Molecular Imbalances Between Striosome and Matrix Compartments Characterize the Pathogenesis and Pathophysiology of Huntington’s Disease Model Mouse
Abstract
1. Introduction
2. Results
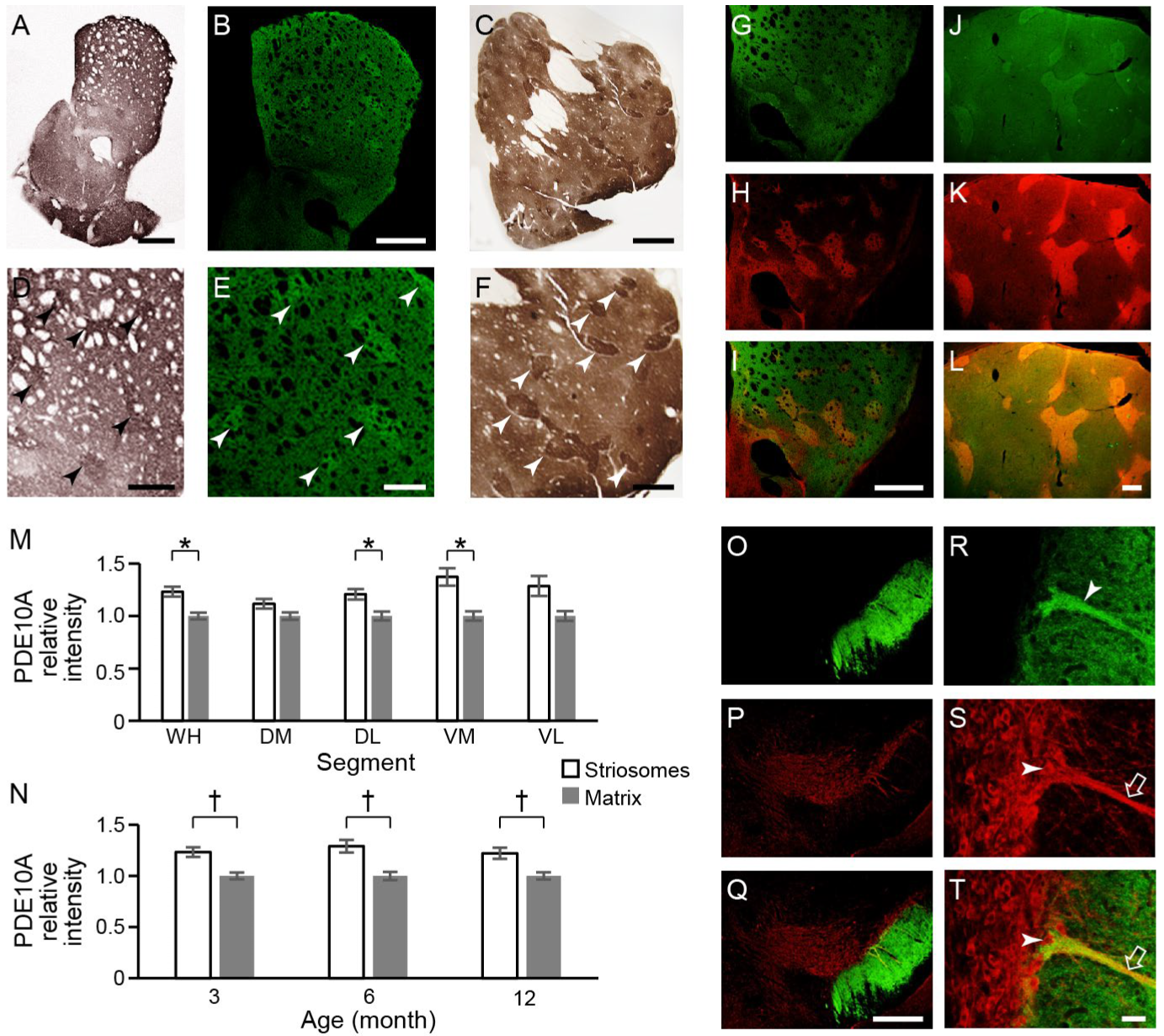
2.1. Striosomal Segmentation Using Deep Learning by a Fully Convolutional Residual Neural Network U-Net
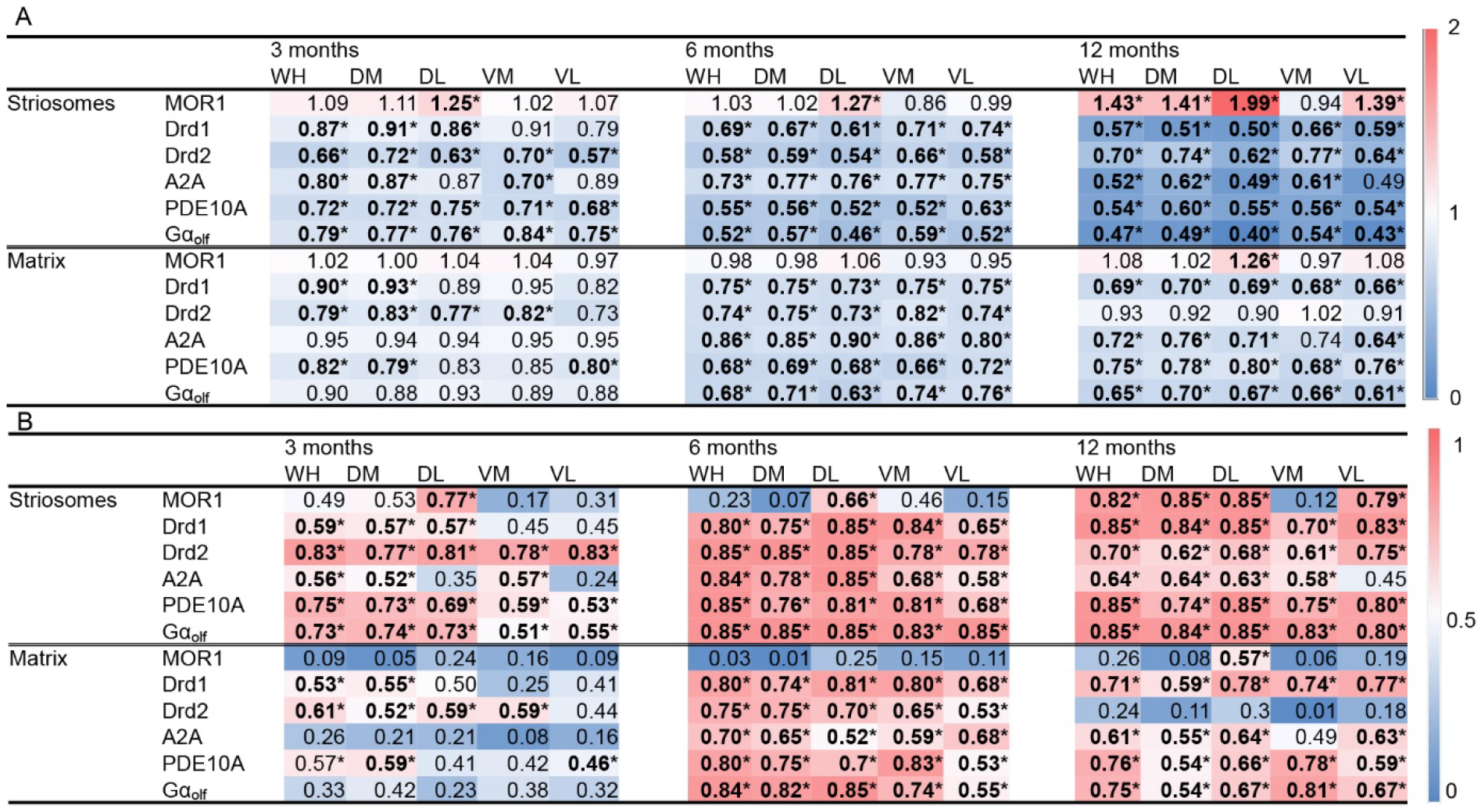
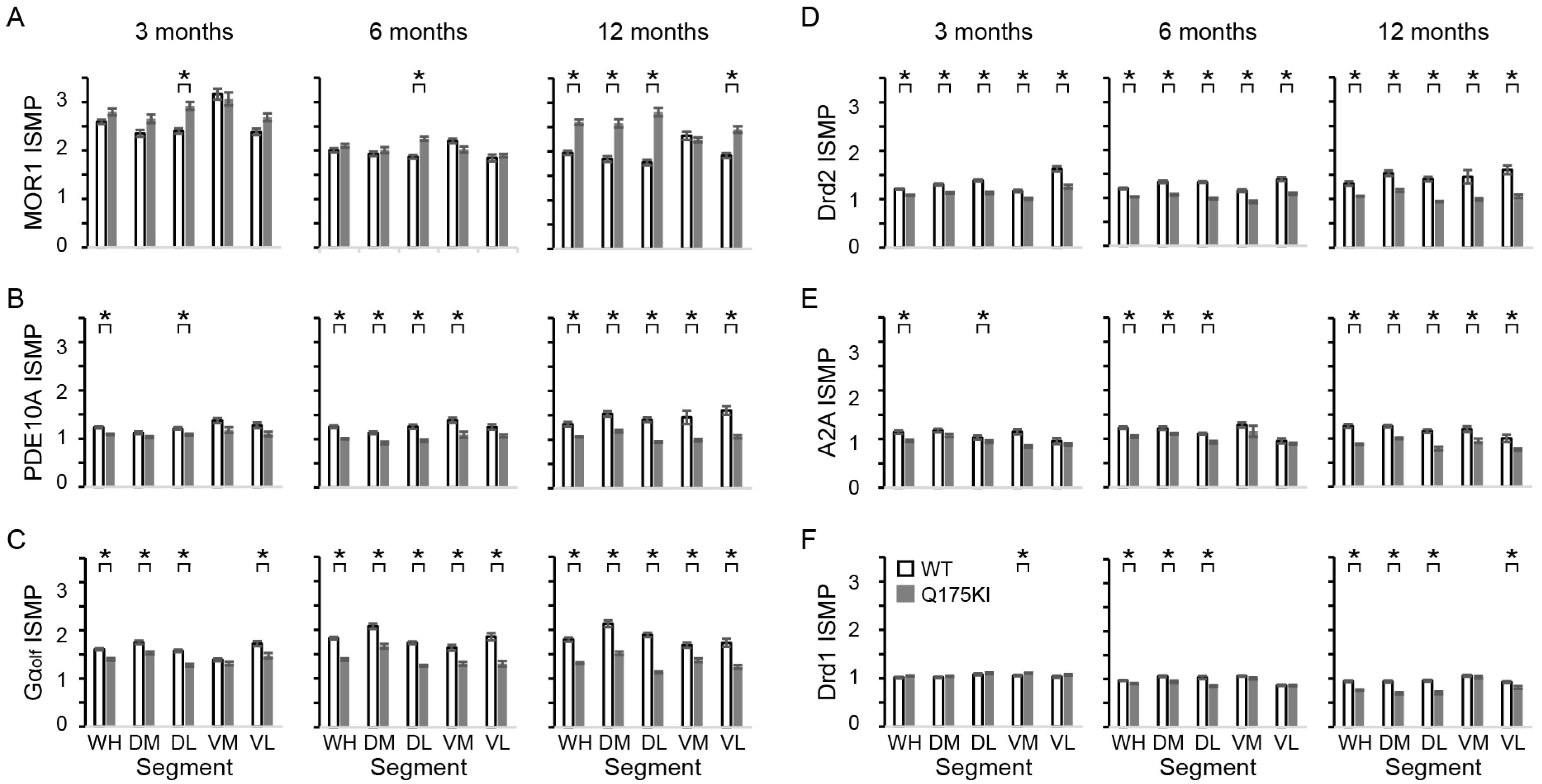
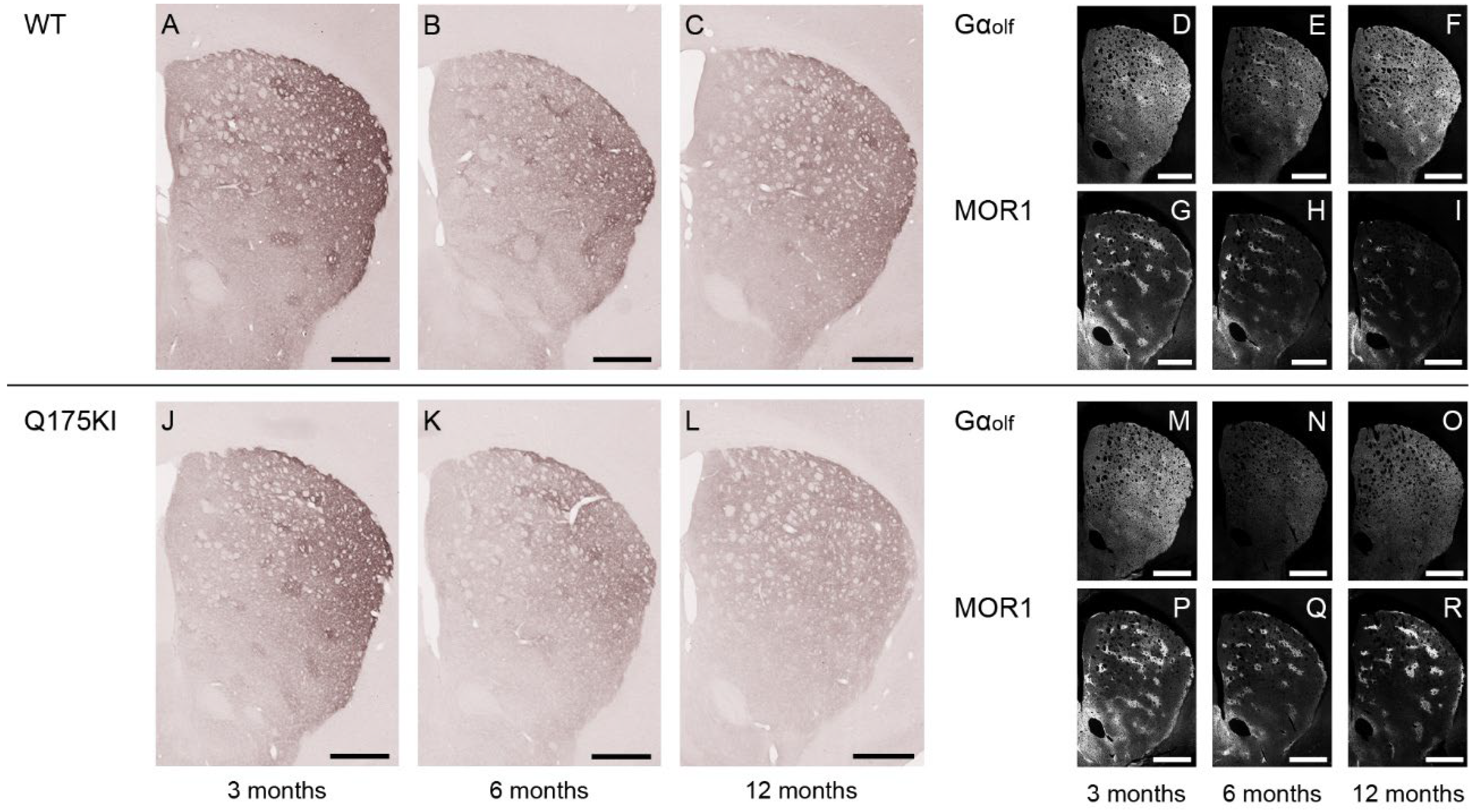
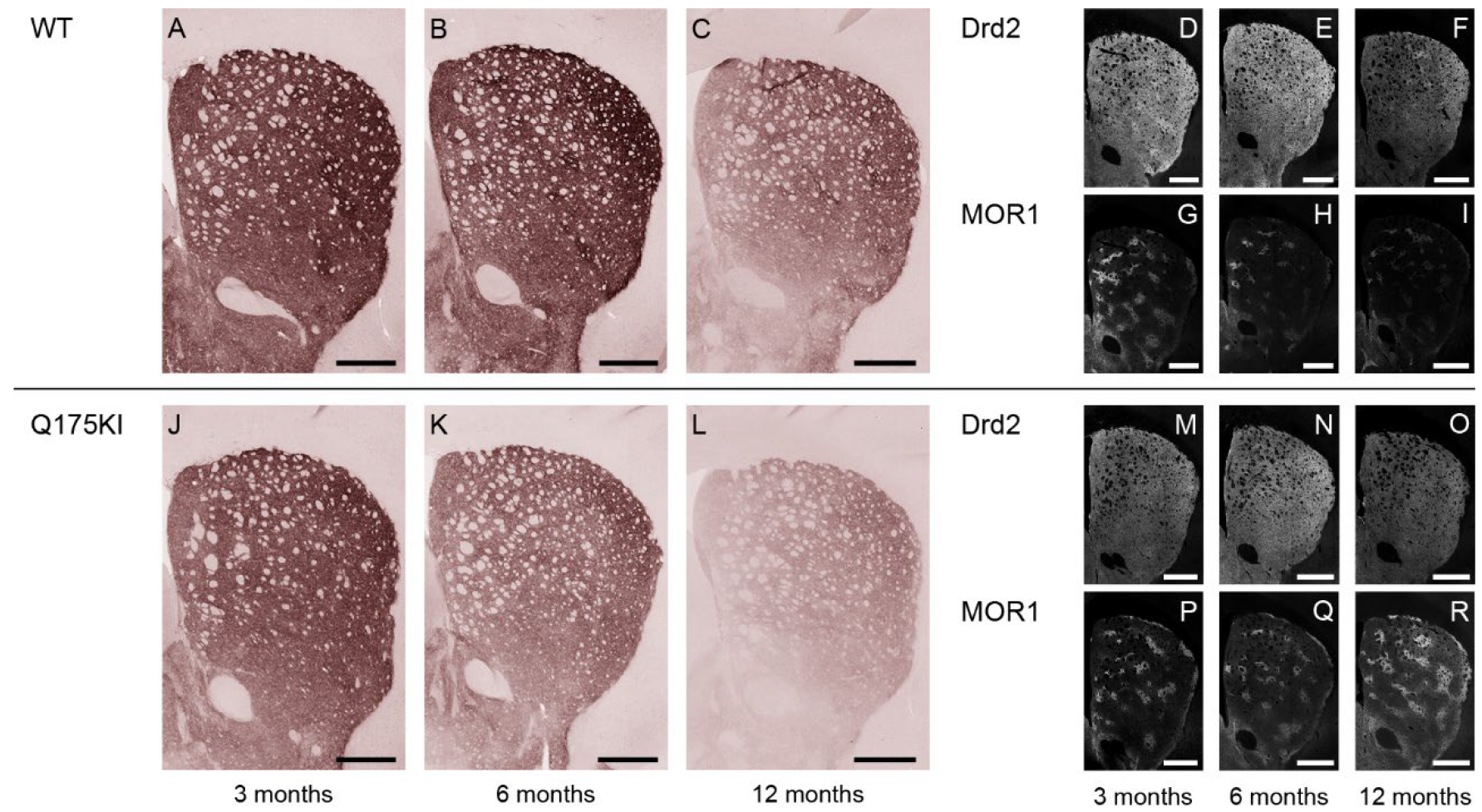
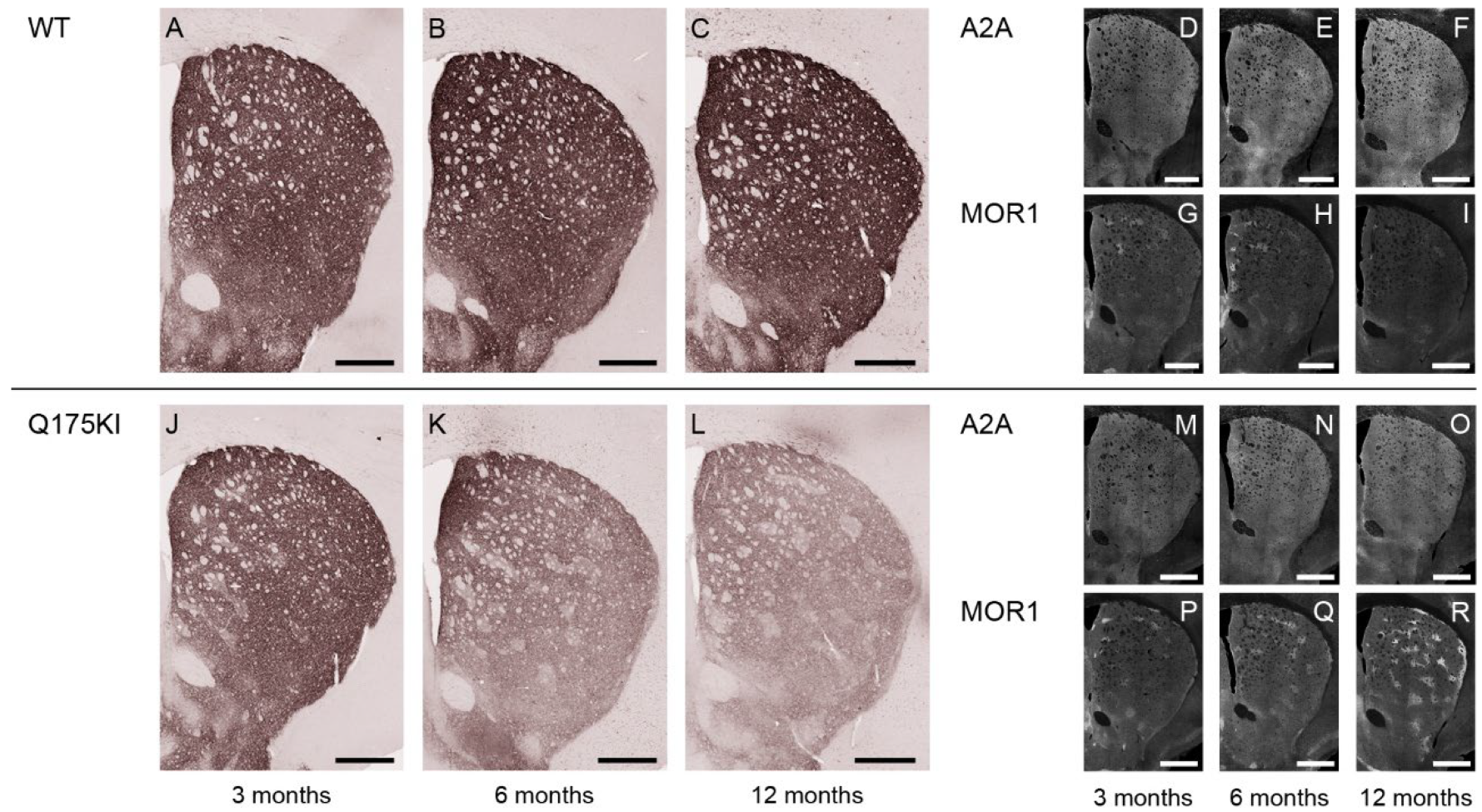
2.2. MOR1 Is Upregulated in the Striatum of the Q175KI Mice
2.3. PDE10A Immunoreactivity over Age
2.3.1. PDE10A Expression Is Relatively Enhanced in the Striosome Compartment in Naïve/WT Mouse and Naïve Monkey
2.3.2. PDE10A Exhibited Decreased Expression in Q175KI Mice
2.4. Gαolf and Dopamine D2 Receptor Expression Largely Decreased as Early as 3 Months of Age
2.5. Adenosine 2A and Dopamine D1 Receptor Expression Exhibited Relatively Gradual Decreases in Q175KI Mice Through Aging
3. Discussion
3.1. Unique Status of MOR1 Protein Expression in HD Mutants
3.2. Clues to the Etiology of Observed Molecular Changes Suggested by Clinical Studies
3.3. Hypothesis: Gαolf as a Primary Etiologic Signal of Abnormality in HD Model Mice
3.4. The Potential Role of PDE10A and Changes in cAMP
4. Material and Methods
4.1. Animals
4.2. Immunizing Peptide-Blocking Assay
4.3. Immunohistochemistry
4.4. Immunohistochemical Detection of PDE10A in Monkeys’ Brain
4.5. Striosomal Segmentation
4.6. Digital Images and Densitometry
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferrante, R.J.; Kowall, N.W.; Beal, M.F.; Richardson, E.P.; Bird, E.D.; Martin, J.B. Selective Sparing of a Class of Striatal Neurons in Huntington’s Disease. Science 1985, 230, 561–563. [Google Scholar] [CrossRef]
- Ferrante, R.J.; Flint Beal, M.; Kowall, N.W.; Richardson, E.P.; Martin, J.B. Sparing of Acetylcholinesterase-Containing Striatal Neurons in Huntington’s Disease. Brain Res. 1987, 411, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Albin, R.L.; Anderson, K.D.; D’Amato, C.J.; Penney, J.B.; Young, A.B. Differential Loss of Striatal Projection Neurons in Huntington Disease. Proc. Natl. Acad. Sci. USA 1988, 85, 5733–5737. [Google Scholar] [CrossRef]
- Reiner, A.; Shelby, E.; Wang, H.; DeMarch, Z.; Deng, Y.; Guley, N.H.; Hogg, V.; Roxburgh, R.; Tippett, L.J.; Waldvogel, H.J.; et al. Striatal Parvalbuminergic Neurons Are Lost in Huntington’s Disease: Implications for Dystonia. Mov. Disord. 2013, 28, 1691–1699. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Hirano, A.; Rojas-Corona, R.R. An Immunohistochemical Investigation of the Human Neostriatum in Huntington’s Disease. Ann. Neurol. 1989, 25, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Albin, R.L.; Reiner, A.; Anderson, K.D.; Penney, J.B.; Young, A.B. Striatal and Nigral Neuron Subpopulations in Rigid Huntington’s Disease: Implications for the Functional Anatomy of Chorea and Rigidity-Akinesia. Ann. Neurol. 1990, 27, 357–365. [Google Scholar] [CrossRef]
- Morton, A.J.; Nicholson, L.F.B.; Faull, R.L.M. Compartmental Loss of NADPH Diaphorase in the Neuropil of the Human Striatum in Huntington’s Disease. Neuroscience 1993, 53, 159–168. [Google Scholar] [CrossRef]
- Hedreen, J.C.; Folstein, S.E. Early Loss of Neostriatal Striosome Neurons in Huntington’s Disease. J. Neuropathol. Exp. Neurol. 1995, 54, 105–120. [Google Scholar] [CrossRef]
- Augood, S.J.; Faull, R.L.M.; Love, D.R.; Emson, P.C. Reduction in Enkephalin and Substance P Messenger RNA in the Striatum of Early Grade Huntington’s Disease: A Detailed Cellularin Situ Hybridization Study. Neuroscience 1996, 72, 1023–1036. [Google Scholar] [CrossRef]
- Cicchetti, F.; Prensa, L.; Wu, Y.; Parent, A. Chemical Anatomy of Striatal Interneurons in Normal Individuals and in Patients with Huntington’s Disease. Brain Res. Rev. 2000, 34, 80–101. [Google Scholar] [CrossRef]
- Tippett, L.J.; Waldvogel, H.J.; Thomas, S.J.; Hogg, V.M.; Roon-Mom, W.V.; Synek, B.J.; Graybiel, A.M.; Faull, R.L.M. Striosomes and Mood Dysfunction in Huntington’s Disease. Brain 2007, 130, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Hedreen, J.C.; Berretta, S.; White Iii, C.L. Postmortem Neuropathology in Early Huntington Disease. J. Neuropathol. Exp. Neurol. 2024, 83, 294–306. [Google Scholar] [CrossRef] [PubMed]
- Matsushima, A.; Pineda, S.S.; Crittenden, J.R.; Lee, H.; Galani, K.; Mantero, J.; Tombaugh, G.; Kellis, M.; Heiman, M.; Graybiel, A.M. Transcriptional Vulnerabilities of Striatal Neurons in Human and Rodent Models of Huntington’s Disease. Nat. Commun. 2023, 14, 282. [Google Scholar] [CrossRef] [PubMed]
- Crittenden, J.R.; Graybiel, A.M. Basal Ganglia Disorders Associated with Imbalances in the Striatal Striosome and Matrix Compartments. Front. Neuroanat. 2011, 5, 59. [Google Scholar] [CrossRef]
- Niccolini, F.; Haider, S.; Reis Marques, T.; Muhlert, N.; Tziortzi, A.C.; Searle, G.E.; Natesan, S.; Piccini, P.; Kapur, S.; Rabiner, E.A.; et al. Altered PDE10A Expression Detectable Early before Symptomatic Onset in Huntington’s Disease. Brain 2015, 138, 3016–3029. [Google Scholar] [CrossRef]
- Wilson, H.; Niccolini, F.; Haider, S.; Marques, T.R.; Pagano, G.; Coello, C.; Natesan, S.; Kapur, S.; Rabiner, E.A.; Gunn, R.N.; et al. Loss of Extra-Striatal Phosphodiesterase 10A Expression in Early Premanifest Huntington’s Disease Gene Carriers. J. Neurol. Sci. 2016, 368, 243–248. [Google Scholar] [CrossRef]
- Russell, D.S.; Barret, O.; Jennings, D.L.; Friedman, J.H.; Tamagnan, G.D.; Thomae, D.; Alagille, D.; Morley, T.J.; Papin, C.; Papapetropoulos, S.; et al. The Phosphodiesterase 10 Positron Emission Tomography Tracer, [18 F]MNI-659, as a Novel Biomarker for Early Huntington Disease. JAMA Neurol. 2014, 71, 1520. [Google Scholar] [CrossRef]
- Kotera, J.; Fujishige, K.; Yuasa, K.; Omori, K. Characterization and Phosphorylation of PDE10A2, a Novel Alternative Splice Variant of Human Phosphodiesterase That Hydrolyzes cAMP and cGMP. Biochem. Biophys. Res. Commun. 1999, 261, 551–557. [Google Scholar] [CrossRef]
- Nishi, A.; Kuroiwa, M.; Miller, D.B.; O’Callaghan, J.P.; Bateup, H.S.; Shuto, T.; Sotogaku, N.; Fukuda, T.; Heintz, N.; Greengard, P.; et al. Distinct Roles of PDE4 and PDE10A in the Regulation of cAMP/PKA Signaling in the Striatum. J. Neurosci. 2008, 28, 10460–10471. [Google Scholar] [CrossRef]
- Xie, Z.; Adamowicz, W.O.; Eldred, W.D.; Jakowski, A.B.; Kleiman, R.J.; Morton, D.G.; Stephenson, D.T.; Strick, C.A.; Williams, R.D.; Menniti, F.S. Cellular and Subcellular Localization of PDE10A, a Striatum-Enriched Phosphodiesterase. Neuroscience 2006, 139, 597–607. [Google Scholar] [CrossRef]
- Esposito, S.; Carecchio, M.; Tonduti, D.; Saletti, V.; Panteghini, C.; Chiapparini, L.; Zorzi, G.; Pantaleoni, C.; Garavaglia, B.; Krainc, D.; et al. A PDE10A de Novo Mutation Causes Childhood-Onset Chorea with Diurnal Fluctuations: PDE10A Mutation. Mov. Disord. 2017, 32, 1646–1647. [Google Scholar] [CrossRef]
- Narayanan, D.L.; Deshpande, D.; Das Bhowmik, A.; Varma, D.R.; Dalal, A. Familial Choreoathetosis Due to Novel Heterozygous Mutation in PDE10A. Am. J. Med. Genet. 2018, 176, 146–150. [Google Scholar] [CrossRef]
- Miyatake, S.; Koshimizu, E.; Shirai, I.; Kumada, S.; Nakata, Y.; Kamemaru, A.; Nakashima, M.; Mizuguchi, T.; Miyake, N.; Saitsu, H.; et al. A Familial Case of PDE10A-associated Childhood-onset Chorea with Bilateral Striatal Lesions. Mov. Disord. 2018, 33, 177–179. [Google Scholar] [CrossRef] [PubMed]
- Goto, S. Striatal Gαolf/cAMP Signal-Dependent Mechanism to Generate Levodopa-Induced Dyskinesia in Parkinson’s Disease. Front. Cell. Neurosci. 2017, 11, 364. [Google Scholar] [CrossRef] [PubMed]
- Carecchio, M.; Panteghini, C.; Reale, C.; Barzaghi, C.; Monti, V.; Romito, L.; Sasanelli, F.; Garavaglia, B. Novel GNAL Mutation with Intra-Familial Clinical Heterogeneity: Expanding the Phenotype. Park. Relat. Disord. 2016, 23, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Dobričić, V.; Kresojević, N.; Westenberger, A.; Svetel, M.; Tomić, A.; Ralić, V.; Petrović, I.; Lukić, M.J.; Lohmann, K.; Novaković, I.; et al. De Novo Mutation in the GNAL Gene Causing Seemingly Sporadic Dystonia in a Serbian Patient: DE NOVO GNAL (DYT25) MUTATION. Mov. Disord. 2014, 29, 1190–1193. [Google Scholar] [CrossRef]
- Fuchs, T.; Saunders-Pullman, R.; Masuho, I.; Luciano, M.S.; Raymond, D.; Factor, S.; Lang, A.E.; Liang, T.-W.; Trosch, R.M.; White, S.; et al. Mutations in GNAL Cause Primary Torsion Dystonia. Nat. Genet. 2013, 45, 88–92. [Google Scholar] [CrossRef]
- Kaur, A. Rare Autosomal Dominant Mutations in GNAL Are Associated with Primary Torsion Dystonia: HotSpots. Clin. Genet. 2013, 84, 211–212. [Google Scholar] [CrossRef]
- Kumar, K.R.; Lohmann, K.; Masuho, I.; Miyamoto, R.; Ferbert, A.; Lohnau, T.; Kasten, M.; Hagenah, J.; Brüggemann, N.; Graf, J.; et al. Mutations in GNAL: A Novel Cause of Craniocervical Dystonia. JAMA Neurol. 2014, 71, 490. [Google Scholar] [CrossRef]
- Masuho, I.; Fang, M.; Geng, C.; Zhang, J.; Jiang, H.; Özgul, R.K.; Yılmaz, D.Y.; Yalnızoğlu, D.; Yüksel, D.; Yarrow, A.; et al. Homozygous GNAL Mutation Associated with Familial Childhood-Onset Generalized Dystonia. Neurol. Genet. 2016, 2, e78. [Google Scholar] [CrossRef]
- Miao, J.; Wan, X.-H.; Sun, Y.; Feng, J.-C.; Cheng, F.-B. Mutation Screening of GNAL Gene in Patients with Primary Dystonia from Northeast China. Park. Relat. Disord. 2013, 19, 910–912. [Google Scholar] [CrossRef]
- Putzel, G.G.; Fuchs, T.; Battistella, G.; Rubien-Thomas, E.; Frucht, S.J.; Blitzer, A.; Ozelius, L.J.; Simonyan, K. GNAL Mutation in Isolated Laryngeal Dystonia: Gnal in Laryngeal Dystonia. Mov. Disord. 2016, 31, 750–755. [Google Scholar] [CrossRef]
- Vemula, S.R.; Puschmann, A.; Xiao, J.; Zhao, Y.; Rudzińska, M.; Frei, K.P.; Truong, D.D.; Wszolek, Z.K.; LeDoux, M.S. Role of Gα(Olf) in Familial and Sporadic Adult-Onset Primary Dystonia. Hum. Human. Mol. Genet. 2013, 22, 2510–2519. [Google Scholar] [CrossRef]
- Hervé, D. Identification of a Specific Assembly of the G Protein Golf as a Critical and Regulated Module of Dopamine and Adenosine-Activated cAMP Pathways in the Striatum. Front. Neuroanat. 2011, 5, 48. [Google Scholar] [CrossRef] [PubMed]
- Corvol, J.C.; Studler, J.M.; Schonn, J.S.; Girault, J.A.; Hervé, D. Gαolf Is Necessary for Coupling D1 and A2a Receptors to Adenylyl Cyclase in the Striatum: Gαolf-Coupled Striatal Adenylyl Cyclase. J. Neurochem. 2001, 76, 1585–1588. [Google Scholar] [CrossRef] [PubMed]
- Sako, W.; Morigaki, R.; Nagahiro, S.; Kaji, R.; Goto, S. Olfactory Type G-Protein α Subunit in Striosome-Matrix Dopamine Systems in Adult Mice. Neuroscience 2010, 170, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, R.; Okita, S.; Goto, S. Dopamine-Induced Changes in Gαolf Protein Levels in Striatonigral and Striatopallidal Medium Spiny Neurons Underlie the Genesis of l-DOPA-Induced Dyskinesia in Parkinsonian Mice. Front. Cell. Neurosci. 2017, 11, 26. [Google Scholar] [CrossRef]
- Goto, S.; Lee, L.V.; Munoz, E.L.; Tooyama, I.; Tamiya, G.; Makino, S.; Ando, S.; Dantes, M.B.; Yamada, K.; Matsumoto, S.; et al. Functional Anatomy of the Basal Ganglia in X-Linked Recessive Dystonia-Parkinsonism. Ann. Neurol. 2005, 58, 7–17. [Google Scholar] [CrossRef]
- Crittenden, J.R.; Cantuti-Castelvetri, I.; Saka, E.; Keller-McGandy, C.E.; Hernandez, L.F.; Kett, L.R.; Young, A.B.; Standaert, D.G.; Graybiel, A.M. Dysregulation of CalDAG-GEFI and CalDAG-GEFII Predicts the Severity of Motor Side-Effects Induced by Anti-Parkinsonian Therapy. Proc. Natl. Acad. Sci. USA 2009, 106, 2892–2896. [Google Scholar] [CrossRef]
- Morigaki, R.; Goto, S. Striatal Vulnerability in Huntington’s Disease: Neuroprotection Versus Neurotoxicity. Brain Sci. 2017, 7, 63. [Google Scholar] [CrossRef]
- Graybiel, A.M.; Canales, J.J.; Capper-Loup, C. Levodopa-Induced Dyskinesias and Dopamine-Dependent Stereotypies: A New Hypothesis. Trends Neurosci. 2000, 23, S71–S77. [Google Scholar] [CrossRef] [PubMed]
- Morigaki, R.; Lee, J.H.; Yoshida, T.; Wüthrich, C.; Hu, D.; Crittenden, J.R.; Friedman, A.; Kubota, Y.; Graybiel, A.M. Spatiotemporal Up-Regulation of Mu Opioid Receptor 1 in Striatum of Mouse Model of Huntington’s Disease Differentially Affecting Caudal and Striosomal Regions. Front. Neuroanat. 2020, 14, 608060. [Google Scholar] [CrossRef] [PubMed]
- Mikula, S.; Parrish, S.K.; Trimmer, J.S.; Jones, E.G. Complete 3-D visualization of primate striosomes by KChIP1 immunostaining. J. Comp. Neurol. 2009, 514, 507–517. [Google Scholar] [CrossRef]
- Crittenden, J.R.; Tillberg, P.W.; Riad, M.H.; Shima, Y.; Gerfen, C.R.; Curry, J.; Housman, D.E.; Nelson, S.B.; Boyden, E.S.; Graybiel, A.M. Striosome–Dendron Bouquets Highlight a Unique Striatonigral Circuit Targeting Dopamine-Containing Neurons. Proc. Natl. Acad. Sci. USA 2016, 113, 11318–11323. [Google Scholar] [CrossRef] [PubMed]
- Lazaridis, I.; Crittenden, J.R.; Ahn, G.; Hirokane, K.; Wickersham, I.R.; Yoshida, T.; Mahar, A.; Skara, V.; Loftus, J.H.; Parvataneni, K.; et al. Striosomes Control Dopamine via Dual Pathways Paralleling Canonical Basal Ganglia Circuits. Curr. Biol. 2024, 34, 5263–5283.e8. [Google Scholar] [CrossRef]
- Taha, A.A.; Hanbury, A. Metrics for evaluating 3D medical image segmentation: Analysis, selection, and tool. BMC Med. Imaging 2015, 15, 29. [Google Scholar] [CrossRef]
- Reinke, A.; Tizabi, M.D.; Baumgartner, M.; Eisenmann, M.; Heckmann-Nötzel, D.; Kvur, A.E.; Rädsch, T.; Sudre, C.H.; Acion, L.; Antonelli, M.; et al. Understanding metric-related pitfalls in image analysis validation. Nat. Methods 2024, 21, 182–194. [Google Scholar] [CrossRef]
- Canales, J.J.; Graybiel, A.M. A Measure of Striatal Function Predicts Motor Stereotypy. Nat. Neurosci. 2000, 3, 377–383. [Google Scholar] [CrossRef]
- Gines, S.; Seong, I.S.; Fossale, E.; Ivanova, E.; Trettel, F.; Gusella, J.F.; Wheeler, V.C.; Persichetti, F.; MacDonald, M.E. Specific progressive cAMP reduction implicates energy deficit in presymptomatic Huntington’s disease knock-in mice. Hum. Mol. Genet. 2003, 12, 497–508. [Google Scholar] [CrossRef]
- Deng, Y.; Wang, H.; Joji, M.; Sekhri, R.; Reiner, A. Progression of basal ganglia pathology in heterozygous Q175 knock-in Huntington’s disease mice. J. Comp. Neurol. 2021, 529, 1327–1371. [Google Scholar] [CrossRef]
- Menalled, L.B.; Kudwa, A.E.; Miller, S.; Fitzpatrick, J.; Watson-Johnson, J.; Keating, N.; Ruiz, M.; Mushlin, R.; Alosio, W.; McConnell, K.; et al. Comprehensive behavioral and molecular characterization of a new knock-in mouse model of Huntington’s disease: zQ175. PLoS ONE 2012, 7, e49838. [Google Scholar] [CrossRef]
- Smith, G.A.; Rocha, E.M.; McLean, J.R.; Hayes, M.A.; Izen, S.C.; Isacson, O.; Hallett, P.J. Progressive axonal transport and synaptic protein changes correlate with behavioral and neuropathological abnormalities in the heterozygous Q175 KI mouse model of Huntington’s disease. Hum. Mol. Genet. 2014, 23, 4510–4527. [Google Scholar] [CrossRef] [PubMed]
- Warner, J.H.; Long, J.D.; Mills, J.A.; Langbehn, D.R.; Ware, J.; Mohan, A.; Sampaio, C. Standardizing the CAP score in Huntington’s disease by predicting age-at-onset. J. Huntingt. Dis. 2022, 11, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Ostlund, S.B.; James, A.S.; Park, C.S.; Ge, W.; Roberts, K.W.; Mittal, N.; Murphy, N.P.; Cepeda, C.; Kieffer, B.L.; et al. Targeted expression of μ-opioid receptors in a subset of striatal direct-pathway neurons restores opiate reward. Nat. Neurosci. 2014, 17, 254–261. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, H.; Morigaki, R.; Okita, S.; Nagahiro, S.; Kaji, R.; Nakagawa, M.; Goto, S. Response of striosomal opioid signaling to dopamine depletion in 6-hydroxydopamine-lesioned rat model of Parkinson’s disease: A potential compensatory role. Front. Cell. Neurosci. 2013, 17, 74. [Google Scholar] [CrossRef]
- Giampà, C.; Laurenti, D.; Anzilotti, S.; Bernardi, G.; Menniti, F.S.; Fusco, F.R. Inhibition of the Striatal Specific Phosphodiesterase PDE10A Ameliorates Striatal and Cortical Pathology in R6/2 Mouse Model of Huntington’s Disease. PLoS ONE 2010, 5, e13417. [Google Scholar] [CrossRef]
- Giralt, A.; Saavedra, A.; Carretón, O.; Arumí, H.; Tyebji, S.; Alberch, J.; Pérez-Navarro, E. PDE10 Inhibition Increases GluA1 and CREB Phosphorylation and Improves Spatial and Recognition Memories in a Huntington’s Disease Mouse Model: Pde10 Inhibition Improves Cognition in Huntington’s Disease. Hippocampus 2013, 23, 684–695. [Google Scholar] [CrossRef]
- Beaumont, V.; Zhong, S.; Lin, H.; Xu, W.; Bradaia, A.; Steidl, E.; Gleyzes, M.; Wadel, K.; Buisson, B.; Padovan-Neto, F.E.; et al. Phosphodiesterase 10A Inhibition Improves Cortico-Basal Ganglia Function in Huntington’s Disease Models. Neuron 2016, 92, 1220–1237. [Google Scholar] [CrossRef]
- Roos, R.A.C.; Buruma, O.J.S.; Bruyn, G.W.; Kemp, B.; Velde, E.A. Tiapride in the Treatment of Huntington’s Chorea. Acta Neurol. Scand. 1982, 65, 45–50. [Google Scholar] [CrossRef]
- Deroover, J.; Baro, F.; Bourguignon, R.P.; Smets, P.h. Tiapride versus Placebo: A Double-Blind Comparative Study in the Management of Huntington’s Chorea. Curr. Med. Res. Opin. 1984, 9, 329–338. [Google Scholar] [CrossRef]
- Quinn, N.; Marsden, C.D. A Double Blind Trial of Sulpiride in Huntington’s Disease and Tardive Dyskinesia. J. Neurol. Neurosurg. Psychiatry 1984, 47, 844–847. [Google Scholar] [CrossRef]
- Koller, W.C.; Trimble, J. The Gait Abnormality of Huntington’s Disease. Neurology 1985, 35, 1450. [Google Scholar] [CrossRef]
- Bonuccelli, U.; Ceravolo, R.; Maremmani, C.; Nuti, A.; Rossi, G.; Muratorio, A. Clozapine in Huntington’s Chorea. Neurology 1994, 44, 821. [Google Scholar] [CrossRef]
- Duff, K.; Beglinger, L.J.; O’Rourke, M.E.; Nopoulos, P.; Paulson, H.L.; Paulsen, J.S. Risperidone and the Treatment of Psychiatric, Motor, and Cognitive Symptoms in Huntington’s Disease. Ann. Clin. Psychiatry 2008, 20, 1–3. [Google Scholar] [CrossRef]
- Schultz, J.L.; Kamholz, J.A.; Nopoulos, P.C.; Killoran, A. Comparing Risperidone and Olanzapine to Tetrabenazine for the Management of Chorea in Huntington Disease: An Analysis from the Enroll-HD Database. Mov. Disord. Clin. Pract. 2019, 6, 132–138. [Google Scholar] [CrossRef]
- Charvin, D.; Roze, E.; Perrin, V.; Deyts, C.; Betuing, S.; Pagès, C.; Régulier, E.; Luthi-Carter, R.; Brouillet, E.; Déglon, N.; et al. Haloperidol Protects Striatal Neurons from Dysfunction Induced by Mutated Huntingtin in Vivo. Neurobiol. Dis. 2008, 29, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.-S.; Chen, X.; Liu, J.; Bezprozvanny, I. Dopaminergic Signaling and Striatal Neurodegeneration in Huntington’s Disease. J. Neurosci. 2007, 27, 7899–7910. [Google Scholar] [CrossRef] [PubMed]
- André, V.M.; Cepeda, C.; Fisher, Y.E.; Huynh, M.; Bardakjian, N.; Singh, S.; Yang, X.W.; Levine, M.S. Differential Electrophysiological Changes in Striatal Output Neurons in Huntington’s Disease. J. Neurosci. 2011, 31, 1170–1182. [Google Scholar] [CrossRef] [PubMed]
- Domenici, M.R.; Scattoni, M.L.; Martire, A.; Lastoria, G.; Potenza, R.L.; Borioni, A.; Venerosi, A.; Calamandrei, G.; Popoli, P. Behavioral and Electrophysiological Effects of the Adenosine A2A Receptor Antagonist SCH 58261 in R6/2 Huntington’s Disease Mice. Neurobiol. Dis. 2007, 28, 197–205. [Google Scholar] [CrossRef]
- Li, W.; Silva, H.B.; Real, J.; Wang, Y.M.; Rial, D.; Li, P.; Payen, M.P.; Zhou, Y.; Muller, C.E.; Tomé, A.R.; et al. Inactivation of adenosine A2A receptors reverses working memory deficits at early stages of Huntington’s disease models. Neurobiol. Dis. 2015, 79, 70–80. [Google Scholar] [CrossRef]
- Hervé, D.; Le Moine, C.; Corvol, J.-C.; Belluscio, L.; Ledent, C.; Fienberg, A.A.; Jaber, M.; Studler, J.-M.; Girault, J.-A. Gαolf Levels Are Regulated by Receptor Usage and Control Dopamine and Adenosine Action in the Striatum. J. Neurosci. 2001, 21, 4390–4399. [Google Scholar] [CrossRef]
- Chen, J.Y.; Wang, E.A.; Cepeda, C.; Levine, M.S. Dopamine imbalance in Huntingoton’s disease: A mechanism for the lack of behavioral flexibility. Front. Neurosci. 2013, 7, 114. [Google Scholar] [CrossRef]
- Cepeda, C.; Murphy, K.P.S.; Parent, M.; Levine, M.S. The role of dopamine in Huntington’s disease. Prog. Brain Res. 2014, 211, 235–254. [Google Scholar] [CrossRef] [PubMed]
- Reiner, A.; Deng, Y.P. Disrupted striatal neuron inputs and outputs in Huntington’s disease. CNS Neurosci. Ther. 2018, 24, 250–280. [Google Scholar] [CrossRef] [PubMed]
- Richfield, E.K.; Maguire-Zeiss, K.A.; Vonkeman, H.E.; Voorn, P. Preferential Loss of Preproenkephalin versus Preprotachykinin Neurons from the Striatum of Huntington’s Disease Patients. Ann. Neurol. 1995, 38, 852–861. [Google Scholar] [CrossRef] [PubMed]
- Albin, R.L.; Reiner, A.; Anderson, K.D.; Dure, L.S.; Handelin, B.; Balfour, R.; Whetsell, W.O.; Penney, J.B.; Young, A.B. Preferential Loss of Striato-external Pallidal Projection Neurons in Presymptomatic Huntington’s Disease. Ann. Neurol. 1992, 31, 425–430. [Google Scholar] [CrossRef]
- Reilmann, R. Parkinsonism in Huntington’s Disease. In International Review of Neurobiology; Elsevier: Amsterdam, The Netherlands, 2019; Volume 149, pp. 299–306. ISBN 978-0-12-817730-3. [Google Scholar]
- Diggle, C.P.; Sukoff Rizzo, S.J.; Popiolek, M.; Hinttala, R.; Schülke, J.-P.; Kurian, M.A.; Carr, I.M.; Markham, A.F.; Bonthron, D.T.; Watson, C.; et al. Biallelic Mutations in PDE10A Lead to Loss of Striatal PDE10A and a Hyperkinetic Movement Disorder with Onset in Infancy. Am. J. Hum. Human Genet. 2016, 98, 735–743. [Google Scholar] [CrossRef]
- Langfelder, P.; Cantle, J.P.; Chatzopoulou, D.; Wang, N.; Gao, F.; Al-Ramahi, I.; Lu, X.-H.; Ramos, E.M.; El-Zein, K.; Zhao, Y.; et al. Integrated Genomics and Proteomics Define Huntingtin CAG Length-Dependent Networks in Mice. Nat. Neurosci. 2016, 19, 623–633. [Google Scholar] [CrossRef]
- Delnomdedieu, M.; Tan, Y.; Ogden, A.; Berger, Z.; Reilmann, R. J06 a randomized, double-blind, placebo-controlled phase ii efficacy and safety study of the PDE10A inhibitor PF-02545920 in huntington disease (amaryllis). J. Neurolgy Neurosurg. Psychiatry 2018, 89, A99–A100. [Google Scholar] [CrossRef]
- Goto, S.; Morigaki, R.; Okita, S.; Nagahiro, S.; Kaji, R. Development of a highly sensitive immunohistochemical method to detect neurochemical molecules in formalin-fixed and paraffin-embedded tissues from autopsied human brains. Front. Neuroanat. 2015, 3, 22. [Google Scholar] [CrossRef]
- Falk, T.; Mai, D.; Bensch, R.; Çiçek, Ö.; Abdulkadir, A.; Marrakchi, Y.; Böhm, A.; Deubner, J.; Jäckel, Z.; Seiwald, K.; et al. U-Net: Deep Learning for Cell Counting, Detection, and Morphometry. Nat. Methods 2019, 16, 67–70. [Google Scholar] [CrossRef]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Franklin, K.B.J.; Paxinos, G. The Mouse Brain in Stereotaxic Coordinates, Compact, 3rd ed.; Elsevier Academic Press: Amsterdam, The Netherlands; Heidelberg, Germany, 2008; ISBN 978-0-12-374244-5. [Google Scholar]
- Goldsmith, P.; Affinito, J.; McCue, M.; Tsai, M.; Roepcke, S.; Xie, J.; Gertsik, L.; Macek, T.A. A Randomized Multiple Dose Pharmacokinetic Study of a Novel PDE10A Inhibitor TAK-063 in Subjects with Stable Schizophrenia and Japanese Subjects and Modeling of Exposure Relationships to Adverse Events. Drugs R&D 2017, 17, 631–643. [Google Scholar] [CrossRef]
- DeMartinis, N.; Lopez, R.N.; Pickering, E.H.; Schmidt, C.J.; Gertsik, L.; Walling, D.P.; Ogden, A. A Proof-of-Concept Study Evaluating the Phosphodiesterase 10A Inhibitor PF-02545920 in the Adjunctive Treatment of Suboptimally Controlled Symptoms of Schizophrenia. J. Clin. Psychopharmacol. 2019, 39, 318–328. [Google Scholar] [CrossRef]
- Meyer-Lindenberg, A.; Nielsen, J.; Such, P.; Lemming, O.M.; Zambori, J.; Buller, R.; Der Goltz, C.V. A Double-Blind, Randomized, Placebo-Controlled Proof of Concept Study of the Efficacy and Safety of Lu AF11167 for Persistent Negative Symptoms in People with Schizophrenia. Eur. Neuropsychopharmacol. 2022, 61, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Mukai, Y.; Lupinacci, R.; Marder, S.; Snow-adami, L.; Voss, T.; Smith, S.M.; Egan, M.F. Effects of PDE10A Inhibitor MK-8189 in People with an Acute Episode of Schizophrenia: A Randomized Proof-of-Concept Clinical Trial. Schizophr. Res. 2024, 270, 37–43. [Google Scholar] [CrossRef]
- Zagorska, A.; Partyka, A.; Bucki, A.; Gawalskax, A.; Czopek, A.; Pawlowski, M. Phosphodiesterase 10 Inhibitors-Novel Perspectives for Psychiatric and Neurodegenerative Drug Discovery. Curr. Med. Chem. 2018, 25, 3455–3481. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Dawkins, E.; Lobo, M.C.; Kaar, S.J.; Beck, K. New Drug Treatments for Schizophrenia: A Review of Approaches to Target Circuit Dysfunction. Biol. Psychiatry 2024, 96, 638–650. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morigaki, R.; Yoshida, T.; Fujikawa, J.; Crittenden, J.R.; Graybiel, A.M. Molecular Imbalances Between Striosome and Matrix Compartments Characterize the Pathogenesis and Pathophysiology of Huntington’s Disease Model Mouse. Int. J. Mol. Sci. 2025, 26, 8573. https://doi.org/10.3390/ijms26178573
Morigaki R, Yoshida T, Fujikawa J, Crittenden JR, Graybiel AM. Molecular Imbalances Between Striosome and Matrix Compartments Characterize the Pathogenesis and Pathophysiology of Huntington’s Disease Model Mouse. International Journal of Molecular Sciences. 2025; 26(17):8573. https://doi.org/10.3390/ijms26178573
Chicago/Turabian StyleMorigaki, Ryoma, Tomoko Yoshida, Joji Fujikawa, Jill R. Crittenden, and Ann M. Graybiel. 2025. "Molecular Imbalances Between Striosome and Matrix Compartments Characterize the Pathogenesis and Pathophysiology of Huntington’s Disease Model Mouse" International Journal of Molecular Sciences 26, no. 17: 8573. https://doi.org/10.3390/ijms26178573
APA StyleMorigaki, R., Yoshida, T., Fujikawa, J., Crittenden, J. R., & Graybiel, A. M. (2025). Molecular Imbalances Between Striosome and Matrix Compartments Characterize the Pathogenesis and Pathophysiology of Huntington’s Disease Model Mouse. International Journal of Molecular Sciences, 26(17), 8573. https://doi.org/10.3390/ijms26178573






