The Role of Vascularization in Nerve Regeneration: Mechanistic and Therapeutic Perspectives
Abstract
1. Introduction
2. Methods
2.1. Literature Search
2.2. Selection Criteria
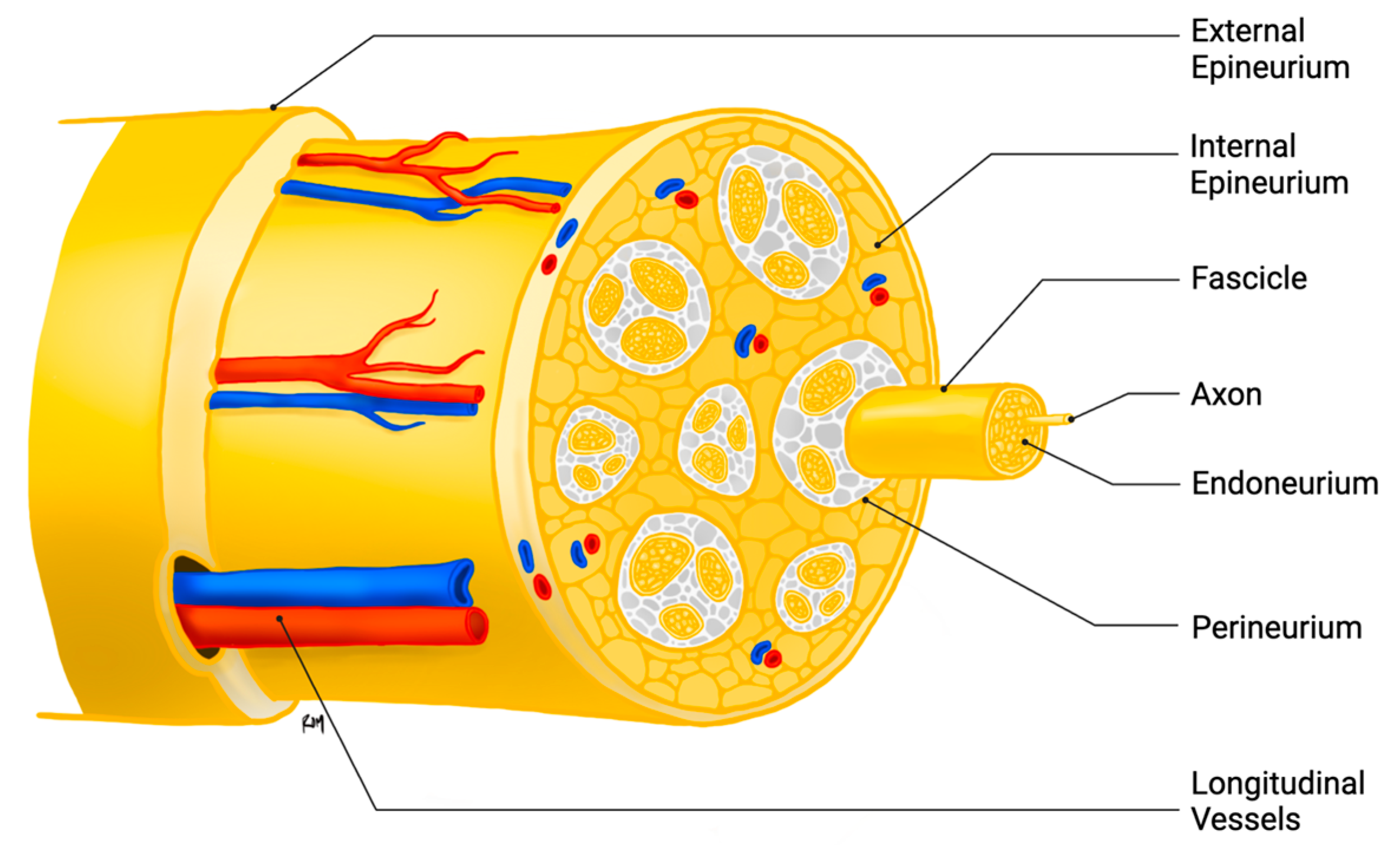
3. Peripheral Nerve Injury and Regeneration
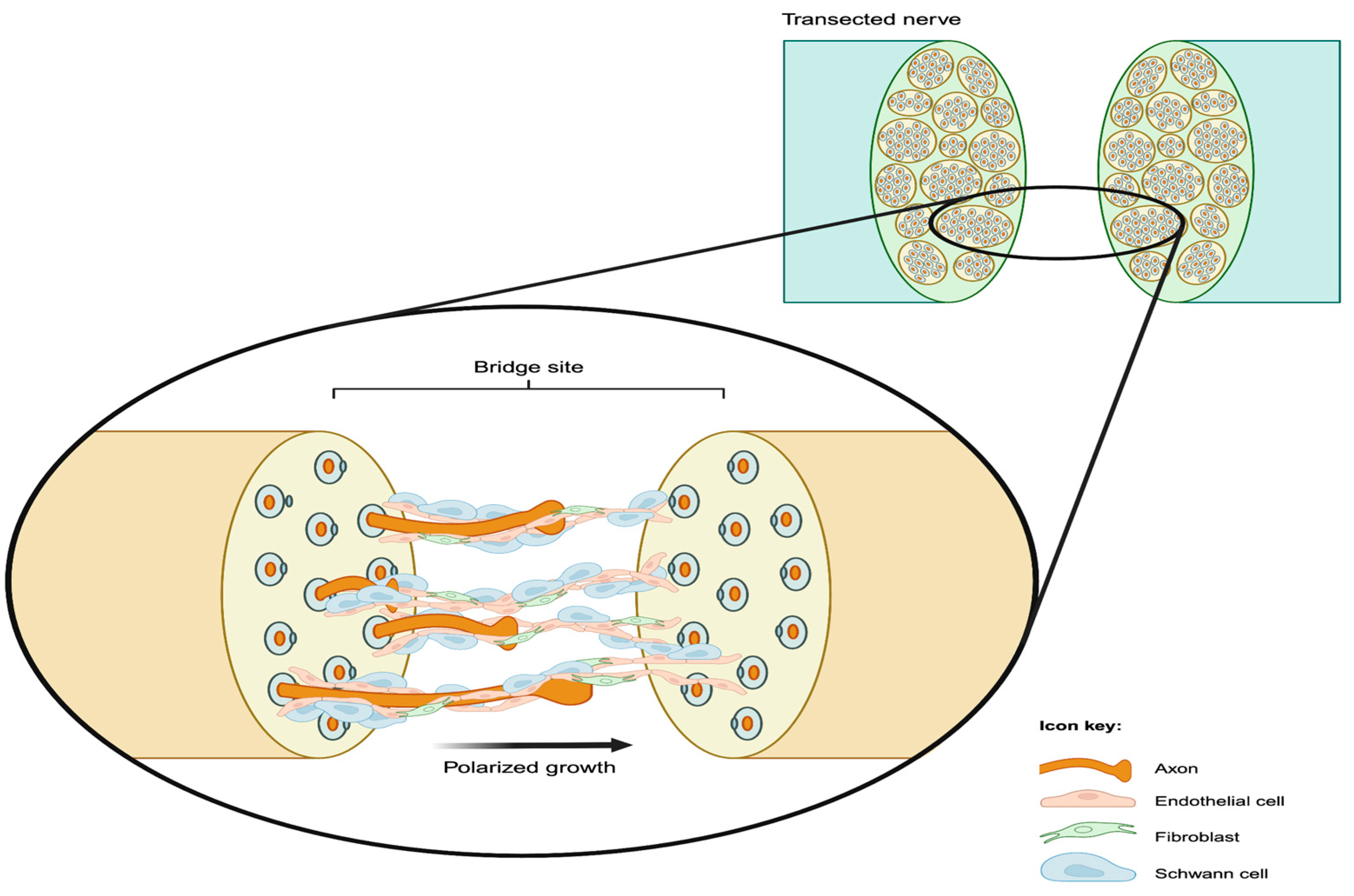
4. Role of Blood Vessels in Nerve Regeneration
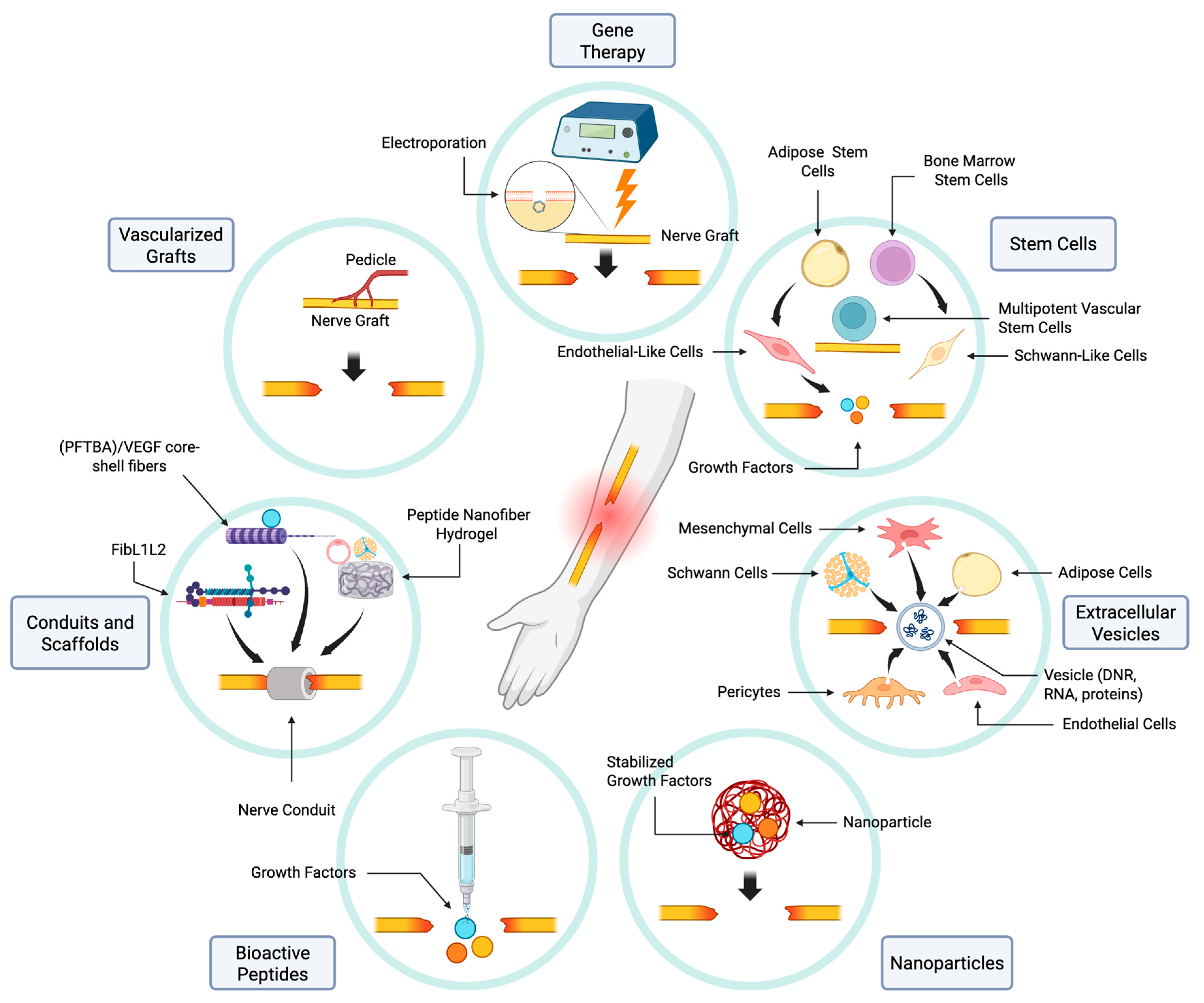
5. Therapeutic Approaches to PNI Repair
5.1. Nerve Graft Vascularization
5.2. Vascularizing Nerve Conduits and Scaffolds
5.3. Bioactive Peptides
5.4. The Role of Nanoparticles in Vascularization and Repair
5.5. Extracellular Vesicles
5.6. Stem Cells
5.7. Gene Therapy
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Magnéli, M.; Axenhus, M. Epidemiology and regional variance of traumatic peripheral nerve injuries in Sweden: A 15-year observational study. PLoS ONE 2024, 19, e0310988. [Google Scholar] [CrossRef]
- Murphy, R.N.A.; de Schoulepnikoff, C.; Chen, J.H.C.; Columb, M.O.; Bedford, J.; Wong, J.K.; Reid, A.J. The incidence and management of peripheral nerve injury in England (2005–2020). J. Plast. Reconstr. Aesthet. Surg. 2023, 80, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Tapp, M.; Wenzinger, E.; Tarabishy, S.; Ricci, J.; Herrera, F.A. The Epidemiology of Upper Extremity Nerve Injuries and Associated Cost in the US Emergency Departments. Ann. Plast. Surg. 2019, 83, 676–680. [Google Scholar] [CrossRef]
- Bailey, R.; Kaskutas, V.; Fox, I.; Baum, C.M.; Mackinnon, S.E. Effect of upper extremity nerve damage on activity participation, pain, depression, and quality of life. J. Hand Surg. Am. 2009, 34, 1682–1688. [Google Scholar] [CrossRef]
- van Hecke, O.; Austin, S.K.; Khan, R.A.; Smith, B.H.; Torrance, N. Neuropathic pain in the general population: A systematic review of epidemiological studies. Pain 2014, 155, 654–662. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.Y.; Gordon, T. Contributing factors to poor functional recovery after delayed nerve repair: Prolonged axotomy. J. Neurosci. 1995, 15 Pt 2, 3876–3885. [Google Scholar] [CrossRef]
- Gordon, T.; Tyreman, N.; Raji, M.A. The basis for diminished functional recovery after delayed peripheral nerve repair. J. Neurosci. 2011, 31, 5325–5334. [Google Scholar] [CrossRef]
- Varadarajan, S.G.; Hunyara, J.L.; Hamilton, N.R.; Kolodkin, A.L.; Huberman, A.D. Central nervous system regeneration. Cell 2022, 185, 77–94. [Google Scholar] [CrossRef] [PubMed]
- Acker-Palmer, A. Guiding axon regeneration: Instructions from blood vessels. Neuron 2024, 112, 175–177. [Google Scholar] [CrossRef]
- Contreras, E.; Bolívar, S.; Navarro, X.; Udina, E. New insights into peripheral nerve regeneration: The role of secretomes. Exp. Neurol. 2022, 354, 114069. [Google Scholar] [CrossRef]
- Bhat, G.P.; Maurizio, A.; Motta, A.; Podini, P.; Diprima, S.; Malpighi, C.; Brambilla, I.; Martins, L.; Badaloni, A.; Boselli, D.; et al. Structured wound angiogenesis instructs mesenchymal barrier compartments in the regenerating nerve. Neuron 2024, 112, 209–229.e211. [Google Scholar] [CrossRef]
- Zochodne, D.W. The challenges and beauty of peripheral nerve regrowth. J. Peripher. Nerv. Syst. 2012, 17, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Zochodne, D. Neurobiology of Peripheral Nerve Regeneration, 1st ed.; Cambridge University Press: Cambridge, UK, 2008. [Google Scholar]
- Cattin, A.L.; Burden, J.J.; Van Emmenis, L.; Mackenzie, F.E.; Hoving, J.J.; Garcia Calavia, N.; Guo, Y.; McLaughlin, M.; Rosenberg, L.H.; Quereda, V.; et al. Macrophage-Induced Blood Vessels Guide Schwann Cell-Mediated Regeneration of Peripheral Nerves. Cell 2015, 162, 1127–1139. [Google Scholar] [CrossRef]
- Sasaki, Y.; Nakagawa, T.; Mao, X.; DiAntonio, A.; Milbrandt, J. NMNAT1 inhibits axon degeneration via blockade of SARM1-mediated NAD. eLife 2016, 5, e19749. [Google Scholar] [CrossRef]
- George, E.B.; Glass, J.D.; Griffin, J.W. Axotomy-induced axonal degeneration is mediated by calcium influx through ion-specific channels. J. Neurosci. 1995, 15, 6445–6452. [Google Scholar] [CrossRef]
- Wu, G.; Wen, X.; Kuang, R.; Lui, K.W.; He, B.; Li, G.; Zhu, Z. Roles of Macrophages and Their Interactions with Schwann Cells After Peripheral Nerve Injury. Cell. Mol. Neurobiol. 2023, 44, 11. [Google Scholar] [CrossRef]
- Jurecka, W.; Ammerer, H.P.; Lassmann, H. Regeneration of a transected peripheral nerve. An autoradiographic and electron microscopic study. Acta Neuropathol. 1975, 32, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Y.; McDonald, D.; Cheng, C.; Magnowski, B.; Durand, J.; Zochodne, D.W. Axon and Schwann cell partnership during nerve regrowth. J. Neuropathol. Exp. Neurol. 2005, 64, 613–622. [Google Scholar] [CrossRef]
- Parrinello, S.; Napoli, I.; Ribeiro, S.; Wingfield Digby, P.; Fedorova, M.; Parkinson, D.B.; Doddrell, R.D.; Nakayama, M.; Adams, R.H.; Lloyd, A.C. EphB signaling directs peripheral nerve regeneration through Sox2-dependent Schwann cell sorting. Cell 2010, 143, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Cattin, A.L.; Lloyd, A.C. The multicellular complexity of peripheral nerve regeneration. Curr. Opin. Neurobiol. 2016, 39, 38–46. [Google Scholar] [CrossRef]
- Sorrell, J.M.; Caplan, A.I. Fibroblasts-a diverse population at the center of it all. Int. Rev. Cell Mol. Biol. 2009, 276, 161–214. [Google Scholar] [CrossRef]
- Lackmann, M.; Boyd, A.W. Eph, a protein family coming of age: More confusion, insight, or complexity? Sci. Signal. 2008, 1, re2. [Google Scholar] [CrossRef]
- Gerber, H.P.; Hillan, K.J.; Ryan, A.M.; Kowalski, J.; Keller, G.A.; Rangell, L.; Wright, B.D.; Radtke, F.; Aguet, M.; Ferrara, N. VEGF is required for growth and survival in neonatal mice. Development 1999, 126, 1149–1159. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef]
- Shibuya, M. Vascular endothelial growth factor and its receptor system: Physiological functions in angiogenesis and pathological roles in various diseases. J. Biochem. 2013, 153, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, H.; Shibuya, M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin. Sci. 2005, 109, 227–241. [Google Scholar] [CrossRef] [PubMed]
- Sondell, M.; Lundborg, G.; Kanje, M. Vascular endothelial growth factor has neurotrophic activity and stimulates axonal outgrowth, enhancing cell survival and Schwann cell proliferation in the peripheral nervous system. J. Neurosci. 1999, 19, 5731–5740. [Google Scholar] [CrossRef]
- Ferrara, N.; Kerbel, R.S. Angiogenesis as a therapeutic target. Nature 2005, 438, 967–974. [Google Scholar] [CrossRef]
- Jiang, M.; Chen, M.; Liu, N. Interactions between Schwann cell and extracellular matrix in peripheral nerve regeneration. Front. Neurol. 2024, 15, 1372168. [Google Scholar] [CrossRef]
- Chen, Z.L.; Strickland, S. Laminin gamma1 is critical for Schwann cell differentiation, axon myelination, and regeneration in the peripheral nerve. J. Cell Biol. 2003, 163, 889–899. [Google Scholar] [CrossRef]
- Broeren, B.O.; Hundepool, C.A.; Kumas, A.H.; Duraku, L.S.; Walbeehm, E.T.; Hooijmans, C.R.; Power, D.M.; Zuidam, J.M.; De Jong, T. The effectiveness of acellular nerve allografts compared to autografts in animal models: A systematic review and meta-analysis. PLoS ONE 2024, 19, e0279324. [Google Scholar] [CrossRef]
- Seckel, B.R.; Ryan, S.E.; Simons, J.E.; Gagne, R.G.; Watkins, E. Vascularized versus nonvascularized nerve grafts: An experimental structural comparison. Plast. Reconstr. Surg. 1986, 78, 211–220. [Google Scholar] [CrossRef]
- Bertelli, J.A.; Taleb, M.; Mira, J.C.; Calixto, J.B. Muscle fiber type reorganization and behavioral functional recovery of rat median nerve repair with vascularized or conventional nerve grafts. Restor. Neurol. Neurosci. 1996, 10, 5–12. [Google Scholar] [CrossRef]
- Donzelli, R.; Capone, C.; Sgulò, F.G.; Mariniello, G.; Maiuri, F. Vascularized nerve grafts: An experimental study. Neurol. Res. 2016, 38, 669–677. [Google Scholar] [CrossRef] [PubMed]
- Hems, T.E.; Glasby, M.A. Comparison of different methods of repair of long peripheral nerve defects: An experimental study. Br. J. Plast. Surg. 1992, 45, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Matsumine, H.; Sasaki, R.; Takeuchi, Y.; Miyata, M.; Yamato, M.; Okano, T.; Sakurai, H. Vascularized versus nonvascularized island median nerve grafts in the facial nerve regeneration and functional recovery of rats for facial nerve reconstruction study. J. Reconstr. Microsurg. 2014, 30, 127–136. [Google Scholar] [CrossRef]
- Ozcan, G.; Shenaq, S.; Mirabi, B.; Spira, M. Nerve regeneration in a bony bed: Vascularized versus nonvascularized nerve grafts. Plast. Reconstr. Surg. 1993, 91, 1322–1331. [Google Scholar] [CrossRef] [PubMed]
- Tark, K.C.; Roh, T.S. Morphometric study of regeneration through vascularized nerve graft in a rabbit sciatic nerve model. J. Reconstr. Microsurg. 2001, 17, 109–114. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, S.; Zhou, S.; Yu, Z.; Tian, Z.; Zhang, C.; Yang, W. Vascularized versus nonvascularized facial nerve grafts using a new rabbit model. Plast. Reconstr. Surg. 2015, 135, 331e–339e. [Google Scholar] [CrossRef] [PubMed]
- Kanaya, F.; Firrell, J.; Tsai, T.M.; Breidenbach, W.C. Functional results of vascularized versus nonvascularized nerve grafting. Plast. Reconstr. Surg. 1992, 89, 924–930. [Google Scholar] [CrossRef]
- Broeren, B.O.; Duraku, L.S.; Hundepool, C.A.; Walbeehm, E.T.; Zuidam, J.M.; Hooijmans, C.R.; De Jong, T. Nerve recovery from treatment with a vascularized nerve graft compared to an autologous non-vascularized nerve graft in animal models: A systematic review and meta-analysis. PLoS ONE 2021, 16, e0252250. [Google Scholar] [CrossRef] [PubMed]
- Terzis, J.K.; Kostopoulos, V.K. Vascularized nerve grafts for lower extremity nerve reconstruction. Ann. Plast. Surg. 2010, 64, 169–176. [Google Scholar] [CrossRef]
- Xu, W.D.; Xu, J.G.; Gu, Y.D. Comparative clinic study on vascularized and nonvascularized full-length phrenic nerve transfer. Microsurgery 2005, 25, 16–20. [Google Scholar] [CrossRef]
- Falco, N.A.; Pribaz, J.J.; Eriksson, E. Vascularization of skin following implantation of an arteriovenous pedicle: Implications in flap prefabrication. Microsurgery 1992, 13, 249–254. [Google Scholar] [CrossRef]
- Ozcan, G.; Shenaq, S.; Spira, M. Vascularized nerve tube: An experimental alternative for vascularized nerve grafts over short gaps. J. Reconstr. Microsurg. 1993, 9, 405–413. [Google Scholar] [CrossRef]
- Lee, J.I.; Park, J.H.; Kim, Y.R.; Gwon, K.; Hwang, H.W.; Jung, G.; Lee, J.Y.; Sun, J.Y.; Park, J.W.; Shin, J.H.; et al. Delivery of nitric oxide-releasing silica nanoparticles for. Neural Regen. Res. 2022, 17, 2043–2049. [Google Scholar] [CrossRef]
- Rovak, J.M.; Mungara, A.K.; Aydin, M.A.; Cederna, P.S. Effects of vascular endothelial growth factor on nerve regeneration in acellular nerve grafts. J. Reconstr. Microsurg. 2004, 20, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Fansa, H.; Schneider, W.; Keilhoff, G. Revascularization of tissue-engineered nerve grafts and invasion of macrophages. Tissue Eng. 2001, 7, 519–524. [Google Scholar] [CrossRef] [PubMed]
- Fornasari, B.E.; Zen, F.; Nato, G.; Fogli, M.; Luzzati, F.; Ronchi, G.; Raimondo, S.; Gambarotta, G. Blood Vessels: The Pathway Used by Schwann Cells to Colonize Nerve Conduits. Int. J. Mol. Sci. 2022, 23, 2254. [Google Scholar] [CrossRef]
- Saffari, T.M.; Mathot, F.; Friedrich, P.F.; Bishop, A.T.; Shin, A.Y. Revascularization patterns of nerve allografts in a rat sciatic nerve defect model. J. Plast. Reconstr. Aesthet. Surg. 2020, 73, 460–468. [Google Scholar] [CrossRef]
- Bedar, M.; Saffari, T.M.; Johnson, A.J.; Shin, A.Y. The effect of mesenchymal stem cells and surgical angiogenesis on immune response and revascularization of acellular nerve allografts in a rat sciatic defect model. J. Plast. Reconstr. Aesthet. Surg. 2022, 75, 2809–2820. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, M. Enhancement of rat peripheral nerve regeneration through artery-including silicone tubing. Exp. Neurol. 1990, 107, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Yoo, J.J.; Atala, A. Peripheral nerve regeneration using acellular nerve grafts. J. Biomed. Mater. Res. A 2004, 68, 201–209. [Google Scholar] [CrossRef]
- Kakinoki, R.; Nishijima, N.; Ueba, Y.; Oka, M.; Yamamuro, T.; Nakamura, T. Nerve regeneration over a 20-mm gap through a nerve conduit containing blood vessels in rats: The influence of interstump distance on nerve regeneration. J. Neurosurg. Sci. 1998, 42, 11–21. [Google Scholar]
- Hibbitts, A.J.; Kočí, Z.; Kneafsey, S.; Matsiko, A.; Žilić, L.; Dervan, A.; Hinton, P.; Chen, G.; Cavanagh, B.; Dowling, J.K.; et al. Multi-factorial nerve guidance conduit engineering improves outcomes in inflammation, angiogenesis and large defect nerve repair. Matrix Biol. 2022, 106, 34–57. [Google Scholar] [CrossRef]
- Ma, T.; Hao, Y.; Li, S.; Xia, B.; Gao, X.; Zheng, Y.; Mei, L.; Wei, Y.; Yang, C.; Lu, L.; et al. Sequential oxygen supply system promotes peripheral nerve regeneration by enhancing Schwann cells survival and angiogenesis. Biomaterials 2022, 289, 121755. [Google Scholar] [CrossRef]
- Donovan, M.J.; Lin, M.I.; Wiegn, P.; Ringstedt, T.; Kraemer, R.; Hahn, R.; Wang, S.; Ibañez, C.F.; Rafii, S.; Hempstead, B.L. Brain derived neurotrophic factor is an endothelial cell survival factor required for intramyocardial vessel stabilization. Development 2000, 127, 4531–4540. [Google Scholar] [CrossRef]
- Gao, H.; You, Y.; Zhang, G.; Zhao, F.; Sha, Z.; Shen, Y. The use of fiber-reinforced scaffolds cocultured with Schwann cells and vascular endothelial cells to repair rabbit sciatic nerve defect with vascularization. Biomed. Res. Int. 2013, 2013, 362918. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Meena, P.; Kakkar, A.; Kumar, M.; Khatri, N.; Nagar, R.K.; Singh, A.; Malhotra, P.; Shukla, M.; Saraswat, S.K.; Srivastava, S.; et al. Advances and clinical challenges for translating nerve conduit technology from bench to bed side for peripheral nerve repair. Cell Tissue Res. 2021, 383, 617–644. [Google Scholar] [CrossRef]
- Akbarian, M.; Khani, A.; Eghbalpour, S.; Uversky, V.N. Bioactive Peptides: Synthesis, Sources, Applications, and Proposed Mechanisms of Action. Int. J. Mol. Sci. 2022, 23, 1445. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Chu, Z.; Li, S.; Zheng, T.; Wei, S.; Zhao, Y.; Liu, P.; Lu, Q. BDNF-loaded chitosan-based mimetic mussel polymer conduits for repair of peripheral nerve injury. Front. Cell Dev. Biol. 2024, 12, 1431558. [Google Scholar] [CrossRef] [PubMed]
- Dixelius, J.; Jakobsson, L.; Genersch, E.; Bohman, S.; Ekblom, P.; Claesson-Welsh, L. Laminin-1 promotes angiogenesis in synergy with fibroblast growth factor by distinct regulation of the gene and protein expression profile in endothelial cells. J. Biol. Chem. 2004, 279, 23766–23772. [Google Scholar] [CrossRef] [PubMed]
- Woolley, A.L.; Hollowell, J.P.; Rich, K.M. First place—Resident Basic Science Award 1990. Fibronectin-laminin combination enhances peripheral nerve regeneration across long gaps. Otolaryngol. Head Neck Surg. 1990, 103, 509–518. [Google Scholar] [CrossRef] [PubMed]
- Fosgerau, K.; Hoffmann, T. Peptide therapeutics: Current status and future directions. Drug Discov. Today 2015, 20, 122–128. [Google Scholar] [CrossRef]
- Craik, D.J.; Fairlie, D.P.; Liras, S.; Price, D. The future of peptide-based drugs. Chem. Biol. Drug Des. 2013, 81, 136–147. [Google Scholar] [CrossRef]
- Giannaccini, M.; Calatayud, M.P.; Poggetti, A.; Corbianco, S.; Novelli, M.; Paoli, M.; Battistini, P.; Castagna, M.; Dente, L.; Parchi, P.; et al. Magnetic Nanoparticles for Efficient Delivery of Growth Factors: Stimulation of Peripheral Nerve Regeneration. Adv. Healthc. Mater. 2017, 6, 1601429. [Google Scholar] [CrossRef]
- Rezaie, J.; Ajezi, S.; Avci, Ç.; Karimipour, M.; Geranmayeh, M.H.; Nourazarian, A.; Sokullu, E.; Rezabakhsh, A.; Rahbarghazi, R. Exosomes and their Application in Biomedical Field: Difficulties and Advantages. Mol. Neurobiol. 2018, 55, 3372–3393. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, L.; Zhou, D.; Li, L.; Zhang, W.; Zhen, Y.; Wang, T.; Su, J.; Chen, D.; Mao, C.; et al. Extracellular vesicles from human umbilical cord mesenchymal stem cells improve nerve regeneration after sciatic nerve transection in rats. J. Cell. Mol. Med. 2019, 23, 2822–2835. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Ren, S.; Duscher, D.; Kang, Y.; Liu, Y.; Wang, C.; Yuan, M.; Guo, G.; Xiong, H.; Zhan, P.; et al. Exosomes from human adipose-derived stem cells promote sciatic nerve regeneration via optimizing Schwann cell function. J. Cell. Physiol. 2019, 234, 23097–23110. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, G.; Li, S.; Li, J.; Wang, W.; Xue, J.; Wang, Y.; Fang, M.; Zhou, N. Endothelial cell-derived exosomes boost and maintain repair-related phenotypes of Schwann cells via miR199-5p to promote nerve regeneration. J. Nanobiotechnol. 2023, 21, 10. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Li, J.; Li, S.; Yang, X.; Huo, N.; Chen, Q.; Wang, W.; Yang, N.; Wang, Y.; Zhou, N. Netrin-1-engineered endothelial cell exosomes induce the formation of pre-regenerative niche to accelerate peripheral nerve repair. Sci. Adv. 2024, 10, eadm8454. [Google Scholar] [CrossRef]
- Yin, G.N.; Shin, T.Y.; Ock, J.; Choi, M.J.; Limanjaya, A.; Kwon, M.H.; Liu, F.Y.; Hong, S.S.; Kang, J.H.; Gho, Y.S.; et al. Pericyte-derived extracellular vesicles-mimetic nanovesicles improves peripheral nerve regeneration in mouse models of sciatic nerve transection. Int. J. Mol. Med. 2022, 49, 18. [Google Scholar] [CrossRef]
- Sun, J.; Zeng, Q.; Wu, Z.; Li, Z.; Gao, Q.; Liao, Z.; Li, H.; Ling, C.; Chen, C.; Wang, H.; et al. Enhancing intraneural revascularization following peripheral nerve injury through hypoxic Schwann-cell-derived exosomes: An insight into endothelial glycolysis. J. Nanobiotechnol. 2024, 22, 283. [Google Scholar] [CrossRef]
- Namini, M.S.; Daneshimehr, F.; Beheshtizadeh, N.; Mansouri, V.; Ai, J.; Jahromi, H.K.; Ebrahimi-Barough, S. Cell-free therapy based on extracellular vesicles: A promising therapeutic strategy for peripheral nerve injury. Stem Cell Res. Ther. 2023, 14, 254. [Google Scholar] [CrossRef]
- Kumar, M.A.; Baba, S.K.; Sadida, H.Q.; Marzooqi, S.A.; Jerobin, J.; Altemani, F.H.; Algehainy, N.; Alanazi, M.A.; Abou-Samra, A.B.; Kumar, R.; et al. Extracellular vesicles as tools and targets in therapy for diseases. Signal Transduct. Target. Ther. 2024, 9, 27. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Jones, S.; Jia, X. Stem Cell Transplantation for Peripheral Nerve Regeneration: Current Options and Opportunities. Int. J. Mol. Sci. 2017, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- Hong, S.J.; Traktuev, D.O.; March, K.L. Therapeutic potential of adipose-derived stem cells in vascular growth and tissue repair. Curr. Opin. Organ. Transplant. 2010, 15, 86–91. [Google Scholar] [CrossRef]
- Rhode, S.C.; Beier, J.P.; Ruhl, T. Adipose tissue stem cells in peripheral nerve regeneration-In vitro and in vivo. J. Neurosci. Res. 2021, 99, 545–560. [Google Scholar] [CrossRef] [PubMed]
- Mathot, F.; Rbia, N.; Bishop, A.T.; Hovius, S.E.R.; Shin, A.Y. Adipose derived mesenchymal stem cells seeded onto a decellularized nerve allograft enhances angiogenesis in a rat sciatic nerve defect model. Microsurgery 2020, 40, 585–592. [Google Scholar] [CrossRef]
- Nakada, M.; Itoh, S.; Tada, K.; Matsuta, M.; Murai, A.; Tsuchiya, H. Effects of hybridization of decellularized allogenic nerves with adipose-derive stem cell sheets to facilitate nerve regeneration. Brain Res. 2020, 1746, 147025. [Google Scholar] [CrossRef] [PubMed]
- Yamakawa, T.; Kakinoki, R.; Ikeguchi, R.; Nakayama, K.; Morimoto, Y.; Nakamura, T. Nerve regeneration promoted in a tube with vascularity containing bone marrow-derived cells. Cell Transplant. 2007, 16, 811–822. [Google Scholar] [CrossRef]
- Kaizawa, Y.; Kakinoki, R.; Ikeguchi, R.; Ohta, S.; Noguchi, T.; Takeuchi, H.; Oda, H.; Yurie, H.; Matsuda, S. A Nerve Conduit Containing a Vascular Bundle and Implanted With Bone Marrow Stromal Cells and Decellularized Allogenic Nerve Matrix. Cell Transplant. 2017, 26, 215–228. [Google Scholar] [CrossRef] [PubMed]
- Han, J.W.; Choi, D.; Lee, M.Y.; Huh, Y.H.; Yoon, Y.S. Bone Marrow-Derived Mesenchymal Stem Cells Improve Diabetic Neuropathy by Direct Modulation of Both Angiogenesis and Myelination in Peripheral Nerves. Cell Transplant. 2016, 25, 313–326. [Google Scholar] [CrossRef]
- Huang, C.W.; Hsueh, Y.Y.; Huang, W.C.; Patel, S.; Li, S. Multipotent vascular stem cells contribute to neurovascular regeneration of peripheral nerve. Stem Cell Res. Ther. 2019, 10, 234. [Google Scholar] [CrossRef]
- Grimoldi, N.; Colleoni, F.; Tiberio, F.; Vetrano, I.G.; Cappellari, A.; Costa, A.; Belicchi, M.; Razini, P.; Giordano, R.; Spagnoli, D.; et al. Stem cell salvage of injured peripheral nerve. Cell Transplant. 2015, 24, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Maldonado, A.A.; Lee, E.Y.; Shin, A.Y.; Spinner, R.J. Stem cell therapy for peripheral nerve injury: Illustrative cases. J. Neurosurg. Case Lessons 2025, 9, CASE24878. [Google Scholar]
- Shimizu, Y.; Ntege, E.H.; Takahara, E.; Matsuura, N.; Matsuura, R.; Kamizato, K.; Inoue, Y.; Sowa, Y.; Sunami, H. Adipose-derived stem cell therapy for spinal cord injuries: Advances, challenges, and future directions. Regen. Ther. 2024, 26, 508–519. [Google Scholar] [CrossRef]
- Holzbach, T.; Miliojcic, R.; Matiasek, K.; Konerding, M.A.; Anton, M.; Feuchtinger, A.; Schlegel, J.; Volkmer, E.; Krug, C.; Giunta, R.E. Vascular Endothelial Growth Factor Gene Therapy Promotes Nerve Regeneration in a Sciatic Nerve Graft Model in Rats. Surg. Res. Updates 2014, 1, 29–38. [Google Scholar] [CrossRef]
- Ansah, E.O. Ethical Challenges and Controversies in the Practice and Advancement of Gene. Adv. Cell Gene Ther. 2022, 2022, 1015996. [Google Scholar] [CrossRef]
- Pereira Lopes, F.R.; Martin, P.K.; Frattini, F.; Biancalana, A.; Almeida, F.M.; Tomaz, M.A.; Melo, P.A.; Borojevic, R.; Han, S.W.; Martinez, A.M. Double gene therapy with granulocyte colony-stimulating factor and vascular endothelial growth factor acts synergistically to improve nerve regeneration and functional outcome after sciatic nerve injury in mice. Neuroscience 2013, 230, 184–197. [Google Scholar] [CrossRef]
- Pereira Lopes, F.R.; Lisboa, B.C.; Frattini, F.; Almeida, F.M.; Tomaz, M.A.; Matsumoto, P.K.; Langone, F.; Lora, S.; Melo, P.A.; Borojevic, R.; et al. Enhancement of sciatic nerve regeneration after vascular endothelial growth factor (VEGF) gene therapy. Neuropathol. Appl. Neurobiol. 2011, 37, 600–612. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malekzadeh, H.; Otto-Moudry, R.; Moore, A.M. The Role of Vascularization in Nerve Regeneration: Mechanistic and Therapeutic Perspectives. Int. J. Mol. Sci. 2025, 26, 8395. https://doi.org/10.3390/ijms26178395
Malekzadeh H, Otto-Moudry R, Moore AM. The Role of Vascularization in Nerve Regeneration: Mechanistic and Therapeutic Perspectives. International Journal of Molecular Sciences. 2025; 26(17):8395. https://doi.org/10.3390/ijms26178395
Chicago/Turabian StyleMalekzadeh, Hamid, Reade Otto-Moudry, and Amy M. Moore. 2025. "The Role of Vascularization in Nerve Regeneration: Mechanistic and Therapeutic Perspectives" International Journal of Molecular Sciences 26, no. 17: 8395. https://doi.org/10.3390/ijms26178395
APA StyleMalekzadeh, H., Otto-Moudry, R., & Moore, A. M. (2025). The Role of Vascularization in Nerve Regeneration: Mechanistic and Therapeutic Perspectives. International Journal of Molecular Sciences, 26(17), 8395. https://doi.org/10.3390/ijms26178395





