The Combined Potential of PRP and Osteoinductive Carrier Matrices for Bone Regeneration
Abstract
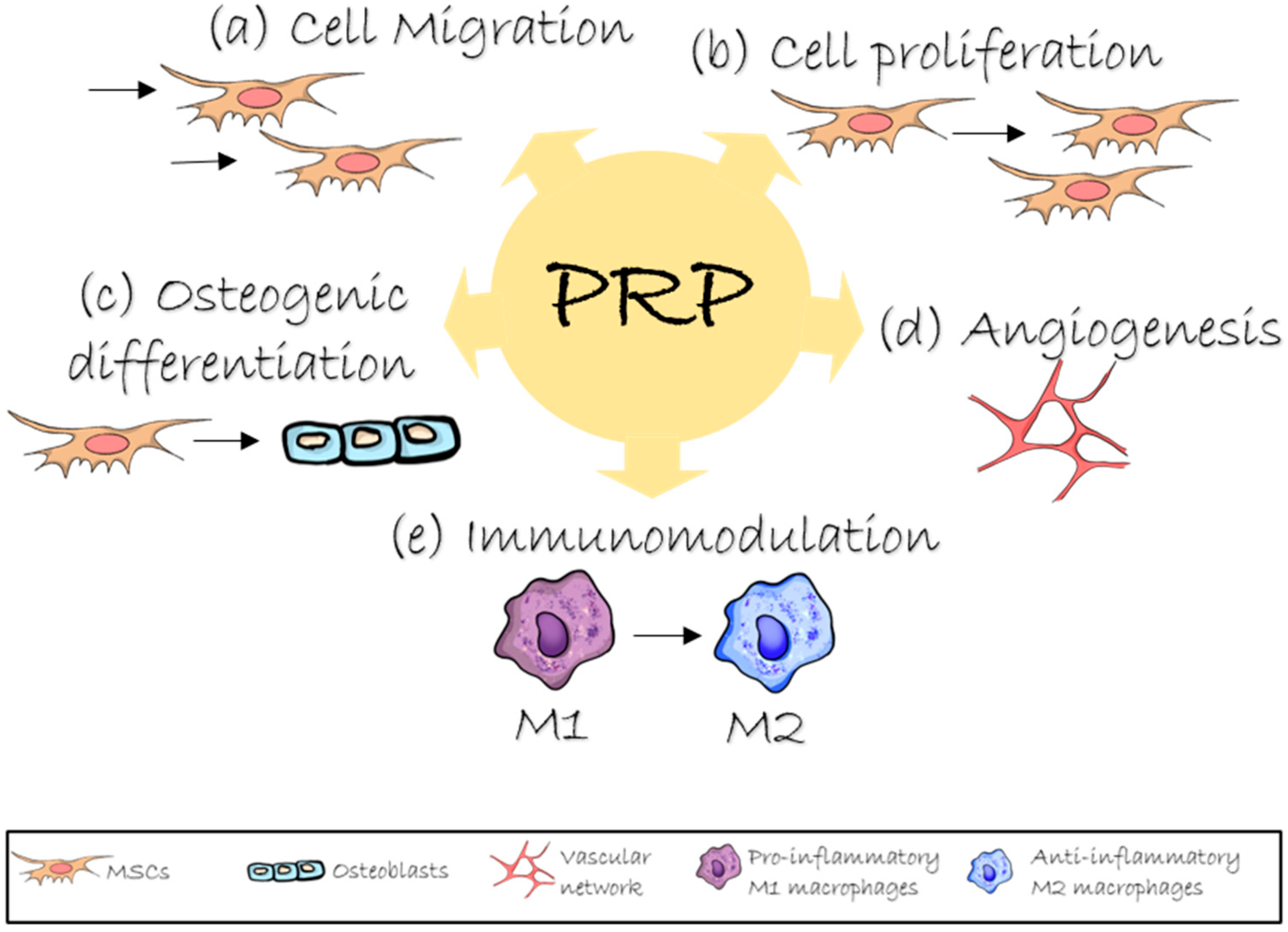
1. Introduction
2. Role of PRP in Bone Regeneration
3. Platelet Concentrates Combined with Biomaterials
4. Platelet Concentrates Combined with BMP-2
5. Platelet Concentrates Combined with Gene Vectors
6. Platelet Concentrates Combined with Stem Cells
7. Platelet Concentrates Combined with Gene-Modified Stem Cells
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ADSCs | Adipose Derived Stem Cells |
| BMPs | Bone Morphogenetic Proteins |
| BMSCs | Bone Marrow Stromal Cells |
| FGF2 | Fibroblast Growth Factor 2 |
| MSCs | Mesenchymal Stem Cells |
| PLA | Poly(Lactic Acid) |
| PRF | Platelet-Rich Fibrin |
| PRP | Platelet-Rich Plasma |
| β-TCP | β-Tricalcium Phosphate |
References
- Jamal, M.S.; Hurley, E.T.; Asad, H.; Asad, A.; Taneja, T. The Role of Platelet Rich Plasma and Other Orthobiologics in Bone Healing and Fracture Management: A Systematic Review. J. Clin. Orthop. Trauma 2022, 25, 101759. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Rasmusson, L.; Albrektsson, T. Classification of Platelet Concentrates: From Pure Platelet-Rich Plasma (P-PRP) to Leucocyte- and Platelet-Rich Fibrin (L-PRF). Trends Biotechnol. 2009, 27, 158–167. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Lin, Y.; Hu, X.; Zhang, Y.; Wu, H. A Comparative Study of Platelet-Rich Fibrin (PRF) and Platelet-Rich Plasma (PRP) on the Effect of Proliferation and Differentiation of Rat Osteoblasts in Vitro. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2009, 108, 707–713. [Google Scholar] [CrossRef]
- Li, Y.; Li, R.; Hu, J.; Song, D.; Jiang, X.; Zhu, S. Recombinant Human Bone Morphogenetic Protein-2 Suspended in Fibrin Glue Enhances Bone Formation during Distraction Osteogenesis in Rabbits. Arch. Med. Sci. 2016, 12, 494–501. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Zhang, X.; Yu, Y.; Wang, J.; Liu, C. Dual-Function Injectable Fibrin Gel Incorporated with Sulfated Chitosan Nanoparticles for RhBMP-2-Induced Bone Regeneration. Appl. Mater. Today 2022, 26, 101347. [Google Scholar] [CrossRef]
- Liebig, B.E.; Kisiday, J.D.; Bahney, C.S.; Ehrhart, N.P.; Goodrich, L.R. The Platelet-Rich Plasma and Mesenchymal Stem Cell Milieu: A Review of Therapeutic Effects on Bone Healing. J. Orthop. Res. 2020, 38, 2539–2550. [Google Scholar] [CrossRef]
- Yang, Y.; Xiao, Y. Biomaterials Regulating Bone Hematoma for Osteogenesis. Adv. Healthc. Mater. 2020, 9, 2000726. [Google Scholar] [CrossRef]
- Bacevich, B.M.; Smith, R.D.J.; Reihl, A.M.; Mazzocca, A.D.; Hutchinson, I.D. Advances with Platelet-Rich Plasma for Bone Healing. Biol. Targets Ther. 2024, 18, 29–59. [Google Scholar] [CrossRef]
- Middleton, K.K.; Barro, V.; Muller, B.; Terada, S.; Fu, F.H. Evaluation of the Effects of Platelet-Rich Plasma (PRP) Therapy Involved in the Healing of Sports-Related Soft Tissue Injuries. Iowa Orthop. J. 2012, 32, 150. [Google Scholar]
- Zhang, N.; Wu, Y.P.; Qian, S.J.; Teng, C.; Chen, S.; Li, H. Research Progress in the Mechanism of Effect of PRP in Bone Deficiency Healing. Sci. World J. 2013, 2013, 134582. [Google Scholar] [CrossRef] [PubMed]
- Everts, P.A.; Mazzola, T.; Mautner, K.; Randelli, P.S.; Podesta, L. Modifying Orthobiological PRP Therapies Are Imperative for the Advancement of Treatment Outcomes in Musculoskeletal Pathologies. Biomedicines 2022, 10, 2933. [Google Scholar] [CrossRef]
- Zhu, L.; Li, P.; Qin, Y.; Xiao, B.; Li, J.; Xu, W.; Yu, B. Platelet-Rich Plasma in Orthopedics: Bridging Innovation and Clinical Applications for Bone Repair. J. Orthop. Surg. 2024, 32, 10225536231224952. [Google Scholar] [CrossRef]
- Escobar, G.; Escobar, A.; Ascui, G.; Tempio, F.I.; Ortiz, M.C.; Pérez, C.A.; López, M.N. Pure Platelet-Rich Plasma and Supernatant of Calcium-Activated P-PRP Induce Different Phenotypes of Human Macrophages. Regen. Med. 2018, 13, 427–441. [Google Scholar] [CrossRef]
- Mariani, E.; Pulsatelli, L. Platelet Concentrates in Musculoskeletal Medicine. Int. J. Mol. Sci. 2020, 21, 1328. [Google Scholar] [CrossRef]
- Lian, S.; Mu, Z.; Yuan, Z.; Shafiq, M.; Mo, X.; Mu, W. Methacrylated Gelatin and Platelet-Rich Plasma Based Hydrogels Promote Regeneration of Critical-Sized Bone Defects. Regen. Biomater. 2024, 11, rbae022. [Google Scholar] [CrossRef]
- Kasten, P.; Vogel, J.; Luginbühl, R.; Niemeyer, P.; Weiss, S.; Schneider, S.; Kramer, M.; Leo, A.; Richter, W. Influence of Platelet-Rich Plasma on Osteogenic Differentiation of Mesenchymal Stem Cells and Ectopic Bone Formation in Calcium Phosphate Ceramics. Cells Tissues Organs 2006, 183, 68–79. [Google Scholar] [CrossRef] [PubMed]
- McLaughlin, M.; Gagnet, P.; Cunningham, E.; Yeager, R.; D’Amico, M.; Guski, K.; Scarpone, M.; Kuebler, D. Allogeneic Platelet Releasate Preparations Derived via a Novel Rapid Thrombin Activation Process Promote Rapid Growth and Increased BMP-2 and BMP-4 Expression in Human Adipose-Derived Stem Cells. Stem Cells Int. 2016, 2016, 7183734. [Google Scholar] [CrossRef] [PubMed]
- Casati, L.; Celotti, F.; Negri-Cesi, P.; Sacchi, M.C.; Castanoy, P.; Colciago, A. Platelet Derived Growth Factor (PDGF) Contained in Platelet Rich Plasma (PRP) Stimulates Migration of Osteoblasts by Reorganizing Actin Cytoskeleton. Cell Adh. Migr. 2014, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Cao, L.; Ye, L.; Du, J.; Shan, G.; Hu, J.; Jiang, C.; Song, W. Autogenous Bone Particles Combined with Platelet-Rich Plasma Can Stimulate Bone Regeneration in Rabbits. Exp. Ther. Med. 2020, 20, 279. [Google Scholar] [CrossRef]
- Dallari, D.; Fini, M.; Stagni, C.; Torricelli, P.; Aldini, N.N.; Giavaresi, G.; Cenni, E.; Baldini, N.; Cenacchi, A.; Bassi, A.; et al. In Vivo Study on the Healing of Bone Defects Treated with Bone Marrow Stromal Cells, Platelet-Rich Plasma, and Freeze-Dried Bone Allografts, Alone and in Combination. J. Orthop. Res. 2006, 24, 877–888. [Google Scholar] [CrossRef]
- Ilgenli, T.; Dündar, N.; Kal, B.I. Demineralized Freeze-Dried Bone Allograft and Platelet-Rich Plasma vs. Platelet-Rich Plasma Alone in Infrabony Defects: A Clinical and Radiographic Evaluation. Clin. Oral Investig. 2007, 11, 51–59. [Google Scholar] [CrossRef]
- Kanthan, S.R.; Kavitha, G.; Addi, S.; Choon, D.S.K.; Kamarul, T. Platelet-Rich Plasma (PRP) Enhances Bone Healing in Non-United Critical-Sized Defects: A Preliminary Study Involving Rabbit Models. Injury 2011, 42, 782–789. [Google Scholar] [CrossRef]
- Hexter, A.T.; Karali, A.; Kao, A.; Tozzi, G.; Heidari, N.; Petrie, A.; Boyd, A.; Kalaskar, D.M.; Pendegrass, C.; Rodeo, S.; et al. Effect of Demineralized Bone Matrix, Bone Marrow Mesenchymal Stromal Cells, and Platelet-Rich Plasma on Bone Tunnel Healing After Anterior Cruciate Ligament Reconstruction: A Comparative Micro-Computed Tomography Study in a Tendon Allograft Sheep Model. Orthop. J. Sports Med. 2021, 9, 23259671211034166. [Google Scholar] [CrossRef]
- Ding, Z.-Y.; Tan, Y.; Peng, Q.; Zuo, J.; Li, N. Novel Applications of Platelet Concentrates in Tissue Regeneration (Review). Exp. Ther. Med. 2021, 21, 226. [Google Scholar] [CrossRef] [PubMed]
- Skwarcz, S.; Bryzek, I.; Gregosiewicz, A.; Warda, E.; Gawȩda, K.; Tarczyńska, M.; Skwarcz, J.; Nadulski, R.; Starek, A.; Sanford, J. The Effect of Activated Platelet-Rich Plasma (PRP) on Tricalcium Hydroxyapatite Phosphate Healing in Experimental, Partial Defects of Long Bone Shafts in Animal Models. Pol. J. Vet. Sci. 2019, 22, 243–250. [Google Scholar] [CrossRef]
- Yilmaz, D.; Dogan, N.; Ozkan, A.; Sencimen, M.; Ora, B.E.; Mutlu, I. Effect of Platelet Rich Fibrin and Beta Tricalcium Phosphate on Bone Healing. A Histological Study in Pigs. Acta Cir. Bras. 2014, 29, 59–65. [Google Scholar] [CrossRef]
- Zhang, Y.D.; Wang, G.; Sun, Y.; Zhang, C.Q. Combination of Platelet-Rich Plasma with Degradable Bioactive Borate Glass for Segmental Bone Defect Repair. Acta Orthop. Belg. 2011, 77, 110–115. [Google Scholar]
- Bahraminasab, M.; Doostmohammadi, N.; Talebi, A.; Arab, S.; Alizadeh, A.; Ghanbari, A.; Salati, A. 3D Printed Polylactic Acid/Gelatin-Nano-Hydroxyapatite/Platelet-Rich Plasma Scaffold for Critical-Sized Skull Defect Regeneration. Biomed. Eng. Online 2022, 21, 86. [Google Scholar] [CrossRef] [PubMed]
- Pryor, M.E.; Polimeni, G.; Koo, K.T.; Hartman, M.J.; Gross, H.; April, M.; Safadi, F.F.; Wikesjö, U.M.E. Analysis of Rat Calvaria Defects Implanted with a Platelet-Rich Plasma Preparation: Histologic and Histometric Observations. J. Clin. Periodontol. 2005, 32, 966–972. [Google Scholar] [CrossRef] [PubMed]
- Abdullah, W.A. Evaluation of Bone Regenerative Capacity in Rats Claverial Bone Defect Using Platelet Rich Fibrin with and without Beta Tri Calcium Phosphate Bone Graft Material. Saudi Dent. J. 2016, 28, 109–117. [Google Scholar] [CrossRef]
- Oley, M.C.; Islam, A.A.; Hatta, M.; Hardjo, M.; Nirmalasari, L.; Rendy, L.; Ana, I.D.; Bachtiar, I. Effects of Platelet-Rich Plasma and Carbonated Hydroxyapatite Combination on Cranial Defect Bone Regeneration: An Animal Study. Wound Med. 2018, 21, 12–15. [Google Scholar] [CrossRef]
- Segundo, F.A.D.S.; Costa, E.I.D.S.; De Azevedo, A.S.; De Araújo, A.L.; Silva, A.C.D.F.; De Lima, G.G.; De Sá, M.J.C. Platelet-Rich Plasma, Hydroxyapatite, and Chitosan in the Bone and Cartilaginous Regeneration of Femoral Trochlea in Rabbits: Clinical, Radiographic, and Histomorphometric Evaluations. J. Healthc. Eng. 2018, 2018, 6917958. [Google Scholar] [CrossRef]
- You, J.S.; Kim, S.G.; Oh, J.S.; Kim, J.S. Effects of Platelet-Derived Material (Platelet-Rich Fibrin) on Bone Regeneration. Implant. Dent. 2019, 28, 244–255. [Google Scholar] [CrossRef]
- Tang, S.; Wang, L.; Zhang, Y.; Zhang, F. A Biomimetic Platelet-Rich Plasma-Based Interpenetrating Network Printable Hydrogel for Bone Regeneration. Front. Bioeng. Biotechnol. 2022, 10, 887454. [Google Scholar] [CrossRef]
- Tatullo, M.; Marrelli, M.; Cassetta, M.; Pacifici, A.; Stefanelli, L.V.; Scacco, S.; Dipalma, G.; Pacifici, L.; Inchingolo, F. Platelet Rich Fibrin (P.R.F.) in Reconstructive Surgery of Atrophied Maxillary Bones: Clinical and Histological Evaluations. Int. J. Med. Sci. 2012, 9, 872. [Google Scholar] [CrossRef]
- Eskan, M.A.; Greenwell, H.; Hill, M.; Morton, D.; Vidal, R.; Shumway, B.; Girouard, M.-E. Platelet-Rich Plasma–Assisted Guided Bone Regeneration for Ridge Augmentation: A Randomized, Controlled Clinical Trial. J. Periodontol. 2014, 85, 661–668. [Google Scholar] [CrossRef]
- Pocaterra, A.; Caruso, S.; Bernardi, S.; Scagnoli, L.; Continenza, M.A.; Gatto, R. Effectiveness of Platelet-Rich Plasma as an Adjunctive Material to Bone Graft: A Systematic Review and Meta-Analysis of Randomized Controlled Clinical Trials. Int. J. Oral Maxillofac. Surg. 2016, 45, 1027–1034. [Google Scholar] [CrossRef] [PubMed]
- Aghaloo, T.L.; Moy, P.K.; Freymiller, E.G. Investigation of Platelet-Rich Plasma in Rabbit Cranial Defects: A Pilot Study. J. Oral Maxillofac. Surg. 2002, 60, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Hakimi, M.; Jungbluth, P.; Sager, M.; Betsch, M.; Herten, M.; Becker, J.; Windolf, J.; Wild, M. Combined Use of Platelet-Rich Plasma and Autologous Bone Grafts in the Treatment of Long Bone Defects in Mini-Pigs. Injury 2010, 41, 717–723. [Google Scholar] [CrossRef]
- Thorwarth, M.; Wehrhan, F.; Schultze-Mosgau, S.; Wiltfang, J.; Schlegel, K.A. PRP Modulates Expression of Bone Matrix Proteins in Vivo without Long-Term Effects on Bone Formation. Bone 2006, 38, 30–40. [Google Scholar] [CrossRef]
- Karayürek, F.; Kadiroğlu, E.T.; Nergiz, Y.; Coşkun Akçay, N.; Tunik, S.; Ersöz Kanay, B.; Uysal, E. Combining Platelet Rich Fibrin with Different Bone Graft Materials: An Experimental Study on the Histopathological and Immunohistochemical Aspects of Bone Healing. J. Cranio-Maxillofac. Surg. 2019, 47, 815–825. [Google Scholar] [CrossRef]
- Berner, A.; Boerckel, J.D.; Saifzadeh, S.; Steck, R.; Ren, J.; Vaquette, C.; Zhang, J.Q.; Nerlich, M.; Guldberg, R.E.; Hutmacher, D.W.; et al. Biomimetic Tubular Nanofiber Mesh and Platelet Rich Plasma-Mediated Delivery of BMP-7 for Large Bone Defect Regeneration. Cell Tissue Res. 2012, 347, 603–612. [Google Scholar] [CrossRef]
- Park, J.H.; Kim, J.W.; Kim, S.J. Does the Addition of Bone Morphogenetic Protein 2 to Platelet-Rich Fibrin Improve Healing After Treatment for Medication-Related Osteonecrosis of the Jaw? J. Oral Maxillofac. Surg. 2017, 75, 1176–1184. [Google Scholar] [CrossRef]
- Elsalanty, M.E.; Por, Y.C.; Genecov, D.G.; Salyer, K.E.; Wang, Q.; Barcelo, C.R.; Troxler, K.; Gendler, E.; Opperman, L.A. Recombinant Human BMP-2 Enhances the Effects of Materials Used for Reconstruction of Large Cranial Defects. J. Oral Maxillofac. Surg. 2008, 66, 277–285. [Google Scholar] [CrossRef]
- Liu, Y.; Lin, D.; Li, B.; Hong, H.; Jiang, C.; Yuan, Y.; Wang, J.; Hu, R.; Li, B.; Liu, C. BMP-2/CPC Scaffold with Dexamethasone-Loaded Blood Clot Embedment Accelerates Clinical Bone Regeneration. Am. J. Transl. Res. 2022, 14, 2874. [Google Scholar] [PubMed]
- Kaipel, M.; Schützenberger, S.; Hofmann, A.T.; Ferguson, J.; Nau, T.; Redl, H.; Feichtinger, G.A. Evaluation of Fibrin-Based Gene-Activated Matrices for BMP2/7 Plasmid Codelivery in a Rat Nonunion Model. Int. Orthop. 2014, 38, 2607–2613. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.-S.; Associate Professor, P.D.; Weber, H.-P.; Raymond, D.J.; Pomfret Nagle Professor, E.; Wright, R.F.; Associate Professor, D.; Mooney, D.J.; Gordon McKay Professor of Bioengineering, P.D. Improved Bone Healing by Angiogenic Factor-Enriched Platelet-Rich Plasma and Its Synergistic Enhancement by Bone Morphogenetic Protein-2. Int. J. Oral Maxillofac. Implant. 2008, 23, 818. [Google Scholar]
- Bukharova, T.B.; Nedorubova, I.A.; Mokrousova, V.O.; Meglei, A.Y.; Basina, V.P.; Nedorubov, A.A.; Vasilyev, A.V.; Grigoriev, T.E.; Zagoskin, Y.D.; Chvalun, S.N.; et al. Adenovirus-Based Gene Therapy for Bone Regeneration: A Comparative Analysis of In Vivo and Ex Vivo BMP2 Gene Delivery. Cells 2023, 12, 1762. [Google Scholar] [CrossRef]
- Meglei, A.Y.; Nedorubova, I.A.; Basina, V.P.; Chernomyrdina, V.O.; Nedorubov, A.A.; Kuznetsova, V.S.; Vasilyev, A.V.; Kutsev, S.I.; Goldshtein, D.V.; Bukharova, T.B. Collagen–Platelet-Rich Plasma Mixed Hydrogels as a PBMP2 Delivery System for Bone Defect Regeneration. Biomedicines 2024, 12, 2461. [Google Scholar] [CrossRef]
- Nedorubova, I.A.; Bukharova, T.B.; Mokrousova, V.O.; Khvorostina, M.A.; Vasilyev, A.V.; Nedorubov, A.A.; Grigoriev, T.E.; Zagoskin, Y.D.; Chvalun, S.N.; Kutsev, S.I.; et al. Comparative Efficiency of Gene-Activated Matrices Based on Chitosan Hydrogel and PRP Impregnated with BMP2 Polyplexes for Bone Regeneration. Int. J. Mol. Sci. 2022, 23, 14720. [Google Scholar] [CrossRef]
- Khorsand, B.; Acri, T.M.; Do, A.V.; Femino, J.E.; Petersen, E.; Fredericks, D.C.; Salem, A.K. A Multi-Functional Implant Induces Bone Formation in a Diabetic Model. Adv. Healthc. Mater. 2020, 9, 2000770. [Google Scholar] [CrossRef]
- Wang, G.; Cui, Y.; Leng, Y.; Sun, S.; Yuan, B.; Liu, H.; Peng, C.; Wu, D. Engineered Three-Dimensional Bioactive Scaffold for Enhanced Bone Regeneration through Modulating Transplanted Adipose Derived Mesenchymal Stem Cell and Stimulating Angiogenesis. Front. Bioeng. Biotechnol. 2024, 12, 1342590. [Google Scholar] [CrossRef]
- Tajima, S.; Tobita, M.; Orbay, H.; Hyakusoku, H.; Mizuno, H. Direct and Indirect Effects of a Combination of Adipose-Derived Stem Cells and Platelet-Rich Plasma on Bone Regeneration. Tissue Eng. Part A 2015, 21, 895–905. [Google Scholar] [CrossRef]
- Almansoori, A.A.; Kwon, O.-J.; Nam, J.-H.; Seo, Y.-K.; Song, H.-R.; Lee, J.-H. Mesenchymal Stem Cells and Platelet-Rich Plasma-Impregnated Polycaprolactone-β Tricalcium Phosphate Bio-Scaffold Enhanced Bone Regeneration around Dental Implants. Int. J. Implant. Dent. 2021, 7, 35. [Google Scholar] [CrossRef]
- Park, C.G.; Joo, M.W.; Jeong, J.; Kang, Y.K.; Lee, D.R. Evaluation of the Effects of the Combination of Autologous Mesenchymal Stem Cells and Platelet-Rich Plasma on Structural Bone Allograft Healing. Cell Tissue Bank. 2017, 18, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Yuan, X.; Fernandes, G.; Dziak, R.; Ionita, C.N.; Li, C.; Wang, C.; Yang, S. The Combination of Nano-Calcium Sulfate/Platelet Rich Plasma Gel Scaffold with BMP2 Gene-Modified Mesenchymal Stem Cells Promotes Bone Regeneration in Rat Critical-Sized Calvarial Defects. Stem Cell Res. Ther. 2017, 8, 122. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Wang, C.; Yuan, X.; Liu, Z.; Dziak, R.; Yang, S. Combination of Controlled Release Platelet-Rich Plasma Alginate Beads and Bone Morphogenetic Protein-2 Genetically Modified Mesenchymal Stem Cells for Bone Regeneration. J. Periodontol. 2016, 87, 470–480. [Google Scholar] [CrossRef]
- Bukharova, T.B.; Volkov, A.V.; Voronin, A.S.; Filimonov, K.A.; Chaplygin, S.S.; Murushidi, M.Y.; Nazaryan, A.K.; Zadorina, A.Y.; Rubinskaya, M.S.; Rozenbaum, A.Y.; et al. Development of Tissue Engineering Construction Based on Multipotent Stromal Cells of Human Adipose Tissue Transfected with the Gene of Bone Morphogenic Protein BMP-2. Clin. Exp. Morphol. 2013, 1, 45–51. Available online: https://elibrary.ru/item.asp?id=18912641 (accessed on 27 August 2025).
| Biomaterial | Model/Defect | Osteoinductor | Results | Reference |
|---|---|---|---|---|
| Calcium phosphate cement, blood clot and dexamethasone | Rats/Calvarial defect; Patients/Tibial plateau fractures or proximal humeral fractures | rhBMP-2 | The addition of a blood clot to the scaffold resulted in more efficient bone formation compared to the scaffold alone. The introduction of dexamethasone effectively facilitated M2 polarization of macrophages | [45] |
| Fibrin gel incorporated with sulfated chitosan nanoparticles | Mice/Femoral defect | rhBMP-2 | Fibrin scaffold combined with an inductive protein demonstrated the highest bone formation when compared to empty defects or protein-only administration. Sulfated chitosan nanoparticles regulate the macrophage polarization | [5] |
| Fibrin gel | Rabbits/Tibial defect | rhBMP-2 | The mineral density and new bone volume were significantly higher in the rhBMP-2 + fibrin gel group compared to other treatment groups | [4] |
| Fibrin gel | Rats/Femoral defect | rhBMP-2 | In the group using fibrin gel in combination with protein, a trend towards increased bone volume was observed compared to fibrin implantation alone | [46] |
| Calcium phosphate-coated nanofiber mesh tube and PRP | Rats/Femoral defect | BMP-7 | Scaffolds containing BMP-7 and PRP resulted in complete defect closure by 12 weeks after implantation compared to both empty scaffolds and PRP only scaffolds | [42] |
| Fibronectin-coated PGA scaffolds and modified PRP | Rats/Calvarial defect | rhBMP-2 | PGA scaffolds combined with both PRP and rhBMP-2 promoted more substantial bone defect healing than either PGA+PRP or PGA+rhBMP-2 alone. Modified PRP induced faster migration of cord blood-derived outgrowth endothelial-like cells and significantly increased numbers of blood vessels | [47] |
| L-PRF and collagen sponge | Patients/Medication-related osteonecrosis of the jaws | rhBMP-2 | The combined application of BMP-2 and L-PRF led to earlier new bone formation relative to L-PRF administration alone | [43] |
| Demineralized bone matrix and PRP | Dogs/Calvarial defect | rhBMP-2 | PRP supplementation showed no significant effect on the regeneration process, regardless of rhBMP-2 co-administration | [44] |
| Biomaterial | Model/Defect | Osteoinductor | Results | Reference |
|---|---|---|---|---|
| Polylactide particles PRP-based fibrin clot | Rats/Calvarial defect | Ad-BMP2 | The gene-activated PLA/PRP-Ad-BMP2 scaffolds promoted more substantial new bone formation compared to empty defects | [48] |
| Collagen-based scaffolds with PRP | Rats/Calvarial defect | pBMP2 | Incorporation of PRP into gene-activated scaffolds resulted in a two-fold increase in newly formed bone volume compared to PRP-free scaffolds | [49] |
| Polylactide particles and PRP-based fibrin clot | Rats/Calvarial defect | pBMP2 | Gene-activated scaffolds incorporating PRP demonstrated enhanced osteogenic differentiation of MSCs and improved healing of critical-sized rat calvarial defects compared to PRP-free gene-activated carriers | [50] |
| PLGA-microparticles embedded in a fibrin gel surrounded by a collagen matrix | Rats/Ectopic osteogenesis | pBMP-2 | The gel-containing material stimulated ectopic bone formation compared to other experimental groups | [51] |
| Fibrin gel | Rats/Femoral defect | pBMP2/7 | The fibrin gel with pBMP2/7 combination showed a trend toward increased bone volume compared to fibrin-only implants | [46] |
| Biomaterial | Model/Defect | Osteoinductor | Results | Reference |
|---|---|---|---|---|
| 3D-porous titanium alloy implants and PRP | Rabbits/Femoral defect | ADSC | Both bone volume and mineral density were significantly higher in the group receiving titanium scaffolds combined with PRP and cells, compared to control groups containing either scaffolds alone or cell-seeded scaffolds. The introduction of PRP significantly increased tube formation, expression of angiogenic markers and CD31+ cells in the defect site | [52] |
| PRP | Rats/Calvarial defect | ADSC | The most pronounced bone tissue regeneration was observed following ADSCs/PRP implantation compared to ADSCs/col1, PRP-only, or type 1 collagen-only groups | [53] |
| Polycaprolactone-β tricalcium phosphate bio-scaffold and PRP | Pigs/Mandibular defect | MSC | Implantation of PCL-TCP + MSCs + PRP scaffolds resulted in significantly greater bone formation area and higher mineral density compared to unseeded scaffold controls | [54] |
| Allogenic bone graft and PRP | Rabbits/Femoral defect | BMSC | The combination of MSCs and PRP enhanced the expression of osteogenic markers compared to the cell-free control group | [55] |
| Autogenous bone particles and PRP | Rabbits/Radial diaphysis defect | BMSC | The newly formed bone fraction area was significantly larger in the group receiving autologous bone chips combined with PRP and cells compared to the group without PRP administration | [19] |
| Biomaterial | Model/Defect | Osteoinductor | Results | Reference |
|---|---|---|---|---|
| Nano-calcium sulfate and PRP | Rats/Calvarial defect | Ad-BMP2- MSC | The combination of BMP2-modified MSCs with nCS/PRP scaffolds resulted in increased volume and mineral density of regenerated bone compared to control groups lacking either PRP or BMP2-modified MSCs | [56] |
| PRP with alginate microspheres | in vitro | Ad-BMP2- MSC | The osteogenic differentiation efficiency was significantly higher in the MSC/BMP2 + PRP group compared to the non-PRP control group | [57] |
| Polylactide particles and PRP-based fibrin clot | Rats/Calvarial defect | Ad-BMP2- MSC | Scaffolds containing transduced MSCs demonstrated faster and more pronounced bone defect regeneration compared to empty defects | [48] |
| Osteomatrix and PRP | in vitro | Ad-BMP2- MSC | The introduction of platelet-rich plasma (PRP) into tissue engineering scaffolds improves cell distribution and viability, while augmenting BMP-2 protein secretion | [58] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meglei, A.Y.; Nedorubova, I.A.; Basina, V.P.; Chernomyrdina, V.O.; Goldshtein, D.V.; Bukharova, T.B. The Combined Potential of PRP and Osteoinductive Carrier Matrices for Bone Regeneration. Int. J. Mol. Sci. 2025, 26, 8457. https://doi.org/10.3390/ijms26178457
Meglei AY, Nedorubova IA, Basina VP, Chernomyrdina VO, Goldshtein DV, Bukharova TB. The Combined Potential of PRP and Osteoinductive Carrier Matrices for Bone Regeneration. International Journal of Molecular Sciences. 2025; 26(17):8457. https://doi.org/10.3390/ijms26178457
Chicago/Turabian StyleMeglei, Anastasiia Yurevna, Irina Alekseevna Nedorubova, Viktoriia Pavlovna Basina, Viktoria Olegovna Chernomyrdina, Dmitry Vadimovich Goldshtein, and Tatiana Borisovna Bukharova. 2025. "The Combined Potential of PRP and Osteoinductive Carrier Matrices for Bone Regeneration" International Journal of Molecular Sciences 26, no. 17: 8457. https://doi.org/10.3390/ijms26178457
APA StyleMeglei, A. Y., Nedorubova, I. A., Basina, V. P., Chernomyrdina, V. O., Goldshtein, D. V., & Bukharova, T. B. (2025). The Combined Potential of PRP and Osteoinductive Carrier Matrices for Bone Regeneration. International Journal of Molecular Sciences, 26(17), 8457. https://doi.org/10.3390/ijms26178457








