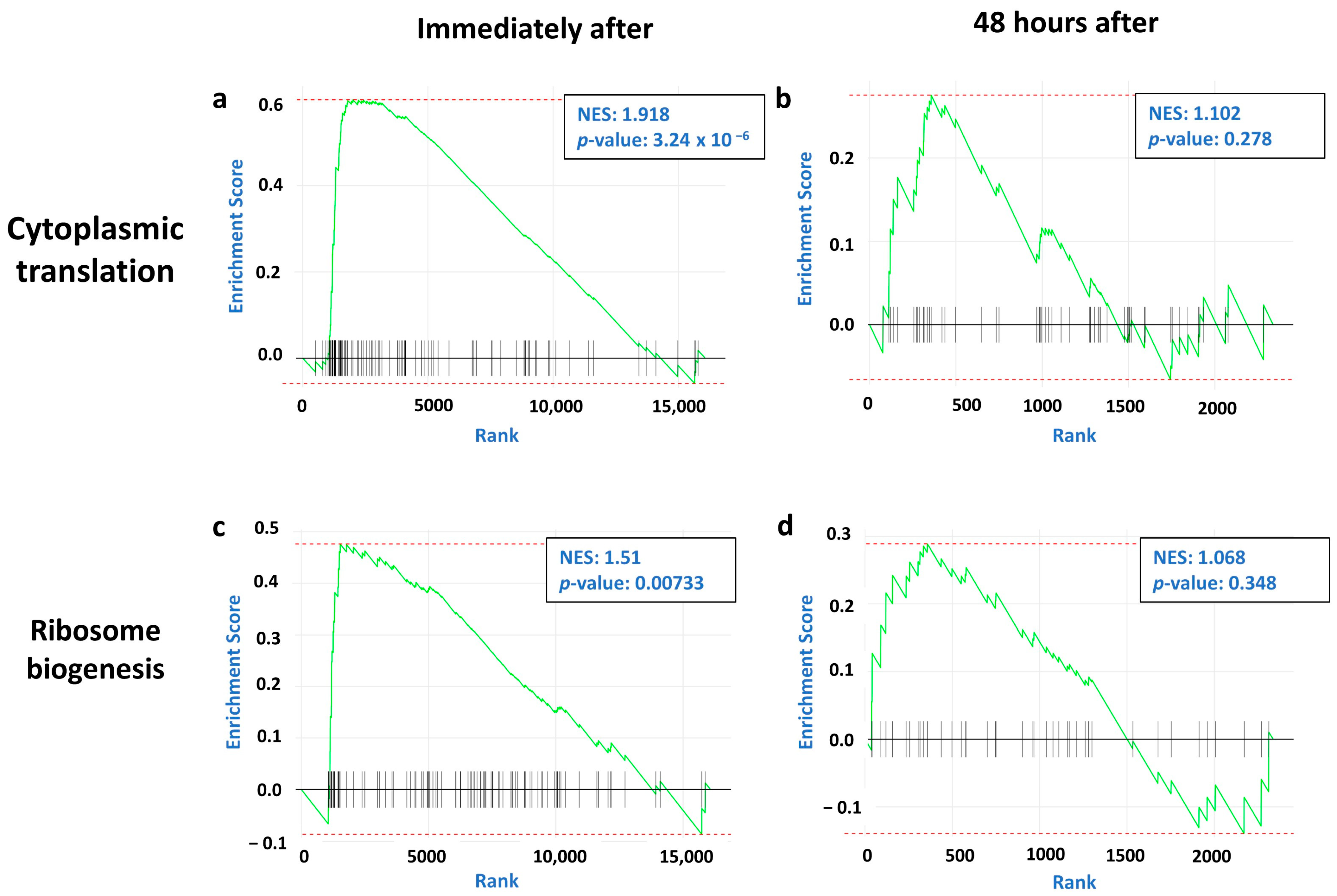
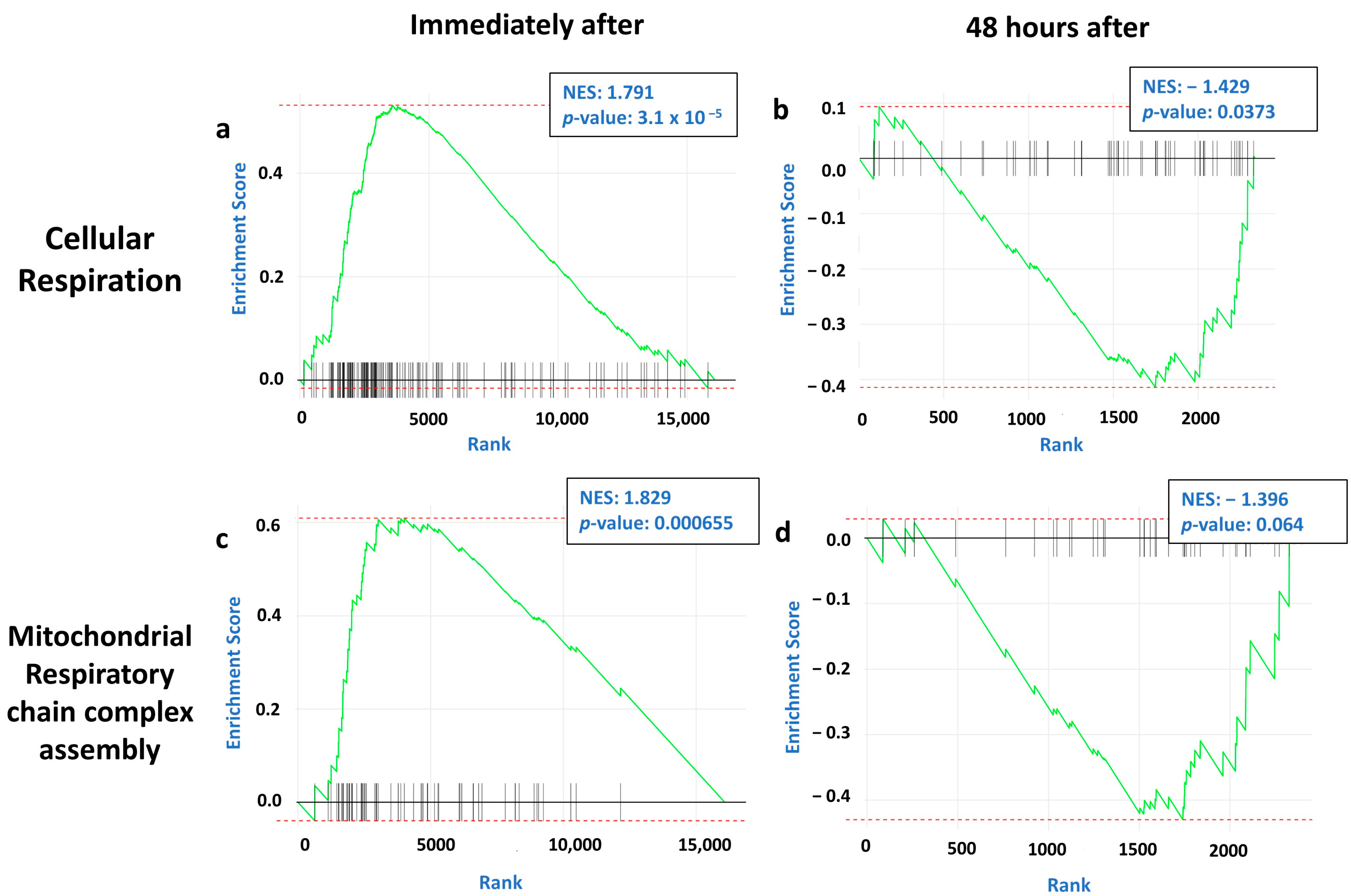
2.2. Immediate and Long-Term Effect of tDCS on Synaptic and Mitochondrial Gene Expression
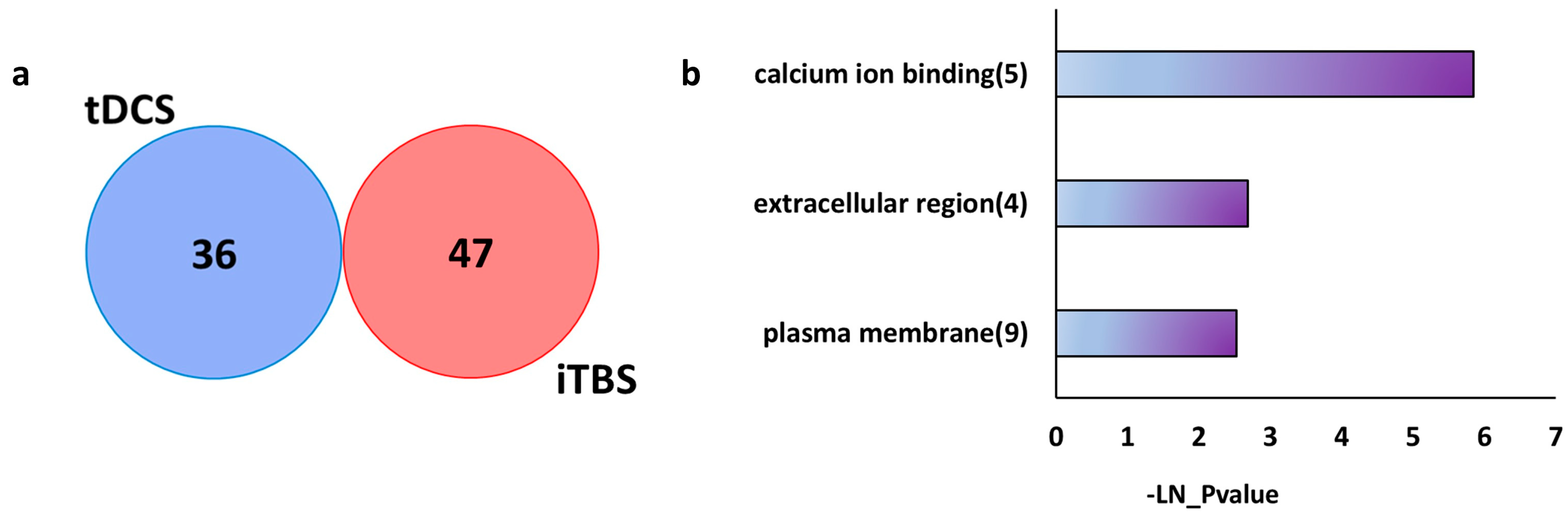
2.3. tDCS and TMS Elicit Distinct Gene Expression Changes in the Parietal Cortex
3. Discussion
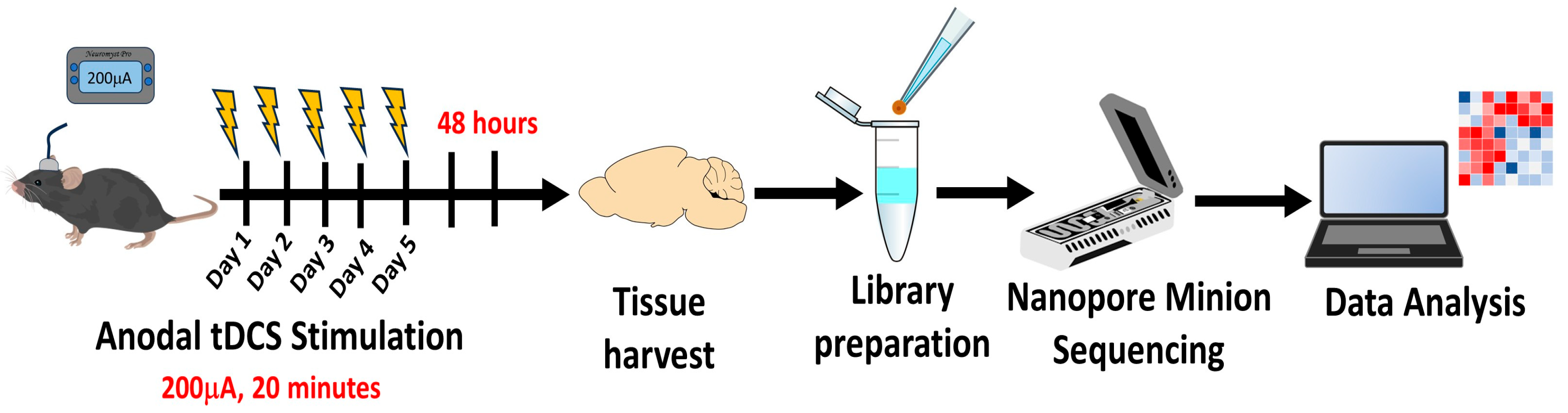
4. Materials and Methods
4.1. Animals
4.2. Surgical Procedure
4.3. tDCS Treatment
4.4. Tissue Harvesting
4.5. Sequencing
4.6. Publicly Available Datasets
4.7. Functional Analysis of Gene Expression
5. Conclusions
6. Limitations of the Study
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| tDCS | Transcranial Direct Current Stimulation |
| TMS | Transcranial Magnetic Stimulation |
| iTBS | Intermittent Theta Burst Stimulation |
| PCA | Principal component analysis |
| GSEA | Gene Set Enrichment Analysis |
References
- Zhao, H.; Qiao, L.; Fan, D.; Zhang, S.; Turel, O.; Li, Y.; Li, J.; Xue, G.; Chen, A.; He, Q. Modulation of brain activity with noninvasive transcranial direct current stimulation (tDCS): Clinical applications and safety concerns. Front. Psychol. 2017, 8, 259023. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J. Physiol. 2000, 527, 633–639. [Google Scholar] [CrossRef] [PubMed]
- Fehring, D.J.; Yokoo, S.; Abe, H.; Buckley, M.J.; Miyamoto, K.; Jaberzadeh, S.; Yamamori, T.; Tanaka, K.; Rosa, M.G.P.; Mansouri, F.A. Direct current stimulation modulates prefrontal cell activity and behaviour without inducing seizure-like firing. Brain 2024, 147, 3751–3763. [Google Scholar] [CrossRef] [PubMed]
- Li, L.M.; Uehara, K.; Hanakawa, T. The contribution of interindividual factors to variability of response in transcranial direct current stimulation studies. Front. Cell. Neurosci. 2015, 9, 181. [Google Scholar] [CrossRef] [PubMed]
- Stagg, C.J.; Jayaram, G.; Pastor, D.; Kincses, Z.T.; Matthews, P.M.; Johansen-Berg, H. Polarity and timing-dependent effects of transcranial direct current stimulation in explicit motor learning. Neuropsychologia 2011, 49, 800–804. [Google Scholar] [CrossRef]
- Yamada, Y.; Sumiyoshi, T. Neurobiological Mechanisms of Transcranial Direct Current Stimulation for Psychiatric Disorders; Neurophysiological, Chemical, and Anatomical Considerations. Front. Hum. Neurosci. 2021, 15, 631838. [Google Scholar] [CrossRef]
- Philip, N.S.; Nelson, B.G.; Frohlich, F.; Lim, K.O.; Widge, A.S.; Carpenter, L.L. Low-Intensity Transcranial Current Stimulation in Psychiatry. Am. J. Psychiatry 2017, 174, 628–639. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Paulus, W. Sustained excitability elevations induced by transcranial DC motor cortex stimulation in humans. Neurology 2001, 57, 1899–1901. [Google Scholar] [CrossRef]
- Stagg, C.J.; Nitsche, M.A. Physiological Basis of Transcranial Direct Current Stimulation. Neuroscientist 2011, 17, 37–53. [Google Scholar] [CrossRef]
- Frase, L.; Mertens, L.; Krahl, A.; Bhatia, K.; Feige, B.; Heinrich, S.P.; Vestring, S.; Nissen, C.; Domschke, K.; Bach, M.; et al. Transcranial direct current stimulation induces long-term potentiation-like plasticity in the human visual cortex. Transl. Psychiatry 2021, 11, 17. [Google Scholar] [CrossRef]
- Monte-Silva, K.; Kuo, M.F.; Hessenthaler, S.; Fresnoza, S.; Liebetanz, D.; Paulus, W.; Nitsche, M.A. Induction of late LTP-like plasticity in the human motor cortex by repeated non-invasive brain stimulation. Brain Stimul. 2013, 6, 424–432. [Google Scholar] [CrossRef]
- Vestring, S.; Wolf, E.; Dinkelacker, J.; Frase, S.; Hessling-Zeinen, C.; Insan, S.; Kumlehn, M.M.; Feige, B.; Domschke, K.; Normann, C.; et al. Lasting effects of transcranial direct current stimulation on the inducibility of synaptic plasticity by paired-associative stimulation in humans. J. Neuroeng. Rehabil. 2024, 21, 162. [Google Scholar] [CrossRef] [PubMed]
- Leffa, D.T.; de Souza, A.; Scarabelot, V.L.; Medeiros, L.F.; de Oliveira, C.; Grevet, E.H.; Caumo, W.; de Souza, D.O.; Rohde, L.A.P.; Torres, I.L.S. Transcranial direct current stimulation improves short-term memory in an animal model of attention-deficit/hyperactivity disorder. Eur. Neuropsychopharmacol. 2016, 26, 368–377. [Google Scholar] [CrossRef] [PubMed]
- Binkofski, F.; Loebig, M.; Jauch-Chara, K.; Bergmann, S.; Melchert, U.H.; Scholand-Engler, H.G.; Schweiger, U.; Pellerin, L.; Oltmanns, K.M. Brain Energy Consumption Induced by Electrical Stimulation Promotes Systemic Glucose Uptake. Biol. Psychiatry 2011, 70, 690–695. [Google Scholar] [CrossRef]
- Rae, C.D.; Lee, V.H.-C.; Ordidge, R.J.; Alonzo, A.; Loo, C. Anodal transcranial direct current stimulation increases brain intracellular pH and modulates bioenergetics. Int. J. Neuropsychopharmacol. 2013, 16, 1695–1706. [Google Scholar] [CrossRef]
- Agrawal, B.; Boulos, S.; Khatib, S.; Feuermann, Y.; Panov, J.; Kaphzan, H. Molecular Insights into Transcranial Direct Current Stimulation Effects: Metabolomics and Transcriptomics Analyses. Cells 2024, 13, 205. [Google Scholar] [CrossRef]
- Lee, S.-B.; Youn, J.; Jang, W.; Yang, H.O. Neuroprotective effect of anodal transcranial direct current stimulation on 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-induced neurotoxicity in mice through modulating mitochondrial dynamics. Neurochem. Int. 2019, 129, 104491. [Google Scholar] [CrossRef]
- Blaschke, S.J.; Vlachakis, S.; Pallast, N.; Walter, H.L.; Volz, L.J.; Wiedermann, D.; Fink, G.R.; Hoehn, M.; Aswendt, M.; Schroeter, M.; et al. Transcranial Direct Current Stimulation Reverses Stroke-Induced Network Alterations in Mice. Stroke 2023, 54, 2145–2155. [Google Scholar] [CrossRef]
- Cherchi, L.; Anni, D.; Buffelli, M.; Cambiaghi, M. Early Application of Ipsilateral Cathodal-tDCS in a Mouse Model of Brain Ischemia Results in Functional Improvement and Perilesional Microglia Modulation. Biomolecules 2022, 12, 588. [Google Scholar] [CrossRef]
- Walter, H.L.; Pikhovych, A.; Endepols, H.; Rotthues, S.; Bärmann, J.; Backes, H.; Hoehn, M.; Wiedermann, D.; Neumaier, B.; Fink, G.R.; et al. Transcranial-Direct-Current-Stimulation Accelerates Motor Recovery After Cortical Infarction in Mice: The Interplay of Structural Cellular Responses and Functional Recovery. Neurorehabil. Neural Repair 2022, 36, 701–714. [Google Scholar] [CrossRef]
- Lloyd, D.M.; Wittkopf, P.G.; Arendsen, L.J.; Jones, A.K.P. Is Transcranial Direct Current Stimulation (tDCS) Effective for the Treatment of Pain in Fibromyalgia? A Systematic Review and Meta-Analysis. J. Pain 2020, 21, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- Woodham, R.D.; Selvaraj, S.; Lajmi, N.; Hobday, H.; Sheehan, G.; Ghazi-Noori, A.-R.; Lagerberg, P.J.; Rizvi, M.; Kwon, S.S.; Orhii, P.; et al. Home-based transcranial direct current stimulation treatment for major depressive disorder: A fully remote phase 2 randomized sham-controlled trial. Nat. Med. 2024, 31, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Valiengo, L.d.C.L.; Goerigk, S.; Gordon, P.C.; Padberg, F.; Serpa, M.H.; Koebe, S.; dos Santos, L.A.; Lovera, R.A.M.; Carvalho, J.B.d.; van de Bilt, M.; et al. Efficacy and Safety of Transcranial Direct Current Stimulation for Treating Negative Symptoms in Schizophrenia. JAMA Psychiatry 2020, 77, 121. [Google Scholar] [CrossRef] [PubMed]
- García-Fernández, L.; Romero-Ferreiro, V.; Padilla, S.; Wynn, R.; Pérez-Gálvez, B.; Álvarez-Mon, M.Á.; Sánchez-Cabezudo, Á.; Rodriguez-Jimenez, R. Transcranial direct current stimulation (tDCS) enhances cognitive function in schizophrenia: A randomized double-blind sham-controlled trial. Psychiatry Res. 2025, 344, 116308. [Google Scholar] [CrossRef]
- Li, X.; Chen, L.; Yu, K.; Zhuang, W.; Zhu, H.; Xu, W.; Yan, H.; Qi, G.; Zhou, D.; Wu, S. Impact of twice-a-day transcranial direct current stimulation intervention on cognitive function and motor cortex plasticity in patients with Alzheimer’s disease. Gen. Psychiatry 2023, 36, e101166. [Google Scholar] [CrossRef]
- Huerta, P.T.; Volpe, B.T. Transcranial magnetic stimulation, synaptic plasticity and network oscillations. J. Neuroeng. Rehabil. 2009, 6, 7. [Google Scholar] [CrossRef]
- Siebner, H.R.; Funke, K.; Aberra, A.S.; Antal, A.; Bestmann, S.; Chen, R.; Classen, J.; Davare, M.; Di Lazzaro, V.; Fox, P.T.; et al. Transcranial magnetic stimulation of the brain: What is stimulated?—A consensus and critical position paper. Clin. Neurophysiol. 2022, 140, 59–97. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; André-Obadia, N.; Antal, A.; Ayache, S.S.; Baeken, C.; Benninger, D.H.; Cantello, R.M.; Cincotta, M.; de Carvalho, M.; De Ridder, D.; et al. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin. Neurophysiol. 2014, 125, 2150–2206. [Google Scholar] [CrossRef]
- Lefaucheur, J.-P.; Antal, A.; Ayache, S.S.; Benninger, D.H.; Brunelin, J.; Cogiamanian, F.; Cotelli, M.; De Ridder, D.; Ferrucci, R.; Langguth, B.; et al. Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin. Neurophysiol. 2017, 128, 56–92. [Google Scholar] [CrossRef]
- Huang, Y.-Z.; Edwards, M.J.; Rounis, E.; Bhatia, K.P.; Rothwell, J.C. Theta Burst Stimulation of the Human Motor Cortex. Neuron 2005, 45, 201–206. [Google Scholar] [CrossRef]
- Cole, E.; O’Sullivan, S.J.; Tik, M.; Williams, N.R. Accelerated Theta Burst Stimulation: Safety, Efficacy, and Future Advancements. Biol. Psychiatry 2024, 95, 523–535. [Google Scholar] [CrossRef]
- Blumberger, D.M.; Vila-Rodriguez, F.; Thorpe, K.E.; Feffer, K.; Noda, Y.; Giacobbe, P.; Knyahnytska, Y.; Kennedy, S.H.; Lam, R.W.; Daskalakis, Z.J.; et al. Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): A randomised non-inferiority trial. Lancet 2018, 391, 1683–1692, Erratum in Lancet 2018, 391, e24. [Google Scholar] [CrossRef]
- Kishi, T.; Ikuta, T.; Sakuma, K.; Hatano, M.; Matsuda, Y.; Wilkening, J.; Goya-Maldonado, R.; Tik, M.; Williams, N.R.; Kito, S.; et al. Theta burst stimulation for depression: A systematic review and network and pairwise meta-analysis. Mol. Psychiatry 2024, 29, 3893–3899. [Google Scholar] [CrossRef] [PubMed]
- Bae, E.B.; Lee, J.H.; Song, J.J. Single-Session of Combined tDCS-TMS May Increase Therapeutic Effects in Subjects With Tinnitus. Front. Neurol. 2020, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Moulier, V.; Isaac, C.; Guillin, O.; Januel, D.; Bouaziz, N.; Rothärmel, M. Effects of the combination of neurostimulation techniques in patients with mental disorders: A systematic review. Asian J. Psychiatr. 2024, 91, 103863. [Google Scholar] [CrossRef] [PubMed]
- McLaren, M.E.; Nissim, N.R.; Woods, A.J. The effects of medication use in transcranial direct current stimulation: A brief review. Brain Stimul. 2018, 11, 52–58. [Google Scholar] [CrossRef]
- Nitsche, M.A.; Fricke, K.; Henschke, U.; Schlitterlau, A.; Liebetanz, D.; Lang, N.; Henning, S.; Tergau, F.; Paulus, W. Pharmacological Modulation of Cortical Excitability Shifts Induced by Transcranial Direct Current Stimulation in Humans. J. Physiol. 2003, 553, 293–301. [Google Scholar] [CrossRef]
- Ma, S.; Kosorok, M.R. Identification of differential gene pathways with principal component analysis. Bioinformatics 2009, 25, 882–889. [Google Scholar] [CrossRef]
- Holmes, B.; Jung, S.H.; Lu, J.; Wagner, J.A.; Rubbi, L.; Pellegrini, M.; Jankord, R. Transcriptomic Modification in the Cerebral Cortex following Noninvasive Brain Stimulation: RNA-Sequencing Approach. Neural Plast. 2016, 2016, 5942980. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef]
- Sergushichev, A.A. An algorithm for fast preranked gene set enrichment analysis using cumulative statistic calculation. bioRxiv 2016, 060012. [Google Scholar] [CrossRef]
- Lu, C.; Wei, Y.; Hu, R.; Wang, Y.; Li, K.; Li, X. Transcranial Direct Current Stimulation Ameliorates Behavioral Deficits and Reduces Oxidative Stress in 1-Methyl-4-Phenyl-1,2,3,6-Tetrahydropyridine-Induced Mouse Model of Parkinson’s Disease. Neuromodulation 2015, 18, 442–446; discussion 447. [Google Scholar] [CrossRef]
- Guo, T.; Fang, J.; Tong, Z.Y.; He, S.; Luo, Y. Transcranial Direct Current Stimulation Ameliorates Cognitive Impairment via Modulating Oxidative Stress, Inflammation, and Autophagy in a Rat Model of Vascular Dementia. Front. Neurosci. 2020, 14, 28. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, J.; Wei, S.; Yu, C.; Wang, D.; Li, Y.; Li, J.; Zhuang, W.; Luo, R.-C.-X.; Li, Y.; et al. Cognitive enhancing effect of rTMS combined with tDCS in patients with major depressive disorder: A double-blind, randomized, sham-controlled study. BMC Med. 2024, 22, 253. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Li, X.; Wei, S.; Yu, C.; Wang, D.; Li, Y.; Li, J.; Liu, J.; Li, S.; Zhuang, W.; et al. Transcranial Direct Current Stimulation Combined With Repetitive Transcranial Magnetic Stimulation for Depression. JAMA Netw. Open 2024, 7, e2444306. [Google Scholar] [CrossRef] [PubMed]
- Weiler, M.; Stieger, K.C.; Shroff, K.; Klein, J.P.; Wood, W.H.; Zhang, Y.; Chandrasekaran, P.; Lehrmann, E.; Camandola, S.; Long, J.M.; et al. Transcriptional changes in the rat brain induced by repetitive transcranial magnetic stimulation. Front. Hum. Neurosci. 2023, 17, 1215291. [Google Scholar] [CrossRef]
- Lebedev, M.; Berryhill, M.; Luczak, A.; Durantin, G.; Teo, W.P.; Schaefer, H.-E.; Donchin, O.; Das, S.; Holland, P.; Frens, M.A. Impact of Transcranial Direct Current Stimulation (tDCS) on Neuronal Functions. Front. Neurosci. 2016, 10, 550. [Google Scholar] [CrossRef]
- Kim, M.S.; Koo, H.; Han, S.W.; Paulus, W.; Nitsche, M.A.; Kim, Y.-H.; Yoon, J.A.; Shin, Y.-I. Repeated anodal transcranial direct current stimulation induces neural plasticity-associated gene expression in the rat cortex and hippocampus. Restor. Neurol. Neurosci. 2017, 35, 137–146. [Google Scholar] [CrossRef]
- Fritsch, B.; Reis, J.; Martinowich, K.; Schambra, H.M.; Ji, Y.; Cohen, L.G.; Lu, B. Direct Current Stimulation Promotes BDNF-Dependent Synaptic Plasticity: Potential Implications for Motor Learning. Neuron 2010, 66, 198–204. [Google Scholar] [CrossRef]
- Virga, D.M.; Hamilton, S.; Osei, B.; Morgan, A.; Kneis, P.; Zamponi, E.; Park, N.J.; Hewitt, V.L.; Zhang, D.; Gonzalez, K.C.; et al. Activity-dependent compartmentalization of dendritic mitochondria morphology through local regulation of fusion-fission balance in neurons in vivo. Nat. Commun. 2024, 15, 2142. [Google Scholar] [CrossRef]
- Pekkurnaz, G.; Wang, X. Mitochondrial heterogeneity and homeostasis through the lens of a neuron. Nat. Metab. 2022, 4, 802. [Google Scholar] [CrossRef]
- Korai, S.A.; Ranieri, F.; Di Lazzaro, V.; Papa, M.; Cirillo, G. Neurobiological After-Effects of Low Intensity Transcranial Electric Stimulation of the Human Nervous System: From Basic Mechanisms to Metaplasticity. Front. Neurol. 2021, 12, 587771. [Google Scholar] [CrossRef]
- Rapid Sequencing DNA V14-Barcoding (SQK-RBK114.24 or SQK-RBK114.96). Available online: https://nanoporetech.com/document/rapid-sequencing-gdna-barcoding-sqk-rbk114 (accessed on 2 March 2025).
- Mus Musculus Genome Assembly GRCm38-NCBI-NLM. Available online: https://www.ncbi.nlm.nih.gov/datasets/genome/GCF_000001635.20/ (accessed on 19 February 2025).
- Li, H. Minimap2: Pairwise alignment for nucleotide sequences. Bioinformatics 2018, 34, 3094–3100. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, J.; Leger, A.; Prawer, Y.D.J.; Lane, T.A.; Harrison, P.J.; Haerty, W.; Clark, M.B. Accurate expression quantification from nanopore direct RNA sequencing with NanoCount. Nucleic Acids Res. 2022, 50, e19. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 323. [Google Scholar] [CrossRef]
- Sherman, B.T.; Hao, M.; Qiu, J.; Jiao, X.; Baseler, M.W.; Lane, H.C.; Imamichi, T.; Chang, W. DAVID: A web server for functional enrichment analysis and functional annotation of gene lists (2021 update). Nucleic Acids Res. 2022, 50, W216–W221. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.-G.; Han, Y.; He, Q.-Y. clusterProfiler: An R Package for Comparing Biological Themes Among Gene Clusters. Omi. A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 2nd ed.; Springer: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Agrawal, B.; Feuermann, Y.; Panov, J.; Kaphzan, H. From Immediate Impact to Enduring Change: A Transcriptomic Comparison of tDCS’s Temporal Effects and Its Long-Term Equivalence with TMS. Int. J. Mol. Sci. 2025, 26, 8634. https://doi.org/10.3390/ijms26178634
Agrawal B, Feuermann Y, Panov J, Kaphzan H. From Immediate Impact to Enduring Change: A Transcriptomic Comparison of tDCS’s Temporal Effects and Its Long-Term Equivalence with TMS. International Journal of Molecular Sciences. 2025; 26(17):8634. https://doi.org/10.3390/ijms26178634
Chicago/Turabian StyleAgrawal, Bhanumita, Yonatan Feuermann, Julia Panov, and Hanoch Kaphzan. 2025. "From Immediate Impact to Enduring Change: A Transcriptomic Comparison of tDCS’s Temporal Effects and Its Long-Term Equivalence with TMS" International Journal of Molecular Sciences 26, no. 17: 8634. https://doi.org/10.3390/ijms26178634
APA StyleAgrawal, B., Feuermann, Y., Panov, J., & Kaphzan, H. (2025). From Immediate Impact to Enduring Change: A Transcriptomic Comparison of tDCS’s Temporal Effects and Its Long-Term Equivalence with TMS. International Journal of Molecular Sciences, 26(17), 8634. https://doi.org/10.3390/ijms26178634





