Brain Metabolism of Allopregnanolone and Isoallopregnanolone in Male Rat Brain
Abstract
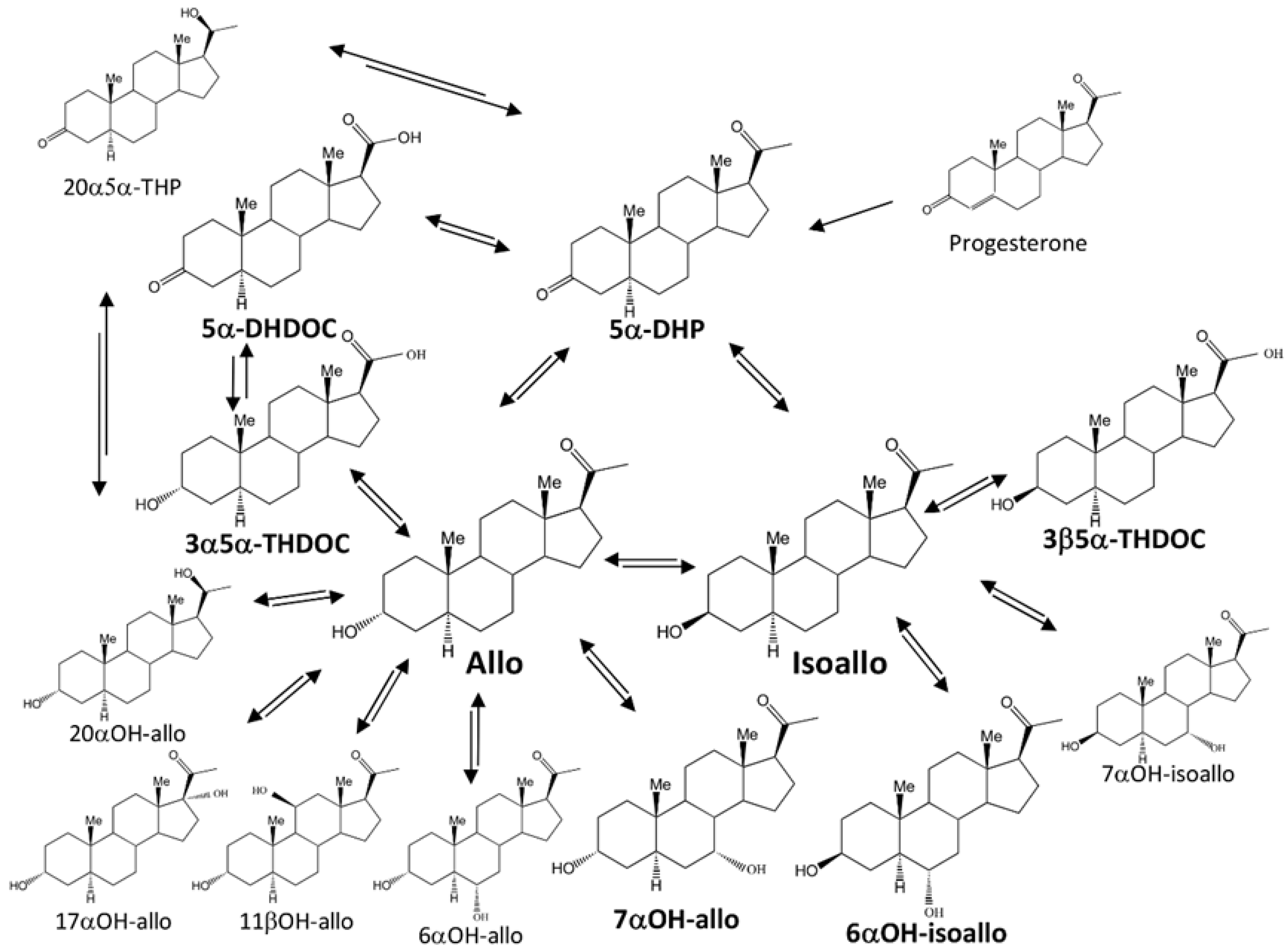
1. Introduction
2. Results
2.1. Method Development for Steroid Analyses
2.2. Steroid Concentrations in Vehicle Treated Animals
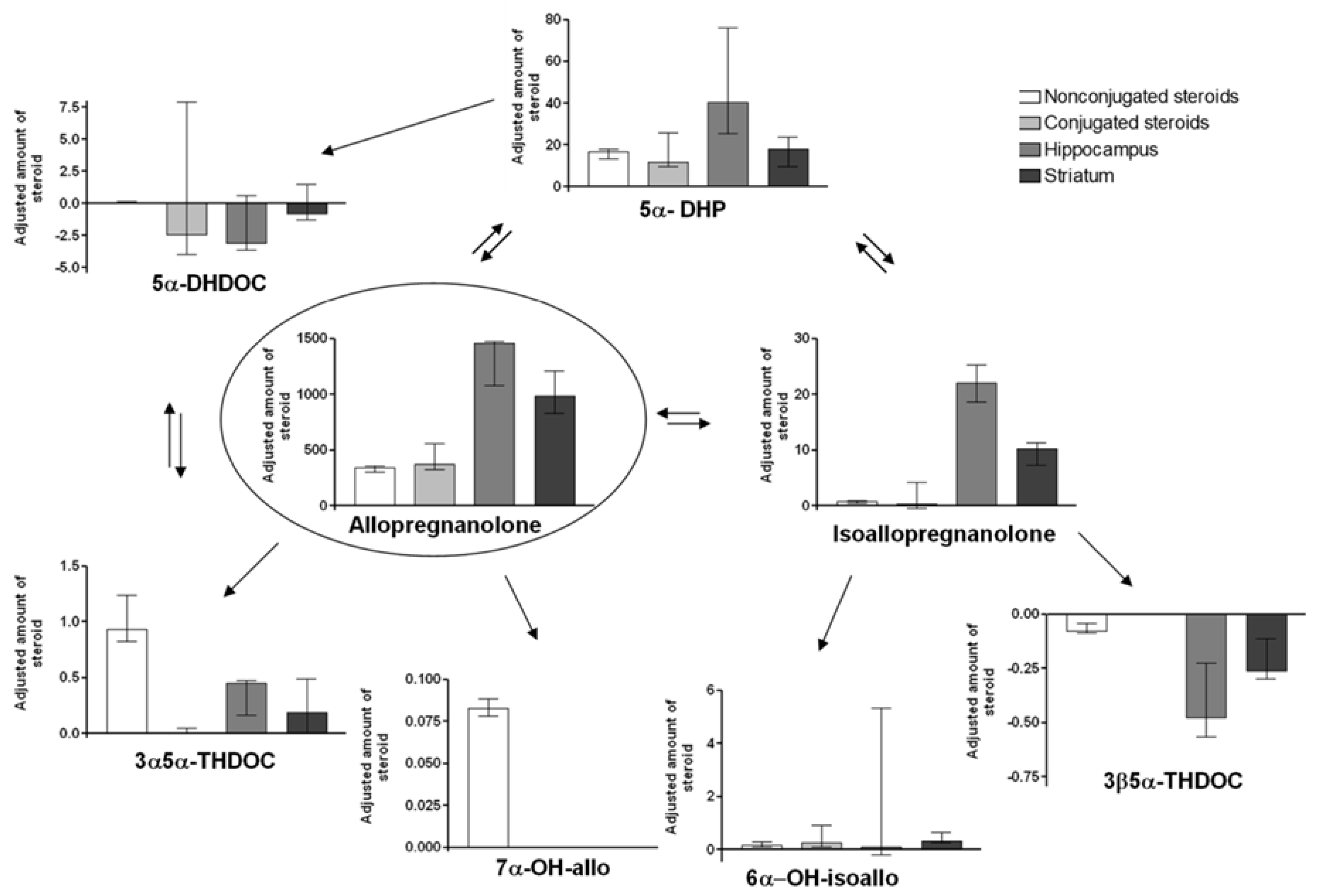
2.3. Steroid Concentrations in Allo Treated Animals
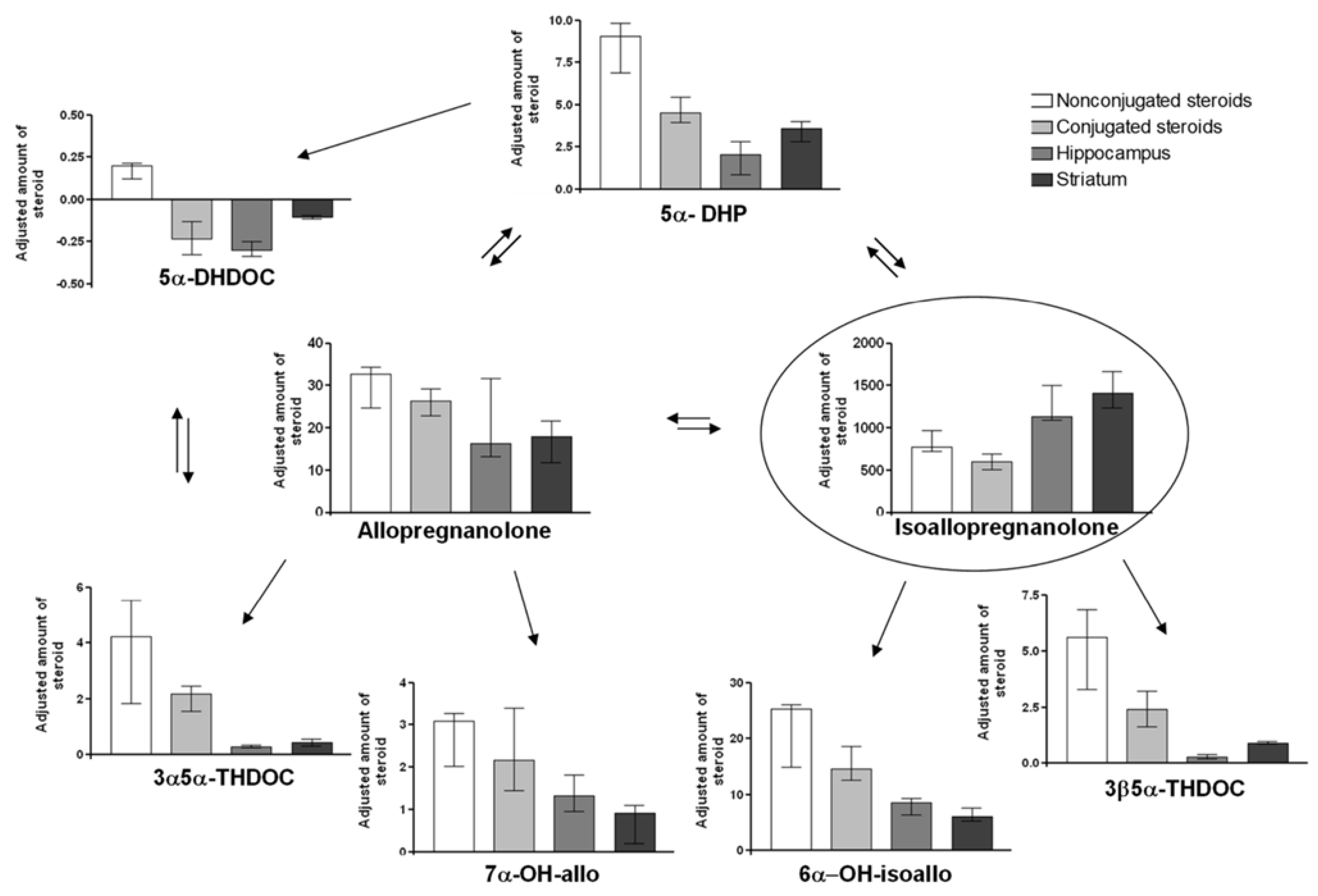
2.4. Steroid Concentrations in Isoallo Treated Animals
2.5. Adjusted Changes of Metabolites
3. Discussion
3.1. Potential Clinical Implications of the Findings
3.2. Future Directions
3.3. Limitations and Strengths of the Study
4. Materials and Methods
4.1. Chemicals
4.2. Animal Studies
4.3. Instruments/Method
4.4. Sample Pre-Treatment
4.5. Data Work up
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Ebner, M.J.; Corol, D.I.; Havlikova, H.; Honour, J.W.; Fry, J.P. Identification of neuroactive steroids and their precursors and metabolites in adult male rat brain. Endocrinology 2006, 147, 179–190. [Google Scholar] [CrossRef]
- Mathur, C.; Prasad, V.V.; Raju, V.S.; Welch, M.; Lieberman, S. Steroids and their conjugates in the mammalian brain. Proc. Natl. Acad. Sci. USA 1993, 90, 85–88. [Google Scholar] [CrossRef]
- Saalmann, Y.B.; Kirkcaldie, M.T.; Waldron, S.; Calford, M.B. Cellular distribution of the GABAA receptor-modulating 3alpha-hydroxy, 5alpha-reduced pregnane steroids in the adult rat brain. J. Neuroendocrinol. 2007, 19, 272–284. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.D.; Harrison, N.L.; Schwartz, R.D.; Barker, J.L.; Paul, S.M. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science 1986, 232, 1004–1007. [Google Scholar] [CrossRef] [PubMed]
- Havlikova, H.; Hill, M.; Kancheva, L.; Vrbikova, J.; Pouzar, V.; Cerny, I.; Kancheva, R.; Starka, L. Serum profiles of free and conjugated neuroactive pregnanolone isomers in nonpregnant women of fertile age. J. Clin. Endocrinol. Metab. 2006, 91, 3092–3099. [Google Scholar] [CrossRef]
- Backstrom, T.; Andersson, A.; Andree, L.; Birzniece, V.; Bixo, M.; Bjorn, I.; Haage, D.; Isaksson, M.; Johansson, I.M.; Lindblad, C.; et al. Pathogenesis in menstrual cycle-linked CNS disorders. Ann. N. Y. Acad. Sci. 2003, 1007, 42–53. [Google Scholar] [CrossRef]
- Lundgren, P.; Stromberg, J.; Backstrom, T.; Wang, M. Allopregnanolone-stimulated GABA-mediated chloride ion flux is inhibited by 3β-hydroxy-5α-pregnan-20-one (isoallopregnanolone). Brain Res. 2003, 982, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Stromberg, J.; Haage, D.; Taube, M.; Backstrom, T.; Lundgren, P. Neurosteroid modulation of allopregnanolone and GABA effect on the GABA-A receptor. Neuroscience 2006, 143, 73–81. [Google Scholar] [CrossRef]
- Backstrom, T.; Wahlstrom, G.; Wahlstrom, K.; Zhu, D.; Wang, M.D. Isoallopregnanolone; an antagonist to the anaesthetic effect of allopregnanolone in male rats. Eur. J. Pharmacol. 2005, 512, 15–21. [Google Scholar] [CrossRef]
- Bengtsson, S.K.; Nyberg, S.; Hedstrom, H.; Zingmark, E.; Jonsson, B.; Backstrom, T.; Bixo, M. Isoallopregnanolone antagonize allopregnanolone-induced effects on saccadic eye velocity and self-reported sedation in humans. Psychoneuroendocrinology 2015, 52, 22–31. [Google Scholar] [CrossRef]
- Wang, M.; He, Y.; Eisenman, L.N.; Fields, C.; Zeng, C.M.; Mathews, J.; Benz, A.; Fu, T.; Zorumski, E.; Steinbach, J.H.; et al. 3beta -hydroxypregnane steroids are pregnenolone sulfate-like GABA(A) receptor antagonists. J. Neurosci. 2002, 22, 3366–3375. [Google Scholar] [CrossRef]
- Huang, X.F.; Luu-The, V. Molecular characterization of a first human 3(α→β)-hydroxysteroid epimerase. J. Biol. Chem. 2000, 275, 29452–29457. [Google Scholar] [CrossRef]
- Huang, X.F.; Luu-The, V. Gene structure, chromosomal localization and analysis of 3-ketosteroid reductase activity of the human 3(α→β)-hydroxysteroid epimerase. Biochim. Biophys. Acta 2001, 1520, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Hung, C.F.; Penning, T.M. Members of the nuclear factor 1 transcription factor family regulate rat 3α-hydroxysteroid/dihydrodiol dehydrogenase (3α-HSD/DD AKR1C9) gene expression: A member of the aldo-keto reductase superfamily. Mol. Endocrinol. 1999, 13, 1704–1717. [Google Scholar] [CrossRef] [PubMed]
- Dam, P.T.M.; Jang, Y.J.; Kim, J.Y.; Choi, S.G.; Park, J.I.; Seo, Y.W.; Chun, S.Y. Expression of aldo-keto reductase family 1, member C14 during ovulation in the rat. Endocr. J. 2017, 64, 797–805. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Epperson, C.N.; Rubinow, D.R.; Meltzer-Brody, S.; Deligiannidis, K.M.; Riesenberg, R.; Krystal, A.D.; Bankole, K.; Huang, M.Y.; Li, H.; Brown, C.; et al. Effect of brexanolone on depressive symptoms, anxiety, and insomnia in women with postpartum depression: Pooled analyses from 3 double-blind, randomized, placebo-controlled clinical trials in the HUMMINGBIRD clinical program. J. Affect. Disord. 2023, 320, 353–359. [Google Scholar] [CrossRef]
- Bixo, M.; Ekberg, K.; Poromaa, I.S.; Hirschberg, A.L.; Jonasson, A.F.; Andreen, L.; Timby, E.; Wulff, M.; Ehrenborg, A.; Backstrom, T. Treatment of premenstrual dysphoric disorder with the GABA(A) receptor modulating steroid antagonist Sepranolone (UC1010)-A randomized controlled trial. Psychoneuroendocrinology 2017, 80, 46–55. [Google Scholar] [CrossRef]
- Backstrom, T.; Ekberg, K.; Hirschberg, A.L.; Bixo, M.; Epperson, C.N.; Briggs, P.; Panay, N.; O’Brien, S. A randomized, double-blind study on efficacy and safety of sepranolone in premenstrual dysphoric disorder. Psychoneuroendocrinology 2021, 133, 105426. [Google Scholar] [CrossRef]
- Hill, M.; Parizek, A.; Kancheva, R.; Duskova, M.; Velikova, M.; Kriz, L.; Klimkova, M.; Paskova, A.; Zizka, Z.; Matucha, P.; et al. Steroid metabolome in plasma from the umbilical artery, umbilical vein, maternal cubital vein and in amniotic fluid in normal and preterm labor. J. Steroid Biochem. Mol. Biol. 2010, 121, 594–610. [Google Scholar] [CrossRef]
- Osborne, L.M.; Etyemez, S.; Pinna, G.; Alemani, R.; Standeven, L.R.; Wang, X.Q.; Payne, J.L. Neuroactive steroid biosynthesis during pregnancy predicts future postpartum depression: A role for the 3α and/or 3β-HSD neurosteroidogenic enzymes? Neuropsychopharmacology 2025, 50, 904–912, Erratum in Neuropsychopharmacology 2025, 50, 1021. [Google Scholar] [CrossRef]
- Mellon, S.H.; Griffin, L.D.; Compagnone, N.A. Biosynthesis and action of neurosteroids. Brain Res. Brain Res. Rev. 2001, 37, 3–12. [Google Scholar] [CrossRef]
- Agis-Balboa, R.C.; Pinna, G.; Zhubi, A.; Maloku, E.; Veldic, M.; Costa, E.; Guidotti, A. Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis. Proc. Natl. Acad. Sci. USA 2006, 103, 14602–14607. [Google Scholar] [CrossRef] [PubMed]
- Caruso, D.; Pesaresi, M.; Abbiati, F.; Calabrese, D.; Giatti, S.; Garcia-Segura, L.M.; Melcangi, R.C. Comparison of plasma and cerebrospinal fluid levels of neuroactive steroids with their brain, spinal cord and peripheral nerve levels in male and female rats. Psychoneuroendocrinology 2013, 38, 2278–2290. [Google Scholar] [CrossRef] [PubMed]
- Giatti, S.; Diviccaro, S.; Serafini, M.M.; Caruso, D.; Garcia-Segura, L.M.; Viviani, B.; Melcangi, R.C. Sex differences in steroid levels and steroidogenesis in the nervous system: Physiopathological role. Front. Neuroendocrinol. 2020, 56, 100804. [Google Scholar] [CrossRef]
- Zamora-Sanchez, C.J.; Camacho-Arroyo, I. Allopregnanolone: Metabolism, Mechanisms of Action, and Its Role in Cancer. Int. J. Mol. Sci. 2023, 24, 560. [Google Scholar] [CrossRef]
- Bengtsson, S.K.S.; Backstrom, T.; Brinton, R.; Irwin, R.W.; Johansson, M.; Sjostedt, J.; Wang, M.D. GABA-A receptor modulating steroids in acute and chronic stress; relevance for cognition and dementia? Neurobiol. Stress 2020, 12, 100206. [Google Scholar] [CrossRef]
- Wang, M.D.; Wahlstrom, G.; Gee, K.W.; Backstrom, T. Potency of lipid and protein formulation of 5α-pregnanolone at induction of anaesthesia and the corresponding regional brain distribution. Br. J. Anaesth. 1995, 74, 553–557. [Google Scholar] [CrossRef] [PubMed]
- Ossewaarde, L.; van Wingen, G.A.; Kooijman, S.C.; Backstrom, T.; Fernandez, G.; Hermans, E.J. Changes in functioning of mesolimbic incentive processing circuits during the premenstrual phase. Soc. Cogn. Affect. Neurosci. 2011, 6, 612–620. [Google Scholar] [CrossRef]
- Stiernman, L.; Dubol, M.; Comasco, E.; Sundstrom-Poromaa, I.; Boraxbekk, C.J.; Johansson, M.; Bixo, M. Emotion-induced brain activation across the menstrual cycle in individuals with premenstrual dysphoric disorder and associations to serum levels of progesterone-derived neurosteroids. Transl. Psychiatry 2023, 13, 124. [Google Scholar] [CrossRef]
- Belyaeva, O.V.; Chetyrkin, S.V.; Clark, A.L.; Kostereva, N.V.; SantaCruz, K.S.; Chronwall, B.M.; Kedishvili, N.Y. Role of microsomal retinol/sterol dehydrogenase-like short-chain dehydrogenases/reductases in the oxidation and epimerization of 3α-hydroxysteroids in human tissues. Endocrinology 2007, 148, 2148–2156. [Google Scholar] [CrossRef]
- Dombroski, R.A.; Casey, M.L.; MacDonald, P.C. 5-Alpha-dihydroprogesterone formation in human placenta from 5alpha-pregnan-3beta/alpha-ol-20-ones and 5-pregnan-3beta-yl-20-one sulfate. J. Steroid Biochem. Mol. Biol. 1997, 63, 155–163. [Google Scholar] [CrossRef]
- Stromstedt, M.; Warner, M.; Banner, C.D.; MacDonald, P.C.; Gustafsson, J.A. Role of brain cytochrome P450 in regulation of the level of anesthetic steroids in the brain. Mol. Pharmacol. 1993, 44, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Vallee, M.; Rivera, J.D.; Koob, G.F.; Purdy, R.H.; Fitzgerald, R.L. Quantification of neurosteroids in rat plasma and brain following swim stress and allopregnanolone administration using negative chemical ionization gas chromatography/mass spectrometry. Anal. Biochem. 2000, 287, 153–166. [Google Scholar] [CrossRef]
- Steckelbroeck, S.; Watzka, M.; Stoffel-Wagner, B.; Hans, V.H.; Redel, L.; Clusmann, H.; Elger, C.E.; Bidlingmaier, F.; Klingmuller, D. Expression of the 17β-hydroxysteroid dehydrogenase type 5 mRNA in the human brain. Mol. Cell Endocrinol. 2001, 171, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Penning, T.M.; Chen, M.; Jin, Y. Promiscuity and diversity in 3-ketosteroid reductases. J. Steroid Biochem. Mol. Biol. 2015, 151, 93–101. [Google Scholar] [CrossRef]
- Penning, T.M.; Wangtrakuldee, P.; Auchus, R.J. Structural and Functional Biology of Aldo-Keto Reductase Steroid-Transforming Enzymes. Endocr. Rev. 2019, 40, 447–475. [Google Scholar] [CrossRef] [PubMed]
- Bixo, M.; Andersson, A.; Winblad, B.; Purdy, R.H.; Backstrom, T. Progesterone, 5alpha-pregnane-3,20-dione and 3alpha-hydroxy-5alpha-pregnane-20-one in specific regions of the human female brain in different endocrine states. Brain Res. 1997, 764, 173–178. [Google Scholar] [CrossRef]
- Wang, M.D.; Wahlstrom, G.; Backstrom, T. The regional brain distribution of the neurosteroids pregnenolone and pregnenolone sulfate following intravenous infusion. J. Steroid Biochem. Mol. Biol. 1997, 62, 299–306. [Google Scholar] [CrossRef]
- Kancheva, R.; Hill, M.; Novak, Z.; Chrastina, J.; Velikova, M.; Kancheva, L.; Riha, I.; Starka, L. Peripheral neuroactive steroids may be as good as the steroids in the cerebrospinal fluid for the diagnostics of CNS disturbances. J. Steroid Biochem. Mol. Biol. 2010, 119, 35–44. [Google Scholar] [CrossRef]
- Qaiser, M.Z.; Dolman, D.E.M.; Begley, D.J.; Abbott, N.J.; Cazacu-Davidescu, M.; Corol, D.I.; Fry, J.P. Uptake and metabolism of sulphated steroids by the blood-brain barrier in the adult male rat. J. Neurochem. 2017, 142, 672–685. [Google Scholar] [CrossRef]
- Chantilis, S.; Dombroski, R.; Shackleton, C.H.; Casey, M.L.; MacDonald, P.C. Metabolism of 5 alpha-dihydroprogesterone in women and men: 3 beta- and 3 alpha-,6 alpha-dihydroxy-5 alpha-pregnan-20-ones are major urinary metabolites. J. Clin. Endocrinol. Metab. 1996, 81, 3644–3649. [Google Scholar]
- Gemzik, B.; Parkinson, A. Hydroxylation of 5α-androstane-3β,17β-diol by rat prostate microsomes: Potent inhibition by imidazole-type antimycotic drugs and lack of inhibition by steroid 5α-reductase inhibitors. Arch. Biochem. Biophys. 1992, 296, 366–373. [Google Scholar] [CrossRef]
- Sundin, M.; Warner, M.; Haaparanta, T.; Gustafsson, J.A. Isolation and catalytic activity of cytochrome P-450 from ventral prostate of control rats. J. Biol. Chem. 1987, 262, 12293–12297. [Google Scholar] [CrossRef] [PubMed]
- Funae, Y.; Kishimoto, W.; Cho, T.; Niwa, T.; Hiroi, T. CYP2D in the brain. Drug Metab. Pharmacokinet. 2003, 18, 337–349. [Google Scholar] [CrossRef]
- Koganti, P.P.; Selvaraj, V. Single cell resolution of neurosteroidogenesis in the murine brain: De novo biosynthesis. J. Endocrinol. 2025, 265, e240318. [Google Scholar] [CrossRef] [PubMed]
- Johansson, I.M.; Birzniece, V.; Lindblad, C.; Olsson, T.; Backstrom, T. Allopregnanolone inhibits learning in the Morris water maze. Brain Res. 2002, 934, 125–131. [Google Scholar] [CrossRef]
- Zhu, D.; Birzniece, V.; Backstrom, T.; Wahlstrom, G. Dynamic aspects of acute tolerance to allopregnanolone evaluated using anaesthesia threshold in male rats. Br. J. Anaesth. 2004, 93, 560–567. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Backstrom, T.; Sundstrom, I.; Wahlstrom, G.; Olsson, T.; Zhu, D.; Johansson, I.M.; Bjorn, I.; Bixo, M. Neuroactive steroids and central nervous system disorders. Int. Rev. Neurobiol. 2001, 46, 421–459. [Google Scholar]
- Kancheva, R.; Hill, M.; Cibula, D.; Vcelakova, H.; Kancheva, L.; Vrbikova, J.; Fait, T.; Parizek, A.; Starka, L. Relationships of circulating pregnanolone isomers and their polar conjugates to the status of sex, menstrual cycle, and pregnancy. J. Endocrinol. 2007, 195, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.S.; Zhang, H.; Kim, H.Y. Profiling neurosteroids in cerebrospinal fluids and plasma by gas chromatography/electron capture negative chemical ionization mass spectrometry. Anal. Biochem. 2000, 277, 187–195. [Google Scholar] [CrossRef]
- Eechaute, W.P.; Dhooge, W.S.; Gao, C.Q.; Calders, P.; Rubens, R.; Weyne, J.; Kaufman, J.M. Progesterone-transforming enzyme activity in the hypothalamus of the male rat. J. Steroid Biochem. Mol. Biol. 1999, 70, 159–167. [Google Scholar] [CrossRef] [PubMed]
- Kudova, E.; Mares, P.; Hill, M.; Vondrakova, K.; Tsenov, G.; Chodounska, H.; Kubova, H.; Vales, K. The Neuroactive Steroid Pregnanolone Glutamate: Anticonvulsant Effect, Metabolites and Its Effect on Neurosteroid Levels in Developing Rat Brains. Pharmaceuticals 2022, 15, 49. [Google Scholar] [CrossRef]
- Landgren, S.; Aasly, J.; Backstrom, T.; Dubrovsky, B.; Danielsson, E. The effect of progesterone and its metabolites on the interictal epileptiform discharge in the cat’s cerebral cortex. Acta Physiol. Scand. 1987, 131, 33–42. [Google Scholar] [CrossRef]
- Do Rego, J.L.; Seong, J.Y.; Burel, D.; Leprince, J.; Luu-The, V.; Tsutsui, K.; Tonon, M.C.; Pelletier, G.; Vaudry, H. Neurosteroid biosynthesis: Enzymatic pathways and neuroendocrine regulation by neurotransmitters and neuropeptides. Front. Neuroendocrinol. 2009, 30, 259–301. [Google Scholar] [CrossRef] [PubMed]
- Steckelbroeck, S.; Jin, Y.; Gopishetty, S.; Oyesanmi, B.; Penning, T.M. Human cytosolic 3α-hydroxysteroid dehydrogenases of the aldo-keto reductase superfamily display significant 3β-hydroxysteroid dehydrogenase activity: Implications for steroid hormone metabolism and action. J. Biol. Chem. 2004, 279, 10784–10795. [Google Scholar] [CrossRef]
- Trauger, J.W.; Jiang, A.; Stearns, B.A.; LoGrasso, P.V. Kinetics of allopregnanolone formation catalyzed by human 3α-hydroxysteroid dehydrogenase type III (AKR1C2). Biochemistry 2002, 41, 13451–13459. [Google Scholar] [CrossRef]
- Rizner, T.L.; Smuc, T.; Rupreht, R.; Sinkovec, J.; Penning, T.M. AKR1C1 and AKR1C3 may determine progesterone and estrogen ratios in endometrial cancer. Mol. Cell Endocrinol. 2006, 248, 126–135. [Google Scholar] [CrossRef]
- Liang, J.J.; Rasmusson, A.M. Overview of the Molecular Steps in Steroidogenesis of the GABAergic Neurosteroids Allopregnanolone and Pregnanolone. Chronic Stress 2018, 2, 1–17. [Google Scholar] [CrossRef]
- Steckelbroeck, S.; Watzka, M.; Reichelt, R.; Hans, V.H.; Stoffel-Wagner, B.; Heidrich, D.D.; Schramm, J.; Bidlingmaier, F.; Klingmuller, D. Characterization of the 5alpha-reductase-3alpha-hydroxysteroid dehydrogenase complex in the human brain. J. Clin. Endocrinol. Metab. 2001, 86, 1324–1331. [Google Scholar]
- Stoffel-Wagner, B.; Watzka, M.; Steckelbroeck, S.; Ludwig, M.; Clusmann, H.; Bidlingmaier, F.; Casarosa, E.; Luisi, S.; Elger, C.E.; Beyenburg, S. Allopregnanolone serum levels and expression of 5α-reductase and 3α-hydroxysteroid dehydrogenase isoforms in hippocampal and temporal cortex of patients with epilepsy. Epilepsy Res. 2003, 54, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Higaki, Y.; Usami, N.; Shintani, S.; Ishikura, S.; El-Kabbani, O.; Hara, A. Selective and potent inhibitors of human 20α-hydroxysteroid dehydrogenase (AKR1C1) that metabolizes neurosteroids derived from progesterone. Chem. Biol. Interact. 2003, 143–144, 503–513. [Google Scholar] [CrossRef] [PubMed]
- Timby, E.; Balgard, M.; Nyberg, S.; Spigset, O.; Andersson, A.; Porankiewicz-Asplund, J.; Purdy, R.H.; Zhu, D.; Backstrom, T.; Poromaa, I.S. Pharmacokinetic and behavioral effects of allopregnanolone in healthy women. Psychopharmacology 2006, 186, 414–424. [Google Scholar] [CrossRef]
- Oakley, J.; Hill, M.; Giess, A.; Tanguy, M.; Elgar, G. Long read sequencing characterises a novel structural variant, revealing underactive AKR1C1 with overactive AKR1C2 as a possible cause of severe chronic fatigue. J. Transl. Med. 2023, 21, 825. [Google Scholar] [CrossRef]
- Chetyrkin, S.V.; Hu, J.; Gough, W.H.; Dumaual, N.; Kedishvili, N.Y. Further characterization of human microsomal 3α-hydroxysteroid dehydrogenase. Arch. Biochem. Biophys. 2001, 386, 1–10. [Google Scholar] [CrossRef]
- Hedström, H.; Bixo, M.; Nyberg, S.; Spigset, O.; Zingmark, E.; Bäckström, T. Studies of pharmacokinetic and pharmacodynamic properties of isoallopregnanolone in healthy women. Psychopharmacology 2009, 203, 85–98. [Google Scholar] [CrossRef]
- Pihlajoki, M.; Dorner, J.; Cochran, R.S.; Heikinheimo, M.; Wilson, D.B. Adrenocortical zonation, renewal, and remodeling. Front. Endocrinol. 2015, 6, 27. [Google Scholar] [CrossRef]
- Rege, J.; Nakamura, Y.; Wang, T.; Merchen, T.D.; Sasano, H.; Rainey, W.E. Transcriptome profiling reveals differentially expressed transcripts between the human adrenal zona fasciculata and zona reticularis. J. Clin. Endocrinol. Metab. 2014, 99, E518–E527. [Google Scholar] [CrossRef]
- Setchell, K.D.; Alme, B.; Axelson, M.; Sjovall, J. The multicomponent analysis of conjugates of neutral steroids in urine by lipophilic ion exchange chromatography and computerised gas chromatography-mass spectrometry. J. Steroid Biochem. 1976, 7, 615–629. [Google Scholar] [CrossRef] [PubMed]
- Dehennin, L.; Lafarge, P.; Dailly, P.; Bailloux, D.; Lafarge, J.P. Combined profile of androgen glucuro- and sulfoconjugates in post-competition urine of sportsmen: A simple screening procedure using gas chromatography-mass spectrometry. J. Chromatogr. B Biomed. Appl. 1996, 687, 85–91. [Google Scholar] [CrossRef] [PubMed]
| Steroid | Effective Mass | Retention Time (min) | |||||
|---|---|---|---|---|---|---|---|
| m/z (Da) | Peak 1 | Peak 2 | σ | ||||
| 17α-estradiol (internal standard) | 231 | 285 | 416 | --- | 8.16 | --- | 0.02 |
| 3β-OH-5β-pregnane-20-one | 241 | 298 | 388 | --- | 9.79 | --- | 0.03 |
| Allopregnanolone (allo) | 241 | 298 | 388 | --- | 10.18 | --- | 0.03 |
| 7α-OH-allo | 358 | 386 | 476 | --- | 10.51 | --- | 0.03 |
| 3α-OH-5β-pregnane-20-one | 241 | 298 | 388 | --- | 10.52 | --- | 0.03 |
| 3β,5β-THDOC | 282 | 386 | 476 | --- | 12.14 | --- | 0.02 |
| Isoallo | 241 | 298 | 388 | 12.25 | --- | 0.02 | |
| 3α5α-THDOC | 476 | --- | --- | --- | 12.34 | --- | 0.02 |
| 3α5β-THDOC | 282 | 358 | 476 | --- | 13.15 | --- | 0.02 |
| 6α-OH-isoallo | 282 | 358 | 386 | 476 | 13.25 | --- | 0.01 |
| 3β5α-THDOC | 358 | 386 | 476 | --- | 13.55 | --- | 0.01 |
| 5β-DHP | 275 | 288 | 343 | --- | 13.69 | 13.74 | 0.01 |
| 5α-DHP | 275 | 288 | 343 | --- | 14.15 | 14.18 | 0.01 |
| 5β-DHDOC | 275 | 288 | 359 | 431 | 14.29 | 14.32 | 0.01 |
| 5α-DHDOC | 431 | --- | --- | --- | 14.79 | --- | 0.02 |
| Steroid | Vehicle Group | Allo Treated Group | Isoallo Treated Group |
|---|---|---|---|
| Allo | 28.5 (17.2, 34.5) | 717 (635, 742) * | 1080 (818, 1130) * |
| Isoallo | 0.108 (0.096, 0.364) | 1.68 (1.05, 1.98) * | 24,700 (23,200, 31,000) * |
| 5α-DHP | 1.72 (1.53, 2.3) | 35 (29, 38.1) * | 292 (223, 317) * |
| 5α-DHDOC | 0.247 (0.176, 0.412) | 0.473 (0.366, 0.568) | 6.69 (4.13, 7.2) * |
| 3α5α-THDOC | 0.342 (0.244, 0.617) | 2.26 (2.05, 2.88) * | 136 (58.7, 177) * |
| 3β5α-THDOC | 0.044 (0.04, 0.638) | 0.084 (0.065, 0.154) | 179 (105, 218) * |
| 7α-OH-allo | 0.053 (0.039, 0.073) | 0.221 (0.211, 0.232) * | 98.8 (64.9, 105) * |
| 6α-OH-isoallo | 0.055 (0.036, 1.02) | 0.673 (0.576, 0.993) | 811 (478, 834) * |
| Allo-C | 37.5 (12.5, 93.9) | 795 (701, 1160) * | 890 (495, 1090) * |
| Isoallo-C | 5.09 (0.506, 17.1) | 4.15 (2.5, 11.9) | 19,300 (34,900, 48,200) * |
| 5α-DHDOC-C | 17.2 (12.1, 59.7) | 10.3 (7.25, 31) | 7.71 (1.86, 4.76) * |
| 3α5α-THDOC-C | ND | ND | ND |
| 3β5α-THDOC-C | ND | ND | ND |
| 7α-OHallo-C | ND | ND | ND |
| 6α-OH-isoallo-C | 0.598 (0.192, 7.33) | 0.981 (0.66, 2.31) | 465 (203, 297) * |
| Steroid | Vehicle Group | Allo Treated Group | Isoallo Treated Group |
|---|---|---|---|
| Allo | 69 (33.7, 110) | 2990 (2230, 3010) * | 596 (590, 666) * |
| Isoallo | 9.61 (4.41, 16.5) | 54.2 (47.5, 60.8) * | 36,300 (35,700, 37,000) |
| 5α-DHP | 4.62 (1.8, 7.07) | 85.1 (55, 156) * | 69.7 (33.4, 90.2) * |
| 5α-DHDOC | 12.5 (8.05, 17.6) | 6.56 (5.45, 13.9) | 3.12 (3.03, 4.53) * |
| 3α5α-THDOC | 0.374 (0.116, 1.26) | 1.19 (0.617, 1.24) | 8.99 (8.24, 11.2) * |
| 3β5α-THDOC | 0.963 (0.515, 2.39) | 0.335 (0.16, 0.841) | 10.8 (10.4, 13.8) * |
| 7α-OHallo | ND | ND | 42.4 (42.2, 57.3) * |
| 6α-OH-isoallo | 0.547 (0.06, 13.1) | 0.549 (0.001, 11) | 274 (210, 292) * |
| Steroid | Vehicle Group | Allo Treated Group | Isoallo # Treated Group |
|---|---|---|---|
| Allo | 40.4 (14.2, 60.9) | 2010 (1690, 2450) * | 611 (413, 731) * |
| Isoallo | 8.43 (5.08, 67.3) | 27.5 (21.7, 29.7) | 45,300 (39,500, 53,400) * |
| 5α-DHP | 1.76 (0.784, 16.8) | 37.3 (20.3, 49) * | 116 (90.9, 129) * |
| 5α-DHDOC | 6.49 (4.81, 10.2) | 4.15 (3.29, 8.77) | 2.37 (2.08, 2.69) * |
| 3α5α-THDOC | 0.553 (0.258, 0.636) | 0.855 (0.485, 1.46) | 14.2 (10.4, 17.9) * |
| 3β5α-THDOC | 0.975 (0.503, 1.15) | 0.354 (0.28, 0.652) | 30.1 (28.7, 32.3) * |
| 7α-OHallo | ND | ND | 29.2 (6.28, 35.1) |
| 6α-OH-isoallo | 0.247 (0.181, 35.5) | 0.893 (0.756, 1.54) | 194 (170, 246) * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öfverman, C.; Hill, M.; Johansson, M.; Bäckström, T. Brain Metabolism of Allopregnanolone and Isoallopregnanolone in Male Rat Brain. Int. J. Mol. Sci. 2025, 26, 8559. https://doi.org/10.3390/ijms26178559
Öfverman C, Hill M, Johansson M, Bäckström T. Brain Metabolism of Allopregnanolone and Isoallopregnanolone in Male Rat Brain. International Journal of Molecular Sciences. 2025; 26(17):8559. https://doi.org/10.3390/ijms26178559
Chicago/Turabian StyleÖfverman, Charlotte, Martin Hill, Maja Johansson, and Torbjörn Bäckström. 2025. "Brain Metabolism of Allopregnanolone and Isoallopregnanolone in Male Rat Brain" International Journal of Molecular Sciences 26, no. 17: 8559. https://doi.org/10.3390/ijms26178559
APA StyleÖfverman, C., Hill, M., Johansson, M., & Bäckström, T. (2025). Brain Metabolism of Allopregnanolone and Isoallopregnanolone in Male Rat Brain. International Journal of Molecular Sciences, 26(17), 8559. https://doi.org/10.3390/ijms26178559







