Immune Checkpoint Inhibition in Patients with Brain Metastases from Non-Small-Cell Lung Cancer: Emerging Mechanisms and Personalized Clinical Strategies
Abstract
1. Introduction
2. Mechanisms of ICI Action in Brain Metastases
2.1. Immune Checkpoint Inhibition and T-Cell Activation
2.1.1. Activation of Peripheral T Cells and Suppression of Extracranial Tumor Cells
2.1.2. TIM-3, LAG-3, and Emerging Checkpoints in Brain Metastases
2.1.3. Activation of Brain-Resident or Infiltrating T Cells
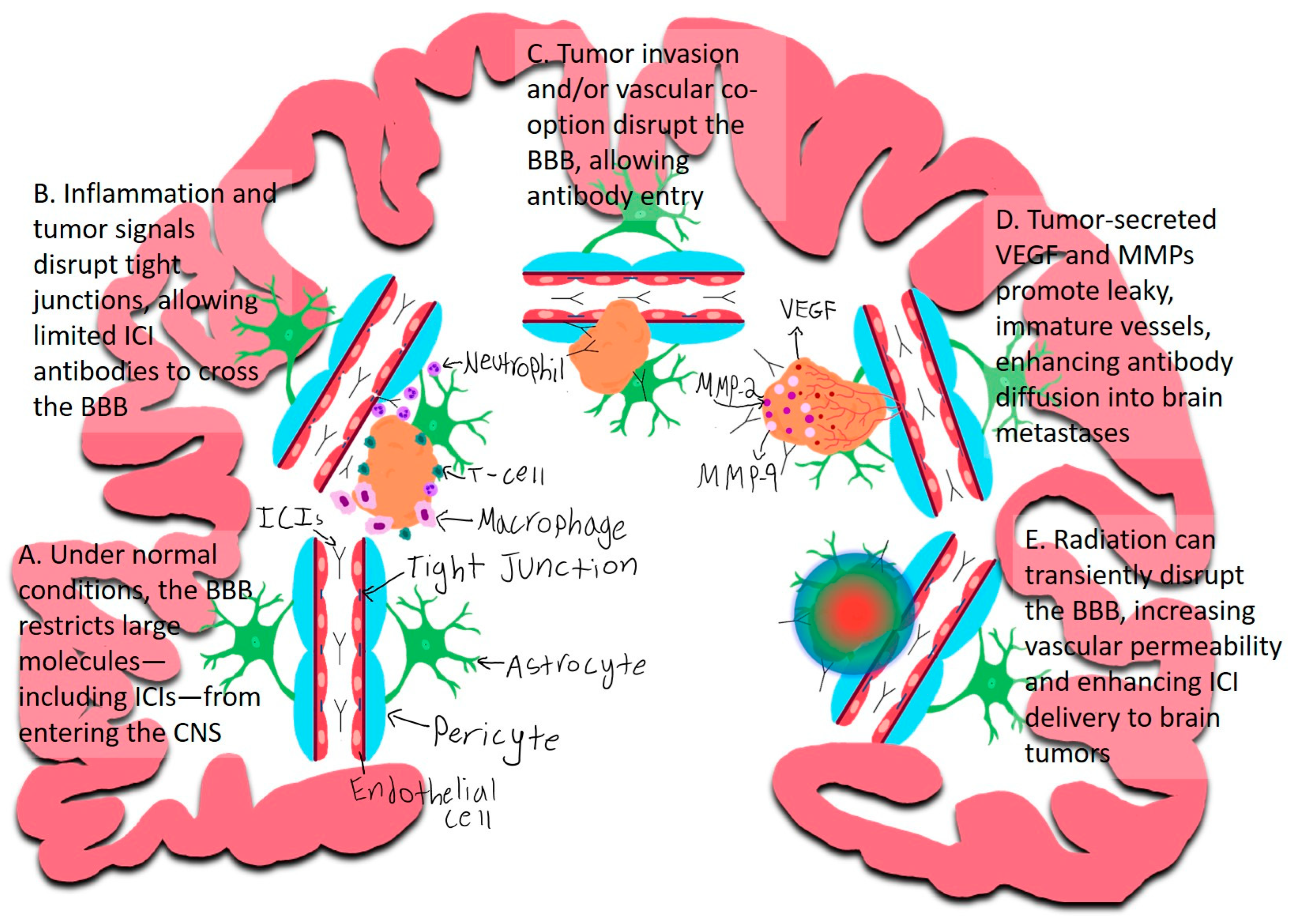
2.2. The Blood–Brain Barrier and Immune Cell Trafficking
2.2.1. Inflammation
2.2.2. Direct Tumor Invasion and/or Vascular Co-Option
2.2.3. Tumor-Induced Angiogenesis and BBB Disruption
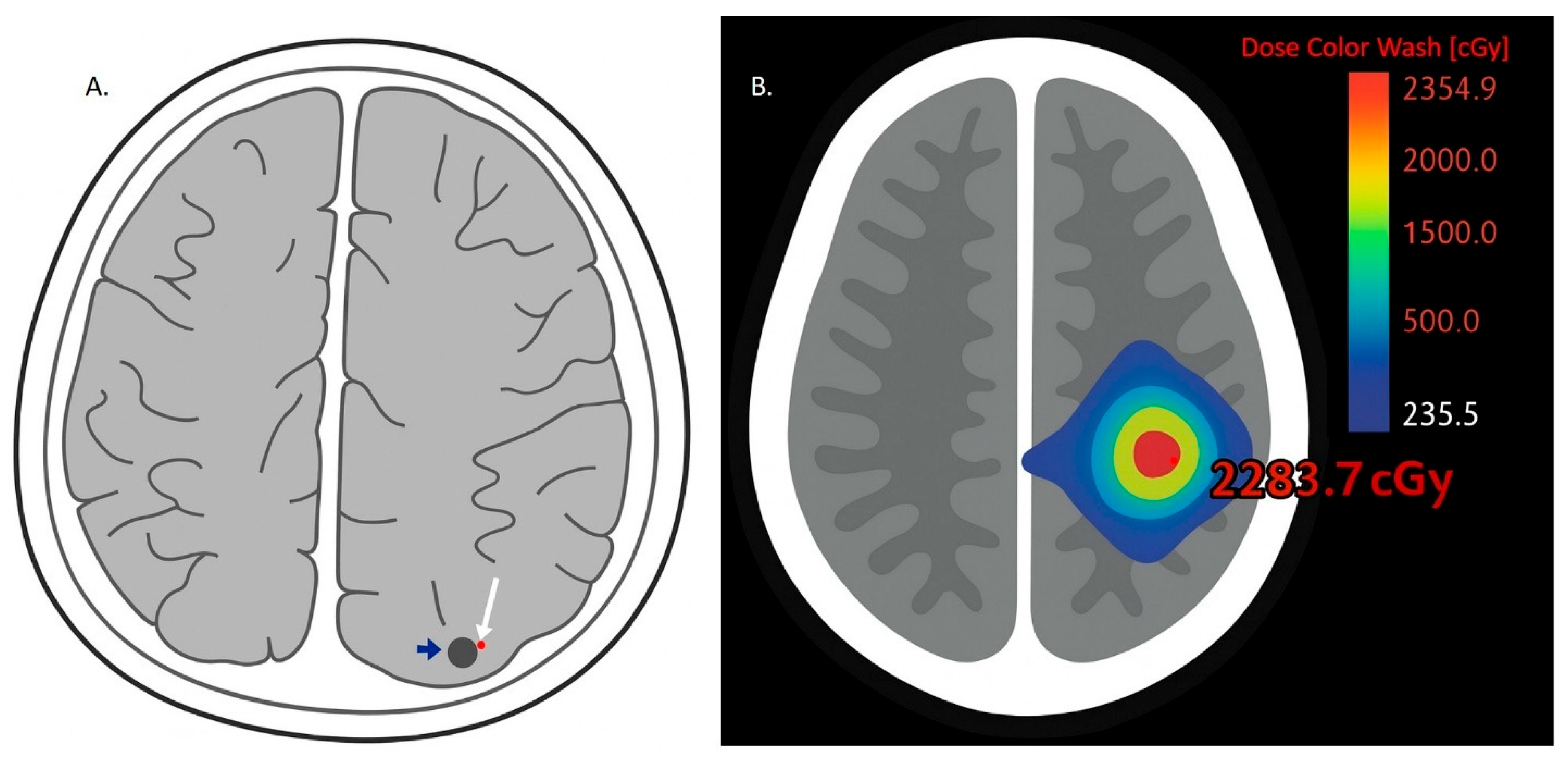
2.2.4. Radiation-Induced BBB Disruption
3. The Tumor Microenvironment in Patients with Brain Metastases
3.1. PD-L1 Expression in the CNS
3.2. Tumor-Associated Macrophages and Dendritic Cells
4. Clinical Evidence of ICI Efficacy in Patients with Brain Metastases from NSCLC
4.1. Clinical Trials and Outcomes
- ○
- CheckMate 017 [93]: This phase III trial evaluated nivolumab in patients with previously treated advanced squamous NSCLC. Patients with treated, stable brain metastases were eligible. The results demonstrated that nivolumab significantly improved overall survival (OS) as compared with docetaxel, with a hazard ratio of 0.59 (95% CI, 0.44 to 0.79). At one year, the overall survival rate was 42% (95% CI, 34% to 50%) with nivolumab as compared with 24% (95% CI, 17% to 31%) with docetaxel. This trial established the efficacy of nivolumab as a second-line therapy for squamous NSCLC [93]. Outcomes in patients with brain metastases were not separately reported.
- ○
- CheckMate 057 [94]: This phase III trial focused on patients with previously treated nonsquamous NSCLC. Similar to CheckMate 017, nivolumab showed a significant improvement in overall survival (OS) as compared with docetaxel. The median OS was 12.2 months (95% CI, 9.7 to 15.1 months) with nivolumab, compared with 9.4 months (95% CI, 8.1 to 10.7 months) with docetaxel, with a hazard ratio (HR) of 0.72 (95% CI, 0.59 to 0.89). At 18 months, the rate of overall survival was 39% (95% CI, 34% to 45%) with nivolumab and 23% (95% CI, 19% to 28%) with docetaxel. Patients with stable brain metastases were eligible to participate if they were asymptomatic and did not require corticosteroids [94]. Outcomes in patients with brain metastases were not separately reported.
- CheckMate 063 and Expanded Access Program (EAP) Findings:
- ○
- CheckMate 063 [101]: This phase II, single-arm trial evaluated the efficacy and safety of nivolumab in patients with squamous non-small-cell lung cancer (NSCLC) who had progressed after two or more lines of therapy [101]. While the trial provided important early evidence of nivolumab’s efficacy in a heavily pretreated patient population, it included only a small number of patients with brain metastases. Consequently, while CheckMate 063 supports the use of nivolumab in advanced squamous NSCLC, its specific findings related to patients with brain metastases are limited, highlighting the need for further research to better understand its effects in this subgroup.
- ○
- Expanded Access Program (EAP) in Italy [99]: The EAP was initiated to provide access to nivolumab for patients with nonsquamous NSCLC, including those with brain metastases, who might not have qualified for clinical trials [99]. Patients with brain metastases were eligible for the EAP if they were asymptomatic, neurologically stable, and either off corticosteroids or on a stable or decreasing dose. The program demonstrated a disease control rate (DCR) of 39% and a median OS of 8.6 months among patients with brain metastases, suggesting that nivolumab can offer clinical benefits even in this challenging patient population with poor prognoses. Although the EAP was not directly started because of CheckMate 063, it was informed by the data from early trials and aimed to provide broader access to nivolumab in a real-world setting [99].
- KEYNOTE-189 Trial [95]: This trial evaluated the combination of pembrolizumab with chemotherapy versus chemotherapy alone in patients with nonsquamous NSCLC, including approximately 17% of patients with previously treated, stable brain metastases. The study demonstrated that pembrolizumab significantly improved overall survival (OS) and progression-free survival (PFS) compared with chemotherapy alone. Importantly, the hazard ratio for OS in patients with brain metastases was 0.41, compared with 0.59 in those without brain metastases, suggesting that ICIs may be beneficial in this subgroup. However, no intracranial-specific response rates or CNS progression data were reported, limiting conclusions about direct ICI activity within brain metastases [95].
- Yale Cancer Center Study on Pembrolizumab in Untreated Brain Metastases from Melanoma and NSCLC [24]: In a nonrandomized, open-label, phase 2 trial conducted at Yale Cancer Center, pembrolizumab was administered to 36 patients with at least one untreated or progressive brain metastasis, ranging between 5 and 20 mm in diameter, without associated neurological symptoms. The trial included both melanoma and NSCLC cohorts, with all patients in the NSCLC cohort testing positive for PD-L1 expression. Pembrolizumab achieved a brain metastasis response in 33% of the NSCLC patients, suggesting its potential as an effective systemic immunotherapy for patients with brain metastases from NSCLC [24]. A recently published updated analysis from the same group, involving 65 patients with NSCLC and melanoma with brain metastases, reported that the median time to progression of metastatic lesions in the brain was 5.7–7 weeks, with metastases smaller than 10 mm more likely to show complete resolution [102].
- Retrospective Multicenter Study by Gauvain et al. [98]: This report, by Gauvain et al., included 43 patients with NSCLC and brain metastases, of whom 79% received local treatment to the metastatic lesions. PD-L1 overexpression was not a requirement for inclusion in the study. All patients were treated with nivolumab. The intracerebral activity of nivolumab was similar to its extracerebral efficacy, with a median intracerebral progression-free survival of 3.9 months and a general progression-free survival of 2.8 months. The authors concluded that nivolumab’s intracerebral activity was similar to its reported extracerebral efficacy [98].
- Tsuchiya-Kawano et al. [97] conducted a multicenter single-arm phase 2 trial involving 30 patients with chemotherapy-naïve advanced NSCLC and at least one untreated brain metastases. The patients received nivolumab plus ipilimumab combined with platinum-doublet chemotherapy for two cycles, followed by nivolumab–ipilimumab alone. The study reported an intracranial response rate of 50% (95% CI, 33.2–66.8%) and a complete response rate of 20%. The median intracranial progression-free survival was 8.1 months, underscoring the promising activity of nivolumab and ipilimumab combined with platinum-based chemotherapy in this challenging patient population [97].
4.2. Interpretation of Clinical Trial Findings in NSCLC Brain Metastases
5. Challenges and Opportunities in Enhancing the Intracranial Efficacy of ICIs
5.1. Blood–Brain Barrier Limitations
- Bradykinin Analogues: Agents such as labradimil (RMP-7), a bradykinin B2 receptor agonist, have been shown to transiently increase BBB permeability by modulating tight junction integrity, thereby enhancing the delivery of chemotherapeutic agents to brain tumors [105].
- Modulation of BBB Transporters: Strategies targeting endogenous transport mechanisms, such as inhibiting efflux transporters or modulating tight junction proteins, are being explored to enhance the permeability of the BBB and facilitate drug delivery [108].
5.2. Heterogeneity of Brain Metastases
5.3. Safety and Toxicity Concerns
5.3.1. Neuroinflammation and Immune-Related Adverse Events
5.3.2. Autoimmune Encephalitis
5.3.3. Radiation Necrosis and Edema
5.3.4. Management Strategies
5.3.5. Balancing Efficacy and Safety
5.4. Surgical Resection of Brain Metastases as a Bridge to ICIs
5.4.1. Immediate Benefits and Impact on the Blood–Brain Barrier (BBB):
5.4.2. Molecular Insights and Personalized Therapy:
5.5. Stereotactic Radiosurgery (SRS) and ICIs for Treatment of Brain Metastases from NSCLC
6. Future Directions and Clinical Implications
6.1. Combination Therapies
6.2. Biomarker Development
6.3. Novel Delivery Methods
7. Conclusions
Funding
Conflicts of Interest
Abbreviations
| NSCLC | non-small-cell lung cancer |
| SRS | stereotactic radiosurgery |
| CNS | central nervous system |
| ICIs | immune checkpoint inhibitors |
| BBB | blood–brain barrier |
| VEGF | vascular endothelial growth factor |
| MMPs | matrix metalloproteinases |
| RT | radiation therapy |
| WBRT | whole-brain radiation therapy |
| n-irAEs | neurologic immune-related adverse events |
| OS | overall survival |
| ctDNA | circulating tumor DNA |
| EMEC | early metastatic epithelial cell clusters |
References
- Ernani, V.; Stinchcombe, T.E. Management of brain metastases in non–small-cell lung cancer. J. Oncol. Pract. 2019, 15, 563–570, Erratum in JCO Oncol. Pract. 2020, 16, 149. https://doi.org/10.1200/JOP.20.00042. [Google Scholar] [CrossRef]
- Sperduto, P.W.; De, B.; Li, J.; Carpenter, D.; Kirkpatrick, J.; Milligan, M.; Shih, H.A.; Kutuk, T.; Kotecha, R.; Higaki, H.; et al. Graded prognostic assessment (GPA) for patients with lung cancer and brain metastases: Initial report of the small cell lung cancer GPA and update of the non-small cell lung cancer GPA including the effect of programmed death ligand 1 and other prognostic factors. Int. J. Radiat. Oncol. Biol. Phys. 2022, 114, 60–74. [Google Scholar] [PubMed]
- Pellerino, A.; Davidson, T.M.; Bellur, S.S.; Ahluwalia, M.S.; Tawbi, H.; Rudà, R.; Soffietti, R. Prevention of Brain Metastases: A New Frontier. Cancers 2024, 16, 2134. [Google Scholar] [CrossRef]
- Neto, E.B.; de Almeida Bastos, D.C.; Yoshikawa, M.H.; Figueiredo, E.G.; de Assis de Souza Filho, F.; Prabhu, S. Short-term predictors of stereotactic radiosurgery outcome for untreated single non-small cell lung cancer brain metastases: A restrospective cohort study. Neurosurg. Rev. 2024, 47, 490. [Google Scholar] [CrossRef] [PubMed]
- Sauvageot, S.; Mollevi, C.; Thomas, Q.D.; Charissoux, M.; Darlix, A.; Rigau, V.; Bauchet, L.; Quantin, X.; Pujol, J.L.; Roch, B.; et al. Prognostic impact of the number and total tumor burden of secondary cerebral lesions in patients with resected brain metastases of non-small cell lung cancers. J. Neurosurg. 2024, 141, 89–99. [Google Scholar] [CrossRef] [PubMed]
- Patel, L.; Kolundzic, N.; Abedalthagafi, M. Progress in personalized immunotherapy for patients with brain metastasis. npj Precis. Oncol. 2025, 9, 31. [Google Scholar] [CrossRef]
- Eskander, R.N.; Sill, M.W.; Beffa, L.; Moore, R.G.; Hope, J.M.; Musa, F.B.; Mannel, R.; Shahin, M.S.; Cantuaria, G.H.; Girda, E.; et al. Pembrolizumab plus Chemotherapy in Advanced Endometrial Cancer. N. Engl. J. Med. 2023, 388, 2159–2170. [Google Scholar] [CrossRef]
- Heijden, M.S.v.d.; Sonpavde, G.; Powles, T.; Necchi, A.; Burotto, M.; Schenker, M.; Sade, J.P.; Bamias, A.; Beuzeboc, P.; Bedke, J.; et al. Nivolumab plus Gemcitabine–Cisplatin in Advanced Urothelial Carcinoma. N. Engl. J. Med. 2023, 389, 1778–1789. [Google Scholar] [CrossRef]
- Powles, T.; Valderrama, B.P.; Gupta, S.; Bedke, J.; Kikuchi, E.; Hoffman-Censits, J.; Iyer, G.; Vulsteke, C.; Park, S.H.; Shin, S.J.; et al. Enfortumab Vedotin and Pembrolizumab in Untreated Advanced Urothelial Cancer. N. Engl. J. Med. 2024, 390, 875–888. [Google Scholar] [CrossRef]
- Choueiri, T.K.; Tomczak, P.; Park, S.H.; Venugopal, B.; Ferguson, T.; Symeonides, S.N.; Hajek, J.; Chang, Y.-H.; Lee, J.-L.; Sarwar, N.; et al. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N. Engl. J. Med. 2024, 390, 1359–1371. [Google Scholar] [CrossRef]
- Blank, C.U.; Lucas, M.W.; Scolyer, R.A.; Wiel, B.A.v.d.; Menzies, A.M.; Lopez-Yurda, M.; Hoeijmakers, L.L.; Saw, R.P.M.; Lijnsvelt, J.M.; Maher, N.G.; et al. Neoadjuvant Nivolumab and Ipilimumab in Resectable Stage III Melanoma. N. Engl. J. Med. 2024, 391, 1696–1708. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Cowey, C.L.; Lao, C.D.; Schadendorf, D.; Dummer, R.; Smylie, M.; Rutkowski, P.; et al. Combined Nivolumab and Ipilimumab or Monotherapy in Untreated Melanoma. N. Engl. J. Med. 2015, 373, 23–34, Erratum in N. Engl. J. Med. 2018, 379, 2185. https://doi.org/10.1056/NEJMx180040. [Google Scholar] [CrossRef] [PubMed]
- Doki, Y.; Ajani, J.A.; Kato, K.; Xu, J.; Wyrwicz, L.; Motoyama, S.; Ogata, T.; Kawakami, H.; Hsu, C.-H.; Adenis, A.; et al. Nivolumab Combination Therapy in Advanced Esophageal Squamous-Cell Carcinoma. N. Engl. J. Med. 2022, 386, 449–462. [Google Scholar] [CrossRef] [PubMed]
- Colombo, N.; Dubot, C.; Lorusso, D.; Caceres, M.V.; Hasegawa, K.; Shapira-Frommer, R.; Tewari, K.S.; Salman, P.; Usta, E.H.; Yañez, E.; et al. Pembrolizumab for Persistent, Recurrent, or Metastatic Cervical Cancer. N. Engl. J. Med. 2021, 385, 1856–1867. [Google Scholar] [CrossRef] [PubMed]
- Nasser, N.J.; Gorenberg, M.; Agbarya, A. First line Immunotherapy for Non-Small Cell Lung Cancer. Pharmaceuticals 2020, 13, 373. [Google Scholar] [CrossRef]
- Cascone, T.; Awad, M.M.; Spicer, J.D.; He, J.; Lu, S.; Sepesi, B.; Tanaka, F.; Taube, J.M.; Cornelissen, R.; Havel, L.; et al. Perioperative Nivolumab in Resectable Lung Cancer. N. Engl. J. Med. 2024, 390, 1756–1769. [Google Scholar] [CrossRef]
- Wakelee, H.; Liberman, M.; Kato, T.; Tsuboi, M.; Lee, S.-H.; Gao, S.; Chen, K.-N.; Dooms, C.; Majem, M.; Eigendorff, E.; et al. Perioperative Pembrolizumab for Early-Stage Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2023, 389, 491–503. [Google Scholar] [CrossRef]
- Herbst, R.S.; Giaccone, G.; Marinis, F.d.; Reinmuth, N.; Vergnenegre, A.; Barrios, C.H.; Morise, M.; Felip, E.; Andric, Z.; Geater, S.; et al. Atezolizumab for First-Line Treatment of PD-L1–Selected Patients with NSCLC. N. Engl. J. Med. 2020, 383, 1328–1339. [Google Scholar] [CrossRef]
- Reck, M.; Remon, J.; Hellmann, M.D. First-Line Immunotherapy for Non-Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 586–597, Erratum in J. Clin. Oncol. 2022, 40, 1265. https://doi.org/10.1200/JCO.22.00560. [Google Scholar] [CrossRef]
- Suay, G.; Garcia-Cañaveras, J.-C.; Aparisi, F.; Garcia, J.; Juan-Vidal, O.; Lahoz, A. Immune checkpoint inhibitors as first-line treatment for brain metastases in stage IV NSCLC patients without driver mutations. Cancer Lett. 2024, 606, 217317. [Google Scholar] [CrossRef]
- Wu, X.; Stabile, L.P.; Burns, T.F. The Emerging Role of Immune Checkpoint Blockade for the Treatment of Lung Cancer Brain Metastases. Clin. Lung Cancer 2024, 25, 483–501. [Google Scholar] [CrossRef]
- Berghoff, A.S.; Venur, V.A.; Preusser, M.; Ahluwalia, M.S. Immune checkpoint inhibitors in brain metastases: From biology to treatment. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e116–e122. [Google Scholar] [CrossRef]
- Lorger, M.; Andreou, T.; Fife, C.; James, F. Immune checkpoint blockade–how does it work in brain metastases? Front. Mol. Neurosci. 2019, 12, 282. [Google Scholar] [CrossRef]
- Goldberg, S.B.; Gettinger, S.N.; Mahajan, A.; Chiang, A.C.; Herbst, R.S.; Sznol, M.; Tsiouris, A.J.; Cohen, J.; Vortmeyer, A.; Jilaveanu, L.; et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: Early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol. 2016, 17, 976–983. [Google Scholar] [CrossRef]
- Yu, C.; Hsieh, K.; Cherry, D.R.; Nehlsen, A.D.; Salgado, L.E.; Lazarev, S.; Sindhu, K.K. Immune Escape in Glioblastoma: Mechanisms of Action and Implications for Immune Checkpoint Inhibitors and CAR T-Cell Therapy. Biology 2023, 12, 1528. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.Y.; Goldberg, S.B. Chemoimmunotherapy for untreated lung cancer brain metastases: Systemic before local therapy? J. Clin. Oncol. 2023, 41, 4462–4464. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Tan, Y. Promising immunotherapy targets: TIM3, LAG3, and TIGIT joined the party. Mol. Ther. Oncol. 2024, 32, 200773. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Wang, C.; Wu, J.; Wang, J.; Ma, T. TIM-3 teams up with PD-1 in cancer immunotherapy: Mechanisms and perspectives. Mol. Biomed. 2025, 6, 27. [Google Scholar] [CrossRef]
- Lu, B.Y.; Gupta, R.; Aguirre-Ducler, A.; Gianino, N.; Wyatt, H.; Ribeiro, M.; Chiang, V.L.; Contessa, J.N.; Adeniran, A.J.; Jilaveanu, L.B.; et al. Spatially resolved analysis of the T cell immune contexture in lung cancer-associated brain metastases. J. Immunother. Cancer 2021, 9, e002684. [Google Scholar] [CrossRef]
- Xiao, G.; Tanzhu, G.; Gao, X.; Li, L.; Liu, Z.; Xia, X.; Zhou, R. An immune scoring system predicts prognosis and immune characteristics in lung adenocarcinoma brain metastases by RNA sequencing. Acta Neuropathol. Commun. 2024, 12, 181. [Google Scholar] [CrossRef]
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood–brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Pandit, R.; Chen, L.; Götz, J. The blood-brain barrier: Physiology and strategies for drug delivery. Adv. Drug Deliv. Rev. 2020, 165–166, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Lokensgard, J.R. Glial cell expression of PD-L1. Int. J. Mol. Sci. 2019, 20, 1677. [Google Scholar] [CrossRef]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596. [Google Scholar] [CrossRef]
- Santos-Lima, B.; Pietronigro, E.C.; Terrabuio, E.; Zenaro, E.; Constantin, G. The role of neutrophils in the dysfunction of central nervous system barriers. Front. Aging Neurosci. 2022, 14, 965169. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive oxygen species in inflammation and tissue injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Yang, C.; Hawkins, K.E.; Doré, S.; Candelario-Jalil, E. Neuroinflammatory mechanisms of blood-brain barrier damage in ischemic stroke. Am. J. Physiol. Cell Physiol. 2019, 316, C135–C153. [Google Scholar] [CrossRef]
- Varatharaj, A.; Galea, I. The blood-brain barrier in systemic inflammation. Brain Behav. Immun. 2017, 60, 1–12. [Google Scholar] [CrossRef]
- Kienast, Y.; von Baumgarten, L.; Fuhrmann, M.; Klinkert, W.E.F.; Goldbrunner, R.; Herms, J.; Winkler, F. Real-time imaging reveals the single steps of brain metastasis formation. Nat. Med. 2010, 16, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Boire, A.; Brastianos, P.K.; Garzia, L.; Valiente, M. Brain metastasis. Nat. Rev. Cancer 2020, 20, 4–11. [Google Scholar] [CrossRef] [PubMed]
- Priego, N.; Zhu, L.; Monteiro, C.; Mulders, M.; Wasilewski, D.; Bindeman, W.; Doglio, L.; Martínez, L.; Martínez-Saez, E.; Ramón, Y.C.S.; et al. STAT3 labels a subpopulation of reactive astrocytes required for brain metastasis. Nat. Med. 2018, 24, 1024–1035, Erratum in Nat. Med. 2018, 24, 1481. https://doi.org/10.1038/s41591-018-0108-5. [Google Scholar] [CrossRef]
- Carbonell, W.S.; Ansorge, O.; Sibson, N.; Muschel, R. The vascular basement membrane as “soil” in brain metastasis. PLoS ONE 2009, 4, e5857. [Google Scholar] [CrossRef]
- Argaw, A.T.; Gurfein, B.T.; Zhang, Y.; Zameer, A.; John, G.R. VEGF-mediated disruption of endothelial CLN-5 promotes blood-brain barrier breakdown. Proc. Natl. Acad. Sci. USA 2009, 106, 1977–1982. [Google Scholar] [CrossRef]
- Jiang, S.; Xia, R.; Jiang, Y.; Wang, L.; Gao, F. Vascular endothelial growth factors enhance the permeability of the mouse blood-brain barrier. PLoS ONE 2014, 9, e86407. [Google Scholar] [CrossRef] [PubMed]
- Rempe, R.G.; Hartz, A.M.S.; Bauer, B. Matrix metalloproteinases in the brain and blood-brain barrier: Versatile breakers and makers. J. Cereb. Blood Flow Metab. 2016, 36, 1481–1507. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Q.; Ma, Y.; Wang, F.; Tan, X.; Song, D.; Hoo, R.L.C.; Wang, Z.; Ge, X.; Han, H.; et al. An MMP-9 exclusive neutralizing antibody attenuates blood-brain barrier breakdown in mice with stroke and reduces stroke patient-derived MMP-9 activity. Pharmacol. Res. 2023, 190, 106720. [Google Scholar] [CrossRef]
- Suzuki, Y.; Nagai, N.; Umemura, K. A Review of the Mechanisms of Blood-Brain Barrier Permeability by Tissue-Type Plasminogen Activator Treatment for Cerebral Ischemia. Front. Cell. Neurosci. 2016, 10, 2. [Google Scholar] [CrossRef]
- Chen, G.; Wu, K.; Li, H.; Xia, D.; He, T. Role of hypoxia in the tumor microenvironment and targeted therapy. Front. Oncol. 2022, 12, 961637. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, R.; Enström, A.; Paul, G. Molecular Regulation of the Response of Brain Pericytes to Hypoxia. Int. J. Mol. Sci. 2023, 24, 5671. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Martínez, T.; Gornatti, D.G.; Ortiz, M.; Cañellas, G.; Heine-Suñer, D.; Vives-Bauzà, C. The Triad of Blood–Brain Barrier Integrity: Endothelial Cells, Astrocytes, and Pericytes in Perinatal Stroke Pathophysiology. Int. J. Mol. Sci. 2025, 26, 1886. [Google Scholar] [CrossRef]
- Hanahan, D.; Monje, M. Cancer hallmarks intersect with neuroscience in the tumor microenvironment. Cancer Cell 2023, 41, 573–580. [Google Scholar] [CrossRef]
- Fang, L.; Zhao, W.; Ye, B.; Chen, D. Combination of immune checkpoint inhibitors and anti-angiogenic agents in brain metastases from non-small cell lung cancer. Front. Oncol. 2021, 11, 670313. [Google Scholar] [CrossRef]
- Yi, M.; Jiao, D.; Qin, S.; Chu, Q.; Wu, K.; Li, A. Synergistic effect of immune checkpoint blockade and anti-angiogenesis in cancer treatment. Mol. Cancer 2019, 18, 60. [Google Scholar] [CrossRef] [PubMed]
- Popp, I.; Hartong, N.E.; Nieder, C.; Grosu, A.L. PRO: Do We Still Need Whole-Brain Irradiation for Brain Metastases? Cancers 2023, 15, 3193. [Google Scholar] [CrossRef] [PubMed]
- Gondi, V.; Bauman, G.; Bradfield, L.; Burri, S.H.; Cabrera, A.R.; Cunningham, D.A.; Eaton, B.R.; Hattangadi-Gluth, J.A.; Kim, M.M.; Kotecha, R.; et al. Radiation Therapy for Brain Metastases: An ASTRO Clinical Practice Guideline. Pract. Radiat. Oncol. 2022, 12, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Nordal, R.A.; Wong, C.S. Molecular targets in radiation-induced blood-brain barrier disruption. Int. J. Radiat. Oncol. Biol. Phys. 2005, 62, 279–287. [Google Scholar] [CrossRef]
- Cheng, J.; Jiang, J.; He, B.; Lin, W.-J.; Li, Y.; Duan, J.; Li, H.; Huang, X.; Cai, J.; Xie, J.; et al. A phase 2 study of thalidomide for the treatment of radiation-induced blood-brain barrier injury. Sci. Transl. Med. 2023, 15, eabm6543. [Google Scholar] [CrossRef]
- Hart, E.; Odé, Z.; Derieppe, M.P.P.; Groenink, L.; Heymans, M.W.; Otten, R.; Lequin, M.H.; Janssens, G.O.R.; Hoving, E.W.; van Vuurden, D.G. Blood-brain barrier permeability following conventional photon radiotherapy—A systematic review and meta-analysis of clinical and preclinical studies. Clin. Transl. Radiat. Oncol. 2022, 35, 44–55. [Google Scholar] [CrossRef]
- Guipaud, O.; Jaillet, C.; Clément-Colmou, K.; François, A.; Supiot, S.; Milliat, F. The importance of the vascular endothelial barrier in the immune-inflammatory response induced by radiotherapy. Br. J. Radiol. 2018, 91, 20170762. [Google Scholar] [CrossRef]
- Christersdottir, T.; Pirault, J.; Gisterå, A.; Bergman, O.; Gallina, A.L.; Baumgartner, R.; Lundberg, A.M.; Eriksson, P.; Yan, Z.-Q.; Paulsson-Berne, G.; et al. Prevention of radiotherapy-induced arterial inflammation by interleukin-1 blockade. Eur. Heart J. 2019, 40, 2495–2503. [Google Scholar] [CrossRef]
- Wijerathne, H.; Langston, J.C.; Yang, Q.; Sun, S.; Miyamoto, C.; Kilpatrick, L.E.; Kiani, M.F. Mechanisms of radiation-induced endothelium damage: Emerging models and technologies. Radiother. Oncol. 2021, 158, 21–32. [Google Scholar] [CrossRef]
- Allen, B.D.; Limoli, C.L. Breaking barriers: Neurodegenerative repercussions of radiotherapy induced damage on the blood-brain and blood-tumor barrier. Free. Radic. Biol. Med. 2022, 178, 189–201. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef]
- Herbst, R.S.; Soria, J.-C.; Kowanetz, M.; Fine, G.D.; Hamid, O.; Gordon, M.S.; Sosman, J.A.; McDermott, D.F.; Powderly, J.D.; Gettinger, S.N.; et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 2014, 515, 563–567. [Google Scholar] [CrossRef]
- Priego, N.; Valiente, M. The Potential of Astrocytes as Immune Modulators in Brain Tumors. Front. Immunol. 2019, 10, 1314. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, X.; Zhang, Y.; Wang, Y. The role of microglia in brain metastases: Mechanisms and strategies. Aging Dis. 2024, 15, 169. [Google Scholar] [CrossRef]
- Rodriguez-Baena, F.J.; Marquez-Galera, A.; Ballesteros-Martinez, P.; Castillo, A.; Diaz, E.; Moreno-Bueno, G.; Lopez-Atalaya, J.P.; Sanchez-Laorden, B. Microglial reprogramming enhances antitumor immunity and immunotherapy response in melanoma brain metastases. Cancer Cell 2025, 43, 413–427.e419. [Google Scholar] [CrossRef]
- Wang, X.; Liang, H.; Tang, X.; Ling, X.; Yang, Y. Single-cell RNA sequencing reveals distinct transcriptomic profiles and evolutionary patterns in lung cancer brain metastasis. Heliyon 2024, 10, e27071. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Hu, F.; Chi, Q. Single-cell RNA sequencing and spatial transcriptome reveal potential molecular mechanisms of lung cancer brain metastasis. Int. Immunopharmacol. 2024, 140, 112804. [Google Scholar] [CrossRef] [PubMed]
- Shi, Z.; Jia, L.; Wang, B.; Wang, S.; He, L.; Li, Y.; Wang, G.; Song, W.; He, X.; Liu, Z.; et al. Integration of Single-Cell and Bulk Transcriptomes to Identify a Poor Prognostic Tumor Subgroup to Predict the Prognosis of Patients with Early-stage Lung Adenocarcinoma. J. Cancer 2025, 16, 1397–1412. [Google Scholar] [CrossRef]
- Lin, Y.; Song, Y.; Zhang, Y.; Li, X.; Kan, L.; Han, S. New insights on anti-tumor immunity of CD8(+) T cells: Cancer stem cells, tumor immune microenvironment and immunotherapy. J. Transl. Med. 2025, 23, 341. [Google Scholar] [CrossRef]
- Alvarez-Breckenridge, C.A.; Markson, S.C.; Stocking, J.H.; Nayyar, N.; Lastrapes, M.; Strickland, M.R.; Kim, A.E.; de Sauvage, M.; Dahal, A.; Larson, J.M. Microenvironmental correlates of immune checkpoint inhibitor response in human melanoma brain metastases revealed by T cell receptor and single-cell RNA sequencing. bioRxiv 2021. bioRxiv:2021.08.25.456956. [Google Scholar] [CrossRef]
- Duchnowska, R.; Pęksa, R.; Radecka, B.; Mandat, T.; Trojanowski, T.; Jarosz, B.; Czartoryska-Arłukowicz, B.; Olszewski, W.P.; Och, W.; Kalinka-Warzocha, E. Immune response in breast cancer brain metastases and their microenvironment: The role of the PD-1/PD-L axis. Breast Cancer Res. 2016, 18, 43. [Google Scholar] [CrossRef]
- Gao, X.; Li, W.; Syed, F.; Yuan, F.; Li, P.; Yu, Q. PD-L1 signaling in reactive astrocytes counteracts neuroinflammation and ameliorates neuronal damage after traumatic brain injury. J. Neuroinflamm. 2022, 19, 43. [Google Scholar] [CrossRef] [PubMed]
- Qian, J.; Wang, C.; Wang, B.; Yang, J.; Wang, Y.; Luo, F.; Xu, J.; Zhao, C.; Liu, R.; Chu, Y. The IFN-γ/PD-L1 axis between T cells and tumor microenvironment: Hints for glioma anti-PD-1/PD-L1 therapy. J. Neuroinflamm. 2018, 15, 290. [Google Scholar] [CrossRef] [PubMed]
- Chen, N.; Zhang, T.; Yang, X.; Wang, D.; Yu, S. Myeloid cells in the microenvironment of brain metastases. Biochim. Biophys. Acta (BBA)—Rev. Cancer 2025, 1880, 189311. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Li, J.; Yao, R.; Liu, J.; Su, L.; You, W. Focusing on the interplay between tumor-associated macrophages and tumor microenvironment: From mechanism to intervention. Theranostics 2025, 15, 7378–7408. [Google Scholar] [CrossRef]
- Larionova, I.; Kazakova, E.; Gerashchenko, T.; Kzhyshkowska, J. New Angiogenic Regulators Produced by TAMs: Perspective for Targeting Tumor Angiogenesis. Cancers 2021, 13, 3253. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, R.; Wa, Y.; Ding, S.; Yang, Y.; Liao, J.; Tong, L.; Xiao, G. Tumor Immune Microenvironment and Immunotherapy in Brain Metastasis From Non-Small Cell Lung Cancer. Front. Immunol. 2022, 13, 829451. [Google Scholar] [CrossRef]
- Economopoulos, V.; Pannell, M.; Johanssen, V.A.; Scott, H.; Andreou, K.E.; Larkin, J.R.; Sibson, N.R. Inhibition of Anti-Inflammatory Macrophage Phenotype Reduces Tumour Growth in Mouse Models of Brain Metastasis. Front. Oncol. 2022, 12, 850656. [Google Scholar] [CrossRef]
- D’Agostino, P.M.; Gottfried-Blackmore, A.; Anandasabapathy, N.; Bulloch, K. Brain dendritic cells: Biology and pathology. Acta Neuropathol. 2012, 124, 599–614. [Google Scholar] [CrossRef]
- Suter, T.; Biollaz, G.; Gatto, D.; Bernasconi, L.; Herren, T.; Reith, W.; Fontana, A. The brain as an immune privileged site: Dendritic cells of the central nervous system inhibit T cell activation. Eur. J. Immunol. 2003, 33, 2998–3006. [Google Scholar] [CrossRef] [PubMed]
- Korn, T.; Kallies, A. T cell responses in the central nervous system. Nat. Rev. Immunol. 2017, 17, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Bowman-Kirigin, J.A.; Desai, R.; Saunders, B.T.; Wang, A.Z.; Schaettler, M.O.; Liu, C.J.; Livingstone, A.J.; Kobayashi, D.K.; Durai, V.; Kretzer, N.M.; et al. The conventional dendritic cell 1 subset primes CD8+ T cells and traffics tumor antigen to drive antitumor immunity in the brain. Cancer Immunol. Res. 2023, 11, 20–37. [Google Scholar] [CrossRef]
- Oscar, B.-G.; Jenni, N.; Liam, H.; Shokoufeh, K.; Mohanraj, R.; Hitesh Bhagavanbhai, M.; Sven, N.; Mats, H. Brain tumors induce immunoregulatory dendritic cells in draining lymph nodes that can be targeted by OX40 agonist treatment. J. Immunother. Cancer 2025, 13, e011548. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Wang, R.; Wang, X.; Yang, H.; Dong, J.; He, X.; Yang, Y.; Guo, J.; Cui, J.; Zhou, Z. Impaired function of dendritic cells within the tumor microenvironment. Front. Immunol. 2023, 14, 1213629. [Google Scholar] [CrossRef]
- Badillo, O.; Helfridsson, L.; Niemi, J.; Hellström, M. Exploring dendritic cell subtypes in cancer immunotherapy: Unraveling the role of mature regulatory dendritic cells. Upsala J. Med. Sci. 2024, 129, e10627. [Google Scholar] [CrossRef]
- Bandola-Simon, J.; Roche, P.A. Dysfunction of antigen processing and presentation by dendritic cells in cancer. Mol. Immunol. 2019, 113, 31–37. [Google Scholar] [CrossRef]
- Ruffell, B.; Chang-Strachan, D.; Chan, V.; Rosenbusch, A.; Ho, C.M.; Pryer, N.; Daniel, D.; Hwang, E.S.; Rugo, H.S.; Coussens, L.M. Macrophage IL-10 blocks CD8+ T cell-dependent responses to chemotherapy by suppressing IL-12 expression in intratumoral dendritic cells. Cancer Cell 2014, 26, 623–637. [Google Scholar] [CrossRef]
- Bala, N.; McGurk, A.I.; Zilch, T.; Rup, A.N.; Carter, E.M.; Leddon, S.A.; Fowell, D.J. T cell activation niches-Optimizing T cell effector function in inflamed and infected tissues. Immunol. Rev. 2022, 306, 164–180. [Google Scholar] [CrossRef]
- Andersen, B.M.; Faust Akl, C.; Wheeler, M.A.; Chiocca, E.A.; Reardon, D.A.; Quintana, F.J. Glial and myeloid heterogeneity in the brain tumour microenvironment. Nat. Rev. Cancer 2021, 21, 786–802. [Google Scholar] [CrossRef]
- Brown, L.J.; Yeo, N.; Gee, H.; Kong, B.Y.; Hau, E.; da Silva, I.P.; Nagrial, A. Immune Checkpoint Inhibitors+/− Chemotherapy for Patients With NSCLC and Brain Metastases: A Systematic Review and Network Meta-Analysis. Thorac. Cancer 2025, 16, e15510. [Google Scholar]
- Brahmer, J.; Reckamp, K.L.; Baas, P.; Crinò, L.; Eberhardt, W.E.E.; Poddubskaya, E.; Antonia, S.; Pluzanski, A.; Vokes, E.E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 123–135. [Google Scholar] [CrossRef]
- Borghaei, H.; Paz-Ares, L.; Horn, L.; Spigel, D.R.; Steins, M.; Ready, N.E.; Chow, L.Q.; Vokes, E.E.; Felip, E.; Holgado, E.; et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non–Small-Cell Lung Cancer. N. Engl. J. Med. 2015, 373, 1627–1639. [Google Scholar] [CrossRef] [PubMed]
- Gadgeel, S.; Rodríguez-Abreu, D.; Speranza, G.; Esteban, E.; Felip, E.; Dómine, M.; Hui, R.; Hochmair, M.J.; Clingan, P.; Powell, S.F.; et al. Updated analysis from KEYNOTE-189: Pembrolizumab or placebo plus pemetrexed and platinum for previously untreated metastatic nonsquamous non–small-cell lung cancer. J. Clin. Oncol. 2020, 38, 1505–1517. [Google Scholar] [CrossRef] [PubMed]
- Descourt, R.; Greillier, L.; Perol, M.; Ricordel, C.; Auliac, J.-B.; Falchero, L.; Gervais, R.; Veillon, R.; Vieillot, S.; Guisier, F.; et al. First-line single-agent pembrolizumab for PD-L1-positive (tumor proportion score ≥ 50%) advanced non-small cell lung cancer in the real world: Impact in brain metastasis: A national French multicentric cohort (ESCKEYP GFPC study). Cancer Immunol. Immunother. 2023, 72, 91–99. [Google Scholar]
- Tsuchiya-Kawano, Y.; Shiraishi, Y.; Tanaka, K.; Tachihara, M.; Saito, R.; Okamoto, T.; Sugasaki, N.; Nakatomi, K.; Kiyomi, F.; Okamoto, I. Nivolumab plus ipilimumab with chemotherapy for non-small cell lung cancer with untreated brain metastases: A multicenter single-arm phase 2 trial (NIke, LOGiK 2004). Eur. J. Cancer 2024, 212, 115052. [Google Scholar] [CrossRef]
- Gauvain, C.; Vauléon, E.; Chouaid, C.; Le Rhun, E.; Jabot, L.; Scherpereel, A.; Vinas, F.; Cortot, A.B.; Monnet, I. Intracerebral efficacy and tolerance of nivolumab in non–small-cell lung cancer patients with brain metastases. Lung Cancer 2018, 116, 62–66, Erratum in Lung Cancer 2019, 136, 159. https://doi.org/10.1016/j.lungcan.2018.10.011. [Google Scholar] [CrossRef]
- Crinò, L.; Bronte, G.; Bidoli, P.; Cravero, P.; Minenza, E.; Cortesi, E.; Garassino, M.C.; Proto, C.; Cappuzzo, F.; Grossi, F.; et al. Nivolumab and brain metastases in patients with advanced non-squamous non-small cell lung cancer. Lung Cancer 2019, 129, 35–40. [Google Scholar] [PubMed]
- Borghaei, H.; Gettinger, S.; Vokes, E.E.; Chow, L.Q.; Burgio, M.A.; de Castro Carpeno, J.; Pluzanski, A.; Arrieta, O.; Frontera, O.A.; Chiari, R.; et al. Five-year outcomes from the randomized, phase III trials checkmate 017 and 057: Nivolumab versus docetaxel in previously treated non–small-cell lung cancer. J. Clin. Oncol. 2021, 39, 723–733, Erratum in J. Clin. Oncol. 2021, 39, 1190. https://doi.org/10.1200/JCO.21.00546. [Google Scholar] [CrossRef]
- Rizvi, N.A.; Mazières, J.; Planchard, D.; Stinchcombe, T.E.; Dy, G.K.; Antonia, S.J.; Horn, L.; Lena, H.; Minenza, E.; Mennecier, B.; et al. Activity and safety of nivolumab, an anti-PD-1 immune checkpoint inhibitor, for patients with advanced, refractory squamous non-small-cell lung cancer (CheckMate 063): A phase 2, single-arm trial. Lancet Oncol. 2015, 16, 257–265. [Google Scholar] [CrossRef]
- Mahajan, A.; Goldberg, S.L.; Weiss, S.A.; Tran, T.; Singh, K.; Joshi, K.; Aboian, M.S.; Kluger, H.M.; Chiang, V.L. Patterns of brain metastases response to immunotherapy with pembrolizumab. J. Neuro-Oncol. 2024, 169, 555–561. [Google Scholar] [CrossRef]
- Meng, Y.; Hynynen, K.; Lipsman, N. Applications of focused ultrasound in the brain: From thermoablation to drug delivery. Nat. Rev. Neurol. 2021, 17, 7–22. [Google Scholar] [CrossRef] [PubMed]
- Sheybani, N.D. How Sound Waves Could Revolutionize Cancer (Immuno) therapy. Acoust. Today 2024, 20, 49. [Google Scholar] [CrossRef]
- Emerich, D.F.; Dean, R.L.; Osborn, C.; Bartus, R.T. The development of the bradykinin agonist labradimil as a means to increase the permeability of the blood-brain barrier: From concept to clinical evaluation. Clin. Pharmacokinet. 2001, 40, 105–123. [Google Scholar] [CrossRef]
- Liu, J.; Wang, Y.; Song, Z.; Zhang, Y. Nanoengineered immune check point inhibitors delivery for targeted brain cancer treatment: Current status and future perspectives. Biochem. Pharmacol. 2025, 233, 116789. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, L. Modern methods for delivery of drugs across the blood–brain barrier. Adv. Drug Deliv. Rev. 2012, 64, 640–665. [Google Scholar] [CrossRef]
- Zhang, Y.; Cheng, R.; Ding, T.; Wu, J. Discrepancies in PD-L1 expression, lymphocyte infiltration, and tumor mutational burden in non-small cell lung cancer and matched brain metastases. Transl. Lung Cancer Res. 2024, 13, 3590–3602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Abdo, R.; Iosef, C.; Kaneko, T.; Cecchini, M.; Han, V.K.; Li, S.S.-C. The spatial transcriptomic landscape of non-small cell lung cancer brain metastasis. Nat. Commun. 2022, 13, 5983. [Google Scholar] [CrossRef]
- Tonse, R.; Rubens, M.; Appel, H.; Tom, M.C.; Hall, M.D.; Odia, Y.; McDermott, M.W.; Ahluwalia, M.S.; Mehta, M.P.; Kotecha, R. Systematic review and meta-analysis of PD-L1 expression discordance between primary tumor and lung cancer brain metastasis. Neuro-Oncol. Adv. 2021, 3, vdab166. [Google Scholar] [CrossRef]
- Chen, Y.; Zhang, Z.; Li, L.; Zhang, M.; Li, Z. Spatial transcriptomics study of Castleman disease. J. Transl. Med. 2025, 23, 459. [Google Scholar] [CrossRef]
- Li, T.; Sun, S.; Li, Y.; Zhang, Y.; Wei, L. Immunotherapy revolutionizing brain metastatic cancer treatment: Personalized strategies for transformative outcomes. Front. Immunol. 2024, 15, 1418580. [Google Scholar] [CrossRef]
- Marini, A.; Bernardini, A.; Gigli, G.L.; Valente, M.; Muñiz-Castrillo, S.; Honnorat, J.; Vogrig, A. Neurologic Adverse Events of Immune Checkpoint Inhibitors. Neurology 2021, 96, 754–766. [Google Scholar] [CrossRef]
- Sandoval, M.; Wechsler, A.H.; Alhajji, Z.; Viets-Upchurch, J.; Brock, P.; Lipe, D.N.; Al-breiki, A.; Yeung, S.-C.J. Evaluation and management of acute high-grade immunotherapy-related neurotoxicity. Heliyon 2023, 9, e13725. [Google Scholar] [CrossRef] [PubMed]
- Velasco, R.; Villagrán, M.; Jové, M.; Simó, M.; Vilariño, N.; Alemany, M.; Palmero, R.; Martínez-Villacampa, M.M.; Nadal, E.; Bruna, J. Encephalitis induced by immune checkpoint inhibitors: A systematic review. JAMA Neurol. 2021, 78, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Vogrig, A.; Fouret, M.; Joubert, B.; Picard, G.; Rogemond, V.; Pinto, A.-L.; Muñiz-Castrillo, S.; Roger, M.; Raimbourg, J.; Dayen, C.; et al. Increased frequency of anti-Ma2 encephalitis associated with immune checkpoint inhibitors. Neurol. Neuroimmunol. Neuroinflamm. 2019, 6, e604. [Google Scholar] [CrossRef]
- Buckley, M.W.; Balaji Warner, A.; Brahmer, J.; Cappelli, L.C.; Sharfman, W.H.; Fuchs, E.; Kang, H.; Forde, P.M.; Gladstone, D.E.; Ambinder, R.; et al. Immune-related encephalitis after immune checkpoint inhibitor therapy. Oncologist 2025, 30, oyae186. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Ren, H.; Fu, J. Treatment of Radiation-Induced Brain Necrosis. Oxidative Med. Cell. Longev. 2021, 2021, 4793517. [Google Scholar] [CrossRef]
- Bernhardt, D.; König, L.; Grosu, A.; Wiestler, B.; Rieken, S.; Wick, W.; Gempt, J.; Krieg, S.; Schmidt-Graf, F.; Sahm, F.; et al. DEGRO practical guideline for central nervous system radiation necrosis part 1: Classification and a multistep approach for diagnosis. Strahlenther. Und Onkol. 2022, 198, 873–883. [Google Scholar] [CrossRef]
- Ji, X.; Wang, L.; Tan, Y.; Shang, Y.; Huo, R.; Fang, C.; Li, C.; Zhang, L. Radionecrosis mimicking pseudo-progression in a patient with lung cancer and brain metastasis following the combination of anti-PD-1 therapy and stereotactic radiosurgery: A case report. Oncol. Lett. 2023, 26, 361. [Google Scholar] [CrossRef] [PubMed]
- Pathak, R.; Amini, A.; Hill, A.; Massarelli, E.; Salgia, R. Immunotherapy in Non-Small Cell Lung Cancer Patients with Brain Metastases: Clinical Challenges and Future Directions. Cancers 2021, 13, 3407. [Google Scholar] [CrossRef]
- Urban, H.; Steidl, E.; Hattingen, E.; Filipski, K.; Meissner, M.; Sebastian, M.; Koch, A.; Strzelczyk, A.; Forster, M.T.; Baumgarten, P.; et al. Immune Checkpoint Inhibitor-Induced Cerebral Pseudoprogression: Patterns and Categorization. Front. Immunol. 2021, 12, 798811. [Google Scholar] [CrossRef]
- Tran, T.T.; Jilaveanu, L.B.; Omuro, A.; Chiang, V.L.; Huttner, A.; Kluger, H.M. Complications associated with immunotherapy for brain metastases. Curr. Opin. Neurol. 2019, 32, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Spain, L.; Tippu, Z.; Larkin, J.M.; Carr, A.; Turajlic, S. How we treat neurological toxicity from immune checkpoint inhibitors. ESMO open 2019, 4, e000540. [Google Scholar] [CrossRef]
- Proescholdt, M.A.; Schödel, P.; Doenitz, C.; Pukrop, T.; Höhne, J.; Schmidt, N.O.; Schebesch, K.-M. The management of brain metastases—Systematic review of neurosurgical aspects. Cancers 2021, 13, 1616. [Google Scholar] [CrossRef] [PubMed]
- Ene, C.I.; Ferguson, S.D. Surgical management of brain metastasis: Challenges and nuances. Front. Oncol. 2022, 12, 847110. [Google Scholar] [CrossRef]
- Verheijden, R.J.; de Groot, J.S.; Fabriek, B.O.; Hew, M.N.; May, A.M.; Suijkerbuijk, K.P. Corticosteroids for immune-related adverse events and checkpoint inhibitor efficacy: Analysis of six clinical trials. J. Clin. Oncol. 2024, 42, 3713–3724. [Google Scholar] [CrossRef]
- Goodman, R.S.; Johnson, D.B.; Balko, J.M. Corticosteroids and cancer immunotherapy. Clin. Cancer Res. 2023, 29, 2580–2587. [Google Scholar] [CrossRef]
- Fernandes, L.F.; Lozano, N.; Peeyatu, C.; Ho, Y.; Thompson, L.; Kostarelos, K.; Kisby, T. JS08.7.A Surgery Transiently Disrupts the Blood Brain Barrier of the Glioblastoma Resection Margin: Opportunities for Early and Selective Postoperative Treatment. Neuro-Oncology 2024, 26, v15. [Google Scholar] [CrossRef]
- Giantini-Larsen, A.M.; Pandey, A.; Garton, A.L.A.; Rampichini, M.; Winston, G.; Goldberg, J.L.; Magge, R.; Stieg, P.E.; Souweidane, M.M.; Ramakrishna, R. Therapeutic manipulation and bypass of the blood-brain barrier: Powerful tools in glioma treatment. Neurooncol. Adv. 2025, 7, vdae201. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Mo, C.; Xu, S.; Chen, L.; Ye, W.; Kang, Y.; Chen, G.; Zhu, T. Research progress on perioperative blood-brain barrier damage and its potential mechanism. Front. Cell Dev. Biol. 2023, 11, 1174043. [Google Scholar] [CrossRef]
- Yang, T.; Velagapudi, R.; Terrando, N. Neuroinflammation after surgery: From mechanisms to therapeutic targets. Nat. Immunol. 2020, 21, 1319–1326. [Google Scholar] [CrossRef]
- Takata, F.; Nakagawa, S.; Matsumoto, J.; Dohgu, S. Blood-brain barrier dysfunction amplifies the development of neuroinflammation: Understanding of cellular events in brain microvascular endothelial cells for prevention and treatment of BBB dysfunction. Front. Cell. Neurosci. 2021, 15, 661838. [Google Scholar] [CrossRef]
- Xiao, M.; Xiao, Z.J.; Yang, B.; Lan, Z.; Fang, F. Blood-Brain Barrier: More Contributor to Disruption of Central Nervous System Homeostasis Than Victim in Neurological Disorders. Front. Neurosci. 2020, 14, 764. [Google Scholar] [CrossRef]
- Adib, E.; Nassar, A.H.; Bou Farhat, E.; Tanguturi, S.K.; Rahman, R.M.; Haas-Kogan, D.A.; Bi, W.L.; Arnaout, O.; Wen, P.Y.; Kwiatkowski, D.J.; et al. PD-L1, Tumor Mutational Burden, and Outcomes in NSCLC With Brain Metastases: A Brief Report. JTO Clin. Res. Rep. 2025, 6, 100797. [Google Scholar] [CrossRef]
- Zgura, A.; Chipuc, S.; Bacalbasa, N.; Haineala, B.; Rodica, A.; Sebastian, V. Evaluating Tumour Mutational Burden as a Key Biomarker in Personalized Cancer Immunotherapy: A Pan-Cancer Systematic Review. Cancers 2025, 17, 480. [Google Scholar] [CrossRef]
- Redmond, K.J.; Gui, C.; Benedict, S.; Milano, M.T.; Grimm, J.; Vargo, J.A.; Soltys, S.G.; Yorke, E.; Jackson, A.; El Naqa, I. Tumor control probability of radiosurgery and fractionated stereotactic radiosurgery for brain metastases. Int. J. Radiat. Oncol. Biol. Phys. 2021, 110, 53–67. [Google Scholar] [CrossRef] [PubMed]
- Mansouri, A.; Ozair, A.; Bhanja, D.; Wilding, H.; Mashiach, E.; Haque, W.; Mikolajewicz, N.; de Macedo Filho, L.; Mahase, S.S.; Machtay, M. Stereotactic radiosurgery for patients with brain metastases: Current principles, expanding indications and opportunities for multidisciplinary care. Nat. Rev. Clin. Oncol. 2025, 22, 327–347. [Google Scholar] [CrossRef]
- Qian, J.M.; Yu, J.B.; Kluger, H.M.; Chiang, V.L. Timing and type of immune checkpoint therapy affect the early radiographic response of melanoma brain metastases to stereotactic radiosurgery. Cancer 2016, 122, 3051–3058. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez Plá, M.; Dualde Beltrán, D.; Ferrer Albiach, E. Immune checkpoints inhibitors and SRS/SBRT synergy in metastatic non-small-cell lung cancer and melanoma: A systematic review. Int. J. Mol. Sci. 2021, 22, 11621. [Google Scholar] [CrossRef]
- Yomo, S.; Oda, K.; Oguchi, K. Synergistic effects of immune checkpoint inhibitors in combination with stereotactic radiosurgery for patients with lung cancer and brain metastases: A propensity score–matched analysis. J. Neurosurg. 2023, 139, 1628–1637. [Google Scholar] [CrossRef] [PubMed]
- Porte, J.; Saint-Martin, C.; Frederic-Moreau, T.; Massiani, M.-A.; Bozec, L.; Cao, K.; Verrelle, P.; Otz, J.; Jadaud, E.; Minsat, M. Efficacy and safety of combined brain stereotactic radiotherapy and immune checkpoint inhibitors in non-small-cell lung cancer with brain metastases. Biomedicines 2022, 10, 2249. [Google Scholar] [CrossRef] [PubMed]
- Suwinski, R. Combination of immunotherapy and radiotherapy in the treatment of brain metastases from non-small cell lung cancer. J. Thorac. Dis. 2021, 13, 3315–3322. [Google Scholar] [CrossRef]
- Sun, C.; Zhou, F.; Li, X.; Zhao, C.; Li, W.; Li, J.; Xiong, A.; Yu, J.; Gao, G.; Wang, Q. PD-1/PD-L1 inhibitor combined with chemotherapy can improve the survival of non-small cell lung cancer patients with brain metastases. OncoTargets Ther. 2020, 13, 12777–12786. [Google Scholar] [CrossRef]
- Ranjan, T.; Podder, V.; Margolin, K.; Velcheti, V.; Maharaj, A.; Ahluwalia, M.S. Immune checkpoint inhibitors in the management of brain metastases from non-small cell lung cancer: A comprehensive review of current trials, guidelines and future directions. Cancers 2024, 16, 3388. [Google Scholar] [CrossRef]
- Ayers, M.; Lunceford, J.; Nebozhyn, M.; Murphy, E.; Loboda, A.; Kaufman, D.R.; Albright, A.; Cheng, J.D.; Kang, S.P.; Shankaran, V. IFN-γ–related mRNA profile predicts clinical response to PD-1 blockade. J. Clin. Investig. 2017, 127, 2930–2940. [Google Scholar] [CrossRef] [PubMed]
- Tumeh, P.C.; Harview, C.L.; Yearley, J.H.; Shintaku, I.P.; Taylor, E.J.M.; Robert, L.; Chmielowski, B.; Spasic, M.; Henry, G.; Ciobanu, V.; et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 2014, 515, 568–571. [Google Scholar] [CrossRef]
- Thommen, D.S.; Koelzer, V.H.; Herzig, P.; Roller, A.; Trefny, M.; Dimeloe, S.; Kiialainen, A.; Hanhart, J.; Schill, C.; Hess, C.; et al. A transcriptionally and functionally distinct PD-1+ CD8+ T cell pool with predictive potential in non-small-cell lung cancer treated with PD-1 blockade. Nat. Med. 2018, 24, 994–1004. [Google Scholar] [CrossRef]
- Wu, J.; Liu, Z.; Huang, T.; Wang, Y.; Song, M.M.; Song, T.; Long, G.; Zhang, X.; Li, X.; Zhang, L. Cerebrospinal fluid circulating tumor DNA depicts profiling of brain metastasis in NSCLC. Mol. Oncol. 2023, 17, 810–824. [Google Scholar] [CrossRef]
- Mehta, A.M.; Sonabend, A.M.; Bruce, J.N. Convection-Enhanced Delivery. Neurotherapeutics 2017, 14, 358–371. [Google Scholar] [CrossRef] [PubMed]
- Chouleur, T. Understanding the biology of lung cancer brain metastases. Nat. Cancer 2025, 6, 560. [Google Scholar] [CrossRef] [PubMed]
- Tagore, S.; Caprio, L.; Amin, A.D.; Bestak, K.; Luthria, K.; D’Souza, E.; Barrera, I.; Melms, J.C.; Wu, S.; Abuzaid, S.; et al. Single-cell and spatial genomic landscape of non-small cell lung cancer brain metastases. Nat. Med. 2025, 31, 1351–1363. [Google Scholar] [CrossRef] [PubMed]

| Trial | ICI Used | Brain Metastases Included | Intracranial Response Rate | Key Findings |
|---|---|---|---|---|
| CheckMate 017 [93] | Nivolumab | Yes (asymptomatic) | Not separately reported | Demonstrated OS benefit; did not report separate intracranial response data |
| CheckMate 057 [94] | Nivolumab | Yes (asymptomatic) | Not separately reported | Demonstrated OS benefit; did not report separate intracranial response data |
| KEYNOTE-189 [95] | Pembrolizumab + chemotherapy | Yes (~17% of patients) | HR for OS in patients with brain metastases: 0.41 | HR for OS in brain metastases: 0.41; suggested benefit, but no intracranial response data provided |
| Yale Phase II (Goldberg et al.) [24] | Pembrolizumab | Yes (untreated, PD-L1+) | 33% | Pembrolizumab active in untreated brain metastases |
| Descourt et al. (French Cohort) [96] | Pembrolizumab | Yes (~20% of patients) | Comparable to nonbrain metastases group | No difference in OS/PFS between brain and nonbrain metastases |
| Tsuchiya-Kawano et al. [97] | Nivolumab + ipilimumab + chemotherapy | Yes (untreated) | 50% (20% CR) | Strong intracranial activity in untreated brain metastases |
| Gauvain et al. [98] | Nivolumab | Yes (79% received local brain therapy) | Intracranial PFS: 3.9 months | Similar intracranial and extracranial efficacy |
| Expanded Access Program (Italy) [99] | Nivolumab | Yes (asymptomatic, stable) | DCR 39%, OS 8.6 months | Real-world evidence of ICI efficacy in brain metastases; supported benefit in patients with poor prognoses |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, N.J.; Sindhu, K.K.; Nasser, L.; Shafaee, Z.; Li, J.; Resende Salgado, L.; Li, B. Immune Checkpoint Inhibition in Patients with Brain Metastases from Non-Small-Cell Lung Cancer: Emerging Mechanisms and Personalized Clinical Strategies. Int. J. Mol. Sci. 2025, 26, 8624. https://doi.org/10.3390/ijms26178624
Nasser NJ, Sindhu KK, Nasser L, Shafaee Z, Li J, Resende Salgado L, Li B. Immune Checkpoint Inhibition in Patients with Brain Metastases from Non-Small-Cell Lung Cancer: Emerging Mechanisms and Personalized Clinical Strategies. International Journal of Molecular Sciences. 2025; 26(17):8624. https://doi.org/10.3390/ijms26178624
Chicago/Turabian StyleNasser, Nicola J., Kunal K. Sindhu, Loor Nasser, Zahra Shafaee, Joshua Li, Lucas Resende Salgado, and Baoqing Li. 2025. "Immune Checkpoint Inhibition in Patients with Brain Metastases from Non-Small-Cell Lung Cancer: Emerging Mechanisms and Personalized Clinical Strategies" International Journal of Molecular Sciences 26, no. 17: 8624. https://doi.org/10.3390/ijms26178624
APA StyleNasser, N. J., Sindhu, K. K., Nasser, L., Shafaee, Z., Li, J., Resende Salgado, L., & Li, B. (2025). Immune Checkpoint Inhibition in Patients with Brain Metastases from Non-Small-Cell Lung Cancer: Emerging Mechanisms and Personalized Clinical Strategies. International Journal of Molecular Sciences, 26(17), 8624. https://doi.org/10.3390/ijms26178624





