Musculoskeletal Complications in COVID-19: Exploring the Role of Key Biomarkers
Abstract
1. Introduction
2. Method
3. COVID-19 and Musculoskeletal Pathology
4. Alteration in Cartilage Biomarkers Post-COVID-19
4.1. Cartilage Oligomeric Matrix Protein (COMP)
4.2. Hyaluronic Acid (HA)
5. Alterations in Bone and Collagen Biomarkers in Post-COVID-19
5.1. PINP (PIIINP)
5.2. Serum Total Alkaline Phosphatase (ALP)
5.3. Osteocalcin
5.4. MMP-3 and MMP-9
5.5. Osteopontin
6. Alteration in Muscle Biomarkers Post-COVID-19
6.1. Myostatin
6.2. IGF-1
6.3. Follistatin
6.4. Creatine Kinase
7. Discussion
8. Potential Translational Implications of Summarize Biomarkers in COVID-19 Infection
9. Impact of COVID-19 Vaccination on MSK
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Muralidar, S.; Ambi, S.V.; Sekaran, S.; Krishnan, U.M. The emergence of COVID-19 as a global pandemic: Understanding the epidemiology, immune response and potential therapeutic targets of SARS-CoV-2. Biochimie 2020, 179, 85–100. [Google Scholar] [CrossRef]
- Khan, M.; Adil, S.F.; Alkhathlan, H.Z.; Tahir, M.N.; Saif, S.; Khan, M.; Khan, S.T. COVID-19: A Global Challenge with Old History, Epidemiology and Progress So Far. Molecules 2020, 26, 39. [Google Scholar] [CrossRef] [PubMed]
- Peters, D.J. Community Susceptibility and Resiliency to COVID-19 Across the Rural-Urban Continuum in the United States. J. Rural. Health 2020, 36, 446–456. [Google Scholar] [CrossRef] [PubMed]
- Perrotta, F.; Corbi, G.; Mazzeo, G.; Boccia, M.; Aronne, L.; D’Agnano, V.; Komici, K.; Mazzarella, G.; Parrella, R.; Bianco, A. COVID-19 and the elderly: Insights into pathogenesis and clinical decision-making. Aging Clin. Exp. Res. 2020, 32, 1599–1608. [Google Scholar] [CrossRef]
- Fejer, R.; Ruhe, A. What is the prevalence of musculoskeletal problems in the elderly population in developed countries? A systematic critical literature review. Chiropr. Man. Therap. 2012, 20, 31. [Google Scholar] [CrossRef]
- Nguyen, A.; Lee, P.; Rodriguez, E.K.; Chahal, K.; Freedman, B.R.; Nazarian, A. Addressing the growing burden of musculoskeletal diseases in the ageing US population: Challenges and innovations. Lancet Healthy Longev. 2025, 6, 100707. [Google Scholar] [CrossRef]
- Brosnahan, S.B.; Jonkman, A.H.; Kugler, M.C.; Munger, J.S.; Kaufman, D.A. COVID-19 and Respiratory System Disorders: Current Knowledge, Future Clinical and Translational Research Questions. Arterioscler. Thromb. Vasc. Biol. 2020, 40, 2586–2597. [Google Scholar] [CrossRef]
- Kapoor, M.C. Respiratory and cardiovascular effects of COVID-19 infection and their management. J. Anaesthesiol. Clin. Pharmacol. 2020, 36, S21–S28. [Google Scholar] [CrossRef]
- Kaur, S.P.; Gupta, V. COVID-19 Vaccine: A comprehensive status report. Virus Res. 2020, 288, 198114. [Google Scholar] [CrossRef]
- Samrat, S.K.; Tharappel, A.M.; Li, Z.; Li, H. Prospect of SARS-CoV-2 spike protein: Potential role in vaccine and therapeutic development. Virus Res. 2020, 288, 198141. [Google Scholar] [CrossRef]
- Baroni, C.; Potito, J.; Perticone, M.E.; Orausclio, P.; Luna, C.M. How Does Long-COVID Impact Prognosis and the Long-Term Sequelae? Viruses 2023, 15, 1173. [Google Scholar] [CrossRef]
- Disser, N.P.; De Micheli, A.J.; Schonk, M.M.; Konnaris, M.A.; Piacentini, A.N.; Edon, D.L.; Toresdahl, B.G.; Rodeo, S.A.; Casey, E.K.; Mendias, C.L. Musculoskeletal Consequences of COVID-19. J. Bone Joint Surg. Am. 2020, 102, 1197–1204. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Li, Z.; Shen, Y.; Qian, S.; Tang, M.; He, J.; Lu, H.; Zhang, N. Long-term effects of COVID-19 infection on bone mineral density. J. Glob. Health 2024, 14, 05029. [Google Scholar] [CrossRef] [PubMed]
- Tuzun, S.; Keles, A.; Okutan, D.; Yildiran, T.; Palamar, D. Assessment of musculoskeletal pain, fatigue and grip strength in hospitalized patients with COVID-19. Eur. J. Phys. Rehabil. Med. 2021, 57, 653–662. [Google Scholar] [CrossRef] [PubMed]
- Berktaş, B.M.; Gökçek, A.; Hoca, N.T.; Koyuncu, A. COVID-19 illness and treatment decrease bone mineral density of surviving hospitalized patients. Eur. Rev. Med. Pharmacol. Sci. 2022, 26, 3046–3056. [Google Scholar] [CrossRef]
- Mao, S.S.; Li, D.; Syed, Y.S.; Gao, Y.; Luo, Y.; Flores, F.; Child, J.; Cervantes, M.; Kalantar-Zadeh, K.; Budoff, M.J. Thoracic Quantitative Computed Tomography (QCT) Can Sensitively Monitor Bone Mineral Metabolism: Comparison of Thoracic QCT vs Lumbar QCT and Dual-energy X-ray Absorptiometry in Detection of Age-relative Change in Bone Mineral Density. Acad. Radiol. 2017, 24, 1582–1587. [Google Scholar] [CrossRef]
- Creecy, A.; Awosanya, O.D.; Harris, A.; Qiao, X.; Ozanne, M.; Toepp, A.J.; Kacena, M.A.; McCune, T. COVID-19 and Bone Loss: A Review of Risk Factors, Mechanisms, and Future Directions. Curr. Osteoporos. Rep. 2024, 22, 122–134. [Google Scholar] [CrossRef]
- Krenytska, D.; Strubchevska, K.; Kozyk, M.; Vovk, T.; Halenova, T.; Kot, L.; Raksha, N.; Savchuk, O.; Falalyeyeva, T.; Tsyryuk, O.; et al. Circulating levels of inflammatory cytokines and angiogenesis-related growth factors in patients with osteoarthritis after COVID-19. Front. Med. 2023, 10, 1168487. [Google Scholar] [CrossRef]
- Gobbi, M.; Bezzoli, E.; Ismelli, F.; Trotti, G.; Cortellezzi, S.; Meneguzzo, F.; Arreghini, M.; Seitanidis, I.; Brunani, A.; Aspesi, V.; et al. Skeletal Muscle Mass, Sarcopenia and Rehabilitation Outcomes in Post-Acute COVID-19 Patients. J. Clin. Med. 2021, 10, 5623. [Google Scholar] [CrossRef]
- Piotrowicz, K.; Gąsowski, J.; Michel, J.P.; Veronese, N. Post-COVID-19 acute sarcopenia: Physiopathology and management. Aging Clin. Exp. Res. 2021, 33, 2887–2898. [Google Scholar] [CrossRef]
- Mi, B.; Xiong, Y.; Zhang, C.; Zhou, W.; Chen, L.; Cao, F.; Chen, F.; Geng, Z.; Panayi, A.C.; Sun, Y.; et al. SARS-CoV-2-induced Overexpression of miR-4485 Suppresses Osteogenic Differentiation and Impairs Fracture Healing. Int. J. Biol. Sci. 2021, 17, 1277–1288. [Google Scholar] [CrossRef]
- Griffith, J.F. Musculoskeletal complications of severe acute respiratory syndrome. Semin. Musculoskelet. Radiol. 2011, 15, 554–560. [Google Scholar] [CrossRef]
- Ciaffi, J.; Vanni, E.; Mancarella, L.; Brusi, V.; Lisi, L.; Pignatti, F.; Naldi, S.; Assirelli, E.; Neri, S.; Reta, M.; et al. Post-Acute COVID-19 Joint Pain and New Onset of Rheumatic Musculoskeletal Diseases: A Systematic Review. Diagnostics 2023, 13, 1850. [Google Scholar] [CrossRef]
- Tamariz, L.; Bast, E.; Abad, M.; Klimas, N.; Caralis, P.; Palacio, A. Post COVID-19 joint pain: Preliminary report of the relationship with antinuclear antibodies and inflammation. J. Med. Virol. 2022, 94, 3479–3481. [Google Scholar] [CrossRef] [PubMed]
- Suchowiecki, K.; Reid, S.P.; Simon, G.L.; Firestein, G.S.; Chang, A. Persistent Joint Pain Following Arthropod Virus Infections. Curr. Rheumatol. Rep. 2021, 23, 26. [Google Scholar] [CrossRef]
- Rousseau, J.; Garnero, P. Biological markers in osteoarthritis. Bone 2012, 51, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Papaneophytou, C.; Alabajos-Cea, A.; Viosca-Herrero, E.; Calvis, C.; Costa, M.; Christodoulides, A.E.; Kroushovski, A.; Lapithis, A.; Lapithi, V.M.; Papayiannis, I.; et al. Associations between serum biomarkers of cartilage metabolism and serum hyaluronic acid, with risk factors, pain categories, and disease severity in knee osteoarthritis: A pilot study. BMC Musculoskelet. Disord. 2022, 23, 195. [Google Scholar] [CrossRef]
- Wisłowska, M.; Jabłońska, B. Serum cartilage oligomeric matrix protein (COMP) in rheumatoid arthritis and knee osteoarthritis. Clin. Rheumatol. 2005, 24, 278–284. [Google Scholar] [CrossRef]
- Mündermann, A.; Dyrby, C.O.; Andriacchi, T.P.; King, K.B. Serum concentration of cartilage oligomeric matrix protein (COMP) is sensitive to physiological cyclic loading in healthy adults. Osteoarthr. Cartil. 2005, 13, 34–38. [Google Scholar] [CrossRef]
- Hoch, J.M.; Mattacola, C.G.; Medina McKeon, J.M.; Howard, J.S.; Lattermann, C. Serum cartilage oligomeric matrix protein (sCOMP) is elevated in patients with knee osteoarthritis: A systematic review and meta-analysis. Osteoarthr. Cartil. 2011, 19, 1396–1404. [Google Scholar] [CrossRef]
- Kluzek, S.; Bay-Jensen, A.C.; Judge, A.; Karsdal, M.A.; Shorthose, M.; Spector, T.; Hart, D.; Newton, J.L.; Arden, N.K. Serum cartilage oligomeric matrix protein and development of radiographic and painful knee osteoarthritis. A community-based cohort of middle-aged women. Biomarkers 2015, 20, 557–564. [Google Scholar] [CrossRef]
- Verma, P.; Dalal, K. Serum cartilage oligomeric matrix protein (COMP) in knee osteoarthritis: A novel diagnostic and prognostic biomarker. J. Orthop. Res. 2013, 31, 999–1006. [Google Scholar] [CrossRef] [PubMed]
- Huet, A.; Tugarov, Y.; Dvorshchenko, K.; Grebinyk, D.; Savchuk, O.; Korotkyi, O.; Ostapchenko, L. TGFB1, FOXO1, and COMP Genes Expression in Blood of Patients with Osteoarthritis after SARS-CoV2 Infection. Cytol. Genet. 2023, 57, 128–133. [Google Scholar] [CrossRef] [PubMed]
- Byeon, S.K.; Madugundu, A.K.; Garapati, K.; Ramarajan, M.G.; Saraswat, M.; Kumar, M.P.; Hughes, T.; Shah, R.; Patnaik, M.M.; Chia, N.; et al. Development of a multiomics model for identification of predictive biomarkers for COVID-19 severity: A retrospective cohort study. Lancet Digit. Health 2022, 4, e632–e645. [Google Scholar] [CrossRef] [PubMed]
- Kong, K.; Chang, Y.; Qiao, H.; Zhao, C.; Chen, X.; Rong, K.; Zhang, P.; Jin, M.; Zhang, J.; Li, H.; et al. Paxlovid accelerates cartilage degeneration and senescence through activating endoplasmic reticulum stress and interfering redox homeostasis. J. Transl. Med. 2022, 20, 549. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- Singh, S.; Kumar, D.; Sharma, N.R. Role of hyaluronic Acid in early diagnosis of knee osteoarthritis. J. Clin. Diagn. Res. 2014, 8, Lc04–Lc07. [Google Scholar] [CrossRef]
- Turan, Y.; Bal, S.; Gurgan, A.; Topac, H.; Koseoglu, M. Serum hyaluronan levels in patients with knee osteoarthritis. Clin. Rheumatol. 2007, 26, 1293–1298. [Google Scholar] [CrossRef]
- Queisser, K.A.; Mellema, R.A.; Middleton, E.A.; Portier, I.; Manne, B.K.; Denorme, F.; Beswick, E.J.; Rondina, M.T.; Campbell, R.A.; Petrey, A.C. COVID-19 generates hyaluronan fragments that directly induce endothelial barrier dysfunction. JCI Insight 2021, 6, e147472. [Google Scholar] [CrossRef]
- Zha, D.; Fu, M.; Qian, Y. Vascular Endothelial Glycocalyx Damage and Potential Targeted Therapy in COVID-19. Cells 2022, 11, 1972. [Google Scholar] [CrossRef]
- Ontong, P.; Prachayasittikul, V. Unraveled roles of hyaluronan in severe COVID-19. Excli J. 2021, 20, 117–125. [Google Scholar] [CrossRef]
- Yamaoka-Tojo, M. Vascular Endothelial Glycocalyx Damage in COVID-19. Int. J. Mol. Sci. 2020, 21, 9712. [Google Scholar] [CrossRef]
- Li, Y.; Cui, X.; Zhu, N.; Lin, Y.; Li, X. Elevated hyaluronic acid levels in severe SARS-CoV-2 infection in the post-COVID-19 era. Front. Cell Infect. Microbiol. 2024, 14, 1338508. [Google Scholar] [CrossRef]
- Beltrán-Camacho, L.; Eslava-Alcón, S.; Rojas-Torres, M.; Sánchez-Morillo, D.; Martinez-Nicolás, M.P.; Martín-Bermejo, V.; de la Torre, I.G.; Berrocoso, E.; Moreno, J.A.; Moreno-Luna, R.; et al. The serum of COVID-19 asymptomatic patients up-regulates proteins related to endothelial dysfunction and viral response in circulating angiogenic cells ex-vivo. Mol. Med. 2022, 28, 40. [Google Scholar] [CrossRef] [PubMed]
- Breisnes, H.W.; Leeming, D.J.; Karsdal, M.A.; Burke, H.; Freeman, A.; Wilkinson, T.; Fazleen, A.; Bülow Sand, J.M. Biomarkers of tissue remodelling are elevated in serum of COVID-19 patients who develop interstitial lung disease—An exploratory biomarker study. BMC Pulm. Med. 2024, 24, 331. [Google Scholar] [CrossRef] [PubMed]
- Moretti, A.; Liguori, S.; Paoletta, M.; Migliaccio, S.; Toro, G.; Gimigliano, F.; Iolascon, G. Bone fragility during the COVID-19 pandemic: The role of macro- and micronutrients. Ther. Adv. Musculoskelet. Dis. 2023, 15, 1759720x231158200. [Google Scholar] [CrossRef] [PubMed]
- Gillett, M.J.; Vasikaran, S.D.; Inderjeeth, C.A. The Role of PINP in Diagnosis and Management of Metabolic Bone Disease. Clin. Biochem. Rev. 2021, 42, 3–10. [Google Scholar] [CrossRef]
- Chavassieux, P.; Portero-Muzy, N.; Roux, J.P.; Garnero, P.; Chapurlat, R. Are Biochemical Markers of Bone Turnover Representative of Bone Histomorphometry in 370 Postmenopausal Women? J. Clin. Endocrinol. Metab. 2015, 100, 4662–4668. [Google Scholar] [CrossRef]
- Alazawi, I.; Mohammed, N.; Jassim, N. Measuring of Specific Bone Alkaline phosphatase (BAP) Bone Remodeling biomarker for Post-COVID Iraqi Patient. J. Fac. Med. Baghdad 2023, 64, 208–213. [Google Scholar] [CrossRef]
- Kerschan-Schindl, K.; Dovjak, P.; Butylina, M.; Rainer, A.; Mayr, B.; Röggla, V.; Haslacher, H.; Weber, M.; Jordakieva, G.; Pietschmann, P. Moderate COVID-19 Disease Is Associated With Reduced Bone Turnover. J. Bone Miner. Res. 2023, 38, 943–950. [Google Scholar] [CrossRef]
- Li, T.; Wang, L.; Wang, H.; Gao, Y.; Hu, X.; Li, X.; Zhang, S.; Xu, Y.; Wei, W. Characteristics of laboratory indexes in COVID-19 patients with non-severe symptoms in Hefei City, China: Diagnostic value in organ injuries. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2447–2455. [Google Scholar] [CrossRef]
- DAvila-Mesquita, C.; Couto, A.E.S.; Campos, L.C.B.; Vasconcelos, T.F.; Michelon-Barbosa, J.; Corsi, C.A.C.; Mestriner, F.; Petroski-Moraes, B.C.; Garbellini-Diab, M.J.; Couto, D.M.S.; et al. MMP-2 and MMP-9 levels in plasma are altered and associated with mortality in COVID-19 patients. Biomed. Pharmacother. 2021, 142, 112067. [Google Scholar] [CrossRef] [PubMed]
- Gelzo, M.; Cacciapuoti, S.; Pinchera, B.; De Rosa, A.; Cernera, G.; Scialò, F.; Comegna, M.; Mormile, M.; Fabbrocini, G.; Parrella, R.; et al. Matrix metalloproteinases (MMP) 3 and 9 as biomarkers of severity in COVID-19 patients. Sci. Rep. 2022, 12, 1212. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Su, M.; Shen, G.; Hu, Y.; Yi, F.; Zeng, Z.; Zhu, P.; Yang, G.; Zhou, H.; Li, Q.; et al. Matrix metalloproteinase 3 as a valuable marker for patients with COVID-19. J. Med. Virol. 2021, 93, 528–532. [Google Scholar] [CrossRef]
- Hayek, S.S.; Roderburg, C.; Blakely, P.; Launius, C.; Eugen-Olsen, J.; Tacke, F.; Ktena, S.; Keitel, V.; Luedde, M.; Giamarellos-Bourboulis, E.J.; et al. Circulating Osteopontin Levels and Outcomes in Patients Hospitalized for COVID-19. J. Clin. Med. 2021, 10, 3907. [Google Scholar] [CrossRef]
- Tonello, S.; D’Onghia, D.; Apostolo, D.; Matino, E.; Costanzo, M.; Casciaro, G.F.; Croce, A.; Rizzi, E.; Zecca, E.; Pedrinelli, A.R.; et al. Baseline Plasma Osteopontin Protein Elevation Predicts Adverse Outcomes in Hospitalized COVID-19 Patients. Viruses 2023, 15, 630. [Google Scholar] [CrossRef]
- Koivula, M.K.; Risteli, L.; Risteli, J. Measurement of aminoterminal propeptide of type I procollagen (PINP) in serum. Clin. Biochem. 2012, 45, 920–927. [Google Scholar] [CrossRef]
- Breisnes, H.W.; Nielsen, S.H.; Bager, C.L.; Leeming, D.J.; Cahn, T.; O’Shea, C.; Siderfin, J.; Hove, K.; Layton, M.; Bay-Jensen, A.C.; et al. Serum collagen biomarkers are related to fatal outcome in patients with severe COVID-19 in a Phase II clinical trial. Mech. Lung Inj. Repair 2023. [Google Scholar]
- Yang, T.; Liu, L.L.; Wu, X.H.; Xue, J.G.; He, C.Y. Serum hyaluronic acid and procollagen III, N-terminal propeptide levels are highly associated with disease severity and predict the progression of COVID-19. Front. Cell Infect. Microbiol. 2023, 13, 1249038. [Google Scholar] [CrossRef]
- Yu, D.; Yin, G.; Lei, J.; Gong, Y.; Zheng, L.; He, D.; Lei, L.; Sun, L. The correlation between serum levels of laminin, type IV collagen, type III procollagen N-terminal peptide and hyaluronic acid with the progression of post-COVID-19 pulmonary fibrosis. Front. Cell Dev. Biol. 2024, 12, 1382244. [Google Scholar] [CrossRef]
- Sharma, U.; Pal, D.; Prasad, R. Alkaline phosphatase: An overview. Indian. J. Clin. Biochem. 2014, 29, 269–278. [Google Scholar] [CrossRef]
- Farley, J.R.; Chesnut, C.H., 3rd; Baylink, D.J. Improved method for quantitative determination in serum of alkaline phosphatase of skeletal origin. Clin. Chem. 1981, 27, 2002–2007. [Google Scholar] [CrossRef]
- Vimalraj, S. Alkaline phosphatase: Structure, expression and its function in bone mineralization. Gene 2020, 754, 144855. [Google Scholar] [CrossRef] [PubMed]
- Romagnoli, E.; Minisola, G.; Carnevale, V.; Scillitani, A.; Frusciante, V.; Aliberti, G.; Minisola, S. Assessment of serum total and bone alkaline phosphatase measurement in clinical practice. Clin. Chem. Lab. Med. 1998, 36, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Negrão, M.R.; Martins, M.J.; Ramos, E.; Barros, H.; Hipólito-Reis, C.; Azevedo, I. Human serum alcaline phosphatase and ageing. Acta Med. Port. 2003, 16, 395–400. [Google Scholar] [PubMed]
- Ralston, S.H.; Corral-Gudino, L.; Cooper, C.; Francis, R.M.; Fraser, W.D.; Gennari, L.; Guañabens, N.; Javaid, M.K.; Layfield, R.; O’Neill, T.W.; et al. Diagnosis and Management of Paget’s Disease of Bone in Adults: A Clinical Guideline. J. Bone Miner. Res. 2019, 34, 579–604. [Google Scholar] [CrossRef]
- Siregar, M.A.G.; Lathifah, D.S.; Anvi, N.M. Pemanfaatan Alkaline Phosphatase sebagai Novel Biomarker Deteksi dalam Diagnosis Osteoporosis selama Pandemi COVID-19. Scr. SCORE Sci. Med. J. 2023. [Google Scholar] [CrossRef]
- Qiao, W.; Lau, H.E.; Xie, H.; Poon, V.K.; Chan, C.C.; Chu, H.; Yuan, S.; Yuen, T.T.; Chik, K.K.; Tsang, J.O.; et al. SARS-CoV-2 infection induces inflammatory bone loss in golden Syrian hamsters. Nat. Commun. 2022, 13, 2539. [Google Scholar] [CrossRef]
- Faruqui, S.; Okoli, F.C.; Olsen, S.K.; Feldman, D.M.; Kalia, H.S.; Park, J.S.; Stanca, C.M.; Figueroa Diaz, V.; Yuan, S.; Dagher, N.N.; et al. Cholangiopathy After Severe COVID-19: Clinical Features and Prognostic Implications. Am. J. Gastroenterol. 2021, 116, 1414–1425. [Google Scholar] [CrossRef]
- Moghaddam, R.R.; Khorasanchi, Z.; Noor, A.R.; Moghadam, M.S.F.; Esfahani, A.J.; Alyakobi, A.K.M.; Alboresha, M.L.; Sharifan, P.; Bahari, A.; Rezvani, R.; et al. High-dose vitamin D supplementation is related to an improvement in serum alkaline phosphatase in COVID-19 patients; a randomized double-blinded clinical trial. J. Health Popul. Nutr. 2023, 42, 71. [Google Scholar] [CrossRef]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocalcin: Skeletal and extra-skeletal effects. J. Cell Physiol. 2013, 228, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Rathore, B.; Singh, M.; Kumar, V.C.S.; Misra, A. Osteocalcin: An emerging biomarker for bone turnover. Int. J. Res. Med. Sci. 2016, 4, 3670–3674. [Google Scholar] [CrossRef]
- Smith, C.; Lin, X.; Parker, L.; Yeap, B.B.; Hayes, A.; Levinger, I. The role of bone in energy metabolism: A focus on osteocalcin. Bone 2024, 188, 117238. [Google Scholar] [CrossRef] [PubMed]
- Booth, S.L.; Centi, A.; Smith, S.R.; Gundberg, C. The role of osteocalcin in human glucose metabolism: Marker or mediator? Nat. Rev. Endocrinol. 2013, 9, 43–55. [Google Scholar] [CrossRef]
- Zoch, M.L.; Clemens, T.L.; Riddle, R.C. New insights into the biology of osteocalcin. Bone 2016, 82, 42–49. [Google Scholar] [CrossRef]
- Martiniakova, M.; Biro, R.; Kovacova, V.; Babikova, M.; Zemanova, N.; Mondockova, V.; Omelka, R. Current knowledge of bone-derived factor osteocalcin: Its role in the management and treatment of diabetes mellitus, osteoporosis, osteopetrosis and inflammatory joint diseases. J. Mol. Med. 2024, 102, 435–452. [Google Scholar] [CrossRef]
- Komori, T. Functions of Osteocalcin in Bone, Pancreas, Testis, and Muscle. Int. J. Mol. Sci. 2020, 21, 7513. [Google Scholar] [CrossRef]
- Arrieta, F.; Martinez-Vaello, V.; Bengoa, N.; Rosillo, M.; de Pablo, A.; Voguel, C.; Pintor, R.; Belanger-Quintana, A.; Mateo-Lobo, R.; Candela, A.; et al. Stress Hyperglycemia and Osteocalcin in COVID-19 Critically Ill Patients on Artificial Nutrition. Nutrients 2021, 13, 3010. [Google Scholar] [CrossRef]
- Burrage, P.S.; Brinckerhoff, C.E. Molecular targets in osteoarthritis: Metalloproteinases and their inhibitors. Curr. Drug Targets 2007, 8, 293–303. [Google Scholar] [CrossRef]
- Wan, J.; Zhang, G.; Li, X.; Qiu, X.; Ouyang, J.; Dai, J.; Min, S. Matrix Metalloproteinase 3: A Promoting and Destabilizing Factor in the Pathogenesis of Disease and Cell Differentiation. Front. Physiol. 2021, 12, 663978. [Google Scholar] [CrossRef]
- Tuncer, T.; Kaya, A.; Gulkesen, A.; Kal, G.A.; Kaman, D.; Akgol, G. Matrix metalloproteinase-3 levels in relation to disease activity and radiological progression in rheumatoid arthritis. Adv. Clin. Exp. Med. 2019, 28, 665–670. [Google Scholar] [CrossRef]
- Singh, S.; Jindal, D.; Khanna, R. Can serum MMP-3 diagnose early knee osteoarthritis? J. Orthop. 2023, 38, 42–46. [Google Scholar] [CrossRef]
- Smith, G.N., Jr. The role of collagenolytic matrix metalloproteinases in the loss of articular cartilage in osteoarthritis. Front. Biosci. 2006, 11, 3081–3095. [Google Scholar] [CrossRef] [PubMed]
- Slovacek, H.; Khanna, R.; Poredos, P.; Poredos, P.; Jezovnik, M.; Hoppensteadt, D.; Fareed, J.; Hopkinson, W. Interrelationship of MMP-9, Proteoglycan-4, and Inflammation in Osteoarthritis Patients Undergoing Total Hip Arthroplasty. Clin. Appl. Thromb. Hemost. 2021, 27, 1076029621995569. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Li, X.; Zhuang, J.; Han, J.; Luo, G.; Yang, F.; Sun, Y.; Liao, P.; Han, Y.; He, Y.; et al. Blocking Matrix Metalloproteinase-9 Abrogates Collagen-Induced Arthritis via Inhibiting Dendritic Cell Migration. J. Immunol. 2018, 201, 3514–3523. [Google Scholar] [CrossRef] [PubMed]
- Savic, G.; Stevanovic, I.; Mihajlovic, D.; Jurisevic, M.; Gajovic, N.; Jovanovic, I.; Ninkovic, M. MMP-9/BDNF ratio predicts more severe COVID-19 outcomes. Int. J. Med. Sci. 2022, 19, 1903–1911. [Google Scholar] [CrossRef]
- Ramezani, S.; Ezzatifar, F.; Hojjatipour, T.; Hemmatzadeh, M.; Shabgah, A.G.; Navashenaq, J.G.; Aslani, S.; Shomali, N.; Arabi, M.; Babaie, F.; et al. Association of the matrix metalloproteinases (MMPs) family gene polymorphisms and the risk of coronavirus disease 2019 (COVID-19); implications of contribution for development of neurological symptoms in the COVID-19 patients. Mol. Biol. Rep. 2023, 50, 173–183. [Google Scholar] [CrossRef]
- Cavalcante, G.L.; Bonifacio, L.P.; Sanches-Lopes, J.M.; Puga, F.G.; de Carvalho, F.S.; Bellissimo-Rodrigues, F.; Tanus-Santos, J.E. Matrix metalloproteinases are associated with severity of disease among COVID-19 patients: A possible pharmacological target. Basic. Clin. Pharmacol. Toxicol. 2024, 134, 727–736. [Google Scholar] [CrossRef]
- Si, J.; Wang, C.; Zhang, D.; Wang, B.; Zhou, Y. Osteopontin in Bone Metabolism and Bone Diseases. Med. Sci. Monit. 2020, 26, e919159. [Google Scholar] [CrossRef]
- Honsawek, S.; Tanavalee, A.; Sakdinakiattikoon, M.; Chayanupatkul, M.; Yuktanandana, P. Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin. Biochem. 2009, 42, 808–812. [Google Scholar] [CrossRef]
- Cheng, C.; Gao, S.; Lei, G. Association of osteopontin with osteoarthritis. Rheumatol. Int. 2014, 34, 1627–1631. [Google Scholar] [CrossRef] [PubMed]
- Lund, S.A.; Giachelli, C.M.; Scatena, M. The role of osteopontin in inflammatory processes. J. Cell Commun. Signal 2009, 3, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Martín-Márquez, B.T.; Sandoval-García, F.; Corona-Meraz, F.I.; Martínez-García, E.A.; Sánchez-Hernández, P.E.; Salazar-Páramo, M.; Fletes-Rayas, A.L.; González-Inostroz, D.; Vazquez-Del Mercado, M. Osteopontin: A Bone-Derived Protein Involved in Rheumatoid Arthritis and Osteoarthritis Immunopathology. Biomolecules 2023, 13, 502. [Google Scholar] [CrossRef] [PubMed]
- Matsui, Y.; Iwasaki, N.; Kon, S.; Takahashi, D.; Morimoto, J.; Matsui, Y.; Denhardt, D.T.; Rittling, S.; Minami, A.; Uede, T. Accelerated development of aging-associated and instability-induced osteoarthritis in osteopontin-deficient mice. Arthritis Rheum. 2009, 60, 2362–2371. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, L.; Zhao, L.; Gao, S.; Han, R.; Su, D.; Lei, G. Phosphorylation of osteopontin in osteoarthritis degenerative cartilage and its effect on matrix metalloprotease 13. Rheumatol. Int. 2013, 33, 1313–1319. [Google Scholar] [CrossRef]
- Lin, C.; Chen, Z.; Guo, D.; Zhou, L.; Lin, S.; Li, C.; Li, S.; Wang, X.; Lin, B.; Ding, Y. Increased expression of osteopontin Osteopontin in subchondral bone promotes bone turnover and remodeling, and accelerates the progression of OA in a mouse model. Aging 2022, 14, 253–271. [Google Scholar] [CrossRef]
- Varım, C.; Demirci, T.; Cengiz, H.; Hacıbekiroğlu, İ.; Tuncer, F.B.; Çokluk, E.; Toptan, H.; Karabay, O.; Yıldırım, İ. Relationship between serum osteopontin levels and the severity of COVID-19 infection. Wien. Klin. Wochenschr. 2021, 133, 298–302. [Google Scholar] [CrossRef]
- Reisner, A.; Blackwell, L.S.; Sayeed, I.; Myers, H.E.; Wali, B.; Heilman, S.; Figueroa, J.; Lu, A.; Hussaini, L.; Anderson, E.J.; et al. Osteopontin as a biomarker for COVID-19 severity and multisystem inflammatory syndrome in children: A pilot study. Exp. Biol. Med. 2022, 247, 145–151. [Google Scholar] [CrossRef]
- Bai, G.; Furushima, D.; Niki, T.; Matsuba, T.; Maeda, Y.; Takahashi, A.; Hattori, T.; Ashino, Y. High Levels of the Cleaved Form of Galectin-9 and Osteopontin in the Plasma Are Associated with Inflammatory Markers That Reflect the Severity of COVID-19 Pneumonia. Int. J. Mol. Sci. 2021, 22, 4978. [Google Scholar] [CrossRef]
- Yoshida, T.; Delafontaine, P. Mechanisms of IGF-1-Mediated Regulation of Skeletal Muscle Hypertrophy and Atrophy. Cells 2020, 9, 1970. [Google Scholar] [CrossRef]
- Sharma, S.; Patil, A.S. Myostatin’s marvels: From muscle regulator to diverse implications in health and disease. Cell Biochem. Funct. 2024, 42, e4106. [Google Scholar] [CrossRef] [PubMed]
- Mińko, A.; Turoń-Skrzypińska, A.; Rył, A.; Mańkowska, K.; Cymbaluk-Płoska, A.; Rotter, I. The Significance of Selected Myokines in Predicting the Length of Rehabilitation of Patients after COVID-19 Infection. Biomedicines 2024, 12, 836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Baczek, J.; Silkiewicz, M.; Wojszel, Z.B. Myostatin as a Biomarker of Muscle Wasting and other Pathologies-State of the Art and Knowledge Gaps. Nutrients 2020, 12, 2401. [Google Scholar] [CrossRef] [PubMed]
- Visconti, N.R.G.; Rocha, N.N.; Nascimento, G.S.; Menário, C.V.B.; Silva, J.D.; Martins, C.M.; Caruso-Neves, C.; Cruz, F.F.; Rocco, P.R.M.; Mello, F.C.Q.; et al. Elevated adipokines and myokines are associated with fatigue in long COVID patients. Front. Med. 2025, 12, 1547886. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Feizollahi, P.; Matin, S.; Roghani, S.A.; Mostafaei, S.; Safarzadeh, E.; Taghadosi, M. Evaluation serum levels of Insulin Growth Factor-1 (IGF-1) and its association with clinical parameters in severe COVID-19. Inflammopharmacology 2022, 30, 199–205. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ilias, I.; Diamantopoulos, A.; Botoula, E.; Athanasiou, N.; Zacharis, A.; Tsipilis, S.; Jahaj, E.; Vassiliou, A.G.; Vassiliadi, D.A.; Kotanidou, A.; et al. COVID-19 and Growth Hormone/Insulin-Like Growth Factor 1: Study in Critically and Non-Critically Ill Patients. Front. Endocrinol. 2021, 12, 644055. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhao, M.; Liu, Z.; Shao, F.; Zhou, W.; Chen, Z.; Xia, P.; Wang, S.; Yang, P. Communication Pattern Changes Along With Declined IGF1 of Immune Cells in COVID-19 Patients During Disease Progression. Front. Immunol. 2022, 12, 729990. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Synolaki, E.; Papadopoulos, V.; Divolis, G.; Tsahouridou, O.; Gavriilidis, E.; Loli, G.; Gavriil, A.; Tsigalou, C.; Tziolos, N.R.; Sertaridou, E.; et al. The Activin/Follistatin Axis Is Severely Deregulated in COVID-19 and Independently Associated With In-Hospital Mortality. J. Infect. Dis. 2021, 223, 1544–1554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Roh, J.D.; Kitchen, R.R.; Guseh, J.S.; McNeill, J.N.; Aid, M.; Martinot, A.J.; Yu, A.; Platt, C.; Rhee, J.; Weber, B.; et al. Plasma Proteomics of COVID-19-Associated Cardiovascular Complications: Implications for Pathophysiology and Therapeutics. JACC Basic. Transl. Sci. 2022, 7, 425–441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Brewster, L.M. Creatine kinase, energy reserve, and hypertension: From bench to bedside. Ann. Transl. Med. 2018, 6, 292. [Google Scholar] [CrossRef]
- Orsucci, D.; Trezzi, M.; Anichini, R.; Blanc, P.; Barontini, L.; Biagini, C.; Capitanini, A.; Comeglio, M.; Corsini, P.; Gemignani, F.; et al. Increased Creatine Kinase May Predict A Worse COVID-19 Outcome. J. Clin. Med. 2021, 10, 1734. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Friedman, S.A.; Charmchi, Z.; Silver, M.; Jacoby, N.; Perk, J.; Anziska, Y. Skeletal Muscle Manifestations and Creatine Kinase in COVID-19. Neurohospitalist 2022, 12, 597–606. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Laches, R.E.; Tillotson, S.; Kaufman, E.; As Sayaideh, M. Extremely Elevated Creatine Kinase in COVID-19-Associated Rhabdomyolysis. Cureus 2023, 15, e45448. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Khoja, O.; Silva Passadouro, B.; Mulvey, M.; Delis, I.; Astill, S.; Tan, A.L.; Sivan, M. Clinical Characteristics and Mechanisms of Musculoskeletal Pain in Long COVID. J. Pain Res. 2022, 15, 1729–1748. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ho, X.D.; Nguyen, H.G.; Trinh, L.H.; Reimann, E.; Prans, E.; Kõks, G.; Maasalu, K.; Le, V.Q.; Nguyen, V.H.; Le, N.T.N.; et al. Analysis of the Expression of Repetitive DNA Elements in Osteosarcoma. Front. Genet. 2017, 8, 193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Reimann, E.; Kõks, S.; Ho, X.D.; Maasalu, K.; Märtson, A. Whole exome sequencing of a single osteosarcoma case--integrative analysis with whole transcriptome RNA-seq data. Hum. Genomics 2014, 8, 20. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Poudel, B.H.; Koks, S. The whole transcriptome analysis using FFPE and fresh tissue samples identifies the molecular fingerprint of osteosarcoma. Exp. Biol. Med. 2024, 249, 10161. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Beladiya, J.; Kumar, A.; Vasava, Y.; Parmar, K.; Patel, D.; Patel, S.; Dholakia, S.; Sheth, D.; Boddu, S.H.S.; Patel, C. Safety and efficacy of COVID-19 vaccines: A systematic review and meta-analysis of controlled and randomized clinical trials. Rev. Med. Virol. 2024, 34, e2507. [Google Scholar] [CrossRef] [PubMed]
- Català, M.; Mercadé-Besora, N.; Kolde, R.; Trinh, N.T.H.; Roel, E.; Burn, E.; Rathod-Mistry, T.; Kostka, K.; Man, W.Y.; Delmestri, A.; et al. The effectiveness of COVID-19 vaccines to prevent long COVID symptoms: Staggered cohort study of data from the UK, Spain, and Estonia. Lancet Respir. Med. 2024, 12, 225–236. [Google Scholar] [CrossRef] [PubMed]
- Kenny, G.; McCann, K.; O’Brien, C.; O’Broin, C.; Tinago, W.; Yousif, O.; O’Gorman, T.; Cotter, A.G.; Lambert, J.S.; Feeney, E.R.; et al. All Ireland Infectious Diseases Cohort Study. Impact of vaccination and variants of concern on long COVID clinical phenotypes. BMC Infect. Dis. 2023, 23, 804. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
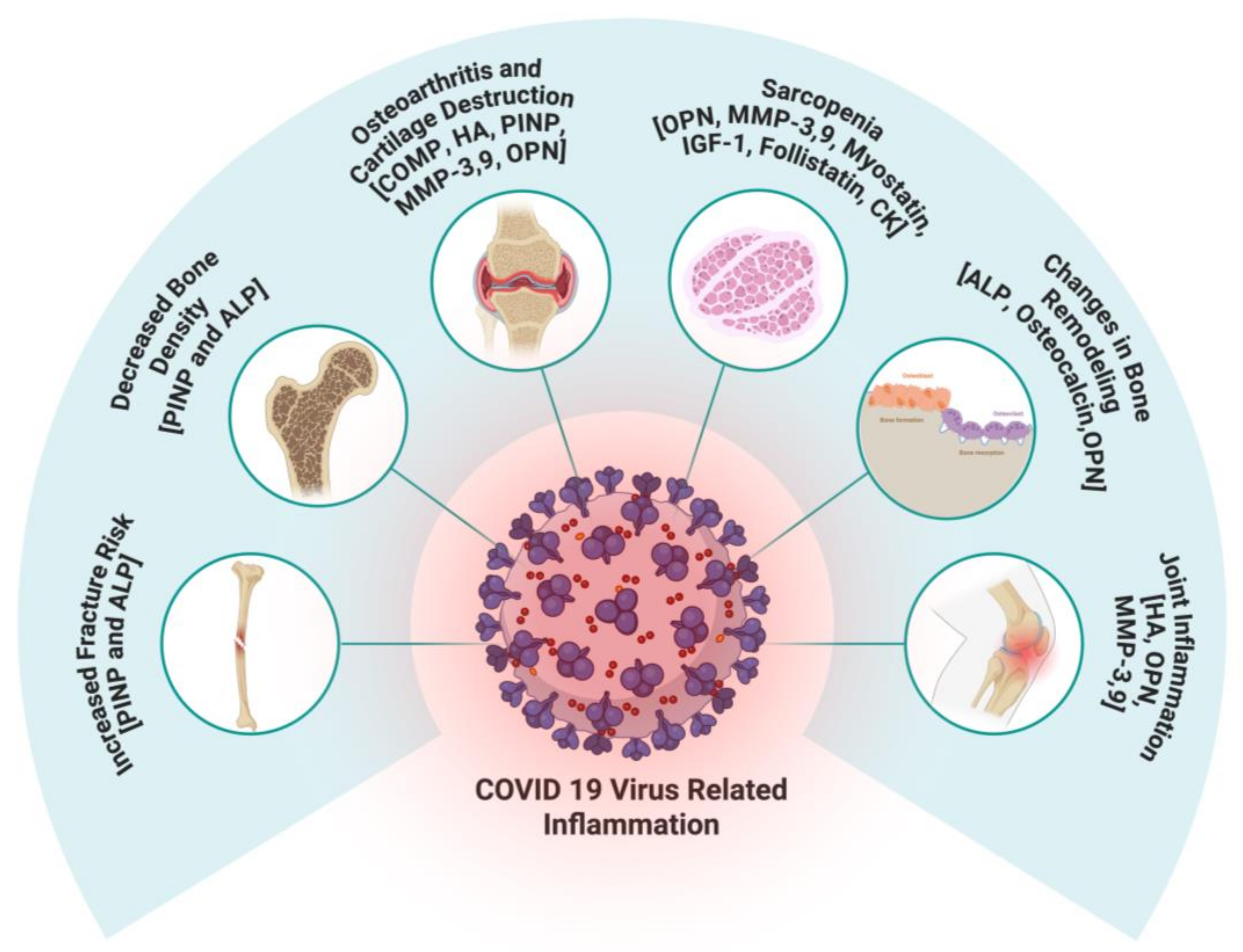
| Biomarker | Role in MSK Health | Alteration in COVID-19 | Implications for MSK Health | References |
|---|---|---|---|---|
| Cartilage Oligomeric Matrix Protein (COMP) | Stabilizes collagen fiber network in articular cartilage; marker of cartilage degradation and osteoarthritis progression | Decreased serum COMP levels, especially in severe COVID-19 cases | Indicates cartilage degradation, increased osteoarthritis progression and joint pain | [26,33,34,35] |
| Hyaluronic Acid (HA) | Maintains synovial fluid viscosity and joint lubrication. | Dysregulated metabolism with accumulation of low-molecular-weight HA fragments | Joint inflammation and pain; exacerbates endothelial dysfunction and inflammatory responses | [26,39,40,41,42,43,44] |
| Procollagen Type I N-terminal Peptide (PINP) | Marker of bone and collagen formation; reflects bone formation rate | Elevated levels in severe COVID-19, especially with interstitial lung disease | Indicates excessive tissue remodeling; altered musculoskeletal remodeling | [45,47,48,58,59,60] |
| Serum Total Alkaline Phosphatase (ALP) | Enzyme involved in bone mineralization and remodeling | Elevated levels in COVID-19 patients | Associated with accelerated bone remodeling, decreased bone mineral density, increased fracture risk | [17,49,61,62,63,64,65,66,67,68,69,70] |
| Osteocalcin | Vitamin K-dependent protein regulating bone mineralization and turnover | Reduced serum levels in moderate to severe COVID-19 patients | Disrupted bone turnover, increased bone fragility, higher fracture risk | [46,50,51,78] |
| Matrix Metalloproteinase-3 (MMP-3) | Degrades extracellular matrix components; involved in cartilage remodeling and inflammation | Significantly elevated in COVID-19; levels increase with disease severity | Contributes to cartilage degradation; potential diagnostic marker for disease severity | [52,53,54,79,80,81,82] |
| Matrix Metalloproteinase-9 (MMP-9) | Degrades collagen types IV and V; linked to inflammation and immune response | Elevated in severe COVID-19; associated with inflammatory responses and mortality risk | Linked to cartilage degradation and inflammation; potential prognostic marker | [52,84,85,86,87,88] |
| Osteopontin (OPN) | Glycoprotein involved in bone resorption, inflammation, and cartilage degradation | Markedly increased in severe COVID-19 patients | Correlates with inflammation, joint damage, and musculoskeletal complications | [55,56,89,90,91,92,93,94,95,96,97,98,99] |
| Myostatin | Muscle-derived factor that inhibits protein synthesis and promotes breakdown; linked to wasting, frailty, and sarcopenia. | Elevated after COVID-19; associated with longer hospital stay, prolonged rehab, and fatigue. | Higher risk of muscle loss, longer recovery, and reduced function. | [101,102,104] |
| IGF-1 | Hormone that promotes cell growth, protein synthesis, and anabolic processes; supports muscle, lung, liver, and brain health. | Reduced levels after COVID-19; lower in severe cases and non-survivors; inversely linked to age and severity. | Low IGF-1 may contribute to muscle loss and sarcopenia in severe/prolonged cases. | [100,105,106,107] |
| Follistatin | Inhibits activin signaling; rises during inflammation as a protective response. | Markedly elevated in acute COVID-19; linked to severity and higher mortality risk. | No direct post-COVID link to sarcopenia, but indicates severe systemic inflammation. | [107,108,109] |
| Creatine Kinase (CK) | Enzyme for ATP regeneration in muscle; marker of muscle injury. | Elevated in acute COVID-19; higher in severe cases; extreme rises in rhabdomyolysis. | Suggests muscle injury; persistent elevation may lead to loss of muscle mass and function. | [111,112,113] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Patel, S.; Foster, C.; Patel, K.; Hunter, M.; Isales, C.M.; Fulzele, S. Musculoskeletal Complications in COVID-19: Exploring the Role of Key Biomarkers. Int. J. Mol. Sci. 2025, 26, 8569. https://doi.org/10.3390/ijms26178569
Patel S, Foster C, Patel K, Hunter M, Isales CM, Fulzele S. Musculoskeletal Complications in COVID-19: Exploring the Role of Key Biomarkers. International Journal of Molecular Sciences. 2025; 26(17):8569. https://doi.org/10.3390/ijms26178569
Chicago/Turabian StylePatel, Sagar, Cameron Foster, Kamal Patel, Monte Hunter, Carlos M. Isales, and Sadanand Fulzele. 2025. "Musculoskeletal Complications in COVID-19: Exploring the Role of Key Biomarkers" International Journal of Molecular Sciences 26, no. 17: 8569. https://doi.org/10.3390/ijms26178569
APA StylePatel, S., Foster, C., Patel, K., Hunter, M., Isales, C. M., & Fulzele, S. (2025). Musculoskeletal Complications in COVID-19: Exploring the Role of Key Biomarkers. International Journal of Molecular Sciences, 26(17), 8569. https://doi.org/10.3390/ijms26178569





