Mitochondria-Associated Pathways in Cancer and Precancerous Conditions: Mechanistic Insights
Abstract
1. Introduction
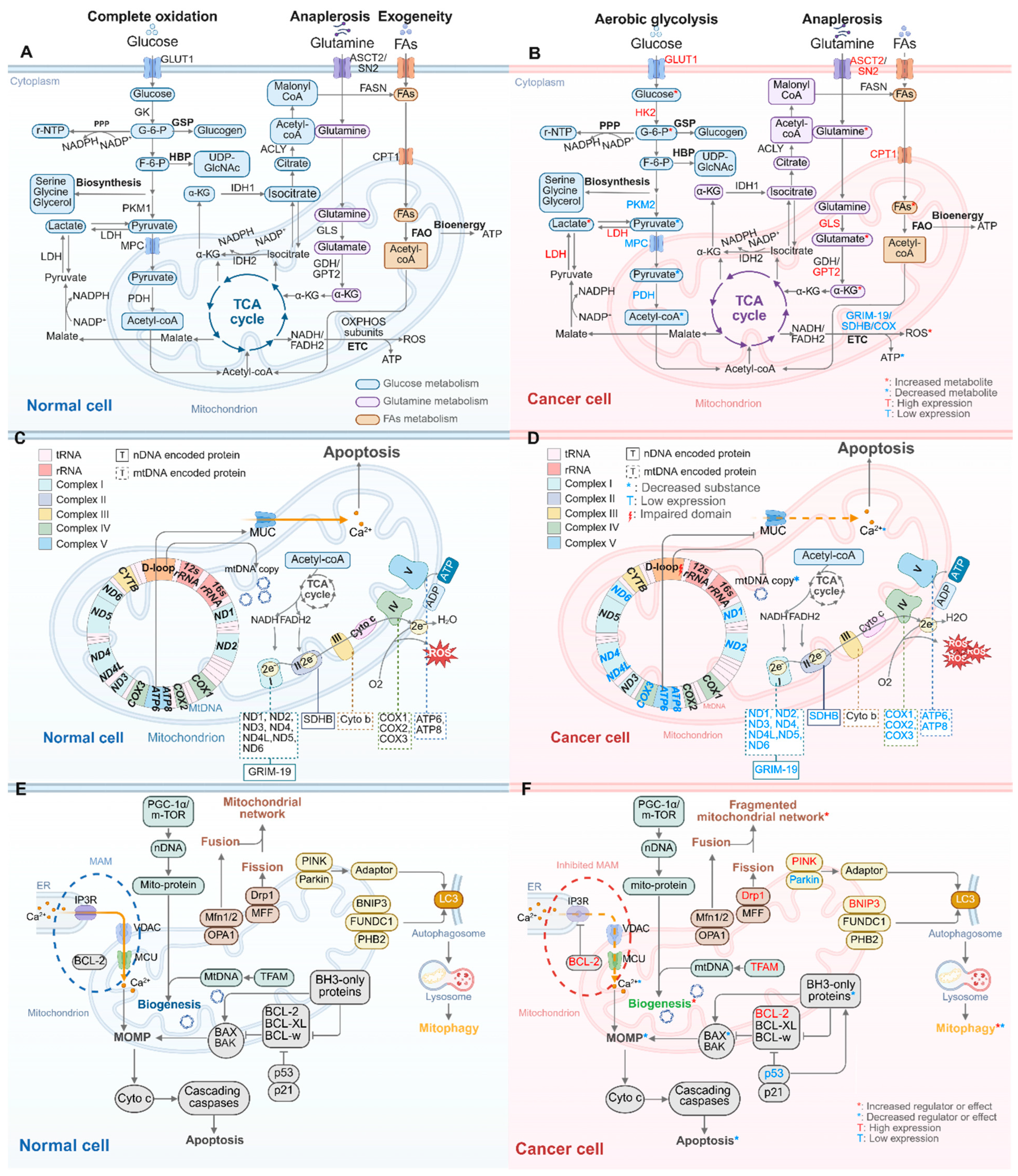
2. Mitochondrial Dysfunctions in Cancer Progression
2.1. Oncogenic Mutations in nDNA Lead to Reprogramming of Mitochondrial Metabolism
2.1.1. Glucose Metabolism Alterations: From Complete Oxidation to Aerobic Glycolysis
2.1.2. Changes in Amino Acid and Lipid Metabolism: Enhanced Glutamine Anaplerosis and FAs Oxidation
2.1.3. Impaired Mitochondrial OXPHOS
2.2. Oncogenic Mutations in nDNA Disrupt the Mitochondrial Regulation of Cell Death and mtQC
2.2.1. Inhibition of Mitochondria-Related Apoptosis Leading to Cancer Cell Immortality
2.2.2. Increased Mitochondrial Biogenesis Providing Enhanced Mitochondrial Mass
2.2.3. Upregulated Mitochondrial Fission Leading to a Fragmented Mitochondrial Network
2.2.4. Bidirectional Regulation of Mitophagy
2.3. Mitochondrial Dysfunctions Driven by Oncogenic mtDNA Mutations
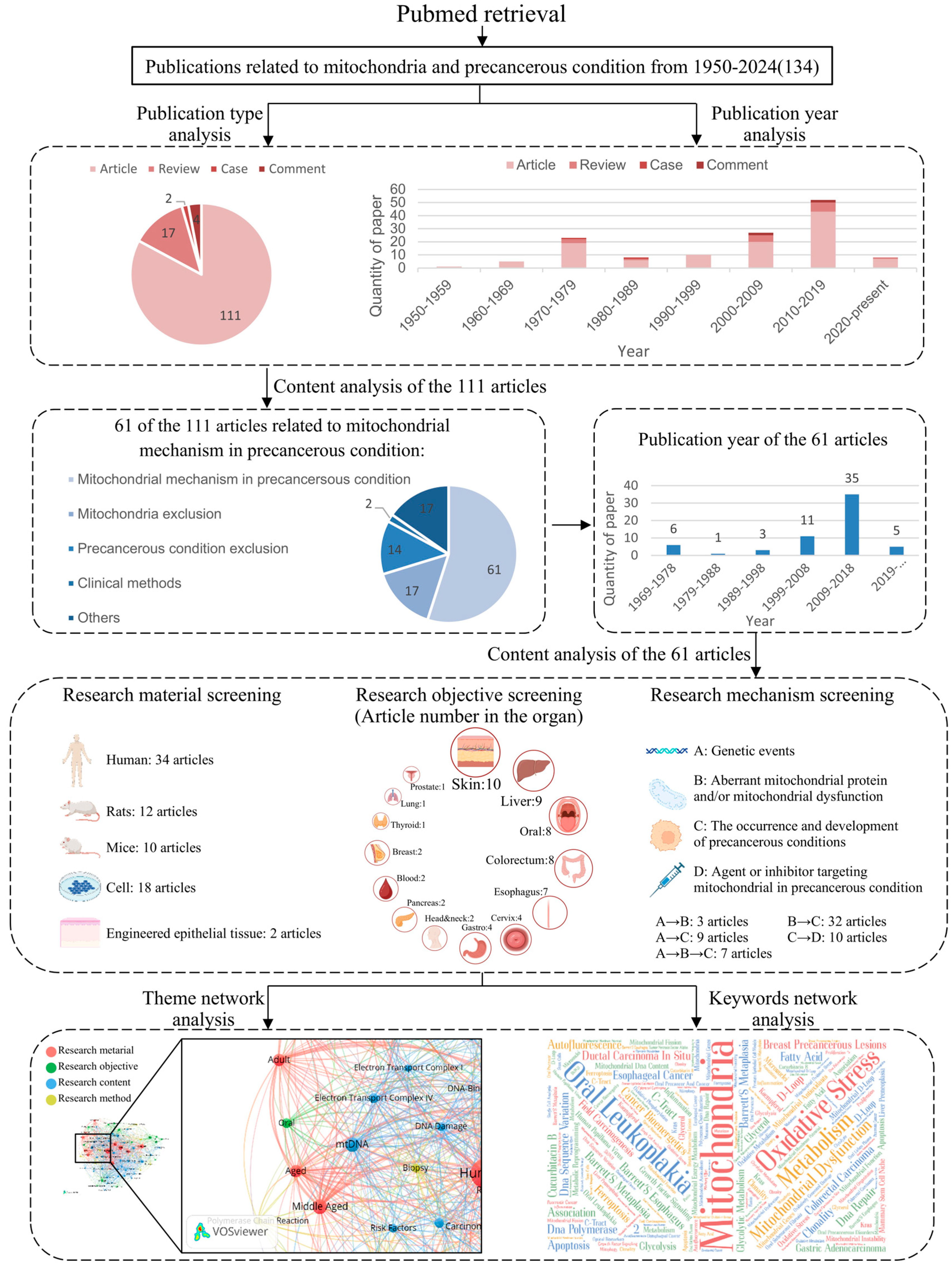
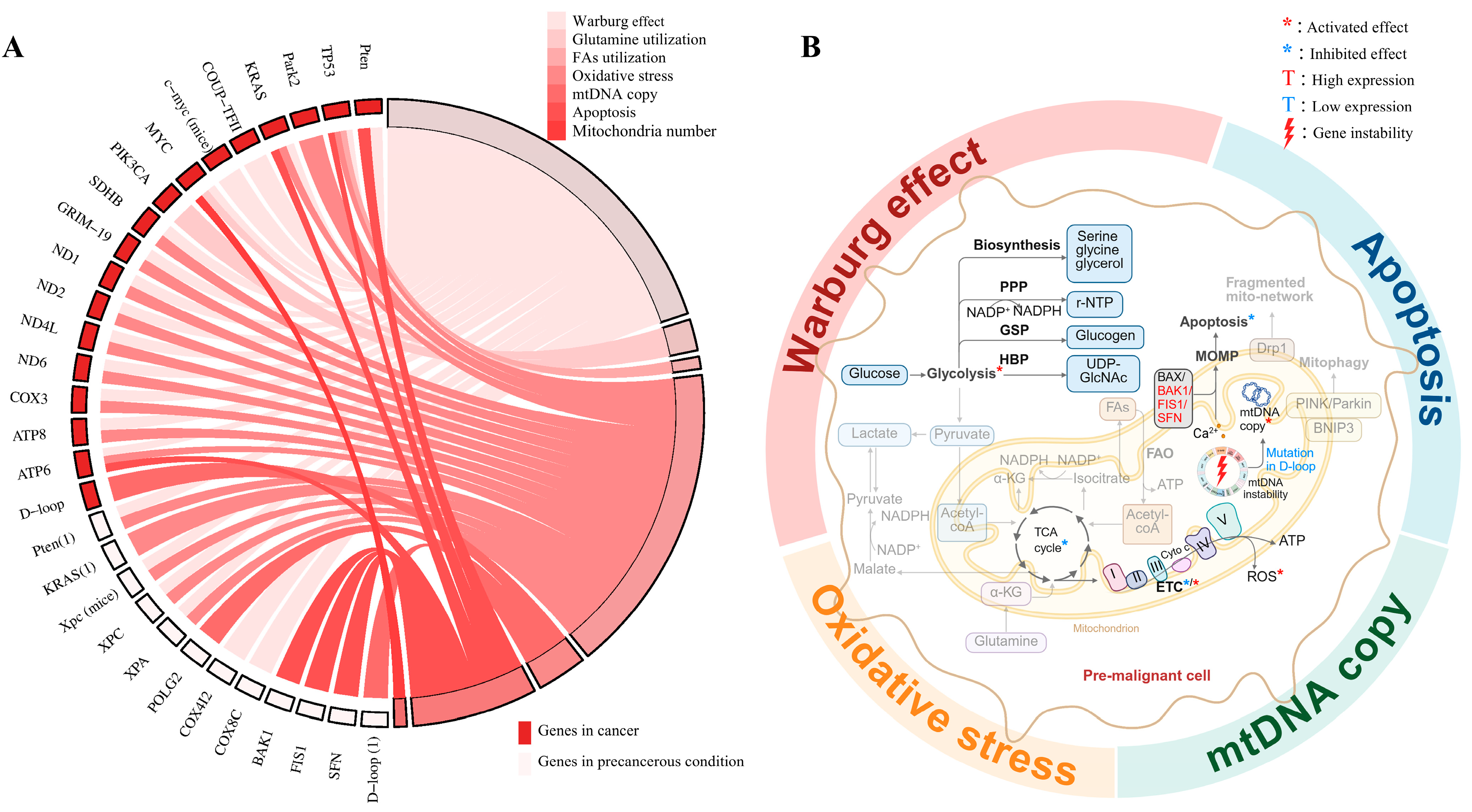
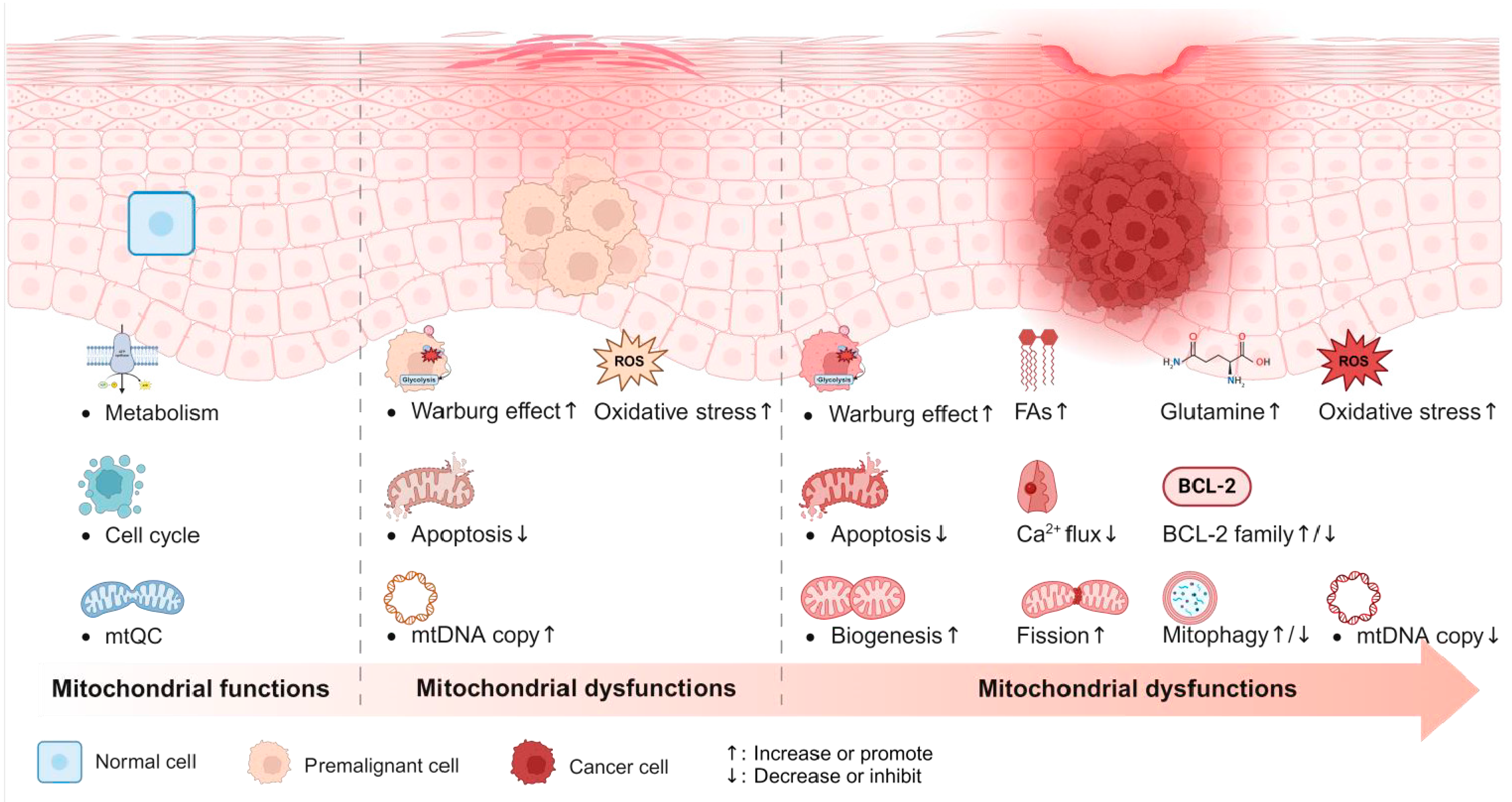
3. Mitochondrial Mechanisms in Precancerous Conditions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| nDNA | nuclear DNA |
| ER | endoplasmic reticulum |
| FAO | fatty acid oxidation |
| IMM | inner mitochondrial membrane |
| MAM | mitochondria-associated ER membrane |
| MMP | mitochondrial membrane potential |
| MOMP | mitochondrial outer membrane permeabilization |
| mtQC | mitochondrial quality control |
| OLK | oral leukoplakia |
| OMM | outer mitochondrial membrane |
| OPMD | oral potential malignant disorder |
| OSCC | oral squamous cell carcinoma |
| VDAC | voltage-dependent anion channel |
References
- Gorman, G.S.; Chinnery, P.F.; DiMauro, S.; Hirano, M.; Koga, Y.; McFarland, R.; Suomalainen, A.; Thorburn, D.R.; Zeviani, M.; Turnbull, D.M. Mitochondrial diseases. Nat. Rev. Dis. Primers 2016, 2, 16080. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. The hallmarks of cancer. Cell 2000, 100, 57–70. [Google Scholar] [CrossRef]
- Sarode, S.C.; Sarode, G.S.; Tupkari, J.V. Oral potentially malignant disorders: A proposal for terminology and definition with review of literature. J. Oral Maxillofac. Pathol. JOMFP 2014, 18 (Suppl. 1), S77–S80. [Google Scholar] [CrossRef]
- Vander Heiden, M.G.; Cantley, L.C.; Thompson, C.B. Understanding the Warburg effect: The metabolic requirements of cell proliferation. Science 2009, 324, 1029–1033. [Google Scholar] [CrossRef]
- Warburg, O. On the origin of cancer cells. Science 1956, 123, 309–314. [Google Scholar] [CrossRef]
- Osthus, R.C.; Shim, H.; Kim, S.; Li, Q.; Reddy, R.; Mukherjee, M.; Xu, Y.; Wonsey, D.; Lee, L.A.; Dang, C.V. Deregulation of glucose transporter 1 and glycolytic gene expression by c-Myc. J. Biol. Chem. 2000, 275, 21797–21800. [Google Scholar] [CrossRef]
- Mathupala, S.P.; Ko, Y.H.; Pedersen, P.L. Hexokinase-2 bound to mitochondria: Cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin. Cancer Biol. 2009, 19, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Levine, A.J.; Puzio-Kuter, A.M. The control of the metabolic switch in cancers by oncogenes and tumor suppressor genes. Science 2010, 330, 1340–1344. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xiong, H.; Wu, F.; Zhang, Y.; Wang, J.; Zhao, L.; Guo, X.; Chang, L.J.; Zhang, Y.; You, M.J.; et al. Hexokinase 2-mediated Warburg effect is required for PTEN- and p53-deficiency-driven prostate cancer growth. Cell Rep. 2014, 8, 1461–1474. [Google Scholar] [CrossRef]
- David, C.J.; Chen, M.; Assanah, M.; Canoll, P.; Manley, J.L. HnRNP proteins controlled by c-Myc deregulate pyruvate kinase mRNA splicing in cancer. Nature 2010, 463, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Singh, M.; Rani, R. Role of LDH in tumor glycolysis: Regulation of LDHA by small molecules for cancer therapeutics. Semin. Cancer Biol. 2022, 87, 184–195. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, M.; Qin, J.; Lin, S.C.; Lee, H.J.; Tsai, S.Y.; Tsai, M.J. MPC1, a key gene in cancer metabolism, is regulated by COUPTFII in human prostate cancer. Oncotarget 2016, 7, 14673–14683. [Google Scholar] [CrossRef] [PubMed]
- Contractor, T.; Harris, C.R. p53 negatively regulates transcription of the pyruvate dehydrogenase kinase Pdk2. Cancer Res. 2012, 72, 560–567. [Google Scholar] [CrossRef] [PubMed]
- DeBerardinis, R.J.; Mancuso, A.; Daikhin, E.; Nissim, I.; Yudkoff, M.; Wehrli, S.; Thompson, C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. USA 2007, 104, 19345–19350. [Google Scholar] [CrossRef]
- Gao, P.; Tchernyshyov, I.; Chang, T.C.; Lee, Y.S.; Kita, K.; Ochi, T.; Zeller, K.I.; De Marzo, A.M.; Van Eyk, J.E.; Mendell, J.T.; et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 2009, 458, 762–765. [Google Scholar] [CrossRef]
- Wise, D.R.; DeBerardinis, R.J.; Mancuso, A.; Sayed, N.; Zhang, X.Y.; Pfeiffer, H.K.; Nissim, I.; Daikhin, E.; Yudkoff, M.; McMahon, S.B.; et al. Myc regulates a transcriptional program that stimulates mitochondrial glutaminolysis and leads to glutamine addiction. Proc. Natl. Acad. Sci. USA 2008, 105, 18782–18787. [Google Scholar] [CrossRef]
- Hao, Y.; Samuels, Y.; Li, Q.; Krokowski, D.; Guan, B.J.; Wang, C.; Jin, Z.; Dong, B.; Cao, B.; Feng, X.; et al. Oncogenic PIK3CA mutations reprogram glutamine metabolism in colorectal cancer. Nat. Commun. 2016, 7, 11971. [Google Scholar] [CrossRef]
- Shen, L.; O’Shea, J.M.; Kaadige, M.R.; Cunha, S.; Wilde, B.R.; Cohen, A.L.; Welm, A.L.; Ayer, D.E. Metabolic reprogramming in triple-negative breast cancer through Myc suppression of TXNIP. Proc. Natl. Acad. Sci. USA 2015, 112, 5425–5430. [Google Scholar] [CrossRef]
- Cheung, E.C.; Vousden, K.H. The role of ROS in tumour development and progression. Nat. Rev. Cancer 2022, 22, 280–297. [Google Scholar] [CrossRef]
- Máximo, V.; Lima, J.; Soares, P.; Silva, A.; Bento, I.; Sobrinho-Simões, M. GRIM-19 in Health and Disease. Adv. Anat. Pathol. 2008, 15, 46–53. [Google Scholar] [CrossRef]
- Vanharanta, S.; Buchta, M.; McWhinney, S.R.; Virta, S.K.; Peçzkowska, M.; Morrison, C.D.; Lehtonen, R.; Januszewicz, A.; Järvinen, H.; Juhola, M.; et al. Early-onset renal cell carcinoma as a novel extraparaganglial component of SDHB-associated heritable paraganglioma. Am. J. Hum. Genet. 2004, 74, 153–159. [Google Scholar] [CrossRef]
- Matoba, S.; Kang, J.G.; Patino, W.D.; Wragg, A.; Boehm, M.; Gavrilova, O.; Hurley, P.J.; Bunz, F.; Hwang, P.M. p53 regulates mitochondrial respiration. Science 2006, 312, 1650–1653. [Google Scholar] [CrossRef]
- Cárdenas, C.; Miller, R.A.; Smith, I.; Bui, T.; Molgó, J.; Müller, M.; Vais, H.; Cheung, K.H.; Yang, J.; Parker, I.; et al. Essential regulation of cell bioenergetics by constitutive InsP3 receptor Ca2+ transfer to mitochondria. Cell 2010, 142, 270–283. [Google Scholar] [CrossRef]
- Shoshan-Barmatz, V.; Gincel, D. The voltage-dependent anion channel: Characterization, modulation, and role in mitochondrial function in cell life and death. Cell Biochem. Biophys. 2003, 39, 279–292. [Google Scholar] [CrossRef]
- Kirichok, Y.; Krapivinsky, G.; Clapham, D.E. The mitochondrial calcium uniporter is a highly selective ion channel. Nature 2004, 427, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Szalai, G.; Krishnamurthy, R.; Hajnóczky, G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999, 18, 6349–6361. [Google Scholar] [CrossRef] [PubMed]
- Rosa, N.; Speelman-Rooms, F.; Parys, J.B.; Bultynck, G. Modulation of Ca(2+) signaling by antiapoptotic Bcl-2 versus Bcl-xL: From molecular mechanisms to relevance for cancer cell survival. Biochim. Biophys. Acta Rev. Cancer 2022, 1877, 188791. [Google Scholar] [CrossRef] [PubMed]
- Cory, S.; Adams, J.M. The Bcl2 family: Regulators of the cellular life-or-death switch. Nat. Rev. Cancer 2002, 2, 647–656. [Google Scholar] [CrossRef]
- Yu, J.; Zhang, L. No PUMA, no death: Implications for p53-dependent apoptosis. Cancer Cell 2003, 4, 248–249. [Google Scholar] [CrossRef]
- Mansour, M.A.; El-Salamoni, M.A.; Mostafa, H.N. Harnessing PUMA’s lethal potential: BCL-2 family dynamics and novel strategies to combat cancer recurrence. Cancer Treat. Res. Commun. 2025, 44, 100975. [Google Scholar] [CrossRef]
- Yang, X.; Zhuang, J.; Song, W.; Wu, W.; Shen, H.; Han, S. Mitochondria-associated endoplasmic reticulum membrane: Overview and inextricable link with cancer. J. Cell. Mol. Med. 2023, 27, 906–919. [Google Scholar] [CrossRef]
- Lindemann, A.; Takahashi, H.; Patel, A.A.; Osman, A.A.; Myers, J.N. Targeting the DNA Damage Response in OSCC with TP53 Mutations. J. Dent. Res. 2018, 97, 635–644. [Google Scholar] [CrossRef]
- Kang, Y.J.; Yang, W.G.; Chae, W.S.; Kim, D.W.; Kim, S.G.; Rotaru, H. Administration of 4-hexylresorcinol increases p53-mediated transcriptional activity in oral cancer cells with the p53 mutation. Oncol. Rep. 2022, 48, 160. [Google Scholar] [CrossRef]
- Kim, E.M.; Jung, C.H.; Kim, J.; Hwang, S.G.; Park, J.K.; Um, H.D. The p53/p21 Complex Regulates Cancer Cell Invasion and Apoptosis by Targeting Bcl-2 Family Proteins. Cancer Res. 2017, 77, 3092–3100. [Google Scholar] [CrossRef]
- Liu, L.; Li, Y.; Chen, G.; Chen, Q. Crosstalk between mitochondrial biogenesis and mitophagy to maintain mitochondrial homeostasis. J. Biomed. Sci. 2023, 30, 86. [Google Scholar] [CrossRef] [PubMed]
- Morita, M.; Gravel, S.P.; Hulea, L.; Larsson, O.; Pollak, M.; St-Pierre, J.; Topisirovic, I. mTOR coordinates protein synthesis, mitochondrial activity and proliferation. Cell Cycle 2015, 14, 473–480. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Wang, Y.; Zeller, K.I.; Potter, J.J.; Wonsey, D.R.; O’Donnell, K.A.; Kim, J.W.; Yustein, J.T.; Lee, L.A.; Dang, C.V. Myc stimulates nuclearly encoded mitochondrial genes and mitochondrial biogenesis. Mol. Cell Biol. 2005, 25, 6225–6234. [Google Scholar] [CrossRef]
- Dang, C.V. MYC, metabolism, cell growth, and tumorigenesis. Cold Spring Harb. Perspect. Med. 2013, 3, a014217. [Google Scholar] [CrossRef]
- Lamb, R.; Bonuccelli, G.; Ozsvári, B.; Peiris-Pagès, M.; Fiorillo, M.; Smith, D.L.; Bevilacqua, G.; Mazzanti, C.M.; McDonnell, L.A.; Naccarato, A.G.; et al. Mitochondrial mass, a new metabolic biomarker for stem-like cancer cells: Understanding WNT/FGF-driven anabolic signaling. Oncotarget 2015, 6, 30453–30471. [Google Scholar] [CrossRef] [PubMed]
- Kraus, F.; Roy, K.; Pucadyil, T.J.; Ryan, M.T. Function and regulation of the divisome for mitochondrial fission. Nature 2021, 590, 57–66. [Google Scholar] [CrossRef]
- Serasinghe, M.N.; Wieder, S.Y.; Renault, T.T.; Elkholi, R.; Asciolla, J.J.; Yao, J.L.; Jabado, O.; Hoehn, K.; Kageyama, Y.; Sesaki, H.; et al. Mitochondrial division is requisite to RAS-induced transformation and targeted by oncogenic MAPK pathway inhibitors. Mol. Cell 2015, 57, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Renault, T.T.; Floros, K.V.; Elkholi, R.; Corrigan, K.A.; Kushnareva, Y.; Wieder, S.Y.; Lindtner, C.; Serasinghe, M.N.; Asciolla, J.J.; Buettner, C.; et al. Mitochondrial shape governs BAX-induced membrane permeabilization and apoptosis. Mol. Cell 2015, 57, 69–82. [Google Scholar] [CrossRef]
- Wang, Y.; Goh, K.Y.; Chen, Z.; Lee, W.X.; Choy, S.M.; Fong, J.X.; Wong, Y.K.; Li, D.; Hu, F.; Tang, H.W. A Novel TP53 Gene Mutation Sustains Non-Small Cell Lung Cancer through Mitophagy. Cells 2022, 11, 3587. [Google Scholar] [CrossRef] [PubMed]
- Humpton, T.J.; Alagesan, B.; DeNicola, G.M.; Lu, D.; Yordanov, G.N.; Leonhardt, C.S.; Yao, M.A.; Alagesan, P.; Zaatari, M.N.; Park, Y.; et al. Oncogenic KRAS Induces NIX-Mediated Mitophagy to Promote Pancreatic Cancer. Cancer Discov. 2019, 9, 1268–1287. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Wang, N.N.; Ma, Q.L.; Chen, Y.; Yao, L.; Zhang, L.; Li, Q.S.; Shi, M.H.; Wang, H.F.; Ying, Z. Somatic and germline mutations in the tumor suppressor gene PARK2 impair PINK1/Parkin-mediated mitophagy in lung cancer cells. Acta Pharmacol. Sin. 2020, 41, 93–100. [Google Scholar] [CrossRef]
- Poole, L.P.; Macleod, K.F. Mitophagy in tumorigenesis and metastasis. Cell Mol. Life Sci. 2021, 78, 3817–3851. [Google Scholar] [CrossRef]
- Wallace, D.C. A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: A dawn for evolutionary medicine. Annu. Rev. Genet. 2005, 39, 359–407. [Google Scholar] [CrossRef]
- Järviaho, T.; Hurme-Niiranen, A.; Soini, H.K.; Niinimäki, R.; Möttönen, M.; Savolainen, E.R.; Hinttala, R.; Harila-Saari, A.; Uusimaa, J. Novel non-neutral mitochondrial DNA mutations found in childhood acute lymphoblastic leukemia. Clin. Genet. 2018, 93, 275–285. [Google Scholar] [CrossRef]
- Yu, M.; Wan, Y.; Zou, Q. Reduced mitochondrial DNA copy number in Chinese patients with osteosarcoma. Transl. Res. 2013, 161, 165–171. [Google Scholar] [CrossRef]
- Yu, M.; Wan, Y.; Zou, Q. Decreased copy number of mitochondrial DNA in Ewing’s sarcoma. Clin. Chim. Acta 2010, 411, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Zhou, Y.; Shi, Y.; Ning, L.; Yang, Y.; Wei, X.; Zhang, N.; Hao, X.; Niu, R. Reduced mitochondrial DNA copy number is correlated with tumor progression and prognosis in Chinese breast cancer patients. IUBMB Life 2007, 59, 450–457. [Google Scholar] [CrossRef] [PubMed]
- Niedzwiecka, K.; Tisi, R.; Penna, S.; Lichocka, M.; Plochocka, D.; Kucharczyk, R. Two mutations in mitochondrial ATP6 gene of ATP synthase, related to human cancer, affect ROS, calcium homeostasis and mitochondrial permeability transition in yeast. Biochim. Biophys. Acta Mol. Cell Res. 2018, 1865, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Antico Arciuch, V.G.; Russo, M.A.; Kang, K.S.; Di Cristofano, A. Inhibition of AMPK and Krebs cycle gene expression drives metabolic remodeling of Pten-deficient preneoplastic thyroid cells. Cancer Res. 2013, 73, 5459–5472. [Google Scholar] [CrossRef]
- Brosens, L.A.; Hackeng, W.M.; Offerhaus, G.J.; Hruban, R.H.; Wood, L.D. Pancreatic adenocarcinoma pathology: Changing “landscape”. J. Gastrointest. Oncol. 2015, 6, 358–374. [Google Scholar] [CrossRef]
- Storz, P.; Crawford, H.C. Carcinogenesis of Pancreatic Ductal Adenocarcinoma. Gastroenterology 2020, 158, 2072–2081. [Google Scholar] [CrossRef]
- Xu, H.N.; Nioka, S.; Chance, B.; Li, L.Z. Heterogeneity of mitochondrial redox state in premalignant pancreas in a PTEN null transgenic mouse model. Adv. Exp. Med. Biol. 2011, 701, 207–213. [Google Scholar] [CrossRef]
- Liou, G.Y.; Döppler, H.; DelGiorno, K.E.; Zhang, L.; Leitges, M.; Crawford, H.C.; Murphy, M.P.; Storz, P. Mutant KRas-Induced Mitochondrial Oxidative Stress in Acinar Cells Upregulates EGFR Signaling to Drive Formation of Pancreatic Precancerous Lesions. Cell Rep. 2016, 14, 2325–2336. [Google Scholar] [CrossRef]
- Hail, N., Jr.; Chen, P.; Rower, J.; Bushman, L.R. Teriflunomide encourages cytostatic and apoptotic effects in premalignant and malignant cutaneous keratinocytes. Apoptosis Int. J. Program. Cell Death 2010, 15, 1234–1246. [Google Scholar] [CrossRef]
- Behar, V.; Pahima, H.; Kozminsky-Atias, A.; Arbel, N.; Loeb, E.; Herzberg, M.; Becker, O.M. A Hexokinase 2 Modulator for Field-Directed Treatment of Experimental Actinic Keratoses. J. Investig. Dermatol. 2018, 138, 2635–2643. [Google Scholar] [CrossRef]
- Mori, M.P.; Costa, R.A.; Soltys, D.T.; Freire, T.S.; Rossato, F.A.; Amigo, I.; Kowaltowski, A.J.; Vercesi, A.E.; de Souza-Pinto, N.C. Lack of XPC leads to a shift between respiratory complexes I and II but sensitizes cells to mitochondrial stress. Sci. Rep. 2017, 7, 155. [Google Scholar] [CrossRef] [PubMed]
- Parlanti, E.; Pietraforte, D.; Iorio, E.; Visentin, S.; De Nuccio, C.; Zijno, A.; D’Errico, M.; Simonelli, V.; Sanchez, M.; Fattibene, P.; et al. An altered redox balance and increased genetic instability characterize primary fibroblasts derived from xeroderma pigmentosum group A patients. Mutat. Res. 2015, 782, 34–43. [Google Scholar] [CrossRef]
- Rothe, M.; Werner, D.; Thielmann, H.W. Enhanced expression of mitochondrial genes in xeroderma pigmentosum fibroblast strains from various complementation groups. J. Cancer Res. Clin. Oncol. 1993, 119, 675–684. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.; Mahfouf, W.; Serrano-Sanchez, M.; Raad, H.; Harfouche, G.; Bonneu, M.; Claverol, S.; Mazurier, F.; Rossignol, R.; Taieb, A.; et al. Premature skin aging features rescued by inhibition of NADPH oxidase activity in XPC-deficient mice. J. Investig. Dermatol. 2015, 135, 1108–1118. [Google Scholar] [CrossRef]
- Rezvani, H.R.; Kim, A.L.; Rossignol, R.; Ali, N.; Daly, M.; Mahfouf, W.; Bellance, N.; Taïeb, A.; de Verneuil, H.; Mazurier, F.; et al. XPC silencing in normal human keratinocytes triggers metabolic alterations that drive the formation of squamous cell carcinomas. J. Clin. Investig. 2011, 121, 195–211. [Google Scholar] [CrossRef]
- Kumari, P.; Debta, P.; Dixit, A. Oral Potentially Malignant Disorders: Etiology, Pathogenesis, and Transformation Into Oral Cancer. Front. Pharmacol. 2022, 13, 825266. [Google Scholar] [CrossRef] [PubMed]
- Garg, A.D.; Bose, M.; Ahmed, M.I.; Bonass, W.A.; Wood, S.R. In vitro studies on erythrosine-based photodynamic therapy of malignant and pre-malignant oral epithelial cells. PLoS ONE 2012, 7, e34475. [Google Scholar] [CrossRef] [PubMed]
- Datta, S.; Ray, A.; Roy, R.; Roy, B. Association of DNA sequence variation in mitochondrial DNA polymerase with mitochondrial DNA synthesis and risk of oral cancer. Gene 2016, 575 Pt 3, 650–654. [Google Scholar] [CrossRef]
- Phelan, J.J.; MacCarthy, F.; Feighery, R.; O’Farrell, N.J.; Lynam-Lennon, N.; Doyle, B.; O’Toole, D.; Ravi, N.; Reynolds, J.V.; O’Sullivan, J. Differential expression of mitochondrial energy metabolism profiles across the metaplasia-dysplasia-adenocarcinoma disease sequence in Barrett’s oesophagus. Cancer Lett. 2014, 354, 122–131. [Google Scholar] [CrossRef]
- Phelan, J.J.; MacCarthy, F.; O’Toole, D.; Ravi, N.; Reynolds, J.V.; O’Sullivan, J. The Mitochondrial Genes BAK1, FIS1 and SFN are Linked with Alterations in Mitochondrial Membrane Potential in Barrett’s Esophagus. Int. J. Mol. Sci. 2018, 19, 3483. [Google Scholar] [CrossRef]
- O’Farrell, N.J.; Feighery, R.; Picardo, S.L.; Lynam-Lennon, N.; Biniecka, M.; McGarrigle, S.A.; Phelan, J.J.; MacCarthy, F.; O’Toole, D.; Fox, E.J.; et al. Changes in mitochondrial stability during the progression of the Barrett’s esophagus disease sequence. BMC Cancer 2016, 16, 497. [Google Scholar] [CrossRef]
- Correa, P.; Shiao, Y.H. Phenotypic and genotypic events in gastric carcinogenesis. Cancer Res. 1994, 54 (Suppl. 7), 1941s–1943s. [Google Scholar] [PubMed]
- Ling, X.L.; Fang, D.C.; Wang, R.Q.; Yang, S.M.; Fang, L. Mitochondrial microsatellite instability in gastric cancer and its precancerous lesions. World, J. Gastroenterol. 2004, 10, 800–803. [Google Scholar] [CrossRef] [PubMed]
- Riddell, R.H.; Goldman, H.; Ransohoff, D.F.; Appelman, H.D.; Fenoglio, C.M.; Haggitt, R.C.; Ahren, C.; Correa, P.; Hamilton, S.R.; Morson, B.C.; et al. Dysplasia in inflammatory bowel disease: Standardized classification with provisional clinical applications. Hum. Pathol. 1983, 14, 931–968. [Google Scholar] [CrossRef]
- Nishikawa, M.; Oshitani, N.; Matsumoto, T.; Nishigami, T.; Arakawa, T.; Inoue, M. Accumulation of mitochondrial DNA mutation with colorectal carcinogenesis in ulcerative colitis. Br. J. Cancer 2005, 93, 331–337. [Google Scholar] [CrossRef]
- Wakae, K.; Nishiyama, T.; Kondo, S.; Izuka, T.; Que, L.; Chen, C.; Kase, K.; Kitamura, K.; Mohiuddin, M.; Wang, Z.; et al. Keratinocyte differentiation induces APOBEC3A, 3B, and mitochondrial DNA hypermutation. Sci. Rep. 2018, 8, 9745. [Google Scholar] [CrossRef]
- Cereser, B.; Jansen, M.; Austin, E.; Elia, G.; McFarlane, T.; van Deurzen, C.H.; Sieuwerts, A.M.; Daidone, M.G.; Tadrous, P.J.; Wright, N.A.; et al. Analysis of clonal expansions through the normal and premalignant human breast epithelium reveals the presence of luminal stem cells. J. Pathol. 2018, 244, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Ha, P.K.; Tong, B.C.; Westra, W.H.; Sanchez-Cespedes, M.; Parrella, P.; Zahurak, M.; Sidransky, D.; Califano, J.A. Mitochondrial C-tract alteration in premalignant lesions of the head and neck: A marker for progression and clonal proliferation. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2002, 8, 2260–2265. [Google Scholar]
- Pandey, R.; Mehrotra, D.; Mahdi, A.A.; Sarin, R.; Kowtal, P. Additional cytosine inside mitochondrial C-tract D-loop as a progression risk factor in oral precancer cases. J. Oral Biol. Craniofacial Res. 2014, 4, 3–7. [Google Scholar] [CrossRef]
- Elamir, A.; ElRefai, S.M.; Ghazy, S.E. Molecular alterations of mitochondrial D-loop in oral leukoplakia. J. Cell. Biochem. 2019, 120, 13944–13951. [Google Scholar] [CrossRef]
- Banerjee, S.; Mukherjee, S.; Mitra, S.; Singhal, P. Comparative Evaluation of Mitochondrial Antioxidants in Oral Potentially Malignant Disorders. Kurume Med. J. 2020, 66, 15–27. [Google Scholar] [CrossRef]
- Pitiyage, G.N.; Slijepcevic, P.; Gabrani, A.; Chianea, Y.G.; Lim, K.P.; Prime, S.S.; Tilakaratne, W.M.; Fortune, F.; Parkinson, E.K. Senescent mesenchymal cells accumulate in human fibrosis by a telomere-independent mechanism and ameliorate fibrosis through matrix metalloproteinases. J. Pathol. 2011, 223, 604–617. [Google Scholar] [CrossRef]
- Benjamin, J.B.; Jayanthi, V.; Devaraj, H. MUC1 expression and its association with other aetiological factors and localization to mitochondria in preneoplastic and neoplastic gastric tissues. Clin. Chim. Acta Int. J. Clin. Chem. 2010, 411, 2067–2072. [Google Scholar] [CrossRef]
- Gruno, M.; Peet, N.; Tein, A.; Salupere, R.; Sirotkina, M.; Valle, J.; Peetsalu, A.; Seppet, E.K. Atrophic gastritis: Deficient complex I of the respiratory chain in the mitochondria of corpus mucosal cells. J. Gastroenterol. 2008, 43, 780–788. [Google Scholar] [CrossRef]
- Hirata, Y.; Sezaki, T.; Tamura-Nakano, M.; Oyama, C.; Hagiwara, T.; Ishikawa, T.; Fukuda, S.; Yamada, K.; Higuchi, K.; Dohi, T.; et al. Fatty acids in a high-fat diet potentially induce gastric parietal-cell damage and metaplasia in mice. J. Gastroenterol. 2017, 52, 889–903. [Google Scholar] [CrossRef]
- Braun, T.; Carvalho, G.; Coquelle, A.; Vozenin, M.C.; Lepelley, P.; Hirsch, F.; Kiladjian, J.J.; Ribrag, V.; Fenaux, P.; Kroemer, G. NF-kappaB constitutes a potential therapeutic target in high-risk myelodysplastic syndrome. Blood 2006, 107, 1156–1165. [Google Scholar] [CrossRef]
- Braun, T.; Carvalho, G.; Grosjean, J.; Ades, L.; Fabre, C.; Boehrer, S.; Debili, N.; Fenaux, P.; Kroemer, G. Differentiating megakaryocytes in myelodysplastic syndromes succumb to mitochondrial derangement without caspase activation. Apoptosis Int. J. Program. Cell Death 2007, 12, 1101–1108. [Google Scholar] [CrossRef]
- Abdelwahab, S.I.; Abdul, A.B.; Devi, N.; Taha, M.M.; Al-zubairi, A.S.; Mohan, S.; Mariod, A.A. Regression of cervical intraepithelial neoplasia by zerumbone in female Balb/c mice prenatally exposed to diethylstilboestrol: Involvement of mitochondria-regulated apoptosis. Exp. Toxicol. Pathol. Off. J. Ges. Toxikol. Pathol. 2010, 62, 461–469. [Google Scholar] [CrossRef]
- Jandová, A.; Pokorný, J.; Kobilková, J.; Janousek, M.; Masata, J.; Trojan, S.; Nedbalová, M.; Dohnalová, A.; Beková, A.; Slavík, V.; et al. Cell-mediated immunity in cervical cancer evolution. Electromagn. Biol. Med. 2009, 28, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Vaish, V.; Tanwar, L.; Kaur, J.; Sanyal, S.N. Chemopreventive effects of non-steroidal anti-inflammatory drugs in early neoplasm of experimental colorectal cancer: An apoptosome study. J. Gastrointest. Cancer 2011, 42, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Cruz, M.D.; Ledbetter, S.; Chowdhury, S.; Tiwari, A.K.; Momi, N.; Wali, R.K.; Bliss, C.; Huang, C.; Lichtenstein, D.; Bhattacharya, S.; et al. Metabolic reprogramming of the premalignant colonic mucosa is an early event in carcinogenesis. Oncotarget 2017, 8, 20543–20557. [Google Scholar] [CrossRef] [PubMed]
- Santhanam, S.; Rajamanickam, S.; Motamarry, A.; Ramakrishna, B.S.; Amirtharaj, J.G.; Ramachandran, A.; Pulimood, A.; Venkatraman, A. Mitochondrial electron transport chain complex dysfunction in the colonic mucosa in ulcerative colitis. Inflamm. Bowel Dis. 2012, 18, 2158–2168. [Google Scholar] [CrossRef]
- Santhanam, S.; Venkatraman, A.; Ramakrishna, B.S. Impairment of mitochondrial acetoacetyl CoA thiolase activity in the colonic mucosa of patients with ulcerative colitis. Gut 2007, 56, 1543–1549. [Google Scholar] [CrossRef]
- Zhang, X. Simultaneous exposure to dietary acrylamide and corn oil developed carcinogenesis through cell proliferation and inhibition of apoptosis by regulating p53-mediated mitochondria-dependent signaling pathway. Toxicol. Ind. Health 2009, 25, 101–109. [Google Scholar] [CrossRef]
- Ussakli, C.H.; Ebaee, A.; Binkley, J.; Brentnall, T.A.; Emond, M.J.; Rabinovitch, P.S.; Risques, R.A. Mitochondria and tumor progression in ulcerative colitis. J. Natl. Cancer Inst. 2013, 105, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- DaCosta, R.S.; Andersson, H.; Cirocco, M.; Marcon, N.E.; Wilson, B.C. Autofluorescence characterisation of isolated whole crypts and primary cultured human epithelial cells from normal, hyperplastic, and adenomatous colonic mucosa. J. Clin. Pathol. 2005, 58, 766–774. [Google Scholar] [CrossRef] [PubMed]
- de Luján Alvarez, M.; Cerliani, J.P.; Monti, J.; Carnovale, C.; Ronco, M.T.; Pisani, G.; Lugano, M.C.; Carrillo, M.C. The in vivo apoptotic effect of interferon alfa-2b on rat preneoplastic liver involves Bax protein. Hepatology 2002, 35, 824–833. [Google Scholar] [CrossRef]
- Schroeder, C.P.; Kadara, H.; Lotan, D.; Woo, J.K.; Lee, H.Y.; Hong, W.K.; Lotan, R. Involvement of mitochondrial and Akt signaling pathways in augmented apoptosis induced by a combination of low doses of celecoxib and N-(4-hydroxyphenyl) retinamide in premalignant human bronchial epithelial cells. Cancer Res. 2006, 66, 9762–9770. [Google Scholar] [CrossRef]
- Capiglioni, A.M.; Lorenzetti, F.; Quiroga, A.D.; Parody, J.P.; Ronco, M.T.; Pisani, G.B.; Carrillo, M.C.; Ceballos, M.P.; Alvarez, M.L. Attenuation of liver cancer development by oral glycerol supplementation in the rat. Eur. J. Nutr. 2018, 57, 1215–1224. [Google Scholar] [CrossRef] [PubMed]
- Metzger, C.; Mayer, D.; Hoffmann, H.; Bocker, T.; Hobe, G.; Benner, A.; Bannasch, P. Sequential appearance and ultrastructure of amphophilic cell foci, adenomas, and carcinomas in the liver of male and female rats treated with dehydroepiandrosterone. Toxicol. Pathol. 1995, 23, 591–605. [Google Scholar] [CrossRef]
- Reznik-Schüller, H.M.; Gregg, M. Sequential morphologic changes during methapyrilene-induced hepatocellular carcinogenesis in rats. J. Natl. Cancer Inst. 1983, 71, 1021–1031. [Google Scholar] [PubMed]
- Kakehashi, A.; Ishii, N.; Shibata, T.; Wei, M.; Okazaki, E.; Tachibana, T.; Fukushima, S.; Wanibuchi, H. Mitochondrial prohibitins and septin 9 are implicated in the onset of rat hepatocarcinogenesis. Toxicol. Sci. Off. J. Soc. Toxicol. 2011, 119, 61–72. [Google Scholar] [CrossRef] [PubMed]
- Mayer, D.; Metzger, C.; Leonetti, P.; Beier, K.; Benner, A.; Bannasch, P. Differential expression of key enzymes of energy metabolism in preneoplastic and neoplastic rat liver lesions induced by N-nitrosomorpholine and dehydroepiandrosterone. Int. J. Cancer 1998, 79, 232–240. [Google Scholar] [CrossRef]
- Poliakov, V.M.; Lankin, V.Z. Changes in phospholipid composition in rat liver microsomes and mitochondria under chemical carcinogenesis. Izmenenie sostava fosfolipidov v mikrosomakh i mitokhondriiakh pecheni krys pri khimicheskom kantserogeneze. Biokhimiia 1977, 42, 799–808. [Google Scholar]
- Anghileri, L.J.; Heidbreder, M.; Weiler, G.; Dermietzel, R. Mitochondrial calcium during liver carcinogenesis due to thioacetamide and 4-dimethylaminoazobenzene. Tumori 1977, 63, 7–14. [Google Scholar] [CrossRef]
- Karasaki, S. The fine structure of proliferating cells in preneoplastic rat livers during azo-dye carcinogenesis. J. Cell Biol. 1969, 40, 322–335. [Google Scholar] [CrossRef]
- Caputo, R.; Califano, A. Ultrastructural changes in the epidermis of Xeroderma pigmentosum lesions in various stages of development. Arch. Dermatol. Forsch. 1971, 241, 364–373. [Google Scholar] [CrossRef] [PubMed]
- Afifi, A.K.; Der Kaloustian, V.M.; Mire, J.J. Muscular abnormality in xeroderma pigmentosum. High resolution light-microscopy and electron-microscopic observations. J. Neurol. Sci. 1972, 17, 435–442. [Google Scholar] [CrossRef]
- Atri, S.K.; Van der Schueren, B.; Creemers, J.; De Loecker, W. Ultrastructural changes in the epidermis of mice induced by methylcholanthrene, croton oil and phorbol. Arch. Int. Physiol. Biochim. 1972, 80, 481–488. [Google Scholar] [CrossRef]
- Xylas, J.; Varone, A.; Quinn, K.P.; Pouli, D.; McLaughlin-Drubin, M.E.; Thieu, H.T.; Garcia-Moliner, M.L.; House, M.; Hunter, M.; Munger, K.; et al. Noninvasive assessment of mitochondrial organization in three-dimensional tissues reveals changes associated with cancer development. Int. J. Cancer 2015, 136, 322–332. [Google Scholar] [CrossRef]
- Wang, J.; Shi, X.; Johnson, R.H.; Kelbauskas, L.; Zhang, W.; Meldrum, D.R. Single-cell analysis reveals early manifestation of cancerous phenotype in pre-malignant esophageal cells. PLoS ONE 2013, 8, e75365. [Google Scholar] [CrossRef]
- Suchorolski, M.T.; Paulson, T.G.; Sanchez, C.A.; Hockenbery, D.; Reid, B.J. Warburg and Crabtree effects in premalignant Barrett’s esophagus cell lines with active mitochondria. PLoS ONE 2013, 8, e56884. [Google Scholar] [CrossRef]
- Verbeek, R.E.; Siersema, P.D.; Vleggaar, F.P.; Ten Kate, F.J.; Posthuma, G.; Souza, R.F.; de Haan, J.; van Baal, J.W. Toll-like Receptor 2 Signalling and the Lysosomal Machinery in Barrett’s Esophagus. J. Gastrointest. Liver Dis. JGLD 2016, 25, 273–282. [Google Scholar] [CrossRef]
- Xu, Y.; Surman, D.R.; Diggs, L.; Xi, S.; Gao, S.; Gurusamy, D.; McLoughlin, K.; Drake, J.; Feingold, P.; Brown, K.; et al. Bile acid-induced “Minority MOMP” promotes esophageal carcinogenesis while maintaining apoptotic resistance via Mcl-1. Oncogene 2020, 39, 877–890. [Google Scholar] [CrossRef]
- Vaughan, R.A.; Garcia-Smith, R.; Trujillo, K.A.; Bisoffi, M. Tumor necrosis factor alpha increases aerobic glycolysis and reduces oxidative metabolism in prostate epithelial cells. Prostate 2013, 73, 1538–1546. [Google Scholar] [CrossRef]
- Cao, J.; Ma, X.; Yan, X.; Zhang, G.; Hong, S.; Ma, R.; Wang, Y.; Ma, M. Kaempferol induces mitochondrial dysfunction and mitophagy by activating the LKB1/AMPK/MFF pathway in breast precancerous lesions. Phytother. Res. PTR 2023, 37, 3602–3616. [Google Scholar] [CrossRef]
- Warnakulasuriya, S.; Kujan, O.; Aguirre-Urizar, J.M.; Bagan, J.V.; González-Moles, M.; Kerr, A.R.; Lodi, G.; Mello, F.W.; Monteiro, L.; Ogden, G.R.; et al. Oral potentially malignant disorders: A consensus report from an international seminar on nomenclature and classification, convened by the WHO Collaborating Centre for Oral Cancer. Oral Dis. 2021, 27, 1862–1880. [Google Scholar] [CrossRef] [PubMed]
- Guan, J.Y.; Luo, Y.H.; Lin, Y.Y.; Wu, Z.Y.; Ye, J.Y.; Xie, S.M.; Li, J. Malignant transformation rate of oral leukoplakia in the past 20 years: A systematic review and meta-analysis. J. Oral Pathol. Med. Off. Publ. Int. Assoc. Oral Pathol. Am. Acad. Oral Pathol. 2023, 52, 691–700. [Google Scholar] [CrossRef] [PubMed]
- Stojanov, I.J.; Woo, S.B. Malignant Transformation Rate of Non-reactive Oral Hyperkeratoses Suggests an Early Dysplastic Phenotype. Head Neck Pathol. 2022, 16, 366–374. [Google Scholar] [CrossRef]
- Brandon, M.; Baldi, P.; Wallace, D.C. Mitochondrial mutations in cancer. Oncogene 2006, 25, 4647–4662. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, E.; Pedersen, P.L. High aerobic glycolysis of rat hepatoma cells in culture: Role of mitochondrial hexokinase. Proc. Natl. Acad. Sci. USA 1977, 74, 3735–3739. [Google Scholar] [CrossRef]
- Gatenby, R.A.; Gillies, R.J. Why do cancers have high aerobic glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Bungard, D.; Fuerth, B.J.; Zeng, P.-Y.; Faubert, B.; Maas, N.L.; Viollet, B.; Carling, D.; Thompson, C.B.; Jones, R.G.; Berger, S.L. Signaling Kinase AMPK Activates Stress-Promoted Transcription via Histone H2B Phosphorylation. Science 2010, 329, 1201–1205. [Google Scholar] [CrossRef]
- Mihara, M.; Erster, S.; Zaika, A.; Petrenko, O.; Chittenden, T.; Pancoska, P.; Moll, U.M. p53 Has a Direct Apoptogenic Role at the Mitochondria. Mol. Cell 2003, 11, 577–590. [Google Scholar] [CrossRef] [PubMed]
- Matissek, K.J.; Mossalam, M.; Okal, A.; Lim, C.S. The DNA Binding Domain of p53 Is Sufficient To Trigger a Potent Apoptotic Response at the Mitochondria. Mol. Pharm. 2013, 10, 3592–3602. [Google Scholar] [CrossRef]
- Goiran, T.; Duplan, E.; Rouland, L.; el Manaa, W.; Lauritzen, I.; Dunys, J.; You, H.; Checler, F.; da Costa, C.A. Nuclear p53-mediated repression of autophagy involves PINK1 transcriptional down-regulation. Cell Death Differ. 2018, 25, 873–884. [Google Scholar] [CrossRef]
- Kashatus, J.A.; Nascimento, A.; Myers, L.J.; Sher, A.; Byrne, F.L.; Hoehn, K.L.; Counter, C.M.; Kashatus, D.F. Erk2 Phosphorylation of Drp1 Promotes Mitochondrial Fission and MAPK-Driven Tumor Growth. Mol. Cell 2015, 57, 537–551. [Google Scholar] [CrossRef]
- Fang, X.; Liu, C.-X.; Zeng, X.-R.; Huang, X.-M.; Chen, W.-L.; Wang, Y.; Ai, F. Orphan nuclear receptor COUP-TFII is an oncogenic gene in renal cell carcinoma. Clin. Transl. Oncol. 2020, 22, 772–781. [Google Scholar] [CrossRef]
- Stine, Z.E.; Walton, Z.E.; Altman, B.J.; Hsieh, A.L.; Dang, C.V. MYC, Metabolism, and Cancer. Cancer Discov. 2015, 5, 1024–1039. [Google Scholar] [CrossRef]
- Petros, J.A.; Baumann, A.K.; Ruiz-Pesini, E.; Amin, M.B.; Sun, C.Q.; Hall, J.; Lim, S.; Issa, M.M.; Flanders, W.D.; Hosseini, S.H.; et al. mtDNA mutations increase tumorigenicity in prostate cancer. Proc. Natl. Acad. Sci. USA 2005, 102, 719–724. [Google Scholar] [CrossRef]
- Lee, H.C.; Li, S.H.; Lin, J.C.; Wu, C.C.; Yeh, D.C.; Wei, Y.H. Somatic mutations in the D-loop and decrease in the copy number of mitochondrial DNA in human hepatocellular carcinoma. Mutat. Res. Mol. Mech. Mutagen. 2004, 547, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Higuchi, M. Regulation of mitochondrial DNA content and cancer. Mitochondrion 2007, 7, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Yu, M. Generation, function and diagnostic value of mitochondrial DNA copy number alterations in human cancers. Life Sci. 2011, 89, 65–71. [Google Scholar] [CrossRef] [PubMed]
| / | Genetic Alteration | Pathway | mt-Related Protein | Alteration of mt-Function | Precancerous Conditions | Feature of Precancerous Conditions | Therapeutic Molecule and Mitochondrial Target in This Disease | Ref |
|---|---|---|---|---|---|---|---|---|
| nDNA | Pten mutation | PI3K ↑ and AMPK ↓ | TCA cycle and OXPHOS gene expression ↓ | Warburg effect ↑ | Thyroid hyperplasia | / | / | [53] |
| / | / | Oxidative stress ↑ | Pancreatic precancerous lesion [54]: PanIN | Low-grade dysplasia, high-grade dysplasia (carcinoma in situ) [55] | / | [56] | ||
| KRAS mutation | EGFR signalling ↑ | / | [57] | |||||
| Xpc (mice) mutation | 1. NOX1 ↑ 2. Reduced GSH/GSSG ratio ↓ | 1. Complex I ↓ 2. COX1, CYTB, and 16S rRNA ↑ | 1. Warburg effete↑ 2. Oxidative stress ↑ | XP | / | TFN: DHODH ↓ [58]; Comp-1: HK2 detachment from the mitochondria [59] | [60,61,62,63,64] | |
| XPC/XPA mutation | ||||||||
| SNP at POLG2 | 1. POLG2 ↑ 2. Enzymes responsible for mtDNA synthesis and transcription ↑ | / | mtDNA copy ↑ | OLK | Hyperplasia, mild dysplasia, moderate dysplasia, severe dysplasia, and carcinoma in situ [65]. | Erythrosine (photosensitizer): mitochondrial accumulation [66] | [67] | |
| COX4I2 and COX8C high expression | / | OXPHOS protein markers (like ATP5B and HSP60) ↑ | Respiration discovery | BE | Normal squamous epithelium, metaplasia, dysplasia, and esophageal adenocarcinoma | / | [68] | |
| BAK1, FIS1, and SFN overexpression | / | / | Apoptosis ↓ | [69] | ||||
| mtDNA | Genome instability in mtDNA | / | / | Oxidative stress ↑ | [70] | |||
| / | / | / | Gastric precancerous lesion | Chronic gastritis, atrophy, intestinal metaplasia, and dysplasia [71] | / | [72] | ||
| / | / | / | UC | Low-grade dysplasia, high-grade dysplasia [73] | / | [74] | ||
| / | / | / | Cervical dysplasia | / | / | [75] | ||
| COX mutation | / | COX ↓ | / | DCIS | / | / | [76]. | |
| Mutation in C-tract | / | / | / | OPMD; Head and neck precancerous lesion | / | / | [77,78] | |
| Mutation in D-loop | / | / | mtDNA copy ↑ | OLK | Hyperplasia, mild dysplasia, moderate dysplasia, severe dysplasia, and carcinoma in situ [65]. | / | [79] | |
| Non-genetic research | / | / | / | Oxidative stress ↓ | OLK, OPL, OSMF | / | / | [80] |
| / | Oxidative stress ↑ | OSMF | / | / | [81] | |||
| / | Apoptosis ↓ | Gastric precancerous lesion | Chronic gastritis, atrophy, intestinal metaplasia, and dysplasia [71] | / | [82] | |||
| / | Warburg effect ↑ | [83] | ||||||
| / | Apoptosis ↑ | [84] | ||||||
| / | Apoptosis ↑ | MDS | / | Bortezomib: NF-κB ↓ and mitochondrial related cell death [85] | [86] | |||
| / | Warburg effect ↑ | Cervical precancerous lesion | / | ZER: BAX ↑ and BCL-2 ↓ [87] | [88] | |||
| HIF-1α, GLUT1, PKM2, and LDHA, Drp1, OPA1, PGC-1α, UCP2 and mtND1 ↑ | 1. Warburg effect ↑ 2. Mitochondria number ↑ 3. mtDNA copy ↑ | Premalignant colorectal lesion | / | NSAIDs (Diclofenac and Celecoxib): BCL-2 ↓ [89] | [90] | |||
| / | Warburg effect ↑ | [91] | ||||||
| / | Oxidative stress ↑ | [92] | ||||||
| / | Apoptosis ↓ | [93] | ||||||
| / | Mitochondria number ↓ | [94] | ||||||
| / | Mitochondria number ↑ | [95] | ||||||
| / | Liver preneoplastic lesion | / | 1. IFN-α2b: BAX ↑ [96]; 2. Combination of celecoxib and synthetic retinoid N-(4-hydroxyphenyl) retinamide (4HPR): BCL-2 ↓ [97]; 3. Glycerol: BAX/BCL-2 ratio ↑, Bad ↑, and PUMA ↑ [98]. | [99,100] | ||||
| Mitochondrial chaperons ↑ | / | [101] | ||||||
| COX, SDH and glycerol-3-phosphate dehydrogenase ↑ | / | [102] | ||||||
| Change in phospholipid composition of mitochondria | / | [103] | ||||||
| / | Apoptosis ↑ | [104] | ||||||
| Changes in mitochondrial morphology | [105] | |||||||
| XP | / | / | [106,107] | |||||
| Engineered precancerous epithelial tissue | / | / | [108] | |||||
| Warburg effect (↑ or ↓) | [109] | |||||||
| 1. mtDNA copy ↑ 2. Apoptosis ↓ | BE | Normal squamous epithelium, metaplasia, dysplasia, and esophageal adenocarcinoma | / | [110] | ||||
| Warburg effect (↑ or ↓) | [111] | |||||||
| Mitochondria number ↑ | [112] | |||||||
| Apoptosis ↓ | [113] | |||||||
| Warburg effect ↑ | Pre-malignant prostate lesion | / | / | [114] | ||||
| / | Breast precancerous lesion | / | Kaempferol: Drp1 ↑ | [115] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, L.; Pan, D.; Ai, R.; Zhou, Y. Mitochondria-Associated Pathways in Cancer and Precancerous Conditions: Mechanistic Insights. Int. J. Mol. Sci. 2025, 26, 8537. https://doi.org/10.3390/ijms26178537
Li L, Pan D, Ai R, Zhou Y. Mitochondria-Associated Pathways in Cancer and Precancerous Conditions: Mechanistic Insights. International Journal of Molecular Sciences. 2025; 26(17):8537. https://doi.org/10.3390/ijms26178537
Chicago/Turabian StyleLi, Ling, Dan Pan, Ruixue Ai, and Yu Zhou. 2025. "Mitochondria-Associated Pathways in Cancer and Precancerous Conditions: Mechanistic Insights" International Journal of Molecular Sciences 26, no. 17: 8537. https://doi.org/10.3390/ijms26178537
APA StyleLi, L., Pan, D., Ai, R., & Zhou, Y. (2025). Mitochondria-Associated Pathways in Cancer and Precancerous Conditions: Mechanistic Insights. International Journal of Molecular Sciences, 26(17), 8537. https://doi.org/10.3390/ijms26178537






